Abstract
Self-preservation is required for life. At the cellular level, this fundamental principle is expressed in the form of molecular mechanisms for preconditioning and tolerance. When the cell is threatened, internal cascades of survival signaling become triggered to protect against cell death and defend against future insults. Recently, however, emerging findings suggest that this principle of self-preservation may involve not only intracellular signals; the release of extracellular signals may provide a way to recruit adjacent cells into an amplified protective program. In the central nervous system where multiple cell types co-exist, this mechanism would allow threatened neurons to “ask for help” from glial and vascular compartments. In this review, we describe this new concept of help-me signaling, wherein damaged or diseased neurons release signals that may shift glial and vascular cells into potentially beneficial phenotypes, and help remodel the neurovascular unit. Understanding and dissecting these non-cell autonomous mechanisms of self-preservation in the CNS may lead to novel opportunities for neuroprotection and neurorecovery.
1. Introduction
Cellular function requires the ability to respond to an existing stimulus and then adapt for future stimuli. Within this broad definition of homeostasis lies the concept of tolerance and preconditioning against injurious stimuli. Preconditioning is a well-defined phenomenon whereby a first sublethal dose of an otherwise harmful stimulus results in tolerance to a second injury stimulus (Stevens et al. 2014). A large amount of data from both experimental models as well as clinical conditions exists for cerebral ischemia. Therefore, this review is focused on signaling cascades for ischemic brain injury.
Ischemic preconditioning in the brain was described in 1990, wherein an initial sublethal ischemic stress induced tolerance in the hippocampal CA1 against subsequent lethal ischemic injury in gerbil models of transient global ischemia (Kitagawa et al. 1990). Then this phenomenon was confirmed for transient global ischemia in the rat brain (Nishi et al. 1993) and other models of focal ischemia (Chen et al. 1996). Several retrospective studies have also suggested that transient ischemic attacks (TIAs) in humans are associated with improved clinical outcome after stroke, perhaps because TIAs are capable of inducing ischemic tolerance (Fu et al. 2008; Moncayo et al. 2000; Wegener et al. 2004; Weih et al. 1999). In the context of stroke, preconditioning induces a transient window of protection that requires gene activation and new protein synthesis (Dirnagl et al. 2009). This reprogrammed response forms the basis for endogenous neuroprotection and provides a conceptual framework for investigating the molecular mechanisms that protect the brain against ischemic injury (Chen et al. 1996; Kapinya et al. 2002; Koerner et al. 2007; Marsh et al. 2009; McCabe and Simon 1993; Stenzel-Poore et al. 2003; Stevens et al. 2011; Truettner et al. 2002; Zimmermann et al. 2001).
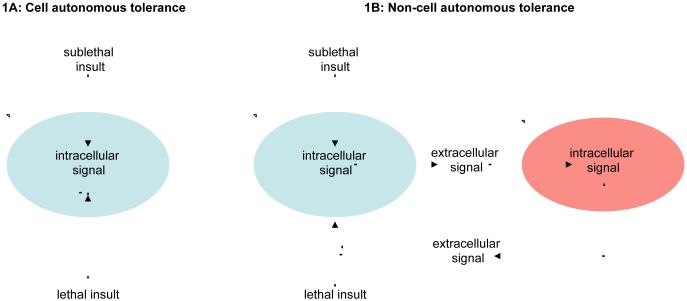
At a cellular level, the ability of preconditioning to trigger endogenous protective mechanisms can be viewed within a conceptually cell autonomous model (Figure 1A). The initial sublethal insult induces intracellular signaling pathways that serve to block the second lethal insult. However, cells do not exist in isolation and beyond a theoretical single cell response, the release of extracellular signals may provide a way to recruit adjacent cells into an amplified protective program (Figure 1B). The initial sublethal insult induces a cascade of intracellular signals that provoke the release of extracellular mediators that affect an adjacent cell. Then this second cell responds by releasing another set of extracellular signals that block a lethal insult against the original cell. This non-cell autonomous model thus sets the stage for the concept of help-me signaling, wherein multiple cells interact to assemble an integrated adaptive and protective response after injury and disease.
Figure 1. Cell autonomous tolerance (A) and non-cell autonomous tolerance (B).
(A) The initial sublethal insult induces intracellular signaling pathways that serve to block the second lethal insult. (B) The initial sublethal insult induces a cascade of intracellular signals that provoke the release of extracellular mediators that affect an adjacent cell. This second cell responds by releasing another set of extracellular signals that then block a lethal insult against the original cell.
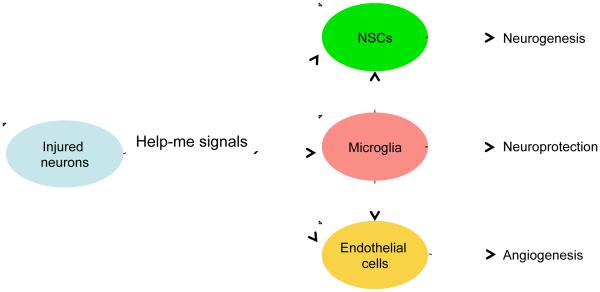
In the brain, these non-cell autonomous interactions should involve multiple cell types. The neurovascular unit is not only an anatomical construct but also serves as a functional unit for the interactions between neurons, glial cells and blood vessels under normal conditions and in response to injury. In this review, we will use the neurovascular unit as a basis to describe this new concept of help-me signaling, wherein damaged or diseased neurons release signals that may shift glial and vascular cells into potentially beneficial phenotypes (Figure 2). Beyond neuronal help-me signals per se, we also discuss three representative classes of extracellular signals, i.e. cytokines, chemokines or growth factors, which are released after ischemia during the acute injury and delayed recovery stages after stroke. Finally, we explore the possibilities to develop potential therapeutic targets by finding new endogenous protective factors and help-me signals using transcriptome and secretome analyses.
Figure 2. Help-me signals.
Damaged or diseased neurons release signals that may shift glial and vascular cells into potentially beneficial phenotypes, i.e. providing neuroprotection and promoting neurogenesis and angiogenesis.
2. Neuronal help-me signals and neuron-immune interactions
The brain is known as an “immune-privileged” organ. This does not imply the lack of an immune system in the brain, but means that brain immunity is kept under tight control to protect vulnerable nervous tissue from potential harmful immune reactions (Biber et al. 2007; Galea et al. 2007). Microglia play central roles to survey and regulate the microenvironment to support homeostasis during CNS development and under normal and diseased conditions (Prinz and Priller 2014). Microglia differ from macrophages that reside in other tissues based on their cell-specific gene expression signatures, distinct ontogeny and differential functions (Butovsky et al. 2012; Gautier et al. 2012; Ginhoux et al. 2010; Kierdorf et al. 2013; Prinz and Priller 2014; Schulz et al. 2012). Microglial activation is restricted in the healthy brain, and shows a more quiescent immunological profile than other tissue macrophages. Once activated, microglial immune function is rapidly turned down to prevent the development of unwanted side effects, particularly secondary neuronal damage (Galea et al. 2007).
Traditionally, neurons are considered to be passive targets of activated microglia. However, accumulating evidence now suggest that neurons actively regulate microglia function and modulate immune pathways in the brain (Biber et al. 2007). Endangered or damaged neurons can release a repertoire of signaling molecules to attract surrounding cells including microglia, control microglial function and regulate microglia-mediated phagocytosis and neuroprotection (Biber et al. 2007). These molecules have traditionally been called “find-me” and “eat-me” signals (Chekeni et al. 2010; Grimsley and Ravichandran 2003; Lu et al. 2011; Napoli and Neumann 2009; Ravichandran 2010).
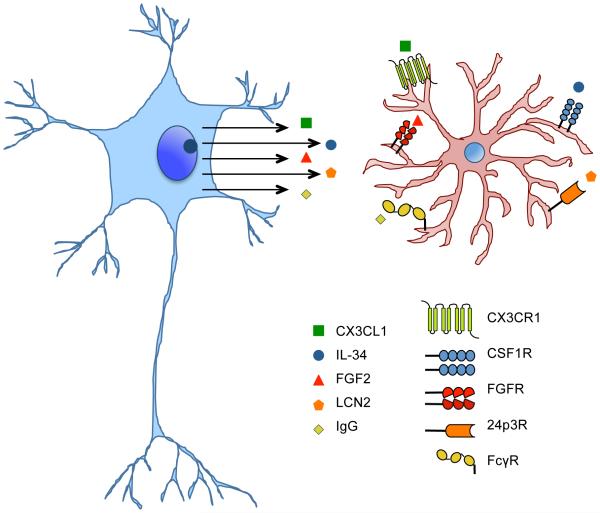
Emerging data now appears to recast some of these extracellular factors as neuronal “help-me” signals (Kyritsis et al. 2012; Mizuno et al. 2011; Noda et al. 2011; Noda et al. 2014; Xing et al. 2014). After brain injury, neurons can release unique “help-me” signals, including chemokines, cytokines, growth factors etc, which will interact with receptors expressed in microglia to guide microglial activation into a beneficial phenotype of neuroprotection and neurorecovery (Figure 3). For example, in the regenerative process of adult zebrafish brain, injured neurons release cysteinyl leukotriene that stimulates resident immune cells (microglia, leukocytes, and other glia) to release beneficial signals that promote neurogenesis (Kyritsis et al. 2012). It has been showed that injury-induced inflammation is sufficient to enhance the neural progenitor proliferation and neurogenesis after traumatic brain injury (Kyritsis et al. 2012).
Figure 3. Neuronal help-me signals are involved in the interaction of neurons and microglia.
After brain injury, neurons can release unique “help-me” signals, such as CX3CL1, IL-34, FGF2, LCN2, and IgG etc, which will interact with receptors expressed in microglia to guide microglial activation into a beneficial phenotype of neuroprotection and neurorecovery.
Mediators from the damage associated molecular pattern family (DAMPs) comprise a set of molecular determinants derived from cellular debris, intracellular proteins/enzymes or nuclear DNA/RNA that are released from injured cells (Seong and Matzinger 2004). Pattern recognition receptors are expressed on innate immune cells and bind DAMPs to initiate non-infectious immune responses in injured tissue. Several DAMPs, including high-mobility group box 1, ATP and S100 have been shown to be necessary for the initiation of immune responses following CNS injury (An et al. 2014). Although both help-me signals and DAMPs are released from injured neurons and may have functional overlap, the concept of help-me signals may fundamentally differ from DAMPs in terms of the balance between benefit versus harm. Damaged neurons can release many factors including DAMPs that activate glia into deleterious forms that worsen neuroinflammation. For example, damaged neurons release glutamate that activate metabotropic receptors on microglia and shift them into neurotoxic phenotypes (Taylor et al. 2005). In contrast, help-me signals released from distressed neurons are proposed to shift glial and vascular cells into potentially beneficial phenotypes. In this section, we briefly survey representative examples of help-me signals that have been described in recent literature.
2.1 CX3CL1/CX3CR1
Chemokines are small, secreted proteins and important inflammatory factors that regulate the attraction and migration of cells, especially immune cells (Conductier et al. 2010; Reaux-Le Goazigo et al. 2013). According to systematic nomenclature, chemokines are subdivided into four families, i.e. CXC, CC, CX3C and C. Chemokine receptors belong to the seven-transmembrane domain G protein coupled receptor superfamily. Neurons and glia constitutively express a wide spectrum of chemokines and their receptors. Thus, chemokines may play a dual role in the CNS, attracting and activating immune cells as well as modulating the survival and function of neurons (Conductier et al. 2010).
CX3CL1 is a transmembrane molecule that was cloned by two independent labs from neurons and endothelium (Bazan et al. 1997; Pan et al. 1997), and originally called neurotactin or fractalkine. When the extreme N-terminal chemokine domain is cleaved from the membrane domain, CX3CL1 can be released as a soluble form into extracellular space (Reaux-Le Goazigo et al. 2013). In the brain, neurons constitutively express high levels of CX3CL1, and its receptor, CX3CR1, is mostly expressed on microglia (Harrison et al. 1998; Nishiyori et al. 1998; Schwaeble et al. 1998). Besides this neuronal expression, CX3CL1 is also constitutively expressed by astrocytes at lower levels in adult mouse, rat and human brain (Hulshof et al. 2003; Sunnemark et al. 2005). Owing to expression patterns in the CNS, CX3CL1/CX3CR1 signaling may be an important pathway that allows neuronal cells to modify microglial functions during development and disease.
The effects of modifying CX3CL1/CX3CR1 pathways may be context dependent (Limatola and Ransohoff 2014). During inflammation post-injury, CX3CL1 may promote microglial activation, while under normal conditions, it may help maintain baseline microglia function (Sheridan and Murphy 2013). Despite controversial reports of benefit versus harm, many studies have provided evidence supporting the neuroprotective roles of CX3CL1. In stroke patients, higher plasma CX3CL1 level was associated with better outcome, and plasma CX3CL1 was inversely associated with systemic inflammatory markers, including white blood cell counts and high-sensitivity C-reactive protein (Donohue et al. 2012). The expression of CX3CL1 significantly increased in ischemic brain (Zhu et al. 2009). Compared to wild-type controls, knockout mice lacking CX3CL1 or CX3CR1 had smaller infarct volumes reduced blood-brain barrier (BBB) leakage, lower mortality and enhanced functional recovery after focal cerebral ischemia (Cipriani et al. 2011; Denes et al. 2008; Soriano et al. 2002). Intracerebroventricular administration of exogenous CX3CL1 to wild type rats subjected to permanent focal cerebral ischemia significantly reduced infarction and neurologic deficits. Correspondingly, exogenous CX3CL1 treatments in CX3CL1-deficient mice aggravated ischemic brain damage (Cipriani et al. 2011). Altogether, these studies suggest that the role of CX3CR1 receptors is dependent on constitutive baseline signaling. When normal CX3CR1-mediated signaling is present in microglia during development, exogenous CX3CL1 protects against cerebral ischemia; but when constitutive CX3CL1/CX3CR1 signaling is not present, further addition of CX3CL1 into the system significantly alters microglial response and exacerbates injury after brain ischemia (Cipriani et al. 2011).
The CX3CL1/CX3CR1 pathway is involved in multiple signaling cascades but its neuroprotective actions may primarily involve its ability to inhibit microglia by regulating the release of proinflammatory substances. CX3CL1 significantly reduced neuronal death induced by lipopolysaccharide (LPS)- or interferon-gamma-activated microglia, and these mechanisms appear to involve the inhibition of nitric oxide (NO) production, tumor necrosis factor α (TNFα) and interleukin (IL)-6. Neutralizing antibodies against endogenous CX3CL1 abrogated this protective effect, suggesting that the function of CX3CL1 as an anti-inflammatory chemokine and an intrinsic inhibitor against neurotoxicity may depend on its ability to control the activation state of microglia (Mizuno et al. 2003; Zujovic et al. 2000). Compared to wild-type, LPS-treated neuron-glial co-cultures prepared from CX3CR−/− mice produced a reduced amount of TNFα, NO and superoxide; however, CX3CL1 treatment inhibited the release of pro-inflammatory factors in wild-type cultures (Mattison et al. 2013). In contrast, microglia from CX3CR1−/− mice produced higher IL-1β after LPS treatment, and was toxic when transplanted into wild-type brains (Cardona et al. 2006). CX3CL1-CX3CR1 signaling is multi-faceted, but clearly, it plays a key role in linking neuronal responses to microglial inflammation.
Normal neurons, not microglia, release CX3CL1, and mild to moderate excitotoxic neuronal damage amplifies CX3CL1 production, consistent with the putative role of CX3CL1 as a “help me” signal to modulate microglia phagocytosis (Noda et al. 2011). In neuron-microglia cocultures, CX3CL1 effectively decreased excitotoxic neuronal death by upregulating the expression of MFG-E8 that may enhance microglial phagocytosis and the clearance of damaged neurons (Fuller and Van Eldik 2008; Leonardi-Essmann et al. 2005; Noda et al. 2011). In addition, CX3CL1 upregulates microglial expression of the antioxidant enzyme heme oxygenase-1, but does not further elevate the production of neurotoxic glutamate, TNF or NO (Noda et al. 2011).
Beyond ischemia and excitotoxicity per se, beneficial effects of CX3CL1 have also been documented in other injury conditions such as 6-hydroxydopamine (6-OHDA) or MPTP models of Parkinson’s disease (Cardona et al. 2006; Pabon et al. 2011). Compared with wild-type mice, CX3CL1−/− or CX3CR1−/−mice displayed similar enhancement of dopaminergic neuronal loss induced by MPTP injection, indicating the perturbation of CX3CL1-mediated modulation of microglial activity worsens neuronal cell death (Cardona et al. 2006). Treatment with exogenous CX3CL1 reduced striatal injury and dopaminergic neuronal losses by suppressing microglia activation in 6-OHDA-damaged rats (Pabon et al. 2011).
The wide spectrum of studies in cell models, transgenic mice and in vivo models of CNS injury and disease, all suggest that CX3CL1 may be a key extracellular mediator that links neuronal perturbations to microglial response. Taken together, these phenomena enable neurons to modify the actions of microglial inflammation, thus allowing CX3CL1 to serve as a candidate help-me signal in the CNS.
2.2 IL-34/CSF1R
The cytokine interleukin-34 (IL-34) is a novel ligand of colony stimulating factor-1 receptor (CSF1R). CSF1R is a member of the platelet-derived growth factor family, and possesses a highly glycosylated extracellular region comprising five immunoglobulin domains, a transmembrane domain, and an intracellular tyrosine kinase domain (Stanley and Chitu 2014). IL-34 and colony-stimulating factor 1 (CSF1) lack sequence homology, but each is secreted as a dimeric glycoprotein and both bind similar regions of the CSF1R with similar affinities (Zelante and Ricciardi-Castagnoli 2012). IL-34 is broadly expressed in various organs including heart, brain, lung, liver, kidney, spleen, and colon (Lin et al. 2008). In the brain, IL-34 is mainly produced by neurons (Greter et al. 2012; Wang et al. 2012). Embryonic mRNA expression of IL-34 occurs before CSF1 expression in most brain regions (Wei et al. 2010), suggesting that IL-34 and CSF1 may play unique non-overlapping roles during development and adulthood (Hamilton and Achuthan 2013).
The major function of IL-34 is to stimulate the proliferation and differentiation of monocytes/macrophages through CSF1R, which is also shared by CSF1 (Mizuno et al. 2011). The development of microglia is independent of CSF but highly dependent on CSF1R signaling, and microglia are present in CSF1-deficient mice but absent from CSF1R-deficient mice (Greter et al. 2012; Wang et al. 2012). Notably, IL-34 but not CSF1, contributes to the development of microglia, and IL-34 deficient mice display a reduction of microglia, whereas monocytes/macrophages and dendritic cells are not affected (Greter et al. 2012; Wang et al. 2012).
IL-34 provides potent neuroprotection via microglia modulation. IL-34 protein in cell lysates was detected primarily in non-treated neurons but not in microglia and astrocytes, and CSF1R was only expressed in microglia, not neurons and astrocytes (Mizuno et al. 2011). The lack of IL-34 and consequent lower abundance of microglia impaired CNS defenses against virus infection (Wang et al. 2012). IL-34 promoted microglial proliferation, and IL-34-treated microglia increased the clearance of β amyloid (Aβ) 1-42 via upregulation of Aβ degrading enzyme insulin-degrading enzyme, and the production of antioxidant heme oxygenase-1 (Mizuno et al. 2011). IL-34-treated microglia could decrease Aβ neurotoxicity in neuron-microglia co-cultures but this protective effect was not observed in neuron cultures alone, which suggests that the protective effect of IL-34 might be indirect and mediated via microglia (Mizuno et al. 2011). In an APP/PS1 transgenic mouse model of Alzheimer’s disease, administrating IL-34 intracerebroventricularly reduced Aβ levels and improved associative learning (Mizuno et al. 2011).
Recently, IL-34 was shown to protect against neurodegeneration, and this effect may be related to CSF1R signaling within the hippocampus and cortex. Neuronal expression of CSF1R is increased after kainic acid injections (Luo et al. 2013). Systemic administration of CSF1 and IL-34 reduced neuronal excitotoxicity and gliosis in wild-type mice, and selective cerebral deletion of CSF1R in mice exacerbated excitotoxic neurodegeneration (Luo et al. 2013). Endogenous CSF1 is upregulated in neurons after excitotoxic injury (Luo et al. 2013), but no studies have described changes of IL-34 in damaged neurons so far. Future studies to map neuronal IL-34 responses are warranted to determine whether these hypothesized mechanisms may be consistent with the role of CSF1R signaling as a help-me pathway in the brain.
2.3 Fibroblast growth factor 2
Fibroblast growth factors (FGFs) are a superfamily of proteins, most of which bind heparin and extracellular heparin sulfate proteoglycans and have a homologous central core of 140 amino acids (Burgess and Maciag 1989). FGF2 is expressed in different isoforms with distinct molecular weights (Forthmann et al. 2015). Signaling of FGF2 occurs through the high-affinity tyrosine kinase receptors FGFR1-4 (Jaye et al. 1992). FGF2 has pleiotropic effects in different tissues and organs, including potent angiogenic effects and an important role in differentiation and function in CNS (Woodbury and Ikezu 2014). In mammalian brain, FGF2 promotes neurogenesis by stimulating the proliferation and differentiation of neural stem cells (Mudo et al. 2009). Here, instead of discussing the well-known effects of FGF2 on neuroprotection, neurogenesis and angiogenesis, we will focus on the novel role of FGF2 as a candidate neuronal help-me signal. Basically, FGF2 can be released from damaged neurons, and mediates crosstalk between degenerating neurons and microglia (Figueiredo et al. 2008; Noda et al. 2014).
Intracerebroventricular administration of FGF2 in rats induced the appearance of reactive microglia with a multipolar and granular morphology, and doubled the number of microglia (Goddard et al. 2002). FGF2 plays a pivotal role in preventing quinolinic acid-induced neurotoxicity via the FGFR1 receptor after being released by neurons in the presence of microglia (Figueiredo et al. 2008). Cerebellar granule neurons became resistant to quinolinic acid-induced cell death when cultured with microglia or in the presence of mixed culture conditioned medium (Figueiredo et al. 2008). FGF2 was upregulated in neurons, not microglia and secreted and enriched in mixed culture conditioned medium, and the protective effect of mixed culture conditioned medium was lost when FGF receptor was impaired or when FGF2 was depleted from the conditioned medium of the mixed culture (Figueiredo et al. 2008).
In another study, damaged neurons rapidly released neuroprotective levels of FGF2 that also augmented microglial migration via FGFR3-Wnt-ERK signaling (Noda et al. 2014). Neurons comprise the major source of stimulated FGF2 release (Noda et al. 2014). In contrast, FGF2 secretion by astrocytes was not enhanced by various stimuli including glutamate, LPS, Aβ, and other proinflammatory cytokines. FGF2 significantly augmented microglial migration, and conditioned media from glutamate-treated neurons could attract microglia (Noda et al. 2014). FGF2 dose-dependently ameliorated neurotoxicity of glutamate in neuron-microglia co-cultures but not in neurons alone, while an anti-FGF2 antibody canceled the effect, suggesting that the neuroprotective affects of FGF2 involves its ability to suppress the production of neurotoxic molecules from activated microglia, such as glutamate and NO (Noda et al. 2014). Taken together, this collection of studies may be consistent with the role of FGF2 as a neuronal help-me signal.
2.4 Lipocalin-2
Lipocalin-2 (LCN2), also known as neutrophil gelatinase-associated lipocalin, siderocalin, uterocalin or 24p3, belongs to the lipocalin superfamily which includes a group of about 20 small secreted lipoproteins. Lipocalin superfamily acts as the transporters of small hydrophobic substances such as prostaglandins, retinoids, arachidonic acid, hormones and fatty acids (Bolignano et al. 2010; Flower 1996). LCN2 captures and transports iron particles to the inner cell by interacting with specific membrane receptors (24p3R or megalin) (Goetz et al. 2000). Although initially identified as an antibacterial factor released from activated neutrophils (Flower 1996; Kjeldsen et al. 1993), LCN2 can be induced by many organs in response to injury and participates in inflammation and tissue remodeling. LCN2 is produced by renal tubular cells during kidney disease, and may be an early and specific biomarker of organ damage and prognosis (Bolignano et al. 2008). LCN2 is also increased after myocardial infarction, in both necrotic and surrounding healthy tissues (Hemdahl et al. 2006; Yndestad et al. 2009).
LCN2 may also be important in CNS disease. In human stroke, serum levels of LCN2 progressively increased following acute ischemia and transient ischemic attacks, and persisted for up to 1 year (Anwaar et al. 1998; Elneihoum et al. 1996). LCN2 levels in CSF were elevated in multiple sclerosis patients (Marques et al. 2012). LCN2 was increased in postmortem brain tissue of Alzheimer’s disease patients (Naude et al. 2012). In rat brain, LCN2 mRNA and protein were upregulated after neuronal injury induced by kainite (Chia et al. 2011) or after neuroinflammation induced by systemic LPS injections (Ip et al. 2011). Some papers showed that astrocytes produced LCN2 (Bi et al. 2013), but other papers showed that LCN2 colocalized with neurons, not astrocytes or microglia (Jeon et al. 2013; Mucha et al. 2011; Skrzypiec et al. 2013). Our data suggest that ischemic neurons (not glia) produced LCN2 in rat and human stroke brains (Xing et al. 2014).
The effects of LCN2 are likely to be disease, model, and species-dependent. In a mouse model of renal damage, LCN2 protected the kidney against ischemia-reperfusion injury (Mori et al. 2005). Macrophages that overexpress anti-inflammatory factor IL-10 were protective in rat models of kidney dysfunction via iron-mediated upregulation of LCN2 and its receptors, eliciting both anti-inflammatory and proliferative responses (Jung et al. 2012). LCN2 is being increasingly explored in CNS disease. In models of amyotrophic lateral sclerosis and spinal cord trauma, LCN2 induced oxidative stress in neurons (Berard et al. 2012; Lee et al. 2009; Rathore et al. 2011). Recently, our findings provided proof of concept that LCN2 was released by injured neurons as a help-me signal that activated microglia and astrocytes into potentially prorecovery phenotypes (Xing et al. 2014). LCN2 was upregulated in injured neurons in rat cerebral ischemia and human stroke patients. The release of LCN2 increased in neuronal conditioned media after oxygen-glucose deprivation (OGD), but not detected in microglial or astrocytic cultures. After OGD in vitro or cerebral ischemia in vivo, LCN2 released by injured-but-not-dead neurons shifted astrocytes and microglia into beneficial phenotypes to protect neurons against OGD and promote neuroplasticity. A recent study showed that astrocytes expressed LCN2 and LCN2 deficiency attenuated neuroinflammation and tissue damage in mouse models of transient cerebral ischemia (Jin et al. 2014a), seemingly at odds with our hypothesis. Here, species differences may be critical because the mouse gene differs from rat and human homologs (Tsaparas et al. 2006; Zhao et al. 2004). Rigorously understanding how LCN2 help-me signaling contributes to the balance between injury and repair should be useful for pursuing future therapeutics for stroke recovery.
2.5 Neuron-derived IgG
Although IgG is most commonly thought of as a blood protein, it can also be produced by neurons. IgG-positive neurons are found in cerebral cortex, hippocampus, mesencephalon, cerebellum, lower brainstem, dentate gyrus, and the spinal cord (Huang et al. 2008; Niu et al. 2011; Yoshimi et al. 2002; Zhang et al. 2013a; Zhang et al. 2013b). Although similar IgG-positive neurons are observed in rabbit, rat, and mouse brains, each species have a characteristic distribution. Compared to rat and mouse brains, IgG-positive neurons are more abundant in rabbit brains (Yoshimi et al. 2002). No positive IgG signals are detected in astrocytes and oligodendrocytes (Zhang et al. 2013b). But a fraction of microglia is IgG positive (Yoshimi et al. 2002; Zhang et al. 2013b). FcγRs (mainly CD64, the receptor with the highest affinity for IgG) is expressed in microglia (Niu et al. 2011; Vedeler et al. 1994; Zhang et al. 2013a). Astrocytes, oligodendrocytes and neurons do not appear to express FcRs (Vedeler et al. 1994).
Neuron-derived IgG protects neurons against cell death through CD64 and TLR4 pathway and attenuating the release of NO by microglia (Zhang et al. 2013a; Zhang et al. 2013b). IgG production increased in primary cultured neurons after exposure to 6-OHDA or complement, and neuron-derived IgG reduced apoptosis of neurons and inhibited secondary NO release from microglia in these models (Zhang et al. 2013a; Zhang et al. 2013b). Neuron-derived IgG triggered microglial activation, including morphological changes and production of TNFα and IL-10 to protective levels via the FcγR I and TLR4 pathways, and this effect could be attenuated by IgG blocking (Zhang et al. 2013b). Cultured microglia also showed FcR-mediated agglutination and phagocytosis of IgG-sensitized erythrocytes (Vedeler et al. 1994). Therefore, it is possible that under some specific circumstances, injured neurons may release extracellular IgG that serve as help-me signals that amplify endogenous protective responses against further neurotoxic stimuli. Further studies are warranted to explore these potential pathways in various models of stroke and brain injury.
3. Extracellular signals within the neurovascular unit for neuroprotection and neurorecovery
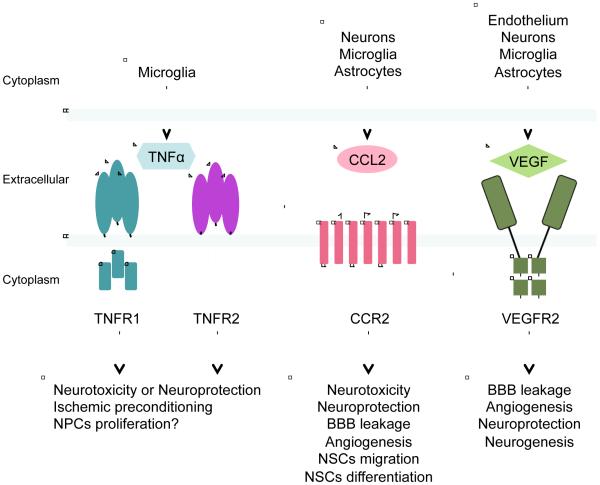
Besides neuronal help-me signals, other extracellular proteins may also be involved in the interaction between different cells in the neurovascular unit. Importantly, many of these mediators are biphasic in nature, comprising a complex mix of help-me signals, DAMPs and PAMPs. Nevertheless, in theory, if one can distinguish the differential signaling mechanisms of detrimental versus beneficial CNS responses, one may design combination therapies to protect and repair the neurovascular unit. Here, we select three representative examples (a cytokine TNFα, a chemokine CCL2, and a growth factor VEGF) to discuss the effects of extracellular help-me signals in non-cell autonomous mechanisms of neuroprotection and neurorecovery after ischemic stroke (Figure 4).
Figure 4. Extracellular signals within the neurovascular unit for neuroprotection and neurorecovery.
Three representative examples (TNFα, CCL2, and VEGF) of extracellular signals may be involved in the interaction between different cells in the neurovascular unit and in non-cell autonomous mechanisms of neuroprotection and neurorecovery after ischemic stroke.
3.1 Cytokines: tumor necrosis factor α
Cytokines comprise a group of multifunctional polypeptides with low molecular weight. Almost all cytokine family members possess broad-spectrum pleiotropic properties including the regulation of cell proliferation, differentiation, and activation (Sriram and O'Callaghan 2007). In the CNS, TNFα is a stereotypical cytokine that is central to multiple physiologic processes as well as classical inflammatory responses (Park and Bowers 2010). In the healthy brain, constitutive TNFα exerts a permissive and regulatory function on crucial physiological processes, such as learning and memory, food and water intake, sleep, and synaptic plasticity, including astrocyte-induced synaptic strengthening (glial transmission) (Santello and Volterra 2012).
3.1.1 TNFα and its receptors
TNFα is synthesized as a 26kDa transmembrane protein precursor. Upon cleavage by TNFα converting enzyme, a soluble form is released as a homotrimer protein of 17kDa subunits that go onto act in various autocrine and paracrine pathways (Sriram and O'Callaghan 2007). TNFα can be produced by many cell types including macrophage, lymphocytes, monocytes, dendritic cells and natural killer cells (Kaltsonoudis et al. 2014). In the brain, microglia are a prominent source of TNFα, although TNFα can also be released by astrocytes and some populations of neurons under specific conditions (Figiel 2008; Montgomery and Bowers 2012).
The biological activities of TNFα take place via two receptors, TNFR1 (p55) and TNFR2 (p75), which belongs to the TNF receptor superfamily which includes other receptors such as Fas, CD40, p75 nerve growth factor receptor, lymphotoxin β receptor, etc (Kaltsonoudis et al. 2014; Sprang 1990; Sriram and O'Callaghan 2007; Tartaglia et al. 1991). TNFR1 may be expressed in most CNS cell types, whereas TNFR2 is mainly found on endothelium and microglia (Santello and Volterra 2012). TNFα signal transduction is complex, and the differential expression pattern of TNF receptors and downstream cascades may play a key role in determining beneficial or detrimental effects of TNFα (Santello and Volterra 2012). TNFα binding to TNFR1 affects biological processes including cell growth, cell death, and inflammation, whereas stimulation of TNFR2 preferentially leads to activation of anti-apoptotic and proinflammatory pathways (Santello and Volterra 2012). Although distinct cellular responses are mediated by different TNFα receptors, emerging data now demonstrate important overlap of two receptors in mediating its varied biological effects (Figiel 2008).
3.1.2 Profiles of TNFα expression after brain ischemia
In ischemic stroke patients, TNFα is elevated in serum, plasma and CSF samples (Intiso et al. 2004; Vila et al. 2000; Zaremba and Losy 2001). In animal models of cerebral ischemia, TNFα levels in the blood were rapidly increased during ischemia and early reperfusion (Lavine et al. 1998). In mouse models of global cerebral ischemia, TNFα increased in the brain 1.5 hours after injury, then decreased at 6 hours followed by a secondary increase again at 3 days (Uno et al. 1997). In models of focal ischemia, TNFα mRNA and protein levels were elevated by 3 hours in the ischemic hemisphere, peaked at 6 to 12 hours followed by a prolonged plateau that can persist for days (Buttini et al. 1996; Gong et al. 1998; Liu et al. 1994). In human ischemic brains, microglia probably constitutes the main cellular source of TNFα (Dziewulska and Mossakowski 2003). In animal models, TNFα may be mainly released from microglia and invading leukocytes (Buttini et al. 1996; Gregersen et al. 2000; Lambertsen et al. 2009; Sairanen et al. 2001). In addition, TNFα immunoreactivity was also showed in neurons, astrocytes, and endothelial cells (Botchkina et al. 1997). TNFα protein is localized with neurons in both infarct core and adjacent tissues at an early stage after ischemia and peaking bilaterally at 2-3 days, while TNFα expression in astrocytes and macrophages may occur in later phases (Gong et al. 1998; Liu et al. 1994; Sairanen et al. 2001).
Focal cerebral ischemia also induced a significant up-regulation of TNF receptors, with an early peak of TNFR1 around 6 hours, and a later peak of TNFR2 around 24 hours post-ischemia (Botchkina et al. 1997). Besides neurons and blood vessels, expression of TNFα receptors may be induced in glial cells (astrocytes and microglia/macrophages) after ischemia (Dziewulska and Mossakowski 2003).
3.1.3 Neurotoxic and neuroprotective effects of TNFα in cerebral ischemia: opposite roles of TNFR1 and TNFR2
Exogenous TNFα increased the infarction induced by transient or permanent focal ischemia in a dose-related manner (Barone et al. 1997). Correspondingly, neutralizing antibodies against TNFα, compounds that inhibit endogenous TNFα synthesis, or soluble TNFR1 to inhibit the activity of TNFα all significantly attenuated microvessel perfusion impairment, enhanced reperfusion, reduced infarct volume, and improved functional outcome (Barone et al. 1997; Dawson et al. 1996; Lavine et al. 1998; Meistrell et al. 1997). In vitro, it seemed that TNFα itself alone failed to kill neurons in cultured cerebellar granule cells (Barone et al. 1997), but it might be harmful to neurons when acting synergistically with other deleterious factors released from glia in cocultures (Zhao et al. 2001).
In spite of these well-documented neurotoxic actions, some studies have suggested that TNFα may also possess neuroprotective effects. TNFα protects cultured hippocampal and cortical neurons and cerebellar granule cells against glucose deprivation, excitotoxicity and Aβ (Barger et al. 1995; Cheng et al. 1994; Kaltschmidt et al. 1999). Compared with wild-type mice, TNF knockout significantly exacerbated neuron damage, infarction and behavioral deficit caused by cerebral ischemia (Bruce et al. 1996; Lambertsen et al. 2009). TNFα may also contribute to neuroprotection by upregulating the expression of neurotrophic factors in astrocytes, including nerve growth factor, brain-derived neurotrophic factor and glial-derived neurotrophic factor (Appel et al. 1997; Hattori et al. 1993; Kuno et al. 2006; Saha et al. 2006).
In many experimental studies, predominant TNFR1 activation was associated with circuit alterations and neuronal damage, whereas TNFR2 activation was protective. However, TNFα/TNFRs action is more complex than initially thought. In primary cortical neurons, TNFα-mediated protection against N-methyl-D-aspartate (NMDA)-mediated excitotoxicity is TNFR2-independent and requires the activation of the TNFR1 plus the release of endogenous TNFα (Carlson et al. 1998). But beneficial effects of TNFα against glutamate excitotoxicity are mediated by TNFR2 (Marchetti et al. 2004). In ischemia-reperfusion-induced retinal damage in mice, absence of TNFR1 potently decreased neuronal death and lack of TNFR2 enhanced neuronal death (Fontaine et al. 2002). However, compared with wild-type and TNFR2 deficient mice, deficiency of TNFR1 significantly increased neuronal death after focal cerebral ischemia-reperfusion, and degeneration of CA3 hippocampal neurons after kainic acid injections (Gary et al. 1998). Compared with TNFR2 knockout and wild-type mice, TNFR1 knockout mice had increased infarction, suggesting that neuroprotective effects of microglial-derived TNF may operate through TNFR1 (Lambertsen et al. 2009).
3.1.4 TNFα and Ischemic preconditioning
Preconditioning with TNFα may be protective against cerebral ischemia. TNFα levels in plasma were higher in acute stroke patients with prior TIA (Castillo et al. 2003). Infarct volumes and the frequency of poor outcome were significantly lower in stroke patients with prior TIA, and the TNFα/IL-6 index was associated with good outcome (Castillo et al. 2003). Preconditioning with intracisternal administration of TNFα significantly reduced infarct volume and inhibited microglial activation in a focal ischemia models (Nawashiro et al. 1997). Pre-exposure to TNFα caused a significant reduction in glutamate-induced Ca2+ influx in hippocampal cultures, and antagonism of TNFα completely reversed this effect (Watters et al. 2011). Ischemic preconditioning upregulated neuronal expression of TNFR1, and TNFR1 antisense oligodeoxynucleotide abolished the ischemic preconditioning-induced protective effect (Pradillo et al. 2005). These findings suggest that TNFα signaling participates in the phenomenon of ischemic tolerance.
TNFα is required for LPS-induced ischemic preconditioning as LPS-precondition was not protective in TNFα null mice cerebral ischemia (Rosenzweig et al. 2007), and treatment with a specific TNF antagonist reversed the protective effect of LPS preconditioning in permanent focal ischemia in mice (Tasaki et al. 1997). TNFα is also required for the preconditioning induced by tPA or TLR9 agonist unmethylated cytosine-phosphate-guanine-rich DNA oligonucleotides against the damaging effects of lethal neuronal hypoxia and cerebral ischemia (Haile et al. 2012; Packard et al. 2012).
3.1.5 Roles of TNFα in neurogenesis and angiogenesis
Neural stem cells or neural progenitor cells (NPCs) express TNFR1 and TNFR2 (Ben-Hur et al. 2003; Keohane et al. 2010)(Bernardino et al., 2008; Keohane et al., 2010), and TNFR1 and TNFR2 are also expressed in progenitor cells from hippocampal and subventricular zone (SVZ) (Iosif et al. 2008; Iosif et al. 2006).
To date, the effects of TNFα on neurogenesis remain controversial. In vitro, TNFα signaling through TNFR2 is required for NPC proliferation while signaling through TNFR1 impairs neural progenitor proliferation and induces cell death (Chen and Palmer 2013; Iosif et al. 2008). TNFα treatment inhibited the proliferation of neurospheres obtained from striatum and SVZ without affecting cell survival and did not affect NPCs lineage fate after differentiation (Ben-Hur et al. 2003; Iosif et al. 2008). In contrast, TNFα treatment promoted NPCs proliferation in culture (Widera et al. 2006). Exposure of NPCs to TNFα enhanced astrogliogenesis and inhibited neuronal differentiation, and percentages of newborn neurons reduced and percentages of astrocytes increased (Keohane et al. 2010; Lan et al. 2012; Liu et al. 2005). In contrast, exposure of NPCs to TNFα resulted in increased neuronal differentiation and axonogenesis, and the proneurogenic effect of TNFα is mediated via TNFR1 (Bernardino et al. 2008).
In vivo, TNFR1 may be involved in the negative regulation of neural progenitor proliferation in both normal and diseased brain. Baseline neurogenesis in the hippocampus elevated in TNFα−/−, TNFR1−/− and TNFR1/R2−/− animals, whereas absence of TNFR2 decreased baseline neurogenesis or showed no significant changes (Chen and Palmer 2013; Iosif et al. 2006). After focal stroke, TNFα promoted the survival of newborn striatal and hippocampal neurons via TNFR2, and TNFα antibody-treated rats showed fewer new striatal and hippocampal neurons (Heldmann et al. 2005). Concommitantly, deficiency of TNFR1 enhanced proliferation and neuroblast formation in the subventricular zones after focal cerebral ischemia (Iosif et al. 2008).
Compared to neurogenesis, the effects of TNFα on angiogenesis are not as well studied. TNFα inhibited endothelial cell proliferation in vitro, including basal and FGF-stimulated proliferation (Frater-Schroder et al. 1987). Surprisingly, in vivo TNFα stimulated neovascularization in the rabbit cornea (Frater-Schroder et al. 1987). In addition, TNFα/TNFR1 signaling was found to upregulate the EPO receptor in endothelium, thus amplifying EPO-mediated activation of VEGF/VEGFR2 and Ang1/Tie2 angiogenic pathways (Wang et al. 2011b). In primary rat cerebral endothelial cultures, TNFα potently increased EPO receptor expression; further exposure to EPO in TNFα-treated cells significantly promoted matrigel tube formation, whereas blocking TNFR1 dampened TNFα-induced EPO receptor levels and prevented EPO-induced tube formation (Wang et al. 2011b). Recently, it has been proposed that microglia enhanced in vitro angiogenesis of brain microvascular endothelial cells by releasing TNFα and upregulating the expression of angiogenic factors ephrin-A3 and ephrin-A4 (Li et al. 2014). Altogether, these data are consistent with the idea that TNFα may act as a remodeling signal within the recovering neurovascular unit.
3.2 Chemokines: CCL2/CCR2
The monocyte chemoattractant proteins (MCPs) belong to the CC-chemokine sub-family. The five MCP members share approximately 60% sequence homology, but MCP-1 (CCL2) is the first discovered and best characterized MCP and signals through its receptor CCR2 (Conductier et al. 2010). CCL2 is a potent chemoattractant for monocytes, memory T-cells and natural killer cells (Allavena et al. 1994; Carr et al. 1994; Sozzani et al. 1994). In this section, we briefly review the roles of CCL2 as an intercellular signal that underlies non-cell autonomous interactions in the neurovascular unit.
3.2.1 Expression: the CCL2/CCR2 network in brain
Constitutive CCL2 expression in neurons has been documented in many brain regions including cortex, hippocampus, substantia nigra, globus pallidus, various nuclei in hypothalamus, and Purkinje cells of the cerebellum (Banisadr et al. 2005a; Banisadr et al. 2002; Coughlan et al. 2000; Gourmala et al. 1997; Stamatovic et al. 2005; van der Meer et al. 2000). Co-distribution of CCL2 with classical neurotransmitters such as acetylcholine and dopamine suggest that CCL2 may modulate neuronal and neuroendocrine functions (Banisadr et al. 2005a; Rostene et al. 2007). Adding CCL2 to dopaminergic neurons amplified dopamine release and neuronal excitability (Guyon et al. 2009). Colocalization of CCL2 with arginine vasopressin neurons and melanin-concentrating hormone expressing neurons in pituitary and hypothalamic areas support an interaction with neuroendocrine regulation (Banisadr et al. 2005a; Callewaere et al. 2007). Furthermore, besides neurons, CCL2 can also be found in astrocytes, endothelium, and perivascular microglia (Andjelkovic and Pachter 2000; Andjelkovic et al. 1999; Barna et al. 1994; Berman et al. 1996; Glabinski et al. 1996; Gourmala et al. 1997; Hanisch 2002; Hayashi et al. 1995; Hurwitz et al. 1995; Kim et al. 1995). Altogether, this specific yet widespread network of CCL2 may be consistent with its key roles in CNS function.
In contrast to the ligand, its receptor CCR2 may participate in immune signaling. Monocytes, activated T cells, and dendritic cells, constitutively express CCR2 (Van Coillie et al. 1999). In the CNS, CCR2 can be found in microglia (Boddeke et al. 1999; Conductier et al. 2010), and reactive astrocytes that arise during neuroinflammation (Andjelkovic et al. 2002; Croitoru-Lamoury et al. 2003). CCR2 is also detected on large number of neurons, including cortex, hippocampus, striatum, hypothalamus, brainstem and cerebellum (Banisadr et al. 2005b; Rostene et al. 2007).
In ischemic stroke patients, CCL2 levels are increased in the blood and CSF (Arakelyan et al. 2005; Losy and Zaremba 2001; Sanchez-Moreno et al. 2004; Worthmann et al. 2010). In animal model of focal ischemia, mRNA and protein levels of CCL2 rapidly increase within hours and then remain elevated for days after ischemic onset (Wang et al. 1995; Yamagami et al. 1999). CCL2 tends to appear first in neurons in early stages of stroke, and then becomes upregulated in astrocytes later (Che et al. 2001).
3.2.2 Neurotoxicity and neuroprotection of CCL2 in ischemic stroke
Compared to normal mice, transgenic mice overexpressing CCL2 show larger infarct volumes and increased perivascular accumulation of neutrophils and macrophages in the brain (Chen et al. 2003). Conversely, focal ischemia in CCL2 deficient mice resulted in reduced infarct size, improved neurological functions, impaired leukocyte infiltration, and reduced inflammatory markers such as IL-6, IL-1β, and G-CSF (Hughes et al. 2002; Kumai et al. 2004; Strecker et al. 2011). CCL2 may be required for recruiting blood cells to damaged brain; neutrophils and macrophage infiltration was reduced in CCL2-deficient animals compared to wild type mice without a detectable effect on microglia (Schilling et al. 2009). Analogous findings have been obtained in receptor mutant mice. Compared with wildtypes, CCR2 knockouts were be protected against ischemia-reperfusion injury, along with a decrease in brain levels of cytokines, neutrophils, and monocytes (Dimitrijevic et al. 2007). However, as with any inflammatory mediator, its ultimate role in brain injury and recovery is complex and may depend on the context of stroke evolution over time. Using MRI, another study suggested that by 3 days after focal ischemia, brain infarction was essentially similar in wild-type mice and CCL2 knockout mice (Andres et al. 2011).
During the acute stage, CCL2-CCR2 signaling may be detrimental due to its effects on leukocyte targeting into damaged brain. But during the recovery stage, emerging data suggest a potentially positive role for this complex signaling network. In primary neurons, CCL2 was protective against oxygen-glucose deprivation or glutamate toxicity, in part by blocking intracellular calcium buildup, dampening secondary glutamate efflux, and ameliorating the subsequent loss of ATP (Madrigal et al. 2009). In mixed neuron-astrocyte cultures, CCL2 was protective against NMDA or Tat neurotoxicity by inhibiting increases in extracellular glutamate and NMDA receptor 1 expression (Bruno et al. 2000; Eugenin et al. 2003). In vivo, exogenous CCL2 was neuroprotective in the wild-type mice after HIV-1 Tat injection into striatum, but not in CCR2 knockout mice, suggesting the neuroprotection of CCL2 is mediated by CCR2 (Yao et al. 2009).
Besides being a direct neuroprotective agent, CCL2 may also be involved in the regulation of other protective factors. The neuroprotective effects of norepinephrine were partially mediated by CCL2 released from astrocyte, and was blocked by neutralizing antibody of CCL2 (Madrigal et al. 2009). In hippocampal neurons subjected to glutamate or oxygen-glucose deprivation, the protective effect of CXCL16 was dramatically smaller when co-administered with CCL2 blocking antibodies (Rosito et al. 2012).
3.2.3 CCL2 and blood-brain barrier permeability and angiogenesis
Like many other factors that promote angiogenesis will increase vascular permeability, such as VEGF (see the discussion in next section), CCL2 may also possess biphasic properties. It may be deleterious in terms of reducing BBB integrity by regulating tight junctions and cytoskeleton. It may be beneficial because it may play a role in promoting neovascularization. CCL2 can affect BBB permeability, in part because of its ability to modulate the actin cytoskeleton and regulate the intracellular versus membrane localization of tight junction proteins such as ZO-1, claudin-5 and occludin (Stamatovic et al. 2003). In astrocyte-endothelial cocultures subjected to OGD, blocking CCL2 with antisense oligonucleotide or neutralizing antibody improved the distribution of tight junction proteins and rescued BBB function (Dimitrijevic et al. 2006). Intracerebral and intracerebroventricular administration of CCL2 induced a significant increase in the BBB permeability, whereas monocytes/macrophages depletion reduced the effect of CCL2 on BBB integrity (Stamatovic et al. 2005). Compared to wild-type mice, CCL2–deficient mice had better preservation of tight junction proteins and BBB function after transient focal cerebral ischemia (Strecker et al. 2013).
CCL2 is a key factor in angiogenesis (Keeley et al. 2008), and its function to promote neovascularization has been demonstrated in a wide spectrum of in vitro and in vivo models (Barcelos et al. 2004; Galvez et al. 2005; Goede et al. 1999; Niu et al. 2008; Salcedo et al. 2000; Stamatovic et al. 2006; Weber et al. 1999). CCL2 can act as a direct angiogenic factor (Salcedo et al. 2000). CCL2 increased the expression, clustering, and activity of membrane type 1-matrix metalloproteinase and promoted tube formation in human endothelium. Blocking membrane type 1-matrix metalloproteinase activity effectively negates the pro-angiogenic actions of CCL2 (Galvez et al. 2005). The transcription factors Ets-1 and MCP-1 induced protein play a critical role in CCL2-induced angiogenesis. CCL2 upregulates both factors, and Ets-1 antisense oligonucleotide or knockdown of MCP-1 induced protein by siRNA suppressed CCL2-induced angiogenesis (Niu et al. 2008; Stamatovic et al. 2006). Finally, CCL2 can also be linked with the two standard networks for angiogenesis, i.e. hypoxia-inducible factor 1α and vascular endothelial growth factor (VEGF) (Hong et al. 2005).
3.2.4 Roles of CCL2 in migration and differentiation of neural stem cells
NPCs are known to respond to chemokine gradients during migration and differentiation. In this context, the expression of CCR2 on NPCs may be relevant (Tran et al. 2004). Using a Boyden chamber assay, NPC migration increased in response to CCL2 in vitro (Magge et al. 2009; Widera et al. 2004). Time-lapse video microscopy visualized the migration of single stem cells from neurospheres in CCL2-treated cultures, whereas no migration occurred in untreated cultures (Widera et al. 2004). In vivo, infusion of CCL2 into the brain induced neuroblasts migration to the infusion site (Magge et al. 2009; Yan et al. 2007).
The putative role of CCL2 in adult neurogenesis has been explored in experimental stroke (Semple et al. 2010). After focal ischemia, neuroblasts derived from SVZ neural progenitors migrate towards the injured brain regions (Arvidsson et al. 2002; Jin et al. 2001a; Parent et al. 2002; Zhang et al. 2004), and CCL2 signaling may be involved in this phenomenon. During the migration of newly formed neuroblasts, CCL2 plays an important role. Transciptional analysis of SVZ NPCs in this model suggest that CCL2 is one of the most robustly upregulated genes after focal cerebral ischemia (Liu et al. 2007). CCL2 expression and the number of CCL2-positive cells were significantly increased in ischemic cortex, striatum and SVZ (Liu et al. 2007; Yan et al. 2007). CCL2 also promotes neuronal differentiation in vitro. Treating NPCs with CCL2 dose-dependently increased the number of Tuj1-positive cells (Liu et al. 2007). Ultimately, the migration and differentiation of NPCs is CCL2/CCR2-dependent since blocking CCL2 with neutralizing antibodies or gene knockdown of either CCL2 or CCR2 almost completely negates this phenomenon (Liu et al. 2007; Yan et al. 2007).
3.3 Vascular endothelial growth factors (VEGF)
VEGF is one of the most well characterized trophic factors in vascular development, homeostasis and pathology. In the context of stroke, brain injury and neurodegeneration, the various isoforms of VEGF have been implicated in atherosclerosis and blood vessel disease, BBB leakage and brain edema, neurogenesis and angiogenesis during CNS repair and remodeling, and the interactions between central and peripheral compartments during cell therapies (Greenberg and Jin 2013). VEGF-A and its receptor VEGFR-2 are considered to be the most active members of VEGF family to mediate these various effects. Here, we survey the literature that supports a role for VEGF as a non-cell autonomous mediator for help-me signaling between different elements in the neurovascular unit.
3.3.1 VEGF and its receptor family
VEGF is a member of the cysteine knot growth factor family (Keck et al. 1989; Leung et al. 1989; Senger et al. 1983). In humans, the VEGF family includes VEGF-A (typically just termed as VEGF in the literature), VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF) (Ma et al. 2012). Human VEGF-A gene contains eight exons and seven introns which undergo extensive alternative splicing following transcription and proteolytic processing, thereby leading to the production of several isoforms (Tischer et al. 1991). Isoforms of VEGF-A include: VEGF121, VEGF121b, VEGF145, VEGF145b, VEGF165, VEGF165b, VEGF183, VEGF189, and VEGF206 (Crafts et al. 2015).
Three different VEGF receptors (VEGFR-1, -2, -3), which belong to tyrosine kinase receptor family, bind differentially to the VEGF peptides. VEGFR-2, known as kinase domain-containing receptor in humans and Flk-1 in murine systems, is the main mediator of angiogenesis and vascular permeability by binding VEGF-A, VEGF-C and VEGF-D (Matsumoto and Claesson-Welsh 2001; Shalaby et al. 1995). VEGFR-1, also known as Flt-1, binds VEGF-A, VEGF-B and PlGF. Although most VEGF signals are pro-angiogenic individually, network function is more complex in its entirety. For example, by acting as a decoy receptor, VEGFR-1 may downregulate angiogenesis by preventing VEGFR-2 from binding VEGF-A (Ferrara et al. 2003; Park et al. 1993; Roskoski 2008). VEGF signals operate outside of the main vascular system per se. VEGFR-3 (also called Flt-4) is involved in lymphangiogenesis (Gordon et al. 2013; Veikkola et al. 2001).
3.3.2 Regulation of VEGF signaling in cerebral ischemia
Changes in VEGF have been described in many studies of cerebral ischemia and brain injury. In focal stroke models, rapid upregulation of VEGF in the penumbra occurs within a few hours, peaks by 24 hours, and then is sustained at a lower elevated level up to a week later (Plate et al. 1999). Cellular distributions are complex. In transient models of focal ischemia, VEGF mRNA and protein levels are significantly increased within 1-3 hours after reperfusion in neurons and pial cells, and then decreased in neurons but sustained in the pia for up to a week post-reperfusion (Hayashi et al. 1997). Other studies have shown an even broader response, and under some conditions, VEGF immunoreactivity can be increased in both ipsilateral and contralateral hemispheres in neurons as well as blood vessels (Lennmyr et al. 1998). In the evolving ischemic penumbra, VEGF mRNA and protein can be detected in astrocytes over 24-48 hors after stroke onset (Cobbs et al. 1998), while another study suggested that microglia and invading macrophages may also upregulate some VEGF isoforms in the ischemic borderzones (Plate et al. 1999).
In concert with ligand responses, various receptors including VEGFR-1 and VEGFR-2 are also altered in cerebral ischemia. After focal stroke, vascular levels of VEGFR-1 may be elevated over days to weeks along with the development of angiogenesis (Kovacs et al. 1996). VEGFR-1 immunoreactivity was widely noted in neurons, glial and endothelial cells, while VEGFR-2 immunoreactivity was most often observed in glial cells (Lennmyr et al. 1998).
3.3.3 Effects of VEGF on vascular permeability and angiogenesis
VEGF was identified originally based on two biological effects - angiogenesis (Leung et al. 1989) and vascular permeability (Keck et al. 1989). In this respect, VEGF is a perfect example of the biphasic properties of non-cell autonomous signals in the recovering neurovascular unit. As a beneficial signal, VEGF may promote recovery angiogenesis by increasing the proliferation and migration of endothelial cells during in the recovery and repair phases of pathological conditions. But as a deleterious signal, VEGF may worsen BBB leakage and induce the formation of brain edema after brain injury. In rat models of stroke, early administration of VEGF (within 1 hour of onset) impaired outcome and exacerbated BBB permeability and hemorrhagic conversion, whereas delayed treatment (48 hours after onset) was beneficial by promoting neurovascular repair and recovery (Zhang et al. 2000). During the acute stage of focal cerebral ischemia, inhibition of VEGF by receptor antagonists or anti-VEGF antibodies prevented BBB leakage, ameliorated hemorrhagic conversion, and improved behavioral outcomes (Chi et al. 2007; Kimura et al. 2005; van Bruggen et al. 1999). Combination of angiopoietin-1 and VEGF or coexpression of angiopoietin-1 with VEGF augmented BBB integrity and reduced edema and brain damage after ischemia, but did not affect the angiogenic effects of VEGF (Shen et al. 2011; Valable et al. 2005; Zhang et al. 2002). Thus further dissection of the complex VEGF signaling networks may ultimately provide ways of separating beneficial and deleterious effects for therapeutic gain.
As a potential pro-recovery molecule, VEGF enhances angiogenesis in the ischemic brain and reduces neurological deficits during delayed stages post-injury. Late intracerebroventricular administration (48 hours after focal ischemia) of VEGF165 dramatically improved microvascular perfusion and angiogenesis in the penumbra and improved neurological recovery (Zhang et al. 2000). Chronic intraventricular infusions of VEGF165 dose-dependently increased microvessel density (Harrigan et al. 2002). In a rat focal ischemia model, intracerebroventricular administration of VEGF between 1-3 days of reperfusion increased the number of von Willebrand factor-immunoreactive endothelial cells in the ischemic striatal core (Sun et al. 2003). Although network interactions between multiple ligand and receptor isoforms are complex, the primary pro-angiogenic effects of VEGF are thought to occur via VEGFR-2 (Ferrara et al. 2003), since VEGFR-2 deficient knockout die in utero because of defects in vasculogenesis (Shalaby et al. 1995).
3.3.4 Effects of VEGF on neuroprotection and neurogenesis
The sum of the literature suggests that VEGF may be a potent neuroprotector against cerebral ischemia. VEGF protected primary cultured neurons from excitotoxicity and OGD (Jin et al. 2000; Matsuzaki et al. 2001; Svensson et al. 2002). Direct VEGF treatments onto rat brain reduced infarct volume and neuronal damage post-ischemia-reperfusion (Hayashi et al. 1998). Intracerebroventricular infusion of VEGF165 after focal cerebral ischemia reduced infarction in a blood flow-independent manner(Harrigan et al. 2003), whereas intraventricular injection of VEGF antibody exacerbated infarction (Bao et al. 1999). Hence, VEGF may have non-vascular actions in the context of CNS injury. Overexpression of VEGF or treatments with VEGF decreased infarction (Wang et al. 2005), and improved functional recovery after focal ischemia by downregulating caspase-3 and preventing neuronal dropout without any direct effects in angiogenesis (Kaya et al. 2005; Sun et al. 2003; Wang et al. 2006).
Beyond angiogenesis per se, VEGF may also have effects in neurogenesis. In cortical neuronal precursors cultures, VEGF increased cell number and 5-bromo-2'-deoxyuridine (BrdU) incorporation, an effect that can be blocked by the VEGFR2 tyrosine kinase inhibitor SU1498 (Jin et al. 2002). In vivo, injections of VEGF into the ventricles increased BrdU-labeled cells in the two primary neurogenic zones, i.e. SVZ and subgranular zones of the dentate gyrus, and these signals were detected in multiple cell types comprising immature and mature neurons, glial cells, and endothelial cells (Jin et al. 2002). In adult rats, VEGF gene transfer into the hippocampus almost doubled rates of neurogenesis and augmented cognition, whereas inhibition of VEGF with RNA interference abolished this neurogenic response (Cao et al. 2004).
VEGF enhances neurogenesis not only in normal brain, but also in ischemic brain. Intraventricular injections of VEGF during early stages of reperfusion after focal stroke enhanced the survival of newborn neurons in the SVZ and dentate zones of neurogenesis (Sun et al. 2003). VEGF overexpression amplified the proliferation of neural progenitors in the SVZ, subgranular zone and dentate gyrus, increased the numbers of immature and mature newborn neurons and significantly improved their migration towards lesioned brain (Li et al. 2009; Wang et al. 2007b). In transgenic mice overexpressing VEGF, SVZ neurogenesis markedly increased at 7-28 days after cerebral ischemia, neuroblasts appeared to extend into cortical penumbral regions, and the number of newly generated neurons may even persist for up to 14-28 days post-ischemia (Wang et al. 2007a).
3.4 Roles of help-me signals in neurogenesis and angiogenesis
The sections above briefly surveyed three representative examples of neurovascular unit signals drawn from cytokine, chemokine and growth factor families. In the context of endogenous protective programs, these various extracellular factors can also be interpreted as adaptive help-me signals that promote recovery by augmenting neurogenesis and angiogenesis in a damaged or diseased brain.
3.4.1 CX3CL1/CX3CR1 and neurogenesis
CX3CL1/CX3CR1 signaling is involved in neuroplasticity. It has been proposed that CX3CR1 deficiency may promote IL-1β signaling, thus interfering with synaptic homeostasis and cognition (Rogers et al. 2011). CX3CL1 is upregulated in the hippocampus during memory-associated synaptic plasticity (Sheridan et al. 2014), and CX3CL1/CX3CR1 signaling regulates hippocampal neurogenesis by directly modifying the niche environment (Bachstetter et al. 2011). Disruption in CX3CL1/CX3CR1 signaling in young adult rodents decreased survival and proliferation of neural progenitors through IL-1β (Bachstetter et al. 2011). Aged rats showed decreased CX3CL1 in hippocampus, and interruption of CX3CR1 in these aged brains did not yield further effects on neurogenesis (Bachstetter et al. 2011). Interestingly, injection of exogenous CX3CL1 reversed these age-related perturbations in hippocampal neurogenesis, but exogenous CX3CL1 did not change neurogenesis in young animals (Bachstetter et al. 2011). If CX3CL1 can be fully defined as a help-me signal, these pathways may provide new leads for regrowing neural circuits in order to repair damaged brain tissue.
3.4.2 IL-34 and blood-brain barrier and angiogenesis
CSF1R is also expressed in microvessel endothelial cells in the CNS (Jin et al. 2014b). A novel function of IL-34 in the BBB has been recently described. IL-34 upregulated the tight junction proteins claudin-5 and occluding, and reversed BBB disruption induced by pro-inflammatory cytokines (IL-1β and TNFα) (Jin et al. 2014b). In addition, IL-34 overexpression is associated with an increase of angiogenesis (Segaliny et al. 2014). In vitro, IL-34 stimulated endothelial cell proliferation and vascular cord formation, and pre-treatment of endothelial cells by chondroitinases/heparinases reduced matrigel tube formation and abolished the associated cell signaling (Segaliny et al. 2014). Hence, promoting IL-34 pathways may augment neurovascular repair.
3.4.3 Lipocalin-2 and angiogenesis
As a candidate help-me factor, LCN2 may also function as an angiogenic factor. LCN2 promoted angiogenesis in human breast cancer cells (Yang et al. 2013), and these effects are thought to occur via the upregulation of VEGF via hypoxia-inducible factor 1α and ERK signaling, suggesting that VEGF may be essential for the angiogenic activity of LCN2 (Yang et al. 2013). LCN2 may also enhance angiogenesis in brain endothelial cells (Wu et al. 2015). LCN2 promoted matrigel tube formation and wound healing migration via iron and ROS-related pathways in rat brain endothelial cells, and ROS scavengers, Nox inhibitors and iron chelators all dampened the ability of LCN2 to enhance in vitro angiogenesis in brain endothelial cells (Wu et al. 2015). Because LCN2 can be released by damaged-but-not-dead neurons as a help-me signal, this factor could potentially serve a critical role not only in modulating neuroinflammation but also as a way for a damaged neurovascular system to repair itself.
4. Endogenous protective mechanisms and secreted help-me signals
In this review, we have attempted to introduce the concept of help-me signaling as a non-cell autonomous mechanism for neuroprotection and neurorepair. The accumulating literature has provided many candidate factors for this phenomenon. However, it is also clear that such signals cannot operate alone. It is likely that help-me signaling involves an integrated and recursive network of mediators. How would one begin to find more factors and build a representation of this network? Here, we propose that analyses of the transcriptome and secretome of the perturbed neurovascular unit may provide a way forward. The transcriptome should provide insight into intercellular mechanisms. The secretome should provide insight into extracellular mechanisms. And together, these databases may allow us to rigorously define the network of help-me signaling for neuroprotection and neurorecovery after stroke and brain injury.
4.1 Mapping the transcriptome
Mechanisms of damage and repair in cerebral ischemia are very complex, and analysis of the transcriptome by microarray is a useful tool for studying molecular pathophysiology and transcriptional changes (Cox-Limpens et al. 2014; Stenzel-Poore et al. 2007; VanGilder et al. 2012). Microarray studies investigating the transcriptome of both focal and global ischemia showed that the differentially expressed genes involved immediate early genes, stress response genes, apoptosis, signal transduction, neurotransmission, ion channels, inflammation, cytoskeleton, ribosome, and neurotrophic factors, et al (Buttner et al. 2009; Cox-Limpens et al. 2014; Gilbert et al. 2003; Hori et al. 2012; Jin et al. 2001b; Lu et al. 2003; Lu et al. 2004; Ramos-Cejudo et al. 2012; Sarabi et al. 2008; Schmidt-Kastner et al. 2002; Soriano et al. 2000; Sun et al. 2007; Tang et al. 2002; Wang et al. 2011a; Yakubov et al. 2004).
Preconditioning activates endogenous protective mechanisms by reprograming the brain transcriptome in order to achieve ischemic tolerance (Stenzel-Poore et al. 2007). Several studies have investigated preconditioning induced gene expression with microarrays (Bernaudin et al. 2002; Cox-Limpens et al. 2013; Feng et al. 2007; Gustavsson et al. 2007; Kawahara et al. 2004; Prasad et al. 2012; Stenzel-Poore et al. 2003; Tang et al. 2006; Truettner et al. 2002). Examining the genomic profile of focal ischemia with and without preconditioning demonstrates expression of similar genes; however, preconditioning results in a substantial down regulation of the common expressed genes (Stenzel-Poore et al. 2004). Severe and damaging levels of ischemia generally upregulated gene expression; whereas ischemic preconditioning followed by a second damaging ischemic challenge generally downregulated overall gene expression (Della-Morte et al. 2012). The genomic profile of ischemic preconditioning is characterized by suppression of gene expression involved in ion channel regulation, control of membrane excitability, metabolism, ATP regulation, cell cycle regulation, immune responses, and decreased blood coagulation (Della-Morte et al. 2012; Van Elzen et al. 2008). In spite of the promise of these array approaches, replication of individual gene responses has not been easy, and may be highly system and model-dependent. For example, a comparison effort based on single-gene analyses revealed that only about 15 genes were common in two studies or more (Cox-Limpens et al. 2014). Further cluster-based investigation into these 15 genes suggested that their common signaling pathways may be related to ERK1/2 networks that underlie cell survival and proliferation (Cox-Limpens et al. 2014). Future studies are warranted to carefully define sources of similarity and variation in the transcriptome response that may require attention to specifics in experimental paradigms, such as age, insult type, gender, the investigated brain region, and selected cellular and functional endpoints (Cox-Limpens et al. 2014).
The experimental study of preconditioning contributes to the knowledge of endogenous neuroprotective mechanisms, which may eventually lead to potential pharmaceutical treatments. Several pharmacological approaches have been suggested, including stimulus mimetics such as PKC modifying agents, thioredoxin 1, resveratrol and statins (Della-Morte et al. 2012). Fundamentally, mapping the individual and integrated transcriptome of neural, glial and vascular cells after IPC should allow us to understand how intercellular mechanisms control the release of extracellular help-me signals that protect against acute damage and promote repair after stroke.
4.2 Mapping the secretome
If the transcriptome provides a window into the molecular mechanisms of intracellular control, then mapping the secretome should allow one to probe the entire network of extracellular factors that underlie non-cell autonomous mechanisms. This may be especially important in the CNS where crosstalk occurs between multiple cell types provide the basis for coordinating the communication between cells. In order to dissect the network of inter-cellular help-me signals and understand how cells to “talk to each other”, mapping the “secretome” (i.e. the subset of the entire cellular proteome) with advanced proteomic methods will be required (Colucci-D'Amato et al. 2011).
One of the initial proteomic maps of the neuronal secretome identified about 34 major secreted proteins belonging to families involved in neurite and axonal maintenance, synaptic transmission, proteases and protease inhibitors, and cell adhesion (Thouvenot et al. 2008). Among these 34 proteins, several proteins are secreted by cells via the classical vesicular pathway and encompassing a signal peptide at their N terminus (e.g., cystatin C, apolipoprotein E, matrix metalloprotease-inhibitor 2, carboxypeptidase E and several complement subunits), whereas a larger set of proteins are released following proteolytic cleavage of the ectodomain of a membrane-bound or a transmembrane precursor (Thouvenot et al. 2008). In addition, the characterization of proteins released from neurons, astrocytes and neural precursor cells shows that the extracellular space within the nervous system has a more diverse protein composition than previously thought (Schubert et al. 2009). Although there is overlap between the different cell types, the extracellular protein pool is likely to be somewhat unique for each cell population. Neurons and neuronal precursor cells release a larger number of proteins with more functional diversity, while astrocytes release a relatively small number of proteins.
Recently, characterization of secretome from primary neurons was used to explore the mechanisms underlying neuronal death (Thouvenot et al. 2012) and to identify novel substrate candidates of protease BACE1 (Kuhn et al. 2012; Zhou et al. 2012). After comparing the secretome of apoptotic and surviving cerebellar granule neurons, 47 proteins in the supernatants were differentially expressed (Thouvenot et al. 2012). Among the 47 proteins, 31 proteins are secreted via either the vesicular pathway or a non-classical mechanism of secretion, while 13 of them, annotated as membrane proteins, might be released following proteolytic cleavage of the ectodomain of a transmembrane precursor (Thouvenot et al. 2012). Functional GO analysis of these 47 proteins revealed the enrichment in proteins residing in the extracellular compartment and in proteins involved in cell adhesion (Thouvenot et al. 2012). Secretome analysis of neuronal BACE1 revealed several novel substrates and suggested that this system may contribute the shedding and release of key inter-cellular signals in the CNS (Kuhn et al. 2012; Zhou et al. 2012), including molecules that may be essential for regulating neurite extension and synaptic integrity (Kuhn et al. 2012). These approaches may ultimately allow one to define novel molecular mechanisms underlying BACE1 activity in the CNS and perhaps even help predict potential side effects in BACE clinical trials for dementia.
Currently, extracellular vesicles (also known as exosomes, microvesicles, and microparticles, or other names) have gained attention as important factors in cell-cell communication. Extracellular vesicles are composed of a lipid bilayer enclosing proteins and RNAs, and modify the state and function of the recipient cells by inducing signaling via receptor-ligand interaction or delivering their content into the recipient cells (Tkach and Thery 2016). Extracellular vesicles can be formed by budding from plasma membrane, or originated from multivesicular endosomes or multivesicular bodies (MVBs) (Tkach and Thery 2016). Neurons can release exosomes that contain functionally active proteins and miRNAs, which can exert a neuroprotective or neurotoxic role (Ghidoni et al. 2011; Janas et al. 2016; Lachenal et al. 2011; Morel et al. 2013). Recent several reviews offer the roles of exosomes and microvesicles in normal function, the development of regeneration of CNS as well as in the onset and progression of of some neurodegenerative and neuroinflammatory diseases (Janas et al. 2016; Porro et al. 2015). As a key component of any cellular secretome, extracellular vesicles may then comprise logical candidates for help-me signaling in the context of damaged neurons. The fact that these vesicles may also be detected in plasma and serum may even pint toward a potential use of measurable biomarkers for measuring the dynamic balance between injury and repair in the CNS.
Of course, the secretome is a dynamic entity. So differential analyses will be necessary in order to investigate the proposed phenomenon of help-me signaling. Each cell type would be mapped under normal, sublethally stimulated, and lethally disrupted conditions. Acute versus chronic secretomes may also differ. And then each secretome “state” would be validated against functional databases for paracrine effects on other cells. Theoretically, an integrated response profile can be built for each secreting cell type and responding cell type over time, and ultimately, the resulting linked database can then be mined for novel candidate help-me signals under various injury and disease conditions.
5. Conclusions and future opportunities
Help-me signals essentially comprise a subset of extracellular signals that reside within the larger family of damage signals (Kono and Rock 2008). Along with various find me signals, eat me signals and clean-up signals, these may form a complex web of interacting and recursive loops that underlie homeostasis in any multicellular system. From an evolutionary perspective, these networks provide a biological system with the ability to respond and adapt to external stimuli. In the context of brain injury and disease, help-me signaling defines a non-cell autonomous basis for preconditioning and tolerance. When applied in stroke, these signals may be essential in neuroprotection and neurorepair.
Standard experimental models have tended to emphasize the deleterious nature of intracellular signals and extracellular factors. Hence, translational research has traditionally focused on finding ways to block receptors or enzymes in order to prevent injury. Ultimately, however, any attempt to develop targeted therapies in brain injury and neurodegeneration must take into account the biphasic nature of all mediators in the entire remodeling neurovascular unit, comprising reactions to injury in neural, glial and vascular compartments. Deleterious mediators co-exist with beneficial ones, and help-me signals may define this dynamic balance between initial injury and subsequent repair. A better understanding of help-me signaling may eventually lead to novel therapeutic approaches for neuroprotection and neurorecovery.
Abbreviations
- Aβ
β amyloid
- BBB
blood brain barrier
- BrdU
5-bromo-2'-deoxyuridine
- CSF
cerebrospinal fluid
- CSF1
colony stimulating factor-1
- CSF1R
colony stimulating factor-1 receptor
- DAMPs
damage associated molecular pattern family
- EPO
erythropoietin
- FGF
fibroblast growth factors
- IL
interleukin
- LCN2
Lipocalin-2
- LPS
lipopolysaccharide
- MCPs
monocyte chemoattractant proteins
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- NPCs
neural progenitor cells
- OGD
oxygen-glucose deprivation
- 6-OHDA
6-hydroxydopamine
- SVZ
subventricular zone
- TIA
transient ischemic attack
- TNFα
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
- ZO
zonula occludens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allavena P, Bianchi G, Zhou D, van Damme J, Jilek P, Sozzani S, et al. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994;24:3233–6. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- An C, Shi Y, Li P, Hu X, Gan Y, Stetler RA, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog Neurobiol. 2014;115:6–24. doi: 10.1016/j.pneurobio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic AV, Pachter JS. Characterization of binding sites for chemokines MCP-1 and MIP-1alpha on human brain microvessels. J Neurochem. 2000;75:1898–906. doi: 10.1046/j.1471-4159.2000.0751898.x. [DOI] [PubMed] [Google Scholar]
- Andjelkovic AV, Spencer DD, Pachter JS. Visualization of chemokine binding sites on human brain microvessels. J Cell Biol. 1999;145:403–12. doi: 10.1083/jcb.145.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic AV, Song L, Dzenko KA, Cong H, Pachter JS. Functional expression of CCR2 by human fetal astrocytes. J Neurosci Res. 2002;70:219–31. doi: 10.1002/jnr.10372. [DOI] [PubMed] [Google Scholar]
- Andres RH, Choi R, Pendharkar AV, Gaeta X, Wang N, Nathan JK, et al. The CCR2/CCL2 interaction mediates the transendothelial recruitment of intravascularly delivered neural stem cells to the ischemic brain. Stroke. 2011;42:2923–31. doi: 10.1161/STROKEAHA.110.606368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwaar I, Gottsater A, Ohlsson K, Mattiasson I, Lindgarde F. Increasing levels of leukocyte-derived inflammatory mediators in plasma and cAMP in platelets during follow-up after acute cerebral ischemia. Cerebrovasc Dis. 1998;8:310–7. doi: 10.1159/000015873. [DOI] [PubMed] [Google Scholar]
- Appel E, Kolman O, Kazimirsky G, Blumberg PM, Brodie C. Regulation of GDNF expression in cultured astrocytes by inflammatory stimuli. Neuroreport. 1997;8:3309–12. doi: 10.1097/00001756-199710200-00023. [DOI] [PubMed] [Google Scholar]
- Arakelyan A, Petrkova J, Hermanova Z, Boyajyan A, Lukl J, Petrek M. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediators Inflamm. 2005;2005:175–9. doi: 10.1155/MI.2005.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, et al. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–44. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Kitabgi P, Rostene W, Parsadaniantz SM. Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: evidence for its colocalization with neurotransmitters and neuropeptides. J Comp Neurol. 2005a;489:275–92. doi: 10.1002/cne.20598. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Rostene W, Kitabgi P, Melik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J Comp Neurol. 2005b;492:178–92. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, et al. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem. 2002;81:257–69. doi: 10.1046/j.1471-4159.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Bao WL, Lu SD, Wang H, Sun FY. Intraventricular vascular endothelial growth factor antibody increases infarct volume following transient cerebral ischemia. Zhongguo Yao Li Xue Bao. 1999;20:313–8. [PubMed] [Google Scholar]
- Barcelos LS, Talvani A, Teixeira AS, Cassali GD, Andrade SP, Teixeira MM. Production and in vivo effects of chemokines CXCL1-3/KC and CCL2/JE in a model of inflammatory angiogenesis in mice. Inflamm Res. 2004;53:576–84. doi: 10.1007/s00011-004-1299-4. [DOI] [PubMed] [Google Scholar]
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci U S A. 1995;92:9328–32. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna BP, Pettay J, Barnett GH, Zhou P, Iwasaki K, Estes ML. Regulation of monocyte chemoattractant protein-1 expression in adult human non-neoplastic astrocytes is sensitive to tumor necrosis factor (TNF) or antibody to the 55-kDa TNF receptor. J Neuroimmunol. 1994;50:101–7. doi: 10.1016/0165-5728(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–44. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–4. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci. 2003;24:623–31. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- Berard JL, Zarruk JG, Arbour N, Prat A, Yong VW, Jacques FH, et al. Lipocalin 2 is a novel immune mediator of experimental autoimmune encephalomyelitis pathogenesis and is modulated in multiple sclerosis. Glia. 2012;60:1145–59. doi: 10.1002/glia.22342. [DOI] [PubMed] [Google Scholar]
- Berman JW, Guida MP, Warren J, Amat J, Brosnan CF. Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J Immunol. 1996;156:3017–23. [PubMed] [Google Scholar]
- Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 2008;26:2361–71. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Tang Y, Reilly M, Petit E, Sharp FR. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem. 2002;277:39728–38. doi: 10.1074/jbc.M204619200. [DOI] [PubMed] [Google Scholar]
- Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, et al. Reactive astrocytes secrete lcn2 to promote neuron death. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4069–74. doi: 10.1073/pnas.1218497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Boddeke EW, Meigel I, Frentzel S, Gourmala NG, Harrison JK, Buttini M, et al. Cultured rat microglia express functional beta-chemokine receptors. J Neuroimmunol. 1999;98:176–84. doi: 10.1016/s0165-5728(99)00096-x. [DOI] [PubMed] [Google Scholar]
- Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Bolignano D, Donato V, Lacquaniti A, Fazio MR, Bono C, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: a new protein enters the scene. Cancer Lett. 2010;288:10–6. doi: 10.1016/j.canlet.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Botchkina GI, Meistrell ME, 3rd, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med. 1997;3:765–81. [PMC free article] [PubMed] [Google Scholar]
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–94. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Bruno V, Copani A, Besong G, Scoto G, Nicoletti F. Neuroprotective activity of chemokines against N-methyl-D-aspartate or beta-amyloid-induced toxicity in culture. Eur J Pharmacol. 2000;399:117–21. doi: 10.1016/s0014-2999(00)00367-8. [DOI] [PubMed] [Google Scholar]
- Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–87. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Appel K, Sauter A, Gebicke-Haerter PJ, Boddeke HW. Expression of tumor necrosis factor alpha after focal cerebral ischaemia in the rat. Neuroscience. 1996;71:1–16. doi: 10.1016/0306-4522(95)00414-9. [DOI] [PubMed] [Google Scholar]
- Buttner F, Cordes C, Gerlach F, Heimann A, Alessandri B, Luxemburger U, et al. Genomic response of the rat brain to global ischemia and reperfusion. Brain Res. 2009;1252:1–14. doi: 10.1016/j.brainres.2008.10.045. [DOI] [PubMed] [Google Scholar]
- Callewaere C, Banisadr G, Rostene W, Parsadaniantz SM. Chemokines and chemokine receptors in the brain: implication in neuroendocrine regulation. J Mol Endocrinol. 2007;38:355–63. doi: 10.1677/JME-06-0035. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–35. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Carlson NG, Bacchi A, Rogers SW, Gahring LC. Nicotine blocks TNF-alpha-mediated neuroprotection to NMDA by an alpha-bungarotoxin-sensitive pathway. J Neurobiol. 1998;35:29–36. doi: 10.1002/(sici)1097-4695(199804)35:1<29::aid-neu3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J, Moro MA, Blanco M, Leira R, Serena J, Lizasoain I, et al. The release of tumor necrosis factor-alpha is associated with ischemic tolerance in human stroke. Ann Neurol. 2003;54:811–9. doi: 10.1002/ana.10765. [DOI] [PubMed] [Google Scholar]
- Che X, Ye W, Panga L, Wu DC, Yang GY. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001;902:171–7. doi: 10.1016/s0006-8993(01)02328-9. [DOI] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467:863–7. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Graham SH, Zhu RL, Simon RP. Stress proteins and tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab. 1996;16:566–77. doi: 10.1097/00004647-199607000-00006. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, et al. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23:748–55. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- Chen Z, Palmer TD. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain Behav Immun. 2013;30:45–53. doi: 10.1016/j.bbi.2013.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–53. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Chi OZ, Hunter C, Liu X, Weiss HR. Effects of anti-VEGF antibody on blood-brain barrier disruption in focal cerebral ischemia. Exp Neurol. 2007;204:283–7. doi: 10.1016/j.expneurol.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Chia WJ, Dawe GS, Ong WY. Expression and localization of the iron-siderophore binding protein lipocalin 2 in the normal rat brain and after kainate-induced excitotoxicity. Neurochem Int. 2011;59:591–9. doi: 10.1016/j.neuint.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Cipriani R, Villa P, Chece G, Lauro C, Paladini A, Micotti E, et al. CX3CL1 is neuroprotective in permanent focal cerebral ischemia in rodents. J Neurosci. 2011;31:16327–35. doi: 10.1523/JNEUROSCI.3611-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs CS, Chen J, Greenberg DA, Graham SH. Vascular endothelial growth factor expression in transient focal cerebral ischemia in the rat. Neurosci Lett. 1998;249:79–82. doi: 10.1016/s0304-3940(98)00377-2. [DOI] [PubMed] [Google Scholar]
- Colucci-D'Amato L, Farina A, Vissers JP, Chambery A. Quantitative neuroproteomics: classical and novel tools for studying neural differentiation and function. Stem Cell Rev. 2011;7:77–93. doi: 10.1007/s12015-010-9136-3. [DOI] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010;224:93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, et al. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Cox-Limpens KE, Gavilanes AW, Zimmermann LJ, Vles JS. Endogenous brain protection: what the cerebral transcriptome teaches us. Brain Res. 2014;1564:85–100. doi: 10.1016/j.brainres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Cox-Limpens KE, Vles JS, Schlechter J, Zimmermann LJ, Strackx E, Gavilanes AW. Fetal brain genomic reprogramming following asphyctic preconditioning. BMC Neurosci. 2013;14:61. doi: 10.1186/1471-2202-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts TD, Jensen AR, Blocher-Smith EC, Markel TA. Vascular endothelial growth factor: Therapeutic possibilities and challenges for the treatment of ischemia. Cytokine. 2015;71:385–93. doi: 10.1016/j.cyto.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Croitoru-Lamoury J, Guillemin GJ, Boussin FD, Mognetti B, Gigout LI, Cheret A, et al. Expression of chemokines and their receptors in human and simian astrocytes: evidence for a central role of TNF alpha and IFN gamma in CXCR4 and CCR5 modulation. Glia. 2003;41:354–70. doi: 10.1002/glia.10181. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett. 1996;218:41–4. doi: 10.1016/0304-3940(96)13116-5. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Guadagni F, Palmirotta R, Ferroni P, Testa G, Cacciatore F, et al. Genetics and genomics of ischemic tolerance: focus on cardiac and cerebral ischemic preconditioning. Pharmacogenomics. 2012;13:1741–57. doi: 10.2217/pgs.12.157. [DOI] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–21. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2006;26:797–810. doi: 10.1038/sj.jcbfm.9600229. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–53. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MM, Cain K, Zierath D, Shibata D, Tanzi PM, Becker KJ. Higher plasma fractalkine is associated with better 6-month outcome from ischemic stroke. Stroke. 2012;43:2300–6. doi: 10.1161/STROKEAHA.112.657411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziewulska D, Mossakowski MJ. Cellular expression of tumor necrosis factor a and its receptors in human ischemic stroke. Clin Neuropathol. 2003;22:35–40. [PubMed] [Google Scholar]
- Elneihoum AM, Falke P, Axelsson L, Lundberg E, Lindgarde F, Ohlsson K. Leukocyte activation detected by increased plasma levels of inflammatory mediators in patients with ischemic cerebrovascular diseases. Stroke. 1996;27:1734–8. doi: 10.1161/01.str.27.10.1734. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D'Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Feng Z, Davis DP, Sasik R, Patel HH, Drummond JC, Patel PM. Pathway and gene ontology based analysis of gene expression in a rat model of cerebral ischemic tolerance. Brain Res. 2007;1177:103–23. doi: 10.1016/j.brainres.2007.07.047. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Figiel I. Pro-inflammatory cytokine TNF-alpha as a neuroprotective agent in the brain. Acta Neurobiol Exp (Wars) 2008;68:526–34. doi: 10.55782/ane-2008-1720. [DOI] [PubMed] [Google Scholar]
- Figueiredo C, Pais TF, Gomes JR, Chatterjee S. Neuron-microglia crosstalk up-regulates neuronal FGF-2 expression which mediates neuroprotection against excitotoxicity via JNK1/2. J Neurochem. 2008;107:73–85. doi: 10.1111/j.1471-4159.2008.05577.x. [DOI] [PubMed] [Google Scholar]
- Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318:1–14. doi: 10.1042/bj3180001. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U. Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22:RC216. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthmann B, Grothe C, Claus P. A nuclear odyssey: fibroblast growth factor-2 (FGF-2) as a regulator of nuclear homeostasis in the nervous system. Cell Mol Life Sci. 2015 doi: 10.1007/s00018-014-1818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frater-Schroder M, Risau W, Hallmann R, Gautschi P, Bohlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987;84:5277–81. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Sun JL, Ma JF, Geng X, Sun J, Liu JR, et al. The neuroprotection of prodromal transient ischaemic attack on cerebral infarction. Eur J Neurol. 2008;15:797–801. doi: 10.1111/j.1468-1331.2008.02188.x. [DOI] [PubMed] [Google Scholar]
- Fuller AD, Van Eldik LJ. MFG-E8 regulates microglial phagocytosis of apoptotic neurons. J Neuroimmune Pharmacol. 2008;3:246–56. doi: 10.1007/s11481-008-9118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–8. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Galvez BG, Genis L, Matias-Roman S, Oblander SA, Tryggvason K, Apte SS, et al. Membrane type 1-matrix metalloproteinase is regulated by chemokines monocyte-chemoattractant protein-1/ccl2 and interleukin-8/CXCL8 in endothelial cells during angiogenesis. J Biol Chem. 2005;280:1292–8. doi: 10.1074/jbc.M408673200. [DOI] [PubMed] [Google Scholar]
- Gary DS, Bruce-Keller AJ, Kindy MS, Mattson MP. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J Cereb Blood Flow Metab. 1998;18:1283–7. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–28. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghidoni R, Paterlini A, Albertini V, Glionna M, Monti E, Schiaffonati L, et al. Cystatin C is released in association with exosomes: a new tool of neuronal communication which is unbalanced in Alzheimer's disease. Neurobiol Aging. 2011;32:1435–42. doi: 10.1016/j.neurobiolaging.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RW, Costain WJ, Blanchard ME, Mullen KL, Currie RW, Robertson HA. DNA microarray analysis of hippocampal gene expression measured twelve hours after hypoxia-ischemia in the mouse. J Cereb Blood Flow Metab. 2003;23:1195–211. doi: 10.1097/01.WCB.0000088763.02615.79. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, et al. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol. 1996;156:4363–8. [PubMed] [Google Scholar]
- Goddard DR, Berry M, Kirvell SL, Butt AM. Fibroblast growth factor-2 induces astroglial and microglial reactivity in vivo. J Anat. 2002;200:57–67. doi: 10.1046/j.0021-8782.2001.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765–70. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Goetz DH, Willie ST, Armen RS, Bratt T, Borregaard N, Strong RK. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry. 2000;39:1935–41. doi: 10.1021/bi992215v. [DOI] [PubMed] [Google Scholar]
- Gong C, Qin Z, Betz AL, Liu XH, Yang GY. Cellular localization of tumor necrosis factor alpha following focal cerebral ischemia in mice. Brain Res. 1998;801:1–8. doi: 10.1016/s0006-8993(98)00489-2. [DOI] [PubMed] [Google Scholar]
- Gordon K, Spiden SL, Connell FC, Brice G, Cottrell S, Short J, et al. FLT4/VEGFR3 and Milroy disease: novel mutations, a review of published variants and database update. Hum Mutat. 2013;34:23–31. doi: 10.1002/humu.22223. [DOI] [PubMed] [Google Scholar]
- Gourmala NG, Buttini M, Limonta S, Sauter A, Boddeke HW. Differential and time-dependent expression of monocyte chemoattractant protein-1 mRNA by astrocytes and macrophages in rat brain: effects of ischemia and peripheral lipopolysaccharide administration. J Neuroimmunol. 1997;74:35–44. doi: 10.1016/s0165-5728(96)00203-2. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70:1753–61. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:53–65. doi: 10.1097/00004647-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–60. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley C, Ravichandran KS. Cues for apoptotic cell engulfment: eat-me, don't eat-me and come-get-me signals. Trends Cell Biol. 2003;13:648–56. doi: 10.1016/j.tcb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Gustavsson M, Wilson MA, Mallard C, Rousset C, Johnston MV, Hagberg H. Global gene expression in the developing rat brain after hypoxic preconditioning: involvement of apoptotic mechanisms? Pediatr Res. 2007;61:444–50. doi: 10.1203/pdr.0b013e3180332be4. [DOI] [PubMed] [Google Scholar]
- Guyon A, Skrzydelski D, De Giry I, Rovere C, Conductier G, Trocello JM, et al. Long term exposure to the chemokine CCL2 activates the nigrostriatal dopamine system: a novel mechanism for the control of dopamine release. Neuroscience. 2009;162:1072–80. doi: 10.1016/j.neuroscience.2009.05.048. [DOI] [PubMed] [Google Scholar]
- Haile WB, Wu J, Echeverry R, Wu F, An J, Yepes M. Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-alpha. J Cereb Blood Flow Metab. 2012;32:57–69. doi: 10.1038/jcbfm.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34:81–9. doi: 10.1016/j.it.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–55. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Harrigan MR, Ennis SR, Masada T, Keep RF. Intraventricular infusion of vascular endothelial growth factor promotes cerebral angiogenesis with minimal brain edema. Neurosurgery. 2002;50:589–98. doi: 10.1097/00006123-200203000-00030. [DOI] [PubMed] [Google Scholar]
- Harrigan MR, Ennis SR, Sullivan SE, Keep RF. Effects of intraventricular infusion of vascular endothelial growth factor on cerebral blood flow, edema, and infarct volume. Acta Neurochir (Wien) 2003;145:49–53. doi: 10.1007/s00701-002-1035-1. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A, Tanaka E, Murase K, Ishida N, Chatani Y, Tsujimoto M, et al. Tumor necrosis factor stimulates the synthesis and secretion of biologically active nerve growth factor in non-neuronal cells. J Biol Chem. 1993;268:2577–82. [PubMed] [Google Scholar]
- Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME. Production and function of monocyte chemoattractant protein-1 and other beta-chemokines in murine glial cells. J Neuroimmunol. 1995;60:143–50. doi: 10.1016/0165-5728(95)00064-9. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18:887–95. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–44. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- Heldmann U, Thored P, Claasen JH, Arvidsson A, Kokaia Z, Lindvall O. TNF-alpha antibody infusion impairs survival of stroke-generated neuroblasts in adult rat brain. Exp Neurol. 2005;196:204–8. doi: 10.1016/j.expneurol.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26:136–42. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105:1405–7. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- Hori M, Nakamachi T, Rakwal R, Shibato J, Nakamura K, Wada Y, et al. Unraveling the ischemic brain transcriptome in a permanent middle cerebral artery occlusion mouse model by DNA microarray analysis. Dis Model Mech. 2012;5:270–83. doi: 10.1242/dmm.008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Sun X, Mao Y, Zhu X, Zhang P, Zhang L, et al. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse brain neurons. Int J Biochem Cell Biol. 2008;40:1604–15. doi: 10.1016/j.biocel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22:308–17. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Hulshof S, van Haastert ES, Kuipers HF, van den Elsen PJ, De Groot CJ, van der Valk P, et al. CX3CL1 and CX3CR1 expression in human brain tissue: noninflammatory control versus multiple sclerosis. J Neuropathol Exp Neurol. 2003;62:899–907. doi: 10.1093/jnen/62.9.899. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Lyman WD, Berman JW. Tumor necrosis factor alpha and transforming growth factor beta upregulate astrocyte expression of monocyte chemoattractant protein-1. J Neuroimmunol. 1995;57:193–8. doi: 10.1016/0165-5728(95)00011-p. [DOI] [PubMed] [Google Scholar]
- Intiso D, Zarrelli MM, Lagioia G, Di Rienzo F, Checchia De Ambrosio C, Simone P, et al. Tumor necrosis factor alpha serum levels and inflammatory response in acute ischemic stroke patients. Neurol Sci. 2004;24:390–6. doi: 10.1007/s10072-003-0194-z. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–12. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, Ahlenius H, Ekdahl CT, Darsalia V, Thored P, Jovinge S, et al. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28:1574–87. doi: 10.1038/jcbfm.2008.47. [DOI] [PubMed] [Google Scholar]
- Ip JP, Nocon AL, Hofer MJ, Lim SL, Muller M, Campbell IL. Lipocalin 2 in the central nervous system host response to systemic lipopolysaccharide administration. J Neuroinflammation. 2011;8:124. doi: 10.1186/1742-2094-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas AM, Sapon K, Janas T, Stowell MH. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim Biophys Acta. 2016;1858:1139–51. doi: 10.1016/j.bbamem.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Jaye M, Schlessinger J, Dionne CA. Fibroblast growth factor receptor tyrosine kinases: molecular analysis and signal transduction. Biochim Biophys Acta. 1992;1135:185–99. doi: 10.1016/0167-4889(92)90136-y. [DOI] [PubMed] [Google Scholar]
- Jeon S, Jha MK, Ock J, Seo J, Jin M, Cho H, et al. Role of lipocalin-2-chemokine axis in the development of neuropathic pain following peripheral nerve injury. The Journal of biological chemistry. 2013;288:24116–27. doi: 10.1074/jbc.M113.454140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001a;98:4710–5. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Eshoo MW, Nagayama T, Minami M, Simon RP, et al. Microarray analysis of hippocampal gene expression in global cerebral ischemia. Ann Neurol. 2001b;50:93–103. doi: 10.1002/ana.1073. [DOI] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–7. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Kim JH, Jang E, Lee YM, Soo Han H, Woo DK, et al. Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2014a doi: 10.1038/jcbfm.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Sonobe Y, Kawanokuchi J, Horiuchi H, Cheng Y, Wang Y, et al. Interleukin-34 restores blood-brain barrier integrity by upregulating tight junction proteins in endothelial cells. PLoS One. 2014b;9:e115981. doi: 10.1371/journal.pone.0115981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Sola A, Hughes J, Kluth DC, Vinuesa E, Vinas JL, et al. Infusion of IL-10-expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int. 2012;81:969–82. doi: 10.1038/ki.2011.446. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Uherek M, Wellmann H, Volk B, Kaltschmidt C. Inhibition of NF-kappaB potentiates amyloid beta-mediated neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:9409–14. doi: 10.1073/pnas.96.16.9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltsonoudis E, Voulgari PV, Konitsiotis S, Drosos AA. Demyelination and other neurological adverse events after anti-TNF therapy. Autoimmun Rev. 2014;13:54–8. doi: 10.1016/j.autrev.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke KF, Isaev NK, et al. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–98. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- Kawahara N, Wang Y, Mukasa A, Furuya K, Shimizu T, Hamakubo T, et al. Genome-wide gene expression analysis for induced ischemic tolerance and delayed neuronal death following transient global ischemia in rats. J Cereb Blood Flow Metab. 2004;24:212–23. doi: 10.1097/01.WCB.0000106012.33322.A2. [DOI] [PubMed] [Google Scholar]
- Kaya D, Gursoy-Ozdemir Y, Yemisci M, Tuncer N, Aktan S, Dalkara T. VEGF protects brain against focal ischemia without increasing blood--brain permeability when administered intracerebroventricularly. J Cereb Blood Flow Metab. 2005;25:1111–8. doi: 10.1038/sj.jcbfm.9600109. [DOI] [PubMed] [Google Scholar]
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–12. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1928–36. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor-alpha impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: Role of Hes1. Mol Cell Neurosci. 2010;43:127–35. doi: 10.1016/j.mcn.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–80. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA, et al. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. J Neuroimmunol. 1995;56:127–34. doi: 10.1016/0165-5728(94)00138-e. [DOI] [PubMed] [Google Scholar]
- Kimura R, Nakase H, Tamaki R, Sakaki T. Vascular endothelial growth factor antagonist reduces brain edema formation and venous infarction. Stroke. 2005;36:1259–63. doi: 10.1161/01.STR.0000165925.20413.14. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, et al. 'Ischemic tolerance' phenomenon found in the brain. Brain Res. 1990;528:21–4. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- Koerner IP, Gatting M, Noppens R, Kempski O, Brambrink AM. Induction of cerebral ischemic tolerance by erythromycin preconditioning reprograms the transcriptional response to ischemia and suppresses inflammation. Anesthesiology. 2007;106:538–47. doi: 10.1097/00000542-200703000-00019. [DOI] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs Z, Ikezaki K, Samoto K, Inamura T, Fukui M. VEGF and flt. Expression time kinetics in rat brain infarct. Stroke. 1996;27:1865–72. doi: 10.1161/01.str.27.10.1865. discussion 72-3. [DOI] [PubMed] [Google Scholar]
- Kuhn PH, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, et al. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. Embo J. 2012;31:3157–68. doi: 10.1038/emboj.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumai Y, Ooboshi H, Takada J, Kamouchi M, Kitazono T, Egashira K, et al. Anti-monocyte chemoattractant protein-1 gene therapy protects against focal brain ischemia in hypertensive rats. J Cereb Blood Flow Metab. 2004;24:1359–68. doi: 10.1097/01.WCB.0000143534.76388.3C. [DOI] [PubMed] [Google Scholar]
- Kuno R, Yoshida Y, Nitta A, Nabeshima T, Wang J, Sonobe Y, et al. The role of TNF-alpha and its receptors in the production of NGF and GDNF by astrocytes. Brain Res. 2006;1116:12–8. doi: 10.1016/j.brainres.2006.07.120. [DOI] [PubMed] [Google Scholar]
- Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, et al. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;338:1353–6. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–18. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–30. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Chen Q, Wang Y, Jia B, Sun L, Zheng J, et al. TNF-alpha affects human cortical neural progenitor cell differentiation through the autocrine secretion of leukemia inhibitory factor. PLoS One. 2012;7:e50783. doi: 10.1371/journal.pone.0050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine SD, Hofman FM, Zlokovic BV. Circulating antibody against tumor necrosis factor-alpha protects rat brain from reperfusion injury. J Cereb Blood Flow Metab. 1998;18:52–8. doi: 10.1097/00004647-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Lee S, Park JY, Lee WH, Kim H, Park HC, Mori K, et al. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci. 2009;29:234–49. doi: 10.1523/JNEUROSCI.5273-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennmyr F, Ata KA, Funa K, Olsson Y, Terent A. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. J Neuropathol Exp Neurol. 1998;57:874–82. doi: 10.1097/00005072-199809000-00009. [DOI] [PubMed] [Google Scholar]
- Leonardi-Essmann F, Emig M, Kitamura Y, Spanagel R, Gebicke-Haerter PJ. Fractalkine-upregulated milk-fat globule EGF factor-8 protein in cultured rat microglia. J Neuroimmunol. 2005;160:92–101. doi: 10.1016/j.jneuroim.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Li SF, Sun YB, Meng QH, Li SR, Yao WC, Hu GJ, et al. Recombinant adeno-associated virus serotype 1-vascular endothelial growth factor promotes neurogenesis and neuromigration in the subventricular zone and rescues neuronal function in ischemic rats. Neurosurgery. 2009;65:771–9. doi: 10.1227/01.NEU.0000349931.61771.52. discussion 9. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu DX, Li MY, Qin XX, Fang WG, Zhao WD, et al. Ephrin-A3 and ephrin-A4 contribute to microglia-induced angiogenesis in brain endothelial cells. Anat Rec (Hoboken) 2014;297:1908–18. doi: 10.1002/ar.22998. [DOI] [PubMed] [Google Scholar]
- Limatola C, Ransohoff RM. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front Cell Neurosci. 2014;8:229. doi: 10.3389/fncel.2014.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–11. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–8. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg SR, Wang L, Yier T, et al. Chemokine ligand 2 (CCL2) induces migration and differentiation of subventricular zone cells after stroke. J Neurosci Res. 2007;85:2120–5. doi: 10.1002/jnr.21359. [DOI] [PubMed] [Google Scholar]
- Liu YP, Lin HI, Tzeng SF. Tumor necrosis factor-alpha and interleukin-18 modulate neuronal cell fate in embryonic neural progenitor culture. Brain Res. 2005;1054:152–8. doi: 10.1016/j.brainres.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Losy J, Zaremba J. Monocyte chemoattractant protein-1 is increased in the cerebrospinal fluid of patients with ischemic stroke. Stroke. 2001;32:2695–6. doi: 10.1161/hs1101.097380. [DOI] [PubMed] [Google Scholar]
- Lu A, Tang Y, Ran R, Clark JF, Aronow BJ, Sharp FR. Genomics of the periinfarction cortex after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:786–810. doi: 10.1097/01.WCB.0000062340.80057.06. [DOI] [PubMed] [Google Scholar]
- Lu XC, Williams AJ, Yao C, Berti R, Hartings JA, Whipple R, et al. Microarray analysis of acute and delayed gene expression profile in rats after focal ischemic brain injury and reperfusion. J Neurosci Res. 2004;77:843–57. doi: 10.1002/jnr.20218. [DOI] [PubMed] [Google Scholar]
- Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS, et al. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat Cell Biol. 2011;13:1076–83. doi: 10.1038/ncb2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Elwood F, Britschgi M, Villeda S, Zhang H, Ding Z, et al. Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J Exp Med. 2013;210:157–72. doi: 10.1084/jem.20120412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zechariah A, Qu Y, Hermann DM. Effects of vascular endothelial growth factor in ischemic stroke. J Neurosci Res. 2012;90:1873–82. doi: 10.1002/jnr.23088. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Leza JC, Polak P, Kalinin S, Feinstein DL. Astrocyte-derived MCP-1 mediates neuroprotective effects of noradrenaline. J Neurosci. 2009;29:263–7. doi: 10.1523/JNEUROSCI.4926-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magge SN, Malik SZ, Royo NC, Chen HI, Yu L, Snyder EY, et al. Role of monocyte chemoattractant protein-1 (MCP-1/CCL2) in migration of neural progenitor cells toward glial tumors. J Neurosci Res. 2009;87:1547–55. doi: 10.1002/jnr.21983. [DOI] [PubMed] [Google Scholar]
- Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869–81. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Marques F, Mesquita SD, Sousa JC, Coppola G, Gao F, Geschwind DH, et al. Lipocalin 2 is present in the EAE brain and is modulated by natalizumab. Front Cell Neurosci. 2012;6:33. doi: 10.3389/fncel.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, et al. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–49. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:re21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, et al. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. Faseb J. 2001;15:1218–20. [PubMed] [Google Scholar]
- Mattison HA, Nie H, Gao H, Zhou H, Hong JS, Zhang J. Suppressed pro-inflammatory response of microglia in CX3CR1 knockout mice. J Neuroimmunol. 2013;257:110–5. doi: 10.1016/j.jneuroim.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe T, Simon RP. Hyperthermia induces 72kDa heat shock protein expression in rat brain in non-neuronal cells. Neurosci Lett. 1993;159:163–5. doi: 10.1016/0304-3940(93)90824-5. [DOI] [PubMed] [Google Scholar]
- Meistrell ME, 3rd, Botchkina GI, Wang H, Di Santo E, Cockroft KM, Bloom O, et al. Tumor necrosis factor is a brain damaging cytokine in cerebral ischemia. Shock. 1997;8:341–8. [PubMed] [Google Scholar]
- Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979:65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Doi Y, Mizoguchi H, Jin S, Noda M, Sonobe Y, et al. Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-beta neurotoxicity. Am J Pathol. 2011;179:2016–27. doi: 10.1016/j.ajpath.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncayo J, de Freitas GR, Bogousslavsky J, Altieri M, van Melle G. Do transient ischemic attacks have a neuroprotective effect? Neurology. 2000;54:2089–94. doi: 10.1212/wnl.54.11.2089. [DOI] [PubMed] [Google Scholar]
- Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012;7:42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, et al. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013;288:7105–16. doi: 10.1074/jbc.M112.410944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha M, Skrzypiec AE, Schiavon E, Attwood BK, Kucerova E, Pawlak R. Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18436–41. doi: 10.1073/pnas.1107936108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudo G, Bonomo A, Di Liberto V, Frinchi M, Fuxe K, Belluardo N. The FGF-2/FGFRs neurotrophic system promotes neurogenesis in the adult brain. J Neural Transm. 2009;116:995–1005. doi: 10.1007/s00702-009-0207-z. [DOI] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–8. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Naude PJ, Nyakas C, Eiden LE, Ait-Ali D, van der Heide R, Engelborghs S, et al. Lipocalin 2: novel component of proinflammatory signaling in Alzheimer's disease. Faseb J. 2012;26:2811–23. doi: 10.1096/fj.11-202457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997;17:483–90. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Nishi S, Taki W, Uemura Y, Higashi T, Kikuchi H, Kudoh H, et al. Ischemic tolerance due to the induction of HSP70 in a rat ischemic recirculation model. Brain Res. 1993;615:281–8. doi: 10.1016/0006-8993(93)90039-p. [DOI] [PubMed] [Google Scholar]
- Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, et al. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429:167–72. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP) J Biol Chem. 2008;283:14542–51. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu N, Zhang J, Guo Y, Zhao Y, Korteweg C, Gu J. Expression and distribution of immunoglobulin G and its receptors in the human nervous system. Int J Biochem Cell Biol. 2011;43:556–63. doi: 10.1016/j.biocel.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Noda M, Doi Y, Liang J, Kawanokuchi J, Sonobe Y, Takeuchi H, et al. Fractalkine attenuates excito-neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. J Biol Chem. 2011;286:2308–19. doi: 10.1074/jbc.M110.169839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Takii K, Parajuli B, Kawanokuchi J, Sonobe Y, Takeuchi H, et al. FGF-2 released from degenerating neurons exerts microglial-induced neuroprotection via FGFR3-ERK signaling pathway. J Neuroinflammation. 2014;11:76. doi: 10.1186/1742-2094-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabon MM, Bachstetter AD, Hudson CE, Gemma C, Bickford PC. CX3CL1 reduces neurotoxicity and microglial activation in a rat model of Parkinson's disease. J Neuroinflammation. 2011;8:9. doi: 10.1186/1742-2094-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard AE, Leung PY, Vartanian KB, Stevens SL, Bahjat FR, Stenzel-Poore MP. TLR9 bone marrow chimeric mice define a role for cerebral TNF in neuroprotection induced by CpG preconditioning. J Cereb Blood Flow Metab. 2012;32:2193–200. doi: 10.1038/jcbfm.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–7. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–26. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal. 2010;22:977–83. doi: 10.1016/j.cellsig.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate KH, Beck H, Danner S, Allegrini PR, Wiessner C. Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol. 1999;58:654–66. doi: 10.1097/00005072-199906000-00010. [DOI] [PubMed] [Google Scholar]
- Porro C, Trotta T, Panaro MA. Microvesicles in the brain: Biomarker, messenger or mediator? J Neuroimmunol. 2015;288:70–8. doi: 10.1016/j.jneuroim.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Pradillo JM, Romera C, Hurtado O, Cardenas A, Moro MA, Leza JC, et al. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:193–203. doi: 10.1038/sj.jcbfm.9600019. [DOI] [PubMed] [Google Scholar]
- Prasad SS, Russell M, Nowakowska M, Williams A, Yauk C. Gene expression analysis to identify molecular correlates of pre- and post-conditioning derived neuroprotection. J Mol Neurosci. 2012;47:322–39. doi: 10.1007/s12031-012-9751-3. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–12. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Ramos-Cejudo J, Gutierrez-Fernandez M, Rodriguez-Frutos B, Exposito Alcaide M, Sanchez-Cabo F, Dopazo A, et al. Spatial and temporal gene expression differences in core and periinfarct areas in experimental stroke: a microarray analysis. PLoS One. 2012;7:e52121. doi: 10.1371/journal.pone.0052121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore KI, Berard JL, Redensek A, Chierzi S, Lopez-Vales R, Santos M, et al. Lipocalin 2 plays an immunomodulatory role and has detrimental effects after spinal cord injury. J Neurosci. 2011;31:13412–9. doi: 10.1523/JNEUROSCI.0116-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807–17. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaux-Le Goazigo A, Van Steenwinckel J, Rostene W, Melik Parsadaniantz S. Current status of chemokines in the adult CNS. Prog Neurobiol. 2013;104:67–92. doi: 10.1016/j.pneurobio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, et al. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31:16241–50. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig HL, Minami M, Lessov NS, Coste SC, Stevens SL, Henshall DC, et al. Endotoxin preconditioning protects against the cytotoxic effects of TNFalpha after stroke: a novel role for TNFalpha in LPS-ischemic tolerance. J Cereb Blood Flow Metab. 2007;27:1663–74. doi: 10.1038/sj.jcbfm.9600464. [DOI] [PubMed] [Google Scholar]
- Rosito M, Deflorio C, Limatola C, Trettel F. CXCL16 orchestrates adenosine A3 receptor and MCP-1/CCL2 activity to protect neurons from excitotoxic cell death in the CNS. J Neurosci. 2012;32:3154–63. doi: 10.1523/JNEUROSCI.4046-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr. VEGF receptor protein-tyrosine kinases: structure and regulation. Biochem Biophys Res Commun. 2008;375:287–91. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol. 2006;1:212–22. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen T, Carpen O, Karjalainen-Lindsberg ML, Paetau A, Turpeinen U, Kaste M, et al. Evolution of cerebral tumor necrosis factor-alpha production during human ischemic stroke. Stroke. 2001;32:1750–8. doi: 10.1161/01.str.32.8.1750. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- Sanchez-Moreno C, Dashe JF, Scott T, Thaler D, Folstein MF, Martin A. Decreased levels of plasma vitamin C and increased concentrations of inflammatory and oxidative stress markers after stroke. Stroke. 2004;35:163–8. doi: 10.1161/01.STR.0000105391.62306.2E. [DOI] [PubMed] [Google Scholar]
- Santello M, Volterra A. TNFalpha in synaptic function: switching gears. Trends Neurosci. 2012;35:638–47. doi: 10.1016/j.tins.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Sarabi AS, Shen H, Wang Y, Hoffer BJ, Backman CM. Gene expression patterns in mouse cortical penumbra after focal ischemic brain injury and reperfusion. J Neurosci Res. 2008;86:2912–24. doi: 10.1002/jnr.21734. [DOI] [PubMed] [Google Scholar]
- Schilling M, Strecker JK, Schabitz WR, Ringelstein EB, Kiefer R. Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice. Neuroscience. 2009;161:806–12. doi: 10.1016/j.neuroscience.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Zhang B, Belayev L, Khoutorova L, Amin R, Busto R, et al. DNA microarray analysis of cortical gene expression during early recirculation after focal brain ischemia in rat. Brain Res Mol Brain Res. 2002;108:81–93. doi: 10.1016/s0169-328x(02)00516-8. [DOI] [PubMed] [Google Scholar]
- Schubert D, Herrera F, Cumming R, Read J, Low W, Maher P, et al. Neural cells secrete a unique repertoire of proteins. J Neurochem. 2009;109:427–35. doi: 10.1111/j.1471-4159.2009.05968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Schwaeble WJ, Stover CM, Schall TJ, Dairaghi DJ, Trinder PK, Linington C, et al. Neuronal expression of fractalkine in the presence and absence of inflammation. FEBS Lett. 1998;439:203–7. doi: 10.1016/s0014-5793(98)01384-2. [DOI] [PubMed] [Google Scholar]
- Segaliny AI, Mohamadi A, Dizier B, Lokajczyk A, Brion R, Lanel R, et al. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int J Cancer. 2014 doi: 10.1002/ijc.29376. [DOI] [PubMed] [Google Scholar]
- Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab. 2010;30:459–73. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–78. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shen F, Walker EJ, Jiang L, Degos V, Li J, Sun B, et al. Coexpression of angiopoietin-1 with VEGF increases the structural integrity of the blood-brain barrier and reduces atrophy volume. J Cereb Blood Flow Metab. 2011;31:2343–51. doi: 10.1038/jcbfm.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan GK, Murphy KJ. Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open Biol. 2013;3:130181. doi: 10.1098/rsob.130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan GK, Wdowicz A, Pickering M, Watters O, Halley P, O'Sullivan NC, et al. CX3CL1 is up-regulated in the rat hippocampus during memory-associated synaptic plasticity. Front Cell Neurosci. 2014;8:233. doi: 10.3389/fncel.2014.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypiec AE, Shah RS, Schiavon E, Baker E, Skene N, Pawlak R, et al. Stress-induced lipocalin-2 controls dendritic spine formation and neuronal activity in the amygdala. PLoS One. 2013;8:e61046. doi: 10.1371/journal.pone.0061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano MA, Tessier M, Certa U, Gill R. Parallel gene expression monitoring using oligonucleotide probe arrays of multiple transcripts with an animal model of focal ischemia. J Cereb Blood Flow Metab. 2000;20:1045–55. doi: 10.1097/00004647-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, et al. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol. 2002;125:59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Zhou D, Locati M, Rieppi M, Proost P, Magazin M, et al. Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3. Similarities and differences with MCP-1. J Immunol. 1994;152:3615–22. [PubMed] [Google Scholar]
- Sprang SR. The divergent receptors for TNF. Trends Biochem Sci. 1990;15:366–8. doi: 10.1016/0968-0004(90)90228-4. [DOI] [PubMed] [Google Scholar]
- Sriram K, O'Callaghan JP. Divergent roles for tumor necrosis factor-alpha in the brain. J Neuroimmune Pharmacol. 2007;2:140–53. doi: 10.1007/s11481-007-9070-6. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction 'opening': signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–28. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Mostarica-Stojkovic M, Andjelkovic AV. CCL2 regulates angiogenesis via activation of Ets-1 transcription factor. J Immunol. 2006;177:2651–61. doi: 10.4049/jimmunol.177.4.2651. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, et al. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014:6. doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Simon RP. Genomics of preconditioning. Stroke. 2004;35:2683–6. doi: 10.1161/01.STR.0000143735.89281.bb. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, King JS, Simon RP. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke. 2007;38:680–5. doi: 10.1161/01.STR.0000251444.56487.4c. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–37. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Stevens SL, Vartanian KB, Stenzel-Poore MP. Reprogramming the response to stroke by preconditioning. Stroke. 2014;45:2527–31. doi: 10.1161/STROKEAHA.114.002879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Leung PY, Vartanian KB, Gopalan B, Yang T, Simon RP, et al. Multiple preconditioning paradigms converge on interferon regulatory factor-dependent signaling to promote tolerance to ischemic brain injury. J Neurosci. 2011;31:8456–63. doi: 10.1523/JNEUROSCI.0821-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker JK, Minnerup J, Gess B, Ringelstein EB, Schabitz WR, Schilling M. Monocyte chemoattractant protein-1-deficiency impairs the expression of IL-6, IL-1beta and G-CSF after transient focal ischemia in mice. PLoS One. 2011;6:e25863. doi: 10.1371/journal.pone.0025863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker JK, Minnerup J, Schutte-Nutgen K, Gess B, Schabitz WR, Schilling M. Monocyte chemoattractant protein-1-deficiency results in altered blood-brain barrier breakdown after experimental stroke. Stroke. 2013;44:2536–44. doi: 10.1161/STROKEAHA.111.000528. [DOI] [PubMed] [Google Scholar]
- Sun SL, Li TJ, Yang PY, Qiu Y, Rui YC. Modulation of signal transducers and activators of transcription (STAT) factor pathways during focal cerebral ischaemia: a gene expression array study in rat hippocampus after middle cerebral artery occlusion. Clin Exp Pharmacol Physiol. 2007;34:1097–101. doi: 10.1111/j.1440-1681.2007.04679.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnemark D, Eltayeb S, Nilsson M, Wallstrom E, Lassmann H, Olsson T, et al. CX3CL1 (fractalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: kinetics and cellular origin. J Neuroinflammation. 2005;2:17. doi: 10.1186/1742-2094-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B, Peters M, Konig HG, Poppe M, Levkau B, Rothermundt M, et al. Vascular endothelial growth factor protects cultured rat hippocampal neurons against hypoxic injury via an antiexcitotoxic, caspase-independent mechanism. J Cereb Blood Flow Metab. 2002;22:1170–5. doi: 10.1097/01.wcb.0000037988.07114.98. [DOI] [PubMed] [Google Scholar]
- Tang Y, Lu A, Aronow BJ, Wagner KR, Sharp FR. Genomic responses of the brain to ischemic stroke, intracerebral haemorrhage, kainate seizures, hypoglycemia, and hypoxia. Eur J Neurosci. 2002;15:1937–52. doi: 10.1046/j.1460-9568.2002.02030.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Pacary E, Freret T, Divoux D, Petit E, Schumann-Bard P, et al. Effect of hypoxic preconditioning on brain genomic response before and following ischemia in the adult mouse: identification of potential neuroprotective candidates for stroke. Neurobiol Dis. 2006;21:18–28. doi: 10.1016/j.nbd.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr., Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991;88:9292–6. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–70. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Jones F, Kubota ES, Pocock JM. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci. 2005;25:2952–64. doi: 10.1523/JNEUROSCI.4456-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouvenot E, Urbach S, Vigy O, Seveno M, Galeotti N, Nguyen G, et al. Quantitative proteomic analysis reveals protein expression changes in the murine neuronal secretome during apoptosis. J Proteomics. 2012;77:394–405. doi: 10.1016/j.jprot.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Thouvenot E, Urbach S, Dantec C, Poncet J, Seveno M, Demettre E, et al. Enhanced detection of CNS cell secretome in plasma protein-depleted cerebrospinal fluid. J Proteome Res. 2008;7:4409–21. doi: 10.1021/pr8003858. [DOI] [PubMed] [Google Scholar]
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–54. [PubMed] [Google Scholar]
- Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226–32. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Truettner J, Busto R, Zhao W, Ginsberg MD, Perez-Pinzon MA. Effect of ischemic preconditioning on the expression of putative neuroprotective genes in the rat brain. Brain Res Mol Brain Res. 2002;103:106–15. doi: 10.1016/s0169-328x(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Tsaparas P, Marino-Ramirez L, Bodenreider O, Koonin EV, Jordan IK. Global similarity and local divergence in human and mouse gene co-expression networks. BMC Evol Biol. 2006;6:70. doi: 10.1186/1471-2148-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Matsuyama T, Akita H, Nishimura H, Sugita M. Induction of tumor necrosis factor-alpha in the mouse hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17:491–9. doi: 10.1097/00004647-199705000-00002. [DOI] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, et al. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–20. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- van der Meer P, Ulrich AM, Gonzalez-Scarano F, Lavi E. Immunohistochemical analysis of CCR2, CCR3, CCR5, and CXCR4 in the human brain: potential mechanisms for HIV dementia. Exp Mol Pathol. 2000;69:192–201. doi: 10.1006/exmp.2000.2336. [DOI] [PubMed] [Google Scholar]
- Van Elzen R, Moens L, Dewilde S. Expression profiling of the cerebral ischemic and hypoxic response. Expert Rev Proteomics. 2008;5:263–82. doi: 10.1586/14789450.5.2.263. [DOI] [PubMed] [Google Scholar]
- VanGilder RL, Huber JD, Rosen CL, Barr TL. The transcriptome of cerebral ischemia. Brain Res Bull. 2012;88:313–9. doi: 10.1016/j.brainresbull.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedeler C, Ulvestad E, Grundt I, Conti G, Nyland H, Matre R, et al. Fc receptor for IgG (FcR) on rat microglia. J Neuroimmunol. 1994;49:19–24. doi: 10.1016/0165-5728(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. Embo J. 2001;20:1223–31. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila N, Castillo J, Davalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–9. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhou C, Wang Z, Liu J, Jing Z, Zhang Z, et al. Dynamic variation of genes profiles and pathways in the hippocampus of ischemic mice: a genomic study. Brain Res. 2011a;1372:13–21. doi: 10.1016/j.brainres.2010.11.099. [DOI] [PubMed] [Google Scholar]
- Wang L, Chopp M, Teng H, Bolz M, Francisco MA, Aluigi DM, et al. Tumor necrosis factor alpha primes cerebral endothelial cells for erythropoietin-induced angiogenesis. J Cereb Blood Flow Metab. 2011b;31:640–7. doi: 10.1038/jcbfm.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yue TL, Barone FC, Feuerstein GZ. Monocyte chemoattractant protein-1 messenger RNA expression in rat ischemic cortex. Stroke. 1995;26:661–5. doi: 10.1161/01.str.26.4.661. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Galvan V, Gorostiza O, Ataie M, Jin K, Greenberg DA. Vascular endothelial growth factor improves recovery of sensorimotor and cognitive deficits after focal cerebral ischemia in the rat. Brain Res. 2006;1115:186–93. doi: 10.1016/j.brainres.2006.07.060. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kilic E, Kilic U, Weber B, Bassetti CL, Marti HH, et al. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain. 2005;128:52–63. doi: 10.1093/brain/awh325. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, et al. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007a;85:740–7. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–60. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YQ, Guo X, Qiu MH, Feng XY, Sun FY. VEGF overexpression enhances striatal neurogenesis in brain of adult rat after a transient middle cerebral artery occlusion. J Neurosci Res. 2007b;85:73–82. doi: 10.1002/jnr.21091. [DOI] [PubMed] [Google Scholar]
- Watters O, Pickering M, O'Connor JJ. Preconditioning effects of tumor necrosis factor-alpha and glutamate on calcium dynamics in rat organotypic hippocampal cultures. J Neuroimmunol. 2011;234:27–39. doi: 10.1016/j.jneuroim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of CCR2 by endothelial cells : implications for MCP-1 mediated wound injury repair and In vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol. 1999;19:2085–93. doi: 10.1161/01.atv.19.9.2085. [DOI] [PubMed] [Google Scholar]
- Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–21. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M, et al. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih M, Kallenberg K, Bergk A, Dirnagl U, Harms L, Wernecke KD, et al. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke. 1999;30:1851–4. doi: 10.1161/01.str.30.9.1851. [DOI] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera D, Holtkamp W, Entschladen F, Niggemann B, Zanker K, Kaltschmidt B, et al. MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol. 2004;83:381–7. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- Woodbury ME, Ikezu T. Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration. J Neuroimmune Pharmacol. 2014;9:92–101. doi: 10.1007/s11481-013-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthmann H, Tryc AB, Goldbecker A, Ma YT, Tountopoulou A, Hahn A, et al. The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc Dis. 2010;30:85–92. doi: 10.1159/000314624. [DOI] [PubMed] [Google Scholar]
- Wu L, Du Y, Lok J, Lo EH, Xing C. Lipocalin-2 enhances angiogenesis in rat brain endothelial cells via reactive oxygen species and iron-dependent mechanisms. J Neurochem. 2015;132:622–8. doi: 10.1111/jnc.13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Wang X, Cheng C, Montaner J, Mandeville E, Leung W, et al. Neuronal production of lipocalin-2 as a help-me signal for glial activation. Stroke. 2014;45:2085–92. doi: 10.1161/STROKEAHA.114.005733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubov E, Gottlieb M, Gil S, Dinerman P, Fuchs P, Yavin E. Overexpression of genes in the CA1 hippocampus region of adult rat following episodes of global ischemia. Brain Res Mol Brain Res. 2004;127:10–26. doi: 10.1016/j.molbrainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Yamagami S, Tamura M, Hayashi M, Endo N, Tanabe H, Katsuura Y, et al. Differential production of MCP-1 and cytokine-induced neutrophil chemoattractant in the ischemic brain after transient focal ischemia in rats. J Leukoc Biol. 1999;65:744–9. doi: 10.1002/jlb.65.6.744. [DOI] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1213–24. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- Yang J, McNeish B, Butterfield C, Moses MA. Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer. Faseb J. 2013;27:45–50. doi: 10.1096/fj.12-211730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Peng F, Dhillon N, Callen S, Bokhari S, Stehno-Bittel L, et al. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29:1657–69. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yndestad A, Landro L, Ueland T, Dahl CP, Flo TH, Vinge LE, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30:1229–36. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- Yoshimi K, Woo M, Son Y, Baudry M, Thompson RF. IgG-immunostaining in the intact rabbit brain: variable but significant staining of hippocampal and cerebellar neurons with anti-IgG. Brain Res. 2002;956:53–66. doi: 10.1016/s0006-8993(02)03479-0. [DOI] [PubMed] [Google Scholar]
- Zaremba J, Losy J. Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurol Scand. 2001;104:288–95. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- Zelante T, Ricciardi-Castagnoli P. The yin-yang nature of CSF1R-binding cytokines. Nat Immunol. 2012;13:717–9. doi: 10.1038/ni.2375. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu N, Li B, McNutt MA. Neuron-derived IgG protects neurons from complement-dependent cytotoxicity. J Histochem Cytochem. 2013a;61:869–79. doi: 10.1369/0022155413504196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu N, Wang M, McNutt MA, Zhang D, Zhang B, et al. Neuron-derived IgG protects dopaminergic neurons from insult by 6-OHDA and activates microglia through the FcgammaR I and TLR4 pathways. Int J Biochem Cell Biol. 2013b;45:1911–20. doi: 10.1016/j.biocel.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Tsang W, Wang L, Chopp M. Down-regulation of p27kip1 increases proliferation of progenitor cells in adult rats. Neuroreport. 2004;15:1797–800. doi: 10.1097/01.wnr.0000135693.81613.cc. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683–7. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–38. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Shetty J, Hou L, Delcher A, Zhu B, Osoegawa K, et al. Human, mouse, and rat genome large-scale rearrangements: stability versus speciation. Genome Res. 2004;14:1851–60. doi: 10.1101/gr.2663304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Bausano B, Pike BR, Newcomb-Fernandez JK, Wang KK, Shohami E, et al. TNF-alpha stimulates caspase-3 activation and apoptotic cell death in primary septo-hippocampal cultures. J Neurosci Res. 2001;64:121–31. doi: 10.1002/jnr.1059. [DOI] [PubMed] [Google Scholar]
- Zhou L, Barao S, Laga M, Bockstael K, Borgers M, Gijsen H, et al. The neural cell adhesion molecules L1 and CHL1 are cleaved by BACE1 protease in vivo. J Biol Chem. 2012;287:25927–40. doi: 10.1074/jbc.M112.377465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhou Z, Liu Y, Zheng J. Fractalkine and CX3CR1 are involved in the migration of intravenously grafted human bone marrow stromal cells toward ischemic brain lesion in rats. Brain Res. 2009;1287:173–83. doi: 10.1016/j.brainres.2009.06.068. [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Ginis I, Furuya K, Klimanis D, Ruetzler C, Spatz M, et al. Lipopolysaccharide-induced ischemic tolerance is associated with increased levels of ceramide in brain and in plasma. Brain Res. 2001;895:59–65. doi: 10.1016/s0006-8993(01)02028-5. [DOI] [PubMed] [Google Scholar]
- Zujovic V, Benavides J, Vige X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–15. [PubMed] [Google Scholar]