Abstract
3-D cell printing, which can accurately deposit cells, biomaterial scaffolds and growth factors in precisely defined spatial patterns to form biomimetic tissue structures, has emerged as a powerful enabling technology to create live tissue and organ structures for drug discovery and tissue engineering applications. Unlike traditional 3-D printing that uses metals, plastics and polymers as the printing materials, cell printing has to be compatible with living cells and biological matrix. It is also required that the printing process preserves the biological functions of the cells and extracellular matrix, and to mimic the cell-matrix architectures and mechanical properties of the native tissues. Therefore, there are significant challenges in order to translate the technologies of traditional 3-D printing to cell printing, and ultimately achieve functional outcomes in the printed tissues. So it is essential to develop new technologies specially designed for cell printing and in-depth basic research in the bioprinted tissues, such as developing novel biomaterials specifically for cell printing applications, understanding the complex cell-matrix remodeling for the desired mechanical properties and functional outcomes, establishing proper vascular perfusion in bioprinted tissues, etc. In recent years, many exciting research progresses have been made in the 3-D cell printing technology and its application in engineering live tissue constructs. This review paper summarized the current development in 3-D cell printing technologies; focus on the outcomes of the live printed tissues and their potential applications in drug discovery and regenerative medicine. Current challenges and limitations are highlighted, and future directions of 3-D cell printing technology are also discussed.
Keywords: Cell printing, tissue engineering, regenerative medicine, 3-D tissue model
Introduction
Recently, three-dimensional (3-D) printing, also called rapid prototyping or additive manufacturing, has emerged as a powerful tool to fabricate physical models of hard tissues, biomaterial scaffolds and custom-shaped tissue implant prostheses. 3-D printing systems build 3-D structures using computer-controlled process to deposit materials onto a moving platform. Current 3-D printing systems build objects through several techniques36: (i) photo-polymerize liquid monomer, (ii) sinter powdered materials, (iii) process material either thermally or chemically as it passes through a nozzle, or (iv) print materials, such as chemical binder onto powder. 3-D printing techniques can be easily automated and integrated with imaging techniques to produce anatomically structures that are customized in size and shape for individual patient. 3-D printing has been very successful in making biomaterial scaffolds with custom-designed geometries and is becoming an important enabling technology for tissue engineering28, 36, 37, 55, 74, 87, 98, 99. These scaffolds can be pre-fabricated and implanted in vivo to allow degradation and remodeling, or cells can be seeded onto the scaffolds to create tissue construct in vitro. However, how to translate the technologies of 3-D printing into building the living tissues is still a daunting task. In most 3-D printing techniques, toxic solvents, high temperatures or strong UV laser are commonly used, thus making them not suitable to build live tissues and limiting their further applications in tissue engineering.
To print 3-D live tissues, recent research focus on developing technology to simultaneously deposit hydrogels with live cells to form 3-D tissue structures16, 45, 56, 61, 62, 67, 75, 97. This new concept, also called cell printing or organ printing, is an advanced form of 3-D printing. The critical components of this technology include the use of biomaterials hydrogels that can undergo phase changing under physiological conditions without harsh chemicals and gentle cell printing technology that does not cause damages to the cells. Current strategies to induce phase change (from liquid to solid after printing) include UV, temperature, pH and ion concentrations, which can be used on a variety of natural and synthetic hydrogels. Cell printing technology is able to simultaneously deposit live cells, growth factors along with biomaterial scaffolds at the accurate location to mimic the native tissue architecture and formation process. 3-D cell printing has great potentials to (i) create fully functional tissues for regenerative medicine, and (ii) fabricate human cell-based tissue models, for applications in disease modeling and drug development. Despite all the promises, 3-D cell printing technology is still at its very early stage, and there are enormous potential for growth in this field. Meanwhile, there are significant challenges that must be solved for this technology to have major impacts to tissue engineering. Compared with traditional 3-D printing, 3-D cell printing involves additional complexities, such as the choice of materials, cell types, growth and differentiation factors, and technical challenges related to the sensitivities of living cells and the construction of tissues. Addressing these complexities requires interdisciplinary approaches that pursue the integration of technologies from the fields of engineering, materials science, biology, chemistry and medicine. For a comprehensive review of 3-D bioprinting technology and issues related to how to integrate all these diverse fields, several excellent review articles have been published recently1, 54, 63. This review summarizes the most recent development of 3-D cell printing technologies to construct live tissues and its applications in disease modeling.
Cell Printing Technologies
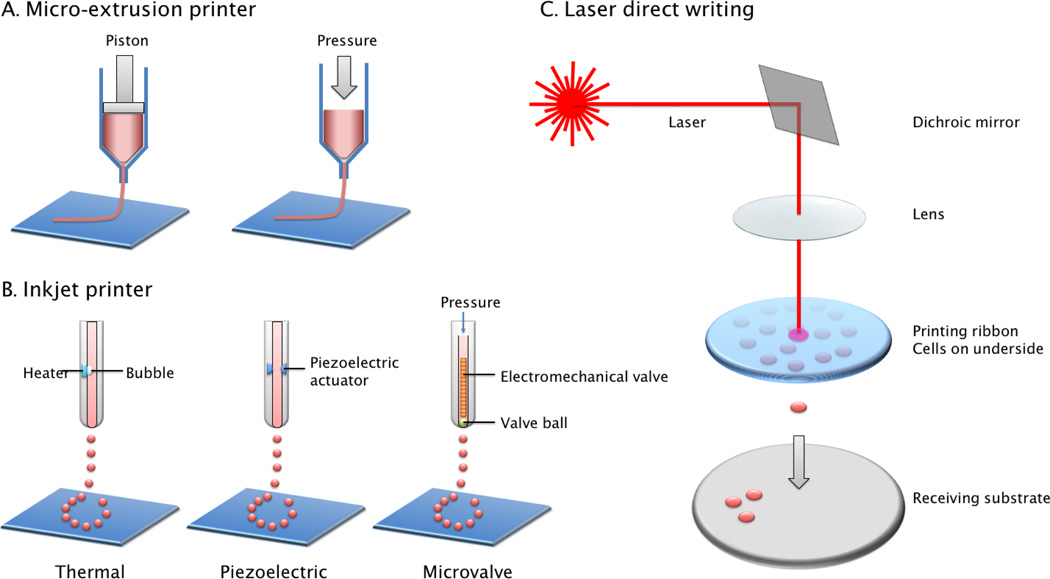
One of the core components for successful cell printing is the dispensing technology that can accurately deposit live cells without causing cellular damages. Several technologies have been developed to dispense cells and biomaterial hydrogels, either as liquid droplets or as continuous stream of viscous gels. Generally, there are three major types of cell printing techniques: inkjet, micro-extrusion, and laser-assisted cell printing63 (Figure 1). In the following section, we will review the main beneficial features of each technology and also their drawbacks. We will also introduce several newly developed cell printing techniques for tissue engineering applications.
Figure 1.
Printing mechanisms of major cell printing techniques. A. Micro-extrusion based cell printer uses computer controlled piston or pneumatic pressure to extrude the materials out of a syringe needle. B. Inkjet printer uses several mechanisms (thermal bubble, piezoelectric or electromechanical valve) to create droplets out of liquid solution. C. Laser direct writing uses the energy of the focused laser beam to generate localized heat to form liquid droplet. Adapted from Malda et al.57
Extrusion-based cell printing
Micro-extrusion is the most commonly used technique for 3D printing of biomaterial scaffolds and can also be adapted for cell printing. Micro-extrusion based bioprinters consist of 3-axis robotic stages and pneumatic or mechanical dispensing system. Under the constant pressure, continuous strands of biomaterials are extruded out from a syringe nozzle (Figure 1A). 3-D constructs are fabricated using a layer-by-layer approach, in which the deposited layer serves as a foundation for the subsequent layer. The amount of dispensed biomaterials can be adjusted by (i) controlling the level of pneumatic or mechanical pressure, (ii) the nozzle size, and (iii) the nozzle moving speed42. Following 2D pattern printing of the hydrogels, these are solidified and stacked layer by layer, to form 3-D structures. Mechanical dispensing systems generally provide more direct control over the material extrusion through the printer nozzle because it can avoid the delay of the gas compression occurred in pneumatic systems. Thus, mechanical dispensing methods are thought to be more suitable for printing highly-viscos materials than pneumatic system.
Extrusion-based printers are the most used printing technique in bio-printing field. There are numerous versions of commercial or custom-made extrusion printers with vast price range. The major advantage of this technique is the printing capability of highly-viscos materials and cell spheroids through the nozzle. Multicellular cell-spheroids, which possess the mechanical and functional properties of the matching tissue, can be deposited using extrusion printer to self-assemble into 3-D structure during post-printing culture39. The major disadvantage of this printer is high shear stress, which tends to kill the cells during or after printing process. Cell survival rates after extrusion printing are generally lower than those seen with the inkjet printers (40–86%), and the survival rate decreases with increased extrusion pressure7, 65.
Inkjet cell printing
Inkjet-based cell printing is a non-contact technique in which droplets of cells or biomaterials are dispensed, driven either by thermal bubble, piezoelectric actuator or electromechanical valve controlled pressure pulse (Figure 1B). The printing parameters of the inkjet can be digitally controlled such as the pressure pulse, temperature, voltage and duration, in which picoliter to microliter volume of aqueous biological materials can be dispensed as liquid droplets. In early days, the commercial desktop inkjet printer was modified for cell printing by replacing the ink cartridge with cell suspension or biological ink93. While this approach has been successfully applied toward printing several tissue constructs10, 75, 97, it lacks the versatility for a variety of cells and hydrogels, and the printing parameters are difficult to control since commercial inkjet printers are not designed to handle cells at first place. Since then, inkjet-based cell printing systems are newly developed to handle a wide range of cells and biomaterials at increasing resolution and speed.
Several mechanisms are used to generate droplet out of the liquid solution. The thermal bubble printers apply heat to the print head, so the pressure generated by bubble can force out bioink in droplet form. During this procedure, 200–300°C of temperature can be generated. However, it lasts for only a few microseconds, therefore the total temperature increase is generally less than 10°C and does not induce any significant damages on cells13, 96. Thermal inkjet printers are of low cost and have high fabrication speeds. However, thermal and mechanical stresses, limitations on material choice, frequent nozzle clogging prevent its further applications in tissue engineering. The piezoelectric 3-D printers respond to the applied electrical signals, generating pulse wave inside print head to break the stream of bioink into droplets77, 90. One major concern in using the piezoelectric mechanism-based bioprinters is that the 15–25 kHz frequencies employed in these printers may induce cellular damage. Another technique of inkjet bioprinting is by electromechanical valve-controlled pressure pulse. In this method, a constant pressure is applied, and the opening and closing of the electromechanical valve under the control of pulsed voltage (~100–500µs) will lead to droplet formation. The droplet size is determined by the valve opening duration, the actuation frequency, material viscosity, and the pressure applied. Compared to thermal inkjet printers, this method does not lead to the heating of the cells, thus allows very gentle deposition of the cells and high post-printing viability51. Recently, newer technology was developed that is able to encapsulate single to few cells via a nozzleless ejection technology using a gentle acoustic field. Various types of cells are encapsulated in acoustic picoliter droplets with frequency of 1–10,000 droplets/second. This technology provides high precision, high viability, and controlled directionality15. In all the inkjet printing system, size of nozzle (printer head) orifice and electrical pulse pattern (duration and amplitude) play main roles in determining the droplet size. The viscosity of bioink has an effect on the droplet size and reproducibility, as highly viscous materials or media suspensions with high cell density often require higher force to be ejected and may cause clogging problems.
Advantages of inkjet printer include availability, low cost and versatility. Inkjet-based 3-D cell printing methods can generate relatively high-resolution structures (20–100µm)66. The drop size (1–300 picoliter) and drop deposition rate (1–10,000 droplets/sec) can be controlled electronically80. The use of gentle pressure guarantees high post-printing viability, showing a great potential of the technique in handling delicate cells such as stem cells, progenitor cells, and embryonic bodies. Due to the dispensing mechanisms and non-contact nature of the inkjet printer, the low viscous aqueous form of hydrogel precursor (viscosity < 10 centi-poise) are preferably used whereas high viscosity materials or high density cell suspension often cause issues including nozzle clogging, irregular droplet size, irregular dispensing trajectory, and premature gelation. Post-printing crosslinking or gelation process is often required. These produces include UV irradiation, temperature changes, or the use of acidic/basic solutions, which may induce various cell damages. The limitation of the biomaterials used implies that the printed structure often has weak mechanical properties10, 51 and high cellular density is still difficult to achieve. For further use of inkjet printer as a tissue engineering tool, the above-mentioned drawbacks need to be addressed.
Laser direct writing
Laser Direct-Write (LDW) is a non-contacting method of material deposition that utilizes laser energy absorption to propel a cell-suspended hydrogel droplet to a receiving surface78. A laser-assisted 3-D bioprinter consists of (i) a pulsed laser beam with a focusing system, (ii) a ‘ribbon’ that has a donor transport support, typically made from glass covered with a laser-energy-absorbing layer (e.g., gold or titanium) and a layer of biological material containing cells and/or hydrogel, and (iii) a receiving substrate facing the ribbon (Figure 1C). The laser is pulsed with a configurable energy and repetition rate through the transparent ribbon. The energy-absorbing layer absorbs the transmitted laser energy, volatizes, and forms a vapor pocket at the ribbon-material interface. This vapor pocket rapidly expands and ejects a droplet of the transfer layer to a receiving substrate.
LDW can deposit cells at a density (<108 cells/ml) with the resolution of a single cell per drop using a laser pulse, at high speed30, 31, 68. These features allow LAB to create high-throughput laser patterning of cells and biomaterials. LDW systems can be set up to visualize cells in real time before and after they are deposited, which no other approach offers. This capability ensures specific cells can be chosen for transfer and confirmed visually post-transfer. By contrast, cells are randomly dispersed in a volume in inkjet printing technique. The number of cells deposited is therefore a function of the probability of the number of cells present in the dispensed volume. LDW is a nozzle-free technique, therefore does not have the problems of nozzle clogging with cells or materials, which are major drawbacks of other bioprinting technologies. Therefore, LDW is compatible with a wide range of biomaterial viscosities (1–300 mPa/sec).
However, LDW may not be appropriate for every application, and its limitations should be considered with other printing approaches. Compared to inkjet printing, LDW has lower throughput, as printing multiple droplets requires movement of the ribbon and receiving stages, and pulsing the laser. The speed of stage movement can limit the rate at which single droplets are deposited. Droplet volume is also generally smaller than droplets printed using inkjet techniques. Smaller droplet volume requires more droplets to cover the same area, and this is also linked to throughput, especially for larger areas.
Stereolithography
Stereolithography is a maskless lithographic technology that utilizes laser-controlled systems for 3-D fabrication of photo-crosslinkable polymers. Stereolithography system consists of light source (laser or digital light), moving stage platform (in z-axis), and container for hydrogel precursor solution. Like other cell printing techniques, stereolithography fabricates 3-D structures through a layer-by-layer approach using pre-defined 3-D design. Ultraviolet (UV) light is illuminated on the photo-crosslinkable precursor solution to solidify patterns of the first plane on stage. Then, the stage moved a defined height in z-axis for the patterning of second layer. This step repeats to create a desired 3-D construct. 2-D pattern on each plane can be created by using either micro-mirror device or 3-axis micro motor stage85.
Stereolithography allows rapid and complex fabrication with micrometer-scale resolution, and has been vastly used for tissue engineering applications. However, the technique is only capable of using photo-sensitive materials, and UV exposure usually causes damage to the embedded cells.
Newly-developed cell printing techniques
There are numerous cell printing techniques that have been invented and improved for tissue engineering applications, however, researchers constantly develop novel cell printing techniques to overcome limitations of current methodologies and to expand its potential in bio-related fields. Most of newly-developed techniques are variations or combinations of existing technologies, thus fall into categories mentioned above. A few examples of the most recent developments will be introduced in the following paragraphs.
Hinton et al. developed a novel printing technique using suspended hydrogel as a supporting material for 3-D extrusion printing34. In their method, bioink extruded from the print head was injected within a secondary thermos-reversible hydrogel bath. This bath contained suspended hydrogel (gelatin slurry in this case), that provides biocompatible support for inject bioink. 3-D structure was built layer-by-layer and, when completed, was released by increasing temperature to 37°C and melting the gelatin slurry. The secondary supporting gel can be supplemented with crosslinking materials. For example, thrombin was added to the bath for fibrinogen printing. They successfully fabricated complex 3-D constructs such as bifurcated tube, embryonic heart model, and human brain model using alginate, fibrinogen, collagen, and matrigel with resolution of ~200µm. During the procedure, the entire structure was immersed in the hydrogel bath and no harsh chemicals used. The hydrated and buffered slurry bath prevents loss of moisture and other harsh situations, which can damage cells during the fabrication process.
Co-axial extrusion technique is another novel printing method that has been actively investigated lately9, 26, 100. Co-axial extrusion is an application of micro-extrusion printing. The print head consists of two needles, small internal needle and larger external needle in co-axial position. Various fiber structures can be fabricated by utilizing different materials for external and internal needles. Alginate filaments with built-in microchannel inside were created by using a co-axial nozzle26. In their experiments, alginate or alginate-cell mixture was printed via external needle, and crosslinking solution (CaCl2) was extruded via internal needle, resulting a filament structure with outer alginate layer and CaCl2 liquid inside. The filaments were printed into CaCl2 liquid reservoir for instantaneous solidification of outer alginate layer. The resolution can be adjusted by controlling concentration and crosslinking densities of hydrogel precursor polymers as well as injection speed. The printed filaments with hollow tube structure allows perfusion through the inside microchannel, thus provide better nutrient delivery for long-term tissue survival and maturation. This approach has a great potential to address vascularization issue in tissue engineering, since endothelial cell seeding and dynamic perfusion can be easily achieved using the embedded microchannels.
Yu et al. utilized two types of extrusion nozzles for scale-up tissue fabrication: a co-axial nozzle for printing tubular vasculature; and a traditional micro-nozzle for printing tissue strands with cell aggregates100. Two units of cell printing platform were used to simultaneously control printing processes of two distinct materials. The tubular strands for vasculature were printed in a continuous single luminal form for the ease of later perfusion. The tissue strands filled the space between vascular channels, thereby created a tissue assembly with perfusable vascular channels. 3-D multilayered scaffold was fabricated by using co-axial printing of alginate solution. Instantaneous gelation when exposed to calcium ions. The coaxial extruder allows forming gel fibers that can be layered according to pre-designed 3-D structure. Resolution can be adjusted by controlling concentration and crosslinking densities of hydrogel precursor polymers.
In another research, printing of two different bioinks through single print head has been demonstrated by utilizing microfluidic platform incorporated to a cell printing system9. This printing approach enables multi-material deposition with high resolution using single extruder print head, thus increases the versatility of cell printing technology for the fabrication of complex heterogeneous structures. The extruded strands consisted of two bioinks that touch each other but do not mix together. In the cross-section of the printed strands, each bioink filled half of the strand, showing clear semicircle form with a clear boundary line.
Biomaterials for cell printing
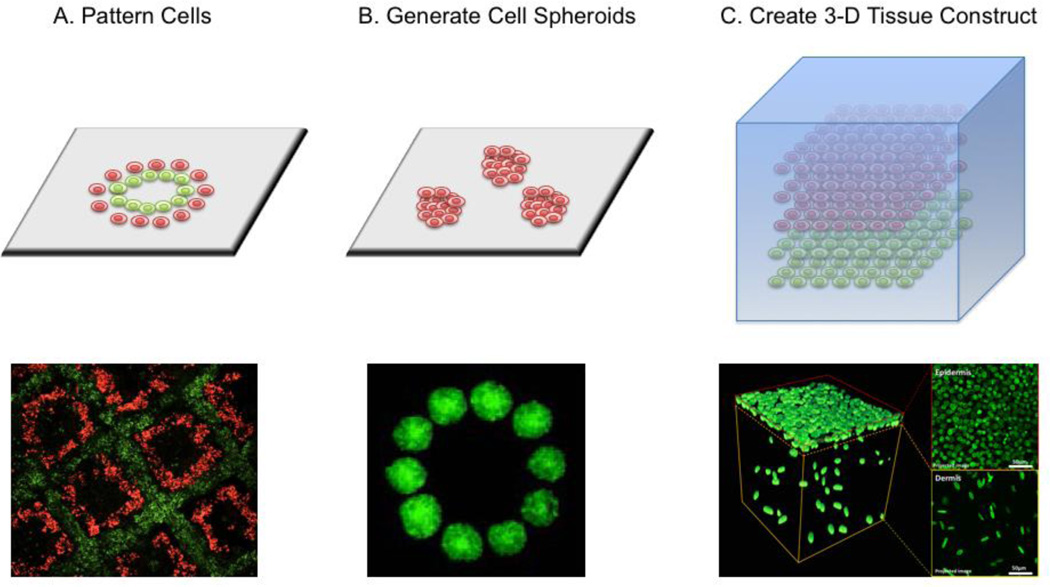
Cell printing technology can be easily applied to create any cellular patterns to study cell-cell interactions (Figure 2A). The printed cellular patterns can be also utilized to develop experimental models for investigating cell-scaffold interactions or cell-soluble factor interactions. The technology also allows efficient generation of 3-D organoid culture by printing high-density cells to allow later cell fusion (Figure 2B). These micro-tissue models, mimicking the complex cell-cell interactions of the in vivo tissues, can be used for basic biology studies as well as high-throughput drug screening. The real power of the cell printing technology, however, is its ability to create 3-D tissue structures which contain various cells and matrix to mimic the native tissues (Figure 2C). Besides a cell compatible dispensing technology, successful implementation of bioprinting relies heavily on the integration with compatible biomaterials (scaffold materials) that are responsible for supporting the cellular components during and after bio-fabrication, and that are also compatible with the cell printing devices. Currently, there is no ideal material specialized for the purpose of cell printing. Most cell printing applications adapt the same biomaterials used in traditional bioengineering81, and sometimes combine them in order to achieve the desired crosslinking and mechanical properties.
Figure 2.
Applications of cell printing: A. pattern the cell-cell interactions21; B. generate cell spheroids to induce cell fusion for organoid culture39; C. create 3-D tissue construct by integrating biomaterial hydrogels48.
With regard to the choice of materials for cell printing, one needs to consider numerous factors such as the printability, rheological properties, the polymerization mechanisms, cytotoxicity, and the material’s compatibility with the printer that will be used. These factors limit options for biomaterials. The biomaterials currently used for cell printing generally fall into two primary categories: (i) curable polymers that form mechanically robust scaffolds after solidification, and (ii) soft hydrogels that provide better microenvironment for residing cells. The curable polymers generally involve a use of harsh polymerization conditions, thus cells need to be seeded after fabrication and washing steps. Soft hydrogels are cytocompatible in most cases, but do not have the same level of mechanical properties as curable polymers. The characteristic properties of printing materials, such as melting points, mechanical properties, and available chemical modifications, and polymerization mechanisms determine the material printability and eventually the quality of resulting products.
Hydrogel is the primarily-used biomaterials for live cell printing64. Hydrogels are composed of polymer or peptide chains. Hydrogels are printed in a liquid precursor form, and then cross-linked to form a solidified macromolecular network. There are two major categories for hydrogel classification: (i) synthetic hydrogels, which exploits polymers that are synthesized in the laboratory, and (ii) naturally-derived hydrogels, which are collected/purified from natural sources and are often further manipulated in the laboratory.
To be considered as cytocompatible materials, these hydrogels should not induce damages on cells, and should provide cell-binding motif to allow cell adherence. Except the stiffest tissue, hydrogels can recapitulate a range of elastic modulus through manipulation of chemistry, crosslinking density, and polymer concentration, thus mimicking the elastic moduli of most the soft tissues in the body. Processing techniques to generate crosslinking reactions can be designed to be non-cytotoxic, allowing 3-D encapsulation of cells within the hydrogel polymer networks at the time of gelation. Because no single hydrogel can meet the multiple requirements of the cell printing process, several different hydrogels can be combined as composite material to achieve the desired properties95. For example, in one study, a bioink that combines the outstanding shear thinning properties of nano-fibrillated cellulose with the fast cross-linking ability of alginate was formulated for the 3D printing of living soft tissue with cells. The shear thinning behavior of the tested bioinks improved the printability and enable the construction of 3-D tissues58.
Polyethylene glycol (PEG)-based materials, such as PEG diacrylate (PEGDA) or polyacrylamide (PAAm) gel, are the most commonly used synthetic hydrogels for the purpose of cell printing. In general, synthetic hydrogels have advantages on fine-tuning of gel properties by adjusting molecular weights, molecular distributions, and crosslinking densities. However, due to the lack of bioactivity through naturally occurring peptide sequences or conformational motifs, PEG-based materials requires additional modifications to establish cell-material interactions that can support biocompatibility and the integration of cells and tissues. In addition, although PEGDA hydrogels are easily crosslinkable via UV exposure and their mechanical properties can be tuned to match various tissue stiffness, the materials are usually very brittle, thus it is very difficult to handle it surgically for clinical translation.
Naturally-derived hydrogels commonly utilized for cell printing include collagen, fibrin, alginate, and Matrigel29, 71, 79. The natural hydrogels have less room for the control of physical properties, but often have an innate bioactivity, which supports cell and tissue integration and biocompatibility1. To crosslink the hydrogel after printing, use of non-cytotoxic crosslinking method is especially desired such as gentle photon-initiated reaction, this works for PEG-based hydrogels but this may not work for some of the natural derived hydrogels. Recently, new photochemistry methods have been developed to modify the natural derived hydrogels such as methacrylated gelatin (GelMA) or hyaluronic acid (HA) so that they can be photo-crosslinkable after printing to initiate phase change and form 3-D structures4, 83.
Regardless of advantages and disadvantages of these hydrogels, neither the synthetic or naturally derived hydrogels can properly replicate the complex composition and architectures of native ECM. This issue prevents the reconstitution of the intrinsic cellular morphologies and functions. Common methods used to overcome this problem include chemical modifications and creating mixture of multiple hydrogels. As mentioned above, synthetic gels have advantages on fine-tuning the mechanical, chemical, physical properties. The biological features are often achieved by the conjugation of functional components to the base hydrogel materials. However, finding optimal properties is challenging because it varies largely depending on the ECM features of target tissue, cell types, and post-printing culture environment. Rutz et al. established a versatile method to create tunable bioinks that permit adjustments of the mechanical, chemical, physical, and biological properties of resulting structures76. They demonstrated 35 formulations of bioinks in order to optimize structural and biological performance while maintaining printability. These bioinks consist of varying materials (PEG, gelatin, and/or fibrinogen), and can be customized with regard to composition, polymer concentration, and crosslinking densities.
Another research group used a blend of alginate (calcium crosslinking) and GelMA (photo-polymerization) to develop a low-viscosity bioink for multi-material deposition9. In general, low-viscosity materials provides better environments for cell proliferation and tissue maturation, as cells can more easily degrade the surrounding matrix and have more access to biological interactions. However, low-viscosity materials often fail to support structural integrity during printing procedure, thus it is challenging to secure the printability of these materials. The bioink mixture that consists of alginate, GelMA, photoinitiator, and cells, was injected into the calcium crosslinking solution using co-axial needle extrusion platform. The bioink was extruded via internal needle, while CaCl2 solution was dispensed via external needle. Physically crosslinked alginate can support low concentration GelMA without collapsing during fabrication process. After printing, GelMA with embedded microfibers was crosslinked by UV exposure. These modification and mixing approaches allows improving the printability of bioink and to provide better functional and architectural features, therefore, to enable the formation of complex heterogeneous structures aimed to meet the requirements of desire tissues.
Decellularized matrix components have been recently suggested as a new type of bioink, as it allows compensating for the lack of functionality to a certain extent. Pati et al. introduced a bioprinting method that utilizes decellularized extracellular matrix (dECM) bioink to construct tissue structures73. Using this method, various tissue-specific bioprinted structures such as adipose, cartilage and heart tissues have been created, demonstrating the versatility of the tissue-specific dECM bioinks. The dECM bioink is capable of providing essential cues for cells engraftment, survival and long-term revelation of biological functions. The results bioprinting with dECM bioinks demonstrated high cell viability and functionality of the printed tissue72, 73.
Besides synthetic and natural-derived bioink, cell-aggregates such as tissue spheroids, cell pellets or tissue strands can also be printed. Tissue spheroids can be considered as “living materials” with certain measurable, evolving and potentially controllable material properties. Three-dimensional functional living macro-tissues and organ constructs can be constructed using tissue spheroids as building blocks. Closely placed tissue spheroids undergo tissue fusion: a process that represents a fundamental biological and biophysical principle of developmental biology-inspired directed tissue self-assembly62. The mechanical properties of tissue spheroids can vary greatly depending on cell/tissue type, seeding density, scaffold materials, culture period, and spheroid size. In general, it is challenging to stack spherical shape of spheroids without using ‘glue’ materials. Hydrogels are commonly used as glue or scaffold materials for successful embedment of tissue spheroids within 3-D constructs. The pace of spheroid fusion highly depends on tissue type, mechanical properties of spheroids, glue materials, and culture condition. The fusion or integration may take several days to several weeks62.
Developments of novel printing materials also influence on the invention of novel cell printing techniques. Typical 3-D cell printing technologies relies on a layer-by-layer stacking approach of a planar pattern (on x-y plane), due to printing mechanism or material limitations. Print head that can freely move on all three x-y-z axis may reduce total printing time, improve biocompatibility during procedure, and allows fabrication of more complex structure. As mentioned above, Hinton et al. developed a novel printing technology to achieve the free movement of print head in 3-D space34. It was accomplished by changing physical state of traditional hydrogel material (from solid gel or liquid state to suspended “slurry” state). Other approaches have been introduced to achieve same goal by resolving limitations of bioink33. Highley et al. developed a cell printing approach in which shear-thinning hydrogel bioink was directly printed into self-healing supporting hydrogels. Both hydrogels are based on modified hyaluronic acid (HA) conjugated with adamantane (bioink) or β-cyclodextrin (supporting gel). The self-healing support gel is deformed when extruder needle was inserted for shear-thinning bioink injection. Then, the support gel rapidly recovers around the injected bioink, resulting stably-retained 3-D printed structure at high resolution (~35um) within the supporting gel. With further HA modification with methacrylates and additional photo-polymerization process, self-supporting 3-D constructs or hydrogel structure with embedded microchannels can be fabricated using this approach.
3-D printed live tissue analogs
3-D cell printing, with its ability to precisely control the geometrical localization of the cells and biomaterials, is especially powerful to create complex 3-D tissue structures. Although there is a lot of work to be done to accomplish a complete fabrication of fully functional human-sized organs, there has been a great progress in generating simplified tissues in smaller scale to mimic the native tissues such as bone, cartilage, skin, and blood vessel. 3-D printed tissue models are valuable in a variety of applications such as tissue regeneration, pathology modeling, drug development, and high-throughput screening. Here we will present some examples of printed live tissues, focusing on the fabrication methods, employed biomaterials, and biological outcomes.
3-D Tumor Model
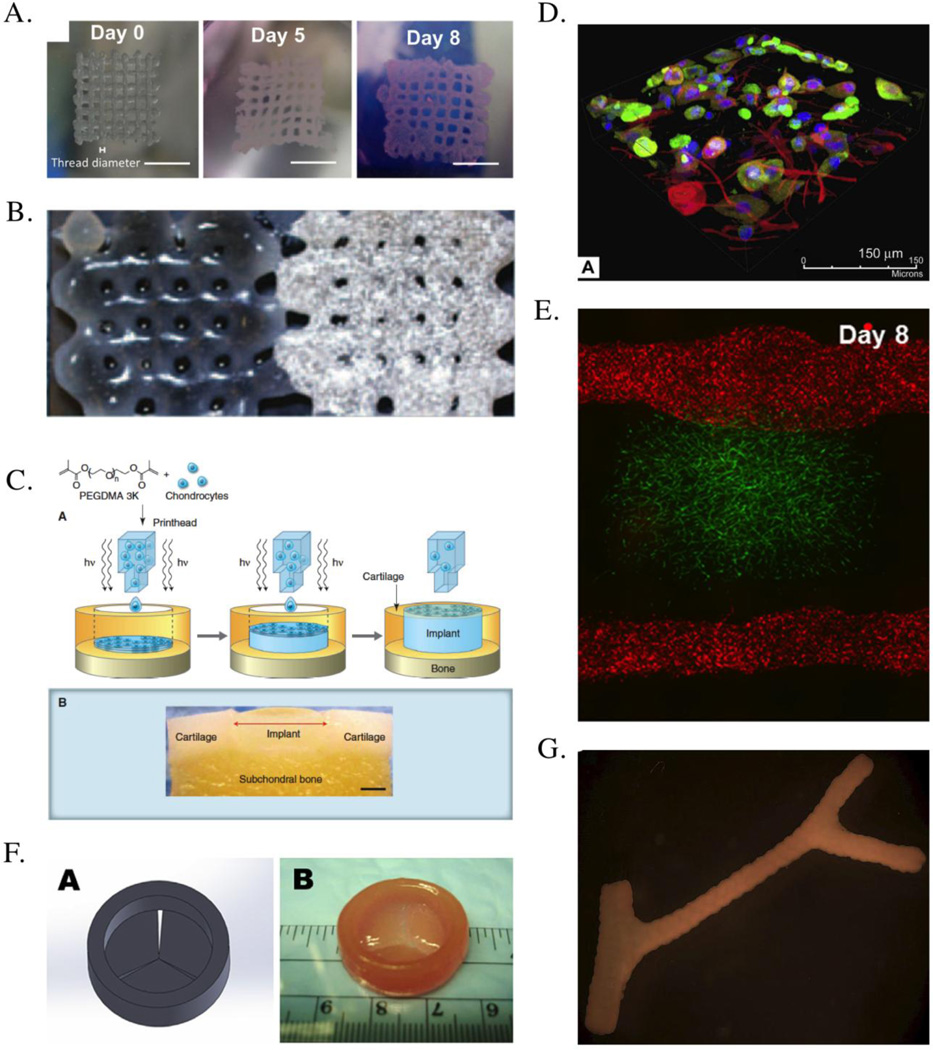
3-D cell printing allows the direct assembly of cells and extracellular matrix to form 3-D tissue models, which are extremely useful for the understanding of basic biology in 3-D environment, as well as for high throughput drug screening. For example, cancer research mostly relies on animal models. While these in vivo models are extremely important, there are significant difference in terms of cancer biology and immunology between human and mice. Therefore, many drugs that work in mice never work in human. Besides, animal models are expensive and the discovery process is very long. Use of the 3-D humanized tumor model derived from individual patient, therefore, offers the tremendous opportunity to advance this field by significantly lower the cost and shorten the time frame of drug discovery. Recently, 3-D cell printing was used to create an in vitro cervical tumor model102. In this study, HeLa cells and gelatin/alginate/fibrinogen hydrogels were used in 3-D printing to create the tumor models (Figure 3. (A)), demonstrating >90% viability. It was demonstrated that there are significant difference between the 2-D and 3-D tumor model. In 3-D printed model, the cell proliferation is higher and form cellular spheroids, in comparison to 2-D culture. 3D printed models also showed higher MMP protein expression and higher resistant to chemotherapy than those in 2D culture. These data indicates the value of the 3-D printing in creating the tumor models, and its clinical significance in drug discovery warrant further studies.
Figure 3.
Examples of tissue constructs created by cell printing. (A) 3-D tumor models were created using HeLa Cells printed in gelatin/alginate/fibrinogen hydrogels, shown here is the top view of 3D HeLa/hydrogel constructs on day 0, day 5 and day 8. Scale bar, 5 mm102. (B) A porous constructs containing osteogenic and endothelial progenitor cells were printed, shown here is the printed graft: 10×20×1mm; (left) endothelial progenitor cell-laden Matrigel part, (right) multipotent stromal cell-laden Matrigel part with added biphasic calcium phosphate20. (C) Bioprinting a cartilage structure, combining inkjet printing with a poly(ethylene glycol) dimethacrylate (PEGDMA) solution containing cells in suspension with a simultaneous photo-polymerization process. (lower panel) Light microscopy image of cell-containing polyethylene hydrogel printed into a defect formed in an osteochondral plug (scale bar, 2 mm)11. (D) 3-D structure of bioprinted multi-layered skin structure consists of fibroblasts layer and keratinocyte layer, shown here is the volume rendered immunofluorescent images of multi-layered printing of skin51. (E) Multi-scale vascular network was created within 3-D hydrogel using cell printing. GFP–HUVECs were embedded within fibrin part for microvascularization. RFP–HUVECs were seeded on the two fluidic channels to form vasculature with mm-scale of lumen size50. (F) Bioprinting of heart valve conduit with encapsulation of human aortic interstitial cells within the leaflets: (left) heart valve model designed by Solidworks, (right) printed valve conduit18. (G) Multicellular spheroids assembled into tubular structures, shown here is the fused branched construct after 6 days of deposition67.
Bone
There are extensive studies of using 3-D printed bone scaffolds without cells, such as incorporating osteogenic factors (e.g. bone morphogenic proteins) in 3-D printed scaffolds, and development of osteoinductive 3-D scaffolds54. However, large-size bone defects hardly heal without cell delivery, thus introducing cells within the scaffolds are necessary. Bone tissue requires the strongest mechanical properties among human tissues. Synthetic hydrogels such as poly(caprolactone) (PCL)91, polylactic acid and polyglycolic acid (PLGA)47, biphasic calcium phosphate (BCP)86, tricalcium phosphate (TCP)89, or combinations of these hydrogels8, 92 have been utilized for bone tissue engineering. Although collagen type I is a major component of bone tissue ECM, collagen I is not a popular scaffold material for bone tissue engineering38 because its mechanical strength is not as good as synthetic hydrogels. Most of the scaffold materials utilized for bone tissue printing are in high concentration and highly-viscos. For this reason, extrusion printing technique is often chosen as a fabrication method. Sintering-based techniques are also popular for scaffolds with even higher mechanical properties6, 54. To promote bone formation, Osteoblasts47, 86, 89 and mesenchymal stem cells (MSCs)8, 92 can be embedded within the scaffolds during printing procedure or seeded on the scaffold surfaces. Growth factors such as BMP-247, 86 and VEGF are also commonly incorporated with the scaffolds to enhance bone formation and bone angiogenesis6.
In an in vivo study, highly porous BCP bone implants were fabricated using 3-D printing and ectopically implanted in the back of rats. The incorporation of osteoblasts seeding and BMP-2 encapsulation created a synergic effect and enhanced bone formation86. In another study using hBMSCs (human bone marrow MSCs), 3-D-printed β-TCP/PLGA scaffolds with bBMSCs seeding were implanted subcutaneously into nude mice for six weeks. The results demonstrated that biomechanical stiffness, radiological densities, and bone ECM accumulation were significantly enhanced after six weeks92.
Anatomically shaped PCL scaffolds with varying porosities were fabricated based on medical imaging data, then used to support the induction of seeded human adipose-derived stem cells to form vascularized bone to repair mandibular and maxillary bone defects91. The findings of this study illustrate the potential of 3-D cell printing technology in engineering autologous, anatomically-shaped bone grafts, which can match the actual defect of patients.
To improve the functionality of the bioprinted structures, multiple cell types can be included in the printing process. In one study, porous constructs containing embedded osteogenic and endothelial progenitor cells were printed together (Figure 3. (B)). Functional osteogenic and endothelial progenitor cells with proper spatial organization were observed within the printed grafts after subcutaneous implantation in immune-deficient mice. It was demonstrated that cell differentiation leading to the expected tissue formation occurs at the site of the deposited progenitor cell type. While perfused blood vessels were formed in the endothelial progenitor cell-laden part of the constructs, bone formation was taking place in the multipotent stromal cell-laden part of the printed grafts20.
Cartilage
Cartilage is aneural, avascular tissue with zonal structure. Articular cartilage shows heterogeneous tissue compositions that vary in different regions, which differs in cell density, morphology, glycosaminoglycan contents, biosynthetic activities, and mechanical properties. The ability to generate precise spatial patterns is one of the advantages of 3-D printing, and can be applied to create zonal gradient of cartilage tissue. It can be achieved by controlling the each printing layer with varying biomaterial properties and cell types. The feasibility of fabricating anatomic cartilage structures was demonstrated by delivering chondrocytes and PEGDA to the precise 3-D locations for mimicking different zone structures of articular cartilage (Figure 3. (C))11, 12, 14, 24, 25.
3-D fiber deposition of PCL- or PEG-based materials was utilized to create cell-laden, heterogeneous hydrogel constructs for potential use as osteochondral grafts11, 12, 14, 20, 24, 25, 46, 88. Scaffolds with varying porosity and elastic modulus were yielded by changing fiber spacing or angle of fiber deposition. Human chondrocytes and osteogenic progenitors were incorporated within different zone regions to mimic the osteochondral tissue structure19.
Mesenchymal stem cells have also been introduced in the 3-D bioprinted construct for cartilage tissue Engineering. A composite of poly-ε-caprolactone and hyaluronic acid (HA) was fabricated and seeded with human bone marrow stem cells (hMSCs). An overlaying layer of poly(ethylene glycol)-based hydrogel encapsulating hMSCs or hMSC-derived chondrocytes was molded into anatomic shape. After six weeks of subcutaneous implantation in athymic rats, hMSCs generated substantially more angiogenesis, whereas the hMSC-derived progenies yielded more mineralized tissue in micro-channels and glycosaminoglycan matrix in the cartilage-like layer46.
In addition to physical distinctions between different zones, a biochemical gradient can be incorporated by printing nanoliter droplets encapsulating human MSCs, bone morphogenetic protein 2 (BMP-2), and transforming growth factor beta1 (TGF-b1). The addition of growth factors enables to more closely mimic an anisotropic biomimetic fibrocartilage microenvironment. The resulting printed tissue constructs displayed multiphasic anisotropy of the incorporated biochemical factors. This leads to simultaneous differentiation of MSC populations into osteogenic phenotype and chondrogenic phenotype32.
In order to create geometrically-reliable patient-specific cartilage tissues, imaging techniques such as MRI or micro CT has been combined with 3-D cell printing. In a study, 3-D printed mold was created using MRI and micro CT images and alginate scaffolds with meniscus cells were injected to fabricate a geometrically-accurate meniscus disc2, 35.
Skin
In skin tissue engineering, unlike traditional strategies, 3-D cell printing of skin takes into consideration of the subtle cell-cell interactions as well as cell-matrix interactions and precise cell layer positioning. Thus, 3-D cell printing could potentially have better performance than the traditional methods. Collagen I, fibrin, and commercially-available acellular dermal substitute (such as Matriderm) are some examples of popular scaffold materials for skin tissue engineering. Most of skin printing approaches incorporate keratinocytes, fibroblasts, and/or stem cell-derived skin cells as5, 43, 44, 48, 51, 54, 59, 82.
Recently, Lee et al. created a 3-D printed skin tissue constructs using an inkjet-based bioprinter. Collagen I, keratinocytes, and fibroblasts were printed in a layer-by-layer manner, mimicking multi-layer structure of skin tissue (Figure 3. (D)). Followed by two weeks of air-liquid interface culture, the printed skin presented a multi-layer structure of dermis and epidermis48, 51.
Koch et al. used a laser-assisted cell printing technique to fabricate 3-D skin grafts consists of collagen, fibroblasts, and keratinocytes44. The formation of the basement membrane and intercellular junctions was observed after in vitro culture. The engineered skin tissue was subsequently tested in vivo, employing the dorsal skin chamber in nude mice. The printed keratinocytes formed a properly-differentiated multi-layered epidermis with stratum corneum. In the mice, some blood vessels were found to grow from the wound sites in direction towards the printed cells, suggesting an integration of the bioprinted skin with the host tissue59.
Vascular
The vascularization of tissue constructs is crucial for survival of thick human-sized tissue or organ. However, the integration of vessels or vessel-like structures within tissue-engineered constructs still remains a major challenge. Cell printing-based approaches for solving this issues utilize sacrificial/fugitive materials3, 49, 52, 60 or construct free-standing tubular structure67 to create hollow channels. The channels can later be seeded with endothelial cells, remodeled into endothelial tubes over culture period. Norotte et al. have used bioprinting to print cell aggregates in a scaffold-free substrate to form branched vessels (Figure 3. (G)) and have demonstrated that the cells will remodel and form a construct similar to a blood vessel67. Another study used 3-D bioprinting to create vessel-like constructs using hyaluronan hydrogels cross-linked with tetrahedral polyethylene glycol tetracrylates84. Both inkjet and laser printing have been employed to print vascular cells in hydrogels to form vascular like patterns. In one study, micron-sized fibrin channels were fabricated using a drop-on-demand polymerization. Human microvascular endothelial cells were printed in conjunction with the fibrin, and the cells aligned themselves inside the channels and proliferated to form confluent linings10. In another study, the biological laser printing was used to fabricate branch structures of human umbilical vein endothelial cells and smooth muscle cells94.
New materials and novel cell printing techniques have been continuously developed in order to address the vascularization issue. Miller et al. developed an extrusion printing technology that can dispense rigid 3-D filament networks of carbohydrate glass. Cell-laden soft hydrogels were subsequently casted to form thick tissues in millimeters-to centimeters-scale. The carbohydrate glass fiber served as a sacrificial template and was dissolved by buffer solutions or culture medium later, resulting perfusable inter-connected channel networks. The channels with endothelial cell seeding were perfused with blood under high-pressure pulsatile flow. The perfused vascular channels sustained the metabolic function of hepatocytes in the vascularized tissue constructs60.
In another proof-of-concept research, bio-printed agarose fibers were used as a sacrificial material to crease microchannel networks for vascularization3. 2–8% of agarose gel was printed using Organovo extrusion printer at 80°C to create microchannels in a 2-D planar orientation or in 3-D lattice architectures. The diameters of microchannels ranged from ~150 µm to ~1000µm. Photo-polymerizable hydrogels (GelMA, SPELA, PEGDMA, and PEGDA; with or without cells embedded) were casted to fully cover the agarose fibers, and then crosslinked by UV light. The agarose fibers are individually removed with a light vacuum or manual pulling. MC3T3 cell-embedded hydrogel with channels presented higher viability and ALP activity level than one without microchannels after 7–14 days of culture.
Perfusable microchannels can be efficiently created by this method without template dissolution or use of harsh chemicals using Bertassoni’s method3. Other types of hydrogel (pH-sensitive, chemically-crosslinkable) can be used as surrounding hydrogels, since photo-polymerization has a limitation of UV length for gel constructs with larger dimensions. In their approach, the agarose fibers need to be removed individually and it may be an obstacle for fabrication of more complex closed-loop network. Although this proof-of-concept research presented a potential of perfusable live tissue fabrication, an actual dynamic perfusion has not been shown in the study.
Newly-developed bioinks with encapsulated human umbilical vein endothelial cell (HUVEC) was deposited using co-axial needle extrusion system9. The cells migrated to outer regions of the printed fibers and formed EC monolayer-covered channels after 10 days of culture. After printing this pre-vascularized structure with endothelial cells, cardiomyocytes were seeded on the surface of the structure. The 3-D HUVEC scaffold supported cardiomyocyte beating, showing its applications for other cell/tissue type.
The vascular printing methods mentioned above presented great results in maintaining tissue viability and representing certain tissue functions. However, integrating vascular hierarchical structures spanning from arteries down to capillaries has been challenging. Lee et al. suggested a cell printing methodology to first create larger vascular channels (0.5–1mm)48, 49, 53, 70, 101, and then, to create adjacent capillary network through a natural maturation process (Figure 3. (E)). This printing approach provides a feasible solution to connect the capillary network to the large perfused vascular channels. In the model, microvascular bed was formed in between two large fluidic vessels, and then connected to the vessels by angiogenic sprouting from the large channel edge50.
Cardiac Tissue
There is very little success in 3-D cell printing for cardiac tissue regeneration due to many significant technical and biological challenges. In one study, HUVEC and MSC were patterned on a polyester-urethane-urea cardiac patch fabricated using the laser induced cell printing technique. The patches were transplanted to the infarction zone of rat heart, resulting increased vessel formation and integration of the transplanted human cells into the connected vessels of the host vascular system21. For the purpose of myocardical tissue repair, Gaetani et al. bioprinted 3-D structures that contain a mixture of human cardiomyocyte progenitor cells (hCMPC) and alginate hydrogel. In their in vivo study, the printed cells retained their commitment for the cardiac lineage and expressed the genes of the early cardiac transcription factors22. In their subsequent study, 3-D bioprinted cardiac patches were fabricated using hCMPC and a hyaluronic acid/gelatin-base matrix and applied on in vivo model for the evaluation and its therapeutic potential. The application of the patch induced a significant reduction in faulty remodeling, preserved cardiac performances, and supported the long-term in vivo survival and implantation of hCMPCs23.
Cardiac Valve
With the frequent damage associated with various pathological conditions, there is great need to develop tissue engineered cardiac valve. Due to the complex geometry, 3-D printing has particular advantage in making the anatomically correct cardiac valves tailored for individual patient. Because cardiac valve tissue needs strong mechanical properties for proper function, only biomaterials that have adequate strength can be used. For this type of biomaterials, micro-extrusion bioprinters is often used to create cardiac valve structures (Figure 3 (F)).17, 35 Duan et al. applied an extrusion-based bioprinting technology into the construction of tri-leaflet heart valve conduit, composed of hybrid hydrogel of hyaluronic acid and gelatin and human aortic valve interstitial cells.18 The printed tri-leaflet heart valve conduit is highly viable and demonstrates great potential for remodeling at 7 days. Subsequent study fabricated an anatomically complex living aortic valve conduit using alginate/gelatin hydrogel containing aortic root sinus smooth muscle cells and aortic valve interst itial cells. These studies demonstrated the possibility that cellularized tissue valves can be created using the bioprinting technology for eventual clinical use.17, 18
In Situ Bioprinting For Clinical Application
Besides building 3-D tissues in the lab for culturing, cell printing technology can also be applied in clinical situations5, 69, 92. in situ bioprinting enables the fast fabrication of thick tissue. With an aid of medical imaging, the architecture of printed tissue can be designed to fit into the defects. Various cells, hydrogels, and soluble factors can be precisely deposited inside defects with desire distributions. The direct printing of tissue constructs into defect sites can minimize the gap space between implant-host interfaces and provide more defined structural stability during the healing process, thus eventually eliminate the need for pre-shaping or re-shaping the scaffold based on the defect geometry. It also allows the growth of thick tissues in critical defects with the help of vascularization driven by nature in lesions, where patient’s body systems act as a natural bioreactor. With appropriate chemical/mechanical cues, the printed tissue can effectively recruit desired cell types from surrounding tissues, lessening a burden of cell source and supply issues.
In situ bioprinting of skin defects and calvaria defects have been tested in mouse model41, 81. Stem cell-derived cells have been directly printed into the large burn wounds on mouse using collagen and fibrin as scaffold materials, demonstrating the potential of in situ skin printing as an effective treatment for large-scale wounds and burns81. In another preliminary proof-of-concept study, nano-hydroxyapatite was directly printed into the mouse calvaria defects using computer-assisted laser direct writing technology41. The results showed the feasibility of in situ bioprinting and its potential applications for regeneration of various body sites, such as dermal wounds, calvarial or craniofacial defects.
Current issues in 3-D cell printing technology and Perspectives
Cell printing technology has a great potential in 3-D tissue engineering. Despite of extensive researches, the tissue constructs created by current printing techniques are relatively simple in terms of architectures, compositions, and biological functions. When it comes to clinical or preclinical studies, 3-D printed tissues tend to be further simplified and most studies are primarily focusing in bone and tooth regeneration, all of which are primarily hard materials. In this type of application, biomaterials can be printed at anatomical geometry and implanted in vivo directly for further remodeling or cells are seeded afterwards in vitro and then implanted in vivo. For bioprinting of soft tissues which contain live cell within the construct, it is still at very early stage. Many obstacles need to be overcome in order to broaden in vivo, in vitro, and clinical applications of 3-D cell printing.
Each of the cell printing technologies has its own strengths and limitations, thus it is important to find ideal applications for each printing technology. However, as the needs for more complex tissue fabrication increases, the limitations in regard of resolution, speed, biocompatibility, and reproducibility need be resolved to meet the needs. One solution to overcome printer-specific disadvantages is to combine different printing technologies27. For instance, both inkjet-based printing and micro-extrusion printing can be utilized for printing of single tissue. Inkjet printer enables fast fabrication of architectures in millimeter- or centimeter-scale. Micro-extrusion that requires longer printing time can be used to construct micro-scale features of the same tissue. This concept of integrating multiple techniques can be expanded to handle different biomaterials with distinctive printing properties such as viscosity, sensitivity to pressure (relevant to cell viability), or gelling mechanism.
Biomaterials, especially scaffold materials used to support 3-D structure, play an essential role in cell printing. However, current choice of printable materials is very limited and existing materials are not capable of fully mimicking the native ECM compositions, which have complex combinations and gradients of numerous ECM types. Therefore, it is crucial to develop new printable biomaterials that can (i) support easy manipulation of structural/functional features by cell printing technology, (ii) maintain structural integrity and cytocompatibility during gelation/crosslinking and later tissue culture. In addition, to accurately print anatomically matched geometries, hard materials without cells are much more developed via processing various polymer scaffolds. However, the available materials for soft tissue bioprinting are very limited. Current 3-D bioprinting of soft tissue uses hydrogel materials such as collagen, fibrin, PEG, alginate, gelatin, hyaluronic acid, etc. The bioprinted constructs from these hydrogel materials, however, are very fragile and are not able to withstand the surgical manipulation and high pulse pressure when implanted in vivo. Therefore, developing biomaterial hydrogels that can be printed together with live cells and at the same time possesses adequate mechanical properties (such as elasticity) for handling is critically needed.
From the basic science point of view, the understanding of the matrix environment in various tissues needs to be improved. To do so, developments of imaging methods will be required to map the physiological ECM components. In addition, there are also needs for establishing methods to reproduce the ECM compositions of native tissues, in order to mimic the native ECM environments. It is also required to improve our understanding of the heterogeneous cell types and their interactions within a specific tissue. Obtaining proper cell types in quantity is another significant issue in cell printing as well as in tissue engineering.
The preclinical and clinical applications of 3-D cell printing are limited due to technical factors such as the survival and directed differentiation of introduced cells, the fidelity of operating steps, and an insufficient rate of vascularization. 3D printing has the potential to create anatomically matched construct for individual patient. However, its application in clinical setting is still at early stage. Especially for soft tissue bioprinting, the available biomaterials that are suitable for handling are very limited. Recently, Dr. Atala’s group has developed a new method to create human-scale tissue constructs with mechanical stability by combining cell-laden hydrogels together with biodegradable polymers in integrated patterns. The polymer provides mechanical support while the hydrogels provide cellular environment. Using this method, anatomical shapes representing clinical imaging data can be printed and is demonstrated by fabricating mandible and calvarial bone, cartilage and skeletal muscle40. This hybrid method may be a more practical approach to solve the problem of mechanical fragility in soft tissue bioprinting before better hydrogel materials are developed specifically for this purpose.
Despite the obstacles, 3-D cell printing technology would be a great help to translate regenerative medicine from lab discovery into the stage of practical application, as it enables a rapid prototyping of patient-specific tissues and also allows a more efficient use of given cells and other biomaterials. Tissue fabrication requires a large number of cells and this cell source issue has been mentioned as one of major challenges in tissue engineering and regenerative medicine. 3-D cell printing may be the most efficient technique for tissue fabrication, since its capability of precise deposition can economize the use of biomaterials. The multidisciplinary collaborations between biologists, bioengineers, and physicians will be required to meet these needs and to further apply 3-D cell printing for the field of drug discovery and regenerative medicine.
Acknowledgments
We acknowledge the support from NSF CBET-1263455, NSF Career-1350240, NIH R01HL118245 and American Heart Association 12SDG12050083.
Footnotes
Conflict of interest declaration:
Both authors declare no conflict of interest associated with this work.
Reference
- 1.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballyns JJ, Gleghorn JP, Niebrzydowski V, Rawlinson JJ, Potter HG, Maher SA, Wright TM, Bonassar LJ. Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Engineering Part A. 2008;14:1195–1202. doi: 10.1089/ten.tea.2007.0186. [DOI] [PubMed] [Google Scholar]
- 3.Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, Barabaschi G, Demarchi D, Dokmeci MR, Yang Y, Khademhosseini A. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–2211. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35:49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 5.Binder KW, Zhao W, Aboushwareb T, Dice D, Atala A, Yoo JJ. In situ bioprinting of the skin for burns. Journal of the American College of Surgeons. 2010;211:S76. [Google Scholar]
- 6.Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Materials Today. 2013;16:496–504. [Google Scholar]
- 7.Chang R, Nam J, Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng Part A. 2008;14:41–48. doi: 10.1089/ten.a.2007.0004. [DOI] [PubMed] [Google Scholar]
- 8.Choi HJ, Kim JM, Kwon E, Che J-H, Lee J-I, Cho S-R, Kang SK, Ra JC, Kang B-C. Establishment of Efficacy and Safety Assessment of Human Adipose Tissue-Derived Mesenchymal Stem Cells (hATMSCs) in a Nude Rat Femoral Segmental Defect Model. Journal of Korean Medical Science. 2011;26:482–491. doi: 10.3346/jkms.2011.26.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, Dokmeci MR, Dentini M, Khademhosseini A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv Mater. 2016;28:677–684. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Cui X, Breitenkamp K, Finn MG, Lotz M, D'Lima DD. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18:1304–1312. doi: 10.1089/ten.tea.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui X, Breitenkamp K, Lotz M, D'Lima D. Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation. Biotechnol Bioeng. 2012;109:2357–2368. doi: 10.1002/bit.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui X, Dean D, Ruggeri ZM, Boland T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol Bioeng. 2010;106:963–969. doi: 10.1002/bit.22762. [DOI] [PubMed] [Google Scholar]
- 14.Cui X, Gao G, Yonezawa T, Dai G. Human cartilage tissue fabrication using three-dimensional inkjet printing technology. J Vis Exp. 2014 doi: 10.3791/51294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demirci U, Montesano G. Single cell epitaxy by acoustic picolitre droplets. Lab Chip. 2007;7:1139–1145. doi: 10.1039/b704965j. [DOI] [PubMed] [Google Scholar]
- 16.Dhariwala B, Hunt E, Boland T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004;10:1316–1322. doi: 10.1089/ten.2004.10.1316. [DOI] [PubMed] [Google Scholar]
- 17.Duan B, Hockaday LA, Kang KH, Butcher JT. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101:1255–1264. doi: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan B, Kapetanovic E, Hockaday LA, Butcher JT. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014;10:1836–1846. doi: 10.1016/j.actbio.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, Malda J, Alblas J, Dhert WJ. Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods. 2012;18:33–44. doi: 10.1089/ten.tec.2011.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedorovich NE, Wijnberg HM, Dhert WJ, Alblas J. Distinct tissue formation by heterogeneous printing of osteo- and endothelial progenitor cells. Tissue Eng Part A. 2011;17:2113–2121. doi: 10.1089/ten.TEA.2011.0019. [DOI] [PubMed] [Google Scholar]
- 21.Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, Gruene M, Toelk A, Wang W, Mark P, Wang F, Chichkov B, Li W, Steinhoff G. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011;32:9218–9230. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 22.Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, Sluijter JP. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012;33:1782–1790. doi: 10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, Giacomello A, Doevendans PA, Sluijter JP. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339–348. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Gao G, Schilling AF, Yonezawa T, Wang J, Dai G, Cui X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J. 2014;9:1304–1311. doi: 10.1002/biot.201400305. [DOI] [PubMed] [Google Scholar]
- 25.Gao G, Yonezawa T, Hubbell K, Dai G, Cui X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol J. 2015 doi: 10.1002/biot.201400635. [DOI] [PubMed] [Google Scholar]
- 26.Gao Q, He Y, Fu JZ, Liu A, Ma L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials. 2015;61:203–215. doi: 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Giannitelli SM, Mozetic P, Trombetta M, Rainer A. Combined additive manufacturing approaches in tissue engineering. Acta Biomater. 2015;24:1–11. doi: 10.1016/j.actbio.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Giordano RA, Wu BM, Borland SW, Cima LG, Sachs EM, Cima MJ. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J Biomater Sci Polym Ed. 1996;8:63–75. doi: 10.1163/156856297x00588. [DOI] [PubMed] [Google Scholar]
- 29.Guillemot F, Mironov V, Nakamura M. Bioprinting is coming of age: Report from the International Conference on Bioprinting and Biofabrication in Bordeaux (3B'09) Biofabrication. 2010;2:010201. doi: 10.1088/1758-5082/2/1/010201. [DOI] [PubMed] [Google Scholar]
- 30.Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, Pippenger B, Bareille R, Remy M, Bellance S, Chabassier P, Fricain JC, Amedee J. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010;6:2494–2500. doi: 10.1016/j.actbio.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 31.Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, Bareille R, Remy M, Bordenave L, Amedee J, Guillemot F. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31:7250–7256. doi: 10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 32.Gurkan UA, El Assal R, Yildiz SE, Sung Y, Trachtenberg AJ, Kuo WP, Demirci U. Engineering anisotropic biomimetic fibrocartilage microenvironment by bioprinting mesenchymal stem cells in nanoliter gel droplets. Mol Pharm. 2014;11:2151–2159. doi: 10.1021/mp400573g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Highley CB, Rodell CB, Burdick JA. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv Mater. 2015;27:5075–5079. doi: 10.1002/adma.201501234. [DOI] [PubMed] [Google Scholar]
- 34.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science Advances. 2015;1 doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hockaday LA, Kang KH, Colangelo NW, Cheung PY, Duan B, Malone E, Wu J, Girardi LN, Bonassar LJ, Lipson H, Chu CC, Butcher JT. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4:035005. doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 37.Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22:354–362. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Kates SL, Awad HA. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci U S A. 2004;101:2864–2869. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 41.Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, Amédée J, Fricain J-C, Catros S. In vivo bioprinting for computer-and robotic-assisted medical intervention: preliminary study in mice. Biofabrication. 2010;2:014101. doi: 10.1088/1758-5082/2/1/014101. [DOI] [PubMed] [Google Scholar]
- 42.Khalil S, Sun W. Bioprinting endothelial cells with alginate for 3D tissue constructs. J Biomech Eng. 2009;131:111002. doi: 10.1115/1.3128729. [DOI] [PubMed] [Google Scholar]
- 43.Killat J, Reimers K, Choi CY, Jahn S, Vogt PM, Radtke C. Cultivation of Keratinocytes and Fibroblasts in a Three-Dimensional Bovine Collagen-Elastin Matrix (Matriderm®) and Application for Full Thickness Wound Coverage in Vivo. International journal of molecular sciences. 2013;14:14460–14474. doi: 10.3390/ijms140714460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koch L, Deiwick A, Schlie S, Michael S, Gruene M, Coger V, Zychlinski D, Schambach A, Reimers K, Vogt PM, Chichkov B. Skin tissue generation by laser cell printing. Biotechnol Bioeng. 2012;109:1855–1863. doi: 10.1002/bit.24455. [DOI] [PubMed] [Google Scholar]
- 45.Landers R, Hubner U, Schmelzeisen R, Mulhaupt R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials. 2002;23:4437–4447. doi: 10.1016/s0142-9612(02)00139-4. [DOI] [PubMed] [Google Scholar]
- 46.Lee CH, Marion NW, Hollister S, Mao JJ. Tissue formation and vascularization in anatomically shaped human joint condyle ectopically in vivo. Tissue Engineering Part A. 2009;15:3923–3930. doi: 10.1089/ten.tea.2008.0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JW, Kang KS, Lee SH, Kim J-Y, Lee B-K, Cho D-W. Bone regeneration using a microstereolithography-produced customized poly(propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorporating BMP-2 loaded PLGA microspheres. Biomaterials. 2011;32:744–752. doi: 10.1016/j.biomaterials.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 48.Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo SS, Dai G, Karande P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods. 2014;20:473–484. doi: 10.1089/ten.tec.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee VK, Kim DY, Ngo H, Lee Y, Seo L, Yoo SS, Vincent PA, Dai G. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials. 2014;35:8092–8102. doi: 10.1016/j.biomaterials.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee VK, Lanzi AM, Haygan N, Yoo SS, Vincent PA, Dai G. Generation of Multi-Scale Vascular Network System within 3D Hydrogel using 3D Bio-Printing Technology. Cell Mol Bioeng. 2014;7:460–472. doi: 10.1007/s12195-014-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee W, Debasitis JC, Lee VK, Lee JH, Fischer K, Edminster K, Park JK, Yoo SS. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials. 2009;30:1587–1595. doi: 10.1016/j.biomaterials.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Lee W, Lee V, Polio S, Keegan P, Lee JH, Fischer K, Park JK, Yoo SS. On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol Bioeng. 2010;105:1178–1186. doi: 10.1002/bit.22613. [DOI] [PubMed] [Google Scholar]
- 53.Lee YB, Polio S, Lee W, Dai G, Menon L, Carroll RS, Yoo SS. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp Neurol. 2010;223:645–652. doi: 10.1016/j.expneurol.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 54.Li J, He L, Zhou C, Zhou Y, Bai YY, Lee FY, Mao JJ. 3D printing for regenerative medicine: From bench to bedside. MRS Bulletin. 2015;40:145–153. [Google Scholar]
- 55.Lin CY, Kikuchi N, Hollister SJ. A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. J Biomech. 2004;37:623–636. doi: 10.1016/j.jbiomech.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 56.Liu Tsang V, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, West JL, Bhatia SN. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21:790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 57.Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJ, Groll J, Hutmacher DW. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25:5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 58.Markstedt K, Mantas A, Tournier I, Martinez Avila H, Hagg D, Gatenholm P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules. 2015 doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 59.Michael S, Sorg H, Peck CT, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One. 2013;8:e57741. doi: 10.1371/journal.pone.0057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21:157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 62.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 64.Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A. 2013;101:272–284. doi: 10.1002/jbm.a.34326. [DOI] [PubMed] [Google Scholar]
- 65.Nair K, Gandhi M, Khalil S, Yan KC, Marcolongo M, Barbee K, Sun W. Characterization of cell viability during bioprinting processes. Biotechnol J. 2009;4:1168–1177. doi: 10.1002/biot.200900004. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura M, Kobayashi A, Takagi F, Watanabe A, Hiruma Y, Ohuchi K, Iwasaki Y, Horie M, Morita I, Takatani S. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2005;11:1658–1666. doi: 10.1089/ten.2005.11.1658. [DOI] [PubMed] [Google Scholar]
- 67.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ovsianikov A, Gruene M, Pflaum M, Koch L, Maiorana F, Wilhelmi M, Haverich A, Chichkov B. Laser printing of cells into 3D scaffolds. Biofabrication. 2010;2:014104. doi: 10.1088/1758-5082/2/1/014104. [DOI] [PubMed] [Google Scholar]
- 69.Ozbolat IT. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015;33:395–400. doi: 10.1016/j.tibtech.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 70.Ozturk MS, Lee VK, Zhao L, Dai G, Intes X. Mesoscopic fluorescence molecular tomography of reporter genes in bioprinted thick tissue. J Biomed Opt. 2013;18:100501. doi: 10.1117/1.JBO.18.10.100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pashuck ET, Stevens MM. Designing regenerative biomaterial therapies for the clinic. Sci Transl Med. 2012;4:160sr164. doi: 10.1126/scitranslmed.3002717. [DOI] [PubMed] [Google Scholar]
- 72.Pati F, Ha DH, Jang J, Han HH, Rhie JW, Cho DW. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials. 2015;62:164–175. doi: 10.1016/j.biomaterials.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 73.Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, Kim DH, Cho DW. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peltola SM, Melchels FP, Grijpma DW, Kellomaki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann Med. 2008;40:268–280. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 75.Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25:3707–3715. doi: 10.1016/j.biomaterials.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 76.Rutz AL, Hyland KE, Jakus AE, Burghardt WR, Shah RN. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv Mater. 2015;27:1607–1614. doi: 10.1002/adma.201405076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saunders RE, Gough JE, Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials. 2008;29:193–203. doi: 10.1016/j.biomaterials.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 78.Schiele NR, Corr DT, Huang Y, Raof NA, Xie Y, Chrisey DB. Laser-based direct-write techniques for cell printing. Biofabrication. 2010;2:032001. doi: 10.1088/1758-5082/2/3/032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seol YJ, Kang HW, Lee SJ, Atala A, Yoo JJ. Bioprinting technology and its applications. Eur J Cardiothorac Surg. 2014;46:342–348. doi: 10.1093/ejcts/ezu148. [DOI] [PubMed] [Google Scholar]
- 80.Singh M, Haverinen HM, Dhagat P, Jabbour GE. Inkjet printing-process and its applications. Adv Mater. 2010;22:673–685. doi: 10.1002/adma.200901141. [DOI] [PubMed] [Google Scholar]
- 81.Skardal A, Atala A. Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng. 2015;43:730–746. doi: 10.1007/s10439-014-1207-1. [DOI] [PubMed] [Google Scholar]
- 82.Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med. 2012;1:792. doi: 10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skardal A, Zhang J, McCoard L, Xu X, Oottamasathien S, Prestwich GD. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A. 2010;16:2675–2685. doi: 10.1089/ten.tea.2009.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Skardal A, Zhang J, Prestwich GD. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31:6173–6181. doi: 10.1016/j.biomaterials.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 85.Skoog SA, Goering PL, Narayan RJ. Stereolithography in tissue engineering. J Mater Sci Mater Med. 2014;25:845–856. doi: 10.1007/s10856-013-5107-y. [DOI] [PubMed] [Google Scholar]
- 86.Strobel LA, Rath SN, Maier AK, Beier JP, Arkudas A, Greil P, Horch RE, Kneser U. Induction of bone formation in biphasic calcium phosphate scaffolds by bone morphogenetic protein-2 and primary osteoblasts. J Tissue Eng Regen Med. 2014;8:176–185. doi: 10.1002/term.1511. [DOI] [PubMed] [Google Scholar]
- 87.Sun W, Darling A, Starly B, Nam J. Computer-aided tissue engineering: overview, scope and challenges. Biotechnol Appl Biochem. 2004;39:29–47. doi: 10.1042/BA20030108. [DOI] [PubMed] [Google Scholar]
- 88.Tao X, Kyle WB, Mohammad ZA, Dennis D, Weixin Z, James JY, Anthony A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5:015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 89.Tarafder S, Balla VK, Davies NM, Bandyopadhyay A, Bose S. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J Tissue Eng Regen Med. 2013;7:631–641. doi: 10.1002/term.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tasoglu S, Demirci U. Bioprinting for stem cell research. Trends Biotechnol. 2013;31:10–19. doi: 10.1016/j.tibtech.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Temple JP, Hutton DL, Hung BP, Huri PY, Cook CA, Kondragunta R, Jia X, Grayson WL. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res A. 2014;102:4317–4325. doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 92.Weinand C, Gupta R, Weinberg E, Madisch I, Neville CM, Jupiter JB, Vacanti JP. Toward regenerating a human thumb in situ. Tissue Eng Part A. 2009;15:2605–2615. doi: 10.1089/ten.TEA.2008.0467. [DOI] [PubMed] [Google Scholar]
- 93.Wilson WC, Jr, Boland T. Cell and organ printing 1: protein and cell printers. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:491–496. doi: 10.1002/ar.a.10057. [DOI] [PubMed] [Google Scholar]
- 94.Wu PK, Ringeisen BR. Development of human umbilical vein endothelial cell (HUVEC) and human umbilical vein smooth muscle cell (HUVSMC) branch/stem structures on hydrogel layers via biological laser printing (BioLP) Biofabrication. 2010;2:014111. doi: 10.1088/1758-5082/2/1/014111. [DOI] [PubMed] [Google Scholar]
- 95.Wust S, Godla ME, Muller R, Hofmann S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014;10:630–640. doi: 10.1016/j.actbio.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 96.Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, Boland T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006;27:3580–3588. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 97.Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26:93–99. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 98.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 2002;8:1–11. doi: 10.1089/107632702753503009. [DOI] [PubMed] [Google Scholar]
- 99.Yeong WY, Chua CK, Leong KF, Chandrasekaran M. Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 2004;22:643–652. doi: 10.1016/j.tibtech.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 100.Yu Y, Zhang Y, Ozbolat IT. A Hybrid Bioprinting Approach for Scale-Up Tissue Fabrication. Journal of Manufacturing Science and Engineering. 2014;136:061013. [Google Scholar]
- 101.Zhao L, Lee VK, Yoo SS, Dai G, Intes X. The integration of 3-D cell printing and mesoscopic fluorescence molecular tomography of vascular constructs within thick hydrogel scaffolds. Biomaterials. 2012;33:5325–5332. doi: 10.1016/j.biomaterials.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Y, Yao R, Ouyang L, Ding H, Zhang T, Zhang K, Cheng S, Sun W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication. 2014;6:035001. doi: 10.1088/1758-5082/6/3/035001. [DOI] [PubMed] [Google Scholar]