Abstract
The farnesoid X receptor (Fxr) controls bile acid homeostasis by coordinately regulating the expression of synthesizing enzymes (Cyp7a1, Cyp8b1), conjugating enzymes (Bal, Baat) and transporters in the ileum (Asbt, Ostα/β) and liver (Ntcp, Bsep, Ostβ). Transcriptional regulation by Fxr can be direct, or through the ileal Fgf15/FGF19 and hepatic Shp pathways. Circulating bile acids are increased during pregnancy due to hormone-mediated disruption of Fxr signaling. While this adaptation enhances lipid absorption, elevated bile acids may predispose women to develop maternal cholestasis. The objective of this study was to determine whether short-term treatment of pregnant mice with GW4064 (a potent FXR agonist) restores Fxr signaling to the level observed in virgin mice. Plasma, liver and ilea were collected from virgin and pregnant mice administered vehicle or GW4064 by oral gavage. Treatment of pregnant mice with GW4064 induced ileal Fgf15, Shp and Ostα/β mRNAs, and restored hepatic Shp, Bal, Ntcp, and Bsep back to vehicle-treated virgin levels. Pregnant mice exhibited 2.5-fold increase in Cyp7a1 mRNA compared to virgin controls, which was reduced by GW4064. Similarly treatment of mouse primary hepatocytes with plasma isolated from pregnant mice induced Cyp7a1 mRNA by nearly 3-fold as compared to virgin plasma, which could be attenuated by co-treatment with either GW4064 or recombinant FGF19 protein. Collectively, these data reveal that repressed activity of intestinal and hepatic Fxr in pregnancy, as previously demonstrated, may be restored by pharmacological activation. This study provides the basis for a novel approach to restore bile acid homeostasis in patients with maternal cholestasis.
Keywords: Fxr, GW4064, pregnancy, Cyp7a1, bile acids
Graphical Abstract
Introduction
Bile acids are required for the absorption of lipids and lipid-soluble vitamins from the intestine. During periods of high nutritional demand such as pregnancy, the body adapts to increase the size of the bile acid pool. While this is necessary for enhanced absorption of nutrients to support placental and fetal growth, hypercholanemia can lead to maternal cholestasis or a condition called intrahepatic cholestasis of pregnancy (ICP). ICP is observed in 0.5–2% of pregnancies in the United States, and carries with it an increased risk of fetal distress, spontaneous preterm delivery and stillbirth (Glantz et al., 2004; Geenes et al., 2014; Henderson et al., 2014; Williamson and Geenes, 2014). Likewise, women with ICP are more likely to have concomitant gestational diabetes or pre-eclampsia, and may be predisposed to develop subsequent liver disease, such as hepatitis C, fibrosis and gallstones (Marschall et al., 2013; Wikstrom Shemer et al., 2013).
The synthesis, metabolism, and excretion of bile acids is tightly regulated by transcription factors, enzymes, transporters, and signaling mediators in the liver and intestine. Only 5% of bile acids are excreted into the feces each day. The remaining 95% are conserved through enterohepatic recirculation (Danielsson and Sjovall, 1975). Bile acids are synthesized in the liver via the classic (key enzymes cholesterol-7α-hydroxylase, Cyp7a1, and sterol 12α-hydroxylase, Cyp8b1) or alternative (key enzyme steroid 27-hydroxylase, Cyp27a1) pathways and released into the intestinal lumen to promote the absorbance of lipids and lipid-soluble vitamins. Bile acids are reabsorbed into enterocytes by the apical sodium-dependent bile acid transporter (Asbt). Bile acids are thought to be bound by intestinal binding proteins such as ileal bile acid binding protein (I-babp), and secreted into the portal circulation by the basolateral organic solute transporter alpha/beta (Ostα/β) heterodimer (Dawson et al., 2005). Portal bile acids are then taken up into hepatocytes predominantly by the sinusoidal transporter Na+-taurocholate cotransporting polypeptide (Ntcp). Removal of bile acids from the liver is mainly accomplished by the canalicular efflux transporters bile salt export pump (Bsep) and multidrug resistance-associated protein (Mrp) 2 (Keppler et al., 1997; Stieger et al., 2007). However, the sinusoidal efflux transporters Ostα/β and Mrp3 can also pump bile acids into the circulation. Sinusoidal efflux of bile acids into the blood can be enhanced and is critical for removing bile acids from the liver during cholestasis (Belinsky et al., 2005; Landrier et al., 2006; Teng and Piquette-Miller, 2007).
The farnesoid X receptor (Fxr) is a bile acid-activated transcription factor that belongs to the nuclear receptor superfamily. Fxr cooperatively regulates the expression of bile acid transporters including intestinal Ostα/β and hepatic Ntcp, Bsep and Ostβ (Lu et al., 2000; Denson et al., 2001; Thomas et al., 2010), as well as the inhibitory hepatic transcription factor small heterodimer partner (Shp) and the intestinal endocrine factor fibroblast growth factor (Fgf) 15 (FGF19 in humans). Fxr-mediated induction of Fgf15 in the ileum of the small intestine has emerged as a major pathway to suppress bile acid synthesis in the liver (Kong et al., 2012). In detail, Fxr activation induces Fgf15/19 in enterocytes, which is secreted into the portal vein and then binds to its cell surface receptor Fgfr4 on hepatocytes where it represses the expression of bile acid synthesizing genes (Cyp7a1 and Cyp8b1) (Inagaki et al., 2005; Yu et al., 2005). In response to bile acid binding in the liver, Fxr up-regulates the transcription of Shp, which works with Fgf15 to suppress the expression of Cyp7a1. Furthermore, Fxr transactivates the Abcb11/Bsep and Slc51/Ostβ genes (Zhu et al., 2011; Kong et al., 2012). During pregnancy, elevated bile acid levels have been attributed to suppressed hepatic Fxr function by increased circulating levels of the steroid hormones estradiol and progesterone, and their metabolites, which results in enhanced synthesis and reduced hepatic uptake and secretion of bile acids (Abu-Hayyeh et al., 2010; Milona et al., 2010; Aleksunes et al., 2012; Abu-Hayyeh et al., 2013).
Despite recent advancements in the ICP and bile acid fields, there are a number of knowledge gaps that remain. It is not known whether intestinal Fxr function is modified during pregnancy. Moreover, the potential to intervene and/or reverse the adaptive modifications to bile acid homeostasis has not been tested in pregnant animals. Therefore, the purposes of this study were to 1) characterize ileal Fxr functions during pregnancy in mice and 2) test the ability to overcome repressed Fxr functions using a selective Fxr agonist. Therefore, pharmacologic approaches to modulate Fxr activity in vivo and ex vivo were used to delineate the molecular mechanisms by which ileal and hepatic Fxr regulate bile acid homeostasis during pregnancy.
Materials and methods
Chemicals
Unless otherwise specified, chemicals were obtained from Sigma-Aldrich (St. Louis, MO). GW4064 was synthesized at the Chemical Discovery Laboratory at the University of Kansas (Lawrence, KS). Recombinant FGF19 protein was synthesized by the laboratory of Dr. Guo at Rutgers University according to a previous report (Kong and Guo, 2014).
Animal treatment
Adult male and female wild-type C57BL/6 mice 8–12 weeks of age were purchased from Charles River Laboratories (Wilmington, MA). Female mice were mated overnight with male mice and checked for the presence of a vaginal sperm plug the next morning (designated gestation day 0). Additional female mice were used as virgin controls. Mice had access to standard chow and water ad libitum. On gestation days 13 and 14, pregnant females (n=5–6) and time-matched virgin females received two doses of vehicle (PBS with 1% Tween-80 and 1% methylcellulose, 10 mL/kg) or the Fxr synthetic agonist GW4064 (100 mg/kg, 10 ml/kg) 12 hours apart by oral gavage. Plasma, whole liver and intestines were collected from vehicle- and GW4064-treated virgin and pregnant mice 3 hours after the second dose on gestation day 14. Small intestines were divided into three equal segments, representing the duodenum, jejunum and ileum. Ileal fragments were utilized for all studies.
Liver and total body weights were recorded for female mice at the time of sacrifice. Tissues were collected within a 60 minute period in the morning (Zhang et al., 2011), snap frozen in liquid nitrogen, and stored at −80°C. All mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care accredited animal care facility in temperature-, light- and humidity-controlled rooms. All animal studies were approved by the Rutgers University Institutional Animal Care and Use Committee, and were in accordance with national guidelines.
Sandwich-cultured primary hepatocytes
Freshly isolated female C57BL/6 mouse hepatocytes (n=3 donors/independent experiments in triplicate) were obtained from Triangle Research Labs (Research Triangle Park, NC). Hepatocytes were sandwich-cultured in 12-well or 6-well plates with a Matrigel overlay, and shipped overnight in cold preservation media. Upon receipt, the preservation media was replaced with serum-free and phenol red-free Williams’ E media. Cells were allowed to recover from shipping for 24 hours at 37°C in an atmosphere containing 5% CO2. After recovery, the hepatocytes were treated with phenol red-free William’s E media containing 20% pooled virgin or pregnant (gestation day 17 or greater) mouse plasma, in the presence of GW4064 (5 μM), recombinant FGF19 protein (20 μg/mL), or vehicle (DMSO), for 1 or 24 hours. Total RNA and protein were isolated.
Plasma analyses
Plasma estradiol and progesterone were quantified using ELISA kits from Calbiotech (Spring Valley, CA) and Genway (San Diego, CA), respectively. Triglycerides and cholesterol were quantified using an enzymatic colorimetric assay from Pointe Scientific (Canton, MI).
RNA isolation and quantitative PCR
Total RNAs were extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA), and complementary DNA (cDNA) was generated using High Capacity cDNA Synthesis (Applied Biosystems, Foster City, CA). RNA purity and concentration were assessed using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Rockford, IL). mRNA expression was quantified by real time-qPCR using SYBR Green-based method (Applied Biosystems) for detection of amplified products. qPCR was performed in a 384-well plate format using the ViiA7 Real Time PCR machine (Life Technologies, Grand Island, NY). Ct values were converted to delta delta Ct values by adjusting to a reference gene (ribosomal protein l13a, Rpl13a) (Livak and Schmittgen, 2001). Primer sequences are listed in Supplemental Table 1.
Western blot analysis
For animal tissues, whole livers and ilea were homogenized in sucrose-Tris buffer (pH=7.4–7.5) using the TissueLyser LT Adapter (Qiagen), per the manufacturer’s protocol. Sandwich-cultured primary mouse hepatocytes were collected in PBS and cell pellets were resuspended in lysis buffer with 1% protease inhibitor. Protein concentrations were determined by BCA assay (Pierce Biotechnology, Rockford, IL). Fifty micrograms of whole liver or ilea homogenate or 30 micrograms of cell lysate protein were loaded onto a SDS-PAGE gel (4–12%, Life Technologies). Semi-quantification of expression was determined using primary antibodies raised against Fgf15 (Sc-27177, 1:1000, Santa Cruz), Ntcp (K4, 1:5000, Dr. Bruno Stieger), Bsep (K44, 1:5000, Dr. Bruno Stieger), phosphor-Erk1/2 (9101, 1:1000, Cell Signaling Technology, Danvers, MA) and Erk1/2 (9102, 1:1000, Cell Signaling Technology) followed by incubation with appropriate secondary antibody. The intensity of band luminescence was acquired using a FluorChem E System Imager (ProteinSimple, Santa Clara, CA). β-Actin (ab8227, Abcam, Cambridge, MA) or Gapdh (ab2302, Abcam) were used as loading controls.
Indirect immunofluorescence
Liver cryosections (6 μm) were fixed in 4% paraformaldehyde for 5 minutes. Sections were blocked with 5% goat serum/PBS with 0.1% Triton X-100 (PBS-Tx) for 1 hour and then incubated with a primary antibody against Bsep diluted 1:100 in 5% goat serum, PBS-Tx for 2 hours at room temperature. Sections were then washed and incubated with an anti-rabbit secondary antibody linked to AlexaFluor488 (Life Technologies). Images were acquired on a Zeiss Observer D1 microscope at 20x using a Jenoptik camera. All sections were stained and imaged under uniform conditions for each antibody. Negative controls without primary antibody were also included (data not shown).
Plasma bile acid profiling
Plasma bile acid extracts were analyzed by a Thermo Accela Ultra Performance Liquid Chromatography system (Thermo Fisher Scientific) coupled with a Thermo LTQ XL Ion Trap Mass Spectrometer (ITMS, Thermo Fisher Scientific). Ionization was accomplished using Electrospray and the ITMS was operated in MS/MS mode with Selective Ion Monitoring. Simultaneous determination of 23 bile acids using commercial standards (Sigma-Aldrich and Steraloids, Newport, RI), was as previously reported (Zhan et al., 2016). The limit of quantification for all bile acids was 3.11 ng/μL plasma.
Statistical analysis
Data are presented as mean ± SE (animal studies) or mean ± SD (hepatocyte studies). Statistical analysis was performed using GraphPad Prism v6 (La Jolla, CA). mRNA and protein expression were analyzed by a 2-way ANOVA or a 1-way ANOVA followed by a Newman-Keul’s multiple comparison post-hoc test when appropriate, to compare overall mean differences between groups. Significance was set at p≤0.05.
Results
Pregnancy outcomes following GW4064 treatment
To determine the effects of Fxr activation on pregnancy adaptations, plasma sex hormone concentrations and other indicators of physiological/pathological changes were quantified in pregnant mice treated with GW4064 (Table 1). As expected, plasma estradiol and progesterone levels increased by 1.5- and 6.5-fold with pregnancy, respectively. Progesterone levels were reduced by 20% with GW4064 treatment (20.3 ng/mL) compared to vehicle-treated pregnant mice (28.0 ng/mL), though still within the normal range for gestation day 14. Pregnancy increased plasma triglycerides by nearly 2-fold, which was not altered by treatment with GW4064. No significant differences were observed in plasma cholesterol levels among treatment groups on gestation day 14. The number of resorption sites at the time of sacrifice was similar between vehicle- and GW4064-treated mice. All fetuses appeared grossly normal. Additionally, both vehicle- and GW4064-treated pregnant mice had increased liver-to-body weight ratios as compared to virgin control mice.
Table 1.
Pregnancy endpoints following GW4064 treatment1.
| Virgin Vehicle | Virgin GW4064 | Pregnant Vehicle | Pregnant GW4064 | |
|---|---|---|---|---|
| Liver: Body weight | 0.0569±0.0019 | 0.0554±0.0017 | 0.0647±0.00070* | 0.0681±0.0010* |
| Resorptions | N/A | N/A | 1.00±0.36 | 0.667±0.33 |
| Estradiol (pg/mL) | 7.91±0.4 | 7.85±1.3 | 12.8±0.8* | 12.1±2.0 |
| Progesterone (ng/mL) | 3.70±1.0 | 2.68±0.6 | 28.0±1.8* | 20.3±3.2*‡ |
| Triglycerides (mg/dL) | 36.9±1.1 | 36.1±2.7 | 65.0±4.2* | 55.7±4.7* |
| Cholesterol (mg/dL) | 91.0±5.7 | 100.7±3.1 | 90.9±3.6 | 92.3±3.1 |
Body weights, liver weights and number of resorptions were recorded at the time of sacrifice. Total litter sizes ranged from 1 to 9 (mean 5.5). Circulating progesterone, estradiol, total bile acids, triglycerides and cholesterol were quantified for vehicle- and GW4064-treated mice on gestation day 14. Data are presented as mean ± SE (n=5–6).
Asterisks (*) represent statistically significant difference (p≤0.05) compared with vehicle-treated virgin mice. Double daggers (‡) represent statistically significant difference (p≤0.05) compared with vehicle-treated pregnant mice. N/A: not applicable.
Expression of hepatic bile acid-related genes in virgin and pregnant mice treated with GW4064
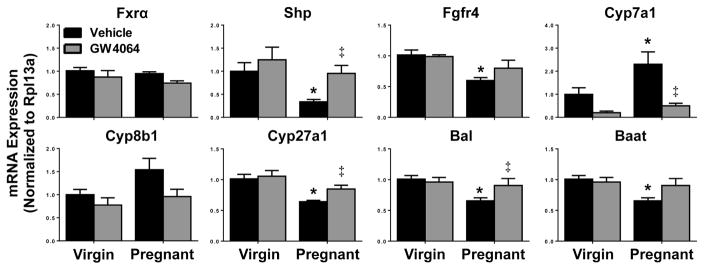
Similar to previously published reports, pregnancy had no effect on Fxr mRNA levels (Fig. 1) (Milona et al., 2010; Aleksunes et al., 2012). Vehicle-treated pregnant mice exhibited a 55% reduction in Shp mRNA, as well as 35 to 40% decreased expression of Fgfr4, Cyp27a1, Bal and Baat mRNAs compared to vehicle-treated virgin mice. A 2-fold increase in Cyp7a1 expression, and a 55% elevation in Cyp8b1, was also observed in pregnant mice. Treatment of pregnant mice with GW4064 restored mRNAs of Shp, Cyp7a1, Cyp27a1 and Bal to virgin control levels.
Fig. 1.
Hepatic mRNA expression in vehicle- or GW4064-treated pregnant mice. Hepatic mRNA expression of Fxr signaling and bile acid synthesis and metabolic enzyme genes was quantified in vehicle- and GW4064-treated virgin and pregnant mice on gestation day 14. Data were normalized to vehicle-treated virgin controls (set to 1.0) and presented as mean relative expression ± SE (n=5–6). Black bars represent vehicle-treated mice and grey bars represent GW4064-treated mice. Asterisks (*) represent statistically significant difference (p≤0.05) compared with virgin mice. Double daggers (‡) represent statistically significant difference (p≤0.05) compared with vehicle-treated pregnant mice.
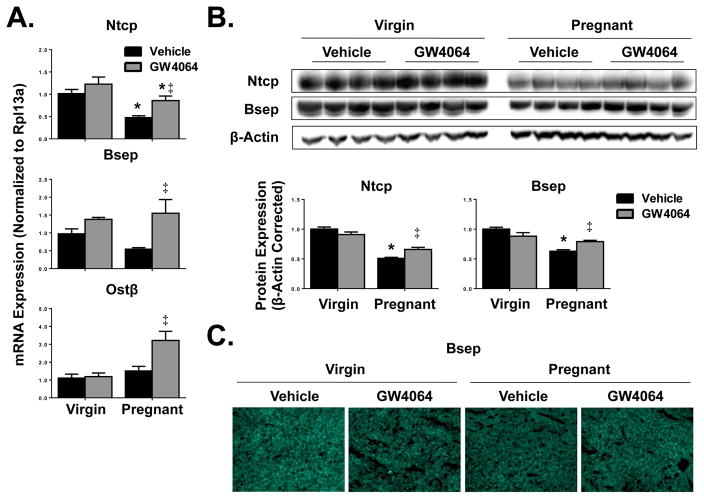
Down-regulation of Ntcp (53%) transporter transcripts was also observed on gestation day 14 compared to virgin controls (Fig. 2A). A 40% decrease in Bsep mRNA of vehicle-treated pregnant mice was noted but not statistically significant when compared to virgin controls. Treatment of pregnant mice with GW4064 up-regulated (25 to 150%) the expression of all hepatic transporters tested. Whereas GW4064 enhanced Ntcp, Bsep and Ostβ expression in pregnant mice, little to no change in transporter expression was observed in virgin mice treated with GW4064.
Fig. 2.
Hepatic transporter expression in vehicle- or GW4064-treated pregnant mice. Hepatic (A) mRNA and (B) protein expression of uptake and efflux transporters were quantified in vehicle- and GW4064-treated virgin and pregnant mice on gestation day 14. Western blots were performed using whole liver homogenates, and protein band intensity was semi-quantified. β-Actin was used as a loading control. Data were normalized to vehicle-treated virgin controls (set to 1.0) and presented as mean relative expression ± SE (n=5–6). Black bars represent vehicle-treated mice and grey bars represent GW4064-treated mice. Asterisks (*) represent statistically significant difference (p≤0.05) compared with virgin mice. Double daggers (‡) represent statistically significant difference (p≤0.05) compared with vehicle-treated pregnant mice. (C) Indirect immunofluorescence against canalicular transporter Bsep (green) was conducted on liver cryosections (6 μm). Representative images are shown. Magnification x100.
At the protein level, down-regulation of Ntcp and Bsep by 50 and 38%, respectively, was confirmed in pregnant vehicle-treated mice (Fig. 2B). GW4064 treatment of pregnant mice moderately increased the expression of both Ntcp (15%) and Bsep (17%) proteins. Additionally, indirect immunofluorescent staining confirmed localization of Bsep to the canaliculi (Fig. 2C). Compared to vehicle-treated virgin mice, pregnancy reduced the intensity of Bsep staining which was restored by GW4064 treatment.
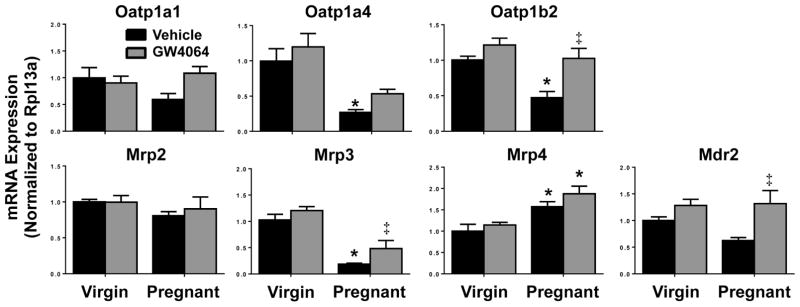
Vehicle-treated pregnant mice had reduced mRNA expression of bile acid transporters not known to be regulated by Fxr, including organic anion-transporting polypeptide (Oatp) 1a4 and 1b2, and Mrp3 (Fig. 3). Alternatively, Mrp4 mRNA expression was enhanced in pregnant mice, regardless of treatment group. Interestingly, GW4064 induced the mRNA expression of Oatp1b2, Mrp3 and Mdr2 in pregnant mice.
Fig. 3.
Hepatic bile acid transporter mRNA expression in vehicle- or GW4064-treated pregnant mice. Hepatic mRNA expression of bile acid uptake and efflux transoprter genes was quantified in vehicle- and GW4064-treated virgin and pregnant mice on gestation day 14. Data were normalized to vehicle-treated virgin controls (set to 1.0) and presented as mean relative expression ± SE (n=5–6). Black bars represent vehicle-treated mice and grey bars represent GW4064-treated mice. Asterisks (*) represent statistically significant difference (p≤0.05) compared with virgin mice. Double daggers (‡) represent statistically significant difference (p≤0.05) compared with vehicle-treated pregnant mice.
Expression of intestinal bile acid-related genes in virgin and pregnant mice treated with GW4064
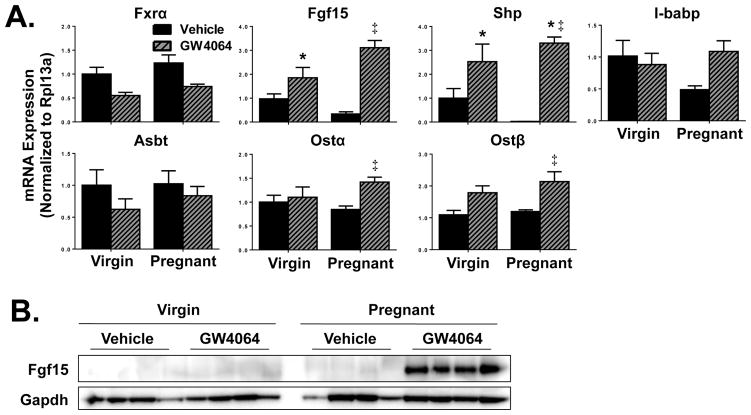
Decreased mRNAs of Fgf15, Shp and I-babp by 65%, 95% and 50%, respectively, were noted on gestation day 14 in vehicle-treated pregnant mice (Fig. 4A). While treatment of virgin mice with GW4064 nearly doubled Fgf15 expression, even greater induction was observed in pregnant mice (9-fold compared to vehicle-treated pregnant mice). GW4064 treatment also increased Ostα and Ostβ mRNA by 67% and 85% compared to virgin controls, respectively. Although I-babp mRNA levels returned to those seen in virgin controls following GW4064 treatment of pregnant mice, these data were not statistically significant. Neither pregnancy nor GW4064 treatment altered expression of Fxr or Asbt transporter mRNAs. Interestingly, Shp mRNA was nearly undetectable in vehicle-treated pregnant mice, but enhanced by 3.3-fold in GW4064-treated pregnant mice as compared to vehicle-treated virgin mice. Similarly, Fgf15 protein was only detectable in pregnant mice following GW4064 treatment (Fig. 4B).
Fig. 4.
Ileal gene and protein expression in vehicle- or GW4064-treated pregnant mice. (A) mRNA and (B) protein expression of Fxr-regulated targets were quantified in ilea from vehicle- and GW4064-treated virgin and pregnant mice on gestation day 14. Western blot staining of Fgf15 protein was performed using whole ileal homogenates. Data were normalized to vehicle-treated virgin controls (set to 1.0) and presented as mean relative expression ± SE (n=5–6). Black bars represent vehicle-treated mice and grey diagonal striped bars represent GW4064-treated mice. Asterisks (*) represent statistically significant difference (p≤0.05) compared with vehicle-treated virgin mice. Double daggers (‡) represent statistically significant difference (p≤0.05) compared with vehicle-treated pregnant mice.
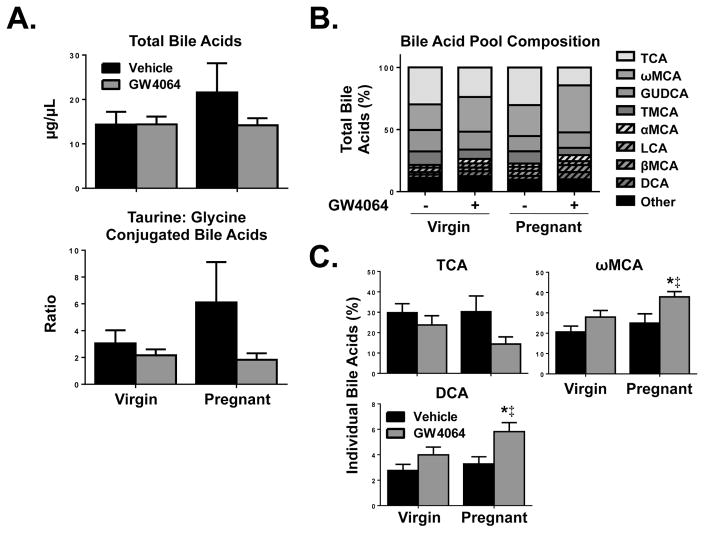
Bile acid pool analysis of plasma from virgin and pregnant mice treated with GW4064
Overall, a trend for increased total bile acids was observed for vehicle-treated pregnant mice as compared to vehicle-treated virgin mice (Fig. 5A). This corresponded with a relative increase (100%) in the ratio of taurine:glycine conjugated bile acids. We further analyzed the bile acid pool composition by comparing relative percentages of individual bile acids (Fig. 5B). Of the bile acids that are most highly concentrated in the plasma of mice, a trend for reduced taurocholic acid (TCA) was observed in pregnant mice with GW4064 treatment compared to vehicle treatment (Fig. 5C). Short-term treatment of pregnant mice with GW4064 increased the relative percentage of the secondary bile acid ω-murcholic acid (ωMCA) compared to vehicle-treated virgin mice (18%) and compared to vehicle-treated pregnant mice (13%). Though deoxycholic acid (DCA) concentrations in the plasma were low in comparison to other bile acids, levels were significantly increased in GW4064-treated pregnant mice as compared to vehicle-treated virgin and pregnant mice (nearly doubled).
Fig. 5.
Plasma bile acid profiling of virgin and pregnant mice after short-term treatment with GW4064. (A) Total and (B, C) individual bile acids were determined in vehicle- and GW4064-treated virgin and pregnant mice on gestation day 14. Individual bile acids are shown as a percentage of total bile acids per group. Data are presented as mean relative expression ± SE (n=5). Black bars represent vehicle-treated mice and grey bars represent GW4064-treated mice. Asterisks (*) represent statistically significant difference (p≤0.05) compared with vehicle-treated virgin mice. Double daggers (‡) represent statistically significant difference (p≤0.05) compared with vehicle-treated pregnant mice.
Modulation of Cyp7a1 expression by pregnant plasma in sandwich-cultured primary mouse hepatocytes
To investigate the hypothesis that circulating factors present in blood lead to pregnancy-related bile acid changes, female naïve sandwich-cultured primary mouse hepatocytes were treated with 20% plasma isolated from virgin or pregnant (gestation day 17 or greater) mice. Treatment with plasma had no effect on hepatocyte cell viability as evidenced by negligible lactate dehydrogenase leakage (data not shown).
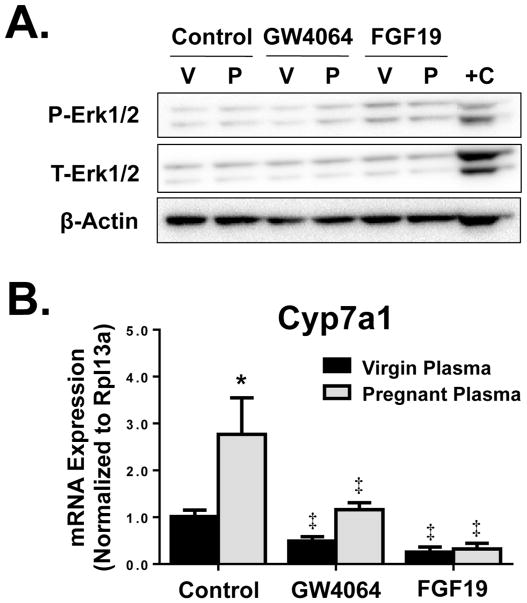
Subsequent studies tested whether the Fxr agonist GW4064 or recombinant FGF19 protein (the human ortholog of mouse Fgf15) could rescue pregnancy-related changes in bile acid synthesis pathways. For this effort, sandwich-cultured primary mouse hepatocytes were concomitantly treated with 20% plasma from virgin and pregnant mice and GW4064 or exogenous recombinant FGF19 protein. Activity of recombinant FGF19 protein was confirmed by increased phosphorylation of Erk1/2 protein after 1 hour of treatment (Fig. 6A) (Kong et al., 2012; Kong and Guo, 2014). Cyp7a1 mRNA expression was induced by 2.7-fold in the presence of pregnant plasma as compared to hepatocytes exposed to virgin plasma for 24 hours (Fig. 6B). Co-treatment of hepatocytes with pregnant plasma and either GW4064 or FGF19 reduced Cyp7a1 mRNA to levels at or below controls. For example, co-treatment with pregnant serum and recombinant FGF19 protein was able to reduce Cyp7a1 mRNA by 70 to 75%.
Fig. 6.
Cyp7a1 regulation in sandwich-cultured primary mouse hepatocytes. (A) Erk1/2 activation as indicated by phospho-Erk1/2 (P-Erk) compared to total Erk1/2 (T-Erk) after 1 hour treatment and (B) mRNA expression of Cyp7a1 after 24 hour treatment of sandwich-cultured primary mouse hepatocytes with media containing 20% pooled virgin or pregnant mouse plasma, in the presence of GW4064 (5 μM), recombinant FGF19 protein (20 μg/ml), or vehicle (DMSO). Western blots were performed using whole cell lysates. V, virgin plasma; P, pregnant plasma, mouse liver positive control. Data are presented as mean relative expression ± SD (n=3 donors/independent experiments in triplicate). Black bars represent virgin mouse plasma-treated hepatocytes and grey bars represent pregnant mouse plasma-treated hepatocytes. Asterisks (*) represent statistically significant difference (p≤0.05) compared with plasma-treated virgin controls. Double daggers (‡) represent statistically significant difference (p≤0.05) compared with plasma-treated pregnant controls.
Discussion
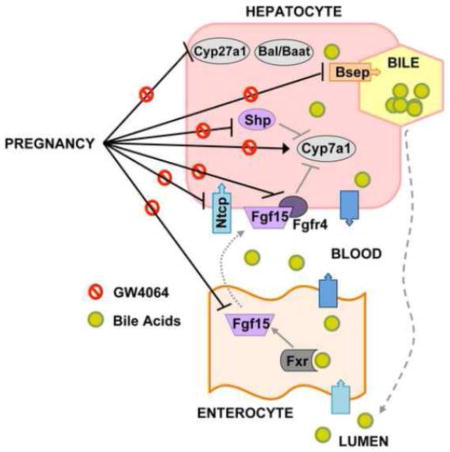
The current study assessed the ability of activated Fxr to modulate hepatic and intestinal regulation of bile acid synthesis and transport pathways during pregnancy. As previously reported, pro-cholestatic adaptive changes in hepatic gene expression have been observed in pregnant mice in the absence of changes in the mRNA expression of Fxr itself (Fig. 1) (Milona et al., 2010; Aleksunes et al., 2012; Song et al., 2014). This included the repression of gene expression of Shp, Fgfr4, Cyp27a1, Bal, Baat, Ntcp, and Mrp3, and induction of the gene expression of Cyp7a1 on gestation day 14. This was further reflected in decreased protein levels of major basolateral and canalicular bile acid transporters, Ntcp and Bsep (Aleksunes et al., 2012). These data support the postulated functional impairment of Fxr activity during pregnancy more than a change in its expression. The coordinated down-regulation of sinusoidal uptake and efflux, as well as canalicular efflux transporters suggests that there is reduced bile acid enterohepatic recirculation, which may contribute to increased plasma bile acid levels late in pregnancy. A trend towards increased total bile acids in plasma was already apparent on gestation day 14 (Fig. 5). Interestingly, treatment of pregnant mice with a specific Fxr agonist GW4064 restored the expression of bile acid synthesis enzymes and transporters towards levels typically observed in virgin mice without negatively impacting plasma hormone and other biochemistries, resorptions or viable fetuses.
It has been demonstrated in the rat that Fxr indirectly suppresses Ntcp expression through the inhibitory transcription factor Shp (Denson et al., 2001). Namely, activation of Fxr in the rat liver induces expression of Shp, which in turn inhibits the gene expression of Ntcp, a bile acid uptake transporter in hepatic sinusoidal membranes (Denson et al., 2001). However, analysis of the same Shp response element in human and mouse suggests this may not be the dominant or singular pathway for bile acid regulation of NTCP/Ntcp in other species (Jung et al., 2004). Previously published literature confirms that during pregnancy, both Shp and Ntcp gene expression are concomitantly reduced (Milona et al., 2010; Aleksunes et al., 2012). In this study, activation of Fxr in pregnant mice with the agonist GW4064 similarly induced both Shp and Ntcp (Fig. 1 and 2). This co-regulation phenomenon for Shp and Ntcp has also been noted in newborn mice with the bile acid surge that initiates Fxr signaling. At birth, a positive correlation between Fxr and Ntcp mRNA is observed (Cui et al., 2012). Though not well-explored, Fxr response elements in the 5′ promoter region of the Ntcp/Slc10a1 gene have been identified in adult mice (Thomas et al., 2010). Thus, a secondary mechanism for Fxr regulation of the bile acid uptake transporter Ntcp may occur in certain physiological states such as pregnancy.
GW4064 was identified and characterized as a potent, nonsteroidal and selective Fxr agonist in 2000 (Maloney et al., 2000). GW4064 is commonly utilized as a pharmacological activator of Fxr in mice. It has been shown to decrease bile acid synthesizing enzymes Cyp7a1 and Cyp8b1 mRNA levels in an Fxr dependent manner, and not in Fxr-null mice (Kong et al., 2012). The ability of GW4064 to reduce expression of Cyp7a1 and Cyp8b1 and induce Shp and Fgf15 mRNA is mitigated by deletion of Fxr in all tissues of mice (Moschetta et al., 2004; Kong et al., 2012). Interestingly, more pronounced increases in intestinal and hepatic mRNA expression of Fxr targets were consistently observed following GW4064 treatment in pregnant mice as compared to virgin mice. This may result from reduced gastrointestinal motility in mice during pregnancy (Datta et al., 1974), similar to humans, which could lead to greater residence time in the intestinal lumen and subsequent absorption into the body. It should be recognized that GW4064 is a chemical probe used for research purposes and possesses little pharmaceutical utility. As a result, pharmacokinetic analysis of GW4064 in pregnant mice is unwarranted. Future studies utilizing long-term treatment of mice with an FDA-approved Fxr agonist, such as obeticholic acid, could help elucidate differences in pharmacokinetics and/or pharmacodynamics as potential mechanisms of action during pregnancy, in addition to providing a clearer understanding of the ability of increased Fxr activation to alter bile acid pool composition.
The current study highlights the relationship between the restoration of intestinal and hepatic Fxr target genes by GW4064. Intestinal Fgf15 was inducible by 9-fold with GW4064 treatment during pregnancy (Fig. 4). This newly identified responsiveness in pregnant mice likely led to the attenuation of Cyp7a1 mRNA induction in the liver (Fig. 1). Further, in an isolated system where only primary mouse hepatocytes were exposed to pregnant plasma in the media, Cyp7a1 mRNAs were induced (Fig. 6). Similar to in vivo results it was observed that this enhanced expression could be abolished by not only a pharmacological Fxr agonist, but also the addition of FGF19, a positively regulated signaling molecule produced by activation of ileal Fxr. The lack of a sensitive method has precluded our ability to quantify Fgf15 protein levels in serum. Nonetheless, this study supports the novel hypothesis that intestinal bile acid pathways can be altered in pregnancy in an Fxr-dependent manner. Further, it is likely that Fxr in the ileum contributes to changes in hepatic gene expression due to the Fgf15/FGF19-mediated communication between the two organs as part of the coordinated regulation of bile acid homeostasis.
Sandwich-cultured fresh primary hepatocytes were utilized in this study because they behave similarly to hepatocytes in vivo and form bile canaliculi in culture to better mimic the liver and bile acid pathway. However, this is still not an ideal system to assess transporter expression and activity, which decrease over time in culture. This was evidenced by higher Ct values of hepatic transporters in sandwich-cultured fresh primary hepatocytes as compared to Ct values in mice (data not shown). Therefore, mRNA expression of uptake and efflux transporters measured in the primary mouse hepatocytes showed limited correlation to data from intact animals.
Data presented in this paper suggest that activation of Fxr can restore bile acid-related gene expression both in the intestine and the liver. Potential mechanisms for the restoration of liver bile acid pathways may occur through 1) activation of hepatic Fxr or 2) suppression of Cyp7a1 by up-regulation of Fgf15 levels. These may be important findings for the treatment of pregnancy-specific cholestatic liver disease. Pharmacological activation of Fxr in rodents prevents or resolves both intra- and extra-hepatic cholestasis (Liu et al., 2003; Moschetta et al., 2004). Other studies have shown that constitutively active Fxr in the intestine can reduce total bile acids and decrease bile acid pool hydrophobicity, and presumably, toxicity (Modica et al., 2012). Intestinal activation of Fxr specifically attenuated liver injury in models of extrahepatic cholestasis (bile duct ligation), as well as intrahepatic cholestasis (treatment with α-naphthylisothiocyanate and genetically induced). In this study, FGF19 treatment abrogated the pregnancy-related increase in Cyp7a1 expression ex vivo. Additional reports in the literature have confirmed that administration of FGF19 not only reduced CYP7A1 activity in healthy human patients but also protected mice from hepatotoxicity due to bile duct ligation and α-naphthylisothiocyanate treatment (Luo et al., 2014). The present study implicates not only hepatic, but for the first time, intestinal Fxr activation as a potential mechanism to regulate bile acid signaling during pregnancy and may represent a new therapeutic target for treating ICP.
Supplementary Material
qPCR Primer Sequences
Highlights.
Ileal bile acid pathways are altered in pregnancy in an Fxr-dependent manner.
Ileal Fxr/Fgf contributes to changes in hepatic bile acid synthesis and transport.
Treatment of pregnant mice with an Fxr agonist restores bile acid homeostasis.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development [Grant F31HD082965], National Institute of Environmental Health Sciences [Grants R01ES020522, T32ES007148, P30ES002022, R25ES005022], National Institute of General Medical Sciences [Grant R01GM104037] and an American Foundation for Pharmaceutical Education Predoctoral Fellowship in Pharmaceutical Science. The authors appreciate antibodies provided by Dr. Bruno Stieger (University Hospital, Zurich, Switzerland), and tissue collections performed by Myrna Trumbauer, Kristin Bircsak, Le Zhan and Jianliang Shen (Dept. of Pharmacology and Toxicology, Rutgers University).
Abbreviations
- Asbt
apical sodium-dependent bile acid transporter
- Baat
bile acid CoA:amino acid N-acetyltransferase
- Bal
bile acid CoA ligase
- Bsep
bile salt export pump
- Cyp
cytochrome P450
- Fgf
fibroblast growth factor
- Fxr
farnesoid X receptor
- I-babp
ileal bile acid binding protein
- ICP
intrahepatic cholestasis of pregnancy
- Mdr
multidrug resistance protein
- Mrp
multidrug resistance-associated protein
- Ntcp
Na+-taurocholate cotransporting polypeptide
- Oatp
organic anion-transporting polypeptide
- Ost
organic solute transporter
- Shp
small heterodimer partner
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Hayyeh S, Martinez-Becerra P, Sheikh Abdul Kadir SH, Selden C, Romero MR, Rees M, Marschall HU, Marin JJ, Williamson C. Inhibition of Na+-taurocholate Co-transporting polypeptide-mediated bile acid transport by cholestatic sulfated progesterone metabolites. J Biol Chem. 2010;285:16504–16512. doi: 10.1074/jbc.M109.072140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Hayyeh S, Papacleovoulou G, Lovgren-Sandblom A, Tahir M, Oduwole O, Jamaludin NA, Ravat S, Nikolova V, Chambers J, Selden C, Rees M, Marschall HU, Parker MG, Williamson C. Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype. Hepatology. 2013;57:716–726. doi: 10.1002/hep.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Yeager RL, Wen X, Cui JY, Klaassen CD. Repression of hepatobiliary transporters and differential regulation of classic and alternative bile acid pathways in mice during pregnancy. Toxicol Sci. 2012;130:257–268. doi: 10.1093/toxsci/kfs248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, Lerro A, Kruh GD. Analysis of the in vivo functions of Mrp3. Mol Pharmacol. 2005;68:160–168. doi: 10.1124/mol.104.010587. [DOI] [PubMed] [Google Scholar]
- Cui JY, Aleksunes LM, Tanaka Y, Fu ZD, Guo Y, Guo GL, Lu H, Zhong XB, Klaassen CD. Bile acids via FXR initiate the expression of major transporters involved in the enterohepatic circulation of bile acids in newborn mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G979–996. doi: 10.1152/ajpgi.00370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson H, Sjovall J. Bile acid metabolism. Annu Rev Biochem. 1975;44:233–253. doi: 10.1146/annurev.bi.44.070175.001313. [DOI] [PubMed] [Google Scholar]
- Datta S, Hey VM, Pleuvry BJ. Effects of pregnancy and associated hormones in mouse intestine, in vivo and in vitro. Pflugers Arch. 1974;346:87–95. doi: 10.1007/BF00587009. [DOI] [PubMed] [Google Scholar]
- Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- Geenes V, Chappell LC, Seed PT, Steer PJ, Knight M, Williamson C. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology. 2014;59:1482–1491. doi: 10.1002/hep.26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467–474. doi: 10.1002/hep.20336. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Shah RR, Gottimukkala S, Ferreira KK, Hamaoui A, Mercado R. Primum non nocere: how active management became modus operandi for intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol. 2014;211:189–196. doi: 10.1016/j.ajog.2014.03.058. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Jung D, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA. Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am J Physiol Gastrointest Liver Physiol. 2004;286:G752–761. doi: 10.1152/ajpgi.00456.2003. [DOI] [PubMed] [Google Scholar]
- Keppler D, Konig J, Buchler M. The canalicular multidrug resistance protein, cMRP/MRP2, a novel conjugate export pump expressed in the apical membrane of hepatocytes. Adv Enzyme Regul. 1997;37:321–333. doi: 10.1016/s0065-2571(96)00013-1. [DOI] [PubMed] [Google Scholar]
- Kong B, Guo GL. Soluble expression of disulfide bond containing proteins FGF15 and FGF19 in the cytoplasm of Escherichia coli. PLoS One. 2014;9:e85890. doi: 10.1371/journal.pone.0085890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- Luo J, Ko B, Elliott M, Zhou M, Lindhout DA, Phung V, To C, Learned RM, Tian H, DePaoli AM, Ling L. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med. 2014;6:247ra100. doi: 10.1126/scitranslmed.3009098. [DOI] [PubMed] [Google Scholar]
- Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis MC, Jones SA, Willson TM. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- Marschall HU, Wikstrom Shemer E, Ludvigsson JF, Stephansson O. Intrahepatic cholestasis of pregnancy and associated hepatobiliary disease: a population-based cohort study. Hepatology. 2013;58:1385–1391. doi: 10.1002/hep.26444. [DOI] [PubMed] [Google Scholar]
- Milona A, Owen BM, Cobbold JF, Willemsen EC, Cox IJ, Boudjelal M, Cairns W, Schoonjans K, Taylor-Robinson SD, Klomp LW, Parker MG, White R, van Mil SW, Williamson C. Raised hepatic bile acid concentrations during pregnancy in mice are associated with reduced farnesoid X receptor function. Hepatology. 2010;52:1341–1349. doi: 10.1002/hep.23849. [DOI] [PubMed] [Google Scholar]
- Modica S, Petruzzelli M, Bellafante E, Murzilli S, Salvatore L, Celli N, Di Tullio G, Palasciano G, Moustafa T, Halilbasic E, Trauner M, Moschetta A. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 2012;142:355–365. e351–354. doi: 10.1053/j.gastro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med. 2004;10:1352–1358. doi: 10.1038/nm1138. [DOI] [PubMed] [Google Scholar]
- Song X, Vasilenko A, Chen Y, Valanejad L, Verma R, Yan B, Deng R. Transcriptional dynamics of bile salt export pump during pregnancy: Mechanisms and implications in intrahepatic cholestasis of pregnancy. Hepatology. 2014 doi: 10.1002/hep.27171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflugers Arch. 2007;453:611–620. doi: 10.1007/s00424-006-0152-8. [DOI] [PubMed] [Google Scholar]
- Teng S, Piquette-Miller M. Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol. 2007;151:367–376. doi: 10.1038/sj.bjp.0707235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Hart SN, Kong B, Fang J, Zhong XB, Guo GL. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology. 2010;51:1410–1419. doi: 10.1002/hep.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom Shemer E, Marschall HU, Ludvigsson JF, Stephansson O. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG. 2013;120:717–723. doi: 10.1111/1471-0528.12174. [DOI] [PubMed] [Google Scholar]
- Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2014;124:120–133. doi: 10.1097/AOG.0000000000000346. [DOI] [PubMed] [Google Scholar]
- Yu C, Wang F, Jin C, Huang X, McKeehan WL. Independent repression of bile acid synthesis and activation of c-Jun N-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. J Biol Chem. 2005;280:17707–17714. doi: 10.1074/jbc.M411771200. [DOI] [PubMed] [Google Scholar]
- Zhan L, Yang I, Kong B, Shen J, Gorczyca L, Memon N, Buckley BT, Guo GL. Dysregulation of bile acid homeostasis in parenteral nutrition mouse model. Am J Physiol Gastrointest Liver Physiol. 2016;310:G93–G102. doi: 10.1152/ajpgi.00252.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Guo GL, Klaassen CD. Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PLoS One. 2011;6:e16683. doi: 10.1371/journal.pone.0016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Li F, Guo GL. Tissue-specific function of farnesoid X receptor in liver and intestine. Pharmacol Res. 2011;63:259–265. doi: 10.1016/j.phrs.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
qPCR Primer Sequences