Abstract
Frontal–thalamic interactions are crucial for bottom‐up gating and top‐down control, yet have not been well studied from brain network perspectives. We applied network modeling of fMRI signals [dynamic causal modeling (DCM)] to investigate frontal–thalamic interactions during an attention task with parametrically varying levels of demand. fMRI was collected while subjects participated in a sustained continuous performance task with low and high attention demands. 162 competing model architectures were employed in DCM to evaluate hypotheses on bilateral frontal–thalamic connections and their modulation by attention demand, selected at a second level using Bayesian model selection. The model architecture evinced significant contextual modulation by attention of ascending (thalamus → dPFC) and descending (dPFC → thalamus) pathways. However, modulation of these pathways was asymmetric: while positive modulation of the ascending pathway was comparable across attention demand, modulation of the descending pathway was significantly greater when attention demands were increased. Increased modulation of the (dPFC → thalamus) pathway in response to increased attention demand constitutes novel evidence of attention‐related gain in the connectivity of the descending attention pathway. By comparison demand‐independent modulation of the ascending (thalamus → dPFC) pathway suggests unbiased thalamic inputs to the cortex in the context of the paradigm. Hum Brain Mapp 37:2557–2570, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: sustained attention, dynamic causal modeling, prefrontal cortex, thalamus, fMRI, adolescence
INTRODUCTION
Sustained attention is generally defined as the ability to maintain consistent (as opposed to transient) task‐related vigilance for extended periods of time, and is one of the most fundamental of sensory‐motor domains [Posner and Rothbart, 1998]. Attention is accomplished in the cortex, in part through synchronization of frontal, parietal, and temporal cortical regions [Gross et al., 2004; Sarter et al., 2001]. However, because the cortex operates under a “high input regime” [Logothetis et al., 2009] from the thalamus, the structure has been identified as key in mediating visual inputs important for subsequent attention related processing [Fan et al., 2005; Gitelman et al., 1999; Lawrence et al., 2003]. In this study, we investigated the network characteristics of attention processing in cortical and thalamic networks. In particular, we applied dynamic causal modelling (DCM) [Stephan et al., 2007] to fMRI data collected during a sustained attention task [Salgado‐Pineda et al., 2004] with variable task demands (low vs. high) [Diwadkar et al., 2011]. Our goal was to assess the effects of attention demand on the contextual modulation of ascending (thalamus → prefrontal cortex) and descending pathways (prefrontal cortex → thalamus). Given the relative roles of each of these regions derived from activation‐based studies [see Bush 2010; Sarter et al. 2001, for reviews], we expected to see distinct effects of attention load in each direction. In particular, we hypothesized that the modulation of the descending pathway would be highly sensitive to increases in attention load, with the combination of ascending and descending effects collectively informing network profiles of attentional gating.
THE ATTENTION NETWORK
The attention network can be classified into sub‐networks with relatively specialized functions [Posner, 2012] and encompassing a combination of ascending and descending mechanisms. Frontal subnetworks that include the dorsal anterior cingulate cortex (dACC), the dorsal prefrontal cortex (dPFC) and the basal ganglia (BG) appear to be central to executive or supervisory mechanisms of attention. These mechanisms include regulation, error monitoring or processing and sustained vigilance [Fan et al., 2008; Posner et al., 2007]. In comparison, regions correlated with modality specific attention processes (e.g., visuospatial attention) include the intra‐parietal sulcus associated with spatial orientation, and the temporal–parietal junction associated with spatial reorientation [Corbetta et al., 1998, 1995]. Higher order mechanisms, relating to control and vigilance are particularly dependent on the brain's frontal regions [Kelley et al., 2008]. The development of the frontal cortex remains dynamic in adolescence [Gogtay et al., 2004], though proficiency associated with basic attention paradigms appears to peak early in this developmental stage [Klenberg et al., 2001] suggesting that basic attention‐related proficiency may be relatively mature mid‐way through this neurodevelopmental period.
The thalamus, the “gateway” to the cortex [Jang et al., 2014], is comprised of various sub‐nuclei involved in attention gating [Brunia 1993; McAlonan et al., 2008]. For example, increased neuronal responses are recorded when a target is inside the receptive field of a neuron but the thalamic reticular nucleus, which inhibits activity of other thalamic nuclei, is concurrently less responsive. Thus, attention to stimuli suppresses neuronal activity of the reticular nucleus over selected relay nuclei, and this disinhibition gates thalamocortical inputs. These functional effects appear to be mediated by anatomical connections between the thalamus (and specific thalamic nuclei) and regions of the frontal lobe, including the dorsal prefrontal cortex and the anterior cingulate cortex. Activation‐based fMRI studies have documented attention‐related responses in all of these regions of the attention network [Fan et al., 2005]. However, effective connectivity techniques such as Dynamic Causal Modeling [Friston et al., 2003] that can assess contextual modulation of network pathways have been under‐utilized.
Like the dPFC, dACC plays a critical role in control mechanisms. These include the integration of goal, motor, and feedback‐related information within the intracortical network. As a critical “control center”, the structure modulates the organization and efficient recruitment of executive brain regions [Bakshi et al., 2011; Bush 2010; Diwadkar 2012; Paus 2001]. The dACC has bilateral connections to the dPFC, an executive brain region responsible for the functions of vigilance, selective and divided attention, executive control, and working memory [Duncan and Owen, 2000]. These cortical regions are also connected to the BG, which receives input from the cortex and deliver output to the thalamus. The BG organizes motivations that lead to the execution of goal‐directed behaviors; for example, pushing a button [Haber, 2003]. The superior parietal cortex (SPC) is involved in the allocation of spatial attention and is innervated by connections from the thalamus [Bush, 2010; Jang et al., 2014; Modha and Singh, 2010]. In addition to sending projections to diverse cortical targets, the thalamus also contains nuclei that form higher order relay centers for indirect cortical‐cortical communication. The processing demand of a stimulus modulates the activity of these relay centers as the thalamus attempts to filter the flow of incoming information for appropriate recruitment of attentional networks [Sherman and Guillery, 2002]. Thus, DCM was used to explore the network dynamics between regions displayed in Figure 1.
Figure 1.
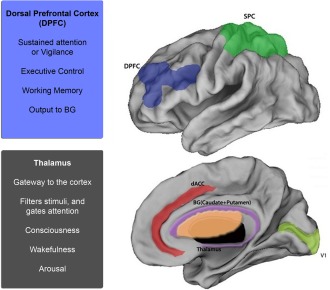
The attention network. Brain regions constituting the sustained attention network are depicted. These include the dorsal anterior cingulate cortex (dACC), dorsal prefrontal cortex (dPFC), superior parietal cortex (SPC), basal ganglia (BG), primary visual cortex (V1), and the thalamus. Regions of particular importance (and focus in the current investigation) are the thalamus and the dPFC. The thalamus is involved in the gating of attention and the dPFC is the executive control center; both play a role in regulating descending and ascending attention‐related mechanisms. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
MATERIALS AND METHODS
Subjects
Methods were approved by the Human Investigation Committee at Wayne State University, and 21 typically developing subjects (11 ≤ age ≤ 19; 6 females; Mean: 14.6 years; SD: 2.45) provided informed consent or assent to participate in fMRI studies, where for assenting participants, the parent provided consent. The mean education level was that of a 9th grader or high school freshman. IQ was assessed using either the WISC or the WAIS (76 ≤ IQ ≤ 124; Mean: 94; SD: 16.5). The participants were free of psychiatric or neurological illness, thereby considered typically developing [Kaufman et al., 1997].
fMRI Task and Data Acquisition
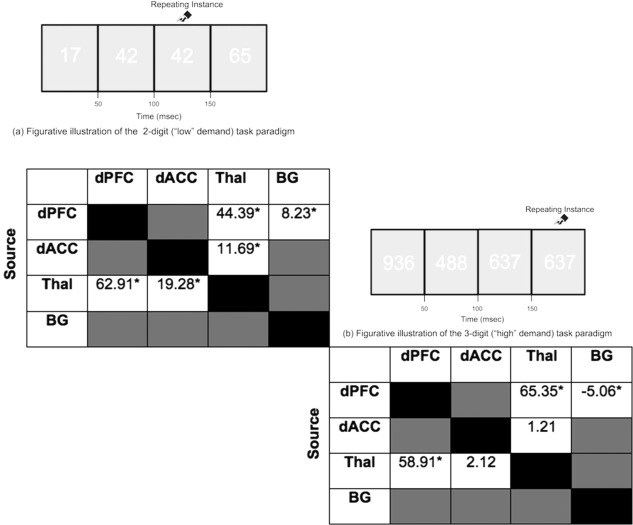
Subjects performed a version of the Continuous Performance Task, Identical pairs version (CPT‐IP) [Diwadkar et al., 2011b; Salgado‐Pineda et al., 2003]. During the study, in a block of trials, either two‐ or three‐digit numbers were sequentially and rapidly presented (50 ms, 250 ms SOA); subjects detected the repetition of numbers in the sequence. Two control blocks were also incorporated into the task, during which subjects passively viewed strings of two or three digit numbers (“00” or “11”; “000” or “111”). Extended block lengths (120 s; 480 stimuli/block; 25% targets) were used to ensure sustained periods of attention related processing. One block of trials for each of the two levels of attention demand was employed (with one block of trials for each of the control conditions). The total scan duration was 560 s (480 s across the four task and control epochs, plus four 20 s rest epochs interspersed throughout) [Diwadkar et al., 2014].
Figure‐ground contrast was manipulated using white numbers (RGB:255,255,255) on an off‐white background (RGB:225,225,225) to ensure increased overall vigilance and preempt potential gains from conditions of maximal contrast [Dresp and Grossberg, 1999]. The manipulation of numerosity (two‐ or three‐digit numbers) constitutes a central manipulation of attention demand [Butterworth, 2005] that exerts cortical processing demands [Cohen Kadosh et al., 2005]. Figure 2 depicts the two classes of task epochs.
Figure 2.
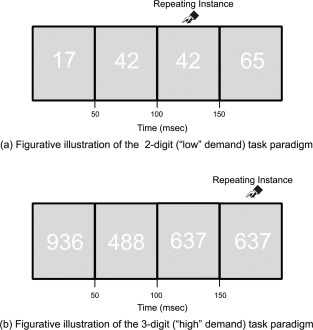
Task schematic. The figure schematically depicts the continuous performance task (identical pairs version, CPT‐IP) employed in the study. Subjects were required to identify consecutively repeated instances of numbers (identical pairs). Attention demand was nominally varied by changing the numerosity of numbers used within an epoch: (a) 2‐digit (“low” demand) task paradigm and (b) 3‐digit (“high” demand) task paradigm.
Functional data were continuously acquired using a full body Bruker MedSpec 4.0 T system running the Siemens Syngo console. Gradient echo planar images (EPI) were collected using an eight‐channel head coil (TR = 2,000 ms; TE = 30 ms; matrix size = 64 × 64; FOV = 240 mm; voxel size = 3.75 × 3.75 × 4 mm). Twenty‐four continuous axial slices per brain volume were collected, positioned parallel to the anterior commissure/posterior commissure (AC–PC) line.
fMRI Processing
Data were processed with Statistical Parametric Mapping (SPM8) using typical methods. Realignment was performed to correct for head motion artifact during the scan. Realigned images were normalized to the Montreal Neurological Institute (MNI) EPI template and voxels resliced (2 × 2 × 2 mm). Normalized images were smoothed using an 8 mm FWHM Gaussian kernel. Images where estimated motion exceeded 4 mm were discarded from the analyses (<1% of all images), and these subjects were excluded from the study. To account for extended block lengths (120 s), a lenient high pass filter was used to remove low frequency drifts and fluctuations allowing us to preserve attention related responses in the fMRI signals.
Dynamic Causal Modeling Analyses
In preparation for DCM analyses, first level GLM analysis employed three separate regressors to represent a) visual and b) attention processing associated with each level of demand. Regressors were modeled as boxcar vectors (representing individual epochs) convolved with a canonical hemodynamic reference waveform.
DCM allows the interpretation of causal interactions between hidden state variables [Friston, 2011; Friston et al., 2012; Stephan et al., 2010]. The brain is viewed as a bilinear system in which the inputs are the experimental conditions and the outputs are the hemodynamic response measured using fMRI. Changes in the neural circuitry are modeled using the following state differential equation:
and are the result of three variables A, B, and C. A represents the endogenous coupling that exists between regions that is seen as independent of task‐imposed experimental conditions. The variable B (j) represents the modulatory response in the network connections due to changes in experimental conditions (uj). Finally, C represents the direct driving input on particular regions as induced by the experimental conditions. All DCM modeling relied on bilinear deterministic DCM (vs. nonlinear, stochastic, two‐state DCM) [Diwadkar et al., 2014, 2012].
Network discovery with DCM relies on the identification of generative network architectures with the highest evidence given the observed fMRI data, thus testing hypotheses on an a priori defined model space. The model space is comprised of neurobiologically plausible competing models, each representing hypotheses on the connective architecture of the investigated neural system.
DCM Model Space
The model space was created to investigate hypotheses associated with the modulation of bi‐directional frontal–thalamic pathways by experimental demand (two‐digit vs. three‐digit numbers). The regions depicted in Figure 1 were incorporated into a six‐region network that included: primary visual cortex (V1), superior parietal cortex (SPC), thalamus, basal ganglia (BG), dorsal anterior cingulate cortex (dACC), and the dorsal prefrontal cortex (dPFC). The fixed and permuted endogenous connections and their contextual modulation were motivated by known anatomical network properties, and hypotheses of interest in the current study.
Eight endogenous connections were common across all models (i.e., no hypotheses were associated with these), specifically between visual regions (V1), regions for spatial attention and orienting (via SPC), and gating (the thalamus). The connections within the frontal–striatal–thalamic circuit were: (thalamus → BG), (BG → thalamus), (dACC → BG), and (BG → dPFC). These connections were motivated by the flow of information by cortical input to the basal ganglia, and striatal output to the cortex via the thalamus. An additional endogenous connection (dPFC → BG) was permuted to assess frontal–striatal interactions.
To summarize we employed (a) Eight fixed endogenous connections, and (b) Four endogenous connections were permuted (dPFC → thalamus, thalamus → dPFC, dACC → thalamus, thalamus → dACC). (c) On Five endogenous connections (dPFC → thalamus, thalamus → dPFC, dACC → thalamus, thalamus → dACC, dPFC → BG), we permuted their contextual modulation by attention. Thus, four connections were 1) permuted for endogenous connectivity that 2) were, or 3) were not contextually modulated; and one endogenous connection (dPFC → BG) was permuted for contextual modulation. The resultant space consisted of 162 (34 × 2) unique models, a combination of three possible modulatory effects on four permuted endogenous connections (i.e., 34 possibilities) and two possible modulatory effects on one fixed endogenous connection (i.e., 2 possibilities).
The primary goal of this study was to examine the contextual modulation of interactions between brain regions as a function of varying attention demands. Therefore, contextual modulation of thalamic–frontal connections constituted a principal hypothesis of interest and was permuted across the model space. Specifically, bidirectional modulation of thalamic–frontal pathways to the dACC and the dPFC as a function of differential attention demand was employed; these connections were (dPFC ↔ thalamus) and (dACC ↔ thalamus). The modulation of thalamic‐frontal pathways is presumed to reflect ascending attention processes engaged by external sensory inputs of salient and novel stimuli (e.g., signals that flow “bottom‐up” regardless of whether two‐ or three‐digit numbers are being presented). In comparison, modulation of frontal–thalamic pathways represents descending attention processes mediated by voluntary shifts of attention based on expectations of goals and rewards [Connor et al., 2004]. The resultant model space included 162 competing models, and the methodology for generating these is illustrated in Figure 3.
Figure 3.
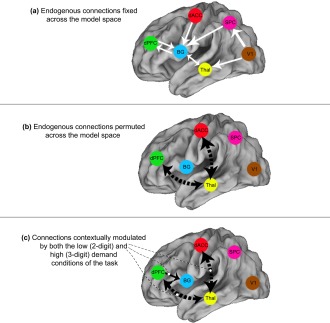
Model space and connections of interest. The figure provides an illustration of the employed model space. (a) White arrows represent endogenous connections that were fixed across the model space. (b) Black arrows represent endogenous connections that were permuted across the model space. (c) These connections were contextually modulated by both the low (2‐digit) and high (3‐digit) demand conditions. Broken black arrows (ascending and descending dPFC ↔ thalamus and dACC ↔ thalamus connections) were permuted for endogenous connectivity AND contextual modulation, whereas the solid black arrow (dPFC → BG connection) was fixed, and was also modulated by task. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Model Estimation
Prior to modeling, time series from each participant were extracted from each region of interest (ROI) using spheres (5 mm radius) centered on the peak of the “effects of interest” (p < 0.05, adjusted for “effects of no interest”) from the participant's first‐level model [Diwadkar et al., 2012]. To determine the most likely generative model, we applied a random effects (RFX) Bayesian model selection (BMS) procedure to all 162 models estimated across all 21 participants. RFX uses a variational Bayes method to estimate posterior probabilities of competing models, given the parameter values determined by DCM, proving a posterior likelihood of a given model being the generative model across all subjects. Empirical use of the RFX has demonstrated its usefulness in studies of cognitive tasks, which can be implemented neuronally in a variety of ways, and thus was ideal given the nature of this task and population [Diwadkar et al., 2014, 2012; Stephan et al., 2010].
Bayesian parameter averages (henceforth BPA) of coupling estimates (with a focus on modulatory coupling) were analyzed to determine potential differences in frontal–thalamic modulation as a function of attention‐related demand. To test for differences in contextual modulation as a function of attention demand, we compared the BPAs across subjects for each condition. This procedure provides posterior densities over the effective connectivity parameters, enabling one to estimate the difference between means and posterior confidence in those differences [shown in terms of a posterior standard error in Figs. 6 and 7]. Statistical significance was assessed using Bonferroni corrected group differences (p < 0.0033 for each of the 15 tests conducted; see Tables 2 and 3) and denoted in the figures (differences from baseline are indicated by *; pairwise differences are represented by ϕ).
Figure 6.
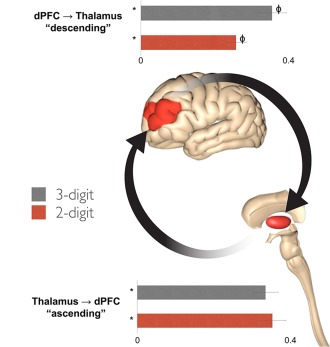
Modulation of frontal–thalamic connections. Contextual modulation of the descending (dPFC → thalamus) and ascending (thalamus → dPFC) pathways are graphically presented. Differences from zero (denoted by *) and among means (denoted by ϕ) were Bonferroni corrected (p < 0.0033, see text). (a) There was a significant increase in the modulation of the descending (dPFC → thalamus) pathway during the 3‐digit condition compared to the 2‐digit condition, suggesting an adaptive and dynamic mechanism for ensuring the required gain related to attention demands of the task. (b) In comparison, there were no differences between conditions in the contextual modulation of the ascending (thalamus → dPFC) pathway. Error bars are ±SD. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 7.
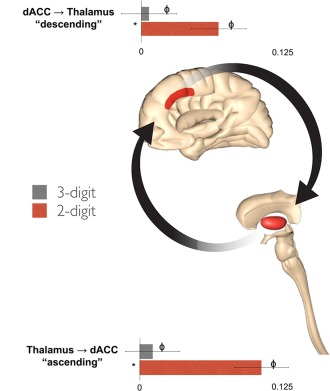
Modulation of Cingulo‐thalamic connections. Contextual modulation of the descending (dACC → thalamus) and ascending (thalamus → dACC) pathways are graphically presented. Both pathways experienced significantly increased positive modulation during the “low” demand two‐digit condition relative to the “high” demand three‐digit condition. This result is notably different from the findings with the dPFC pathway. Error bars are ±SD. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Table depicts statistical information (t scores) associated with estimates of significant contextual modulation (i.e., different than zero) during the (a) “low” (2‐digit; left) and (b) “high” (3‐digit; right) demand condition
|
Source regions for each pathway are listed by row; targets are listed by column. Significant differences (p < 0.05, Bonferroni) are denoted*
Table 3.
Table depicts statistical information (t scores) associated with differences between contextual modulation during the “low” (2‐digit) and “high” (3‐digit) demand conditions. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]

|
Source regions for each pathway are listed by row; targets are listed by column. Significant differences (p < 0.05, Bonferroni) are denoted*. Cells with increased modulation during the high demand condition are shaded red. Cells with increased modulation during the low demand condition are shaded blue.
RESULTS
Behavioral Performance
Behavioral performance was assessed in terms of sensitivity to distinguish targets from distracters using d' [Macmillan and Creelman, 2005], an established metric in Signal Detection theory [Green and Swets, 1966; Wickens, 2001]. The metric incorporates the hit‐rate (e.g., the rate of responding “same” to successively presented stimuli in the same valence category) and the false alarm rate (e.g., the rate of responding “different” to successively presented stimuli in different valence categories), and is based on the difference between the inverse function of the cumulative Gaussian distribution applied to each; a higher d' reflects greater sensitivity to the task.
Three tests were employed to assess the effects of demand and the effects of age on behavioral performance, subsumed under a single repeated measures analysis of covariance. In this analysis, attention load was modeled as the single repeated measure, with age as covariate (Family p < 0.05, p < 0.017 for each analysis), allowing us to assess the effects of load, age, and the interaction between load and age. The only significant effect was that of attention load [(mean (±SD); 3 Digit = 3.89 (0.87) < 2 Digit = 4.45 (1.18)]. Participants were less sensitive to discriminating targets from distracters in the more demanding condition, F(1,19) = 12.21, p < 0.002, MSe = 0.237. The load by age interaction and the effect of age were not significant (ps > 0.10).
fMRI Activation Data
Activation analyses revealed significant clusters of activation in the anatomically defined attention‐related network in both task‐related conditions and in their conjunction. Figure 4 shows significant clusters projected to the bilateral dorsal and serial axial views of the cortical surface (see Table 1 for statistical information). Consistent with previous imaging studies, attention related activation was observed in the network of interest: dorsal prefrontal cortex, the basal ganglia, the parietal cortex, the dorsal ACC and the thalamus [Bush, 2010; Fan et al., 2005; Posner, 2012].
Figure 4.
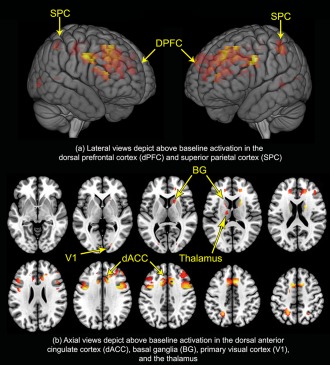
Activation in the attention network. The results of activation analyses depict engagement of the sustained attention network represented in a conjunction analyses (2‐digit ∩ 3‐digit). (a) Surface projections depict suprathreshold activation in the dorsal prefrontal cortex (dPFC) and superior parietal cortex (SPC). (b) Axial views depict suprathreshold activation in the dorsal anterior cingulate cortex (dACC), basal ganglia (BG), primary visual cortex (V1), and thalamus. These results (consistent with previously published evidence) demonstrate that conventional image analytic methods identify engagement of the attention network. Our focus turns to the network interactions latent within these activations. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
Information on the observed peaks for the conjunction analysis (2‐digit ∩ 3‐digit) (p < 0.05, cluster level, see Fig. 4) is depicted
| Region | Peak (x, y, z) MNI coordinates | t‐statistic at peak |
|---|---|---|
| dACC | (−6, 10, 52) | 4.51 |
| dPFC | (−42, 0, 36) | 4.73 |
| V1 | (22, −88, −2) | 3.01 |
| Superior parietal | (−22, −58, 46) | 3.49 |
| Basal ganglia | (30, 22, 2) | 5.57 |
| Thalamus | (−18, −8, 10) | 1.83 |
DCM Results
The presentation of DCM results is sequentially organized to emphasize the BMS results and the effects associated with contextual modulation by attention demand of thalamocortical pathways. First, we present the results of Bayesian Model Selection, used to identify the most likely generative model(s) of the data [Stephan et al., 2010]. We then provide an account of the model structure of the winning model, which constitutes the winner amongst competing hypotheses associated with the network space and the modulation of network pathways by task conditions. We follow the presentation of model details with statistical inference on model parameters, focusing on differences in the contextual modulation by task (2 digit vs. 3 digit) [Friston et al., 2003] of ascending (thalamus → dPFC) and descending (dPFC → thalamic) pathways.
Model Selection
BMS identified a single winning model with notably higher exceedence probabilities than priors and its relative competitors. Figure 5 depicts exceedence probabilities across the model space with the winning model and the model structure as determined by BMS RFX, clearly identified.
Figure 5.
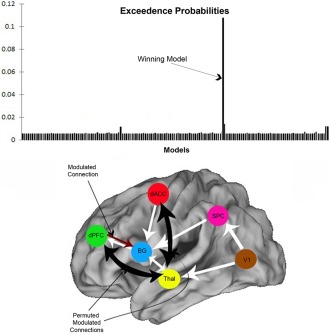
BMS results and winning model architecture. Exceedence probabilities (relative likelihood) of each competing model are illustrated on the bar graph (depicting a single winning model). The architecture of the winning model is depicted below the graph: The permuted modulated connections that emerged were (dPFC ↔ thalamus) and (dACC ↔ thalamus). Note that contextual modulation of (dPFC → BG) was not permuted across the model space. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Endogenous Connections
The winning model contained the bilaterally permuted endogenous connections (thalamus ↔ dPFC) and (thalamus ↔ dACC), consistent with prior evidence of anatomical connections between the thalamus and the cortex [Saalmann and Kastner, 2009) (see Supporting Information Table I for BPAs for the endogenous connections in the winning model).
The primary hypotheses across permuted models focused on contextual modulation of frontal–thalamic pathways during the two task epochs, and potential differences in parameter estimates associated with the varying demands on attention processing. We next present the observed parameter estimates for frontal–thalamic (dPFC ↔ thalamus) pathways and cingulo‐thalamic (dACC ↔ thalamus) pathways.
Contextual Modulation of the dPFC ↔ Thalamus Pathway by Attention
Modulation of ascending thalamic‐frontal pathways and descending frontal–thalamic pathways was observed to be different; these results are depicted in Figure 6. Analysis of BPA estimates revealed no significant differences in the modulation of the ascending (thalamus → dPFC) pathway between the high (3‐digit) and low (2‐digit) demand conditions; this connection was modulated by attention‐related load to a similar degree in both conditions. Contrasting results were obtained for the descending (dPFC → thalamus) pathway; a statistically significant effect of differing modulation was observed between the two conditions. BPA estimates indicate this pathway was characterized by greater modulation during the high demand (3‐digit) condition relative to low demand (2‐digit).
Contextual Modulation of dACC ↔ Thalamus Pathway by Attention
Pathways to the dorsal anterior cingulate cortex (dACC), evinced a different pattern of load‐related modulation than those observed with the dorsal prefrontal cortex; our findings are depicted in Figure 7. Unlike the asymmetry identified in the modulation of ascending and descending frontal–thalamic pathways (dPFC ↔ thalamus), a consistent pattern of modulation was uncovered for the ascending (thalamus → dACC) and descending (dACC → thalamus) pathways during conditions of low and high demand. BPA estimates signify the ascending (thalamus → dACC) pathway evinced significantly increased modulation under low demand (2‐digit) relative to high demand (3‐digit); likewise, the descending (dACC → thalamus) pathway was modulated to a greater degree during the low demand (2‐digit) condition relative to high demand (3‐digit). Statistical information associated with all of the analyses presented in Figures 6 and 7 is depicted in Tables 2 and 3.
Contextual Modulation of dPFC → BG Pathway by Attention
The endogenous descending (dPFC → BG) connection was fixed across all models and was analyzed for potential differences in modulation resulting from differences in attention‐related load. This pathway was differently modulated during conditions of low and high demand; positive modulation was observed during the low demand condition (2‐digit). In contrast, negative modulation was observed during the high demand condition of high (3‐digit).
Developmental Effects
The relationships between age and each of the eight parameters associated with contextual modulation of cortical–thalamic pathways by attention were investigated in a bivariate cross‐correlation matrix. For this family of 72 tests, the overall family‐wise probability was set at p < 0.05 (p < 0.0007 for each of the individual tests).
Two results were in evidence: First, age was not correlated with parameter estimates of contextual modulation (0.004 ≤ r 2 ≤ 0.20, n.s.) in any of the eight analyses. This negative result implies that developmental effects did not underlie the estimates of significant contextual modulation observed in our data. However, parallel analyses within pairs in the cross‐correlation matrix were meaningfully significant. Specifically, on the ascending pathway to the dPFC (thalamus → dPFC; Fig. 6), the degree of contextual modulation between the low and the high demand attention conditions was strongly positively correlated (r = 0.782, p < 0.0001). This statistical relationship between patterns of modulation of the ascending thalamic‐frontal pathway under each of the attention conditions indicates that the results depicted in Figure 6 were highly consistent within participants.
DISCUSSION
In this study, we employed dynamic causal modeling to investigate effective connectivity within an a priori cortical–striatal–thalamic circuit subserving sustained attention. Hypotheses regarding the modulation by attention of the effective connectivity of frontal–thalamic and cortical–thalamic pathways were assessed. Our principal results were these:
Activation analysis results revealed significant regional activity in dACC, dPFC, BG, SPC, V1, and thalamus. These results are consistent with previous activation‐based assessments of the attention network [Diwadkar et al., 2014; Fan et al., 2005].
BMS revealed a winning network architecture that included the permuted bilateral dPFC ↔ thalamus and dACC ↔ thalamus endogenous connections, consistent with the existence of known cortical connections between these regions [Barnea‐Goraly et al., 2005; Herrero et al., 2002; Saalmann and Kastner, 2009].
Attention‐related demand modulated cortical–thalamic pathways in systematic and distinct ways:
Attention demand exerted asymmetric effects on the contextual modulation of frontal–thalamic (“descending”) and thalamic‐frontal (“ascending”) pathways. There was no demand‐related difference in modulation of the ascending thalamic–frontal pathway (thalamus → dPFC). However, the high‐demand (3‐digit) condition resulted in greater modulation of the descending frontal–thalamic (dPFC → thalamus) pathway.
In comparison to the dPFC ↔ thalamus pathway, the dACC ↔ thalamus pathway experienced greater modulation during the low demand (2‐digit) condition.
Below, following interpretation of the observed network effects from systems perspectives, we speculate on the reasons for the negative findings related to age‐related changes, suggesting that network mechanisms subserving attention may be in a relatively stable state by the second decade of life.
Modulation of the dPFC ↔ Thalamus Pathway
Here we address differentiated roles of the dPFC and the thalamus in the context of sustained attention, the functional significance of ascending (thalamus → dPFC) and descending (dPFC → thalamus) attention pathways, and interpretation of asymmetric modulation of these pathways by attention load. The asymmetric modulation of the dPFC ↔ thalamus pathway provides a basis for understanding functional integration of fMRI signals between the thalamus and the dPFC, and descending mechanisms of attention‐related gain in cortical–thalamic processing units.
Initially seen as a passive relay to the cortex, the thalamus is now strongly associated with gating of sensory inputs [Briggs and Usrey, 2008; McCormick and Bal, 1994; Sherman, 2007] by virtue of thalamic nuclei exerting dynamic control over information relayed upward [Basso et al., 2005]. Studies have revealed the existence of ascending connections that project topographically to the cortex, as well as those that are arranged diffusely with overlap in multiple cortical regions. A similar organization of descending pathways projecting to the thalamus has been identified; moreover, these connections have been shown to disperse activity across both thalamic and cortical neurons [Jones, 2001]. Descending cortical–thalamic connections directly excite relay neurons or indirectly inhibit them via GABAergic neurons of the thalamic reticular nucleus. This ability in combination with the widespread distribution of connectivity and versatile polysynaptic receptors enabling tonic and burst modes of firing [Sherman, 2001] precipitates the evolution of intrinsic oscillatory circuitry with the capacity to dynamically modify corticothalamic synchrony; synchronization of the thalamus with the cortex is accompanied by discrete conscious events [Jones, 2002]. Thus, this framework of nonreciprocal connections in addition to reciprocal coupling subserves the repertoire to carry out a variety of cognitive functions [Haber and Calzavara, 2009].
What ostensible network signatures of such interactions have been identified in adulthood or adolescence? Network signatures of cortical–thalamic processing have been detected primarily using electrophysiological techniques. Intracranial recordings in humans reveal cross‐frequency cortical–thalamic coupling [Fitzgerald et al., 2013; Malekmohammadi et al., 2014] hypothesized to sub‐serve information transfer. Indeed, in task‐active states, cortical–thalamic synchrony is increased during periods of sustained vigilance [Steriade 1997]. Moreover, inhibiting the activity of neurons in the medial–dorsal nucleus of the thalamus impairs frontal–thalamic synchrony, and cognitive functions that are reliant on this synchrony [Parnaudeau et al., 2013]. fMRI studies have confirmed that thalamic activity is modulated during directed attention processing [Buchel et al., 1998; O'Connor et al., 2002; Schneider and Kastner 2009], further supporting a model of descending cortical inputs within cortical–thalamic processing units [Saalmann and Kastner, 2009].
What effect does attention demand exert on these interactions? Attention demands result in bursting or tonic activity in the lateral geniculate nuclei, conveying information in an ascending fashion to the cortex [Sherman and Guillery, 2002]. These ascending projections from the thalamus are complemented by cortical inputs from multiple sensory, motor, and frontal regions to diverse thalamic nuclei including the ventral and posteromedial complexes [Klein et al., 2010; Li et al., 2013; Ray and Price, 1993]. Descending projections from sensory regions of the cortex appear to shape the properties of thalamic neurons [Alitto and Usrey, 2003; Sillito and Jones, 2002]. In particular, attention modulates the responses of neurons in the lateral geniculate nucleus; this amplification of thalamic responses may optimize the ascending flow of highly relevant sensory information [McAlonan et al., 2008]. It appears therefore that these closed cortical–thalamic loops form processing units underlying sensorimotor function [Scannell et al., 1999]. Though unproven, it is reasonable to expect that these fundamental mechanisms may well be in place in adolescence.
In the current experiment, the ascending (thalamus → dPFC) pathway was modulated during conditions of both low (2‐digit) and high (3‐digit) demand (Fig. 6), but no significant differences in modulation were observed between the levels of demand. As the ascending connection is not sensitive to variations in attention‐related demand, attention may tune the ascending relay of task‐relevant information to the cortex in an unbiased manner. We propose this is a principal contribution of the ascending (thalamus → dPFC) connection in corticothalamic processing units subserving sustained attention.
In contrast to the function of thalamic nuclei, a signature of the selective and executive role played by the prefrontal cortex may be revealed by enhanced gain on descending pathways during demanding attention commitments. Variations in sustained attention demand modulate engagement of the prefrontal and anterior cingulate cortices [Culham et al., 1998; Diwadkar et al., 2000; Petersen and Posner, 2012], considered regions at the executive core of the attention network [Diwadkar et al., 2011b; Rueda et al., 2005], but in fact may more likely modulate the effective connectivity of efferent pathways from the dPFC. Selective enhancement of responses to target stimuli [Connor et al., 2004] and heightened activity in cortical neurons under taxing attention conditions have been demonstrated [Sarter et al., 2001]. Thus, cortical neurons seem to be involved in feedback pathways that broadly assume flexible functional states in compliance with task‐relevant demands [Gilbert and Li, 2013]; i.e., cortical neurons participate in the allocation of descending attention resources directed towards target stimuli and modulate their own responses according to task variations.
In demonstrating greater modulation of the descending (dPFC → thalamus) pathway under conditions of increased load (3‐digit), our results contend that the descending connection indeed adapts to meet dynamic changes in task demands. This increase in effective connectivity of the (dPFC → thalamus) pathway provides relatively direct evidence (at the systems level) that the descending pathway implements attention‐related gain by modifying dynamic connectivity in response to attention demands. This effect is consistent with the prescribed role of the dPFC in enhancing control mechanisms for attention [Rowe et al., 2002]. In Figure 8, we provide evidence of significantly greater activation in both the thalamus and the dPFC under conditions of increased demand, thus corroborating this notion. Thus greater BOLD response observed in the thalamus may be a consequence of enhanced effective connectivity from the dPFC to the thalamus in response to greater attention demand. In this way, dynamic responses in the dPFC are able to modulate activity in thalamic nuclei in order to meet task demands. In comparison, effective connectivity of the ascending pathway shows comparable increases during each level of attention demand, consistent with the prescribed role of the thalamus in gating.
Figure 8.
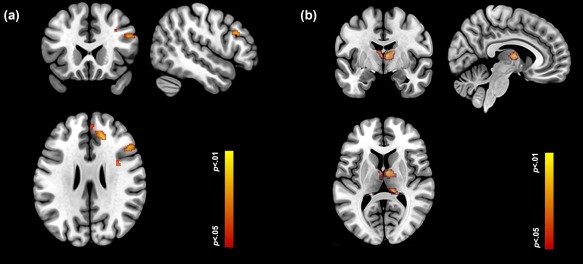
Both the dPFC (a) and the thalamus (b) showed increased activation during the 3 Digit (relative to the 2 Digit) condition. Loci are depicted on orthoviews for each structure. These activation profiles underpin the effective connectivity analyses of the descending (dPFC → thalamus) pathway further supporting the idea that dynamic changes in brain subnetworks are responsive to changes in task demand. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To summarize, in addition to the highly specialized roles of the dPFC and the thalamus, the individual functions supported by ascending and descending connections between these two regions engender cortical–thalamic processing units that are instrumental in various cognitive functions. In the context of sustained attention with varying attention loads, the ascending (thalamus → dPFC) pathway facilitates the relay of task‐relevant sensory information to the cortex with no effect of demand; whereas the descending (dPFC → thalamus) pathway assumes the responsibility of adapting to task‐relevant requirements. Thus, dynamic interactions between the dPFC and the thalamus are driven by the nature of attention demand, and collectively function to sustain attention. That the dPFC ↔ thalamic interactions appear privileged is suggested by evidence of entirely distinct patterns of modulation of the dACC ↔ thalamus pathway, a difference that may represent the dACC's role in error monitoring and error avoidance [Carter et al., 1998; Magno et al., 2006], and the structure's sensitivity to task performance. Thalamic nuclei (ventral and anterior) projecting to the dACC support the maintenance of attention when appropriate with task‐relevant demands; increases in demand results in increased error (also observed in our study). Increased errors have been associated with decreased neuronal activity within these thalamic nuclei [Schiff et al., 2013], and with reduced engagement of the thalamus and anterior cingulate cortex [Paus et al., 1997]. The patterns of contextual modulation observed in the dACC ↔ thalamus pathway are broadly consistent with this view. When demand was low (and therefore error rates were low), the contextual modulation of the descending and ascending pathway was higher. Moreover these DCM related effects are consistent with behavioral performance (see “Results”) showing lower sensitivity during the more demanding attention condition. Furthermore, we also specifically evaluated miss rates associated with the low and high demand conditions, and these additional analyses indicated that miss rates were marginally increased in the demanding condition (0.15 vs. 0.10; t 20 = 1.68, p < 0.05, one‐tailed). Therefore, we speculate that these distinct patterns of contextual modulation of the descending and ascending pathways between each of the dPFC and the dACC with the thalamus reflect distinct processing mechanisms in these subcircuits.
Developmental Effects
Several aspects within our data suggest that neurodevelopmental considerations exerted limited influence on our results. First, age did not exert significant behavioral effects on performance (see “Results”). This is notable as it is consistent with behavioral data demonstrating that both levels of the task were tractable enough to be performed across a cross‐section of ages in adolescence. This evidence of behavioral proficiency is an important constraint in interpreting neuroimaging results in adolescent and clinical populations [Carter et al., 2008; Casey et al., 2005]. Nevertheless, structural MRI and activation studies have suggested that many of the regions assessed in our network analyses undergo developmental changes through adolescence [Gogtay et al., 2004; Luna et al., 2004; Rubia et al., 2007; Yurgelun‐Todd, 2007]. Changes in effective connectivity from adolescence to adulthood have been demonstrated in complex domains including reasoning [Bazargani et al., 2014] and processes related to language [Booth et al., 2008]. Other investigations in more fundamental domains such as reward processing, have provided negative results [Cho et al., 2013]. In general, there is little doubt that the brain remains plastic in adolescence but the relationship between this plasticity, psychological domains, and effective connectivity between brain networks may not be straightforward. It is tempting to conclude that our results suggest that networks related to attention during adolescence are stable, and that this stability transcends structural changes. However, we admit this is an open question, more conclusive answers to which will elucidate understanding of both normal and pathological neurodevelopmental trajectories.
SUMMARY
The results provide compelling evidence of the value of dynamic causal modeling in recovering brain network dynamics, particularly when applied to core attention circuits. Understanding how at any time, complex function arises from the brain's largely fixed structural connections is a fundamental and challenging problem [Park and Friston, 2013]. Characterizing dynamic changes in effective connectivity in simple domains such as attention provides an opportunity for estimating how connectivity in critical subnetworks is modulated by variations in task conditions. Our results establish a framework for characterizing network dynamics underlying normative frontal–thalamic function. In addition to attention‐deficit hyperactivity disorder, attention‐related deficits are implicated in a cross‐section of neuropsychiatric illnesses including schizophrenia and mood disorders [Diwadkar, 2012; Diwadkar et al., 2011a, 2011b; 2014; Epstein et al., 2009; Fleck et al., 2012], and understanding frontal–thalamic network dynamics may be crucial in identifying the neural underpinnings of these deficits from in vivo acquired fMRI signals.
Supporting information
Supporting Information
ACKNOWLEDGMENT
We thank Dalal Khatib for assistance with data collection, and Simon Eickhoff, Robert Langner, Steven Bressler, Mihály Bányai, László Négyessy, and Péter Érdi for helpful discussions.
REFERENCES
- Alitto HJ, Usrey WM (2003): Corticothalamic feedback and sensory processing. Curr Opin Neurobiol 13:440–445. [DOI] [PubMed] [Google Scholar]
- Bakshi N, Pruitt P, Radwan J, Keshavan MS, Rajan U, Zajac‐Benitez C, Diwadkar VA (2011): Inefficiently increased anterior cingulate modulation of cortical systems during working memory in young offspring of schizophrenia patients. J Psychiatr Res 45:1067–1076. [DOI] [PubMed] [Google Scholar]
- Barnea‐Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL (2005): White matter development during childhood and adolescence: A cross‐sectional diffusion tensor imaging study. Cereb Cortex 15:1848–1854. [DOI] [PubMed] [Google Scholar]
- Basso MA, Uhlrich D, Bickford ME (2005): Cortical function: A view from the thalamus. Neuron 45:485–488. [DOI] [PubMed] [Google Scholar]
- Bazargani N, Hillebrandt H, Christoff K, Dumontheil I (2014): Developmental changes in effective connectivity associated with relational reasoning. Hum Brain Mapp 35:3262–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Mehdiratta N, Burman DD, Bitan T (2008): Developmental increases in effective connectivity to brain regions involved in phonological processing during tasks with orthographic demands. Brain Res 1189:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM (2008): Emerging views of corticothalamic function. Curr Opin Neurobiol 18:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunia CHM (1993): Gating in attention and motor preparation. Psychophysiology 30. [DOI] [PubMed] [Google Scholar]
- Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ (1998): The functional anatomy of attention to visual motion. A functional MRI study. Brain 121:1281–1294. [DOI] [PubMed] [Google Scholar]
- Bush G (2010): Attention‐deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology 35:278–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B (2005): The development of arithmetical abilities. J Child Psychol Psychiatry 46:3–18. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280:747–749. [DOI] [PubMed] [Google Scholar]
- Carter CS, Heckers S, Nichols T, Pine DS, Strother S (2008): Optimizing the design and analysis of clinical functional magnetic resonance imaging research studies. Biol Psychiatry 64:842–849. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S (2005): Imaging the developing brain: What have we learned about cognitive development? Trends Cogn Sci 9:104–110. [DOI] [PubMed] [Google Scholar]
- Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, Ernst M (2013): Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. NeuroImage 66:508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh R, Henik A, Rubinsten O, Mohr H, Dori H, van de Ven V, Zorzi M, Hendler T, Goebel R, Linden DE (2005): Are numbers special? The comparison systems of the human brain investigated by fMRI. Neuropsychologia 43:1238–1248. [DOI] [PubMed] [Google Scholar]
- Connor CE, Egeth HE, Yantis S (2004): Visual attention: bottom‐up versus top‐down. Curr Biol 14:R850–R852. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL (1998): A common network of functional areas for attention and eye movements. Neuron 21:761–773. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE (1995): Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science 270:802–805. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB (1998): Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol 80:2657–2670. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA (2012): Adolescent risk pathways toward schizophrenia: Sustained attention and the brain. Curr Top Med Chem 12:2339–2347. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Bakshi N, Gupta G, Pruitt P, White R, Eickhoff SB (2014): Dysfunction and dysconnection in cortical–striatal networks during sustained attention: Genetic risk for schizophrenia or bipolar disorder and its impact on brain network function. Front Psychiatry 5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Carpenter PA, Just MA (2000): Collaborative activity between parietal and dorso‐lateral prefrontal cortex in dynamic spatial working memory revealed by fMRI. NeuroImage 12:85–99. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Goradia D, Hosanagar A, Mermon D, Montrose DM, Birmaher B, Axelson D, Rajarathinem R, Haddad L, Amirsadri A, Zajac‐Benitez C, Rajan U, Keshavan MS (2011a): Working memory and attention deficits in adolescent offspring of schizophrenia or bipolar patients: comparing vulnerability markers. Prog Neuropsychopharmacol Biol Psychiatry 35:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Segel J, Pruitt P, Murphy ER, Keshavan MS, Radwan J, Rajan U, Zajac‐Benitez C (2011b): Hypo‐activation in the executive core of the sustained attention network in adolescent offspring of schizophrenia patients mediated by pre‐morbid functional deficits. J Psychiatric Res Neuroimaging 192:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Wadehra S, Pruitt P, Keshavan MS, Rajan U, Zajac‐Benitez C, Eickhoff SB (2012): Disordered cortico‐limbic interactions during affective processing in children and adolescents at risk for schizophrenia revealed by fMRI and Dynamic Causal Modeling. Arch Gen Psychiatry 69:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresp B, Grossberg S (1999): Spatial facilitation by color and luminance edges: Boundary, surface, and attentional factors. Vis Res 39:3431–3443. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AD (2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23:475–483. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Delbello MP, Adler CM, Altaye M, Kramer M, Mills NP, Strakowski SM, Holland S (2009): Differential patterns of brain activation over time in adolescents with and without attention deficit hyperactivity disorder (ADHD) during performance of a sustained attention task. Neuropediatrics 40:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Hof PR, Guise KG, Fossella JA, Posner MI (2008): The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex 18:796–805. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI (2005): The activation of attentional networks. NeuroImage 26:471–479. [DOI] [PubMed] [Google Scholar]
- Fitzgerald TH, Valentin A, Selway R, Richardson MP (2013): Cross‐frequency coupling within and between the human thalamus and neocortex. Front Hum Neurosci 7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck DE, Eliassen JC, Durling M, Lamy M, Adler CM, DelBello MP, Shear PK, Cerullo MA, Lee JH, Strakowski SM (2012): Functional MRI of sustained attention in bipolar mania. Mol Psychiatry 17:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ (2011): Functional and effective connectivity: A review. Brain Connect 1:13–36. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W (2003): Dynamic causal modelling. NeuroImage 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Li B, Daunizeau J, Stephan KE (2012): Network discovery with DCM. NeuroImage 56:1202–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Li W (2013): Top‐down influences on visual processing. Nat Neurosci 14:350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M (1999): A large‐scale distributed network for covert spatial attention: Further anatomical delineation based on stringent behavioural and cognitive controls. Brain 122:1093–1106. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd Herman DH, Clasen LS, Toga AW, Rapoport JL Thompson PM (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA (1966): Signal Detection Theory and Psychophysics. New York: Wiley. [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A (2004): Modulation of long‐range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci USA 101:13050–13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2003): The primate basal ganglia: Parallel and integrative networks. J Chem Neuroanat 26:317–330. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R (2009): The cortico‐basal ganglia integrative network: The role of the thalamus. Brain Res Bull 78:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM (2002): Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst 18:386–404. [DOI] [PubMed] [Google Scholar]
- Jang SH, Lim HW, Yeo SS (2014): The neural connectivity of the intralaminar thalamic nuclei in the human brain: A diffusion tensor tractography study. Neurosci Lett 579:140–144. [DOI] [PubMed] [Google Scholar]
- Jones EG (2001): The thalamic matrix and thalamocortical synchrony. Trends Neurosci 24:595–601. [DOI] [PubMed] [Google Scholar]
- Jones EG (2002): Thalamic circuitry and thalamocortical synchrony. Philos Trans R Soc Lond B Biol Sci 357:1659–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997): Schedule for Affective Disorders and Schizophrenia for School‐Age Children‐Present and Lifetime Version (K‐SADS‐PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S (2008): Cortical mechanisms for shifting and holding visuospatial attention. Cereb Cortex 18:114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JC, Rushworth MF, Behrens TE, Mackay CE, de Crespigny AJ, D'Arceuil H, Johansen‐Berg H (2010): Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. NeuroImage 51:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenberg L, Korkman M, Lahti‐Nuuttila P (2001): Differential development of attention and executive functions in 3‐ to 12‐year‐old Finnish children. Dev Neuropsychol 20:407–428. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA (2003): Multiple neuronal networks mediate sustained attention. J Cogn Neurosci 15:1028–1038. [DOI] [PubMed] [Google Scholar]
- Li W, Qin W, Liu H, Fan L, Wang J, Jiang T, Yu C (2013): Subregions of the human superior frontal gyrus and their connections. NeuroImage 78:46–58. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA (2004): Maturation of cognitive processes from late childhood to adulthood. Child Dev 75:1357–1372. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD (2005) Detection Theory: A Users Guide. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H (2006): The anterior cingulate and error avoidance. J Neurosci 26:4769–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekmohammadi M, Elias WJ, Pouratian N (2014): Human thalamus regulates cortical activity via spatially specific and structurally constrained phase‐amplitude coupling. Cereb Cortex 25:1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH (2008): Guarding the gateway to cortex with attention in visual thalamus. Nature 456:391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T (1994): Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol 4:550–556. [DOI] [PubMed] [Google Scholar]
- Modha DS, Singh R (2010): Network architecture of the long‐distance pathways in the macaque brain. Proc Natl Acad Sci USA 107:13485–13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Fukui MM, Pinsk MA, Kastner S (2002): Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci 5:1203–1209. [DOI] [PubMed] [Google Scholar]
- Park HJ, Friston K (2013): Structural and functional brain networks: From connections to cognition. Science 342:1238411. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S, O'Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, Balsam PD, Gordon JA, Kellendonk C (2013): Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 77:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T (2001): Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci 2:417–424. [DOI] [PubMed] [Google Scholar]
- Paus T, Zatorre RJ, Hofle N, Caramanos Z, Gotman J, Petrides M, Evans AC (1997): Time‐related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J Cogn Neurosci 9:392–408. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI (2012): The attention system of the human brain: 20 years after. Annu Rev Neurosci 35:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI (2012): Imaging attention networks. NeuroImage 61:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y (2007): The anterior cingulate gyrus and the mechanism of self‐regulation. Cogn Affect Behav Neurosci 7:391–395. [DOI] [PubMed] [Google Scholar]
- Ray JP, Price JL (1993): The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 337:1–31. [DOI] [PubMed] [Google Scholar]
- Rowe J, Friston K, Frackowiak R, Passingham R (2002): Attention to action: Specific modulation of corticocortical interactions in humans. NeuroImage 17:988–998. [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M (2007): Linear age‐correlated functional development of right inferior fronto‐striato‐cerebellar networks during response inhibition and anterior cingulate during error‐related processes. Hum Brain Mapp 28:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI (2005): Training, maturation, and genetic influences on the development of executive attention. Proc Natl Acad Sci USA 102:14931–14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S (2009): Gain control in the visual thalamus during perception and cognition. Curr Opin Neurobiol 19:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado‐Pineda P, Baeza I, Perez‐Gomez M, Vendrell P, Junque C, Bargallo N, Bernardo M (2003): Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic‐naive schizophrenic patients. NeuroImage 19:365–375. [DOI] [PubMed] [Google Scholar]
- Salgado‐Pineda P, Junque C, Vendrell P, Baeza I, Bargallo N, Falcon C, Bernardo M (2004): Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. NeuroImage 21:840–847. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP (2001): The cognitive neuroscience of sustained attention: where top‐down meets bottom‐up. Brain Res Brain Res Rev 35:146–160. [DOI] [PubMed] [Google Scholar]
- Scannell JW, Burns GA, Hilgetag CC, O'Neil MA, Young MP (1999): The connectional organization of the cortico‐thalamic system of the cat. Cereb Cortex 9:277–299. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Shah SA, Hudson AE, Nauvel T, Kalik SF, Purpura KP (2013): Gating of attentional effort through the central thalamus. J Neurophysiol 109:1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KA, Kastner S (2009): Effects of sustained spatial attention in the human lateral geniculate nucleus and superior colliculus. J Neurosci 29:1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM (2001): Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci 24:122–126. [DOI] [PubMed] [Google Scholar]
- Sherman SM (2007): The thalamus is more than just a relay. Curr Opin Neurobiol 17:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW (2002): The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond 357:1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Jones HE (2002): Corticothalamic interactions in the transfer of visual information. Philos Trans R Soc Lond 357:1739–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Harrison LM, Kiebel SJ, David O, Penny WD, Friston KJ (2007): Dynamic causal models of neural system dynamics:current state and future extensions. J Biosci 32:129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ (2010): Ten simple rules for dynamic causal modeling. NeuroImage 49:3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M (1997): Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex 7:583–604. [DOI] [PubMed] [Google Scholar]
- Wickens TD (2001): Elementary Signal Detection Theory. Oxford: Oxford University Press. [Google Scholar]
- Yurgelun‐Todd D (2007): Emotional and cognitive changes during adolescence. Curr Opin Neurobiol 17:251–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


