Abstract
Background and aims
Endothelial-mesenchymal transitions (EndMTs) in endothelial cells (ECs) contribute to vascular disease.
Methods
We used ApoE−/− mice fed a high-fat/high-cholesterol diet.
Results
We reported evidence of EndMT in atherosclerotic lesions contributing to calcification. Stem cell and mesenchymal markers, including sex-determining region Y-box 2 (Sox2), were upregulated in aortic ECs of fat-fed ApoE−/− mice. Limiting Sox2 decreased marker expression and calcification in ApoE−/− aortas. Furthermore, a complex of serine proteases was upregulated in ApoE−/− aortic ECs. Blockade of these proteases reduced expression of Sox2 and atherosclerotic lesion calcification.
Conclusions
Together, our data suggest that EndMTs contribute to atherosclerotic lesion calcification.
Keywords: Endothelial-mesenchymal transition, atherosclerotic lesion calcification, sex-determining region Y-box 2, serine protease, endothelial cells
INTRODUCTION
Vascular calcification is a common feature of atherosclerosis (1–4), associated with increased plaque burden and poor clinical prognosis (5). Vascular calcification is a regulated process that requires systemic and local factors, responding to proatherogenic oscillatory shear stress, oxidative stress and proinflammatory cytokines (4, 6–8). Bone morphogenetic proteins (BMPs) are important pro-calcific factors, found at high levels in calcified atherosclerotic lesions (9, 10). Overexpression of a BMP inhibitor, matrix Gla protein (MGP), limits atherosclerotic lesion calcification in ApoE−/− mice (11). An inhibitor of BMP type I receptor kinases also reduces the lesion calcification in Ldlr−/− mice (12). Recently, it was found that excess BMP activity triggers EndMT allowing the endothelium to contribute cells to the calcifying process (10, 13, 14).
EndMT is a process through which ECs transit into mesenchymal stem cells and gain multipotency (15), prior to differentiating into various cell lineages (13). EndMT has been shown in normal development, such as neural crest formation and cardiogenesis (16, 17). In disease, EndMTs contribute to cardiac and renal fibrosis (18, 19), fibrodysplasia ossificans progressive (20), cancer progression (21) and pulmonary hypertension (22). Although EndMTs have been found to occur in atherosclerotic lesions (23), it is still unclear whether EndMTs contribute to atherosclerotic lesion calcification.
MATERIALS AND METHODS
Animals
ApoE−/− (B6.129P2-ApoEtm1Unc/J) mice were obtained from the Jackson Laboratory. Genotypes were confirmed by PCR (24) and experiments were performed with generations F4-F6. All mice were fed a standard chow diet (Diet 8604, HarlanTeklad Laboratory). At 8 to 10 weeks of age, all ApoE−/− mice were switched to a high-fat/high-cholesterol diet (Western diet) (Research Diets, New Brunswick, NJ, diet #D12108, containing 21% fat, 1.25% cholesterol) for 16 weeks. Diisopropylfluorophosphate (DFP) (Sigma-Aldrich), serpina1 (Origene) or Sox2 shRNA were injected via tail vein or retro-orbital injection (20–50 ng/g, daily) as in previous studies (13). Injections in ApoE−/− mice were started at 16 to 18 weeks of age, and continued for 8 weeks. The studies were reviewed and approved by the Institutional Review Board and conducted in accordance with the animal care guideline set by the University of California, Los Angeles. The investigation conformed to the National Research Council, Guide for the Care and Use of Laboratory Animals, Eighth Edition (Washington, DC: The National Academies Press, 2011).
RNA analysis
Real-time PCR analysis was performed as previously described (25). Primers and probes for mouse Sox2, Kruppel-like factor 4 (Klf4), snail family zinc finger 2 (Slug or Snail2), stem cell antigen 1 (Sca1), cluster of differentiation (CD)10, CD44, CD71, CD90, c-kit (also referred to as CD117), and all elastases and kallikreins were obtained from Applied Biosystems as part of Taqman® Gene Expression Assays.
Immunoblotting
Immunoblotting was performed as previously described (13). Blots were incubated with specific antibodies to c-kit (200 ng/ml; Cell Signaling Technology), Sca1 (200 ng/ml; Merck Millipore), CD90 (both 200 ng/ml; Abcam), CD71 (1:200; ThermoFisher). β-actin (1:5000 dilution; Sigma-Aldrich) was used as loading control.
Quantification
Lesion calcification was quantified as previously described (13).
Statistical analysis
Data were analyzed for statistical significance by ANOVA with post hoc Tukey’s analysis. The analyses were performed using GraphPad Instat®, version 3.0 (GraphPad Software). Data represent mean ± SD. p<0.05 was considered significant, and experiments were performed a minimum of three times.
RESULTS
Induction of Sox2 and EndMTs in calcified atherosclerotic lesions
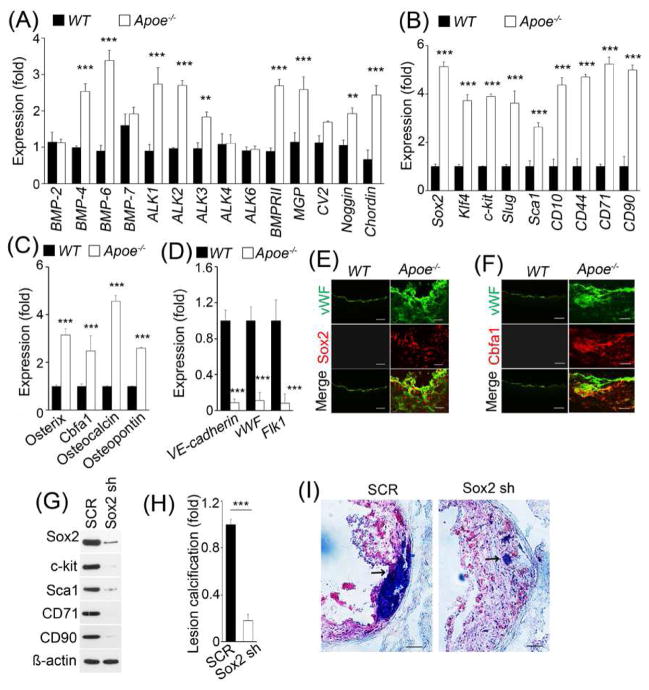
Our previous studies showed increased BMP activity in atherosclerotic lesions in ApoE−/− mice (9). Here, we confirmed high expression of BMP in aortic ECs isolated from ApoE−/− mice fed a western diet for 16 weeks. We found significant induction of BMP ligands BMP4 and 6, receptors activin receptor-like kinase (ALK) 1, 2 and 3, BMP type II receptor and inhibitors matrix gla protein (MGP), noggin and chordin (Figure 1A). Enhanced BMP activity has been reported to induce EndMTs and allow ECs to undergo osteogenic differentiation (10, 14, 20). Therefore, we examined stem cell and mesenchymal markers, and endothelial and osteogenic markers in aortic ECs isolated from the fat-fed ApoE−/− mice. We found strong induction of Sox2, Klf4, c-kit, Slug, Sca1, CD10, CD44, CD71, and CD90 (Fig. 1B), and osterix, Cbfa1, osteocalcin and osteopontin (Fig. 1C) with reduction of endothelial markers VE-cadherin, von Willebrand fatctor (vWF) and Flk1 (Fig. 1D). Immunostaining showed that Sox2 and the osteogenic marker Cbfa1 co-localized with vWF in calcified atherosclerotic lesions (Fig. 1E and F), suggesting that EndMTs occur in atherosclerotic calcification.
Fig. 1. Sox2 and EndMTs in atherosclerotic lesion calcification of ApoE−/− mice.
(A–D) Expression of BMP components (A), stem cell and mesenchymal markers (B), osteogenic markers (C) and EC markers (D) in isolated aortic ECs of wild type (WT) and ApoE−/− mice, as determined by real-time PCR. (E–F) The EC marker vWF co-localizes with Sox2 (E) and bone marker Cbfa1 (F) in calcified atherosclerotic lesions. (G–I) ApoE−/− mice were fed a western diet and treated with scrambled shRNA (SCR) or Sox2 shRNA (sh) for 16 weeks. Aortic expression of Sox2, c-kit, Sca1, CD71 and CD90 was examined by immunoblotting (G). The volume of calcification in atherosclerotic lesions was examined (n=5) (H). Representative Oil Red O stained aortic sinus sections (I). Arrows indicate calcification. Scale bar (b, c, f), 100 μm. ***p <0.001.
Limiting Sox2 reduced EndMTs and lesion calcification
Since depletion of Sox2 reduces EndMTs (13), we treated ApoE−/− mice with Sox2 shRNA and fed them a western diet for 8 weeks before examining the expression of stem cell and mesenchymal markers and lesion calcification. We found that treatment with Sox2 shRNA decreased both marker expression and lesion calcification in the fat-fed ApoE−/− mice (Fig. 1G–I). There was no significant change in serum lipid levels and size of atherosclerotic lesions, as measured in the aortic sinuses (data not shown), although the study was not powered for lesion size but for calcification. The results suggest that Sox2 is a mediator of EndMTs in lesion calcification.
Serine proteases were upregulated in calcified lesions
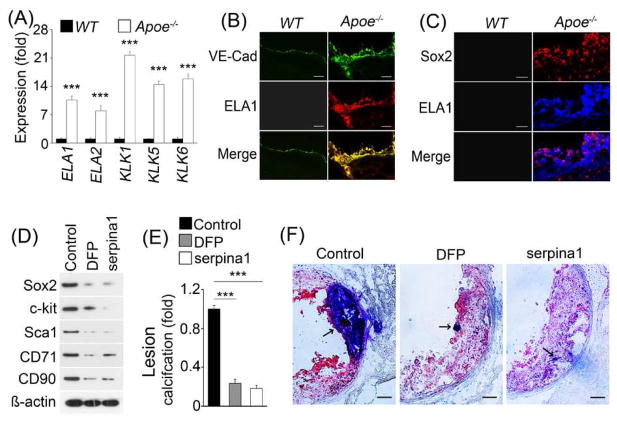
We previously showed that a complex of serine proteases, including elastase 1, 2 and kallikrein 1, 5, and 6, regulate Sox2 in EndMTs (13). Therefore, we examined the expression of elastases and kallikreins, and found a strong induction of elastase 1, 2 and kallikrein 1, 5, 6 in the aortic ECs of fat-fed ApoE−/− mice (Fig. 2A, right panel). Induced elastase 1, as an example of the group, co-localized with vWF and Sox2 in calcified lesions (Fig. 2B and C), supporting that activation of elastases and kallikreins also occurs in ECs in atherosclerotic lesions.
Fig. 2. Elastases and kallikreins in EndMTs in calcified atherosclerotic lesions of ApoE−/− mice.
(A) Expression of elastases and kallikreins in isolated aortic ECs of wild type (WT) and ApoE−/− mice as shown by real-time PCR. (B–C) Elastase (ELA) 1 co-localized with EC marker VE-cadherin (VE-Cad) (B), and Sox2 (C) in calcified atherosclerotic lesions. (D–F) ApoE−/− mice were fed a western diet and treated with control, diisopropylfluorophosphate (DFP) and serpina1. Immunoblotting showed the expression of Sox2, c-kit, Sca1, CD71 and CD90 in aortic tissues (n=5) (D). The volume of calcification in atherosclerotic lesions was examined (n=5) (E). Representative Oil Red O stained aortic sinus sections (F). Arrows indicate calcification. Scale bar (b, c, f), 100 μm. ***p<0.001.
Inhibition of serine proteases decreased calcification in atherosclerotic lesions
We then determined whether protease inhibition reduced Sox2, EndMTs, and calcification by using the inhibitors diisopropylfluorophosphate or serpina1, similar to our previous study (13). We treated ApoE−/− mice for 8 weeks with diisopropylfluorophosphate or serpina1, respectively, together with western diet. We found that protease inhibition reduced both marker expression and lesion calcification in the fat-fed ApoE−/− mice (Fig. 2D–F). No significant changes in serum lipid levels or size of atherosclerotic lesions were detected (data not shown). The data suggest that the activation of elastases and kallikreins triggers Sox2 expression and EndMTs in ECs in atherosclerosis and contributes to the lesion calcification.
Thus, the same mechanism previously detected in MGP-deficient mice is also activated in the calcification of atherosclerotic lesions.
DISCUSSION
We previously found EndMTs contributing to aortic calcification in mice lacking MGP, a vascular BMP inhibitor, where BMP activation is increased due to lack of BMP inhibition (13). In this study, we explored whether similar EndMTs occur in atherosclerotic lesions in fat-fed ApoE−/− mice. MGP, as a BMP inhibitor, is induced in aortic endothelium of fat-fed ApoE−/− mice (Fig. 1) (9). Our studies suggest that increased BMP activity is the primary force to drive EndMTs leading to calcification in both atherosclerotic lesions and Mgp−/− mice. However, the mechanism by which high BMP activity is achieved differs between atherosclerotic lesions where increased expression of BMPs and BMP receptors enhances BMP signaling, and Mgp−/− mice where BMP signaling increases due to less BMP inhibition (9, 13). Furthermore, MGP is induced in atherosclerotic lesions (9), but not enough to overcome BMP induction. Enhancement of the MGP response does suppress the excessive BMP activity (9). In addition, inhibition of serine proteases or reduction of Sox2 expression, both of which decreased calcification in Mgp−/− mice (13), reduced EndMTs and calcification in ApoE−/− mice. However, we did not detect any decrease in the atherosclerotic lesions from these interventions. It may be due to the limited size of the study, which was focused on and powered for changes in calcification. Overall, the results suggest that the same mechanism promoting calcification in Mgp−/− mice is activated in atherosclerotic lesion.
Endothelial-mesenchymal transitions (EndMTs) in atherosclerotic lesions contribute to calcification.
Stem cell and mesenchymal markers, including sex-determining region Y-box 2 (Sox2), are upregulated in aortic endothelial cells (ECs) of fat-fed ApoE−/− mice.
Limiting Sox2 decreases the stem cell and mesenchymal markers and calcification in ApoE−/− aortas.
A complex of serine proteases is upregulated in ApoE−/− aortic ECs.
Blockade of these proteases reduces expression of Sox2 and atherosclerotic lesion calcification.
Acknowledgments
FINANCIAL SUPPORT
Funding for this work was provided in part by NIH grants NS79353, HL30568, HL81397 and HL112839, and the American Heart Association (Western Affiliate).
Footnotes
CONFLICT OF INTEREST
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–85. [PubMed] [Google Scholar]
- 2.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003;108(13):1655–61. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 3.Wu KK, Huan Y. Diabetic atherosclerosis mouse models. Atherosclerosis. 2007;191(2):241–9. doi: 10.1016/j.atherosclerosis.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nature reviews Cardiology. 2010;7(9):528–36. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishbein MC, Fishbein GA. Arteriosclerosis: facts and fancy. Cardiovasc Pathol. 2015;24(6):335–42. doi: 10.1016/j.carpath.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Bostrom K, Demer LL. Regulatory mechanisms in vascular calcification. Crit Rev Eukaryot Gene Expr. 2000;10(2):151–8. [PubMed] [Google Scholar]
- 7.Moonen JR, Lee ES, Schmidt M, Maleszewska M, Koerts JA, Brouwer LA, van Kooten TG, van Luyn MJ, Zeebregts CJ, Krenning G, et al. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res. 2015;108(3):377–86. doi: 10.1093/cvr/cvv175. [DOI] [PubMed] [Google Scholar]
- 8.Krenning G, Barauna VG, Krieger JE, Harmsen MC, Moonen JR. Endothelial Plasticity: Shifting Phenotypes through Force Feedback. Stem cells international. 2016 doi: 10.1155/2016/9762959. 9762959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107(4):485–94. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Y, Jumabay M, Ly A, Radparvar M, Cubberly MR, Bostrom KI. A role for the endothelium in vascular calcification. Circ Res. 2013;113(5):495–504. doi: 10.1161/CIRCRESAHA.113.301792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 107(4):485–94. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, Yu PB. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(3):613–22. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao J, Guihard PJ, Blazquez-Medela AM, Guo Y, Moon JH, Jumabay M, Bostrom KI, Yao Y. Serine Protease Activation Essential for Endothelial-Mesenchymal Transition in Vascular Calcification. Circ Res. 2015;117(9):758–69. doi: 10.1161/CIRCRESAHA.115.306751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yung LM, Sanchez-Duffhues G, Ten Dijke P, Yu PB. Bone morphogenetic protein 6 and oxidized low-density lipoprotein synergistically recruit osteogenic differentiation in endothelial cells. Cardiovasc Res. 2015;108(2):278–87. doi: 10.1093/cvr/cvv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch-Reardon KM, Wu N, Hughes CC. A role for partial endothelial-mesenchymal transitions in angiogenesis? Arterioscler Thromb Vasc Biol. 2015;35(2):303–8. doi: 10.1161/ATVBAHA.114.303220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Current opinion in cell biology. 2003;15(6):740–6. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation. 2012;125(14):1795–808. doi: 10.1161/CIRCULATIONAHA.111.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011;121(2):468–74. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16(12):1400–6. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Science signaling. 2014;7(344):re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Pechoux C, Bogaard HJ, Dorfmuller P, Remy S, Lecerf F, Plante S, et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131(11):1006–18. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 23.Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125(12):4514–28. doi: 10.1172/JCI82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992;89(10):4471–5. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bostrom K, Zebboudj AF, Yao Y, Lin TS, Torres A. Matrix GLA protein stimulates VEGF expression through increased transforming growth factor-beta1 activity in endothelial cells. J Biol Chem. 2004;279(51):52904–13. doi: 10.1074/jbc.M406868200. [DOI] [PubMed] [Google Scholar]