Abstract
Backgrund and aims
Resistin has been implicated in cardiovascular disease and poor interventional cardiovascular outcomes. Previous studies by our group demonstrated resistin promoted vascular smooth muscle cell (VSMC) migration through protein kinase C epsilon (PKCε) pathways, while few others showed that resistin induced reactive oxygen species (ROS) generation in various cell types. In this study, we aim to systemically examine the functional role of resistin at the cellular and tissue levels as well as the potential mechanistic relationship between resistin-induced PKCε activation and ROS production.
Methods
Plasma collected from patients undergoing carotid interventions was analyzed for resistin level and ROS. VSMCs were treated with resistin in the presence or absence of PKCε and NADPH oxidase (Nox)-specific inhibitors. Intracellular ROS production was analyzed using confocal microscopy and Nox activity with chemiluminescence. In vivo studies were performed in apolipoprotein E knock out (ApoE−/−) mice to determine therapeutic effects of PKCε-specific inhibitor, using the guide-wire injury model.
Results
We observed significant correlation between plasma resistin and circulating levels of oxidative stress in patients with severe atherosclerotic disease. We also demonstrated that resistin induced ROS production via PKCε-mediated Nox activation. Resistin-induced ROS production was time-dependent, and Nox4 was the primary isoform involved. Inhibition of Nox completely abolished resistin-exaggerated VSMC proliferation, migration and dedifferentiation, as well as pro-inflammatory cytokine release. Upstream modulation of PKCε significantly reduced resistin-mediated cytosolic ROS, Nox activity and VSMC dysfunction. Moreover, PKCε-specific inhibitor mitigated resistin-induced Nox activation and intimal hyperplasia in ApoE−/− mice.
Conclusions
Resistin-associated VSMC dysfunction and intimal hyperplasia are related to PKCε-dependent Nox activation and ROS generation. Targeting the PKCε-Nox pathway may represent a novel strategy in managing resistin-associated atherosclerotic complications.
Keywords: resistin, protein kinase C epsilon, NADPH-oxidase, vascular smooth muscle cell, inflammation, intimal hyperplasia
Introduction
Resistin, an adipokine expressed primarily in cells of monocyte/macrophage lineage in humans, is elevated in obese and diabetic individuals1,2 and has been associated with cardiovascular disease (CVD) and poor clinical outcomes.3 Serum resistin levels increase with exacerbated coronary artery disease (CAD) in patients with carotid artery stenosis.4 Additionally increasing clinical evidence shows that resistin is an independent predictor of major adverse cardiovascular events including restenosis, myocardial infarction, and death in patients undergoing coronary interventions.5 Elevated plasma resistin has been associated as a risk factor for CVD in a European population study and shown to be high in patients with carotid artery disease,6 but some studies did not find an association.7,8 Abundant resistin has also been detected in atherosclerotic regions of the human carotid artery and aorta.9 There is thus a large body of clinical data suggesting a potential role of resistin in atherosclerosis. However, direct evidence on the effect of resistin on clinical outcomes is still largely unexplored.
Controversial findings with respect to resistin’s mode of action in different species and cells types exist.10,11 However increasing evidence suggests that its inflammatory properties may be responsible for its effects in the human vasculature and atherosclerosis.12 Resistin has several features in common with pro-inflammatory cytokines.13,14 It promotes inflammation through induction of other cytokines, and the expression of resistin itself is up-regulated in peripheral blood mononuclear cells in response to stimulation by pro-inflammatory cytokines, such as IL-6, TNF-α, IL-1β, and LPS.15–16 In humans, elevated levels of resistin are frequently found in association with autoimmune diseases and inflammation.17,18 Resistin at a pathological concentration promotes vascular cell dysfunction, which includes endothelial cell activation,18 monocyte-endothelial cell adhesion,19 and vascular smooth muscle cell (VSMC) proliferation and migration.20–22 However, the underlying mechanisms of resistin-induced cytokine secretions and whether the cellular effect can be translated in vivo, are largely unknown.
We previously have demonstrated that protein kinase C epsilon (PKCε) is a novel upstream modulator for resistin-induced VSMC migration.20 Others have shown that resistin induces ROS production in various cell types.23–24 We hypothesized that PKCε is a key mediator for resistin-stimulated ROS production and subsequent inflammation and cellular dysfunction in VSMCs. In this study, we determined the role of resistin in atherosclerois through mechanistic evaluations, by: 1) examining relationships between ROS and resistin in human plasma; 2) performing in vitro studies in a human VSMC model; and 3) verifying the applicability of our observations in an in vivo gene knockout murine model.
Materials and methods
Human plasma analysis
Human plasma were collected from 99 elderly patients (mean age: 69.3 years) who underwent carotid interventions following an established protocol (IRB 23476). Samples were stored at −80 °C and analyzed with a Luminex magnetic beads-based assay for circulating resistin levels. Plasma carbonyl levels were measured as described later in this section.
Cell culture and in vitro treatments
Human coronary artery smooth muscle cells (HCASMC) or VSMCs from Genlantis25 were used at passage 5 to 8 for experiments. We chose a pathological resistin level of 40 ng/mL for our studies, based on published reports of resistin in human subjects.25–28 Cells were treated for various time points with or without resistin at 40 ng/mL in the presence or absence of 1 μM PKCε-specific peptide inhibitor, εV1-2, or Nox inhibitors VAS-2870 (10 μM) and DPI (5 μM). Inhibitors of other oxidases: rotenone (mitochondrial electron transport chain), and allopurinol (xanthine oxidase) were both used at 1 μM. Cells were pre-treated with inhibitors for 30 min before addition of resistin.
Cytosolic ROS measurement
VSMCs grown on coverslips were either treated with or without resistin and in the presence or absence of inhibitors for the different time points. Cells were incubated with 2 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA, Invitrogen) in HBSS at 37 °C for 30 min (for cytosolic ROS)29 and imaged using confocal microscopy (ZEISS Confocal LSM 710).
Nox activity
Nox activity was measured by the lucigenin-enhanced chemiluminescence method as described.30 Briefly, cultured VSMCs were homogenized in lysis buffer followed by sonication and centrifugation at 8100g at 4°C for 10 min. Nox specific activity was measu red in the presence of VAS-2870 or DPI. Apocynin was not tested because of its reported inactivity in vascular cells.31 Nox dependent superoxide anion production was expressed as relative chemiluminescence (light) units (RLU)/mg of protein. Results are expressed as fold change in Nox activity compared to control.
Real-time polymerase chain reaction (RT-PCR)
Total RNA from VSMCs was isolated using TRIzol according to standard protocols. SYBR green PCR master mix was used for real time PCR. Human primers are listed in Table 1.32 RT-PCR was performed in a Mastercycler RT-PCR detection system (Eppendorf, Westbury, NY). The relative level of target (Nox isoforms) gene in each group was normalized against internal housekeeping gene 18S rRNA using the calculation formula of 2ΔCt[18SrRNA-Nox]. The target gene levels in drug treated groups were further normalized against the control group.
Table 1.
Primers for real time PCR.
| Gene | Forward | Reverse | GenBank No. |
|---|---|---|---|
| NOX1 | 5′-TTCACCAATTCCCAGGATTG AAGTGGATGGTC-3′ |
5′-GACCTGTCACGATGTCAGT GGCCTTGTCAA-3′ |
AF127763 |
| GP91phox | 5′-GTCACACCCTTCGCATCCA TTCTCAAGTCAGT-3′ |
5′-CTGAGACTCATCCCAGCCAGTG-3′ | NM_000397 |
| NOX3 | 5′-ATGAACACCTCTGGGGT CAGCTGA-3′ |
5′-GGATCGGAGTCACTCCC TTCGCTG-3′ |
AF190122 |
| NOX4 | 5′-CTCGAGGAGCTGGCTCGC CAACGAAG-3′ |
5′-GTGATCATGAGGAATAGCA CCACCACCATGCAG-3′ |
AF261943 |
| P22phox | 5′-AACGAGCAGGCGCTGGCGT CCG-3′ |
5′-GCTTGGGCTCGATGGGCGTC CACT-3′ |
NM_000101 |
| P47phox | 5′-AGTCCTGACGAGACGGAAGA-3′ | 5′-GGACGGAAAGTAGCCTGTGA-3′ | NM_000265 |
| P67phox | 5′-GGAGTGTGTCTGGAAGCAG-3′ | 5′-AGTGTGTAGGGCATGGAAC-3′ | NM_000433 |
| 18SrRNA | 5′-GTAACCCGTTGAACCCCATT-3′ | 5′-CCATCCAATCGGTAGTAGCG-3′ | M10098 |
| SM22α | 5′-AACAGCCTGTACCCTGATGG -3′ | 5′-CGGTAGTGCCCATCATTCTT -3′ | NP_003177.2 |
| SM-MHC | 5′-GCTGGAAGACACACTGGACA-3″ | 5′-CCAGGTCTGCGTTCTCTTTC -3 | AAI43365.1 |
| α-ACTIN | 5′-AGGTAACGAGTCAGAGCTTTGGC-3′ | 5′-CTCTCTGTCCACCTTCCAGCAG–3′ | X13839.1 |
| SMOOTHELIN | 5′-TTGGACAAGATGCTGGATCA -3′ | 5′-CGCTGGTCTCTCTTCCTTTG-3′ | NM_198501.2 |
| CALPONIN | 5′-CATGACGGTGTATGGGCTGCCA-3′ | 5′-TAGGCGG AATTGTAGTAGTTGT-3′ | BC138864.1 |
| GAPDH | 5′-TGCACCACCAACTGCTTAGC-3′ | 5′GGCATGGACTGTGGTCATGAG-3′ | GI:182976 |
PKC activity
Total cell lysates were collected and the quantification of PKCε specific activity was carried out using the PKC activity kit (Enzo Life sciences), according to the manufacturer’s instructions. Cell samples were assayed in the presence and absence of εV1-2 (1 μM) and the difference in values is used to calculate PKCε-specific activity. The assay was quantified with a spectrophotometric microplate reader (iMark, Bio-Rad) at a dual wavelength of 450/595 nm. Data is presented as amount of active PKC/mg protein.
Cytokine measurement
0.2×106 VSMCs were plated in 24 well plates and serum starved overnight. Cells were then treated with resistin (40 ng/ml) for different time periods in the absence or presence of PKCε and Nox inhibitors. Supernatants were collected and analyzed for TNF-α, IL-1β and IL-6 using standard ELISA manufacturer’s protocols (Thermo Scientific).
Cell growth for extended time points using growth curves
To assay cell proliferation kinetics, cells were seeded at a density of 3×103/cm2 and grown in the same medium and conditions, as described above. Three wells of plated cells per each condition were counted after each time point (0–7 days) on a TC10 automated cell counter (BioRad). Cell number was graphed vs time to obtain the growth curves and exponential growth curve fitting was performed using the Prism 6.0 software (GraphPad Inc).
Cell migration using scratch wound assay
VSMCs were grown to confluence in a 6-well plate. A transverse scratch wound on each monolayer of VSMC was made using a sterilized 200 μl-tip. The scratch wounded monolayers were then stimulated with or without resistin (40 ng/ml) in the presence or absence of εV1-2 (1 μM) and Nox inhibitor VAS-2870 (10 μM) or DPI (5 μM) for an additional 24 h, at which point the transverse scratch wounds were re-examined for cell migration. Pictures were captured with a phase-contrast microscope and migration was quantified using ImageJ software. Cell migration was calculated as the percent of wound closure relative to controls.
Cell dedifferentiation and Western blot
Cell lysate samples (15 μg of protein) separated on a 4–20% polyacrylamide gel for electrophoresis were transferred onto nitrocellulose membranes using Bio-Rad Mini-Trans-Blot system. The blocked membrane was incubated with primary antibodies overnight at 4°C and with secondary antibody for 1 hour at room temperature. The immunoreactive bands were detected using a BIO-RAD chemiluminescence system, and the bands were captured and intensity quantified by with BIO-RAD ChemiDoc XRS+ camera and Image Lab software respectively.
Guide wire injury and ultrasound evaluation
6–8 week old male ApoE−/− mice on C57BL/6 background (Jackson Labs) were subjected to guide wire injury as described earlier.33 The animals were randomly divided into four treatment groups (n=6–8 per group): vehicle control (normal saline), resistin (1 μg/day), εV1-2 (3 mg/kg/day), or combined resistin and εV1-2 (Resistin+εV1-2), and drugs were delivered using an mini-osmotic pump (ALZET, Model 2004, CA) implanted subcutaneously. All mice underwent carotid evaluations using a VisualSonics Vevo 770 high-resolution ultrasound micro-image system and once every 2 weeks afterwards. Diameters of bilateral CCA were measured at distal and proximal CCAs in both longitudinal and transverse views. After four weeks post-injury, the mice were sacrificed and bilateral CCAs were harvested for TBARS and protein carbonyl assay. The animal IACUC protocol (ACORP1480) was approved.
Protein carbonyl assay
Cell extracts were mixed with 1 volume of 20% trichloroacetic acid to precipitate proteins. Carbonyl content was determined by following the standard spectrophotometric assay procedure. The protocol involves measurement of absorbance at 276 nm of the DNPH-derivatized samples in 6 M guanidine, using 50,000 for molar absorptivity and quantifying for protein.34
Plasma isoprostane analysis
Plasma from human subjects was purified on SPE cartridges before analysis for isoprostane using the Cayman kit according to the manufacturer’s instructions. Isoprostane levels were calculated and expressed in pg/mL.
Statistical analysis
All experiments were performed at least four times (n=4 to 6) in duplicate. The results are expressed as the mean ± SEM. Statistical analyses were performed using GraphPad Prism 6.0 software. We performed parametric or non-parametric analyses on the data sets and the appropriate statistical test used. Time-dependent data from ROS, PKC activity and cell proliferation were compared by one-factor analysis of variance (ANOVA) followed by Dunnett’s test. Effect of inhibitor data from Nox and PKCε inhibitor experiments on ROS and proliferation were analyzed using two-factor ANOVA followed by Tukey’s test. Results from migration, Nox activity and real time PCR did not follow a normal distribution and was analyzed using the non-parametric Mann Whitney test. Similarly data from in vivo experiments were analyzed using the non-parametric test. Statistical significance was considered if the p-value was < 0.05.
Results
Correlation of systemic resistin levels to ROS
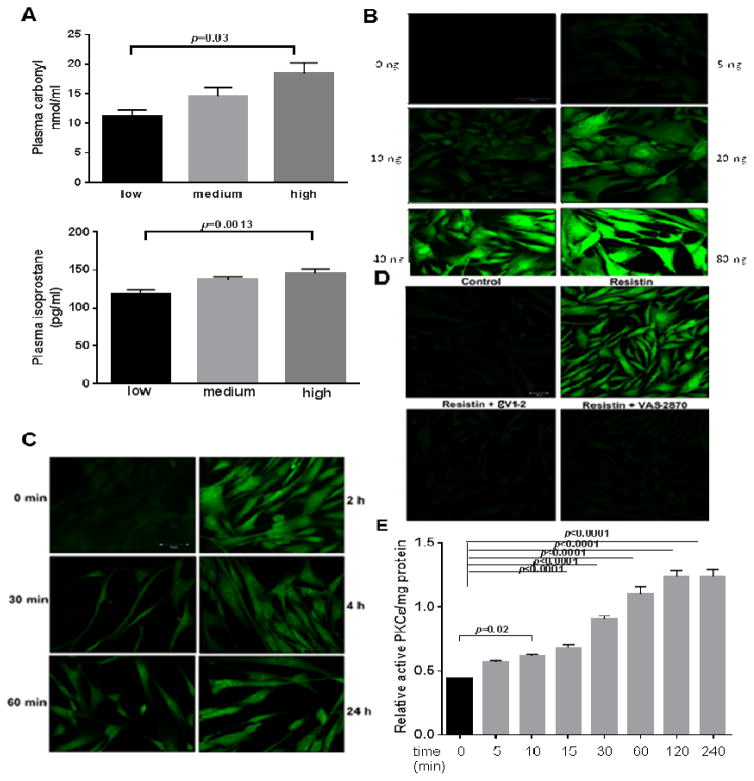
Patients were divided into tertiles based on plasma resistin concentration (n=33 in each group): Low: 2.3 ng/mL (1.0–3.4 ng/mL); Medium: 4.2 ng/mL (3.4–4.9ng/mL); and High: 7.3 ng/mL (4.9–13.3 ng/mL). Circulating plasma carbonyl levels and isoprostane levels correlate with resistin levels (Fig. 1A), showing that patients with higher circulating resistin levels have higher oxidative stress as shown by significantly higher levels of plasma protein carbonyl (top panel) as well as isoprostane (low panel). The highest circulating level of resistin in our patient cohort is 13ng/mL. However, other studies suggest much greater levels, up to 100ng/mL in laboratory investigations.21,35 We have also previously shown that 40ng/mL of resistin significantly stimulates VSMC migration. To be consistent with our previous study and others,20,24 and after evaluating a dose-dependent response curve, we chose 40 ng/ml of resistin for the rest of this study.
Fig. 1. Resistin is associated with ROS in human plasma, and it activates specific Nox isoforms in VSMCs via PKCε.
(A) Human plasma levels of resistin were associated to plasma carbonyls and isoprostane levels. (N=20) analyzed for protein carbonyl and isoprostane in plasma. Resistin levels corresponds to low (1–3.4ng/ml); medium (3.4–4.9 ng/ml) and high (4.9–13.3 ng/ml) respectively. VSMCs treated with different concentration of resistin for various time periods were analyzed for cytosolic ROS by (B and C) confocal microscopy. Effects of Nox inhibitor (VAS 2870; 10 μM) and PKCε inhibitor (eV1-2; 1 μM) on resistin-induced cytosolic ROS are shown in (D). Cells treated with 40ng/mL resistin and Nox activity shown as fold change based on control (E). (F) Change in expression of Nox subunits as analyzed by real time PCR is shown. Specific PKCε activity was measured (G). Effect of Nox4 siRNA inhibition on resistin induced ROS is shown in (H). Data are shown as mean ± S.E.M of at least 4 independent experiments in duplicate. *p<0.05; ***p<0.0005; #p<0.0001 by 1-way or 2-way ANOVA. Values are representative of at least 4 independent experiments.
Resistin dose, time-dependently induces ROS production
Dose-dependent increases in ROS production was observed in VSMCs within 2 hours of resistin treatment (Fig. 1B).
Significant increase in ROS was evident when VSMCs were treated with as low as 10 ng/mL of resistin. Cytosolic ROS production in VSMCs was seen as early as 30 minutes of resistin (40 ng/mL) treatment and increased in a time-dependent manner up to 24 hours as evidenced from Fig. 1C. Long duration of treatment, up to 7 days, still showed increased ROS levels as compared to untreated controls (data not shown), suggesting that both acute and chronic resistin treatment induces oxidative stress in VSMCs. Specific inhibitors of oxidases viz., rotenone (200 nM), allopurinol (250 μM), DPI (5 μM), and VAS-2870 (10 μM) were used to identify potential oxidases involved. VAS-2870 completely inhibited resistin-induced ROS production (DPI did the same; data not shown), as measured by fluorescence intensity using a plate reader (Supplementary Fig. 1A and B) and confocal microscopy (Fig. 1D). On the other hand, neither rotenone nor allopurinol had significant effect, suggesting that Nox is the major cytosolic source of ROS, and that SOD or xanthine oxidase were not involved (data not shown). Inhibitor concentrations used were based on the Ki values. For all our experiments both Nox inhibitors (VAS-2870 and DPI) were tested and found to have similar effects although only results from VAS-2870 are shown.
Resistin activates NOX via PKCε
We know from earlier investigations that PKCε mediates VSMC migration triggered by resistin. PKCε activation in VSMCs was evaluated using a PKC activity kit, as well as immunofluorescence detection of phosphorylated PKCε (phosphoS729-PKCε). PKCε specific activity was determined using the PKCε specific inhibitor, εV1-2.20,36 Fig. 1E shows the time-course of PKCε activation in VSMCs following resistin treatment. Significant increase in activity is observed as early as 5 min and up to 4 hours using a PKC activity kit. Increased phosphorylation of PKCε was observed as early as 10 min, which was inhibited when cells were pre-treated with εV1-2 prior to resistin treatment (Supplementary Fig. 2A and B).
We investigated the role of PKCε in resistin-induced ROS generation and observed that the PKCε inhibitor, εV1-2, completely blocks resistin-induced ROS production. Having identified Nox as the primary oxidase involved in VSMC-resistin interaction, change in chemiluminescence in the presence and absence of VAS-2870 or DPI was used to calculate Nox specific activity in cell homogenates. Resistin (40 ng/mL) significantly induced activation of Nox compared to the control (17.5 nmol/mg protein/min vs.8.2 nmol/mg protein/min P<0.001) (Fig. 1F). Nox activity was markedly suppressed by εV1-2. Differential regulation of Nox homologues viz. Nox1, Nox2, Nox3, Nox4 and subunits p22phox and p47phox in VSMCs were studied by real time PCR. Resistin upregulated the expression of Nox4 and p22phox in VSMCs (Fig. 1G), while the levels of Nox1, Nox2 and the other subunits were unchanged. εV1-2 reversed resistin-associated Nox4 and p22phox up-regulation, suggesting that PKCε is an upstream mediator for resistin-induced Nox activation and ROS production. Involvement of Nox4 was tested using siRNA approach which almost completely quenched resistin induced increase in ROS (Fig. 1H). Efficacy of siRNA inhibition of Nox4 was tested by real time PCR as shown in Supplementary Fig. 3A. Nox 4 siRNA completely inhibited resistin induced proliferation as shown in Supplementary Fig. 3B.
PKCε and Nox modulation mitigates resistin-induced VSMC dysfunction
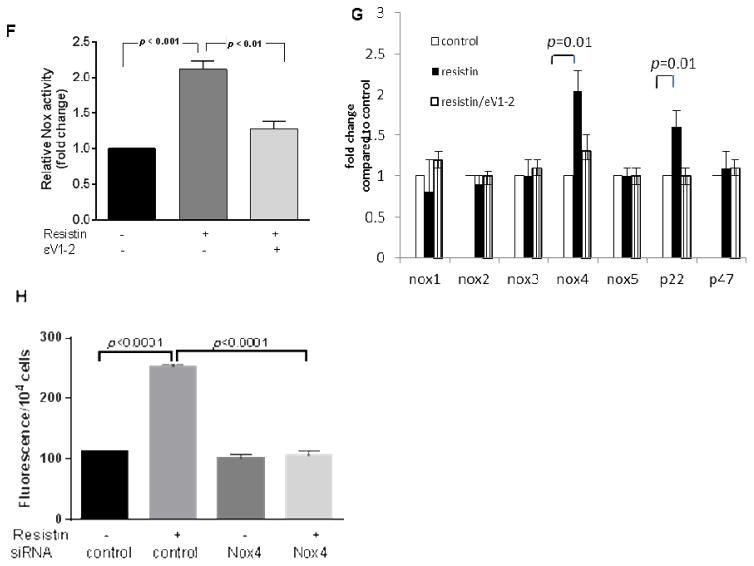
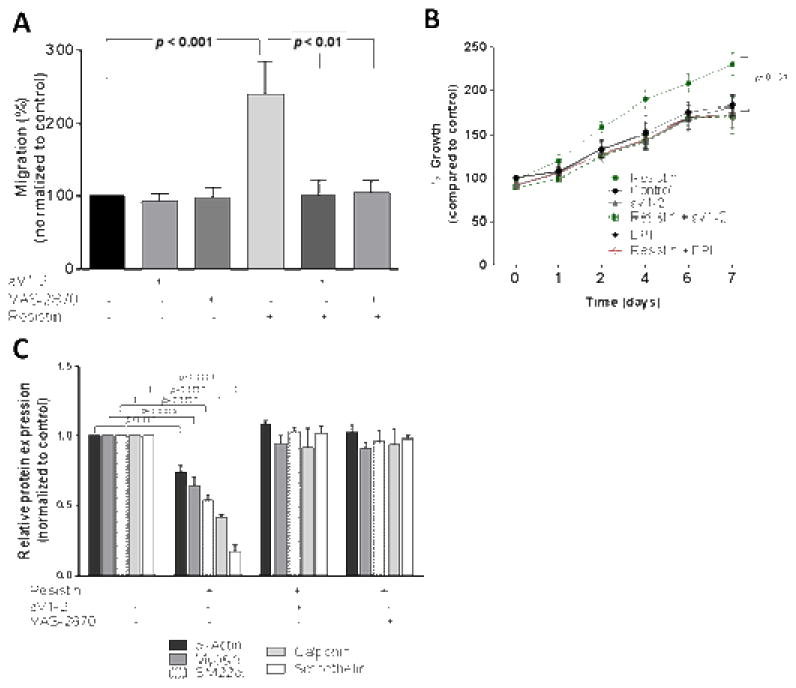
Crucial functions of VSMC in atherosclerotic plaque formation were evaluated, including migration, proliferation, and dedifferentiation. Resistin treatment increased VSMC migration, and it was suppressed by VAS-2870 and εV1-2 as shown in Fig. 2A (images shown in Supplementary Fig. 4A). The inhibitors alone did not have visible effect on VSMC migration. Cell proliferation was measured using the MTT assay and the growth curve method. Cell viability after 24 hours of resistin treatment was significantly higher (~1.5 fold) than untreated cells (p <0.0001) based on the MTT assay (Supplementary Fig. 4B) and inhibited by ROS scavenger (Supplementary Fig. 5). Growth curve of resistin treated cells also showed increased VSMC growth in time-course ranging from 0 to 7 days (Fig. 2B). Both VAS-2870 and εV1-2 decreased the growth of VSMCs significantly. Most healthy VSMCs in vivo exhibit the contractile phenotype while the de-differentiated, synthetic-phenotype is often displayed during atherosclerotic plaque development. De-differentiated VSMCs lose their primary contractile markers including smooth muscle α-actin (α-SMA), smooth muscle myosin heavy chain (SM-MHC), smoothelin, SM22α and calponin-1.37 We observed that resistin promoted VSMC dedifferentiation after 7 days of treatment (Fig. 2C) as evident by reduction in the contractile phenotype markers following resistin treatment compared to the control. Representative blots for the different proteins and gene expression profile are shown in (Supplementary Fig. 4C and D). Resistin-associated VSMC dedifferentiation was reversed by VAS-2870 and εV1-2. Collectively, the data suggests PKCε and Nox mediate resistin-associated smooth muscle cell dysfunction.
Fig. 2. Resistin affects VSMC function.
VSMCs were treated with Nox inhibitor (DPI or VAS-2870) or PKCε inhibitor (εV1-2) in the presence or absence of resistin and analyzed for migration (A), proliferation using growth curves (B) and dedifferentiation by western blot quantification shown in (C). Data are shown as mean ± S.E.M of at least 4 independent experiments in duplicate analyzed by 1-way or 2-way ANOVA. Values are representative of at least 4 independent experiments.
PKCε mediates resistin-induced cytokine release by VSMCs
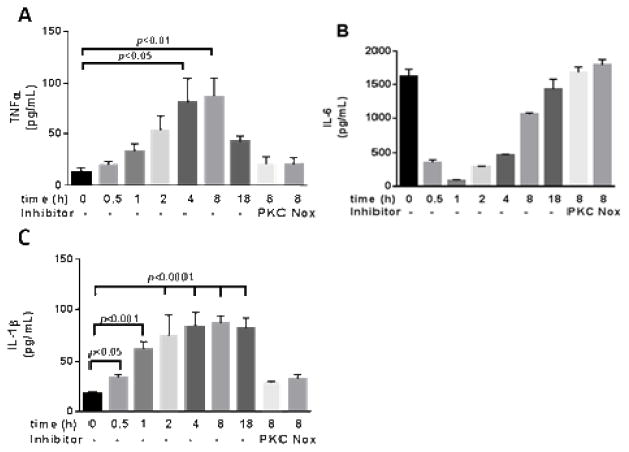
We see a time dependent increase in TNFα and IL-1β in VSMCs with a peak level reached at 4–8 hours after resistin treatment. Inhibition of PKCε and Nox during the 8 hours of treatment totally abolishes resistin-induced cytokine release as shown in Fig. 3A and C. The trend of IL-6 was however different and was not affected by either inhibitors (Fig. 3B), suggesting that resistin induces acute-phase inflammatory cytokines, but not chronic inflammation in VSMCs.
Fig. 3. Resistin induces inflammatory cytokine secretion from VSMCs mitigated by PKCe and Nox.
VSMCs (equal number) were treated with resistin in the absence or presence of εV1-2 or VAS-2870 or DPI for 8h and conditioned media collected was analyzed for TNFα, IL-6 and IL-1β as shown in (A–C). Data are shown as mean ± S.E.M. analyzed by non-parametric Mann Whitney test
Resistin exacerbates intimal hyperplasia in denuded mouse carotid arteries
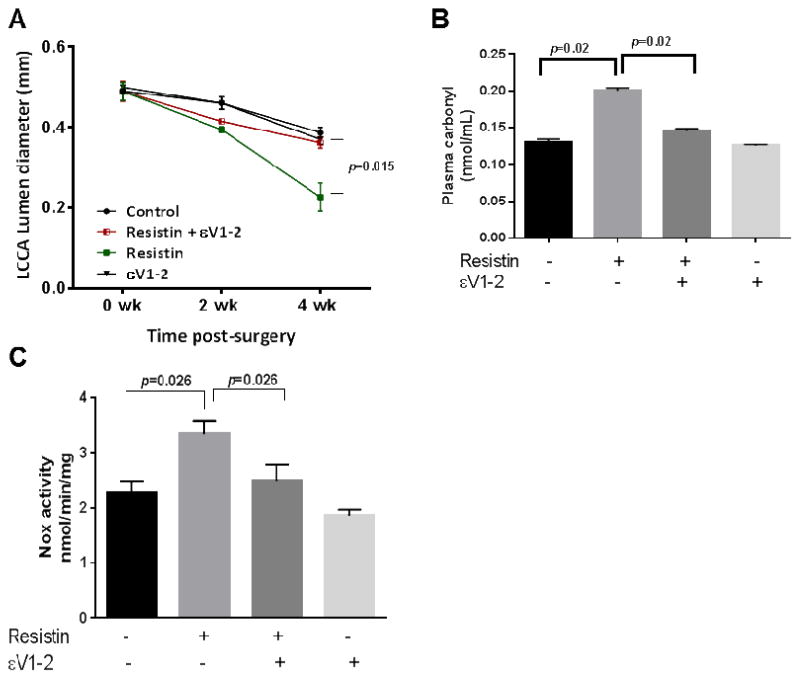
ApoE−/− mice fed on western diet received carotid denudation as previously described.33 We observed progressive luminal reduction and significant injury-induced neointimal hyperplasia in the injured carotid artery 4 weeks post-surgery. Treatment with resistin further decreased the lumen diameter compared to the saline-treated mice (Fig. 4A and Supplementary Fig. 6). Resistin-mediated luminal-narrowing was prevented by continuous subcutaneous εV1-2 treatment (Fig. 4A). Change in lumen diameter in mice treated with εV1-2 alone was not different compared to saline treated (control) mice. Plasma analyses from the mice showed significant increase in systemic ROS levels in resistin-treated mice compared to saline controls (Supplementary Fig. 7A and B), and there was no difference between saline treated and εV1-2/resistin-treated mice. Analyses of plasma showed 4.5 fold higher resistin levels in mice implanted with resistin pump. Elevated levels of tissue ROS were also observed in the carotid-artery tissue samples isolated from mice treated with resistin compared to the saline control (Fig. 4B and Supplementary Fig. 7C). There was no significant increase in carotid tissue ROS in the resistin/εV1-2 group compared to the control. Mice treated with εV1-2 treated alone did not show any increase in systemic or tissue ROS. Based on our in vitro results we tested Nox activity in the injured carotid artery in the 4 groups of animals. As shown in Fig. 4C, resistin infusion increases Nox activity by ~1.6 fold and this is inhibited by PKCε inhibitor. Additionally, superoxide derived ROS in the injured arteries was confirmed in the presence of catalase as shown in Supplementary Fig. 8.
Fig. 4. Resistin exacerbates guidewire injury induced intimal hyperplasia in ApoE−/−mice.
4 groups of ApoE−/− mice treated either with saline, resistin, resistin/εV1-2 and εV1-2 underwent wire injury in the left carotid artery. Ultrasound imaging was performed to measure the narrowing of the artery every other week post-surgery with at least 6 to 8 mice in each group. The luminal diameter of the left carotid artery is shown in (A). Oxidative stress (B) and Nox activity (C) in tissue as shown. Data are shown as mean ± S.E.M by non-parametric Mann Whitney test.
Discussion
To our knowledge this is the first comprehensive study to evaluate the roles that resistin-mediated ROS generation plays in atherosclerosis, in the context of molecular interaction, cellular effects, and in vivo influence. We show that systemic resistin correlates to circulating oxidative stress in patients with severe atherosclerosis, induces VSMC dysfunction in vitro, and exaggerates intimal hyperplasia in vivo. Mechanistically, we observe that resistin promotes dose and time-dependent ROS generation and acute-phase inflammatory cytokine production in VSMCs, and that PKCε mediates resistin-induced cellular and molecular effects. Both chronic and acute effects of resistin contribute to the novel mechanistic basis for resistin-associated cardiovascular effects.
Resistin has been shown to act on a variety of cell types including VSMCs.18–20,38,39 However, research to date primarily focused on the phenotypical properties of VSMCs. We also observed phenotypical changes in VSMCs following resistin treatment, implying its passive role in atherosclerosis. We saw, for the first time, that resistin also stimulates VSMCs to release inflammatory cytokines, a function normally attributed only to inflammatory cells. In addition, we demonstrated that PKCε-mediated Nox activation also governs resistin-induced inflammation in VSMCs and subsequent VSMC dysfunction, suggesting a direct role of resistin on VSMCs, and an active role of VSMC in inflammatory process. Both TNFα and IL-1β are considered potent stimuli of the acute-phase reaction and important activators of lymphocytes, proposing that VSMCs play a dynamic role in atherosclerosis.40–42
Chronic inflammatory cascades in atherosclerosis43 have been shown to be mediated by oxidative stress. Only one previous study by Gan et al. has shown that resistin induces ROS generation via Nox activation in VSMCs in a monocyte-SMC co-culture system.24 Consistently, we too observed that resistin significantly triggered ROS generation. In addition, we further demonstrated a time-dependent and dose-dependent increase in ROS following resistin treatment. Unlike Gan et al., we observed Nox4 being the primary isoform activated by resistin after 2 hours of treatment as opposed to Nox1. It is possible that Nox 4 is activated early whereas Nox 1 activation follows later and happens only in the presence of monocytes or in extremely high concentration of resistin, such as at 100ng/mL used by Gan et al. Our observations of sustained ROS levels suggest involvement of other ROS species in addition to the superoxide generated Nox.
Furthermore, in this study we identified an upstream regulator PKCε, suggesting that ROS production is inherently coupled to PKCε activation, thereby regulating pathways associated with VSMC dysfunction. Time course studies reveal that PKCε activation occurs as early as 5 minutes of resistin treatment while cytosolic ROS generation is seen 30 minutes following resistin treatment, confirming that this member of the novel PKC family is indeed a master regulator. Receptor-mediated action has been reported for resistin and TLR4 has been reported by several groups.44,45 Crosstalk between TLR4 and Nox has also been shown in different cell types including macrophages, monocytes, and VSMCs.46,47 Nox activation, however, is not restricted only to TLR4.48 We speculate that TLR4 may likely be a receptor for resistin in VSMCs. Our preliminary observations suggest such interaction, though further investigations are needed.
Nox activation produces superoxide, which is usually the first stage in the ROS-forming cascade, producing other species of ROS thereafter. Superoxide dismutase activity was however not affected by resistin. It is still possible that hydrogen peroxide, formed from Nox generated superoxide, can produce highly reactive radicals leading to sustained ROS. Based on earlier studies we speculate that the possible downstream targets are MMP9 (in migration) and Erk and Akt (proliferation). Here, we observed that inhibition of Nox, downstream of PKCε, blocked VSMC migration as well as MMP activation (Supplementary Fig. 9). This observation is consistent with our previous findings in PKCε inhibition.21 Similarly, quenching ROS inhibits resistin-induced proliferation via Erk and Akt signaling pathways (data not shown). Besides, ROS may regulate additional signaling pathways such as JNK and NF-κB, which also govern inflammation and cell survival.49 Taken together, these results suggest that VSMC dysfunction mediated by resistin is involved in the onset of pathological states and is primarily regulated by oxidative stress.
An earlier study reported that neointimal hyperplasia in rats exposed to balloon injury was mediated by resistin-induced oxidative stress.50 Our study differs in that it is conducted in a transgenic murine model i.e. ApoE−/−, known to develop advanced atherosclerotic lesions resembling humans, and that the model is consistent with a clinical scenario in which in-line blood flow is maintained during and after vessel injury. In addition, our findings on the role of PKCε in resistin-induced oxidative stress and intimal hyperplasia are novel. While ApoE−/− mice are known to be atherogenic and the effectiveness of guide-wire injury has been demonstrated in our previous study,34 we observed exaggerated intimal hyperplasia after systemic resistin treatment regulated by PKCε, possibly via Nox as evidenced by the upregulation of Nox activity in the injured carotid artery (Fig. 4C). At this juncture, we cannot rule out the effects of resistin on other functions. More focused studies involving a Nox isoform gene knock-out mice or RNA silencing studies need to be performed and are beyond the scope of this investigation.
In conclusion, this study reveals novel molecular links between PKCε, ROS, and inflammation within vascular smooth muscle cells that are relevant in the pathogenesis of atherosclerosis. As an upstream modulator, PKCε may be a potentially novel therapeutic target in resistin-associated poor cardiovascular outcomes, and this new therapeutic option have the potential to translate into reduced morbidities and mortality for elderly patients with atherosclerotic disease.
Supplementary Material
highlights.
Higher resistin levels are associated with increased systemic oxidative stress in humans undergoing cardiovascular interventions
Resistin induces ROS in vascular smooth muscle cells via PKCε mediated activation of NADPH oxidase
NADPH oxidase 4 isoform is involved in the process confirmed using siRNA studies
Resistin potentiates vascular smooth muscle cell dysfunction and inhibition of PKCε completely revokes the effect
Resistin exacerbates carotid thickening in a ApoE knock-out mouse model of guide wire injury induced hyperplasia via PKCε
Acknowledgments
Financial support
This work was supported by the United States Department of Veterans Affairs [grant number I01BX001398] and NIH/NINDS (ZhouR01NS070308).
We thank Dr. Daria Mochly-Rosen (Stanford University) for providing the PKCε inhibitor utilized in this investigation.
Abbreviations
- VSMC
vascular smooth muscle cell
- PKCε
protein kinase C epsilon
- ROS
reactive oxygen species
- Nox
NADPH oxidase
- ApoE−/−
apolipoprotein E knockout
- CVD
cardiovascular disease
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819–1824. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 2.Vozarova de Courten B, Degawa-Yamauchi M, Considine RV, Tataranni PA. High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in pima indians. Diabetes. 2004;53:1279–1284. doi: 10.2337/diabetes.53.5.1279. [DOI] [PubMed] [Google Scholar]
- 3.Owens CD, Kim JM, Hevelone ND, Hamdan A, Raffetto JD, Creager MA, Conte MS. Novel adipokines, high molecular weight adiponectin and resistin, are associated with outcomes following lower extremity revascularization with autogenous vein. Journal of vascular surgery. 2010;51:1152–1159. doi: 10.1016/j.jvs.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Chen DY, Cao J, He ZY, Zhu BP, Long M. High serum resistin level may be an indicator of the severity of coronary disease in acute coronary syndrome. Chinese Medical Sciences Journal. 2009;24(3):161–166. doi: 10.1016/s1001-9294(09)60082-1. [DOI] [PubMed] [Google Scholar]
- 5.On YK, Park HK, Hyon MS, Jeon E. Serum resistin as a biological marker for coronary artery disease and restenosis in type 2 diabetic patients. Circulation Journal. 2007;71:868–873. doi: 10.1253/circj.71.868. [DOI] [PubMed] [Google Scholar]
- 6.Menzaghi C, Bacci S, Salvemini L, Mendonca C, Palladino G, Fontana A, De Bonis C, Marucci A, Goheen E, Prudente S, Morini E. Serum resistin, cardiovascular disease and all-cause mortality in patients with type 2 diabetes. PLoS One. 2013;8(6):e64729. doi: 10.1371/journal.pone.0064729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaturu S, Daberry RP, Rains J, Jain S. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine. 2006;34(3):219–223. doi: 10.1016/j.cyto.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Piestrzeniewicz K, Łuczak K, Komorowski J, Maciejewski M, Wika JJ, Goch JH. Resistin increases with obesity and atherosclerotic risk factors in patients with myocardial infarction. Metabolism. 2008;57(4):488–493. doi: 10.1016/j.metabol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, Devaney JM, Fishman C, Stamou S, Canos D, Zbinden S, Clavijo LC, Jang GJ, Andrews JA, Zhu J, Epstein SE. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 11.Benomar Y, Gertler A, De Lacy P, Crépin D, Hamouda HO, Riffault L, Taouis M. Central resistin overexposure induces insulin resistance through Toll-like receptor 4. Diabetes. 2013;62(1):102–114. doi: 10.2337/db12-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 13.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 14.Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Comm. 2003;309(2):286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Filkova M, Haluzik M, Gay S, Senolt L. The role of resistin as a regulator of inflammation: implications for various human pathologies. Clin Immunol. 2009;133:157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, et al. Resistin promotes endothelial cell activation: further evidence of adipokineendothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 17.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 18.Peter Libby, Clinton Steven K. The role of macrophages in atherogenesis. Current Opinion in Lipidology. 1993;4(5):355–363. [Google Scholar]
- 19.Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, Mickle DA. Resistin promotes endothelial cell activation: Further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 20.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: A new insight into adipocytokine–endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 21.Ding Q, Chai H, Mahmood N, Tsao J, Mochly-Rosen D, Zhou W. Matrix metalloproteinases modulated by protein kinase cε mediate resistin-induced migration of human coronary artery smooth muscle cells. Journal of vascular surgery. 2011;53:1044–1051. doi: 10.1016/j.jvs.2010.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 23.Scott TA, Babayeva O, Banerjee S, Zhong W, Francis SC. SGK1 is modulated by resistin in vascular smooth muscle cells and in the aorta following diet-induced obesity. Obesity. 2016;24(3):678–686. doi: 10.1002/oby.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamaluddin MS, Yan S, Lü J, Liang Z, Yao Q, Chen C. Resistin increases monolayer permeability of human coronary artery endothelial cells. PloS one. 2013;8(12):e84576. doi: 10.1371/journal.pone.0084576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan AM, Pirvulescu MM, Stan D, Simion V, Calin M, Manduteanu I, Butoi E. Monocytes and smooth muscle cells cross-talk activates STAT3 and induces resistin and reactive oxygen species and production. J Cell Biochem. 2013;114:2273–2283. doi: 10.1002/jcb.24571. [DOI] [PubMed] [Google Scholar]
- 26.Clinton SK, Underwood R, Hayes L, Sherman ML, Kufe DW, Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. The American journal of pathology. 1992;140(2):301. [PMC free article] [PubMed] [Google Scholar]
- 27.Ding RQ, Tsao J, Chai H, Mochly-Rosen D, Zhou W. Therapeutic potential for protein kinase C inhibitor in vascular restenosis. J Cardiovasc Pharmacol Ther. 2011;16:160–167. doi: 10.1177/1074248410382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manal Mohsen, et al. Study of possible relation between maternal serum resistin and insulin resistance in patients with preeclampsia. Egyptian Journal of Obesity, Diabetes and Endocrinology. 2015;1(2):77. [Google Scholar]
- 29.Koichiro A, Katsukawa F, Oguchi S, Murata M, Yamazaki H, Shimada A, Saruta T. Correlation between serum resistin level and adiposity in obese individuals. Obesity research. 2003;11(8):997–1001. doi: 10.1038/oby.2003.137. [DOI] [PubMed] [Google Scholar]
- 30.Wu D, Yotnda P. Production and detection of reactive oxygen species (ROS) in cancers. J Vis Exp. 2011;57:pii: 3357. doi: 10.3791/3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nature Reviews Drug Discovery. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51(2):211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 33.Juhasz A, Ge Y, Markel S, et al. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radic Res. 2009;43(6):523–532. doi: 10.1080/10715760902918683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chai H, Dong Y, Wang X, Zhou W. Ginsenoside Rb1 attenuates homocysteine-augmented guidewire injury-induced intimal hyperplasia in mice. J Surg Res. 2009;157(2):193–198. doi: 10.1016/j.jss.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clinica chimica acta. 2003;329(1):23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 36.Ghaemmaghami S, Hedayati M, Mohaddes SM, Mohammadi MG, Barkhordari A. Resistin Effect on HCT-116 Colorectal Cancer Cells Proliferation and Telomerase Expression. Scimetr. 2014;2(2) [Google Scholar]
- 37.Qvit N, Mochly-Rosen D. Highly specific modulators of protein kinase C localization: applications to heart failure. Drug Discovery Today: Disease Mechanisms. 2010;7(2):e87–e93. doi: 10.1016/j.ddmec.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Zheng J, Du Y, Huang Y, Li J, Liu B, Liu C, Zhu Y, Gao Y, Xu Q, Kong W, Wang X. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ Res. 2010;106:514–525. doi: 10.1161/CIRCRESAHA.109.202762. [DOI] [PubMed] [Google Scholar]
- 39.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 40.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-α and IL-12 in macrophages by NF-κB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 41.Kaser S, Kaser A, Sandhofer A, Ebenbichler C, Tilg H, Patsch J. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Le JM, Vilcek Jan. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Laboratory investigation; a journal of technical methods and pathology. 1989;61(6):588. [PubMed] [Google Scholar]
- 43.Mathew RD, Berman B. Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. Journal of Investigative Dermatology. 1991;97(4):686–692. doi: 10.1111/1523-1747.ep12483971. [DOI] [PubMed] [Google Scholar]
- 44.Munro JM, Cotran RS. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Laboratory investigation; a journal of technical methods and pathology. 1988;58(3):249–261. [PubMed] [Google Scholar]
- 45.Amine H, Benomar Y, Gertler A, Taouis M. THR-631: Palmitic Acid Enhances TLR4 Expression and Promotes Resistin/TLR4 Signaling. 2015. [Google Scholar]
- 46.Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. Journal of cellular and molecular medicine. 2010;14(6b):1419–1431. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cellular & molecular immunology. 2015;12(1):5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang D, Li D, Cao L, Wang L, Zhu S, Xu T, … Pan D. Positive feedback regulation of proliferation in vascular smooth muscle cells stimulated by lipopolysaccharide is mediated through the TLR 4/Rac1/Akt pathway. PloS one. 2014;9(3):e92398. doi: 10.1371/journal.pone.0092398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, … Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nature immunology. 2005;6(6):587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- 50.Shyu KG, Lien LM, Wang BW, Kuan P, Chang H. Resistin contributes to neointimal formation via oxidative stress after vascular injury. Clinical Science. 2011;120(3):121–129. doi: 10.1042/CS20100226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.