Abstract
Background
Approximately 20% of women select autologous tissue for postmastectomy breast reconstruction, and most commonly choose the abdomen as the donor site. An increasing proportion of women are seeking muscle-sparing procedures but the benefit remains controversial. It is therefore important to determine whether better outcomes are associated with these techniques, thereby justifying longer operative times and increased costs.
Methods
Patients from five North American centers were eligible if they had reconstruction using the deep inferior epigastric artery perforator flap (DIEP), muscle sparing free transverse abdominis myocutaneous flap (msf-TRAM), free transverse abdominis myocutaneous flap (f-TRAM), or the pedicled transverse abdominis myocutaneous flap (p-TRAM) with minimum one-year follow-up. Patients were sent the BREAST-Q©. Demographics and complications were collected by chart review.
Results
We analyzed 1790 charts representing 670 DIEP, 293 msf-TRAM, 683 p-TRAM, and 144 f-TRAM patients with average follow up of 5.5 years. Flap loss did not differ by flap type. Partial flap loss was higher in p-TRAM compared to DIEP (p=0.002). Fat necrosis was higher in p-TRAM compared to DIEP and msf-TRAM (p<0.001). Hernia/bulge was highest in p-TRAM (p<0.001). Physical Well-Being (Abdomen) scores were higher in DIEP compared to p-TRAM controlling for age, follow-up, BMI, laterality, abdominal surgery, mesh, radiation, income, and education.
Conclusions
Complications and patient-reported outcomes differ when comparing abdominally-based breast reconstruction techniques. The results of this study show that the DIEP was associated with the highest abdominal well-being and the lowest abdominal morbidity when compared to the p-TRAM, but did not differ from msf-TRAM and f-TRAM.
INTRODUCTION
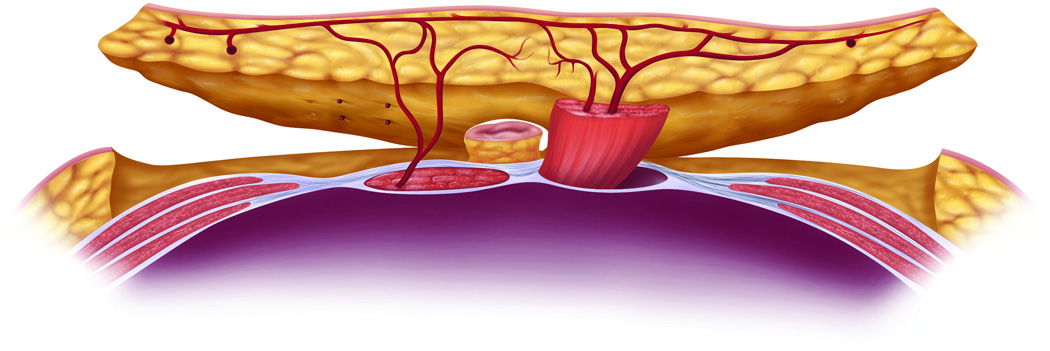
Approximately 12% of women will be diagnosed with breast cancer at some point during their lifetime.1 Of women who elect to have breast reconstruction following mastectomy, 13% undergo autologous tissue reconstruction utilizing tissue from the lower abdomen.2 Surgical techniques that sacrifice only part of the rectus abdominis muscle such as the msf-TRAM and the DIEP have been developed to potentially decrease abdominal wall morbidity. The abdominal tissues used for reconstruction include either only a small piece (msf-TRAM) or none (DIEP) of the rectus abdominis muscle or the majority of the rectus muscle (f-TRAM, p-TRAM) with the overlying skin and fat (Figure 1).
Figure 1.
The abdominal tissues used for breast reconstruction include subcutaneous fat +/− skin +/− rectus abdominis muscle.
Abdominal wall weakness may result from removing or injuring the muscle and muscular fascia or denervating the intercostal nerves. Reports have described abdominal wall hernia and bulge rates as high as 62% for p-TRAM3, 27% for f-TRAM4, 11% for msf-TRAM5, and 10% for DIEP flaps.6,7 While advances in abdominal wall closure techniques may minimize hernia and bulge complications, these are unlikely to decrease abdominal wall weakness and a patient’s ability to conduct activities of daily life without discomfort.6 Multiple studies evaluating objective abdominal wall strength with functional dynamometry have demonstrated significant differences between different types of flaps.8,9,10,11,12,13 In these studies, patients with f-TRAM and p-TRAM reconstruction have been found to have more abdominal wall weakness relative to msf-TRAM and DIEP groups. Additionally, patients who have f-TRAM or msf-TRAM reconstruction also appear to have decreased abdominal muscle strength relative to DIEP patients.
However, the benefit of muscle-sparing procedures remains controversial and it is unclear whether any clinically meaningful differences exist for the patients who undergo the different techniques.14 Furthermore, muscle-sparing procedures are often associated with longer operative times and require specialized surgeon training. Longer operative times may impart greater peri-operative risk to patients and are associated with increased health care costs. Additionally, it has been purported that muscle-sparing techniques are associated with more flap-related complications compared to non-muscle-sparing techniques.15,16 For these and other reasons, North American plastic surgeons are performing fewer microsurgical breast reconstructions.17 It is therefore important to quantify the differences in outcomes associated with the various procedures.
METHODS
Ethics
Ethics approval was obtained from Research Ethics Boards at University of British Columbia (Vancouver), University Health Network (Toronto), Dartmouth University (Lebanon), the New York University (New York) and the Memorial Sloan Kettering Cancer Center (New York).
Patient Selection
Women who underwent abdominally-based reconstruction were recruited at five sites: i) University of British Columbia; Vancouver, BC; ii) University of Toronto; Toronto, ON; iii) Dartmouth College; Lebanon, NH; iv) New York University; New York, NY; and v) Memorial Sloan Kettering Cancer Center; New York, NY. Inclusion criteria were: i) abdominal flap reconstruction one to seven years prior to study initiation (range: 2002–2012); ii) p-TRAM, f-TRAM, msf-TRAM, or DIEP reconstruction; iii) patients at least 21 years of age. Patients were excluded if they were unable to read or speak English, if their address was unavailable, if they had a combination of two of the above flaps in a bilateral case, if they were deceased or refused to participate. The type of flap the patient had was determined by how the reconstruction was coded within each database at each individual institution.
Study Design
This was a cross-sectional study. Patients were sent the BREAST-Q© questionnaire, a self-addressed, postage-paid return envelope and a $5 Starbucks card as an incentive to respond. One additional copy of the questionnaire was distributed to non-responders three months after the first mail-out.18 Chart review was performed in order to compile demographic and surgical data.
Questionnaire
The BREAST-Q© was developed at the Memorial Sloan Kettering Cancer Center and the University of British Columbia.19,20,21 This instrument measures patient satisfaction and health-related quality of life following breast surgery. Details of the BREAST-Q© development are previously published.19,22,23 The BREAST-Q© scales selected for use in the study were 1) Physical well-being: Abdomen; 2) Satisfaction with Breasts; 3) Satisfaction with Outcome; 4) Psychosocial well-being; and 5) Sexual well-being. Scoring was performed using QScore which was developed according to the Rasch model.24,25 All scales were scored on a 0–100 point scale with higher scores indicating greater satisfaction.
Statistical Analysis
Differences in patient characteristics between groups were compared using Pearson's chi-square test (categorical variables) and analysis of variance (ANOVA; continuous variables). Statistically significant differences on ANOVA comparisons for the 4 surgical groups were explored using appropriate follow-up tests (i.e., Tukey's or Games-Howell tests) for pairwise comparisons. Mean scores and standard deviations on the BREAST-Q© subscales were calculated for each group; the group means for each subscale were compared using ANOVA with appropriate follow-up tests. Analyses of covariance (ANCOVAs) were used to examine the same mean differences on the BREAST-Q© subscales controlling for age, time since surgery, BMI, laterality, mesh, history of abdominal surgery, radiation, income and education level. Differences in abdominal morbidity scores between each group were assessed using Pearson’s chi-square test. Hierarchical logistic regressions were then used to examine these differences controlling for the same covariates. Given the relatively high number of analyses, a more conservative alpha of .010 was used to determine statistical significance.
RESULTS
Response Rate
2031 charts were reviewed. 241 patients were excluded for follow up time < 1 year, combination of different flaps if a bilateral procedure, no patient address, patient death and patient refusal to participate. The remaining 1790 patients were contacted by mail and 943 responded (53% overall response rate). Chart review was performed on all 943 responders and 847 non-responders.
Patient Demographics
The demographic and surgical characteristics for each patient group (responders and nonresponders) were compared (Tables 1 and 2), Patients in the p-TRAM group were significantly older and had lower BMI than patients in the other groups. Length of follow up was longest in the f-TRAM group. Patient groups also differed for stage of disease, receipt of radiation therapy, and smoking status (highest percentage of smokers in the DIEP group). Additionally, patient groups differed for immediate versus delayed surgery, laterality, mesh placement (highest in msf-TRAM and lowest in DIEP) and type of suture used for abdominal fascial repair. Patients differed across all socioeconomic variables except for marital status and employment (Table 3).
Table 1.
Demographics – Clinical
| Variable |
DIEP n=670 Mean (SD) |
msTRAM n=293 Mean (SD) |
pTRAM n=683 Mean (SD) |
fTRAM n=144 Mean (SD) |
p |
| Age at surgery | 49.0 (8.4) | 49.6 (7.9) | 50.3 (8.2) | 47.8 (7.7) | .003* |
| Length of follow-up (months) | 53.7 (27.8) | 61.4 (24.2) | 71.2 (28.8) | 87.1 (36.7) | <.001* |
| BMI | 27.3 (4.8) | 28.4 (4.9) | 26.7 (4.2) | 28.3 (5.3) | <.001* |
| Variable |
DIEP n=670 n (%) |
msTRAM n=293 n (%) |
pTRAM n=683 n (%) |
fTRAM n=144 n (%) |
p |
| Pathology | <.001* | ||||
| Invasive | 452 (67.8) | 196 (68.5) | 404 (65.5) | 86 (60.6) | |
| In situ | 156 (23.4) | 76 (26.6) | 196 (31.8) | 50 (35.2) | |
| Prophylactic | 57 (8.5) | 13 (4.5) | 14 (2.3) | 6 (4.2) | |
| Noncancerous | 2 (0.3) | 1 (0.3) | 3 (0.5) | 0 (0) | |
| History of Abdominal Surgery | .044 | ||||
| Yes | 301 (46.8) | 122 (44.4) | 345 (52.8) | 74 (52.9) | |
| No | 342 (53.2) | 153 (55.6) | 309 (47.2) | 66 (47.1) | |
| Chemotherapy | .340 | ||||
| Yes | 344 (51.4) | 149 (51.0) | 371 (55.6) | 72 (50.3) | |
| No | 325 (48.6) | 143 (49.0) | 296 (44.4) | 71 (49.7) | |
| Pre-operative chemotherapy | .015 | ||||
| Yes | 293 (43.8) | 106 (36.3) | 284 (42.5) | 45 (31.7) | |
| No | 376 (56.2) | 186 (63.7) | 384 (57.5) | 97 (68.3) | |
| Post-operative chemotherapy | <.001* | ||||
| Yes | 59 (8.8) | 48 (16.4) | 111 (16.7) | 30 (21.1) | |
| No | 610 (91.2) | 244 (83.6) | 553 (83.3) | 112 (78.9) | |
| Radiation | .008* | ||||
| Yes | 281 (42.0) | 130 (44.5) | 328 (49.2) | 51 (35.9) | |
| No | 388 (58.0) | 162 (55.5) | 339 (50.8) | 91 (64.1) | |
| Pre-operative radiation | .023 | ||||
| Yes | 262 (39.2) | 123 (42.1) | 268 (40.2) | 39 (27.5) | |
| No | 406 (60.8) | 169 (57.9) | 399 (59.8) | 103 (72.5) | |
| Post-operative radiation | <.001* | ||||
| Yes | 21 (3.1) | 8 (2.7) | 65 (9.8) | 12 (8.5) | |
| No | 647 (96.9) | 284 (97.3) | 597 (90.2) | 130 (91.5) | |
| Smoker | .010* | ||||
| Yes | 69 (10.5) | 16 (5.5) | 42 (6.2) | 10 (7.0) | |
| No | 589 (89.5) | 277 (94.5) | 636 (93.8) | 132 (93.0) | |
| Diabetic | .035 | ||||
| Yes | 19 (2.8) | 15 (5.1) | 24 (3.6) | 0 (0) | |
| No | 650 (97.2) | 278 (94.9) | 649 (96.4) | 143 (100) | |
| History of pregnancy | .780 | ||||
| Yes | 505 (87.2) | 221 (84.7) | 404 (86.3) | 117 (87.3) | |
| No | 74 (12.8) | 40 (15.3) | 64 (13.7) | 17 (12.7) | |
Table 2.
Demographics - Surgical
| Variable | DIEP n=670 n (%) |
msTRAM n=293 n (%) |
pTRAM n=683 n (%) |
fTRAM n=144 n (%) |
p |
|---|---|---|---|---|---|
| Unilateral | 383 (57.2) | 185 (63.1) | 541 (79.2) | 114 (79.2) | <.001* |
| Bilateral | 286 (42.8) | 108 (36.9) | 142 (20.8) | 30 (20.8) | |
| Timing | <.001* | ||||
| Immediate | 389 (58.2) | 190 (65.1) | 482 (70.7) | 103 (71.5) | |
| Delayed | 248 (37.1) | 99 (33.9) | 191 (28.0) | 40 (27.8) | |
| Both | 31 (4.6) | 3 (1.0) | 9 (1.3) | 1 (0.7) | |
| Mesh | <.001* | ||||
| Yes | 123 (18.5) | 159 (54.3) | 232 (35.3) | 47 (32.9) | |
| No | 543 (81.5) | 134 (45.7) | 426 (64.7) | 96 (67.1) | |
| Suture | <.001* | ||||
| Absorbable only | 94 (14.3) | 18 (6.2) | 30 (4.6) | 1 (0.7) | |
| Non-absorbable only | 284 (43.2) | 102 (35.2) | 205 (31.2) | 38 (26.4) | |
| Both | 280 (42.6) | 170 (58.6) | 422 (64.2) | 105 (72.9) | |
Table 3.
Demographics – Socioeconomic Status
| Variable | DIEP n=670 n (%) |
msTRAM n=293 n (%) |
pTRAM n=683 n (%) |
fTRAM n=144 n (%) |
p |
|---|---|---|---|---|---|
| Ethnicity | <.001* | ||||
| South Asian | 8 (2.1) | 1 (0.9) | 7 (2.0) | 0 (0) | |
| Asian | 22 (5.9) | 4 (3.4) | 21 (5.9) | 0 (0) | |
| Black | 13 (3.5) | 7 (6.0) | 9 (2.5) | 5 (7.1) | |
| Hispanic | 5 (1.3) | 0 (0) | 2 (0.6) | 0 (0) | |
| White | 313 (83.2) | 93 (80.2) | 296 (83.6) | 57 (81.4) | |
| White/Hispanic | 9 (2.4) | 10 (8.6) | 5 (1.4) | 8 (11.4) | |
| Native | 6 (1.6) | 1 (0.9) | 14 (4.0) | 0 (0) | |
| Education | <.001* | ||||
| Some high school | 12 (3.1) | 3 (2.5) | 17 (4.7) | 1 (1.4) | |
| High school diploma | 40 (10.5) | 11 (9.2) | 72 (19.9) | 8 (10.8) | |
| Some college | 58 (15.2) | 19 (15.8) | 76 (21.1) | 13 (17.6) | |
| College diploma | 158 (41.4) | 42 (35.0) | 134 (37.1) | 28 (37.8) | |
| Some Masters | 25 (6.5) | 9 (7.5) | 17 (4.7) | 5 (6.8) | |
| Masters | 89 (23.3) | 36 (30.0) | 45 (12.5) | 19 (25.7) | |
| Marital status | .562 | ||||
| Married | 280 (73.1) | 78 (65.5) | 264 (73.3) | 48 (64.9) | |
| Living with other | 30 (7.8) | 7 (5.9) | 18 (5.0) | 6 (8.1) | |
| Widowed | 12 (3.1) | 4 (3.4) | 14 (3.9) | 2 (2.7) | |
| Separated | 9 (2.3) | 3 (2.5) | 8 (2.2) | 3 (4.1) | |
| Divorced | 33 (8.6) | 14 (11.8) | 38 (10.8) | 9 (12.2) | |
| Single | 19 (5.0) | 13 (10.9) | 18 (5.0) | 6 (8.1) | |
| Annual income | <.001* | ||||
| < 20K | 17 (4.7) | 4 (3.6) | 27 (8.1) | 2 (3.0) | |
| 20–40K | 29 (8.0) | 12 (10.7) | 65 (19.4) | 7 (10.6) | |
| 40–60K | 35 (9.7) | 14 (12.5) | 61 (18.2) | 17 (25.8) | |
| 60–80K | 48 (13.3) | 17 (15.2) | 54 (16.1) | 2 (3.0) | |
| 80–100K | 61 (16.9) | 9 (8.0) | 38 (11.3) | 12 (18.2) | |
| > 100K | 172 (47.5) | 56 (50.0) | 90 (26.9) | 26 (39.4) | |
| Employment | .017 | ||||
| Full-time | 201 (52.5) | 66 (55.5) | 168 (46.7) | 41 (55.4) | |
| Part-time | 58 (15.1) | 15 (12.6) | 56 (15.6) | 5 (6.8) | |
| Volunteer | 5 (1.3) | 4 (3.4) | 10 (2.8) | 3 (4.1) | |
| Homemaker | 40 (10.4) | 9 (7.6) | 29 (8.1) | 7 (9.5) | |
| Student | 2 (0.5) | 1 (0.8) | 2 (0.6) | 1 (1.4) | |
| Retired | 57 (14.9) | 13 (10.9) | 73 (20.3) | 13 (17.6) | |
| Disabled | 14 (3.7) | 4 (3.4) | 20 (5.6) | 4 (5.4) | |
| Unemployed | 6 (1.6) | 7 (5.9) | 2 (0.6) | 0 (0) | |
Complications
Tables 4a and 5a show the flap- and donor site-related complication profiles across patient groups including both responders and nonresponders. Fat necrosis and infection were highest in the p-TRAM group (25% and 15.7% respectively). Pairwise comparison (Tables 4b, 5b) revealed these differences to be significant when comparing the p-TRAM to the DIEP and msf-TRAM groups only (p<0.001). Partial flap loss was also highest in the p-TRAM group (8.9%) and reached statistical significance only when comparing p-TRAM to DIEP (p=0.002). Total flap loss did not differ between groups and was universally low (1–2%) as was the occurrence of deep vein thrombosis and pulmonary embolism (1–1.6%). DIEP patients had the highest occurrence of hematoma (8.4%) and this reached significance only when comparing the DIEP to p-TRAM groups (p=0.001). The p-TRAM bulge rate (11%) and the bulge/hernia rate (16.6%) were highest on comparison to all other groups (p<0.001). The rate of hernia and hernia/bulge requiring repair were lowest in the DIEP group (p<0.001), but this only reached significance when comparing DIEP to p-TRAM. All significant results remained after controlling for age, time since surgery, BMI, laterality, history of abdominal surgery, mesh placement, radiation, income, and education level.
Table 4.
| a Complications – Flap | |||||
|---|---|---|---|---|---|
| Variable | DIEP n=670 n (%) |
msTRAM n=293 n (%) |
pTRAM n=683 n (%) |
fTRAM n=144 n (%) |
P |
| Hematoma (any) requiring surgery |
.001* | ||||
| Yes | 56 (8.4) | 13 (4.4) | 26 (3.8) | 5 (3.5) | |
| No | 614 (91.6) | 280 (95.6) | 655 (96.2) | 139 (96.5) | |
| Seroma (any) | <.001* | ||||
| Yes | 34 (5.1) | 20 (6.8) | 78 (11.5) | 15 (10.4) | |
| No | 636 (94.9) | 273 (93.2) | 598 (88.5) | 129 (89.6) | |
| Fat necrosis | <.001* | ||||
| Yes | 109 (16.3) | 44 (15.0) | 171 (25.3) | 24 (16.7) | |
| No | 561 (83.7) | 249 (85.0) | 505 (74.7) | 120 (83.3) | |
| Flap loss | .820 | ||||
| Yes | 11 (1.6) | 4 (1.4) | 8 (1.2) | 3 (2.1) | |
| No | 659 (98.4) | 289 (98.6) | 668 (98.8) | 141 (97.9) | |
| Partial flap loss | .002* | ||||
| Yes | 47 (4.0) | 14 (4.8) | 60 (8.9) | 11 (7.6) | |
| No | 643 (96.0) | 279 (95.2) | 616 (91.1) | 133 (92.4) | |
| DVT or PE | .929 | ||||
| Yes | 8 (1.2) | 4 (1.4) | 11 (1.6) | 2 (1.4) | |
| No | 660 (98.8) | 289 (98.6) | 665 (98.4) | 142 (98.6) | |
| Infection (any) | <.001* | ||||
| Yes | 42 (6.3) | 21 (7.2) | 106 (15.7) | 14 (9.7) | |
| No | 628 (93.7) | 272 (92.8) | 569 (84.3) | 130 (90.3) | |
| b Pairwise Comparisons: Patient Surgical Complications | ||||||
|---|---|---|---|---|---|---|
| Variable | DIEP n=670 (%) |
pTRAM n=683 (%) |
fTRAM n=144 (%) |
msTRAM n=293 (%) |
Χ2 | p |
| Hematoma requiring surgery |
||||||
| Yes | 56 (8.4)a | 26 (3.8)b | 5 (3.5)ab | 13 (4.4)ab | 15.7 | .001 |
| No | 614 (91.6) | 655 (96.2) | 139 (96.5) | 280 (95.6) | ||
| Seroma | ||||||
| Yes | 34 (5.1)a | 78 (11.5)b | 15 (10.4)ab | 20 (6.8)ab | 20.3 | <.001 |
| No | 636 (94.9) | 598 (88.5) | 129 (89.6) | 273 (93.2) | ||
| Fat necrosis | ||||||
| Yes | 109 (16.3)a | 171 (25.3)b | 24 (16.7)ab | 44 (15.0)a | 23.4 | <.001 |
| No | 561 (83.7) | 505 (74.7) | 120 (83.3) | 249 (85.0) | ||
| MFN requiring dressings |
||||||
| Yes | 79 (11.8)a | 72 (10.7)a | 12 (8.3)a | 35 (11.9)a | 1.8 | .617 |
| No | 591 (88.2) | 604 (89.3) | 132 (91.7) | 258 (88.1) | ||
| MFN requiring surgery |
||||||
| Yes | 55 (8.2)ab | 82 (12.1)b | 6 (4.2)a | 21 (7.2)ab | 13.5 | .004 |
| No | 615 (91.8) | 594 (87.9) | 138 (95.8) | 271 (92.8) | ||
| Wound healing delay |
||||||
| Yes | 103 (18.5)a | 174 (28.2)b | 26 (22.4)ab | 51 (26.7)ab | 16.2 | .001 |
| No | 454 (81.5) | 443 (71.8) | 90 (77.6) | 140 (73.3) | ||
| Flap loss | ||||||
| Yes | 11 (1.6)a | 8 (1.2)a | 3 (2.1)a | 4 (1.4)a | 0.9 | .820 |
| No | 659 (98.4) | 668 (98.8) | 141 (97.9) | 289 (98.6) | ||
| Partial flap loss | ||||||
| Yes | 47 (4.0)a | 60 (8.9)b | 11 (7.6)ab | 14 (4.8)ab | 15.1 | .002 |
| No | 643 (96.0) | 616 (91.1) | 133 (92.4) | 279 (95.2) | ||
| DVT or PE | ||||||
| Yes | 8 (1.2)a | 11 (1.6)a | 2 (1.4)a | 4 (1.4)a | 0.5 | .929 |
| No | 660 (98.8) | 665 (98.4) | 142 (98.6) | 289 (98.6) | ||
| Infection | ||||||
| Yes | 42 (6.3)a | 106 (15.7)b | 14 (9.7)ab | 21 (7.2)a | 36.4 | <.001 |
| No | 628 (93.7) | 569 (84.3) | 130 (90.3) | 272 (92.8) | ||
Groups with different superscript letters (a, b, c) are significantly different from each other.
Table 5.
| a Complications – Donor Site | |||||
|---|---|---|---|---|---|
| Variable | DIEP n=670 n (%) |
msTRAM n=293 n (%) |
pTRAM n=683 n (%) |
fTRAM n=144 n (%) |
P |
| Abdominal hernia | <.001* | ||||
| Yes | 13 (1.9) | 14 (4.8) | 46 (6.7) | 4 (2.8) | |
| No | 657 (98.1) | 279 (95.2) | 636 (93.3) | 140 (97.2) | |
| Abdominal bulge | <.001* | ||||
| Yes | 18 (2.7) | 15 (5.1) | 74 (10.9) | 5 (3.5) | |
| No | 652 (97.3) | 278 (94.9) | 608 (89.1) | 139 (96.5) | |
| Abdominal bulge or hernia | <.001* | ||||
| Yes | 28 (4.2) | 24 (8.2) | 113 (16.6) | 8 (5.6) | |
| No | 642 (95.8) | 269 (91.8) | 569 (83.4) | 136 (94.4) | |
| Abdominal bulge or hernia requiring surgery |
<.001* | ||||
| Yes | 21 (3.1) | 18 (6.1) | 68 (10.0) | 5 (3.5) | |
| No | 649 (96.9) | 275 (93.9) | 614 (90.0) | 139 (96.5) | |
| b Pairwise Comparisons: Donor Site Complications | ||||||
|---|---|---|---|---|---|---|
| Variable | DIEP n=670 (%) |
msTRAM n=293 (%) |
pTRAM n=683 (%) |
fTRAM n=144 (%) |
Χ2 | p |
| Abdominal hernia | ||||||
| Yes | 13 (1.9)a | 4 (4.8)ab | 46 (6.7)b | 4 (2.8)ab | 19.9 | <.001 |
| No | 657 (98.1) | 279 (95.2) | 636 (93.3) | 140 (97.2) | ||
| Abdominal bulge | ||||||
| Yes | 18 (2.7)a | 15 (5.1)a | 74 (10.9)b | 5 (3.5)a | 41.6 | <.001 |
| No | 652 (97.3) | 278 (94.9) | 608 (89.1) | 139 (96.5) | ||
| Abdominal bulge or hernia |
||||||
| Yes | ||||||
| No | 28 (4.2)a | 24 (8.2)a | 113(16.6)b | 8 (5.6)a | 63.8 | <.001 |
| 642 (95.8) | 269 (91.8) | 569 (83.4) | 136 (94.4) | |||
| Abdominal bulge or hernia requiring surgery |
||||||
| Yes | 21 (3.1)a | 18 (6.1)ab | 68 (10.0)b | 5 (3.5)ab | 29.1 | <.001 |
| No | 649 (96.9) | 275 (93.9) | 614 (90.0) | 139 (96.5) | ||
Groups with different superscript letters (a, b, c) are significantly different from each other.
BREAST-Q© Scores
Mean scores from the 5 BREAST-Q© scales were compared across the four flap groups (Table 6). Using pairwise analysis there were significantly higher scores in the DIEP group compared to the p-TRAM group for the Physical Well-Being Abdomen scale (83.5 vs. 76.2; p<0.001), and this remained significant after controlling for confounders. There was a trend towards significance for higher Satisfaction with Outcome in the DIEP group compared to the p-TRAM group (p=0.015). The other group differences were not significantly different. Patients scored similarly on the Satisfaction with Breasts, Psychosocial well-being, and Sexual well-being scales.
Table 6.
BREAST-Q scores
| Variable | DIEP n=387 Mean (SD) |
msTRAM n=123 Mean (SD) |
pTRAM n=359 Mean (SD) |
fTRAM n=74 Mean (SD) |
p |
|---|---|---|---|---|---|
| Physical well being – abdomen | 83.5 (17.4) | 78.1 (22.8) | 76.2 (21.8) | 78.6 (23.4) | <.0011 |
| Satisfaction with outcome | 78.6 (19.3) | 72.9 (23.3) | 73.9 (24.2) | 76.4 (22.2) | .0152 |
| Satisfaction with breasts | 71.9 (17.3) | 68.7 (18.7) | 69.8 (20.7) | 71.7 (21.0) | .319 |
| Psychosocial well being | 79.9 (18.4) | 75.9 (22.7) | 79.6 (20.4) | 79.1 (21.7) | .307 |
| Sexual well being | 59.0 (21.5) | 56.0 (23.8) | 57.3 (24.0) | 59.4 (25.0) | .538 |
Games-Howell testing revealed that the statistically significant difference is between DIEP and pTRAM
Games-Howell testing revealed that the statistically significant difference is between DIEP and pTRAM
Responders vs. Non-Responders
Compared to non-responders, DIEP responders were significantly older, had less follow up time, were less likely to have had mesh placed and less likely to have had an absorbable suture placed for abdominal fascial repair. p-TRAM responders and non-responders differed for cancer pathology, receipt of pre-operative chemotherapy (higher in the non-responders), and receipt of radiation (higher in the non-responders). There were no differences between responders and non-responders in the msf-TRAM and f-TRAM groups (Table 7).
Table 7.
Demographics for Responders vs. Non-Responders
| DIEP | ||||
| Variable |
Non-responders (N=304) Mean (SD) |
Responders (N=354) Mean (SD) |
F | P |
| Age at surgery | 47.7 (8.1) | 50.0 (8.5) | 12.0 | .001* |
| Length of follow-up (months) | 59.0 (29.4) | 50.1 (26.1) | 16.3 | <.001* |
| BMI | 27.7 (4.9) | 27.1 (4.7) | 2.2 | .143 |
| Variable |
Non-responders - n (%) |
Responders - n (%) |
Χ2 | P |
| Pathology | 2.2 | .526 | ||
| Invasive | 185 (66.3) | 264 (68.9) | ||
| In situ | 68 (24.4) | 87 (22.7) | ||
| Prophylactic | 26 (9.3) | 30 (7.8) | ||
| Noncancerous | 0 (0) | 2 (0.5) | ||
| History of abdominal surgery | 0.1 | .786 | ||
| Yes | 124 (45.9) | 173 (47.0) | ||
| No | 146 (54.1) | 195 (53.0) | ||
| Chemotherapy | 3.4 | .064 | ||
| Yes | 132 (47.1) | 209 (54.4) | ||
| No | 148 (52.9) | 175 (45.6) | ||
| Pre-operative chemotherapy | 5.9 | .015 | ||
| Yes | ||||
| No | 107 (38.2) | 183 (47.7) | ||
| 173 (61.8) | 201 (52.3) | |||
| Post-operative chemotherapy | 0.1 | .757 | ||
| Yes | ||||
| No | 26 (9.3) | 33 (8.6) | ||
| 254 (90.7) | 351 (91.4) | |||
| Radiation | 1.0 | .321 | ||
| Yes | 111 (39.6) | 167 (43.5) | ||
| No | 169 (60.4) | 217 (56.5) | ||
| Pre-operative radiation | 0.5 | .480 | ||
| Yes | ||||
| No | 105 (37.5) | 154 (40.2) | ||
| 175 (62.5) | 229 (59.8) | |||
| Post-operative radiation | 0.2 | .696 | ||
| Yes | 8 (2.9) | 13 (3.4) | ||
| No | 272 (97.1) | 370 (96.6) | ||
| Smoker | 0.3 | .614 | ||
| Yes | 31 (11.1) | 37 (9.9) | ||
| No | 248 (88.9) | 337 (90.1) | ||
| Diabetic | 0.1 | .739 | ||
| Yes | 7 (2.5) | 11 (2.9) | ||
| No | 272 (97.5) | 363 (97.1) | ||
| History of pregnancy | 0.1 | .714 | ||
| Yes | 219 (86.9) | 284 (87.9) | ||
| No | 33 (13.1) | 39 (12.1) | ||
| Unilateral vs. bilateral |
169 (60.1) | 213 (55.5) | 1.5 | .229 |
| 112 (39.9) | 171 (44.5) | |||
| Timing | 5.9 | .053 | ||
| Immediate | 162 (57.7) | 223 (58.4) | ||
| Delayed | 112 (39.9) | 135 (35.3) | ||
| Both | 7 (2.5) | 24 (6.3) | ||
| Mesh | 18.1 | <.001* | ||
| Yes | 73 (26.0) | 50 (13.0) | ||
| No | 208 (74.0) | 334 (87.0) | ||
| Suture | 10.3 | .006* | ||
| Absorbable | 42 (15.2) | 52 (13.7) | ||
| Non- Absorbable |
100 (36.1) | 184 (48.4) | ||
| Both | 135 (48.7) | 144 (37.9) | ||
| p-TRAM | ||||
| Variable |
Non-responders (N=304) Mean (SD) |
Responders (N=354) Mean (SD) |
F | P |
| Age at surgery | 49.6 (8.3) | 51.1 (8.2) | 5.5 | .019 |
| Length of follow-up (months) | 74.3 (29.2) | 70.0 (28.5) | 3.9 | .048 |
| BMI | 26.6 (4.3) | 26.8 (4.1) | 0.8 | .392 |
| Variable |
Non-responders (N=304) Mean (SD) |
Responders (N=354) Mean (SD) |
Χ2 | P |
| Pathology | 12.7 | .005* | ||
| Invasive | 204 (72.3) | 194 (59.5) | ||
| In situ | 73 (25.9) | 120 (36.8) | ||
| Prophylactic | 5 (1.8) | 9 (2.8) | ||
| Noncancerous | 0 (0) | 3 (0.9) | ||
| History of abdominal surgery | 6.1 | .014 | ||
| Yes | 138 (46.9) | 194 (56.7) | ||
| No | 156 (53.1) | 148 (43.3) | ||
| Chemotherapy | 4.5 | .033 | ||
| Yes | 175 (59.5) | 179 (51.1) | ||
| No | 119 (40.5) | 171 (48.9) | ||
| Pre-operative chemotherapy | 11.8 | .001* | ||
| Yes | ||||
| No | 144 (48.8) | 124 (35.4) | ||
| 151 (51.2) | 226 (64.6) | |||
| Post-operative chemotherapy | 0.9 | .355 | ||
| Yes | ||||
| No | 45 (15.4) | 63 (18.1) | ||
| 248 (84.6) | 285 (81.9) | |||
| Radiation | 6.9 | .008* | ||
| Yes | 160 (54.4) | 154 (44.0) | ||
| No | 134 (45.6) | 196 (56.0) | ||
| Pre-operative radiation | 9.0 | .003* | ||
| Yes | ||||
| No | 135 (45.9) | 120 (34.3) | ||
| 159 (54.1) | 230 (65.7) | |||
| Post-operative radiation | 0.2 | .624 | ||
| Yes | ||||
| No | 31 (10.7) | 33 (9.5) | ||
| 260 (89.3) | 315 (90.5) | |||
| Smoker | 0.1 | .730 | ||
| Yes | 20 (6.6) | 21 (6.0) | ||
| No | 282 (93.4) | 331 (94.0) | ||
| Diabetic | 0.6 | .426 | ||
| Yes | 12 (4.0) | 10 (2.9) | ||
| No | 288 (96.0) | 339 (97.1) | ||
| History of pregnancy | 0.9 | .343 | ||
| Yes | ||||
| No | 170 (84.6) | 227 (87.6) | ||
| 31 (15.4) | 32 (12.4) | |||
| Unilateral vs. bilateral |
252 (82.9) | 269 (76.0) | 4.7 | .030 |
| 52 (17.1) | 85 (24.0) | |||
| Timing | 2.2 | .331 | ||
| Immediate | 217 (71.6) | 246 (69.5) | ||
| Delayed | 84 (27.7) | 101 (28.5) | ||
| Both | 2 (0.7) | 7 (2.0) | ||
| Mesh | 3.7 | .053 | ||
| Yes | 119 (39.1) | 113 (31.9) | ||
| No | 185 (60.9) | 241 (68.1) | ||
| Suture | 8.9 | .012 | ||
| Absorbable | 17 (5.6) | 13 (3.7) | ||
| Non-Absorbable | 110 (36.3) | 95 (27.1) | ||
| Both | 176 (58.1) | 243 (69.2) | ||
| msf-TRAM | ||||
| Variable |
Non-responders (N=170) - Mean (SD) |
Responders (N=123) Mean (SD) |
F | P |
| Age at surgery | 49.0 (8.0) | 50.5 (7.8) | 2.7 | .102 |
| Length of follow-up (months) | 61.6 (24.6) | 61.0 (23.7) | 0.1 | .828 |
| BMI | 29.0 (5.0) | 27.7 (4.5) | 5.1 | .025 |
| Variable |
Non-responders - n (%) |
Responders - n (%) |
Χ2 | P |
| Pathology | 2.6 | .452 | ||
| Invasive | 112 (67.9) | 84 (69.4) | ||
| In situ | 47 (28.5) | 29 (24.0) | ||
| Prophylactic | 6 (3.6) | 7 (5.8) | ||
| Noncancerous | 0 (0) | 1 (0.8) | ||
| History of abdominal surgery | 0.2 | .661 | ||
| Yes | 71 (45.5) | 51 (42.9) | ||
| No | 85 (54.5) | 68 (57.1) | ||
| Chemotherapy | 0.2 | .678 | ||
| Yes | 85 (50.0) | 64 (52.5) | ||
| No | 85 (50.0) | 58 (47.5) | ||
| Pre-operative chemotherapy | 0.1 | .943 | ||
| Yes | ||||
| No | 62 (36.5) | 44 (36.1) | ||
| 108 (63.5) | 78 (63.9) | |||
| Post-operative chemotherapy | 0.9 | .346 | ||
| Yes | ||||
| No | 25 (14.7) | 23 (18.9) | ||
| 145 (85.3) | 99 (81.1) | |||
| Radiation | 2.3 | .132 | ||
| Yes | 82 (48.2) | 48 (39.3) | ||
| No | 88 (51.8) | 74 (60.7) | ||
| Pre-operative radiation | 2.4 | .125 | ||
| Yes | ||||
| No | 78 (45.9) | 45 (36.9) | ||
| 92 (54.1) | 77 (63.1) | |||
| Post-operative radiation | 0.2 | .633 | ||
| Yes | 4 (2.4) | 4 (3.3) | ||
| No | 166 (97.6) | 118 (96.7) | ||
| Smoker | 1.4 | .234 | ||
| Yes | 7 (4.1) | 9 (7.3) | ||
| No | 163 (95.9) | 114 (92.7) | ||
| Diabetic | 0.5 | .486 | ||
| Yes | 10 (5.9) | 5 (4.1) | ||
| No | 160 (94.1) | 118 (95.9) | ||
| History of pregnancy | 0.1 | .912 | ||
| Yes | 125 (84.5) | 96 (85.0) | ||
| No | 23 (15.5) | 17 (15.0) | ||
| Unilateral vs. bilateral | 113 (66.5) | 72 (58.5) | 1.9 | .165 |
| 57 (33.5) | 51 (41.5) | |||
| Timing | 0.2 | .910 | ||
| Immediate | 111 (65.7) | 79 (64.2) | ||
| Delayed | 56 (33.1) | 43 (35.0) | ||
| Both | 2 (1.2) | 1 (0.8) | ||
| Mesh | 1.6 | .212 | ||
| Yes | 87 (51.2) | 72 (58.5) | ||
| No | 83 (48.8) | 51 (41.5) | ||
| Suture | 0.1 | .951 | ||
| Absorbable | 11 (6.5) | 7 (5.8) | ||
| Non-Absorbable | 60 (35.5) | 42 (34.7) | ||
| Both | 98 (58.0) | 72 (59.5) | ||
| f-TRAM | ||||
| Variable |
Non-responders (N= 70) Mean (SD) |
Responders (N=73) Mean (SD) |
F | P |
| Age at surgery | 46.3 (7.6) | 49.3 (7.6) | 5.8 | .018 |
| Length of follow-up (months) | 90.6 (35.8) | 83.5 (37.6) | 1.2 | .275 |
| BMI | 27.6 (5.1) | 28.8 (5.3) | 2.0 | .162 |
| Variable |
Non-responders (N= 70) Mean (SD) |
Responders (N=73) Mean (SD) |
Χ2 | P |
| Pathology | 0.9 | .645 | ||
| Invasive | 45 (64.3) | 41 (56.9) | ||
| In situ | 22 (31.4) | 28 (38.9) | ||
| Prophylactic | 3 (4.3) | 3 (4.2) | ||
| Noncancerous | 0 (0) | 0 (0) | ||
| History of abdominal surgery | 0.02 | .888 | ||
| Yes | 35 (52.2) | 39 (53.4) | ||
| No | 32 (47.8) | 34 (46.6) | ||
| Chemotherapy | 0.7 | .403 | ||
| Yes | 33 (47.1) | 39 (54.2) | ||
| No | 37 (52.9) | 33 (45.8) | ||
| Pre-operative chemotherapy | 0.7 | .398 | ||
| Yes | ||||
| No | 20 (28.6) | 25 (35.2) | ||
| 50 (71.4) | 46 (64.8) | |||
| Post-operative chemotherapy | 1.4 | .234 | ||
| Yes | 12 (17.1) | 18 (25.4) | ||
| No | 58 (82.9) | 53 (74.6) | ||
| Radiation | 0.3 | .556 | ||
| Yes | 27 (38.6) | 24 (33.8) | ||
| No | 43 (61.4) | 47 (66.2) | ||
| Pre-operative radiation | 1.9 | .171 | ||
| Yes | 23 (32.9) | 16 (22.5) | ||
| No | 47 (67.1) | 55 (77.5) | ||
| Post-operative radiation | 1.4 | .237 | ||
| Yes | 4 (5.7) | 8 (11.3) | ||
| No | 66 (94.3) | 63 (88.7) | ||
| Smoker | 1.8 | .174 | ||
| Yes | 7 (10.0) | 3 (4.2) | ||
| No | 63 (90.0) | 69 (95.8) | ||
| Diabetic | -- | -- | ||
| Yes | 0 (0) | 0 (0) | ||
| No | 70 (100) | 72 (100) | ||
| History of pregnancy | 0.4 | .517 | ||
| Yes | 58 (89.2) | 59 (85.5) | ||
| No | 7 (10.8) | 10 (14.5) | ||
| Unilateral vs. bilateral | 56 (80.0) | 57 (78.1) | 0.1 | .778 |
| 14 (20.0) | 16 (21.9) | |||
| Timing | 2.3 | .320 | ||
| Immediate | 53 (75.7) | 50 (68.5) | ||
| Delayed | 16 (22.9) | 23 (31.5) | ||
| Both | 1 (1.4) | 0 (0) | ||
| Mesh | 1.1 | .286 | ||
| Yes | 26 (37.1) | 21 (28.8) | ||
| No | 44 (62.9) | 52 (71.2) | ||
| Suture | 1.1 | .582 | ||
| Absorbable | 1 (1.4) | 0 (0) | ||
| Non-Absorbable | 18 (25.7) | 20 (27.4) | ||
| Both | 51 (72.9) | 53 (72.6) | ||
DISCUSSION
In the setting of breast reconstruction surgery, patient satisfaction and quality of life (QOL) may be the most important outcome variables in evaluating surgical success. When using patient-reported QOL as an outcome measure, Atisha et al. have shown that autologous reconstruction is the gold standard. In a study of over 7000 breast cancer patients these authors identified a spectrum of post-operative satisfaction scores. Using breast conserving surgery (BCS) as a reference, patients that underwent mastectomy and no reconstruction scored lowest, patients that underwent reconstruction with implants scored on average 8.6 points lower than those with BCS and patients that underwent abdominal flap reconstruction scored 5.6 points higher than BCS.26 Since the original description of the p-TRAM by Hartrampf27, refinements in abdominally-based autologous reconstruction have led to inclusion of less rectus abdominis muscle and overlying fascia.28,29,30,31 Previous studies have shown advantages of the DIEP flap when compared to the f-TRAM flap for abdominal strength, postoperative pain, and cost.10,32,33,34,35 Similarly, advantages of the DIEP have been shown when comparing it to the p-TRAM for occurrence of hernia/bulge, lower rate of fat necrosis, lower hospital stay, better abdominal contour and decreased postoperative pain.7,26,36 However, many authors have suggested that the higher rate of flap loss in the more difficult microsurgical reconstructions (DIEP) should be considered as a downside when choosing between the different abdominally-based techniques.
The most hotly debated issue is by far that of abdominal wall morbidity. Arguably, the best way to determine if there is a difference in abdominal wall morbidity between the different techniques is to study surgical outcomes and patient reported experience. We have studied patients from five North American centers representing the largest cohort of patients to be compared for results following abdominally-based breast reconstruction. The BREAST-Q© was chosen to determine differences in patient experience because it provides a scientifically rigorous and clinically valid means to examine the impact of breast reconstruction from the patients’ perspective.22,37 Our study has documented experiential differences in satisfaction with DIEP patients expressing significantly higher physical well-being of the abdomen and a trend to higher overall satisfaction with outcome when compared to the p-TRAM reconstruction patients. This finding did not reach significance when comparing the DIEP to the msf-TRAM and f-TRAM groups. BREAST-Q© data which address issues such as “tightness or pulling in the abdomen” and “difficulty doing everyday activities requiring the use of your abdominal muscles” are powerful because the questions asked relate directly to the types of problems patients face as they move on after breast reconstruction. By using a sensitive outcome measure (i.e. calibrated to detect a difference in our target population), we feel confident in reporting that a real difference exists in long-term postoperative satisfaction for abdominal well-being and overall outcome when comparing the DIEP to the p-TRAM patient.
When looking at clinical measures of abdominal wall morbidity, we found DIEP patients to display the lowest rate of hernia/bulge requiring surgery (3.1%) and this reached significance when comparing the DIEP to the p-TRAM. p-TRAM patients had the highest rate of bulge and hernia/bulge compared to all other flap types. Previous studies have corroborated our results. Garvey et al. compared 94 p-TRAM flaps to 96 DIEP flaps and showed a 16% incidence of hernia in the p-TRAM group versus a 1% incidence of hernia in the DIEP group. They however found a high incidence of bulge in both the DIEP (9.4%) and p-TRAM groups (14.9%).7 Knox et al. compared 165 DIEP procedures to 443 p-TRAMs and found eight times the odds of hernia/bulge in the p-TRAM group after controlling for confounders.36 These studies support our finding that DIEP reconstruction confers a lower risk of hernia/bulge when compared to the p-TRAM reconstruction.
Comparisons of the DIEP flap to the f-TRAM flap and the msf-TRAM flap are more variable. A meta-analysis conducted by Man et al. (861 flaps) found DIEP patients to display a 3.1% incidence of bulge and TRAM patients 5.9%; DIEP patients had a 0.8% incidence of hernia compared to 3.9% in the TRAM patients.15 However in this analysis f-TRAM and msf-TRAM patients were combined in one group. A systematic analysis that exclusively compared DIEP to f-TRAM showed no significant difference in the incidence of bulge, however the total number of patients in each group was small.38 Vyas et al. compared 37 f-TRAM flaps to 182 msf-TRAM and 128 DIEP flaps.39 They found a 13.5% incidence of hernia/bulge in the f-TRAM group compared to 3.9% in the DIEP group. msf-TRAM patients had a 6.6% incidence of hernia/bulge and they concluded that while there was a significant difference between the f-TRAM and DIEP groups, there was no difference between msf-TRAM and DIEP groups. Another study by Nelson et al. (n=144) showed no difference in hernia formation when comparing DIEP (0%) to msf-TRAM (4%).40 A recent study by Zhong et al. used propensity to scores to control for selection bias. This method considers potential confounders that may affect outcome, but also factors that contribute to selection of the reconstruction procedure. For example, a planned DIEP flap may be converted to an msf-TRAM procedure if there is no single dominant perforator and the patient is a smoker or diabetic. This may lead to an increased number of msf-TRAM flaps in the smoking or diabetic population who may be at inherently higher risk for hernia/bulge. After controlling for confounders and using propensity score analysis, this group found a 2.7 times increased risk of abdominal wall hernia or bulge in the msf-TRAM group compared to the DIEP group.41 While we found that DIEP flaps conferred a lower risk of abdominal wall morbidity compared to p-TRAM, differences between the DIEP and f-TRAM and the DIEP and msf-TRAM were non-significant. Additionally, hernia/bulge and bulge rates are lower in all groups compared to the p-TRAM group, but there was no significant difference on comparison between the DIEP, msf-TRAM and f-TRAM groups. It is unclear why abdominal wall morbidity is worse in the p-TRAM compared to the f-TRAM given that both approaches sacrifice the entire rectus muscle. This may be a result of the fact that the rectus fascia is not completely closed in the pedicled technique which may alter the mechanical advantage of the internal oblique muscles.
We have shown that across 1790 patients there is no difference in total flap loss between the DIEP, msf-TRAM, f-TRAM and p-TRAM groups. Fat necrosis was highest in the pTRAM group (25%) compared to the DIEP and msf-TRAM. This is likely a result of the use of the dominant blood supply to the flap in the latter three reconstruction techniques. A similar mechanism can be inferred to explain the higher rate of partial flap loss in the p-TRAM group (8.9%) compared to the DIEP (4%).
In general, studies have shown increased satisfaction with autologous reconstruction compared to implant reconstruction.25,42,43 It is thus important to note that if a surgeon’s preference is for the p-TRAM flap, this may still be considered preferable to an alloplastic reconstruction. While our study suggests that DIEP reconstruction leads to less abdominal morbidity, it must be acknowledged that microsurgical breast reconstruction is not available in many centers in North America. Microsurgical breast reconstruction requires a dedicated perioperative environment including sophisticated monitoring and postoperative care. It is therefore difficult to incorporate free flap breast reconstruction techniques into many community medical centers.44,45,46 In these practice settings, patients should still be encouraged to consider p-TRAM reconstruction. Furthermore, while p-TRAMs can be planned pre-operatively, performing a DIEP flap must take into consideration intraoperative factors. If a DIEP flap is planned and there is no single dominant perforator then conversion to an msf-TRAM or a f-TRAM may be indicated. If the patient is diabetic, or has other factors that may affect perfusion to the flap a f-TRAM reconstruction may be preferable. Keeping these factors in mind, we engage the patient in an extensive preoperative discussion outlining our algorithm for reconstructive choice and are clear about the expected outcomes of each reconstructive technique.
Limitations of this study include the response rate which is lower than the average proposed rate of 60% considered optimal for clinical research.47,48 Analysis of non-responders showed differences in characteristics between groups and we controlled for these differences by entering factors assumed to have an impact on outcome into our logistic regression model. Differences in surgeon technique and pre- and intra-operative decision-making cannot be accounted for in our retrospective analysis. For example, patients coded as a DIEP at one institution may have been considered an msf-TRAM at another institution thereby confounding our results. Additionally there was a disproportionately higher usage of mesh at the MSKCC site when compared to other sites. This may have affected abdominal wall morbidity outcomes. We attempted to control for this by including mesh usage in our regression analysis. Lastly, the cross-sectional nature of this study provides information about patients at a single time point (on average 5.5 years post reconstruction) and does not account for dynamic changes in patient perception. A multi-center prospective study with strictly defined preoperative and intraoperative decision-making algorithms would provide a more accurate comparison of the four studied breast reconstruction techniques.
Conclusion
This is the first study to use the BREAST-Q© to compare patient-reported outcomes in patients undergoing breast reconstruction utilizing the four most common abdominally-based breast reconstruction techniques. We have shown that patients who undergo DIEP flap reconstruction have higher satisfaction with abdominal physical well-being when compared to patients that undergo p-TRAM reconstruction, but do not differ from msf-TRAM and f-TRAM patients. p-TRAM patients were found to have the highest rates of hernia/bulge compared to msf-TRAM, f-TRAM and DIEP, but there was no difference between the other groups. There was no difference in total flap failure when comparing the four groups in this large sample of patients. While choice of autologous technique will depend on surgeon training and intra-operative decision-making, the differences we have found in patient-reported symptoms and abdominal donor site outcomes may shift the practice of plastic surgeons towards utilizing methods with lower donor site morbidity and higher patient-reported satisfaction.
Acknowledgments
This study is funded by a generous grant from the Canadian Breast Cancer Foundation.
Footnotes
Presented at the 69th Annual Canadian Society of Plastic Surgery Meeting June 6, 2015.
Financial Disclosure:
Dr. Pusic is a co-developer of the BREAST-Q© which is owned by Memorial Sloan Kettering Cancer Center and the University of British Columbia. When the BREAST-Q is used in for-profit clinical trials, Dr. Pusic receives a share of any license revenues based on the inventor sharing policies of these two institutions.
Author Roles
Dr Sheina Macadam: Lead investigator. Responsible for overall management of study sites, research assistants and allocation of grant funds. Oversaw patient recruitment, creation and mailing of all patient questionnaire packages, data collection/entry. Responsible for compilation of data, initial statistical analysis and writing of manuscript including tables.
Dr Toni Zhong: Responsible for contribution of patient data and editing of manuscript.
Dr Katie Weichman: Responsible for data collection and ethics approval for New York University site.
Dr Peter Lennox: Responsible for contribution of patient data.
Dr Nancy Van Laeken: Responsible for contribution of patient data.
Dr Michael Papsdorf: Responsible for overall statistical analysis and preparation of tables (paid consultant).
Dr Evan Matros: Responsible for contribution of patient data.
Dr Dale Vidal: Responsible for contribution of patient data.
Dr Alexes Hazen: Responsible for contribution of patient data.
Dr Anne Klassen: Responsible for advice conceptual plan and methods.
Dr Stefan Cano: Responsible for scoring of BREASTQ© data (paid consultant).
Dr Joseph Disa: Responsible for contribution of patient data.
Dr Babak Mehrara: Responsible for contribution of patient data.
Dr Peter Cordeiro: Responsible for contribution of patient data.
Dr Andrea Pusic: Responsible for original concept and study design, data review and editing of manuscript.
REFERENCES
- 1.Surveillance, Epidemiology and End Results Program. Based on 2009–2011 data. [Accessed January 2, 2015]; http://seer.cancer.gov/statfacts/html/breast.html.
- 2.American Society of Plastic Surgeons Annual Plastic Surgery Statistics Report. [Accessed January 2, 2015]; http://www.plasticsurgery.org/Documents/news-resources/statistics/2013-statistics/plastic-surgery-statistics-full-report-2013.pdf. [Google Scholar]
- 3.Edsander-Nord A, Jurell G, Wickman M. Donor-site morbidity after pedicled or free TRAM flap surgery: A prospective and objective study. Plast Reconstr Surg. 1998;102:1508–1516. doi: 10.1097/00006534-199810000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Kroll SS, Marchi M. Comparison of strategies for preventing abdominal wall weakness after TRAM flap breast reconstruction. Plast Reconstr Surg. 1992;89(6):1045–1051. [PubMed] [Google Scholar]
- 5.Wan DC, Tseng CY, Anderson-Dam J, et al. Inclusion of mesh in donor-site repair of free TRAM and muscle-sparing free TRAM flaps yields rates of abdominal complications comparable to those of DIEP flap reconstruction. Plast Reconstr Surg. 2010;126(2):367–374. doi: 10.1097/PRS.0b013e3181de1b7e. [DOI] [PubMed] [Google Scholar]
- 6.Takeishi M, Fujimoto M, Ishida K, Makino Y. Muscle sparing-2 transverse rectus abdominis musculocutaneous flap for breast reconstruction: A comparison with deep inferior epigastric perforator flap. Microsurgery. 2008;28:650–655. doi: 10.1002/micr.20563. [DOI] [PubMed] [Google Scholar]
- 7.Garvey PB, Buchel EW, Pockaj BA, et al. DIEP and pedicled TRAM flaps: A comparison of outcomes. Plast Reconstr Surg. 2006;117:1711–1719. doi: 10.1097/01.prs.0000210679.77449.7d. [DOI] [PubMed] [Google Scholar]
- 8.Dulin WA, Avila RA, Verheyden CN, et al. Evaluation of abdominal wall strength after TRAM flap surgery. Plast Reconstr Surg. 2004;113(6):1662–1665. doi: 10.1097/01.prs.0000117197.77201.14. discussion 1666–1667. [DOI] [PubMed] [Google Scholar]
- 9.Bonde CT, Lund H, Fridberg M, et al. Abdominal strength after breast reconstruction using a free abdominal flap. J Plast, Reconstr Aesth Surg. 2007;60(5):519–523. doi: 10.1016/j.bjps.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Futter CM, Webster M, Hagen S, Mitchell SL. A retrospective comparison of abdominal muscle strength following breast reconstruction with a free TRAM or DIEP flap. Brit J Plast Surg. 2000;53(7):578–583. doi: 10.1054/bjps.2000.3427. [DOI] [PubMed] [Google Scholar]
- 11.Futter CM, Weiler-Mithoff E, Hagen S, et al. Do pre-operative abdominal excercises prevent post-operative donor site complications for women undergoing DIEP flap breast-reconstruction? A two-centre, prospective randomised controlled trial. Brit J Plast Surg. 2003;56(7):674–683. doi: 10.1016/s0007-1226(03)00362-x. [DOI] [PubMed] [Google Scholar]
- 12.Kind GM, Rademaker AW, Mustoe TA. Abdominal-wall recovery following TRAM flap: A functional outcome study. Plast Reconstr Surg. 1997;99(2):417–428. doi: 10.1097/00006534-199702000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Atisha DM, Alderman AK. A Systematic review of abdominal wall function following abdominal flaps for post-mastectomy breast reconstruction. Annals Plast Surg. 2009;63(2):222–230. doi: 10.1097/SAP.0b013e31818c4a9e. [DOI] [PubMed] [Google Scholar]
- 14.Chun YS, Sinha I, Turko A, et al. Comparison of morbidity, functional outcome, and satisfaction following bilateral TRAM versus bilateral DIEP flap breast reconstruction. Plast Reconstr Surg. 2010;126(4):1133–1141. doi: 10.1097/PRS.0b013e3181ea42d3. [DOI] [PubMed] [Google Scholar]
- 15.Man LX, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: A meta-analysis and critical review. Plast Reconstr Surg. 2009;124(3):752–764. doi: 10.1097/PRS.0b013e31818b7533. [DOI] [PubMed] [Google Scholar]
- 16.Blondeel PN, Arnstein M, Verstraete K, et al. Venous congestion and blood flow in free transverse rectus abdominis myocutaneous and deep inferior epigastric argery perforator flap surgery. Plast Reconstr Surg. 2000;106:1295–1299. doi: 10.1097/00006534-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni AR, Sears ED, Atisha DM, et al. Use of autologous and microsurgical reconstruction by US plastic surgeons. Plast Reconstr Surg. 2013;132(3):534–541. doi: 10.1097/PRS.0b013e31829ae03e. [DOI] [PubMed] [Google Scholar]
- 18.Dillman D, Smyth J, Christian L. Internet, Mail, and Mixed- Mode Surveys: The Tailored Design Method. 3rd. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 19.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient reported outcome measure for breast surgery: The BREAST-Q. Plast Reconstr Surg. 2009;124:345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 20.Scientific Advisory Committee of the Medical Outcomes Trust. Assessing health status and quality of life instruments: Attributes and review criteria. Qual Life Res. 2002;11:193–205. doi: 10.1023/a:1015291021312. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration. Patient reported outcome measures: Use in medical product development to support labeling claims. [Accessed June 1, 2010];2006 Availble at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM 193282.pdf.
- 22.Cano SJ, Klassen AF, Scott AM, et al. The BREAST-Q: Further validation in independent clinical samples. Plast Reconstr Surg. 2012;129(2):293–302. doi: 10.1097/PRS.0b013e31823aec6b. [DOI] [PubMed] [Google Scholar]
- 23.Klassen A, Pusic AL, Scott A, Klok J, Cano S. Satisfaction and quality of life in women who undergo breast surgery: A qualitative study. BMC Women's Health. 2009;1(9):11. doi: 10.1186/1472-6874-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. http://www.mskcc.org/mskcc/shared/Breast-Q/scoreBQ.html. [Google Scholar]
- 25. http://www.rasch-analysis.com. [Google Scholar]
- 26.Atisha DM, Rushing CN, Samsa GP, et al. A national snapshot of satisfaction with breast cancer procedures. Ann Surg Oncol. 2015;22(2):361–369. doi: 10.1245/s10434-014-4246-9. [DOI] [PubMed] [Google Scholar]
- 27.Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69:216–225. doi: 10.1097/00006534-198202000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Grotting JC, Urist MM, Maddox WA, Vasconez LO. Conventional TRAM flap versus free microsurgical TRAM flap for immediate breast reconstruction. Plast Reconstr Surg. 1989;83:828–841. doi: 10.1097/00006534-198905000-00009. discussion 842–824. [DOI] [PubMed] [Google Scholar]
- 29.Nahabedian MY, Dooley W, Singh N, Manson PN. Contour abnormalities of the abdomen after breast reconstruction with abdominal flaps: the role of muscle preservation. Plast Reconstr Surg. 2002;109:91–101. doi: 10.1097/00006534-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42:645–648. doi: 10.1016/0007-1226(89)90075-1. [DOI] [PubMed] [Google Scholar]
- 31.Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32:32–38. doi: 10.1097/00000637-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan JL, Allen RJ. Cost-based comparison between perforator flaps and TRAM flaps for breast reconstruction. Plast Reconstr Surg. 2000;105:943–948. doi: 10.1097/00006534-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Kroll SS, Reece GP, Miller MM, et al. Comparison of cost for DIEP and free TRAM flap breast reconstruction. Plast Reconstr Surg. 2001;107:1413–1418. doi: 10.1097/00006534-200105000-00014. discussion 1417–1418. [DOI] [PubMed] [Google Scholar]
- 34.Kroll SS, Sharma S, Koutz C, et al. Postoperative morphine requirements of free TRAM and DIEP flaps. Plast Reconstr Surg. 2001;107:338–341. doi: 10.1097/00006534-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Blondeel PN, Vanderstraeten GG, Monstrey SJ, et al. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br J Plast Surg. 1997;50:322–330. doi: 10.1016/s0007-1226(97)90540-3. [DOI] [PubMed] [Google Scholar]
- 36.Knox ADC, Ho AL, Tashakkor Y, et al. Comparison of Outcomes Following Autologous Breast Reconstruction using the DIEP and Pedicled TRAM Flap: A 12 Year Clinical Retrospective Study and Literature Review (Unpublished data. In Press: Plast Reconstr Surg) doi: 10.1097/PRS.0000000000001747. [DOI] [PubMed] [Google Scholar]
- 37.Pusic AL, Lemaine V, Klassen AF, et al. Patient reported outcome measures in plastic surgery: Use and interpreation in evidence-based medicine. Plast Reconstr Surg. 2011;127(3):1361–1367. doi: 10.1097/PRS.0b013e3182063276. [DOI] [PubMed] [Google Scholar]
- 38.Sailon AM, Schachar JS, Levine JP. The transverse rectus abdominis myocutaneous and deep inferior epigastrci perforator flaps for breast reconstruction: A systematic review of flap complication rates and donor-site morbidity. Ann Plast Surg. 2009;62(5):560–563. doi: 10.1097/SAP.0b013e31819faf0d. [DOI] [PubMed] [Google Scholar]
- 39.Vyas RM, Dickinson BP, Fastekjian JH, et al. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg. 2008;121(5):1519–1526. doi: 10.1097/PRS.0b013e31816b1458. [DOI] [PubMed] [Google Scholar]
- 40.Nelson JA, Guo Y, Sonnad SS, et al. A comparison between DIEP and muscle-sparing free TRAM flaps in breast reconstruction: A single surgeon’s recent experience. Plast Reconstr Surg. 2010;126(5):1428–1435. doi: 10.1097/PRS.0b013e3181ef8b20. [DOI] [PubMed] [Google Scholar]
- 41.Zhong T, Novak CB, Gagher S. Using propensity score analysis to compare major complications between DIEP and muscle-sparing TRAM flap breast reconstructions. Plast Reconstr Surg. 2014;133(4):774–782. doi: 10.1097/PRS.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, Zhuang Y, Momeni A, et al. Quality of life and patient satisfaction after microsurgical abdominal flap versus staged expander/implant breast reconstruction: A critical study of unilateral immediate breast reconstruction using patient-reported outcomes instrument BREAST-Q. Breast Cancer Res & Treat. 2014;146(1):117–126. doi: 10.1007/s10549-014-2981-z. [DOI] [PubMed] [Google Scholar]
- 43.Alderman AK, Kuhn LE, Lowery JC, et al. Does patient satisfaction with breast reconstruction change over time? Two year results of the Michigan breast reconstruction outcomes study. J Am Coll Surg. 2007;204(7):7–12. doi: 10.1016/j.jamcollsurg.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 44.Kanchwala SK, Bucky LP. Optimizing pedicled transverse rectus abdominis muscle flap breast reconstruction. The Cancer J. 2008;14(4):236–240. doi: 10.1097/PPO.0b013e318180bce5. [DOI] [PubMed] [Google Scholar]
- 45.Kim EK, Eom JS, Ahn SH, et al. Evolution of the pedicled TRAM flap: A prospective study of 500 consecutive cases by a single surgeon in Asian patients. Ann Pl Surg. 2009;63(4):378–382. doi: 10.1097/SAP.0b013e3181951708. [DOI] [PubMed] [Google Scholar]
- 46.Buck DW, Fine NA. The pedicled transverse rectus abdominis myocutaneous flap: Indications, techniques and outcomes. Plast Reconstr Surg. 2009;124(4):1047–1054. doi: 10.1097/PRS.0b013e3181b457b2. [DOI] [PubMed] [Google Scholar]
- 47.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–1136. doi: 10.1016/s0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]
- 48.Badger F, Werrett J. Room for improvement? Reporting response rates and recruitment in nursing research in the past decade. J Adv Nurs. 2005;51:502–510. doi: 10.1111/j.1365-2648.2005.03521.x. [DOI] [PubMed] [Google Scholar]