Abstract
Increasing knowledge about the influence of genetic variation on human health and growing availability of reliable, cost-effective genetic testing have spurred the implementation of genomic medicine in the clinic. As defined by the National Human Genome Research Institute (NHGRI), genomic medicine uses an individual’s genetic information in his or her clinical care, and has begun to be applied effectively in areas such as cancer genomics, pharmacogenomics, and rare and undiagnosed diseases. In 2011 NHGRI published its strategic vision for the future of genomic research, including an ambitious research agenda to facilitate and promote the implementation of genomic medicine. To realize this agenda, NHGRI is consulting and facilitating collaborations with the external research community through a series of “Genomic Medicine Meetings,” under the guidance and leadership of the National Advisory Council on Human Genome Research. These meetings have identified and begun to address significant obstacles to implementation, such as lack of evidence of efficacy, limited availability of genomics expertise and testing, lack of standards, and diffficulties in integrating genomic results into electronic medical records.
The six research and dissemination initiatives comprising NHGRI’s genomic research portfolio are designed to speed the evaluation and incorporation, where appropriate, of genomic technologies and findings into routine clinical care. Actual adoption of successful approaches in clinical care will depend upon the willingness, interest, and energy of professional societies, practitioners, patients, and payers to promote their responsible use and share their experiences in doing so.
Keywords: Genomics, Pharmacogenomics, Clinical care
1. Introduction
Growing understanding of the role of genetic variants in human health and disease, and improved technologies for measuring these variants rapidly at large scale, have opened the door to increased use of genomic information in clinical care [1]. While individual, high-impact genetic variants, such as the cystic fibrosis conductance transmembrane regulator (CFTR) ΔF508 variant in cystic fibrosis and the beta hemoglobin (HBB) β6 glutamic acid to valine substitution in sickle cell anemia, have long been known to clinical medicine, the advent of high-throughput assay techniques has enabled consideration of much larger numbers of genes and variants in an individual in a more multi-factorial, truly genomic, approach. Although some have argued that the difference between “genetics” and “genomics” is merely two letters, and the terms do tend to be used interchangeably, “genetics” as often used refers to the study of heredity with a focus on a specific and limited number of genes with known function in disease. “Genomics,” in contrast, refers to the totality of an individual’s genetic make-up, their “genome,” and has become much more prominent clinically as technologies and understanding have advanced. Here the focus will largely be on “genomics,” though in any given clinical setting involving a specific disease or drug, emphasis will necessarily sharpen to one or a few individual genes or variants within them.
“Genomic medicine,” another core concept for consideration here, has been defined as the use of an individual patient’s genotypic information in his or her clinical care [2]. While this definition encompasses both Mendelian and multigenic complex diseases, emphasis is shifting to assaying and using multiple variants simultaneously in clinical care for the reasons noted above—an expanding knowledge base and improved measurement techniques that permit a more holistic approach to incorporating genomic findings into patient care.
1.1. Vision for the future of genomic research
The application of genomic information in clinical care has been an increasing focus of the research programs of the National Human Genome Research Institute (NHGRI) in the past several years. In 2011 NHGRI published its strategic vision for the future of genomic research [3], proposing an ambitious research agenda to facilitate and promote the implementation of genomic medicine. This vision is framed around five research domains, three of which (investigating genome structure, genome biology, and the genomic biology of disease) reflect fundamental technologic and basic science pursuits that have long been key components of the Institute’s core mission. For the first time, however, the 2011 strategic vision extended NHGRI’s research agenda to include genomics research to advance the science of medicine and improve the effectiveness of healthcare. These new emphasis areas differ from the disease-association, discovery-focused research that previously came to mind when considering the application of genomics to the study of health and disease. Specifically, genomic medicine at NHGRI would now move beyond demonstrating genotype-phenotype associations, typical of the Biology of Disease domain, to assess and demonstrate improved outcomes for patients and the healthcare system after using genomic information to guide clinical care (Table 1). In this way, NHGRI proposed to initiate concerted efforts to apply genomics for improving the prevention, diagnosis, and treatment of human disease and to evaluate its effectiveness in doing so. Many other genes and variants have been recommended for implementation since then, particularly in the pharmacogenomics realm [8,9].
Table 1.
Disease-related genomics research across three NHGRI research domains. Adapted from Ref. [4].
| NHGRI domain | Biology of disease | Science of medicine | Effectiveness of healthcare |
|---|---|---|---|
| Common rubric | Discovery research | Clinical validation | Clinical implementation |
| General goal | Demonstrate genotype-phenotype associations | Assess outcomes after using genomic information to direct clinical care | Demonstrate improved healthcare with the use of genomic information |
| Specific examples |
|
|
|
Shortly after the 2011 strategic plan was made public, NHGRI consulted many of the nearly 30 Institutes and Centers comprising the U.S. National Institutes of Health to identify genomic research projects related to disease prevention, diagnosis, or treatment that were ready, or nearly ready, for implementation in actual clinical care. Few such projects were found, however, with most Institutes and Centers focused on projects falling more in the realm of genotype-phenotype association studies. Only a handful of studies involved examining the impact of using individual patients’ genomic information in their medical care, and almost none focused on demonstrating the utility of genomics for actually improving the care of patients. This last domain encompasses much of what is referred to as “implementation research”— the study of methods that promote the systematic uptake of proven interventions into routine clinical care [5]. One project that did seem ready for clinical application was the implementation of a newly-developed targeted sequencing panel of 84 pharmacologically important genes in 9000 patients in the multi-site Electronic Medical Records and Genomics (eMERGE) network [6,7]. Of interest, the three drug-gene pairs that nearly all nine eMERGE sites agreed were ready for clinical implementation involved treatments for cardiovascular disease: CYP2C19 variants and clopidogrel treatment for prevention of instent restenosis; SLCO1B1 variants and simvastatin therapy; and CYP2C9 and VKORC1 variants for warfarin treatment in atrial fibrillation.
1.2. NHGRI genomic medicine working group and genomic medicine meetings
In parallel, NHGRI also consulted and facilitated collaborations with the external research community by convening a series of “Genomic Medicine Meetings” (Table 2), under the guidance and leadership of the Genomic Medicine Working Group of the National Advisory Council on Human Genome Research [10]. Although considerable doubt had been voiced during preparation of the 2011 strategic plan as to whether a critical mass of researchers actively engaged in genomic medicine implementation even existed in the U.S., the first genomic medicine meeting in June 2011 quickly laid these to rest. Representatives of 20 groups attended this first meeting on short notice and at their own expense, and described a host of implementation efforts going on within their centers. Commonalities and duplications across these efforts became readily apparent, including similar obstacles encountered and solutions developed, often quite independently. A summary of these efforts and the major lessons learned by early adopters was published as an “implementation roadmap” [2], and plans were made to facilitate collaborations and to address the critical need for a consensus process to identify clinically actionable genomic variants.
Table 2.
NHGRI Genomic Medicine Meetings and related workshops. Adapted from Ref. [4].
| Meeting | Dates | Emphasis | Products | Meeting URL |
|---|---|---|---|---|
| Genomic medicine symposium [2] | June 29, 2011 | Academic medical centers | Implementation roadmap [2], clinaction workshop | https://www.genome.gov/27547270 |
| Clinaction workshop | December 2–3, 2011 | Methods for identifying clinically actionable variants | RFA HG-12-016, clinically relevant genetic variants resource; clingen consortium [9] | http://www.genome.gov/27546546 |
| Genomic medicine II | December 5–6, 2011 | Pilot demonstration projects | RFA HG-12-006 and –007, genomic medicine pilot demonstration projects; IGNITE consortium | https://www.genome.gov/27546373 |
| Genomic medicine III | May 3–4, 2012 | Working with laboratories and payers | Payers’ meeting | https://www.genome.gov/27548693 |
| Genomic medicine IV | January 28–29, 2013 | Physician education in genomics | Inter-society coordinating committee for practitioner education in genomics (ISCC), white pper [11] | https://www.genome.gov/27552294 |
| Genomic medicine V | May 28–29, 2013 | Working with federal stakeholders | Exploratory implementation project in collaboration with VA and military medical services, now supplanted by precision medicine initiative | https://www.genome.gov/27553865 |
| Inter-society coordinating committee | September 19–20, 2013 and ongoing | Physician competencies and shared educational materials across professional societies | Entrustable professional activities [13], web-accessible educational products for use across multiple disciplines [14] | https://www.genome.gov/27554614 |
| Genomic medicine VI | January 8–9, 2014 | Developing international collaborations | Global genomic medicine collaborative (G2MC), white paper [17] | https://www.genome.gov/27555775 |
| Genomic medicine VII | October 2–3, 2014 | Define research agenda for genomic clinical decision support | Collaboration with institute of medicine genomics roundtable and DIGITizE effort [101]; white paper [102] | https://www.genome.gov/27558904 |
| Research directions in genetically-mediated SJS/TEN | March 3–4, 2015 | Identify gaps and priorities for future research to eliminate genetically mediated SJS/TEN | Working group to improve case definition and phenotyping, white paper [19] | https://www.genome.gov/27560487 |
| Genomic medicine VIII | June 8–9, 2015 | Review NHGRI’s genomic medicine research portfolio | Identified need for increased interaction with basic scientists | https://www.genome.gov/27561558 |
| Genomic medicine IX | April 19–20, 2016 | Increase interactions between basic scientists and clinicians | Approach for prioritizing clinically relevant genes for functional investigation | https://www.genome.gov/27564185 |
Additional meetings in December 2011 addressed facilitating collaborations and identifying actionable variants, leading to the release of several NHGRI funding solicitations and ultimately the funding of two new consortia, the Clinical Genome Resource (ClinGen) [11] and the Implementing Genomics in Practice (IGNITE) Network [12]. Later meetings focused on issues relevant to laboratories and payers (May 2012), professional societies (January 2013), and federal agencies (May 2013). Each of these led to follow-up discussions regarding potential collaborative research projects with payers and/or with multiple federal healthcare providers. The January 2013 meeting was particularly productive, with the professional societies urging NHGRI to establish and co-lead an Inter-Society Coordinating Committee on Practitioner Education in Genomics (ISCC) that would facilitate the efforts of professional societies in developing and sharing genomic educational materials and standards for physicians and other health practitioners [13,14]. The ISCC has quickly grown to include over 35 professional societies and nearly 20 NIH Institutes and other Government agencies, and has produced a framework for key genomic medicine competencies for physicians [15] and accessible educational products for use across multiple disciplines and professional organizations [16], as well as adapting a short course in genomics and personalized medicine originally developed for pathology residents to a wide number of specialties [17,18].
The sixth genomic medicine meeting in January 2014 explored genomic medicine implementation efforts internationally and the potential for collaborations among them, similar to the U.S.-focused exploration comprising the first genomic medicine meeting. Also similar to that meeting, it identified numerous related but isolated efforts worldwide, but also revealed a number of innovative projects feasible within unified and smaller, more nimble health systems that are nearly impossible to consider in the U.S. at present [19]. One project in particular, a simple pharmacogenetics card given to patients genetically at risk of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN) in an innovative program to reduce risk of this devastating adverse drug reaction in Thailand, so caught the imagination of the participants that a global effort to eradicate genetically-mediated SJS/TENS was proposed. A subsequent workshop on research and implementation needs in SJS/TEN drew international participation and attention to the rich opportunities for prevention afforded by recent discoveries of genetic variants that can increase the risk of this dreaded condition by over 100-fold [20,21]. Similar to the outcome of the GMIV meeting on physician education, participants agreed to form a Global Genomic Medicine Collaborative (G2MC) that continues to explore opportunities for sharing best practices and for collaboratively addressing obstacles to genomic medicine implementation [22].
Three subsequent meetings through April 2016 have focused on genomic clinical decision support, research needs in genomic medicine in the context of NHGRI’s genomic medicine research portfolio, and enhanced collaborations between basic scientists and clinical genomicists to speed implementation of genomic discoveries in clinical care. All meetings except the first have been live-streamed and web-archived on the NHGRI Genomic Medicine site [10] along with all the slide presentations and meeting summaries and executive summaries. Subsequent meetings addressing key research needs and opportunities are anticipated to be held roughly every 9–12 months.
2. Opportunities for genomic medicine implementation related to atherosclerosis
One of the earliest direct implementation efforts of genomics in all of medicine arose from a relatively uncommon Mendelian condition leading to early onset of severe atherosclerosis, early myocardial infarction, and death. Identification of the LDL-receptor and of the dysfunctional protein product of the mutated LDL-receptor gene (LDLR) in patients with familial hypercholesterolemia (FH) also revealed the pivotal role of repression by intracellular cholesterol of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase, the rate-limiting step in cholesterol synthesis [23]. This led to the development of HMG CoA reductase inhibitors, or statins, now one of the most widely prescribed medications in the world for prevention of coronary atherosclerosis and used predominantly in people with completely normal LDL cholesterol metabolism. Later studies of persons with very low cholesterol levels identified another key gene in the cholesterol pathway, proprotein convertase subtilisin/kexin-type 9 (PCSK9), loss of function mutations in which produce lifelong low cholesterol levels, resistance to coronary disease, and seemingly no other ill effects [24]. This discovery has led to the development of monoclonal antibodies to inhibit the PCSK9 protein, such as evolucumab and alirocumab, that effectively lower LDL-cholesterol levels in persons who have not reached target levels on conventional therapy with diet and statins [25].
These therapies, though dramatic and highly effective, do not actually represent use of an individual’s genomic information in their own clinical care, since these drugs can be used largely independently of a patient’s LDLR or PCSK9 variant status. Examples of true genomic medicine applications in the care of cardiovascular disease are less common than in fields such as cancer [26] and undiagnosed diseases [27], and are even less common for atherosclerotic cardiovascular disease. Genetically determined fatal arrhythmia syndromes such as long QT syndrome, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia, as well gene mutations altering the function of a variety of cardiac ion channel and transporter-associated proteins, are increasingly being sought in clinical practice to identify patients with indications for drug treatment, implantation of cardiac defibrillators, and/or cascade screening of family members [28–30]. Four ventricular arrhythmia-related genes, KCNQ1, KCNH2, SCN5A, and RYR2, are among those recommended by the American College of Medical Genetics and Genomics (ACMG) for reporting as incidental or secondary potentially actionable findings when inactivating variants are found in the course of clinically-indicated genomic sequencing [31]. Although these recommendations have been debated [32], they provide a professional guideline from an expert body in an emerging area with little other guidance and are increasingly being implemented as standard of care.
In addition to genes proven to cause Mendelian arrhythmia syndromes, several genes clearly established as causing hypertrophic or dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy are also screened for in genetic testing panels developed for cardiomyopathy patients [33–35]. Sixteen cardiomyopathy genes (MYBPC3, MYH7, TNNT2, TNNI3, TPM1, MYL3, ACTC1, PRKAG2, GLA, MYL2, LMNA, PKP2, DSP, DSC2, TMEM43, and DSG2) are also on the ACMG list for reporting to patients, as are five genes for arrhythmogenic right ventricular cardiomyopathy (PKP2, DSP, DSC2, TMEM43, and DSG2) and seven genes causing familial aneurysm syndromes (FBN1, TGFBR1, TGFBR2, SMAD3, ACTA2, MYLK, and MYH11). Increased identification of inactivating variants in these genes in patients undergoing testing specifically for these syndromes, and in those in whom these variants present as secondary findings, should help to clarify the predictive and prognostic implications of such findings, especially to the degree they are shared through large databases such as the National Center for Biotechnology’s ClinVar [36] as described further below.
In contrast, relatively few Mendelian syndromes have been described for atherosclerosis, although familial hypercholesterolemia is a prominent example not only in atherosclerosis but in cardiovascular genetics and in medical genetics in general. This is due not only to its paradigmatic role in developing effective treatments for non-Mendelian forms of atherosclerotic cardiovascular disease, as described above, but also to its relatively high frequency and strong penetrance in the heterozygous state, making cascade screening of family members cost-effective and highly productive [37]. These characteristics were capitalized upon by the late Roger Williams and others in the “Make Early Diagnosis to Prevent Disease” (MEDPED) project [38], an archetype of implementation of genetic screening on a population basis. Inactivating mutations in the LDLR gene are included in the ACMG recommendations for reporting of secondary findings, as are two other genes, APOB and PCSK9, but aside from those three there are few hereditary atherosclerotic syndromes that are recommended for investigation in common clinical practice. Rare Mendelian syndromes leading to severe atherosclerosis, such as Tangier disease and Hutchinson-Gilford progeria, are of course well-described but are sufficiently rare not to rise to the level of recognition of most clinical practitioners.
Atherosclerotic coronary disease does provide another paradigm for the use of genomics in clinical care, that of a “genetic risk score” for multiple contributing genetic variants leading to a common, complex disease. Several such scores have been developed based largely on alleles associated with risk of coronary disease or myocardial infarction from genome-wide association studies (GWAS), and have shown modest improvements in predicting coronary events independent of family history or cardiovascular risk factors in some studies [39,40] but not in others [41,42]. Still, such scores have been used to identify individuals at intermediate risk, where scores tend to have their greatest impact on reclassification, and to compare the effectiveness of risk reduction strategies in patients (and their clinicians) who were provided genetic risk information vs. those in whom it was withheld [43,44]. One such trial demonstrated greater LDL-cholesterol lowering in patients receiving a combined genetic + clinical risk score vs. clinical risk score alone, and this overall difference appeared to be driven by increased statin use in participants with high genetic risk scores [45]. This may suggest that high genetic risk information is somehow particularly motivating to patients or their physicians, though such results need to be replicated before efforts at widespread application are undertaken.
Lastly, we should not forget the oldest genomic risk assay in clinical medicine, the family history. Again and in particular for coronary atherosclerotic disease, family history is a powerful predictor of coronary disease risk, especially (as with many inherited diseases) when multiple close relatives are affected and their disease onset is early in life [46,47]. Several user-friendly, patient-facing family history tools are available to simplify data collection for busy clinicians [48–50], and implementation in clinical settings has been successfully achieved [51,52]. Though devoid of the seductive, high-tech nature of other genomic technologies, patient-entered family history provides in essence a “bioassay” of the effect of a patient’s genetic variants in other patients most likely to carry them—their relatives. It is also easy and inexpensive to collect and has demonstrated reliability. It should not be overlooked.
3. Genomic medicine research programs of the NHGRI
3.1. Research gaps circa 2011
Genomic medicine was truly in its infancy at the time NHGRI’s 2011 strategic plan was published; indeed, considerable debate during the development of the plan centered around whether genomic medicine was ready for clinical implementation at all. This controversy lingered despite the handful of genomic applications already in clinical practice in 2011, such as use of specific tumor mutations in cancer treatment, HLA testing prior to abacavir use [3], and clear evidence of early adopter institutions launching successful implementation programs [2,53–56]. Substantial research would still be needed, however, to bring new genomic discoveries into clinical care, including studies to demonstrate the generalizability of genomic findings across ancestrally diverse populations and clinical settings and to generate evidence of the efficacy of using genomic information for clinical care [4]. Research on. integrating genomic information into EMRs, maintaining patient privacy, and providing computerized decision support for practicing clinicians was needed to facilitate genomic medicine implementation in large integrated healthcare systems, which are also ideal for acting rapidly on genomic knowledge in “learning healthcare systems” [57].
Applying the rapid advances in “next-generation” DNA sequencing technologies to challenging clinical problems such as optimizing management of patients with rare disorders [58] and evaluation of patients with undiagnosed conditions [59], raised questions about the feasibility of such approaches outside of highly specialized centers [4]. Extensive genomic and phenotypic characterization also raised challenging issues relating to data sharing, informed consent, and the reporting of incidental genomic findings unrelated to the index condition but with potential implications for clinical care. The potential of genome sequencing to augment or even replace standard approaches to screening for hereditary diseases in newborns raised numerous questions about efficacy, feasibility, and psychosocial impact that also needed to be addressed [60]. Meanwhile, most physicians and other healthcare professionals were largely unaware of genomic advances that might be relevant to their patients and were generally intimidated by the rapidly emerging discipline of genomic medicine, with few feeling competent to use genomics in their practices [61].
3.2. NHGRI’s research programs addressing these gaps
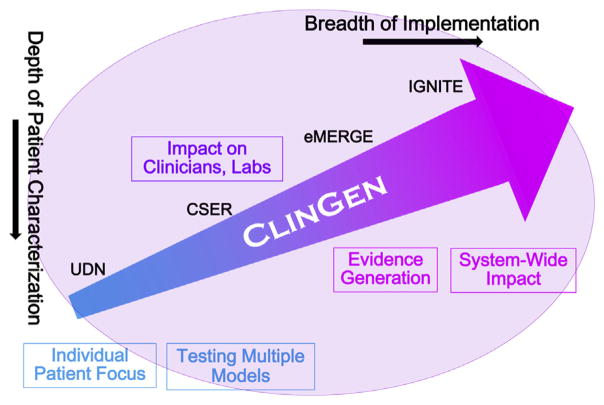
In close consultation with the genomic research and clinical communities, and shaped by critical input from the National Advisory Council on Human Genome Research and its Genomic Medicine Working Group, NHGRI moved quickly to extend existing research programs into genomic medicine implementation and to develop others to fill critical gaps. These programs can be viewed along a continuum from those highly focused on in-depth characterization of and interaction with individual patients and their clinicians to programs addressing broader implementation and system-wide research questions (Fig. 1). Underpinning them all are critical infrastructure programs for knowledge synthesis and integration such as the Clinical Genome Resource (ClinGen [62]), and continued major efforts in understanding the structure and function of the genome and its role in health and disease [63,64]. NIH funding for these programs is expected to total at least $401 million ($M) across fiscal years 2007 through 2018, inclusive (Table 3). This has steadily grown from roughly $6 M in fiscal 2007 to roughly $86 M expected in 2016 (ending September 30, 2016). Amounts for new or renewed programs in 2017 and 2018 have not yet been determined but continued commitments for ongoing programs are included in the $401 M total.
Fig. 1.
NHGRI genomic medicine implementation programs by depth of patient characterization and breadth of implementation.
Table 3.
NHGRI genomic medicine research programs and associated NIH funding and fiscal years of support, 2007–2018 (projected). Amounts for FY2017 and FY2018 are estimates and do not include possible renewals of expiring programs such as UDN, CSER, and ClinGen.
| Program NIH funding and fiscal years (FY) of support | Objectives | Components | Website URL |
|---|---|---|---|
| Undiagnosed diseases network (UDN)a [66] $121 M, FY2013–FY2017 |
|
7 Clinical sites 1 Coordinating center 2 Sequencing centers model organism core metabolomics core |
https://undiagnosed.hms.harvard.edu/ |
| Newborn sequencing in genomic medicine and public health (NSIGHT)b $20 M, FY2013–FY2017 |
|
4 Clinical sites | https://www.genome.gov/27558493/newborn-sequencing-in-genomic-medicine-and-public-health-nsight/ |
| Clinical sequencing exploratory research (CSER) [69] c $83 M, FY2012–FY2016 |
|
9 Clinical sites 1 Coordinating center |
https://cser-consortium.org/projects |
| Electronic medical records and genomics network (eMERGE) [103] d $135 M, FY2007–2018 |
|
9 Clinical sites 1 Coordinating center 2 Sequencing centers |
https://emerge.mc.vanderbilt.edu/ |
| Implementing genomics in practice (IGNITE) [12] $32 M, FY2013–FY2018 |
|
6 Clinical sites 1 Coordinating center |
https://ignite-genomics.org/ |
| Clinical genome resource (ClinGen) [62,82]e $28 M, FY2013–FY2016 |
|
3 Study investigator sites NCBI’s ClinVar |
https://www.clinicalgenome.org/ |
Supported by the NIH Common Fund.
Co-funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Co-funded by the National Cancer Institute.
Co-funded by the NIH Office of the Director.
Co-funded by the National Cancer Institute and the NIH Office of the Director.
In-depth characterization of individual patients was best exemplified by the NIH Undiagnosed Diseases Program (UDP), a collaborative effort of the NIH Office of Rare Disease Research and intramural NHGRI begun in 2008 to establish diagnoses for patients who remain undiagnosed after exhaustive medical workups and to discover new disorders and insights into disease mechanisms [65]. By 2011, the UDP had established diagnoses in nearly a quarter of the patients evaluated and identified several new disorders, but the transferability of the program outside the unique setting of the NIH Clinical Center was unclear. With support from the NIH Common Fund, NHGRI worked with several other NIH Institutes and Centers to expand the UDP into the multi-center Undiagnosed Diseases Network (UDN), involving seven clinical sites, a coordinating center, two DNA sequencing cores, a metabolomics core, a model organism screening center, a central biorepository, and several affiliated gene function studies [66]. The UDN is testing several innovative approaches to undiagnosed diseases, including sharing of detailed phenotypic and genotypic data on individual patients across clinical sites and basic labs, in a manner compliant with the Health Insurance Portability and Accountability Act (HIPAA); a streamlined online application process through the UDN Gateway; a central Institutional Review Board (IRB) housed at the NIH; a weekly case conference for discussion of patients to be admitted to the program; and a comparison of the yield of exome and genome sequencing in undiagnosed diseases. Strong international interest in UDN protocols and methods, and in critically important sharing of case information to enable identification of similar cases to improve diagnoses, led to the establishment of the Undiagnosed Diseases Network International (UDNI [67]).
The Newborn Sequencing in Genomic Medicine and Public Health (NSIGHT) program was established in 2013 to explore the implications, challenges and opportunities associated with the possible use of genomic sequence information in the newborn period. Growing out of a 2010 NIH workshop [60] and funded in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NSIGHT involves four clinical sites across the U.S. Each site addresses three aims: 1) acquisition and analysis of large-scale genomic datasets in newborns; 2) clinical research on specific disorders identifiable via newborn screening through promising new DNA-based analysis; and 3) research in the ethical, legal and social implications (ELSI) of the possible implementation of genomic sequencing of newborns. NSIGHT investigators have already demonstrated the high diagnostic yield (57%) and impact on management of sequencing sick newborns in neonatal intensive care units and enabled dramatic reductions in the turnaround time of such information to a mere 26 h [68].
The Clinical Sequencing Exploratory Research (CSER) consortium was established in 2011 to explore the potential for clinicians to utilize genome sequence data for the care of their patients. Like NSIGHT, CSER combines a defined clinical genomic study utilizing exome or genome sequencing for diagnosis and management in a wide variety of clinical contexts (such as pre-conception screening, intellectual disability, cancer, and healthy adults), with acquisition and analysis of large-scale genomic datasets and investigation of related ELSI issues [69]. To date CSER has recruited over 5000 participants and demonstrated the feasibility of implementing a clinical workflow that recruits, consents, and educates patients and providers and that generates, interprets, and returns relevant genomic information. CSER has disseminated widely applicable best practices including models for genomics-oriented informed consent tailored to the clinical setting, models to improve the consistency of genomic variant interpretation, and approaches to the disclosure of primary pediatric and tumor findings and secondary findings. CSER investigators have also been heavily involved in the development and refinement of clinical guidelines including co-leading and contributing gene-annotation resources to key ACMG recommendations on secondary findings [31], variant interpretation [70], and clinical laboratory standards [71] and initiating studies to assess real-world application of these guidelines. NHGRI recently announced plans to build on the success of CSER with the Clinical Sequencing Evidence-generating Research consortium (CSER2), to generate and analyze evidence of the clinical utility of genome sequencing in multiple clinical contexts; investigate critical interactions among patients, family members, health practitioners, and clinical laboratories to better inform implementation of the clinical genome sequencing process; and explore the feasibility of exchanging genomic, clinical, and health utilization data within existing healthcare systems to build a shared evidence base for clinical decision-making [72,73]. A companion funding opportunity seeks to stimulate investigator-initiated research that informs the implementation of genome sequencing in clinical care, including studies of whether and how clinical genome sequencing impacts disease diagnosis and treatment, studies that address current barriers to the implementation of clinical genome sequencing, and studies of approaches to improve the identification and interpretation of genomic variants for dissemination in clinical settings [74]. Award and initiation of these programs is expected in mid-2017.
The Electronic Medical Records and Genomics (eMERGE) network works more at the level of hospital and academic healthcare systems and their role in generating evidence of the impact of genomic medicine implementation. Established in 2007 to explore how best to use biorepositories linked with EMRs in genomic research projects, eMERGE investigators initially demonstrated the research value of such biorepositories particularly in the validity of electronic phenotyping [75,76]. A second phase was funded in 2011 to expand into genomic medicine implementation studies such as the effects of returning high-risk CFH, HFE, and FVL variants on physician and patient behaviors, and of genomic versus clinical risk assessments in managing coronary disease as described above [45]. Widely available tools for genomic medicine implementation developed by eMERGE include PheKB, a phenotype algorithm repository; Natural Language Processing (NLP) tools such as cTakes and MedEx; the eMERGE InfoButton pointing physicians to clinical decision support (CDS) resources; the pharmacogenetics variant and phenotype data repository (SPHINX); and MyResults. org, an online educational resource on genetic testing for patients [77]. In 2012 eMERGE began a collaboration with the Pharmacogenetics Research Network to perform targeted sequencing of 84 pharmacologically important genes in 9000 patients [78]. The large scale of this project has been especially illuminating, not only in the processes for consent, clinical workflow, and approval at ten diverse institutions, but also in the number of potentially actionable variants found. Over 2% of the first 2022 patients studied, for example, carry rare “known or expected pathogenic variants” in two arrhythmia genes, SCN5A and KCNH2; yet familial arrhythmia syndromes are known to be much less prevalent [79]. Subsequent investigation identified widely differing interpretation of these variants by different clinical labs, a problem also identified in CSER and by clinicians active in this area, leading to increased efforts to standardize variant interpretation and provide needed reference databases for doing so [62,70]. In 2015 a third phase of eMERGE was initiated to detect rare variants presumed to affect gene function, assess the penetrance of these variants, report actionable variants to patients and clinicians to improve clinical care and ultimately health outcomes, and assess the health impact and cost-effectiveness of reporting these variants on a broader population scale. This information will be critical in moving genome sequencing into wide clinical use, as institutions are increasingly concerned about their obligations for following up such results. Without accurate estimates of penetrance and pathogenicity to target feedback only to patients truly at high risk, institutional responsibilities for curating, counseling, and following up these variants will not be sustainable.
The Implementing Genomics in Practice (IGNITE) network brings evidence generation and assessment of impact beyond the level of individual institutions, using a “hub and spoke” model to transport effective implementation efforts from early adopter institutions to diverse sets of partner sites with less specialized genomics expertise and to expand and link existing genomic medicine efforts. Initiated in 2013, IGNITE is developing, assessing, and disseminating successful genomic medicine practice models that integrate genomic data seamlessly into the EMR and deploy clinical decision support tools for point-of-care decision making [12]. Similar to NHGRI’s other collaborative networks, individual IGNITE sites collaborate in their approaches to testing and evaluating these models, but each conducts an individual project that varies in topic and scope, including using APOL1 variants as genetic markers for disease risk prediction and prevention, implementing patient-facing tools for using family history data, incorporating pharmacogenomic data into clinical care, refining diagnosis of diabetes using sequence-based mutation discovery, and creating novel educational approaches [12]. Valuable products to date include the “Supporting Practice through Application, Resources, and Knowledge (SPARK)” Toolbox of nearly 50 tools for clinicians, investigators, educators, and patients to facilitate incorporating genomics into patient care [80].
Lastly, the Clinical Genome Resource (ClinGen) arose directly from NHGRI’s first genomic medicine symposium to fill an urgent need for a systematic approach to developing and disseminating consensus information about genomic variants relevant for clinical care. ClinGen is developing a comprehensive knowledge base that captures genetic variants, their phenotypic associations, and other pertinent phenotypic information and is openly accessible to clinical groups attempting to interpret sequencing data [81]. To date, such efforts have mostly been pursued independently by individual groups, with investigators often evaluating the same assays, assessing the same evidence, and in most cases coming to the same conclusions, all in a highly duplicative fashion within and across sites. ClinGen was established to develop a consensus process for identifying genomic variants that are relevant for clinical care and to incorporate this information into a comprehensive, accessible electronic resource. Initiated in 2013, it is based on the publicly accessible ClinVar database which serves as the primary site for archiving of information about genomic variation and its relationship to human health [82]. Given the many conflicting interpretations of the pathogenicity of genomic variants, ClinVar uses a rating system to assess the quality and consistency of variant assertions submitted by over 500 participating clinical and research laboratories. Assertions receiving the highest ratings are those endorsed by published practice guidelines, such as the 56 ACMG genes [31], followed by interpretations provided by a ClinGen-approved expert panel. ClinVar assertions follow the ACMG recommendations for variant interpretation and classify variants as one of five (often collapsed to three) classes: pathogenic and likely pathogenic; uncertain significance; and likely benign or benign. ClinGen uses the ClinVar variant archive and annotations as well as published literature and clinical experience to assess the evidence of association between genes and genetic disorders. ClinGen has established several working groups in clinical domains such as cardiovascular disease, inborn errors of metabolism, and hereditary cancer syndromes, which classify available evidence of gene-disease associations as definitive, strong, moderate, limited, or even disputed or refuted. These classifications are based on five key evidence types including the number of unrelated probands with clinically associated variants, amount of functional data, number of publications describing patients with variants, time since first publication, and strength of refuting evidence. A crucial clinical question arising when variants are encountered in a specific patient is whether their presence should change management (are “actionable”) and thus they should be reported to the patient and his/her clinicians. This challenging issue is being addressed by ClinGen’s Actionability Working Group, which has developed a semiquantitative assessment that involves disease severity and the availability, efficacy, and invasiveness of interventions [83]. ClinGen is rapidly becoming a definitive resource for assessment of variant pathogenicity and actionability, and is increasingly being looked to by the U.S. Food and Drug Administration in its efforts to regulate and advance next-generation sequencing based diagnostics into clinical care [84].
The system of open sharing of clinically interpreted genomic data supported by ClinGen and ClinVar opens a new era of transparency and dissemination of genomic knowledge painstakingly gained patient-by-patient with the potential rapidly to inform and enhance clinical care [62]. Our understanding of the role of genetic variation (particularly rare variation) in disease depends critically upon sharing data on these variants and their associated phenotypes among clinicians, clinical laboratories, professional organizations, and existing data bases. Initiatives to enhance sharing of genomic data with or without clinical information are growing in number and reach, and include the Beacon and BRCA Challenge efforts of the Global Alliance for Genomics and Health (GA4GH) [85], the Exome Aggregation Consortium (ExAC) [86], and the Clinical Pharmacogenomic Implementation Consortium (CPIC) [87]. ClinVar and ClinGen actively reach out to groups worldwide who are collecting and characterizing human variation and encourage open data sharing (as consistent with patient/participant consent), use of standard methods and ontologies, and comparison of approaches and results [62]. Through these efforts ClinGen works to maximize the efficiency and expertise of its clinical domain working groups, reduce or eliminate redundancies in classification efforts, and resolve conflicting classifications. All clinical and research laboratories are strongly urged to contribute data on sequence variants and their phenotypic manifestations, along with the labs’ determinations of pathogenicity, to ClinVar to facilitate correct classification and consistent interpretation of these variants in clinical care [36]. The importance of periodic reinterrogation of these databases and updating of variant classifications as knowledge accrues [88] cannot be over-emphasized.
4. Genomic medicine implementation research outside of NHGRI
Many of the multicenter programs described here were modeled upon or informed by smaller, single-site projects at early adopter institutions such as those attending NHGRI’s first genomic medicine meeting [2]. These include (among many) Children’s Mercy Hospital’s neonatal intensive care sequencing project [89], Marshfield Clinic’s Personalized Medicine Research Program [90], Northwestern University’s EHR-linked biobank (NUgene) and personalized medicine pilot project [91], St. Jude Children’s Research Hospital’s PG4KDS program [92], Vanderbilt University’s Pharmacogenomic Resource for Enhanced Decisions in Care and Treatment (PREDICT) initiative for preemptive genotyping of pharmacogenetic variants and provision of associated clinical decision support [93]. Other programs outside the U.S. include the Genomics England effort to sequence 100,000 genomes for the care of patients with rare diseases and cancer [94], the Estonian Genome Center’s effort to link genetic data with national health registries in piloting personalized medicine [95], the European Union’s Ubiquitous Pharmacogenomics project to implement clinical pharmacogenomics in seven nations [96], Singapore’s Personalized OMIC Lattice for Advanced Research and Improving Stratification (POLARIS) project to assess genetic risk for stromal corneal dystrophies [97], and Thailand’s innovative Pharmacogenomics and Personalized Medicine program and pharmacogenetics card [98]. In addition, the U.S. Precision Medicine Initiative will collect genomic and EHR data on one million or more Americans to implement personalized medicine in close partnership with study participants who will have access to their individual data [99]. While these programs are too varied and complex to describe here, they address important aspects of genomic medicine implementation at both small and large scale; the free exchange of information and experience derived from them will vastly accelerate the evaluation and implementation of genomic medicine on a global scale.
5. Summary
The research and dissemination initiatives of the National Human Genome Research Institute and other groups described here are designed to speed the evaluation and incorporation, where appropriate, of genomic technologies and findings into routine clinical care. We believe these approaches to have considerable potential for personalizing medical treatments and enhancing the effectiveness of heath care; at present however, aside from specific applications in cancer genomics, pharmacogenomics, and undiagnosed diseases, this belief remains largely a hypothesis waiting to be tested. Integration with other –omics technologies such as epigenomics and transcriptomics and the application of novel bioinformatics and systems medicine approaches may bring further advances. It is indeed possible that the use of genomic information may not improve clinical outcomes, and almost certainly will not in every instance. Evidence supporting the utility of genomic information thus needs to be generated systematically and assessed dispassionately, while at the same time avoiding unreasonable expectations for exhaustive evidence or randomized clinical trial assessment of every genomic variant that may influence human health and disease. Potential misuses of genomic information that can cause unnecessary anxiety, discrimination, increased medical costs, or diverted resources also need to be recognized and avoided [100]. The research programs outlined above, in conjunction with additional future projects now in early design phases, are expected to address many of questions and barriers associated with genomic medicine implementation. These efforts will provide a valuable complement to the highly successful basic research enterprise that has made such genomic advances conceivable. Actual adoption of successful approaches in clinical care will depend upon the willingness, interest, and energy of professional societies, practitioners, patients, and payers to promote their responsible use and share their experiences in doing so.
Footnotes
Conflict of interest
The author declared he does not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- 1.McCarthy JJ, McLeod HL, Ginsburg GS. Genomic medicine: a decade of successes, challenges, and opportunities. Sci Transl Med. 2013 Jun 12;5(189):189sr4. doi: 10.1126/scitranslmed.3005785. [DOI] [PubMed] [Google Scholar]
- 2.Manolio TA, Chisholm RL, Ozenberger B, Roden DM, Williams MS, Wilson R, Bick D, Bottinger EP, Brilliant MH, Eng C, Frazer KA, Korf B, Ledbetter DH, Lupski JR, Marsh C, Mrazek D, Murray MF, O’Donnell PH, Rader DJ, Relling MV, Shuldiner AR, Valle D, Weinshilboum R, Green ED, Ginsburg GS. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013 Apr;15(4):258–267. doi: 10.1038/gim.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green ED, Guyer MS National Human Genome Research Institute. Charting a course for genomic medicine from base pairs to bedside. Nature. 2011 Feb 10;470(7333):204–213. doi: 10.1038/nature09764. [DOI] [PubMed] [Google Scholar]
- 4.Manolio TA, Green ED. Leading the way to genomic medicine. Am J Med Genet C Semin Med Genet. 2014;166:1–7. doi: 10.1002/ajmg.c.31384. [DOI] [PubMed] [Google Scholar]
- 5.Eccles MP, Foy R, Sales A, Wensing M, Mittman B. Implementation science six years on–our evolving scope and common reasons for rejection without review. Implement Sci. 2012;2012(7):71. doi: 10.1186/1748-5908-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon AS, Fulton RS, Qin X, Mardis ER, Nickerson DA, Scherer S. PGRNseq: a targeted capture sequencing panel for pharmacogenetic research and implementation. Pharmacogen Genom. 2016;26:161–168. doi: 10.1097/FPC.0000000000000202. http://dx.doi.org/10.1097/FPC.0000000000000202 [Available on 2017-07-05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen-Torvik LJ, Stallings SC, Gordon AS, Almoguera B, Basford MA, Bielinski SJ, Brautbar A, Brilliant M, Carrell DS, Connolly J, Crosslin DR, Doheny KF, Gallego CJ, Gottesman O, Kim DS, Leppig KA, Li R, Lin S, Manzi S, Mejia AR, Pacheco JA, Pan V, Pathak J, Perry CL, Peterson JF, Prows CA, Ralston J, Rasmussen LV, Ritchie MD, Sadhasivam S, Scott SA, Smith M, Vega A, Vinks A, Volpi S, Wolf W, Bottinger E, Chisholm RL, Chute CG, Haines JL, Harley JB, Keating B, Holm IA, Kullo IJ, Jarvik GP, Larson EB, Manolio T, McCarty CA, Nickerson DA, Scherer SE, Williams MS, Roden DM, Denny JC. Design and anticipated outcomes of the eMERGE-PGx project: a multi-center pilot for pre-emptive pharmacogenomics in electronic health record systems. Clin Pharmacol Ther. 2014;96:482–489. doi: 10.1038/clpt.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CPIC, Clinical Pharmacogenetics Implementation Consortium. [accessed 05.21.16]; https://cpicpgx.org/
- 9.PharmGKB. [accessed 05.21.16];DPWG: Dutch Pharmacogenetics Working Group. https://www.pharmgkb.org/page/dpwg.
- 10.National Human Genome Research Institute, Genomic Medicine Working Group. [accessed 04.12.16]; https://www.genome.gov/27549220/genomic-medicine-working-group/
- 11.Ramos EM, Din-Lovinescu C, Berg JS, Brooks LD, Duncanson A, Dunn M, Good P, Hubbard T, Jarvik GP, O’Donnell C, Sherry ST, Aronson N, Biesecker L, Blumberg B, Calonge N, Colhoun HM, Epstein RS, Flicek P, Gordon ES, Green ED, Green RC, Hurles M, Kawamoto K, Knaus W, Ledbetter DH, Levy HP, Lyon E, Maglott D, McLeod HL, Rahman N, Randhawa G, Wicklund C, Manolio TA, Chisholm RL, Williams MS. Characterizing genetic variants for clinical action. Am J Med Genet C Semin Med Genet. 2014;166:93–104. doi: 10.1002/ajmg.c.31386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weitzel KW, Alexander M, Bernhardt BA, Calman N, Carey DJ, Cavallari LH, Field JR, Hauser D, Junkins HA, Levin PA, Levy K, Madden EB, Manolio TA, Odgis J, Orlando LA, Pyeritz R, Wu RR, Shuldiner AR, Bottinger EP, Denny JC, Dexter PR, Flockhart DA, Horowitz CR, Johnson JA, Kimmel SE, Levy MA, Pollin TI, Ginsburg GS IGNITE network. The IGNITE network: a model for genomic medicine implementation and research. BMC Med Genom. 2016 Jan 5;9(1):1. doi: 10.1186/s12920-015-0162-5. http://dx.doi.org/10.1186/s12920-015-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manolio TA, Murray MF. Inter-Society Coordinating Committee for Practitioner Education in Genomics, The growing role of professional societies in educating clinicians in genomics. Genet Med. 2014 Aug;16(8):571–572. doi: 10.1038/gim.2014.6. http://dx.doi.org/10.1038/gim.2014.6. [DOI] [PubMed] [Google Scholar]
- 14.National Human Genome Research Institute, Inter-Society Coordinating Committee for Practitioner Education in Genomics. [accessed 04.12.16]; http://www.genome.gov/27554614.
- 15.Korf BR, Berry AB, Limson M, Marian AJ, Murray MF, O’Rourke PP, Passamani ER, Relling MV, Tooker J, Tsongalis GJ, Rodriguez LL. Framework for development of physician competencies in genomic medicine: report of the competencies working group of the inter-society coordinating committee for physician education in genomics. Genet Med. 2014 Nov;16(11):804–809. doi: 10.1038/gim.2014.35. http://dx.doi.org/10.1038/gim.2014.35. [DOI] [PubMed] [Google Scholar]
- 16.National Human Genome Research Institute, Genomics and Genetics Competency Center (G2C2) [accessed 04.12.16]; http://g-2-c-2.org//discipline/physician.
- 17.Haspel RL. Teaching residents genomic pathology: a novel approach for new technology. Adv Anat Pathol. 2013 Mar;20(2):125–129. doi: 10.1097/PAP.0b013e31828629b2. http://dx.doi.org/10.1097/PAP.0b013e31828629b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Society of Clinical Pathologists. [accessed 04.12.16];Training Residents in Genomics. http://www.pathologylearning.org/trig/about.
- 19.Manolio TA, Abramowicz M, Al-Mulla F, Anderson W, Balling R, Berger AC, Bleyl S, Chakravarti A, Chantratita W, Chisholm RL, Dissanayake VHW, Dunn M, Dzau VJ, Han B-G, Hubbard T, Kolbe A, Korf B, Kubo M, Lasko P, Leego E, Mahasirimongkol S, Majumdar PP, Matthijs G, McLeod HL, Metspalu A, Meulien P, Miyano S, Naparstek Y, O’Rourke PP, Patrinos GP, Rehm HL, Relling MV, Rennert G, Rodriguez LL, Roden DM, Shuldiner AR, Sinha S, Tan P, Ulfendahl M, Ward R, Williams MS, Wong JEL, Green ED, Ginsburg GS. Global implementation of genomic medicine: we are not alone. Sci Transl Med. 2015;7:ps13. doi: 10.1126/scitranslmed.aab0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Human Genome Research Institute. [accessed 04.16.16];Research Directions in Genetically-Mediated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. https://www.genome.gov/27560487.
- 21.Manolio TA, Hutter C, Vigan MA, Cibotti R, Davis RL, Denny JC, La Grenade L, Wheatley LM, Carrington MN, Chantratita W, Chung WH, Dalton AD, Hung SI, Lee MT, Leeder JS, Lertora JJL, Mahasirimongkol S, McLeod HL, Mockenhaupt M, Pacanowski M, Phillips EJ, Pinheiro S, Pirmohamed M, Sung C, Suwankesawong W, Trepanier L, Tumminia SJ, Veenstra D, Yuliwulandari R, Shear NH. Research directions in genetically mediated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. doi: 10.1002/cpt.890. Submitted, Science Translational Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Academies, Global Genomic Medicine Collaborative (G2MC) [accessed 04.16.16]; http://www.nationalacademies.org/hmd/Activities/Research/GenomicBasedResearch/Innovation-Collaboratives/Global_Genomic_Medicine_Collaborative.aspx.
- 23.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009 Apr;29(4):431–438. doi: 10.1161/ATVBAHA.108.179564. http://dx.doi.org/10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. New Eng J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 25.Marais AD, Kim JB, Wasserman SM, Lambert G. PCSK9 inhibition in LDL cholesterol reduction: genetics and therapeutic implications of very low plasma lipoprotein levels. Pharmacol Ther. 2015 Jan;145:58–66. doi: 10.1016/j.pharmthera.2014.07.004. http://dx.doi.org/10.1016/j.pharmthera.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Brennan P, Wild CP. Genomics of cancer and a new era for cancer prevention. PLoS Genet. 2015 Nov 5;11(11):e1005522. doi: 10.1371/journal.pgen.1005522. http://dx.doi.org/10.1371/journal.pgen.1005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu X, Petrovski S, Xie P, Ruzzo EK, Lu YF, McSweeney KM, Ben-Zeev B, Nissenkorn A, Anikster Y, Oz-Levi D, Dhindsa RS, Hitomi Y, Schoch K, Spillmann RC, Heimer G, Marek-Yagel D, Tzadok M, Han Y, Worley G, Goldstein J, Jiang YH, Lancet D, Pras E, Shashi V, McHale D, Need AC, Goldstein DB. Whole-exome sequencing in undiagnosed genetic diseases: interpreting 119 trios. Genet Med. 2015 Oct;17(10):774–781. doi: 10.1038/gim.2014.191. http://dx.doi.org/10.1038/gim.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackerman MJ, Mohler PJ. Defining a new paradigm for human arrhythmia syndromes: phenotypic manifestations of gene mutations in ion channel-and transporter-associated proteins. Circ Res. 2010 Aug 20;107(4):457–465. doi: 10.1161/CIRCRESAHA.110.224592. http://dx.doi.org/10.1161/CIRCRESAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackerman MJ. My Approach to treatment of the congenital long QT syndromes. Trends Cardiovasc Med. 2015 Jan;25(1):67–69. doi: 10.1016/j.tcm.2014.07.007. http://dx.doi.org/10.1016/j.tcm.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Itoh H, Crotti L, Aiba T, Spazzolini C, Denjoy I, Fressart V, Hayashi K, Nakajima T, Ohno S, Makiyama T, Wu J, Hasegawa K, Mastantuono E, Dagradi F, Pedrazzini M, Yamagishi M, Berthet M, Murakami Y, Shimizu W, Guicheney P, Schwartz PJ, Horie M. The genetics underlying acquired long QT syndrome: impact for genetic screening. Eur Heart J. 2016 May 7;37(18):1456–1464. doi: 10.1093/eurheartj/ehv695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, McGuire AL, Nussbaum RL, O’Daniel JM, Ormond KE, Rehm HL, Watson MS, Williams MS, Biesecker LG. American College of Medical Genetics and Genomics, ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013 Jul;15(7):565–574. doi: 10.1038/gim.2013.73. http://dx.doi.org/10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darnell AJ, Austin H, Bluemke DA, Cannon RO, 3rd, Fischbeck K, Gahl W, Goldman D, Grady C, Greene MH, Holland SM, Hull SC, Porter FD, Resnik D, Rubinstein WS, Biesecker LG. A clinical service to support the return of secondary genomic findings in human research. Am J Hum Genet. 2016 Mar 3;98(3):435–441. doi: 10.1016/j.ajhg.2016.01.010. http://dx.doi.org/10.1016/j.ajhg.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83–99. doi: 10.1016/j.jacc.2014.05.003. [published correction appears in J Am Coll Cardiol. 2014;64:1188] http://dx.doi.org/10.1016/j.jacc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Morales A, Hershberger RE. The rationale and timing of molecular genetic testing for dilated cardiomyopathy. Can J Cardiol. 2015 Nov;31(11):1309–1312. doi: 10.1016/j.cjca.2015.06.034. http://dx.doi.org/10.1016/j.cjca.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poloni G, De Bortoli M, Calore M, Rampazzo A, Lorenzon A. Arrhythmogenic right-ventricular cardiomyopathy: molecular genetics into clinical practice in the era of next generation sequencing. J Cardiovasc Med Hagerst. 2016 Jun;17(6):399–407. doi: 10.2459/JCM.0000000000000385. http://dx.doi.org/10.2459/JCM.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 36.Harrison SM, Riggs ER, Maglott DR, Lee JM, Azzariti DR, Niehaus A, Ramos EM, Martin CL, Landrum MJ, Rehm HL. Using Clinvar as a resource to support variant interpretation. Curr Protoc Hum Genet. 2016 Apr 1;89:8-16.1–8.16.23. doi: 10.1002/0471142905.hg0816s89. http://dx.doi.org/10.1002/0471142905.hg0816s89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadfield GS, Humphries SE. Familial hypercholesterolaemia: cascade testing is tried and tested and cost effective. BMJ. 2007 Oct 6;335(7622):683. doi: 10.1136/bmj.39353.483160.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams RR, Hopkins PN, Stephenson S, Wu L, Hunt SC. Primordial prevention of cardiovascular disease through applied genetics. Prev Med. 1999 Dec;29(6 Pt 2):S41–S49. doi: 10.1006/pmed.1999.0513. [DOI] [PubMed] [Google Scholar]
- 39.Kathiresan S, Melander O, Anevski D, et al. Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med. 2008;358:1240–1249. doi: 10.1056/NEJMoa0706728. [DOI] [PubMed] [Google Scholar]
- 40.Brautbar A, Pompeii LA, Dehghan A, Ngwa JS, Nambi V, Virani SS, Rivadeneira F, Uitterlinden AG, Hofman A, Witteman JC, Pencina MJ, Folsom AR, Cupples LA, Ballantyne CM, Boerwinkle E. A genetic risk score based on direct associations with coronary heart disease improves coronary heart disease risk prediction in the atherosclerosis risk in communities (ARIC), but not in the Rotterdam and Framingham offspring. Stud Atheroscler. 2012 Aug;223(2):421–426. doi: 10.1016/j.atherosclerosis.2012.05.035. http://dx.doi.org/10.1016/j.atherosclerosis.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labos C, Martinez SC, Leo Wang RH, Lenzini PA, Pilote L, Bogaty P, Brophy JM, Engert JC, Cresci S, Thanassoulis G. Utility of a genetic risk score to predict recurrent cardiovascular events 1 year after an acute coronary syndrome: a pooled analysis of the RISCA, PRAXY, and TRIUMPH cohorts. Atherosclerosis. 2015 Sep;242(1):261–267. doi: 10.1016/j.atherosclerosis.2015.07.029. http://dx.doi.org/10.1016/j.atherosclerosis.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krarup NT, Borglykke A, Allin KH, Sandholt CH, Justesen JM, Andersson EA, Grarup N, Jørgensen T, Pedersen O, Hansen T. A genetic risk score of 45 coronary artery disease risk variants associates with increased risk of myocardial infarction in 6041 Danish individuals. Atherosclerosis. 2015 Jun;240(2):305–310. doi: 10.1016/j.atherosclerosis.2015.03.022. http://dx.doi.org/10.1016/j.atherosclerosis.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Kullo IJ, Jouni H, Olson JE, Montori VM, Bailey KR. Design of a randomized controlled trial of disclosing genomic risk of coronary heart disease: the Myocardial Infarction Genes (MI-GENES) study. BMC Med Genom. 2015 Aug 15;8:51. doi: 10.1186/s12920-015-0122-0. http://dx.doi.org/10.1186/s12920-015-0122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knowles JW1, Assimes TL, Kiernan M, Pavlovic A, Goldstein BA, Yank V, McConnell MV, Absher D, Bustamante C, Ashley EA, Ioannidis JP. Randomized trial of personal genomics for preventive cardiology: design and challenges. Circ Cardiovasc Genet. 2012 Jun;5(3):368–376. doi: 10.1161/CIRCGENETICS.112.962746. http://dx.doi.org/10.1161/CIRCGENETICS.112.962746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kullo IJ, Jouni H, Austin EE, Brown SA, Kruisselbrink TM, Isseh IN, Haddad RA, Marroush TS, Shameer K, Olson JE, Broeckel U, Green RC, Schaid DJ, Montori VM, Bailey KR. Incorporating a genetic risk score into coronary heart disease risk estimates: effect on low-density lipoprotein cholesterol levels (the MI-GENES clinical trial) Circulation. 2016 Mar 22;133(12):1181–1188. doi: 10.1161/CIRCULATIONAHA.115.020109. http://dx.doi.org/10.1161/CIRCULATIONAHA.115.020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shea S, Ottman R, Gabrieli C, Stein Z, Nichols A. Family history as an independent risk factor for coronary artery disease. J Am Coll Cardiol. 1984 Oct;4(4):793–801. doi: 10.1016/s0735-1097(84)80408-8. [DOI] [PubMed] [Google Scholar]
- 47.Sesso HD, Lee IM, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001 Jul 24;104(4):393–398. doi: 10.1161/hc2901.093115. [DOI] [PubMed] [Google Scholar]
- 48.Global Alliance for Genomics and Health (GA4GH) [accessed 05.13.16];Family History Tool Inventory. https://genomicsandhealth.org/work-products-demonstration-projects/work-products/family-history-tool-inventory.
- 49.U.S. Surgeon General. My Family Health Portrait. https://familyhistory.hhs.gov/FHH/html/index.html.
- 50.Orlando LA, Buchanan AH, Hahn SE, Christianson CA, Powell KP, Skinner CS, Chesnut B, Blach C, Due B, Ginsburg GS, Henrich VC. Development and validation of a primary care-based family health history and decision support program (MeTree) N C Med J. 2013 Jul-Aug;74(4):287–296. [PMC free article] [PubMed] [Google Scholar]
- 51.Edelman EA, Lin BK, Doksum T, Drohan B, Edelson V, Dolan SM, Hughes KS, O’Leary J, Galvin SL, Degroat N, Pardanani S, Feero WG, Adams C, Jones R, Scott J. Implementation of an electronic genomic and family health history tool in primary prenatal care. Am J Med Genet C Semin Med Genet. 2014 Mar;166C(1):34–44. doi: 10.1002/ajmg.c.31389. http://dx.doi.org/10.1002/ajmg.c.31389. [DOI] [PubMed] [Google Scholar]
- 52.Qureshi N, Armstrong S, Dhiman P, Saukko P, Middlemass J, Evans PH, Kai J ADDFAM (Added Value of Family History in CVD Risk Assessment) Study Group. Effect of adding systematic family history enquiry to cardiovascular disease risk assessment in primary care: a matched-pair, cluster randomized trial. Ann Int Med. 2012 Feb 21;156(4):253–262. doi: 10.7326/0003-4819-156-4-201202210-00002. http://dx.doi.org/10.7326/0003-4819-156-4-201202210-00002. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JA, Gong L, Whirl-Carrillo M, Gage BF, Scott SA, Stein CM, Anderson JL, Kimmel SE, Lee MT, Pirmohamed M, Wadelius M, Klein TE, Altman RB Clinical Pharmacogenetics Implementation Consortium. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011 Oct;90(4):625–629. doi: 10.1038/clpt.2011.185. http://dx.doi.org/10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fackler JL, McGuire AL. Paving the way to personalized genomic medicine: steps to successful implementation. Curr Pharmacogn Pers Med. 2009 Jun 1;7(2):125. doi: 10.2174/187569209788653998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biesecker LG, Mullikin JC, Facio FM, Turner C, Cherukuri PF, Blakesley RW, Bouffard GG, Chines PS, Cruz P, Hansen NF, Teer JK, Maskeri B, Young AC, Manolio TA, Wilson AF, Finkel T, Hwang P, Arai A, Remaley AT, Sachdev V, Shamburek R, Cannon RO, Green ED NISC Comparative Sequencing Program. The clinSeq project: piloting large-scale genome sequencing for research in genomic medicine. Genome Res. 2009 Sep;19(9):1665–1674. doi: 10.1101/gr.092841.109. http://dx.doi.org/10.1101/gr.092841.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Damani SB, Topol EJ. Emerging genomic applications in coronary artery disease. JACC Cardiovasc Interv. 2011 May;4(5):473–482. doi: 10.1016/j.jcin.2010.12.016. http://dx.doi.org/10.1016/j.jcin.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ginsburg GS, Staples J, Abernethy AP. Academic medical centers: ripe for rapid-learning personalized health care. Sci Transl Med. 2011 Sep 21;3(101):101cm27. doi: 10.1126/scitranslmed.3002386. http://dx.doi.org/10.1126/scitranslmed.3002386. [DOI] [PubMed] [Google Scholar]
- 58.Bainbridge MN, Wiszniewski W, Murdock DR, Friedman J, Gonzaga-Jauregui C, Newsham I, Reid JG, Fink JK, Morgan MB, Gingras MC, Muzny DM, Hoang LD, Yousaf S, Lupski JR, Gibbs RA. Whole-genome sequencing for optimized patient management. Sci Transl Med. 2011;3:87re3. doi: 10.1126/scitranslmed.3002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum RL, Kleta R, Gahl WA, Boehm M. NT5E mutations and arterial calcifications. N Engl J Med. 2011;364:432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Human Genome Research Institute, National Institute for Child Health and Human Development, Office of Rare Disease Research. [accessed 05.14.16];Newborn Screening in the Genomic Era: Setting a Research Agenda. http://www.nichd.nih.gov/about/meetings/2010/pages/121410.aspx.
- 61.Feero WG, Green ED. Genomics education for health care professionals in the 21st century. JAMA. 2011;306:989–990. doi: 10.1001/jama.2011.1245. [DOI] [PubMed] [Google Scholar]
- 62.Rehm HL, Berg JS, Brooks LD, Bustamante CD, Evans JP, Landrum MJ, Ledbetter DH, Maglott DR, Martin CL, Nussbaum RL, Plon SE, Ramos EM, Sherry ST, Watson MS ClinGen. ClinGen–the clinical genome resource. N Engl J Med. 2015 Jun 4;372(23):2235–2242. doi: 10.1056/NEJMsr1406261. http://dx.doi.org/10.1056/NEJMsr1406261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kellis M, Wold B, Snyder MP, Bernstein BE, Kundaje A, Marinov GK, Ward LD, Birney E, Crawford GE, Dekker J, Dunham I, Elnitski LL, Farnham PJ, Feingold EA, Gerstein M, Giddings MC, Gilbert DM, Gingeras TR, Green ED, Guigo R, Hubbard T, Kent J, Lieb JD, Myers RM, Pazin MJ, Ren B, Stamatoyannopoulos JA, Weng Z, White KP, Hardison RC. Defining functional DNA elements in the human genome. Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6131–6138. doi: 10.1073/pnas.1318948111. http://dx.doi.org/10.1073/pnas.1318948111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Cancer Institute, National Human Genome Research Institute, The Cancer Genome Atlas (TCGA) [accessed 05.14.16]; http://cancergenome.nih.gov/
- 65.Gahl WA, Markello TC, Toro C, Fajardo KF, Sincan M, Gill F, Carlson-Donohoe H, Gropman A, Pierson TM, Golas G, Wolfe L, Groden C, Godfrey R, Nehrebecky M, Wahl C, Landis DM, Yang S, Madeo A, Mullikin JC, Boerkoel CF, Tifft CJ, Adams D. The national institutes of health undiagnosed diseases program: insights into rare diseases. Genet Med. 2012;14:51–59. doi: 10.1038/gim.0b013e318232a005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gahl WA, Wise AL, Ashley EA. The undiagnosed diseases network of the national institutes of health: a national extension. JAMA. 2015 Nov 3;314(17):1797–1798. doi: 10.1001/jama.2015.12249. http://dx.doi.org/10.1001/jama.2015.12249. [DOI] [PubMed] [Google Scholar]
- 67.Taruscio D, Groft SC, Cederroth H, Melegh B, Lasko P, Kosaki K, Baynam G, McCray A, Gahl WA. Undiagnosed Diseases Network International (UDNI): white paper for global actions to meet patient needs. Mol Genet Metab. 2015 Dec;116(4):223–225. doi: 10.1016/j.ymgme.2015.11.003. http://dx.doi.org/10.1016/j.ymgme.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Petrikin JE, Willig LK, Smith LD, Kingsmore SF. Rapid whole genome sequencing and precision neonatology. Semin Perinatol. 2015 Dec;39(8):623–631. doi: 10.1053/j.semperi.2015.09.009. http://dx.doi.org/10.1053/j.semperi.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Green RC, Goddard KA, Jarvik GP, Amendola LM, Appelbaum PS, Berg JS, Bernhardt BA, Biesecker LG, Biswas S, Blout CL, Bowling KM, Brothers KB, Burke W, Caga-Anan CF, Chinnaiyan AM, Chung WK, Clayton EW, Cooper GM, East K, Evans JP, Fullerton SM, Garraway LA, Garrett JR, Gray SW, Henderson GE, Hindorff LA, Holm IA, Lewis MH, Hutter CM, Janne PA, Joffe S, Kaufman D, Knoppers BM, Koenig BA, Krantz ID, Manolio T, McCullough L, McEwen J, McGuire A, Muzny D, Myers RM, Nickerson DA, Ou J, Parsons DW, Petersen GM, Plon SE, Rehm HL, Roberts JS, Robinson D, Salama JS, Scollon S, Sharp RR, Shirts B, Spinner NB, Tabor HK, Tarczy-Hornoch P, Veenstra DL, Wagle N, Weck K, Wilfond BS, Wilhelmsen K, Wolf SM, Wynn J, Yu JH for the CSER Consortium. The clinical sequencing exploratory research consortium: accelerating the evidence-based practice of genomic medicine. Am J Hum Genet. 2016;98(6):1051–1066. doi: 10.1016/j.ajhg.2016.04.011. http://dx.doi.org/10.1016/j.ajhg.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015 May;17(5):405–424. doi: 10.1038/gim.2015.30. http://dx.doi.org/10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rehm HL, Bale SJ, Bayrak-Toydemir P, Berg JS, Brown KK, Deignan JL, Friez MJ, Funke BH, Hegde MR, Lyon E. Working group of the American college of medical genetics and genomics laboratory quality assurance committee. ACMG clinical laboratory standards for next-generation sequencing. Genet Med. 2013 Sep;15(9):733–747. doi: 10.1038/gim.2013.92. http://dx.doi.org/10.1038/gim.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.National Human Genome Research Institute. [accessed 05.15.16];Clinical Sequencing Evidence-Generating Research (CSER2) - Clinical Sites. http://grants.nih.gov/grants/guide/rfa-files/RFA-HG-16-010.html.
- 73.National Human Genome Research Institute. [accessed 05.15.16];Clinical Sequencing Evidence-Generating Research (CSER2) - Clinical Sites with Enhanced Diversity. http://grants.nih.gov/grants/guide/rfa-files/RFA-HG-16-011.html.
- 74.National Human Genome Research Institute. [accessed 5.15.16];Investigator-Initiated Clinical Sequencing Research. http://grants.nih.gov/grants/guide/pa-files/PAR-16-209.html.
- 75.Denny JC, Bastarache L, Ritchie MD, Carroll RJ, Zink R, Mosley JD, Field JR, Pulley JM, Ramirez AH, Bowton E, Basford MA, Carrell DS, Peissig PL, Kho AN, Pacheco JA, Rasmussen LV, Crosslin DR, Crane PK, Pathak J, Bielinski SJ, Pendergrass SA, Xu H, Hindorff LA, Li R, Manolio TA, Chute CG, Chisholm RL, Larson EB, Jarvik GP, Brilliant MH, McCarty CA, Kullo IJ, Haines JL, Crawford DC, Masys DR, Roden DM. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Bio-technol. 2013 Dec;31(12):1102–1110. doi: 10.1038/nbt.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ritchie MD, Denny JC, Crawford DC, Ramirez AH, Weiner JB, Pulley JM, Basford MA, Brown-Gentry K, Balser JR, Masys DR, Haines JL, Roden DM. Robust replication of genotype-phenotype associations across multiple diseases in an electronic medical record. Am J Hum Genet. 2010;86:560–572. doi: 10.1016/j.ajhg.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vanderbilt University. [accessed 5.15.16];The eMERGE Network. https://emerge.mc.vanderbilt.edu/
- 78.Rasmussen-Torvik LJ, Stallings SC, Gordon AS, Almoguera B, Basford MA, Bielinski SJ, Brautbar A, Brilliant MH, Carrell DS, Connolly JJ, Crosslin DR, Doheny KF, Gallego CJ, Gottesman O, Kim DS, Leppig KA, Li R, Lin S, Manzi S, Mejia AR, Pacheco JA, Pan V, Pathak J, Perry CL, Peterson JF, Prows CA, Ralston J, Rasmussen LV, Ritchie MD, Sadhasivam S, Scott SA, Smith M, Vega A, Vinks AA, Volpi S, Wolf WA, Bottinger E, Chisholm RL, Chute CG, Haines JL, Harley JB, Keating B, Holm IA, Kullo IJ, Jarvik GP, Larson EB, Manolio T, McCarty CA, Nickerson DA, Scherer SE, Williams MS, Roden DM, Denny JC. Design and anticipated outcomes of the eMERGE-PGx project: a multicenter pilot for preemptive pharmacogenomics in electronic health record systems. Clin Pharmacol Ther. 2014 Oct;96(4):482–489. doi: 10.1038/clpt.2014.137. http://dx.doi.org/10.1038/clpt.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Driest SL, Wells QS, Stallings S, Bush WS, Gordon A, Nickerson DA, Kim JH, Crosslin DR, Jarvik GP, Carrell DS, Ralston JD, Larson EB, Bielinski SJ, Olson JE, Ye Z, Kullo IJ, Abul-Husn NS, Scott SA, Bottinger E, Almoguera B, Connolly J, Chiavacci R, Hakonarson H, Rasmussen-Torvik LJ, Pan V, Persell SD, Smith M, Chisholm RL, Kitchner TE, He MM, Brilliant MH, Wallace JR, Doheny KF, Shoemaker MB, Li R, Manolio TA, Callis TE, Macaya D, Williams MS, Carey D, Kapplinger JD, Ackerman MJ, Ritchie MD, Denny JC, Roden DM. Association of arrhythmia-related genetic variants with phenotypes documented in electronic medical records. JAMA. 2016 Jan 5;315(1):47–57. doi: 10.1001/jama.2015.17701. http://dx.doi.org/10.1001/jama.2015.17701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.IGNITE Research Network. [accessed 05.15.16];Supporting Practice through Application, Resources, and Knowledge (SPARK) Toolbox. https://ignite-genomicmedicine.org/spark-toolbox/
- 81.Ramos EM, Din-Lovinescu C, Berg JS, Brooks LD, Duncanson A, Dunn M, Good P, Hubbard TJ, Jarvik GP, O’Donnell C, Sherry ST, Aronson N, Biesecker LG, Blumberg B, Calonge N, Colhoun HM, Epstein RS, Flicek P, Gordon ES, Green ED, Green RC, Hurles M, Kawamoto K, Knaus W, Ledbetter DH, Levy HP, Lyon E, Maglott D, McLeod HL, Rahman N, Randhawa G, Wicklund C, Manolio TA, Chisholm RL, Williams MS. Characterizing genetic variants for clinical action. Am J Med Genet C Semin Med Genet. 2014 Mar;166C(1):93–104. doi: 10.1002/ajmg.c.31386. http://dx.doi.org/10.1002/ajmg.c.31386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Hoover J, Jang W, Katz K, Ovetsky M, Riley G, Sethi A, Tully R, Villamarin-Salomon R, Rubinstein W, Maglott DR. Clinvar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016 Jan 4;44(D1):D862–D868. doi: 10.1093/nar/gkv1222. http://dx.doi.org/10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hunter JE, Irving SA, Biesecker LG, Buchanan A, Jensen B, Lee K, Martin CL, Milko L, Muessig K, Niehaus AD, O’Daniel J, Piper MA, Ramos EM, Schully SD, Scott AF, Slavotinek A, Sobreira N, Strande N, Weaver M, Webber EM, Williams MS, Berg JS, Evans JP, Goddard KA. A standardized, evidence-based protocol to assess clinical actionability of genetic disorders associated with genomic variation. Genet Med. 2016 Apr 28; doi: 10.1038/gim.2016.40. http://dx.doi.org/10.1038/gim.2016.40. [DOI] [PMC free article] [PubMed]
- 84.Altman RB, Prabhu S, Sidow A, Zook JM, Goldfeder R, Litwack D, Ashley E, Asimenos G, Bustamante CD, Donigan K, Giacomini KM, Johansen E, Khuri N, Lee E, Liang XS, Salit M, Serang O, Tezak Z, Wall DP, Mansfield E, Kass-Hout T. A research roadmap for next-generation sequencing informatics. Sci Transl Med. 2016 Apr 20;8(335):335ps10. doi: 10.1126/scitranslmed.aaf7314. http://dx.doi.org/10.1126/scitranslmed.aaf7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.The Global Alliance for Genomics and Health. A federated ecosystem for sharing genomic, clinical data. Science. 2016 Jun 10;352(6291):1278–1280. doi: 10.1126/science.aaf6162. http://dx.doi.org/10.1126/science.aaf6162. [DOI] [PubMed] [Google Scholar]
- 86.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Cooper DN, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won H-H, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Roberto E, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016 Aug 17;536(7616):285–291. doi: 10.1038/nature19057. http://dx.doi.org/10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Relling MV, Klein TE. CPIC: clinical pharmacogenetics implementation consortium of the pharmacogenomics research network. Clin Pharmacol Ther. 2011 Mar;89(3):464–467. doi: 10.1038/clpt.2010.279. http://dx.doi.org/10.1038/clpt.2010.279. Epub 2011 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aronson SJ, Clark EH, Varugheese M, Baxter S, Babb LJ, Rehm HL. Communicating new knowledge on previously reported genetic variants. Genet Med. 2012;14:713–719. doi: 10.1038/gim.2012.19. http://dx.doi.org/10.1038/gim.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, Andraws N, Patterson ML, Krivohlavek LA, Fellis J, Humphray S, Saffrey P, Kingsbury Z, Weir JC, Betley J, Grocock RJ, Margulies EH, Farrow EG, Artman M, Safina NP, Petrikin JE, Hall KP, Kingsmore SF. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012 Oct 3;4(154):154ra135. doi: 10.1126/scitranslmed.3004041. http://dx.doi.org/10.1126/scitranslmed.3004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCarty CA, Wilke RA, Giampietro PF, Wesbrook SD, Caldwell MD. Marshfield clinic Personalized Medicine Research Project (PMRP): design, methods and recruitment for a large population-based biobank. Per Med. 2005;2:49–79. doi: 10.1517/17410541.2.1.49. [DOI] [PubMed] [Google Scholar]
- 91.Northwestern University Feinberg School of Medicine Center for Genetic Medicine. [accessed 05.21.2016];The NUgene Project. http://www.cgm.northwestern.edu/cores/nugene/index.html.
- 92.Hoffman JM, Haidar CE, Wilkinson MR, Crews KR, Baker DK, Kornegay NM, Yang W, Pui CH, Reiss UM, Gaur AH, Howard SC, Evans WE, Broeckel U, Relling MV. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014 Mar;166C(1):45–55. doi: 10.1002/ajmg.c.31391. http://dx.doi.org/10.1002/ajmg.c.31391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pulley JM, Denny JC, Peterson JF, Bernard GR, Vnencak-Jones CL, Ramirez AH, Delaney JT, Bowton E, Brothers K, Johnson K, Crawford DC, Schildcrout J, Masys DR, Dilks HH, Wilke RA, Clayton EW, Shultz E, Laposata M, McPherson J, Jirjis JN, Roden DM. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin Pharmacol Ther. 2012 Jul;92(1):87–95. doi: 10.1038/clpt.2011.371. http://dx.doi.org/10.1038/clpt.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. [accessed 05.21.16];Genomics England. http://www.genomicsengland.co.uk/
- 95.Leitsalu L, Alavere H, Tammesoo ML, Leego E, Metspalu A. Linking a population biobank with national health registries-the estonian experience. J Pers Med. 2015 Apr 16;5(2):96–106. doi: 10.3390/jpm5020096. http://dx.doi.org/10.3390/jpm5020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. [accessed 05.21.16];U-PGx Ubiquitous Pharmacogenomics. http://upgx.eu/
- 97.Agency for Science, Technology and Research (A*STAR) [accessed 05.21.16];Personalised OMIC Lattice for Advanced Research and Improving Stratification (POLARIS) http://research.singhealth.com.sg/Pages/polaris.aspx.
- 98.Sukasem C, Chantratita W. A success story in pharmacogenomics: genetic ID card for SJS/TEN. Pharmacogenomics. 2016 Apr;17(5):455–458. doi: 10.2217/pgs-2015-0009. http://dx.doi.org/10.2217/pgs-2015-0009. [DOI] [PubMed] [Google Scholar]
- 99.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015 Feb 26;372(9):793–795. doi: 10.1056/NEJMp1500523. http://dx.doi.org/10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McGuire AL, Burke W. Health system implications of direct-to-consumer personal genome testing. Public Health Genomics. 2011;14(1):53–58. doi: 10.1159/000321962. http://dx.doi.org/10.1159/000321962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.National Academies. [accessed 05.21.16];DIGITizE: Displaying and Integrating Genetic Information Through the EHR. http://www.nationalacademies.org/hmd/Activities/Research/GenomicBasedResearch/Innovation-Collaboratives/EHR.aspx.
- 102.Williams MS, Masys DR, Freimuth RR, Ostell JM, Butte AJ, Peterson JF, Kawamoto K, Overby CL, Welch BM, Manolio TA, Middleton B. Genomic clinical decision support: From desiderata to action. Submitted, Clinical Pharmacology and Therapeutics. [Google Scholar]
- 103.Gottesman O, Kuivaniemi H, Tromp G, Faucett WA, Li R, Manolio TA, Sanderson SC, Kannry J, Zinberg R, Basford MA, Brilliant M, Carey DJ, Chisholm RL, Chute CG, Connolly JJ, Crosslin D, Denny JC, Gallego CJ, Haines JL, Hakonarson H, Harley J, Jarvik GP, Kohane I, Kullo IJ, Larson EB, McCarty C, Ritchie MD, Roden DM, Smith ME, Böttinger EP, Williams MS eMERGE Network. The electronic medical records and genomics (eMERGE) network: past, present, and future. Genet Med. 2013 Oct;15(10):761–771. doi: 10.1038/gim.2013.72. http://dx.doi.org/10.1038/gim.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]