Abstract
Diabetes is increasing in prevalence and is the leading cause of end-stage renal disease in the United States. Diabetic kidney disease is considered a proteinuric glomerular disease. Although the glomerulus is composed of various cell types, research suggests that podocytes are most important to overall glomerular health. Podocyte injury has been identified as a pivotal event resulting in proteinuric kidney disease, glomerulosclerosis, and loss of renal function. Thus, understanding the signaling mechanisms that trigger podocyte injury in diabetic kidney disease might allow for the development of targeted therapeutics to prevent or ameliorate progression to end-stage renal failure. This review focuses on the role of podocytes in diabetic kidney disease.
Keywords: Diabetes, podocytes, albuminuria, Notch, glomerulosclerosis
INTRODUCTION
Diabetic kidney disease (DKD) is the most common cause of kidney failure in the United States, and is responsible for more than 40% of new end-stage renal disease cases [1-3]. DKD develops in 30-40% of patients with diabetes mellitus [1]. Due to the greater prevalence of T2DM, these patients constitute the majority of DKD cases and represent the bulk of diabetic patients on dialysis [4]. Poor glycemic control, hypertension, smoking, and family history increase one’s risk of nephropathy development [5-8]. DKD, like diabetic retinopathy, is a microvascular complication of diabetes [9-11].
Diabetes causes functional changes in the kidney including hyperfiltration, albuminuria, and decline in glomerular filtration rate (GFR). Clinically, DKD is diagnosed by the presence of albuminuria in patients with long-standing histories of diabetes. Albuminuria is not only the earliest marker of diabetic renal pathology, but is also independently associated with increased cardiovascular morbidity and mortality and is possibly the best predictor of later decline in GFR [12, 13].
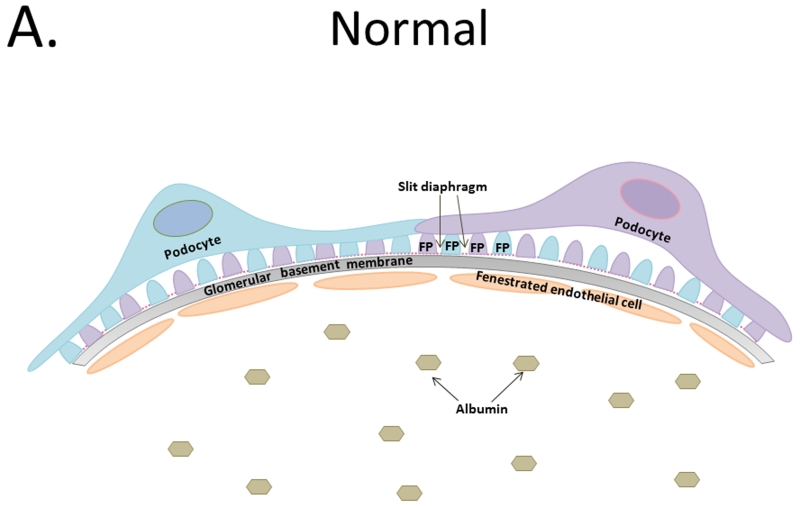
DKD is a glomerular disease that disrupts the glomerular filtration barrier (GFB). This barrier is a tripartite system that consists of fenestrated endothelial cells, the glomerular basement membrane (GBM), and terminally differentiated epithelial cells termed podocytes that work in tandem to allow for selective filtration of water and solutes, while restricting the flow of large macromolecules like albumin (Figure 1a.). Histological changes in the glomerulus are relatively specific for diabetes and they can be used to diagnose DKD. Early changes to the glomeruli are characterized by basement membrane thickening, followed by mesangial expansion and nodular sclerosis [14, 15]. Glomerulosclerosis is frequently associated with tubulointerstitial fibrosis, arterial hyalinosis, and a progressive decrease in GFR [16, 17].
Figure 1.
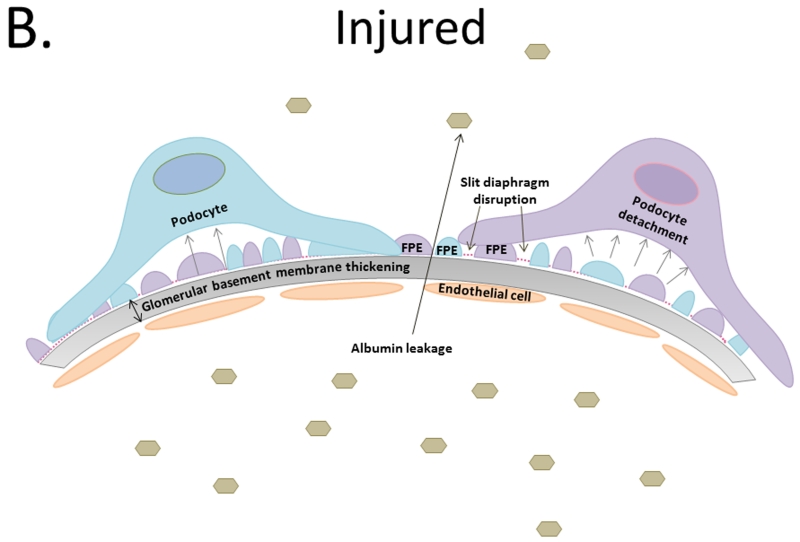
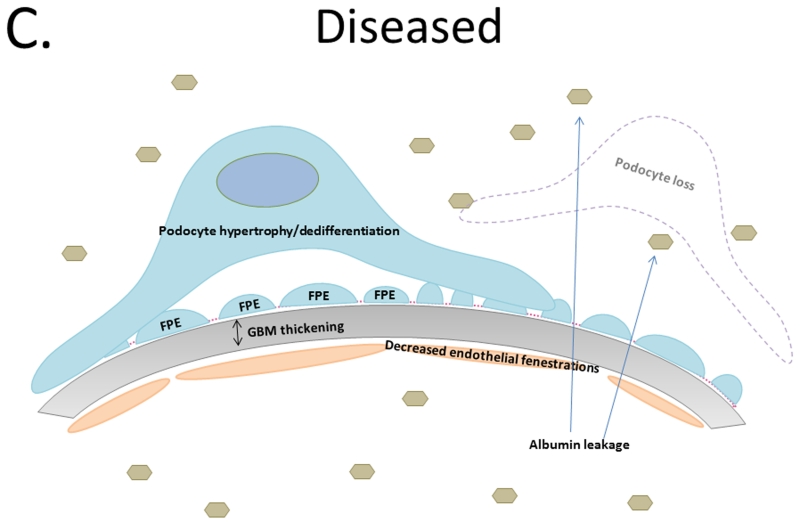
A. Tripartite glomerular filtration barrier includes fenestrated glomerular endothelial cells, glomerular basement membrane, and podocytes. Foot processes interdigitate with neighboring podocyte foot processes creating slit diaphragms. B. Diabetic kidney disease results in glomerular basement membrane thickening and injury to the podocytes depicted by foot process effacement, detachment, and disruption of the slit diaphragm resulting in albumin leakage across the glomerular filtration barrier. C. Podocyte loss results in hypertrophy and/or dedifferentiation, increase albuminuria, and functional kidney decline.
DIABETIC MILIEU
The duration of diabetes strongly correlates with the risk of DKD development. Hyperglycemia can be directly toxic to cells, primarily via the increased generation of reactive oxygen radicals. In addition, prolonged hyperglycemia promotes the generation of advanced glycation end products (AGEs) which bind to AGE receptors (RAGEs) opening the floodgate of deleterious downstream signals including increased reactive oxygen species (ROS) production, inflammatory cell activation, inappropriate increase of angiotensin II (Ang II), and release of growth factors [18]. These products and factors, in various permutations, contribute to DKD pathogenesis and associated loss of filtration capacity.
The renin angiotensin system (RAS) is one of the most important pathways in DKD pathophysiology. Hyperglycemia, AGEs, and mechanical stress increase Ang II, resulting in proteinuria, vascular endothelial and transforming growth factor stimulation, and later, decrease in GFR [19]. Treatment of DKD patients with angiotensin receptor blockers or angiotensin converting enzyme inhibitors, which directly target the RAS, are currently the only medical therapies available that can ameliorate proteinuria and delay the progression of chronic kidney disease [20, 21].
One growth factor altered by the diabetic environment is vascular endothelial growth factor-A (VEGF-A), a podocyte-derived signaling protein. VEGF-A is not only required for the development of normal glomerular capillaries and their fenestrated endothelial phenotype, but also necessary for podocyte maintenance via Akt signaling. In the early stages of DKD, hyperglycemia inhibits endothelial cell production of nitric oxide, thus stimulating podocytes to produce excess VEGF-A to induce growth and proliferation of mesangial and endothelial cells as a compensatory mechanism. However, as DKD progresses, decreased podocyte number results in reduced VEGF-A production, affecting endothelial fenestration, GBM composition, and podocyte health [22-24].
Transforming growth factor-beta (TGF-β) is a hypertrophic, pro-sclerotic cytokine that can be stimulated by many elements of the diabetic milieu including hyperglycemia, Ang II, AGEs, VEGF-A, and ROS [25]. Through Smad signaling, activation of TGF-β in mesangial cells results in renal hypertrophy, accumulation of mesangial extracellular matrix (ECM) components, GBM thickening, and podocyte detachment [26]. Furthermore, TGF-β can increase VEGF-A production perpetuating the cycle of glomerular damage [27]. Current research is focused on therapies that specifically target these signaling pathways to deter the progression of DKD [28].
GBM THICKENING: AN EARLY SIGN OF DIABETIC KIDNEY DISEASE
Glomerular basement thickening is one of the earliest histopathological findings in DKD, and is attributed to the abnormal turnover of ECM proteins [15, 29]. This process, in combination with mesangial expansion, contributes to glomerular hypertrophy [30]. In patients with T1DM, GBM thickening could be detected as early as 1.5 years after T1DM onset, followed by mesangial expansion at 5 years [29]. GBM changes are more heterogeneous in T2DM patients. This has partially been attributed to various comorbid conditions including atherosclerosis and hypertension [17, 31].
Normal GBM is composed of type-IV collagen (mainly α3, α4, and α5), laminin, fibronectin, entactin, and proteoglycans [30, 32]. In DKD there is increased expression and accumulation of type-IV α3 and α4 collagen, and decreased heparan sulfate proteoglycan [33, 34]. Mouse podocytes cultured in high extracellular glucose directly increased specific collagen mRNA and protein production, likely directly contributing to GBM thickening [27, 35]. Other mediators of DKD, such as Ang II and TGF-β, have similar effects on matrix production [35, 36]. In addition to direct synthesis of new collagen, alterations in collagen turnover also contribute to GBM thickening. Matrix metalloproteinases (MMPs) play essential roles in the degradation and remodeling of type-IV collagen. Urinary MMP-9 is increased in DKD patients and correlates with the degree of albuminuria [37]. Ultimately, hyperglycemia causes an imbalance between the synthesis and degradation of ECM components. GBM thickening is not only an early DKD finding: it may also predict which patients with diabetes will eventually develop progressive nephropathy [17, 38].
PODOCYTES
The role of podocytes in DKD pathogenesis became prominent after a landmark observational study by Pagtalunan et al. in which it was demonstrated that reduced podocyte number correlated strongly with albuminuria and loss of GFR in Pima Indians with T2DM [39]. Multiple other clinical studies have since confirmed the correlation between podocyte loss, proteinuria, and glomerulosclerosis, indicating that podocyte loss is potentially a key factor that contributes to DKD progression [23, 40, 41]. Podocytes are terminally differentiated epithelial cells; their loss represents an irreversible event that leads to a decline in GFB function [42].
Podocytes are highly specialized cells that consist of a cell body, primary processes, and branching foot processes. Podocytes encircle glomerular capillaries while their foot processes interdigitate with neighboring podocyte foot processes, creating evenly spaced areas covered by slit-diaphragm (SD) proteins that allow for podocyte-to-podocyte contact. The SD is a multi-protein complex involved in blood filtration and podocyte signal transduction [43]. Injury, stress, or changes in the environmental milieu can result in podocyte cytoskeletal rearrangement reflected by foot-process flattening, widening, and retraction. This phenomenon is termed “effacement” and signifies podocyte injury (Figure 1b) [44]. Foot process effacement (FPE) disrupts the tripartite GFB by weakening its integrity, resulting in albuminuria. FPE is the sine qua non of albuminuria and is seen in all conditions associated with it.
MECHANISM OF EFFACEMENT: NEPHRIN
Disruption of SD proteins such as nephrin in DKD has been suggested to play a role in albuminuria. Nephrin is a transmembrane protein that functions as both a structural and signaling protein in podocytes. Its extracellular domain forms the SD zipper-like structure, while its intracellular domain functions as a signaling molecule. Nephrin is not only an essential protein in the SD complex, but is also involved in podocyte survival [45]. Nephrin mutation in humans causes congenital nephrotic syndrome of the Finnish type [46]. Acquired loss of nephrin is a sensitive marker of podocyte injury.
Nephrin may contribute to the pathogenesis of DKD in several ways. First, nephrin functions as an intracellular signaling scaffold that recruits proteins, such as phosphoinositide-3-kinase, the Src family kinase Fyn, and phospholipase Cγ1 to its tyrosine phosphorylated cytoplasmic tail [47-50]. Binding of these proteins allows nephrin to regulate the podocyte cytoskeleton through Nck adaptor proteins [50-52]. In DKD patients, nephrin mRNA and protein expression are decreased, and recent studies using fly-model systems suggest that Ebf2 transcription plays an important role in hyperglycemia-induced nephrin regulation [53]. This dysregulation of nephrin production might result in aberrant actin rearrangement and breakdown of the SD with resulting FPE [54].
Second, nephrin may be important in regulating podocyte insulin sensitivity. Nephrin’s cytoplasmic domain enables the docking of insulin receptors GLUT1 and GLUT4 with vesicle-associated membrane protein-2, enabling insulin recognition and intracellular signaling [55]. In normoglycemic conditions, podocyte-specific insulin-receptor knockout mice developed albuminuria and histopathological features similar to DKD [54, 56]. Furthermore, Ang II and VEGF have been shown to cause nephrin downregulation [54, 57]. In animal studies, irbesartan, an Ang II type-1 receptor antagonist, was effective in normalizing nephrin levels and decreasing albuminuria [50, 58]. Nephrin is functionally important in podocyte cytoskeletal and insulin signaling, as well as in maintaining overall podocyte health [45, 50, 58].
MECHANISM OF EFFACEMENT: RHO-GTPases
The actin cytoskeletal changes that take place in injured podocytes ultimately result in FPE. The Rho-family of small GTPases, RhoA, Cdc42, and Rac1, are key regulators of actin cytoskeleton remodeling. They function as molecular switches, transitioning between active and inactive states to coordinate signaling to promote cell motility or inhibit cell migration [59]. Imbalance of these small GTPases can result in an undesirable cellular phenotype. In podocyte-specific Rac1 knockout mice, Rac1 deletion was protective in an acute podocyte injury model [59]. Excessive Rac1 activity in vivo caused rapid-onset proteinuria with FPE [60], indicating that Rac1 is both sufficient and necessary for FPE. Manipulation of upstream signaling pathways, such as phosphatase and tensin homolog, or mutations in specific guanine nucleotide exchange factors and GTPase activating proteins of the Rho-family GTPases, can also result in cytoskeletal rearrangement and FPE [61-63]. Components of the diabetic milieu such as hyperglycemia, AGEs, ROS, activation of hexosamine pathway, and oxidized LDL have all been shown to stimulate Rho-GTPase activity [64, 65]. Dysregulation of the Rho family GTPases through these stimuli may promote podocyte actin remodeling and proteinuria in DKD [59].
PODOCYTE LOSS BY DETACHMENT
While foot process changes in podocytes are reversible, podocyte loss represents an irreversible event. Both living and dead podocytes can be recovered from urine, indicating that podocyte loss is a result of both detachment and death [66]. Podocytes normally adhere to the GBM via α3β1 integrin and dystroglycans (DG) [67]. Studies showed that α3β1 integrin expression is decreased in patients with diabetes and in streptozotocin-induced diabetic rats, resulting in focal detachment of podocytes from the GBM [68, 69]. Others then demonstrated that integrin β1 subunit mRNA and protein expression were increased in high extracellular glucose cultured podocytes exposed to Ang II. While these results seem contradictory, it is possible that after initial podocyte loss, there is increased adhesion of the remaining cells.
PODOCYTE LOSS BY DEATH
Several cell-death pathways lead to podocyte death. In 2006 Susztak et al. showed that podocytes cultured under high-glucose conditions died via apoptosis [70]. Mechanistically they showed that release of mitochondrial and plasma membrane ROS played an important role in the p38MAPK process [70]. Future studies then suggested that the plasma membrane NADPH oxidase (NOX) pathways were also involved. These studies confirmed that podocyte apoptosis led to DKD progression, which preceded podocyte depletion, increased urine albumin excretion, and mesangial matrix expansion in both type I and II diabetic animal models [71, 72].
The TGF-β pathway directly and indirectly results in podocyte apoptosis. Upregulation of TGF-β in DKD induces podocyte apoptosis by activating caspase3 via p38MAPK and Smad7 [73, 74]. Notch and Wnt/β-catenin are induced by TGF-β [75]. Induction of these developmental pathways increases the expression of target genes (e.g., Snail1, which suppresses nephrin) as well as podocyte anti-apoptotic signaling. Alternative TGF-β-induced podocyte apoptosis mechanisms have also been described [73, 76, 77].
Alterations in autophagy have recently been proposed to play a role in DKD. Autophagy is a mechanism essential for protein and organelle turnover and quality control. In 2010 Hartleben et al. showed that autophagy flux in podocytes was lower in DKD [78]. Decreased utilization of the autophagy-lysosome pathway shifts the degradation pathways toward the ubiquitin-proteasome system (UPS). Due to podocyte longevity, a defect in autophagy and inability for the UPS system to compensate, results in the accumulation of faulty proteins. This increases susceptibility to injury and could contribute to podocyte death [79].
Recent studies indicate that inflammatory cell death pathways also likely contribute to podocyte loss in diabetes [80]. DKD is an inflammatory disease and diabetic patients have increased caspase1 levels, a marker of inflammation [80]. Caspase1 cleaves interleukin-1β, resulting in inflammatory cell death, termed pyroptosis. Hyperglycemia and increased ROS generation contribute to increased activation of nucleotide-binding domain and leucine-rich repeat pyrin 3 domain (Nlrp3) inflammasome [81]. Nlrp3-inflammasome activation promotes DKD development, and neutralizing interleukin-1β in diabetic mice has been shown to ameliorate diabetic nephropathy, suggesting that this pathway has a functional role in DKD development [80, 82].
ADAPTATION: HYPERTROPHY AND DEDIFFERENTIATION
When podocytes are lost the remaining podocytes adapt to extend coverage over the newly denuded GBM (Figure 1c). Activation of mammalian target of rapamycin (mTOR) appears to be responsible for this podocyte hypertrophy [83]. In general, mTOR signaling is required for cellular development and regeneration. Mammalian target of rapamycin complex 1 (mTORC1) regulates protein translation, ribosomal biogenesis, cell growth, and autophagy [82, 83]. Mammalian target of rapamycin complex 2 (mTORC2) regulates cell survival, metabolism, and cytoskeletal rearrangement [82, 83]. In DKD patients and db/db mice, increased mTOR activity is associated with autophagy and Notch reactivation [82]. Counterintuitively, genetically decreasing podocyte mTORC1 signaling in mice prevented glomerulosclerosis and slowed the progression of DKD [82]. While it seems that hypertrophy is essential for podocyte survival, limiting it might ameliorate disease. Understanding the complex signaling resulting in mTOR over-activation or elimination may be essential for podocyte health [82].
Increased expression and activity of Notch and Wnt/β-catenin genes have been described in podocytes of DKD patients and animals. Notch and Wnt are developmental pathways that regulate kidney development and nephron endowment. Interestingly, their expressions in the adult kidney are near none-existent in healthy individuals, but are increased in proteinuric diseases. Increased expression of Notch is both sufficient and necessary to induce albuminuria and glomerulosclerosis via TGF-β [84]. This interaction creates a positive feedback loop where TGF-β transcriptionally upregulates the Notch ligand Jagged1 and Notch activation increases TGF-β expression [84]. In addition to TGF-β, VEGF is another important regulator of Notch expression and activity. Treating diabetic rats with a γ-secretase inhibitor decreased albuminuria, normalized VEGF and nephrin expression, and ameliorated kidney disease in vivo [85]. However, not all Notch proteins are functionally similar. In a recent study by Sweetwyne et al., Notch1 and Notch2 had varying involvement in disease development [86]. Clarification of specific Notch roles will be necessary moving forward to study their involvement in DKD.
Wnt signaling is equally important in podocyte development. Increased Wnt expression has been observed in patients with DKD and diabetic mouse models. Mice with podocyte-specific expression of stabilized β-catenin (Ctnnb1) presented with early DKD findings and increased susceptibility to glomerulosclerosis [87]. However, increased Wnt activity is also associated with resistance to apoptosis and dedifferentiation. Podocyte-specific Ctnnb1 knockout mice displayed increased podocyte differentiation markers WT1, nephrin, podocalyxin, and synaptopodin [87]. Altogether, these studies suggest that the reemergence of developmental genes in differentiated cells occurs as an adaptive mechanism to cell injury and death. On the other hand, active Ctnnb1 and Notch expression inhibits terminal differentiation of cells and indirectly promotes disease development.
GLOMERULOSCLEROSIS: THE VICIOUS CYCLE OF PODOCYTE LOSS
When podocyte loss reaches 20% the remaining cells die and glomerulosclerosis develops even when the instigating factor is no longer present. In 2005 Ichikawa et al. showed that podocytes not only transmit damage to other glomerular cells, but also from podocyte to podocyte. Using chimeric mice made up of either NEP25 podocytes sensitive to Pseudomonas immunotoxin or immunotoxin-insensitive wild-type cells, injection of the recombinant immunotoxin revealed that both NEP25 podocytes and wild-type podocytes were injured [88]. Similar depletion models demonstrated that adiponectin levels modulated podocyte recovery and glomerular remodeling [89]. These depletion studies suggest that there are delicate intra-glomerular cellular interactions. Severe podocyte damage results in loss of cell-to-cell communication pathways. VEGF is a classic example of this concept. VEGF is made by podocytes, but plays a critical role in endothelial cell maintenance [24]. As podocytes are unable to renew, therapeutics targeted at early phases of podocyte injury will be crucial to prevent functional renal decline among diabetic patients.
CONCLUSION
Podocyte injury is a critical factor in DKD progression. Observational and cohort studies suggest that glomerular podocyte density is a key determinant of albuminuria development in patients with diabetes, and albuminuria is one of the best predictors of GFR decline in DKD [39]. Albuminuria is not only important in assessing kidney function, but in 2010 Hemmelgarn et al. showed that higher levels of albuminuria were associated with increased risks of myocardial infarction and mortality [90]. Patients with DKD and nephrotic-range albuminuria are overrepresented in phase 2 and 3 clinical studies particularly since clinical end points (such as doubling of serum creatinine or ESRD) are more likely to occur in persons with higher albumin excretion rates. It is unclear, however, whether the pathogenesis of DKD in patients with and without high-grade albuminuria is the same.
Human and mouse genetic studies have demonstrated beyond a reasonable doubt that podocyte damage results in nephrotic-range albuminuria [60-63]. All identified mutations in nephrotic syndrome patients localize to podocytes [91]. Genetic manipulations of specific pathways in podocytes can both cause and lessen proteinuria. Since podocytes are one of few cell types in the human body that have no regenerative capacity, once they are lost, the remaining cells have increased susceptibility to stress and injury. Recently, studies have suggested that parietal cells might serve as a podocyte reservoir [92-97]. This might be helpful in situations where the injury is limited, but it remains to be determined if parietal epithelial cells can fully compensate for podocyte loss.
While podocyte injury appears to be a key factor in DKD pathogenesis, glomerular (i.e., endothelial, mesangial, and epithelial) cells are highly interdependent. For example, endothelial health is determined by factors secreted by podocytes such as VEGF; whereas hyperglycemia-induced mesangial production of TGF-β can directly and indirectly result in podocyte loss, death, and dedifferentiation. Overall, there is overwhelming human and diabetic animal model data that implicate podocyte injury and loss as a major mechanism in the pathogenesis of DKD.
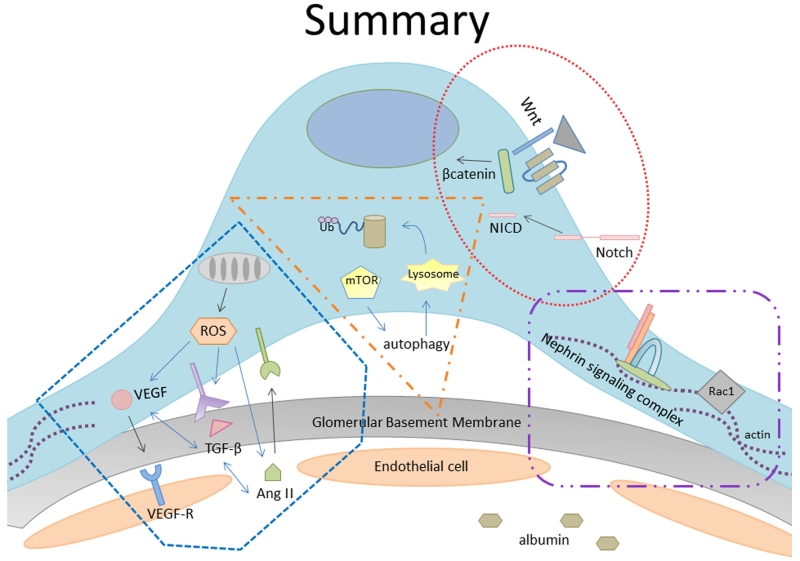
In conclusion, diabetes-induced podocyte injury results in several phenotypic changes including FPE, hypertrophy, detachment, death, and/or dedifferentiation. Ultimately, these signaling mechanisms appear to alter podocyte characteristics in an attempt to increase podocyte survival (Figure 2). In vivo models indicate that genetic manipulations of these delicately balanced pathways can alter disease severity. Altogether, these data support pharmacologically targeting mechanisms involved in podocyte remodeling as potentially successful avenues in DKD treatment. All current evidence points to podocytes as being one of the weakest links in DKD.
Figure 2.
Various complex inter- and intracellular signaling mechanisms have been described in diabetes-induced podocyte injury.
Acknowledgments
Funding
Work in the Susztak lab is supported by the National Institute of Health, Juvenile Diabetes Research Foundation, American Diabetes Association, Boehringer Ingelheim, Biogen, and Lilly. Dr. Lin is supported by NIDDK Ruth L. Kirschstein National Research Service Award institutional research training and post-doctoral fellowship grant.
Footnotes
Conflict of Interest
Jamie S. Lin declares that she has no conflict of interest. Katalin Susztak reports that work in her lab is supported by Biogen, Boehringer Ingelheim, Biogen and Lilly.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
REFERENCES
Recently published papers of particular interest are highlighted as:
• Of importance
•• Of major importance
- 1.Molitch ME, et al. Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79–83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 2.Reddy GR, et al. The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens. 2008;17(1):32–6. doi: 10.1097/MNH.0b013e3282f2904d. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Renal Data System . USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. 2015. [Web] cited 2015 15 Jan. [Google Scholar]
- 4.Incidence of end-stage renal disease attributed to diabetes among persons with diagnosed diabetes --- United States and Puerto Rico, 1996-2007. MMWR Morb Mortal Wkly Rep. 2010;59(42):1361–6. [PubMed] [Google Scholar]
- 5.Zhang L, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis. 2008;51(3):373–84. doi: 10.1053/j.ajkd.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Haroun MK, et al. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14(11):2934–41. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 7.Lu JL, et al. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol. 2015;3(9):704–14. doi: 10.1016/S2213-8587(15)00128-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brancati FL, et al. Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. Multiple Risk Factor Intervention Trial. Jama. 1997;278(23):2069–74. [PubMed] [Google Scholar]
- 9.Cruickshanks KJ, et al. The association of microalbuminuria with diabetic retinopathy. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 1993;100(6):862–7. doi: 10.1016/s0161-6420(93)31562-9. [DOI] [PubMed] [Google Scholar]
- 10.Chavers BM, et al. Relationship between retinal and glomerular lesions in IDDM patients. Diabetes. 1994;43(3):441–6. doi: 10.2337/diab.43.3.441. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, et al. The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: the Renin-Angiotensin System Study. Diabetes. 2005;54(2):527–33. doi: 10.2337/diabetes.54.2.527. [DOI] [PubMed] [Google Scholar]
- 12.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157(13):1413–8. [PubMed] [Google Scholar]
- 13.Parving HH, et al. Early detection of patients at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh) 1982;100(4):550–5. doi: 10.1530/acta.0.1000550. [DOI] [PubMed] [Google Scholar]
- 14.Mauer SM, et al. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74(4):1143–55. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond K, Mauer M. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes. 2002;51(5):1580–7. doi: 10.2337/diabetes.51.5.1580. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56(5):1627–37. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 17*.Ponchiardi C, Mauer M, Najafian B. Temporal profile of diabetic nephropathy pathologic changes. Curr Diab Rep. 2013;13(4):592–9. doi: 10.1007/s11892-013-0395-7. This study describes the natural history of diabetic kidney lesions.
- 18.Tan AL, Forbes JM, Cooper ME. AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol. 2007;27(2):130–43. doi: 10.1016/j.semnephrol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Leehey DJ, et al. Role of angiotensin II in diabetic nephropathy. Kidney Int Suppl. 2000;77:S93–8. doi: 10.1046/j.1523-1755.2000.07715.x. [DOI] [PubMed] [Google Scholar]
- 20.Lewis EJ, et al. The Collaborative Study Group The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329(20):1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 21.Lewis EJ, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 22.Veron D, et al. Podocyte vascular endothelial growth factor (Vegf(1)(6)(4)) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia. 2011;54(5):1227–41. doi: 10.1007/s00125-010-2034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weil EJ, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012;82(9):1010–7. doi: 10.1038/ki.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimoto H, et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278(15):12605–8. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto T, et al. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993;90(5):1814–8. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimoto M, et al. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem Biophys Res Commun. 2003;305(4):1002–7. doi: 10.1016/s0006-291x(03)00885-4. [DOI] [PubMed] [Google Scholar]
- 27.Iglesias-de la Cruz MC, et al. Effects of high glucose and TGF-beta1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 2002;62(3):901–13. doi: 10.1046/j.1523-1755.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- 28**.Hathaway CK, et al. Low TGFbeta1 expression prevents and high expression exacerbates diabetic nephropathy in mice. Proc Natl Acad Sci U S A. 2015;112(18):5815–20. doi: 10.1073/pnas.1504777112. This study suggests that blocking or decreasing TGFbeta1 might be of therapeutic value in diabetic kidney disease.
- 29.osterby R. Early phases in the development of diabetic glomerulopathy. Acta Med Scand Suppl. 1974;574:3–82. [PubMed] [Google Scholar]
- 30.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14(5):1358–73. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 31.Gambara V, et al. Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3(8):1458–66. doi: 10.1681/ASN.V381458. [DOI] [PubMed] [Google Scholar]
- 32.Gunwar S, et al. Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of alpha3, alpha4, and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J Biol Chem. 1998;273(15):8767–75. doi: 10.1074/jbc.273.15.8767. [DOI] [PubMed] [Google Scholar]
- 33.Zeisberg M, et al. Differential expression of type IV collagen isoforms in rat glomerular endothelial and mesangial cells. Biochem Biophys Res Commun. 2002;295(2):401–7. doi: 10.1016/s0006-291x(02)00693-9. [DOI] [PubMed] [Google Scholar]
- 34.Yagame M, et al. Differential distribution of type IV collagen chains in patients with diabetic nephropathy in non-insulin-dependent diabetes mellitus. Nephron. 1995;70(1):42–8. doi: 10.1159/000188542. [DOI] [PubMed] [Google Scholar]
- 35.Ziyadeh FN, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci U S A. 2000;97(14):8015–20. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S, et al. Angiotensin II stimulates alpha3(IV) collagen production in mouse podocytes via TGF-beta and VEGF signalling: implications for diabetic glomerulopathy. Nephrol Dial Transplant. 2005;20(7):1320–8. doi: 10.1093/ndt/gfh837. [DOI] [PubMed] [Google Scholar]
- 37.Bai Y, et al. High ambient glucose levels modulates the production of MMP-9 and alpha5(IV) collagen by cultured podocytes. Cell Physiol Biochem. 2006;17(1-2):57–68. doi: 10.1159/000091464. [DOI] [PubMed] [Google Scholar]
- 38**.Caramori ML, Parks A, Mauer M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol. 2013;24(7):1175–81. doi: 10.1681/ASN.2012070739. This study showed that GBM thickness might be able to predict which T1DM patient will develop proteinuria and ESRD.
- 39.Pagtalunan ME, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342–8. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiggins JE, et al. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16(10):2953–66. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 41.Wharram BL, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16(10):2941–52. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 42.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83(1):253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 43.Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens. 2005;14(3):211–6. doi: 10.1097/01.mnh.0000165885.85803.a8. [DOI] [PubMed] [Google Scholar]
- 44.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13(12):3005–15. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 45*.Li X, et al. Nephrin Preserves Podocyte Viability and Glomerular Structure and Function in Adult Kidneys. J Am Soc Nephrol. 2015;26(10):2361–77. doi: 10.1681/ASN.2014040405. Inducible RNAi-mediated nephrin knockdown mice showed that short versus long-term nephrin knockdown resulted in different histological outcomes when subjected to glomerular injury models. They found decreased AKT phosphorylation in both short- and long-term nephrin knockdown mice.
- 46.Beltcheva O, et al. Mutation spectrum in the nephrin gene (NPHS1) in congenital nephrotic syndrome. Hum Mutat. 2001;17(5):368–73. doi: 10.1002/humu.1111. [DOI] [PubMed] [Google Scholar]
- 47.Simons M, et al. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol. 2001;159(3):1069–77. doi: 10.1016/S0002-9440(10)61782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu J, et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73(5):556–66. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 49.Li H, et al. SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol. 2004;15(12):3006–15. doi: 10.1097/01.ASN.0000146689.88078.80. [DOI] [PubMed] [Google Scholar]
- 50.Verma R, et al. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116(5):1346–59. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tryggvason K, Pikkarainen T, Patrakka J. Nck links nephrin to actin in kidney podocytes. Cell. 2006;125(2):221–4. doi: 10.1016/j.cell.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Jones N, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440(7085):818–23. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 53.Na J, et al. Diet-Induced Podocyte Dysfunction in Drosophila and Mammals. Cell Rep. 2015;12(4):636–47. doi: 10.1016/j.celrep.2015.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doublier S, et al. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52(4):1023–30. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 55.Coward RJ, et al. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes. 2007;56(4):1127–35. doi: 10.2337/db06-0693. [DOI] [PubMed] [Google Scholar]
- 56.Welsh GI, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12(4):329–40. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veron D, et al. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 2010;77(11):989–99. doi: 10.1038/ki.2010.64. [DOI] [PubMed] [Google Scholar]
- 58.Huber TB, et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23(14):4917–28. doi: 10.1128/MCB.23.14.4917-4928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blattner SM, et al. Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int. 2013;84(5):920–30. doi: 10.1038/ki.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu H, et al. Rac1 activation in podocytes induces rapid foot process effacement and proteinuria. Mol Cell Biol. 2013;33(23):4755–64. doi: 10.1128/MCB.00730-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin JS, et al. Loss of PTEN promotes podocyte cytoskeletal rearrangement, aggravating diabetic nephropathy. J Pathol. 2015 doi: 10.1002/path.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akilesh S, et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest. 2011;121(10):4127–37. doi: 10.1172/JCI46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gee HY, et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest. 2013;123(8):3243–53. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danesh FR, et al. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/ p21 signaling pathway: Implications for diabetic nephropathy. Proc Natl Acad Sci U S A. 2002;99(12):8301–5. doi: 10.1073/pnas.122228799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng F, et al. RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes. 2008;57(6):1683–92. doi: 10.2337/db07-1149. [DOI] [PubMed] [Google Scholar]
- 66.Vogelmann SU, et al. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285(1):F40–8. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathew S, et al. Integrins in renal development. Pediatr Nephrol. 2012;27(6):891–900. doi: 10.1007/s00467-011-1890-1. [DOI] [PubMed] [Google Scholar]
- 68.Chen HC, et al. Altering expression of alpha3beta1 integrin on podocytes of human and rats with diabetes. Life Sci. 2000;67(19):2345–53. doi: 10.1016/s0024-3205(00)00815-8. [DOI] [PubMed] [Google Scholar]
- 69.Regoli M, Bendayan M. Alterations in the expression of the alpha 3 beta 1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia. 1997;40(1):15–22. doi: 10.1007/s001250050637. [DOI] [PubMed] [Google Scholar]
- 70.Susztak K, et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–33. [PubMed] [Google Scholar]
- 71.Eid AA, et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58(5):1201–11. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72**.Eid AA, et al. Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes. 2013;62(8):2935–47. doi: 10.2337/db12-1504. This study showed that mTOR drives production of Nox-4 derived ROS generation and podocyte death. Inhibiting mTOR or NADPH oxidase was shown to be beneficial in animal models.
- 73.Schiffer M, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108(6):807–16. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li JH, et al. Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. Faseb j. 2004;18(1):176–8. doi: 10.1096/fj.02-1117fje. [DOI] [PubMed] [Google Scholar]
- 75.Li Y, et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172(2):299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abe Y, et al. TGF-beta1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway. Am J Physiol Renal Physiol. 2013;305(10):F1477–90. doi: 10.1152/ajprenal.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Das R, et al. Upregulation of mitochondrial Nox4 mediates TGF-beta-induced apoptosis in cultured mouse podocytes. Am J Physiol Renal Physiol. 2014;306(2):F155–67. doi: 10.1152/ajprenal.00438.2013. [DOI] [PubMed] [Google Scholar]
- 78.Hartleben B, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120(4):1084–96. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lenoir O, et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy. 2015;11(7):1130–45. doi: 10.1080/15548627.2015.1049799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80*.Shahzad K, et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015;87(1):74–84. doi: 10.1038/ki.2014.271. This study showed that Nlrp3-inflammasome activation aggrevates diabetic nephropathy. Blocking ROS and Nlrp3-inflammasome might be protective in diabetic kidney disease.
- 81.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–5. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 82.Godel M, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121(6):2197–209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inoki K, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121(6):2181–96. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niranjan T, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14(3):290–8. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 85.Lin CL, et al. Modulation of notch-1 signaling alleviates vascular endothelial growth factor-mediated diabetic nephropathy. Diabetes. 2010;59(8):1915–25. doi: 10.2337/db09-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86*.Sweetwyne MT, et al. Notch1 and Notch2 in Podocytes Play Differential Roles During Diabetic Nephropathy Development. Diabetes. 2015;64(12):4099–111. doi: 10.2337/db15-0260. This study looked at different Notch receptors in vivo and demonstrated that Notch1 and Notch2 to have divergent roles in diabetic nephropathy.
- 87.Kato H, et al. Wnt/beta-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem. 2011;286(29):26003–15. doi: 10.1074/jbc.M111.223164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ichikawa I, et al. Podocyte damage damages podocytes: autonomous vicious cycle that drives local spread of glomerular sclerosis. Curr Opin Nephrol Hypertens. 2005;14(3):205–10. doi: 10.1097/01.mnh.0000165884.85803.e1. [DOI] [PubMed] [Google Scholar]
- 89.Sharma K, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118(5):1645–56. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hemmelgarn BR, et al. Relation between kidney function, proteinuria, and adverse outcomes. Jama. 2010;303(5):423–9. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 91.Malaga-Dieguez L, Susztak K. ADCK4 “reenergizes” nephrotic syndrome. J Clin Invest. 2013;123(12):4996–9. doi: 10.1172/JCI73168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hackl MJ, et al. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med. 2013;19(12):1661–6. doi: 10.1038/nm.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eng DG, et al. Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis. Kidney Int. 2015;88(5):999–1012. doi: 10.1038/ki.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berger K, et al. The regenerative potential of parietal epithelial cells in adult mice. J Am Soc Nephrol. 2014;25(4):693–705. doi: 10.1681/ASN.2013050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ronconi E, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20(2):322–32. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guhr SS, et al. The expression of podocyte-specific proteins in parietal epithelial cells is regulated by protein degradation. Kidney Int. 2013;84(3):532–44. doi: 10.1038/ki.2013.115. [DOI] [PubMed] [Google Scholar]
- 97.Appel D, et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20(2):333–43. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]