Abstract
OBJECTIVE
Sleep disturbances are prevalent, persistent, and impairing features of bipolar disorder. However, the near-term and cumulative impact of the severity and variability of sleep disturbances on symptoms and functioning remains unclear. We examined self-reported daily sleep duration and variability in relation to mood symptoms, medication adherence, cognitive functioning, and concurrent daily affect.
METHODS
Forty-one outpatients diagnosed with bipolar disorder were asked to provide daily reports of sleep duration and affect collected via ecological momentary assessment with smartphones over eleven weeks. Measures of depressive and manic symptoms, medication adherence, and cognitive function were collected at baseline and concurrent assessment of affect were collected daily. Analyses examined whether sleep duration or variability were associated with baseline measures and changes in same-day or next-day affect.
RESULTS
Greater sleep duration variability (but not average sleep duration) was associated with greater depressive and manic symptom severity, and lower medication adherence at baseline, and with lower and more variable ratings of positive affect and higher ratings of negative affect. Sleep durations shorter than 7-8 hours were associated with lower same-day ratings of positive and higher same-day ratings of negative affect, however this did not extend to next-day affect.
CONCLUSIONS
Greater cumulative day-to-day sleep duration variability, but not average sleep duration, was related to more severe mood symptoms, lower self-reported medication adherence and higher levels of negative affect. Bouts of short- or long-duration sleep had transient impact on affect. Day-to-day sleep variability may be important to incorporate into clinical assessment of sleep disturbances in bipolar disorder.
Keywords: sleep, bipolar disorder, affective states
INTRODUCTION
Disturbances in the quantity of sleep are common among patients diagnosed with bipolar disorder. Between 70-99% of bipolar patients experience a reduced need for sleep at some time during the course of their illness (Harvey et al., 2009). Many patients also report difficulties with falling asleep or staying asleep over the course of illness resulting in reduced or variable sleep duration. The influence of sleep duration on mood symptoms in bipolar disorder is complex—some studies show reduced and more variable sleep duration precede manic or depressive episodes (Barbini et al., 1996; Fava and Kellner, 1991; Gruber et al., 2011; Jackson et al., 2003; Perlman et al., 2006), and are evident during mood episodes (Cassidy et al., 1998), suggesting that abnormal sleep duration can be both a risk marker and a concomitant of bipolar episodes.
The majority of studies examining sleep in bipolar disorder have employed retrospective global measures of sleep duration collected over relatively short periods of observation. Some studies have employed prospective designs in which sleep and affect are measured concurrently over periods of one or several weeks (Bauer et al., 2006; Gershon et al., 2012; Gonzalez et al., 2014). These studies suggest that sleep and circadian disruptions are predictive of mood changes among people with bipolar disorder, which, in turn, has informed the basis of psychosocial interventions designed to stabilize sleep-wake patterns (Frank et al., 2005). Consistent with the idea that more variable sleep predicts more symptoms in bipolar disorder, Gruber et al. (2011) found in a sample of 196 remitted patients reporting on the maximum and minimum duration of sleep obtained in the previous week, that greater variability in sleep duration was associated with worsening of depression and mania across a one-year follow up period. Seemingly consistent with these data on individuals with bipolar disorder, among healthy individuals, reduced or more variable sleep duration over time is associated with a worsening of mood (Dinges et al., 1997), diminished well-being (Drake et al., 2001) and cognitive impairment (Boland and Alloy, 2013). Poor sleep has also been linked to lower medication adherence in individuals with serious medical conditions (Phillips et al., 2005), although no studies have examined this potential link, to our knowledge, in bipolar disorder. It remains unclear if between person differences in sleep duration variability are adequately captured in brief observation periods and across a range of levels of depressive and manic symptom severity. Additionally, more studies are needed to assess the dimensional components of bipolar disorder (Phillips and Kupfer, 2013). For example, depression has been characterized by low levels of positive affect, rather than high levels of negative affect (Dunn et al., 2004), whereas mania is characterized by high levels of positive affect or irritability, but not necessarily low levels of negative affect. Examining mood symptoms alone may obscure the subtleties of these affective dimensions in bipolar disorder.
Moreover, past studies have not yielded opportunities to examine proximal associations between sleep and mood. Sleep duration and variability has typically been evaluated with cross-sectional surveys (Goossens et al., 2010), and longitudinal studies with time points spaced months, or even years, apart (Gruber et al., 2011; Perlman et al., 2006; Saunders et al., 2015). Studies have employed actigraphy to measure sleep in bipolar patients, but sleep duration in these studies was only measured for one or two weeks (Harvey et al., 2005; Jones et al., 2005; Millar et al., 2004) and concurrent mood ratings were collected at one time point (Harvey et al., 2005; Jones et al., 2005) or by daily mood diary entries (Gershon et al., 2012; Millar et al., 2004). Mobile technology and ecological momentary assessment, the frequent real time and concurrent assessment of naturalistic behavior and affective experience, affords the ability to examine proximal associations between day-to-day sleep duration and variability and concurrent positive and negative affect. In addition, lagged models enable understanding of the potential carryover effects of impaired sleep on affect.
Data from a clinical trial in bipolar disorder with a comprehensive baseline characterization and 11-weeks of daily assessments of sleep and affect via smart phone surveys allowed us to more thoroughly examine the relationship of sleep duration and variability to symptom severity, medication adherence, global cognitive functioning, and longitudinally assessed positive and negative affect. We hypothesized lower average sleep duration would be positively correlated with baseline manic symptom severity and inversely correlated with baseline depressive symptom severity. We also predicted higher day-to-day variability in sleep duration would be associated with greater baseline manic and depressive symptom severity, lower medication adherence, and greater cognitive impairment. Finally, we hypothesized that day-to-day change in sleep duration and in how atypical the day's sleep duration was compared to the person's norm would predict increases in same-day and next-day negative affect and decreases in same-day and next-day positive affect.
MATERIALS AND METHODS
Parent study
Data came from a randomized controlled trial of outpatients with bipolar I and II disorder in San Diego, which compared use of an automated mobile device-delivered intervention following brief psychoeducation with brief psychoeducation alone (Depp et al., 2015; 2012). Only subjects in the active arm (n=41) were included. This study was carried out in accordance with the Declaration of Helsinki, and informed consent was obtained by all participants. The study was approved by the University of California, San Diego (UCSD) Institutional Review Board, and registered in Clinicaltrials.gov (NCT01670123).
Participants
Participants were recruited through flyers/advertisements, online communities, community treatment settings, bipolar disorder support groups, and community outpatient treatment clinics. Diagnoses (current and lifetime psychiatric and substance use disorders) were determined through structured clinician interview with the Mini-International Neuropsychiatric Interview for DSM-IV (Sheehan et al., 1998) and obtained medical records. Diagnoses were confirmed in consensus conferences. Eligibility requirements included: 1) a bipolar I or II disorder diagnosis; 2) being aged 18+; 3) receiving outpatient medication treatment for bipolar disorder; 4) free of visual or manual dexterity disabilities precluding operation of a touch screen device. Exclusion criteria included: 1) diagnosis of alcohol/substance use disorder in prior 3 months; 2) psychiatric hospitalization in prior month; or 3) a score of severe depression severity on the Montgomery Asberg Depression Rating Scale or the Young Mania Rating Scale (defined as a score >32 or >20, respectively). We also excluded participants who were currently experiencing a severe mood state requiring more intense treatment. All participants provided written, informed consent, and were compensated for assessment visits ($25 for each completed assessment with a maximum of $100), but not treatment sessions.
Data Collection
Each participant received an internet-enabled Samsung Fascinate smartphone, and was instructed to respond to study surveys. Surveys were sent twice daily at random times over 3-4 hour blocks in the morning and evening for 11 weeks. To ensure that participants’ daily activities and habits, as well as sleep/wake patterns, were not disturbed, participants were asked at study entry to indicate the times during which they were available to receive surveys. Participants were asked to fill out a web-based survey at the time it was sent, and if they did not respond, they were sent a reminder after 15 minutes. If the participant did not respond within 2 hours, the survey expired. Individuals did not have to complete the entire survey for the data to be captured. Study staff called participants every two weeks to discuss experiences using the device (including technical difficulties), and to remind them about the next assessment visit.
This study reports only on participants who received the mobile device intervention and examines only the morning responses since the sleep survey was only administered in the morning. Available for analyses were 1,882 survey elements —with an average of 45.9 days (range: 8 – 74) per participant. The average response rate was 63% (SD=24%).
Measures
Baseline psychological assessments
Participants were assessed using the Montgomery–Åsberg Depression Rating Scale (MADRS) (Williams and Kobak, 2008), the Young Mania Rating Scale (YMRS) (Young et al., 1978), the Morisky Medication Adherence Scale (MMAS) (Morisky et al., 2008), and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph et al., 1998). The MADRS includes 10-items that assess the severity of bipolar depression. The YMRS is an 11-item interview measure of mania symptom severity, with scores ranging from 0 to 60 (higher scores indicating greater symptom severity). MMAS measures various levels of medication adherence, and consists of four dichotomous (e.g., yes/no) items (forgetting to take medication, careless when taking medication, stop taking if feel worse, and stop taking when feeling better) yielding a total score ranging from 0-4 (higher scores indicate less adherence). RBANS is administered in 12 subtests that measure various domains of cognition (e.g., Immediate Memory, Visuospatial/Construction)—a total score is computed with higher scores indicating worse cognition.
Daily sleep duration
Participants were asked to report sleep duration obtained the previous night. Response options included “<5 hours”, “5-6 hours”, “7-8 hours”, “9-10 hours”, “>10 hours,” and were coded with values ranging from 1 (<5 hours) to 5 (>10 hours).
Daily positive and negative affect
Participants rated how relaxed, happy, energetic, sad or depressed, angry or upset, anxious or nervous, stressed, and impulsive they currently felt using a scale ranging from 1 (“not at all”) to 7 (“extremely”).
Analyses
First, we calculated each participant's average (mean) and within-person standard deviation (variability) of sleep duration across all study days. Spearman correlations were used to examine whether mean or variability of sleep duration were associated with demographic variation and baseline symptom severity, medication adherence, and cognitive functioning. We also tested whether mean and variability of sleep duration differed between sex and minority status using t-tests. Spearman correlations were used to examine the association between mean and variability of sleep duration with mean and variability (calculated as the standard deviation across all study days) of the positive and negative affect ratings.
Second, to assess the prospective impact of daily sleep duration on daily affect, we used multi-level models with fixed effects of sleep duration as predictor and daily affect as outcomes, participant as random intercept, and day on study as random slope. The sleep duration was entered into the model using dummy variables; 7-8 hours of sleep served as the reference (National Heart, Lung, and Blood Institute, 2012). We assessed the impact of sleep duration on same-day and next-day (i.e., lagged) affect ratings. Since ratings are made in the morning regarding the preceding night's sleep, same-day ratings show the effect of the previous night's duration on morning affect while next-day ratings show the effect of sleep duration 2 nights before on morning affect. One individual in our sample had no two-day consecutive records and was thus excluded from lagged analyses.
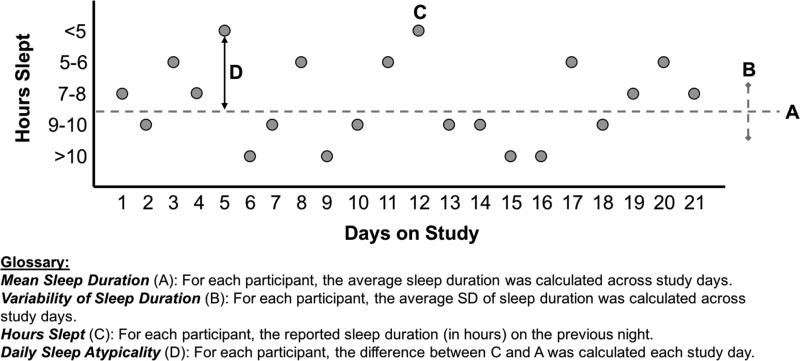
Third, to assess the role of sleep variability on daily affect ratings, we conducted similar analyses, but calculated a “daily sleep atypicality” variable by subtracting each subject's mean sleep duration from the current day's sleep duration and squaring the result. Smaller values indicate typical sleep and larger values indicate atypical sleep (either longer or shorter than usual). This was entered into the model as a continuous variable. We assessed the impact of sleep atypicality on both same-day and next-day affect ratings. Figure 1 displays a visual depiction of how we characterized sleep in this study.
Figure 1.
Calculation of sleep parameters in this study.
To assess the impact of non-random missing data, we assessed whether greater or less adherence to the study protocol influenced recordings of sleep duration and baseline symptom severity. Specifically, we assessed the correlation between number of mobile surveys completed and mean and standard deviation of the sleep duration as well as the baseline measures. The number of mobile surveys completed was neither correlated with mean (r=.00, p=.999), nor standard deviation of the sleep duration (r=.01, p=.949). The number of completed surveys did not correlate with scores on cognitive abilities (RBANS; r=.09, p=.574), depressive (MADRS; r=.25, p=.122) and manic symptomatology (YMRS; r=.08, p=.612), and medication adherence (MMAS; r=−.14, p=.401). We did not impute data on sleep and affect for missed days of mobile surveys because mixed models are robust to missing data and allow for uneven spacing of repeated measurements.
RESULTS
Demographic characteristics are displayed in Table 1. Participant ages were on average in the mid- to late-40s. Participants were balanced by sex, and the majority were White, few were married, and most were living independently in the community. The vast majority of participants were diagnosed with Bipolar I (vs. II), and generally had an age of onset of bipolar disorder in the early 20s. Most participants were medicated.
Table 1.
Sample Characteristics (n=41)
| Mean (SD) or % | |
|---|---|
| Age | 46.9 (11.8) |
| Sex (% female) | 53.7% |
| Ethnicity | |
| White | 78.0% |
| African-American | 9.8% |
| Asian | 2.4% |
| Latino/Hispanic | 4.9% |
| More than one ethnicity | 4.9% |
| Education (Years) | 14.9 (2.1) |
| Marital Status (% Married) | 14.6% |
| Living Situation | |
| Independent living, in community | 90.2% |
| Residential facility | 4.9% |
| Homeless | 4.9% |
| Bipolar I (vs II) | 87.8% |
| Age of First Onset of Mood | 21.9 (10.4) |
| Symptoms | |
| Medications Prescribed | |
| Mood stabilizer | 75.0% |
| Antipsychotic | 46.3% |
| Antidepressant | 56.1% |
| Baseline RBANS Total Score | 84.9 (13.9) |
| Baseline MADRS Total Score | 11.9 (9.0) |
| Baseline YMRS Total Score | 7.2 (5.3) |
| Baseline MMAS Score | 1.3 (1.3) |
Note: RBANS = Repeatable Battery for the Assessment of Neuropsychological Status, MADRS = Montgomery Asberg Depression Rating Scale, YMRS = Young Mania Rating Scale, MMAS = Morisky Medication Adherence Scale.
Average sleep duration and variability
The average mean sleep value of participants in this study was 2.89 (SD=0.81). Older patients had a shorter mean sleep duration, but sleep duration was not correlated with sex, minority status, and education or any baseline measures (symptoms, medication adherence, cognitive functioning) (Table 2). Younger patients and patients with more severe scores at baseline on the MADRS, YMRS, and MMAS showed more variability in sleep duration. People with higher sleep duration variability also had higher variability in ratings of daily energy level, sad or depressed affect, and impulsivity. Similarly, patients with higher sleep duration variability across the evaluation period also had greater mean daily ratings of being angry or upset, anxious or nervous, stressed, and impulsive over the same time frame (Table 3). In contrast, there were no correlations between mean sleep duration and mean and standard deviation of daily positive and negative affect ratings.
Table 2.
Correlations of mean and standard deviation of sleep duration with baseline measures
| Mean Sleep Duration | Sleep Duration Standard Deviation | |
|---|---|---|
| Spearman's Rho | Spearman's Rho | |
| Age | −.39, p=.016 | −.45, p=.004 |
| Sex, t-test | t=.64, p=.525 | t=−.40, p=.692 |
| Minority status, t-test | t=1.96, p=.058 | t=.22, p=.823 |
| Education (Years) | .07, p=.693 | −.14, p=.418 |
| Baseline RBANS Total Score | .10, p=.566 | −.10, p=.532 |
| Baseline MADRS Total Score | .06, p=.712 | .37, p=.022 |
| Baseline YMRS Total Score | −.28, p=.091 | .39, p=.016 |
| Baseline MMAS Score | −.01, p=.964 | .40, p=.012 |
Note: All statistics reported are from Spearman correlations unless otherwise specified; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status, MADRS = Montgomery Asberg Depression Rating Scale, YMRS = Young Mania Rating Scale, MMAS = Morisky Medication Adherence Scale; Bolded results are statistically significant at p<0.05.
Table 3.
Correlations of mean and standard deviation of sleep duration with concurrent affect ratings
| Mean Sleep Duration | Sleep Duration Standard Deviation | |
|---|---|---|
| Spearman's Rho | Spearman's Rho | |
| Positive Affect | ||
| How relaxed are you? | ||
| Mean | .20, p=.206 | −.15, p=.334 |
| Standard Deviation | −.07, p=.666 | .12, p=.444 |
| How happy are you? | ||
| Mean | .05, p=.733 | −.13, p=.413 |
| Standard Deviation | −.16, p=.314 | .20, p=.222 |
| How energetic are you? | ||
| Mean | −.18, p=.268 | −.29, p=.066 |
| Standard Deviation | −.10, p=.519 | .38, p=.015 |
| Negative Affect | ||
| How sad or depressed are you? | ||
| Mean | .07, p=.685 | .26, p=.103 |
| Standard Deviation | −.10, p=.518 | .36, p=.021 |
| How angry or upset are you? | ||
| Mean | −.05, p=.744 | .34, p=.031 |
| Standard Deviation | −.13, p=.432 | .18, p=.253 |
| How anxious or nervous are you? | ||
| Mean | .15, p=.366 | .34, p=.032 |
| Standard Deviation | .10, p=.528 | .26, p=.107 |
| How stressed are you? | ||
| Mean | .11, p=.484 | .33, p=.034 |
| Standard Deviation | .00, p=.983 | .20, p=.207 |
| How impulsive do you feel? | ||
| Mean | −.06, p=.729 | .38, p=.014 |
| Standard Deviation | −.21, p=.182 | .34, p=.029 |
Note: All correlations reported are from Spearman correlations; Bolded results are statistically significant at p<0.05
Day-to-day sleep duration and atypicality
We examined the same-day and next-day effects of sleep duration and atypicality of duration on affect. Compared to participants who reported 7-8 hours of sleep, participants who reported <5 hours of sleep the night before also reported lower same-day ratings of positive affect, and higher same-day ratings on negative affect (with the exception of impulsivity ratings) (Table 4). Participants who reported 5-6 hours of sleep also reported lower same-day ratings of feeling relaxed and happy, but higher same-day ratings on feeling stressed. Participants who reported 9-10 hours of sleep reported lower same-day ratings on feeling happy and energetic, and people reporting >10 hours of sleep reported lower same-day ratings on feeling energetic, but higher ratings for sad or depressed affect, compared to people reporting 7-8 hours of sleep. Fewer effects were seen when examining next-day affect ratings, participants reporting <5 hours reported lower next-day scores on relaxed and energetic compared to people reporting 7-8 hours. People reporting 9-10 hours of sleep reported lower next-day ratings of angry or upset, anxious or nervous, and impulsive.
Table 4.
Association between daily sleep duration and positive and negative affect ratings.
| Positive Affect | Negative Affect | |||||||
|---|---|---|---|---|---|---|---|---|
| Relaxed | Happy | Energetic | Sad or Depressed | Angry or Upset | Anxious or Nervous | Stressed | Impulsive | |
| Sleep Duration | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
| Same Day | ||||||||
| <5 hours | −0.58 (−0.84, −0.33) | −0.39 (−0.62, −0.16) | −0.35 (−0.60, −0.11) | 0.27 (0.03, 0.51) | 0.25 (0.02, 0.48) | 0.31 (0.06, 0.55) | 0.37 (0.11, 0.62) | 0.15 (−0.07, 0.36) |
| 5-6 hours | −0.33 (−0.53, −0.12) | −0.20 (−0.38, −0.02) | −0.15 (−0.34, 0.05) | 0.06 (−0.13, 0.25) | 0.09 (−0.09, 0.27) | 0.18 (−0.01, 0.38) | 0.23 (0.02, 0.43) | 0.13 (−0.04, 0.29) |
| 7-8 hours | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 9-10 hours | 0.11 (−0.10, 0.33) | −0.20 (−0.39, −0.01) | −0.22 (−0.42, −0.01) | 0.14 (−0.06, 0.34) | −0.10 (−0.29, 0.09) | 0.01 (−0.20, 0.21) | −0.02 (−0.23, 0.19) | −0.10 (−0.27, 0.08) |
| >10 hours | 0.25 (−0.02, 0.52) | −0.17 (−0.41, 0.08) | −0.53 (−0.80, −0.27) | 0.26 (0.01, 0.52) | −0.07 (−0.31, 0.17) | −0.09 (−0.35, 0.17) | −0.09 (−0.36, 0.18) | −0.11 (−0.33, 0.12) |
|
Next Daya | ||||||||
| <5 hours | −0.29 (−0.58, −0.01) | −0.19 (−0.44, 0.07) | −0.30 (−0.58, −0.02) | 0.05 (−0.23, 0.32) | 0.14 (−0.12, 0.39) | 0.10 (−0.17, 0.37) | 0.11 (−0.18, 0.39) | 0.05 (−0.19, 0.29) |
| 5-6 hours | −0.06 (−0.29, 0.16) | 0.09 (−0.11, 0.29) | −0.08 (−0.30, 0.14) | −0.15 (−0.37, 0.06) | −0.01 (−0.22, 0.19) | −0.20 (−0.41, 0.02) | −0.08 (−0.31, 0.14) | −0.04 (−0.23, 0.15) |
| 7-8 hours | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 9-10 hours | 0.19 (−0.05, 0.42) | 0.17 (−0.04, 0.38) | 0.02 (−0.21, 0.25) | −0.22 (−0.45, 0.00) | −0.21 (−0.42, 0.00) | −0.40 (−0.62, −0.17) | −0.17 (−0.40, 0.06) | −0.21 (−0.41, −0.01) |
| >10 hours | −0.01 (−0.31, 0.30) | −0.15 (−0.42, 0.13) | −0.03 (−0.33, 0.26) | 0.00 (−0.29, 0.30) | 0.00 (−0.27, 0.27) | −0.27 (−0.56, 0.02) | −0.18 (−0.48, 0.12) | −0.07 (−0.32, 0.19) |
Note: All statistics come from multi-level models adjusted for participants and days on study as random effects; β=beta coefficient for fixed effect of sleep duration on positive or negative affect; CI = Confidence Interval; bolded values are statistically significant at p<0.05.
The sleep duration value was lagged one day.
Finally, we examined whether daily deviations from one's average sleep level were related to same-day and next-day affect. Few effects emerged. Greater atypicality of sleep duration predicted lower same-day ratings of feeling energetic, but higher same-day rating of sad or depressed affect. For next-day ratings of affect, people with greater atypicality of reported sleep duration 2 nights before reported lower ratings of anxious or nervous affect.
Secondary analyses
To assess if sleep duration ratings were related to the previous day's affect ratings (and thus possibly confounding the relationships we observed with the current day's affect) we conducted similar analyses to the lagged day-to-day analyses described above, but instead set the outcome as affect from the previous day. For duration analyses, there were no relationships with happiness, sadness/depression, anger, impulsivity or energy from the previous day; only previous day ratings of relaxation, anxiousness/nervousness, and stress were related to duration of sleep. Specifically, compared to those reporting 7-8 hours of sleep, those reporting 9-10 hours reported the previous day feeling more relaxed (B=0.24; 95% CI=0.00, 0.47; p=0.048), less anxious or nervous (B=−0.25; 95% CI=−0.48, −0.03; p=0.027), and less stressed (B=−0.31; 95% CI=−0.54, −0.07; p=0.010), and those reporting >10 hours reported being less anxious (B=−0.30; 95% CI=−0.59, −0.02; p=0.036). No associations were observed for atypicality analyses, suggesting that the previous day's affect did not influence the atypicality of sleep duration (or the extent to which that night's sleep duration was unusual).
DISCUSSION
This study assessed how sleep duration and variability are related to mood symptoms, medication adherence, cognitive functioning and daily affect using ecological momentary assessment. We found greater person-averaged sleep variability was associated with greater depression and mania symptom severity and with lower self-reported medication adherence as measured at baseline, but not with global cognitive function. In other words, more severely symptomatic and less adherent patients reported more variability in sleep duration over a subsequent 11-week period. People with higher variability in sleep duration also had higher person-averaged levels of most of the negative affective states assessed, as well as greater variability in daily ratings of energy, sad mood, and impulsivity measured over the same evaluation period. In contrast, person-averaged sleep duration bore few associations with baseline characteristics or the mean or variability of affect ratings across the study. We also found patients who reported sleep durations shorter than 7-8 hours or >10 hours in the preceding night also reported lower positive affect and higher negative affect on that survey. However, sleep duration was not generally associated with ratings of affect made the morning of the next day (i.e., after an intervening night of sleep). The degree to which a given night's sleep was atypical for the patient was not strongly associated with same-day or next-day positive or negative affect. Taken together, the daily impact of sleep duration on affective ratings was somewhat transient, whereas the cumulative impact of sleep variability was more clinically relevant. Consistently sleeping very few or very many hours over the study period, however, did not seem to be related to baseline symptoms or concurrent affect.
Findings extend previous studies showing sleep variability is associated with mood symptoms (Gruber et al., 2011; Saunders et al., 2015). However, while we found overall variability in sleep duration was associated with more negative and less positive affect ratings, day-to-day atypicality relative to one's average sleep duration was not associated with day-to-day affect ratings. Duration of sleep overall did not impact outcomes, while day-to-day sleep duration was related to impaired same day positive and negative affect. Overall, shorter sleep duration was associated with higher same-day negative affect and lower same-day positive affect. This finding confirms laboratory studies examining sleep deprivation on mood in healthy samples, which show greater levels of depression and anxiety, as well as increased agitation with fewer hours of sleep (Franzen et al., 2008). Surprisingly, with the exception of happy, energetic, and sad or depressed, we did not see worse ratings of affect for hours of sleep >8 hours. Some studies have shown that health outcomes (primarily related to physical health) with sleep duration follow a U-shaped curve with worse outcomes occurring for both short and long sleep duration (Ohkuma et al., 2014). Studies using objective measures of sleep (e.g., wrist actigraphy) are needed to more accurately assess the impact of long-duration sleep.
The reason sleep duration overall did not impact overall affect and scores on psychological assessments is puzzling in light of studies linking sleep and mood (Barbini et al., 1996; Fava and Kellner, 1991; Gruber et al., 2011; Jackson et al., 2003; Perlman et al., 2006). One reason may be that sleep in past studies was measured globally, whereas our study averaged multiple daily sleep duration ratings. Additionally, our study was over a longer period as compared to studies that examining daily sleep over a one- or two-week interval, resulting in an average sleep duration that may be agnostic to external factors affecting sleep. Regardless, our study demonstrates the importance of examining sleep in bipolar disorder over longer follow-up time periods.
A novel finding was that sleep variability was negatively correlated with medication adherence. Clearly, regular maintenance of medications is key to sustaining successful treatment outcomes (Montes et al., 2013), so poor adherence can substantially worsen bipolar symptomatology. Adherence improvement has been an intense area of research in recent years (Clatworthy et al., 2009; Colom et al., 2005). It is unclear if patients who are less adherent experience less regular sleep due to diminished exposure to medication, or if sleep variability leads to diminished adherence. To the latter possibility, sleep variability may limit the degree to which behavioral routines around medications are maintained, particularly given that medications are frequently linked to morning and nighttime administrations (Wagner and Ryan, 2004).
Findings from this study have several clinical implications. The frequent clinical question “How many hours of sleep do you typically get?” may be insensitive to psychopathology and related morbidity in bipolar disorder. Structured self-report surveys of sleep frequently elicit average sleep duration but not day to day variability in sleep. Sleep variability may be important to assess in routine clinical settings. Given the challenge of retrospective estimation of sleep variability, daily monitoring of sleep through mobile technology may provide useful data to clinicians. Daily diaries, or tracking of sleep duration and quality, have been shown to be useful clinical tools. Recently, there has been a growth in consumer-based personal fitness trackers that provide measurements of daily activity (i.e., steps) and sleep quality (Voets, 2013). While these products have been marketed toward healthy populations, there is growing interest in using these in clinical settings (Albert et al., 2014; Cook et al., 2013) and with bipolar patients, in particular (Puiatti et al., 2011). Findings are also consistent with tenets of Interpersonal and Social Rhythms Therapy (IPSRT), that train regularization of social activity and sleep timing (Frank et al., 2007). Clinical trials of IPSRT indicate that increasing regularization is associated with a preventative impact on mood episode recurrence in patients with bipolar I disorder (Frank et al., 2005). There are also medications and other chronotherapies shown to be highly effective in regulating circadian rhythms (Dallaspezia and Benedetti, 2015), possibly resulting in less variable sleep durations. It is possible that a moving average of variability obtained through unobtrusive self-monitoring devices could inform or enhance such therapies.
This study's strengths include the longitudinal design over a longer duration than previous studies and concurrent ratings of sleep duration/variability and affect. However, this study has limitations. First, we lacked sleep disturbance indicators beyond duration. For example, we did not assess the quality of sleep or more specific sleep difficulties (e.g., trouble with falling asleep or staying asleep). Sleep duration was self-reported, which, although collected shortly after awakening, is still subject to reporting biases. In addition, there was missing data on self-reported sleep and affect (yet the proportion of missing data was not associated with average sleep duration and variability). Second, data on sleep duration were not collected using objective sleep measurements such as actigraphy. Incorporating such measurements may reduce the potential for subjective biases in self-reported data as well as the likelihood of missing data. Third, we did not assess napping during the day. Napping may have influenced how much sleep the participant obtained during the night. Notably, one study found napping during the day to decrease total sleep time by 48 minutes compared to no napping conditions (Monk et al., 2001). Fourth, our sample were outpatients who experienced mild-moderate depression and minimal mania symptoms, on average. Therefore, these findings may not generalize to more severely ill samples. On a related note, the majority of our participants were Bipolar I patients. It is possible that the effects of sleep duration on affect differ for those diagnosed with Bipolar I than for those diagnosed with Bipolar II. For example, one study found that insomnia was associated with depression in Bipolar II patients, whereas hypersomnia was associated with depression in Bipolar I patients (Steinan et al., 2016). Unfortunately, because so few of our participants were diagnosed with Bipolar II, we did not have adequate power to detect such differences. Fifth, participants were all enrolled in a clinical trial. Although the intervention examined in the clinical trial did not directly target sleep, involvement in the trial may have altered participants’ reported affect and may also have limited generalizability of current findings to untreated participants.
Despite this study's limitations, findings add to literature on the relationship of sleep duration variability to clinical severity, treatment adherence, and day-to-day affective experience among people with bipolar disorder. New models of assessing and regularizing sleep variability (including mobile technology), may help improve a variety of outcomes in bipolar disorder.
Table 5.
Association between daily atypicalitya from mean sleep duration and ratings of positive and negative affect.
| Positive Affect | Negative Affect | |||||||
|---|---|---|---|---|---|---|---|---|
| Relaxed | Happy | Energetic | Sad or Depressed | Angry or Upset | Anxious or Nervous | Stressed | Impulsive | |
| Sleep Atypicality | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
| Same Day | 0.01 (−0.05, 0.06) | −0.01 (−0.06, 0.04) | −0.07 (−0.12, −0.02) | 0.07 (0.01, 0.12) | 0.01 (−0.04, 0.06) | 0.02 (−0.03, 0.07) | 0.02 (−0.03, 0.08) | 0.01 (−0.04, 0.06) |
| Next Dayb | −0.01 (−0.07, 0.05) | −0.02 (−0.07, 0.03) | −0.02 (−0.08, 0.04) | 0.00 (−0.06, 0.06) | −0.03 (−0.08, 0.02) | −0.07 (−0.12, −0.01) | −0.05 (−0.10, 0.01) | −0.01 (−0.06, 0.04) |
Note: All statistics come from multi-level models adjusted for participants and days on study as random effects; β=beta coefficient for fixed effect of variation of sleep duration on positive or negative affect; CI = Confidence Interval; bolded values are statistically significant at p<0.05.
Daily atypicality from average sleep duration defined by subtracting participant's mean sleep value from their daily sleep rating and squaring the result.
The sleep variability value was lagged one day.
Acknowledgments
This study received funding from the National Institute of Mental Health (Grant #'s: T32MH019934, K23MH077225, R34MH091260, R01MH100417, K01MH100433, R01MH103318). The funding agencies listed did not have any role in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Dr. Kaufmann wrote the manuscript and conducted statistical analyses. Dr. Gershon wrote the manuscript and provided feedback on statistical analyses. Dr. Eyler wrote the manuscript and provided feedback on statistical analyses. Dr. Depp wrote the manuscript, provided feedback on statistical analyses, and served as the parent study Principle Investigator. All authors have approved the final submitted article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albert MV, Deeny S, McCarthy C, Valentin J, Jayaraman A. Monitoring daily function in persons with transfemoral amputations using a commercial activity monitor: a feasibility study. PM R. 2014;6:1120–1127. doi: 10.1016/j.pmrj.2014.06.006. doi:10.1016/j.pmrj.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Barbini B, Bertelli S, Colombo C, Smeraldi E. Sleep loss, a possible factor in augmenting manic episode. Psychiatry Res. 1996;65:121–125. doi: 10.1016/s0165-1781(96)02909-5. [DOI] [PubMed] [Google Scholar]
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord. 2006;8:160–167. doi: 10.1111/j.1399-5618.2006.00294.x. doi:10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Boland EM, Alloy LB. Sleep disturbance and cognitive deficits in bipolar disorder: toward an integrated examination of disorder maintenance and functional impairment. Clin Psychol Rev. 2013;33:33–44. doi: 10.1016/j.cpr.2012.10.001. doi:10.1016/j.cpr.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy F, Murry E, Forest K, Carroll BJ. Signs and symptoms of mania in pure and mixed episodes. J Affect Disord. 1998;50:187–201. doi: 10.1016/s0165-0327(98)00016-0. [DOI] [PubMed] [Google Scholar]
- Clatworthy J, Bowskill R, Parham R, Rank T, Scott J, Horne R. Understanding medication non-adherence in bipolar disorders using a Necessity-Concerns Framework. J Affect Disord. 2009;116:51–55. doi: 10.1016/j.jad.2008.11.004. doi:10.1016/j.jad.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Colom F, Vieta E, Tacchi MJ, Sánchez-Moreno J, Scott J. Identifying and improving non-adherence in bipolar disorders. Bipolar Disord 7 Suppl. 2005;5:24–31. doi: 10.1111/j.1399-5618.2005.00248.x. doi:10.1111/j.1399-5618.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Thompson JE, Prinsen SK, Dearani JA, Deschamps C. Functional recovery in the elderly after major surgery: assessment of mobility recovery using wireless technology. Ann. Thorac. Surg. 2013;96:1057–1061. doi: 10.1016/j.athoracsur.2013.05.092. doi:10.1016/j.athoracsur.2013.05.092. [DOI] [PubMed] [Google Scholar]
- Dallaspezia S, Benedetti F. Chronobiology of bipolar disorder: therapeutic implication. Curr Psychiatry Rep. 2015;17:606. doi: 10.1007/s11920-015-0606-9. doi:10.1007/s11920-015-0606-9. [DOI] [PubMed] [Google Scholar]
- Depp CA, Ceglowski J, Wang VC, Yaghouti F, Mausbach BT, Thompson WK, Granholm EL. Augmenting psychoeducation with a mobile intervention for bipolar disorder: a randomized controlled trial. J Affect Disord. 2015;174:23–30. doi: 10.1016/j.jad.2014.10.053. doi:10.1016/j.jad.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp CA, Kim DH, de Dios LV, Wang V, Ceglowski J. A pilot study of mood ratings captured by mobile phone versus paper-and-pencil mood charts in bipolar disorder. J Dual Diagn. 2012;8:326–332. doi: 10.1080/15504263.2012.723318. doi:10.1080/15504263.2012.723318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Drake CL, Roehrs TA, Burduvali E, Bonahoom A, Rosekind M, Roth T. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology. 2001;38:979–987. doi: 10.1111/1469-8986.3860979. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD, Cusack R, Ogilvie AD. Categorical and dimensional reports of experienced affect to emotion-inducing pictures in depression. J Abnorm Psychol. 2004;113:654–660. doi: 10.1037/0021-843X.113.4.654. doi:10.1037/0021-843X.113.4.654. [DOI] [PubMed] [Google Scholar]
- Fava GA, Kellner R. Prodromal symptoms in affective disorders. Am J Psychiatry. 1991;148:823–830. doi: 10.1176/ajp.148.7.823. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, Grochocinski V, Houck P, Scott J, Thompson W, Monk T. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch. Gen. Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. doi:10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- Frank E, Swartz HA, Boland E. Interpersonal and social rhythm therapy: an intervention addressing rhythm dysregulation in bipolar disorder. Dialogues Clin Neurosci. 2007;9:325–332. doi: 10.31887/DCNS.2007.9.3/efrank. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. J Sleep Res. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. doi:10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon A, Thompson WK, Eidelman P, McGlinchey EL, Kaplan KA, Harvey AG. Restless pillow, ruffled mind: sleep and affect coupling in interepisode bipolar disorder. J Abnorm Psychol. 2012;121:863–873. doi: 10.1037/a0028233. doi:10.1037/a0028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Tamminga CA, Tohen M, Suppes T. The relationship between affective state and the rhythmicity of activity in bipolar disorder. J Clin Psychiatry. 2014;75:e317–22. doi: 10.4088/JCP.13m08506. doi:10.4088/JCP.13m08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens PJJ, Kupka RW, Beentjes TAA, van Achterberg T. Recognising prodromes of manic or depressive recurrence in outpatients with bipolar disorder: a cross-sectional study. Int J Nurs Stud. 2010;47:1201–1207. doi: 10.1016/j.ijnurstu.2010.01.010. doi:10.1016/j.ijnurstu.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Gruber J, Miklowitz DJ, Harvey AG, Frank E, Kupfer D, Thase ME, Sachs GS, Ketter TA. Sleep matters: sleep functioning and course of illness in bipolar disorder. J Affect Disord. 2011;134:416–420. doi: 10.1016/j.jad.2011.05.016. doi:10.1016/j.jad.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarnà A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. doi:10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Talbot LS, Gershon A. Sleep disturbance in bipolar disorder across the lifespan. Clin Psychol (New York) 2009;16:256–277. doi: 10.1111/j.1468-2850.2009.01164.x. doi:10.1111/j.1468-2850.2009.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. J Affect Disord. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7:176–186. doi: 10.1111/j.1399-5618.2005.00187.x. doi:10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Millar A, Espie CA, Scott J. The sleep of remitted bipolar outpatients: a controlled naturalistic study using actigraphy. J Affect Disord. 2004;80:145–153. doi: 10.1016/S0165-0327(03)00055-7. doi:10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Carrier J, Billy BD, Rose LR. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001;24:680–687. doi: 10.1093/sleep/24.6.680. [DOI] [PubMed] [Google Scholar]
- Montes JM, Maurino J, de Dios C, Medina E. Suboptimal treatment adherence in bipolar disorder: impact on clinical outcomes and functioning. Patient Prefer Adherence. 2013;7:89–94. doi: 10.2147/PPA.S39290. doi:10.2147/PPA.S39290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- National Heart, Lung, and Blood Institute [10.22.15];2012 http://www.nhlbi.nih.gov/health/health-topics/topics/sdd/howmuch/
- Ohkuma T, Fujii H, Iwase M, Ogata-Kaizu S, Ide H, Kikuchi Y, Idewaki Y, Jodai T, Hirakawa Y, Nakamura U, Kitazono T. U-shaped association of sleep duration with metabolic syndrome and insulin resistance in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Metab. Clin. Exp. 2014;63:484–491. doi: 10.1016/j.metabol.2013.12.001. doi:10.1016/j.metabol.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disord. 2006;8:271–274. doi: 10.1111/j.1399-5618.2006.00330.x. doi:10.1111/j.1399-5618.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- Phillips KD, Moneyham L, Murdaugh C, Boyd MR, Tavakoli A, Jackson K, Vyavaharkar M. Sleep disturbance and depression as barriers to adherence. Clin Nurs Res. 2005;14:273–293. doi: 10.1177/1054773805275122. doi:10.1177/1054773805275122. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Kupfer DJ. Bipolar disorder diagnosis: challenges and future directions. Lancet. 2013;381:1663–1671. doi: 10.1016/S0140-6736(13)60989-7. doi:10.1016/S0140-6736(13)60989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puiatti A, Mudda S, Giordano S, Mayora O. Presented at the 2011 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2011. Smartphone-centred wearable sensors network for monitoring patients with bipolar disorder; pp. 3644–3647. doi:10.1109/IEMBS.2011.6090613. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. doi:10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Saunders EFH, Fernandez-Mendoza J, Kamali M, Assari S, McInnis MG. The effect of poor sleep quality on mood outcome differs between men and women: A longitudinal study of bipolar disorder. J Affect Disord. 2015;180:90–96. doi: 10.1016/j.jad.2015.03.048. doi:10.1016/j.jad.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl. 1998;20 22–33–quiz 34–57. [PubMed] [Google Scholar]
- Steinan MK, Scott J, Lagerberg TV, Melle I, Andreassen OA, Vaaler AE, Morken G. Sleep problems in bipolar disorders: more than just insomnia. Acta Psychiatr Scand. 2016;133:368–377. doi: 10.1111/acps.12523. doi:10.1111/acps.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets J. [11.30.15];Study Shows that Over 252 Million Activity Trackers Will Ship Globally in the Coming Five Years. 2013 http://numrush.com/2013/05/17/study-shows-that-over-252-million-activity-trackers-will-ship-globally-in-the-coming-five-years/
- Wagner GJ, Ryan GW. Relationship between routinization of daily behaviors and medication adherence in HIV-positive drug users. AIDS Patient Care STDS. 2004;18:385–393. doi: 10.1089/1087291041518238. doi:10.1089/1087291041518238. [DOI] [PubMed] [Google Scholar]
- Williams JBW, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192:52–58. doi: 10.1192/bjp.bp.106.032532. doi:10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]