Abstract
Anxiety is a risk factor for many adverse neuropsychiatric and socioeconomic outcomes, and has been linked to functional and structural changes in the ventromedial prefrontal cortex (VMPFC). However, the nature of these differences, as well as how they develop in children and adolescents, remains poorly understood. More effective interventions to minimize the negative consequences of anxiety require better understanding of its neurobiology in children. Recent research suggests that structural imaging studies may benefit from clearly delineating between cortical surface area and thickness when examining these associations, as these distinct cortical phenotypes are influenced by different cellular mechanisms and genetic factors. The present study examined relationships between cortical surface area and thickness of the VMPFC and a self-report measure of anxiety (SCARED-R) in 287 youths aged 7–20 years from the Pediatric Imaging, Neurocognition, and Genetics (PING) study. Age and gender interactions were examined for significant associations in order to test for developmental differences. Cortical surface area and thickness were also examined simultaneously to determine whether they contribute independently to the prediction of anxiety. Anxiety was negatively associated with relative cortical surface area of the VMPFC as well as with global cortical thickness, but these associations diminished with age. The two cortical phenotypes contributed additively to the prediction of anxiety. These findings suggest that higher anxiety in children may be characterized by both delayed expansion of the VMPFC and an altered trajectory of global cortical thinning. Further longitudinal studies will be needed to confirm these findings.
Keywords: Anxiety, Brain development, Cortical surface area, Cortical thickness, Magnetic resonance imaging, Ventromedial prefrontal cortex
Introduction
Individuals differ widely in their tendency to experience anxiety. These individual differences can be detected from a very early age, and they are believed to be relatively stable over time (Kagan and Snidman 1991). Some individuals experience fear, worry, and anxiety at relatively higher intensities, or in response to a wider range of stimuli, than others. This anxious phenotype can be detected on various measures of personality and trait anxiety in adults (Spielberger et al. 1983; Costa and McCrae 1992) as well as on measures of temperament and anxiety in children (Spielberger 1973; Rothbart et al. 2001; Vreeke et al. 2012), and it exhibits moderate heritability (Stein et al. 1999). Females typically report higher levels of anxiety than males (Armstrong and Khawaja 2002). In children and adolescents, this phenotype has been associated with higher risk for a number of different anxiety disorders (Biederman et al. 1993), which in turn are linked to depression, drug use, and academic underachievement (Woodward and Fergusson 2001). In adults, trait anxiety has been linked to increased incidence of depression (Aben et al. 2002), stroke (McCarron et al. 2003), and myocardial infarction (Bonaguidi et al. 1994).
In recent years, the neural circuitry relevant to anxious phenotypes has been the subject of active investigation. There is now an extensive literature linking anxiety and fear extinction to the ventromedial prefrontal cortex (VMPFC) in both animals (Milad and Rauch 2007; Shin and Liberzon 2010) and adult humans (Kent and Rauch 2003; Deckersbach et al. 2006; Sehlmeyer and Dannlowski 2011). A prominent model of the neural circuitry of anxiety is that the VMPFC exerts top-down inhibitory influences that suppress amygdala activity resulting from alarming environmental cues (Milad and Rauch 2007). It is widely believed that the VMPFC is hypoactive (Indovina et al. 2011) in anxious individuals. Structural studies have shown that the retention of fear extinction is associated with thicker VMPFC (Milad et al. 2005; Hartley et al. 2011). However, others suggest a more complex role for the VMPFC, pointing to evidence that it appears to be linked to inhibition of anxiety in some circumstances and enhancement in others (Myers-Schulz and Koenigs 2012). Similarly, in two studies of the structural correlates of anxiety, cortical thickness in the orbitofrontal cortex correlated positively with anxiety in one (Blackmon et al. 2011) and negatively in the other (Kühn et al. 2011).
Little is known about how the neural circuitry of anxiety develops throughout childhood and adolescence. Brain regions implicated in anxiety in children and adolescents have generally been the same as those in adults (Strawn et al. 2012). Failure to habituate to fear stimuli has been associated with decreased functional connectivity between the VMPFC and the amygdala in adolescence (Hare et al. 2008). However, as in adults, results of structural studies have been inconsistent. For example, in one study, preadolescents with anxiety disorders had marginally reduced volumes in the ventrolateral (but not ventromedial) prefrontal cortex (Milham et al. 2005), while in another no prefrontal differences were found (De Bellis et al. 2002). Both human and animal studies have shown that specific neurobiological correlates of fear extinction may be age-dependent, suggesting that age could be an important moderating factor accounting for some of the inconsistencies in the developmental structural imaging literature (Guyer et al. 2008; Kim et al. 2009; Kim and Richardson 2010). To date, only one study (Ducharme et al. 2014) has examined the relationship between anxiety and the structure of the VMPFC as a function of age. This cohort-sequential study examined the relationship between anxious/depressed scores on the Child Behavior Checklist (a parent questionnaire), and cortical thickness in the VMPFC as a function of age in typically developing children. A negative association was observed between anxious/depressed scores and thickness in the right VMPFC in younger children but a positive association was observed in adolescents, with a slower rate of cortical thinning in children with higher anxious/depressed scores. These results suggest that age provides crucial context for understanding the relationship between frontolimbic anatomy and the behavioral phenomenon of anxiety, and they provide initial evidence that individuals who experience higher levels of anxiety may also display a different pattern of frontolimbic development. Recent initiatives such as the Research Domain Criteria (RDoC) Framework (Insel et al. 2010) have highlighted the importance of this type of neurodevelopmental work focusing on dimensional constructs in typically developing youth for our understanding of the neurobiological bases of psychopathology (Casey et al. 2014). However, no studies have distinguished between the effects of cortical surface area and thickness on anxiety, or examined both relationships as a function of age. This is likely to be an important distinction, as the developmental trajectories for these two cortical phenotypes exhibit striking differences (Brown et al. 2012), and previous research suggests little overlap between the genetic factors that influence variability in cortical surface area and cortical thickness (Panizzon et al. 2009). Therefore, a fuller understanding of the relationship between anxiety and the developing ventromedial prefrontal cortex requires an examination of both forms of cortical development in the same cohort.
A recent initiative called Pediatric Imaging, Neurocognition, and Genetics (PING) created a pediatric imaging-genomics database consisting of demographic, biographical, neuropsychological, social/emotional, neuroimaging, and genomic information in typically developing youths aged 3–20 at nine sites across the United States (see Brown et al. (2012) and Akshoomoff et al. (2014) for more detailed descriptions of PING). The PING initiative provides an excellent opportunity to advance the goals of RDoC by examining relationships between a continuous measure of generalized anxiety and the two forms of cortical development described above in typically developing children and adolescents. Given the relatively limited and inconsistent structural findings outlined above, our primary hypothesis was that high self-reported generalized anxiety would be associated with both decreased relative surface area and thickness of the VMPFC. We further hypothesized that these alterations would contribute independently to predictions of generalized anxiety. Finally, we sought to determine the extent to which these associations were moderated by age or gender.
Materials and methods
Participants
Participants for the current study were a subset of the larger PING study of typically developing youth. Children, adolescents, and young adults were included in PING if they were between the ages of 3 and 20, and fluent in English. Exclusion criteria included (a) neurological disorders; (b) history of head trauma; (c) preterm birth (less than 36 weeks); (d) diagnosis of an autism spectrum disorder, bipolar disorder, schizophrenia, or mental retardation; (e) pregnancy; and (f) daily illicit drug use by the mother for more than one trimester. More common forms of psychopathology such as anxiety, depression, and attention-deficit/hyperactivity disorder were not excluded from this sample because the recruitment strategy was designed to be representative of the general population. The overall PING sample consists of 1493 participants (780 males) who were recruited by nine sites across the United States, and acceptable imaging data were acquired for 1239 participants (645 males). Written parental informed consent was obtained for all PING subjects below the age of 18, and child assent was also obtained for all children between the ages of 7 and 17. Written informed consent was obtained directly from all participants aged 18 years or older. The study was approved by the IRBs at each of the participating sites. For more information about the PING cohort, see Brown et al. (2012) and Akshoomoff et al. (2014).
The ongoing PING study was included in the PhenX RISING project (McCarty et al. 2014), an initiative aimed at sharing and implementing measures from the PhenX (consensus measures of phenotypes and exposures) Toolkit (http://www.phenxtoolkit.org). As part of this initiative, PING participants above the age of 8 were asked to complete a set of web-based self-report measures, including the generalized anxiety measure used in the current study, using a secure data collection tool called Assessment Center (http://www.assessmentcenter.net). PING participants who completed this measure and whose MRI scan yielded acceptable imaging data were included in the current study. For more details on the role of PING in the PhenX RISING project, see McCarty et al. (2014).
Anxiety measure
The anxiety measure used in the current study is the Generalized Anxiety Disorder (GAD) domain score on the revised version of the Screen for Child Anxiety Related Emotional Disorders (SCARED-R; Muris et al. 1998). The SCARED-R is a self-report rating scale containing 66 items, 9 of which load on the GAD domain scale. This subscale was specifically chosen because, of the measures available in the PING data set, it was the most sensitive to the subjective experience of anxious worry that we expected to correlate with frontolimbic structure. Examples of items on this scale include “I am nervous”, “I worry about being as good as other kids”, and “I worry about things not working out for me”. Participants were asked to rate how frequently they experience each item on a 3-point scale, using the response options, “almost never”, “sometimes”, and “often”. These response options correspond to value ratings of 0, 1, and 2, respectively. Domain scores were computed by summing item response scores within each domain. The range of possible scores on this subscale is 0–18. One participant left one item blank from this subscale, and this value was imputed using the mean of all other items on the scale.
Studies using the GAD scale on the SCARED-R have demonstrated good reliability and validity, including high estimates of internal consistency (Cronbach’s alpha ranging from 0.77 to 0.80), test–retest reliability (0.68), and concurrent validity (0.75; Muris et al. 1999). A recent study found that children with generalized anxiety disorder had a mean score of 7.8 (SD = 4.5) while controls had a mean score of 3.7 (SD = 3.2), and that a score of 8 represented the best cut-off by which to discriminate the clinical sample form controls (Bodden et al. 2009).
Imaging measures
Participants completed a 1-h imaging session as part of the PING protocol that included acquisition of T1, T2, and diffusion-weighted images. Details of the image acquisition and processing protocols are provided in Brown et al. (2012) and on the PING portal (http://pingstudy.ucsd.edu). Briefly, all data included in the current study underwent detailed evaluation to assess the quality and accuracy of the brain measures made across the ages studied here. Standardized quality control procedures were followed for both raw data and data at various processing stages. This included visual inspection ratings by trained imaging technicians and automated quality control algorithms, both testing general image characteristics as well as aspects specific to each imaging modality, such as contrast properties, registrations, and artifacts from motion and other sources. Morphometric analysis of structural MRI data was performed using a specialized processing stream developed for PING that is based upon FreeSurfer, with additional corrections and analyses developed at UCSD Multimodal Imaging Laboratory (see Brown et al. (2012) and http://pingstudy.ucsd.edu for further details).
The VMPFC ROI was defined with a novel genetically informed cortical parcellation scheme developed by Chen et al. (2012) in a study of 406 monozygotic and dizygotic twins. These authors applied conventional twin methodology to compute the vertex-to-vertex genetic correlations among measures of cortical surface expansion at 2500 vertices. This large matrix of genetic correlations was then further analyzed using a data-driven, fuzzy clustering method to identify 12 sets (or clusters) of vertices with relatively higher genetic inter-correlations and relatively lower genetic correlations with other sets (or clusters). These clusters represent regions of the cortex where the twin data suggest that individual differences in relative surface area expansion are related to distinct sets of underlying genetic factors. The method yields a novel set of cortical parcels, one driven by analysis and simplification of the genetic architecture. The 12-cluster solution, chosen on the basis of silhouette coefficients, produced parcels that are largely bilaterally symmetric, each corresponding closely to meaningful structural and functional brain regions. For the current study, partial membership-weighted averages of voxel expansion factors were computed for the cluster labeled 4 by Chen et al. (see Figure S1 in the Online Resource), a cluster that is defined here as the VMPFC.
Genetic ancestry factors (GAFs)
A set of genetic ancestry measures was provided by the PING genomics core based on a subset of the variants obtained from the genotyping analyses; see Akshoomoff et al. (2014) for a more detailed discussion of the genetic ancestry factors. Briefly, values for each of the six GAFs ranged from 0 to 1.0 and each estimated the degree of genetic similarity of the participant to one of 6 reference populations, and thus, 6 GAFs (summing to 1.0) were computed for each participant.
Statistical analyses
The two primary hypotheses were that (1) relatively decreased VMPFC surface area and (2) decreased VMPFC thickness would be associated with higher self-reported generalized anxiety. These hypotheses were tested by estimating the effects of each of the VMPFC ROI measures in separate models predicting the GAD scale scores and including age, gender, the ROI measures and the interactions of these variables as predictors in the models. Additional covariates of no interest were also included. Because PING data collection took place at a number of different sites across the USA, scanner ID was entered as a covariate in all models to correct for scanner effects. The set of genetic ancestry factors (GAFs) was also included to control for race and ethnic factors related to genetic ancestry. The model examining effects of VMPFC surface area also included total cortical surface area as a covariate because the hypothesis was that relative expansion of VMPFC (regionalization) would be related to generalized anxiety. Because we tested two primary hypotheses, we used a Bonferroni-corrected p value of 0.025 as the threshold to indicate significance on these primary tests.
Preliminary analyses examined the effects of socioeconomic status, as well as a quadratic term for age, on anxiety scores. Based on these analyses, socioeconomic status was not included as a covariate in the models because these measures contributed nothing to predictions of anxiety, and because due to missing data, including them would have resulted in the loss of 25 subjects. The quadratic term for age was also not included in the models because it contributed nothing to predictions of anxiety and because age effects on cortical thickness and on relative VMPFC surface area were both linear. Additional analyses were run with the same models used in this study examining associations in the left and right hemispheres separately, and no hemispheric differences in these associations were found.
Results
A total of 287 participants (151 males) completed the SCARED-R and the neuroimaging protocol. These individuals ranged in age from 7 to 20 years (M = 13.8; SD = 3.66) at the imaging session (see Figure S2 in the Online Resource for a more detailed age distribution). According to self or parent reports, the sample was 44.9 % White, 12.9 % Asian, 7.7 % African American, 1.0 % Native American, 0.4 % Pacific Islander, and 23.7 % mixed race. The remaining 9.4 % of the sample did not report race. The sample was 24.0 % Hispanic or Latino ethnicity by self or parent report. Scores on the SCAREDR spanned the full possible range from 0 to 18 (M = 5.65; SD = 4.37), with 70 participants (24.4 %) scoring above 8 (the previously suggested cut-off for a GAD diagnosis (Bodden et al. 2009)).
A simple regression model first estimated the effects of age and gender on anxiety without including imaging variables in the model. This base model consisted of age, gender, their interaction term, and the set of GAFs (as covariates of no interest). The model (F(8, 278) = 5.48, p < 0.0001) revealed strong age (t = 4.42, p < 0.0001) and gender (t = 4.03, p < 0.0001) effects on anxiety. Specifically, older children and females endorsed higher levels of anxiety. The age by gender interaction did not reach significance in this model. Subsequent analyses examining the effects of cortical surface area and thickness included the variables in this simple model as well as scanner as a covariate of no interest.
Next we tested for the hypothesized association between relative areal expansion of VMPFC and anxiety by adding VMPFC surface area and its interactions with age and gender to the above model (Table 1). Total surface area was also included in this model as a covariate. As predicted a significant main effect of VMPFC surface area was observed (t = −3.07, p = 0.0023), with higher anxiety associated with relatively smaller VMPFC surface area. However, a significant interaction between VMPFC area and age was also observed (t = 2.45, p = 0.0148). To illustrate this interaction, we plotted the relationship between relative VMPFC surface area and anxiety separately for “younger” and “older” children by computing the slope for each at the first and third quantile for age, respectively (Fig. 1a). The plot shows that the negative association is attributable to the relationship in younger participants. Total surface area was positively associated with anxiety in this model. Relative surface area of the VMPFC accounted for 8.8 % of the variability in generalized anxiety in children under the age of 14, while it only accounted for 3.2 % of the variability in youth 14 and older.
Table 1.
Regression models predicting anxiety from relative VMPFC and total surface area
| Term | B | SE | t | p |
|---|---|---|---|---|
| VMPFC surface area | ||||
| Age | 0.3507 | 0.0836 | 4.20 | <0.0001 |
| Gender | 1.1489 | 0.2954 | 3.89 | 0.0001 |
| Age × gender | 0.1528 | 0.0752 | 2.03 | 0.0430 |
| Total area | 0.0001 | 3.78e-5 | 3.61 | 0.0004 |
| VMPFC area | −37.336 | 12.148 | −3.07 | 0.0023 |
| VMPFC area × age | 3.9513 | 1.6106 | 2.45 | 0.0148 |
| VMPFC area × gender | −1.2909 | 5.6751 | −0.23 | 0.8202 |
| VMPFC area × age × gender | 0.9116 | 1.6009 | 0.57 | 0.5696 |
| Total surface area | ||||
| Age | 0.3118 | 0.0881 | 3.54 | 0.0005 |
| Gender | 1.2923 | 0.3009 | 4.29 | <0.0001 |
| Age × gender | 0.1497 | 0.0816 | 1.83 | 0.0679 |
| Total area | 3.95e-5 | 1.90e-5 | 2.08 | 0.0384 |
| Total area × age | 8.11e-6 | 5.53e-6 | 1.46 | 0.1442 |
| Total area × gender | 2.10e-5 | 1.82e-5 | 1.15 | 0.2504 |
| Total area × age × gender | 3.94e-6 | 5.63e-6 | 0.70 | 0.4846 |
Both models controlled for scanner and GAFs
VMPFC ventromedial prefrontal cortex
Terms in bold italics were significant at p < 0.01 and terms in bold only were significant at p < 0.05.
Fig. 1.
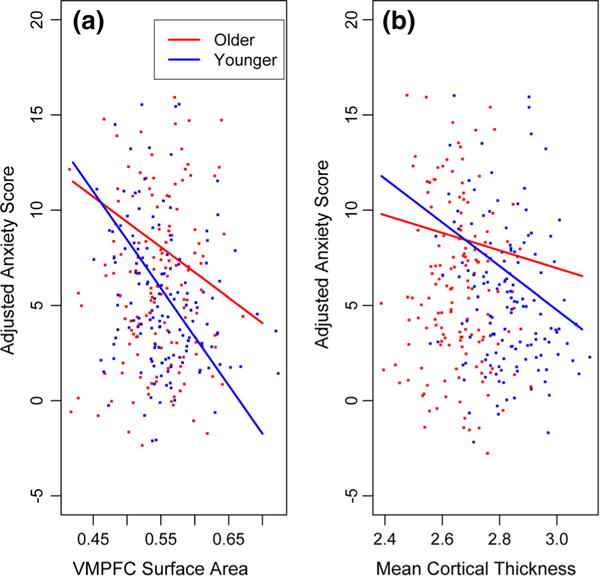
Effects of ROI measures on anxiety as a function of age. Anxiety scores are adjusted for scanner, GAFs, gender, and all interactions between ROI measures, age, and gender. It is also adjusted for total cortical area in the VMPFC surface area model. Slopes for younger and older participants were computed at the first and third quantile for age, respectively. VMPFC surface area units are expressed in terms of mean partial membership-weighted voxel expansion factors. Mean cortical thickness units are in mm. a The relationship between VMPFC surface area and anxiety scores in younger vs. older participants. b The relationship between mean cortical thickness and anxiety scores in younger vs. older participants
Because total surface area was significant in the previous model, we similarly tested the effect of this global measure without including VMPFC surface area in the model (Table 1). There was a modest positive association between total surface area and anxiety (t = 2.08, p = 0.0384). None of the interactions in this model were significant.
In order to test the hypothesized association between VMPFC thickness and anxiety, as well as the interactions with age and gender, we ran another multiple regression model predicting anxiety from the base model and covariates above, as well as VMPFC thickness and its interactions with age and gender (see Table S1 in the Online Resource). As predicted, thickness in the VMPFC was significantly negatively associated with anxiety (t = −2.66, p = 0.0083). No VMPFC thickness by age interaction was observed (t = 0.98, p = 0.3291). In order to determine whether there was a disproportionate effect in VMPFC, or conversely whether the effect of VMPFC thickness was mediated by the global thickness effect, we ran this model again controlling for mean thickness (Table 2). In this model, the mean thickness effects remained marginally significant, while the VMPFC thickness effect disappeared entirely. Again, no interaction effects were observed in this model.
Table 2.
Regression models predicting anxiety from relative VMPFC and mean thickness
| Term | B | SE | t | p |
|---|---|---|---|---|
| VMPFC thickness | ||||
| Age | 0.0354 | 0.1012 | 0.35 | 0.7277 |
| Gender | 0.9448 | 0.3125 | 3.02 | 0.0027 |
| Age × gender | 0.1373 | 0.0867 | 1.58 | 0.1145 |
| Mean thickness | −8.2502 | 4.7649 | −1.73 | 0.0845 |
| VMPFC thickness | −0.3268 | 4.8545 | −0.07 | 0.9464 |
| VMPFC thickness × age | 0.6799 | 0.6387 | 1.06 | 0.2881 |
| VMPFC thickness × gender | 2.2896 | 2.5453 | 0.90 | 0.3692 |
| VMPFC thickness × age × gender | 0.1193 | 0.6413 | 0.19 | 0.8526 |
| Mean thickness | ||||
| Age | 0.0642 | 0.1010 | 0.64 | 0.5256 |
| Gender | 1.1822 | 0.3134 | 3.77 | 0.0002 |
| Age × gender | 0.2035 | 0.0922 | 2.21 | 0.0281 |
| Mean thickness | −7.6904 | 2.6701 | −2.88 | 0.0043 |
| Mean thickness × age | 1.1093 | 0.5516 | 2.01 | 0.0453 |
| Mean thickness × gender | 4.3676 | 2.4540 | 1.78 | 0.0763 |
| Mean thickness × age × gender | 0.7229 | 0.5455 | 1.33 | 0.1863 |
Both models controlled for scanner and GAFs
VMPFC ventromedial prefrontal cortex
Terms in bold italics were significant at p < 0.01 and terms in bold only were significant at p < 0.05
Because global (mean) thickness mediated the relationship between VMPFC thickness and anxiety, we then tested the association between mean thickness and anxiety without VMPFC thickness in the model (Table 2). Results showed a significant effect of this global variable (t = −2.88, p = 0.0043). Lower mean thickness was associated with higher anxiety. Consistent with the base model described above, gender was significantly associated with anxiety in this model, with females endorsing higher levels of anxiety. In contrast, age was not a significant predictor of anxiety. However, the age by gender interaction was significant, with females showing a sharper increase in anxiety with age. Additionally, the mean thickness by age interaction was significant. Figure 1b shows this interaction by plotting the association between mean cortical thickness and anxiety at the first and third quantile for age as described above, and demonstrates that the association of higher anxiety with thinner cortex was stronger in younger participants. Mean cortical thickness accounted for 6.0 % of the variability in generalized anxiety in children under the age of 14, while it only accounted for 0.2 % of the variability in youth 14 and older.
Thus the results were that, as hypothesized, reduced VMPFC surface area relative to total surface area was significantly associated with higher generalized anxiety levels, and this effect interacted with age. Furthermore, although the hypothesized association between reduced VMPFC thickness and higher anxiety was also observed, this association appeared to be entirely mediated by reduction of global cortical thickness, and this association with global thickness also appeared to interact with age. In order to test whether the mean cortical thickness and VMPFC surface area effects on anxiety were additive, both measures were entered into a simultaneous regression model (Table 3) together with the interaction terms that were significant in the earlier models. The main effects of relative VMPFC surface area (t = −2.67, p = 0.0080) and mean thickness (t = −2.18, p = 0.0303) were both significant in this model; their interactions with age both approached significance (ps < 0.10). Together, these cortical phenotypes accounted for 13.1 % of the variability in generalized anxiety in children under the age of 14, while they only accounted for 3.3 % of the variability in youth 14 and older.
Table 3.
Simultaneous regression model predicting anxiety from VMPFC surface area and thickness
| Term | B | SE | t | p |
|---|---|---|---|---|
| Age | 0.1821 | 0.1060 | 1.72 | 0.0868 |
| Gender | 1.1284 | 0.2924 | 3.86 | 0.0001 |
| Age × gender | 0.1363 | 0.0741 | 1.84 | 0.0670 |
| Total area | 0.0001 | 3.79e-5 | 3.17 | 0.0017 |
| VMPFC area | −32.190 | 12.044 | −2.67 | 0.0080 |
| VMPFC area × age | 3.0736 | 1.5951 | 1.93 | 0.0551 |
| Mean thickness | −5.8841 | 2.7019 | −2.18 | 0.0303 |
| Mean thickness × age | 0.9078 | 0.5422 | 1.67 | 0.0953 |
Model controlled for scanner and GAFs
VMPFC ventromedial prefrontal cortex
Terms in bold italics were significant at p < 0.01 and terms in bold only were significant at p < 0.05
To explore differences in apparent neurodevelopmental trajectories as a function of anxiety level, we reversed the models to predict relative VMPFC surface area and mean thickness from the anxiety measure. These models are presented in Table S2 of the Online Resource. To illustrate the differences in these apparent trajectories visually, we plotted the age effects on relative VMPFC surface area and mean cortical thickness separately for “low” and “high” anxiety by computing the slope for each at the first and third quantile for anxiety, respectively. Figure 2a suggests that, while surface area in the VMPFC relative to the cortex as a whole increases with age in both groups, high anxiety children have relatively less cortical surface area within VMPFC at younger ages, but they show a steeper increase in relative VMPFC surface area over the age range. Figure 2b suggests that high anxiety children have a thinner cortex early on, but low anxiety children have a steeper apparent trajectory of cortical thinning over this age range, such that there is no difference by early adulthood.
Fig. 2.
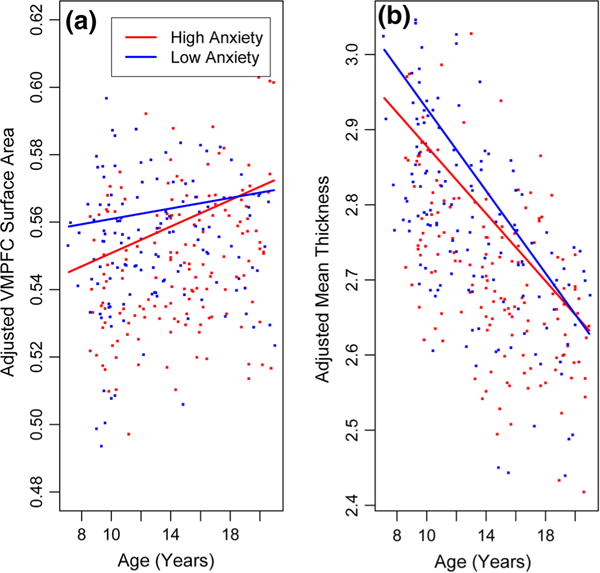
Apparent age trajectories of cortical development by anxiety level. ROI measures are adjusted for scanner, GAFs, gender, and all interactions between anxiety, age and gender. VMPFC surface area is also adjusted for total cortical area. Slopes for low and high anxiety were computed at the first and third quantile for anxiety, respectively. a VMPFC surface area changes with age in low vs. high anxiety children. b Mean thickness changes with age in low vs. high anxiety children
The results of our planned analyses confirmed the association of generalized anxiety with reduced VMPFC area and ruled out the possibility that global surface area reduction might mediate this association. In contrast, while the anxiety measure was also associated with reduced VMPFC thickness, there was strong evidence that this association was fully mediated by the measure of global thinning. The statistical analyses performed to test these a priori hypotheses do not allow further inferences about anatomical specificity, although as discussed in (Jernigan et al. 2003), surface-based analyses provide additional information about effect size variability across the map. Therefore, using the PING Data Exploration Portal (Bartsch et al. 2014), a web-based tool for modeling PING data, we generated post hoc vertex-wise maps of the variability of the size of the generalized anxiety effect across the surface of the cortex, both for relative surface area and for thickness. That is, we mapped, at each vertex, the uncorrected p associated with generalized anxiety level, adjusting for covariates, for both relative cortical surface area and cortical thickness. Since the significant age by anxiety level interactions in the primary analyses suggested that the strength of the associations diminished with age, we computed the maps centered at ages 7, 14, and 21 (Fig. 3). This allowed us to visualize variability in the effect of anxiety across the cortex for each phenotype separately, while also showing how these patterns change across the age range. The relative surface area maps suggest a somewhat complex relationship between anxiety and surface arealization. As expected from the analyses above, high generalized anxiety is associated with relatively smaller VMPFC bilaterally, with this effect diminishing by age 21. A small region of unpredicted negative associations of similar magnitude is observed around the cuneus and precuneus. However, other regions such as the inferior parietal, supramarginal, post-central, and superior temporal gyri exhibit apparent positive relationships with anxiety of a similar magnitude to the predicted negative associations. These relationships appear to persist across the age range in the right hemisphere, but diminish in the left hemisphere. The thickness maps illustrate the global thickness effect shown in the mean thickness analyses above, with high anxiety associated with thinner cortex throughout most of the map. They also illustrate the age interaction, showing that this association is much reduced by age 21. Note that the color scale was chosen to reveal both the magnitude of the effects and to provide as much information about the anatomical variability as possible.
Fig. 3.
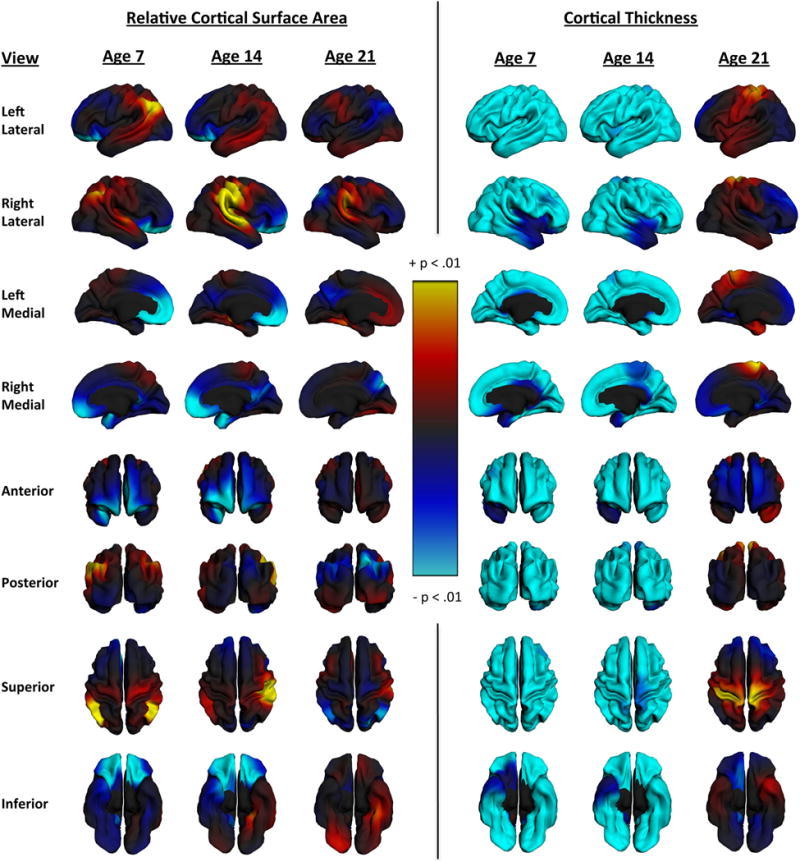
Anxiety scores mapped onto cortical surface expansion and thickness. Maps show the vertex-wise variability of effects on surface area and thickness at ages 7, 14, and 21 in order to visualize the moderating effect of age on the relationship between these structural phenotypes and anxiety. The models used to compute these maps controlled for scanner, GAFs, and gender. Surface expansion maps also controlled for total surface area. Vertex maps labeled “Age 14” were actually computed at the mean age for the sample (13.84) in order to be able to visualize the main effect for anxiety with age held constant
Discussion
We examined the developing architecture of the cortex in a subset of the PING cohort (Brown et al. 2012; Bartsch et al. 2014) and observed that children with higher generalized anxiety differed from their peers on two distinct phenotypes. The study focused on the VMPFC, specifically on a region defined with analyses of twin data (Chen et al. 2012) that produced genetically informed cortical parcels. However, it is useful to review the pattern of developmental change in cortical architecture generally. Cortical maturation during childhood and adolescence involves expansion followed by contraction of cortical surface area in a pattern that is heterochronous across different cortical regions (Brown and Jernigan 2012). In contrast, apparent thickness of the cortex (on MRI) declines continuously across this age range. Early methods for mapping cortical development measured cortical volume, which conflates these two phenotypes, and this led to some confusion about the time course and regional variability of cortical maturation. Here we applied surface-based methods that clearly delineate area and thickness to determine whether either of these phenotypes differs in children with high generalized anxiety levels, and whether interactions with age suggest distinct neurodevelopmental trajectories in these children.
We found evidence that relative surface area of the VMPFC was reduced in children with higher generalized anxiety, and that this association was stronger in children and younger adolescents than in older adolescents and young adults. It should be noted that across the late childhood and adolescent years, some late expanding areas of cortex are peaking and/or show little contraction, while some earlier expanding regions are exhibiting more rapid contraction. The net effect of these changes throughout the cortex is that VMPFC surface area represents a gradually increasing proportion of the cortical surface over a protracted period. These data suggest that, in children with higher generalized anxiety, this increase in VMPFC “territory” may be somewhat delayed relative to that in less anxious children. If this interpretation is correct, attenuation of the effect into adolescence and adulthood could suggest that early structural alterations of the VMPFC give rise to downstream effects (e.g., altered connections with other regions) that persist and account for the maintenance of generalized anxiety symptoms even after this region develops a more mature surface area phenotype.
We also observed that the cortex was thinner in more anxious children and that, again, the apparent trajectory of cortical thinning appeared to differ as a function of gen-eralized anxiety levels; however, this effect was more global; there was no evidence in our study that VMPFC thickness was disproportionately affected. These results resembled in some ways, but also differed from, those obtained in a similar previous study (Ducharme et al. 2014). These authors examined only thickness, and reported no main effect of anxiety levels in the full sample; but they also observed an interaction, such that the association of anxiety levels and thickness differed in younger and older participants. They also reported what appeared to be more anatomically circumscribed effects in VMPFC and related areas. A number of potentially significant differences in the methods and modeling applied in the two studies could account for these differences. First, in the previous study the effects were not estimated for a specific VMPFC thickness measure, but were instead identified in a vertex-wise analysis. The effect of a global thickness measure was therefore not examined in that study, so it is not possible to determine if the reported effects would have remained if mean thickness had been modeled as a covariate. It is interesting that clear evidence for effects that peak in VMPFC were observed in our study for surface area, and not thickness. One factor that may have contributed to the discrepancies is the manner in which the methods used in the previous study, relative to those used in the present study, separate effects of surface area from thickness variability during image processing. In summary, the present results support the conclusion of Ducharme et al. (2014) that the relationship between cortical maturation and anxiety levels varies across this developmental age range, but our results implicate cortical surface area expansion in VMPFC and a relatively independent, and more global, cortical thickness phenotype, both of which relate to high generalized anxiety in youth. These distinctions between effects involving thickness and surface area are important, because both the cellular mechanisms and the set of genetic factors linked to these two aspects of the cortical architecture differ (Panizzon et al. 2009; Chen et al. 2012).
As expected from previous research, female participants of this study endorsed higher levels of generalized anxiety than male participants, and in this sample self-reported generalized anxiety levels increased with age. This increase with age appeared to be more pronounced in female than in male participants; the age by gender interaction was significant in some of the anatomical models of anxiety. However, gender did not significantly moderate the relationships between anxiety and any measure of cortical surface area or thickness.
The hypotheses of the present study were tested with measures of surface area and thickness in a specific cortical region (VMPFC); however, the PING Data Exploration Portal provides a statistical interface for visualizing the vertex-wise variability of effects on surface area and thickness (as well as other phenotypes estimated at each vertex in the PING dataset). Thus, as a post hoc analysis we mapped the associations with generalized anxiety levels across the cortical surface for each phenotype, and displayed the results so that the observed moderating effects of age were visible. These maps are entirely exploratory, but they provide additional information about the anatomical variability of the effects. As expected from our ROI analyses, anxiety was linked to apparent cortical thinning globally, and this association was attenuated by adulthood. For surface area, associations between reduced area and higher anxiety were, as hypothesized, largest and most extensive in the VMPFC, and these were also clearly moderated by age. However, there was a smaller, unpredicted area with a similar level of negative association with anxiety in the precuneus region, but only in the older participants. Ducharme et al. (2014) also observed effects of anxiety in this region, also only in the older participants of their study, though the effects were on thickness. These authors speculated that this effect might be related to increasing interactions between VMPFC and precuneus as the default network develops. Other research implicates the precuneus in metacognitive processes related to contextual memory retrieval and self-reflection (Lundstrom et al. 2005; Cavanna and Trimble 2006; Duarte et al. 2011; McCurdy et al. 2013). One of these studies (McCurdy et al. 2013) even shows a specific anatomical coupling of the precuneus with the anterior prefrontal cortex (aPFC) such that individuals with larger aPFC tend to have larger precuneus. Given the protracted course of development of these metacognitive skills (Kuhn 2000), and the relevance of metacognition to anxious worrying (Wells 2005), it is perhaps not surprising to see this association between anxiety and the precuneus at a later point in development. Finally, there was some indication for unexpected associations of higher anxiety with relative increases in surface area in the left inferior parietal region and the right supramarginal and superior temporal gyri. These effects do not appear to be strongly moderated by age. Given that these apparent effects outside of the VMPFC were not hypothesized a priori, they require replication in prospective studies.
The results of this study suggest that generalized anxiety levels in childhood may be linked to varying trajectories of cortical maturation, and implicate cortical expansion of the VMPFC for the first time. However, the associations have several possible interpretations. Altered trajectories of VMPFC expansion or cortical thinning could represent effects of genetic risk factors for anxious temperament, and if so, they could alternatively represent mediating endophenotypes or irrelevant phenotypic manifestations due to pleiotropy. The associations could also reflect effects of stress and fear on the developing neural circuitry (Gee et al. 2013) through activity-dependent mechanisms; and these effects could be independent of or interactive with genetic risk. A significant limitation of the present study is that the data are entirely cross-sectional, and thus the results are only suggestive of developmental trends. In addition, the current study was limited by the types of measures available in the PING data resource and the number of children administered each of these measures. Having additional measures of anxiety from multiple informants would certainly help to bolster these findings and clarify the specific contexts in which they occur. However, PING imaging methods were a clear strength in this study. Previous work from this data set has already contributed to our understanding of typical trajectories of cortical development using measures of surface area and thickness (Brown and Jernigan 2012). The current study took advantage of a specific measure of anxiety available in this data set (i.e., the Generalized Anxiety Disorder index on the SCARED-R) that best represented the anxious phenotype of interest to begin to address questions about the relationship between anxiety and the developing architecture of the cortex using these two phenotypes. It should be noted that these results may not generalize to other manifestations of anxiety (e.g., social phobia, panic disorder, etc.), and these should be examined separately in future studies. Additionally, future longitudinal studies of even larger samples of developing children with richer assessments of social/emotional functioning are needed so that more complete models of the effects of genetic variants, environmental stressors, and concurrent affective experiences can be constructed. Critically, information is needed about the degree to which both neurodevelopmental and behavioral trajectories can be modified with preventative interventions that reduce adverse outcomes associated with high anxiety.
Supplementary Material
Acknowledgments
This work was supported by the National Institute On Drug Abuse (grant number RC2 DA029475), the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (Grant Number R01 HD061414), and the National Institute of General Medical Sciences (Grant Number R01 GM104400) of the National Institutes of Health. The content presented herein is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. These data are freely available on the PING portal: http://pingstudy.ucsd.edu
Data reported herein represent a subset of the data collected in the PING study. PING is a multisite initiative consisting of six infrastructure cores and 10 data collection sites. The following is a description of key personnel in the infrastructure cores: Coordinating Core, Core PI and Co-PI of PING Terry L. Jernigan, Ph.D., and Connor McCabe, B.S., UC San Diego; Assessment Core, Core PI and Co-PI of PING Linda Chang, M.D., U Hawaii, Natacha Akshoomoff, Ph.D., UC San Diego, Erik Newman, Ph.D., UC San Diego; MRI Post-processing Core, Core PI and Co-PI of PING Anders M. Dale, Ph.D., UC San Diego; MRI Acquisition Core, Core PI and Co-PI of PING Thomas Ernst, Ph.D., U Hawaii, Core Co-PI Anders M. Dale, Ph.D., UC San Diego, Peter Van Zijl, Ph.D., KKI, Joshua Kuperman, Ph.D., UC San Diego; Genetics Core, Core PI and Co-PI of PING Sarah Murray, Ph.D., Cinnamon Bloss, Ph.D., and Nicholas J. Schork, Ph.D., Scripps Translational Science Institute; Informatics and Biostatistics, Mark Appelbaum, Ph.D., Anthony Gamst, Ph.D., Wesley Thompson, Ph.D., and Hauke Bartsch, Ph.D., UC San Diego. The following is a description of the key personnel at the data collection sites: University of California, San Diego, Site PI Terry L. Jernigan, Ph.D., Anders M. Dale, Ph.D., Natacha Akshoomoff, Ph.D.; University of Hawaii, Site PI Linda Chang, M.D., Thomas Ernst, Ph.D., Brian Keating, Ph.D.; University of California, Davis, Site PI David Amaral, Ph.D.; University of California, Los Angeles, Site PI Elizabeth Sowell, Ph.D.; Kennedy Krieger Institute, Johns Hopkins University, Site PIs Walter Kaufmann, M.D. and Stewart Mostofsky, M.D., Peter Van Zijl, Ph.D.; Sackler Institute, Weill Cornell Medical College, Site PI B.J. Casey, Ph.D., Erika J. Ruberry, B.A., Alisa Powers, B.A.; Massachusetts General Hospital, Harvard University, Site PIs Bruce Rosen, M.D., Ph.D. and Tal Kenet, Ph.D.; University of Massachusetts, Site PIs Jean Frazier, M.D. and David Kennedy, Ph.D.; Yale University, Site PI Jeffrey Gruen, M.D.
Footnotes
For the Pediatric Imaging, Neurocognition, and Genetics Study.
Electronic supplementary material The online version of this article (doi:10.1007/s00429-015-1085-9) contains supplementary material, which is available to authorized users.
Conflicts of interest Jean A. Frazier has received research support from GlaxoSmithKline, Pfizer, Inc., Neuren, Roche, and Seaside Therapeutics, NICHD, NIMH, NINDS and has served on a Data Safety Monitoring Board for Forest Pharmaceuticals. Anders M. Dale is a founder of and holds equity interest in CorTechs Labs, La Jolla, CA and serves on its scientific advisory board. The terms of this arrangement have been reviewed and approved by UC San Diego, in accordance with its conflict of interest policies. All other authors reported no biomedical financial interests or potential conflicts of interest.
Research involving human participants All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- Aben I, Denollet J, Lousberg R, et al. Personality and vulnerability to depression in stroke patients: a 1-year prospective follow-up study. Stroke. 2002;33:2391–2395. doi: 10.1161/01.str.0000029826.41672.2e. [DOI] [PubMed] [Google Scholar]
- Akshoomoff N, Newman E, Thompson WK, et al. The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING) Neuropsychology. 2014;28:1–10. doi: 10.1037/neu0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong KA, Khawaja NG. Gender differences in anxiety: an investigation of the symptoms, cognitions, and sensitivity towards anxiety in a nonclinical population. Behav Cognit Psychother. 2002;30:227–231. [Google Scholar]
- Bartsch H, Thompson WK, Jernigan TL, Dale AM. A web-portal for interactive data exploration, visualization, and hypothesis testing. Front Neuroinform. 2014;8:25. doi: 10.3389/fninf.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Rosenbaum JF, Bolduc-Murphy EA, et al. A 3-year follow-up of children with and without behavioral inhibition. J Am Acad Child Adolesc Psychiatry. 1993;32:814–821. doi: 10.1097/00004583-199307000-00016. [DOI] [PubMed] [Google Scholar]
- Blackmon K, Barr WB, Carlson C, et al. Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiatry Res. 2011;194:296–303. doi: 10.1016/j.pscychresns.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodden DHM, Bögels SM, Muris P. The diagnostic utility of the Screen for Child Anxiety Related Emotional Disorders-71 (SCARED-71) Behav Res Ther. 2009;47:418–425. doi: 10.1016/j.brat.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Bonaguidi F, Trivella MG, Carpeggiani C, et al. Personality and acute myocardial infarction: distinctive traits. G Ital Cardiol. 1994;24:745–753. [PubMed] [Google Scholar]
- Brown TT, Jernigan TL. Brain development during the preschool years. Neuropsychol Rev. 2012;22:313–333. doi: 10.1007/s11065-012-9214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, et al. Neuroanatomical assessment of biological maturity. Curr Biol. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the Research Domain Criteria (RDoC) framework. Biol Psychiatry. 2014;76:350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen C-H, Gutierrez ED, Thompson W, et al. Hierarchical genetic organization of human cortical surface area. Science. 2012;335:1634–1636. doi: 10.1126/science.1215330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor Inventory (NEO-FFI) Psychological Assessment Resources; Odessa: 1992. [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2002;51:553–562. doi: 10.1016/s0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Dougherty DD, Rauch SL. Functional imaging of mood and anxiety disorders. J Neuroimaging. 2006;16:1–10. doi: 10.1177/1051228405001474. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. Stimulus content and the neural correlates of source memory. Brain Res. 2011;1373:110–123. doi: 10.1016/j.brainres.2010.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Hudziak JJ, et al. Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb Cortex. 2014;24:2941–2950. doi: 10.1093/cercor/bht151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, et al. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb Cortex. 2011;21:1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Núñez-Elizalde AO, et al. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69:563–571. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Fennema-Notestine C, Ostergaard AL. More “mapping” in brain mapping: statistical comparison of effects. Hum Brain Mapp. 2003;19:90–95. doi: 10.1002/hbm.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Temperamental factors in human development. Am Psychol. 1991;46:856–862. doi: 10.1037//0003-066x.46.8.856. [DOI] [PubMed] [Google Scholar]
- Kent JM, Rauch SL. Neurocircuitry of anxiety disorders. Curr Psychiatry Rep. 2003;5:266–273. doi: 10.1007/s11920-003-0055-8. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. Metacognitive development. Cur Dir Psychol Sci. 2000;9:178–181. [Google Scholar]
- Kühn S, Schubert F, Gallinat J. Structural correlates of trait anxiety: reduced thickness in medial orbitofrontal cortex accompanied by volume increase in nucleus accumbens. J Affect Disord. 2011;134:315–319. doi: 10.1016/j.jad.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage. 2005;27:824–834. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- McCarron P, Gunnell D, Harrison GL, et al. Temperament in young adulthood and later mortality: prospective observational study. J Epidemiol Community Health. 2003;57:888–892. doi: 10.1136/jech.57.11.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty CA, Huggins W, Aiello AE, et al. PhenX RISING: real world implementation and sharing of PhenX measures. BMC Med Genomics. 2014;7:16. doi: 10.1186/1755-8794-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy LY, Maniscalco B, Metcalfe J, et al. Anatomical coupling between distinct metacognitive systems for memory and visual perception. J Neurosci. 2013;33:1897–1906. doi: 10.1523/JNEUROSCI.1890-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. The Role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, et al. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005;57:961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, Mayer B, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED) and traditional childhood anxiety measures. J Behav Ther Exp Psychiatry. 1998;29:327–339. doi: 10.1016/s0005-7916(98)00023-8. [DOI] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, van Brakel A, Mayer AB. The revised version of the screen for child anxiety related emotional disorders (SCARED-R): further evidence for its reliability and validity. Anxiety Stress Coping. 1999;12:411–425. doi: 10.1080/10615809908249319. [DOI] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: the Children’s Behavior Questionnaire. Child Dev. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Dannlowski U. Neural correlates of trait anxiety in fear extinction. Psychol Med. 2011;41:789–798. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State trait anxiety inventory for children. Consulting Psychologists Press; Palo Alto: 1973. [Google Scholar]
- Spielberger R, Gorsuch R, Lushene R, et al. Manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- Stein MB, Jang KL, Livesey DJ. Heritability of anxiety sensitivity: a twin study. Am J Psychiatry. 1999;156:246–251. doi: 10.1176/ajp.156.2.246. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Wehry AM, DelBello MP, et al. Establishing the neurobiologic basis of treatment in children and adolescents with generalized anxiety disorder. Depress Anxiety. 2012;29:328–339. doi: 10.1002/da.21913. [DOI] [PubMed] [Google Scholar]
- Vreeke LJ, Muris P, Mayer B, et al. The assessment of an inhibited, anxiety-prone temperament in a Dutch multi-ethnic population of preschool children. Eur Child Adolesc Psychiatry. 2012;21:623–633. doi: 10.1007/s00787-012-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. The metacognitive model of GAD: assessment of meta-worry and relationship with DSM-IV generalized anxiety disorder. Cogn Therapy Res. 2005;29:107–121. [Google Scholar]
- Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40:1086–1093. doi: 10.1097/00004583-200109000-00018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


