Abstract
Aim: Cholesteryl ester transfer protein (CETP) is an important lipid transfer factor in plasma that enhances prothrombinase activity in purified systems. This study was conducted to test the association of plasma CETP activity with venous thrombosis (VTE) and to address the procoagulant mechanism of CETP activity in prothrombinase assays.
Methods: We measured CETP lipid transfer activity in plasmas of 49 male VTE patients and in plasmas of matched controls. CETP procoagulant activity was tested in purified prothrombinase systems.
Results: CETP lipid transfer activity levels were significantly higher in VTE patients than in controls (p = 0.0008). A subset of patients carrying the CETP mutations Ala373Pro and Arg451Gln, which were also linked to the VTE risk, showed significantly higher plasma CETP activity than the non-carriers. The plasma CETP activity negatively correlated with APTT, suggesting that the CETP activity is associated with plasma coagulability. Recombinant (r) CETP bound to both factor Xa (Kd = 15 nM) and Gla-domainless factor Xa (Kd = 59 nM), whereas rCETP enhanced prothrombin activation by factor Xa, but not by Gla-domainless factor Xa. rCETP also required factor Va for enhancement of prothrombinase activity. When we addressed the effects of mutations in CETP on prothrombinase activity, Gln451-rCETP was found to have five-fold higher thrombin generation activity than wt-rCETP or Pro373-rCETP.
Conclusions: Elevated CETP lipid transfer activity in plasma was associated with the risk of VTE. Gln451-CETP, which is linked to VTE, has much higher procoagulant activity than wt-CETP. CETP might act as a physiologic procoagulant by mechanisms that involve its direct binding to factor Xa.
Keywords: Cholesteryl ester transfer protein, Venous thrombosis, Prothrombinase
See editorial vol. 23: 1144–1146
Introduction
Cholesteryl ester transfer protein (CETP) is a plasma glycoprotein that plays a pivotal role in lipoprotein metabolism by transferring lipids between various donors and acceptors. CETP transfers neutral lipids and phospholipids between lipoproteins, e.g., the transfer of cholesteryl ester from high density lipoprotein (HDL) to apolipoprotein B-containing lipoproteins (i.e., to very low density lipoprotein (VLDL) and low density lipoprotein (LDL) in exchange for triglyceride1, 2). As a result of the transferring of lipids, the plasma level of HDL decreases, especially large HDL particles, which are linked to cardiovascular disease and venous thrombosis (VTE)3–5). To reduce the risk of cardiovascular diseases by increasing HDL levels, CETP inhibitors have been developed by inhibiting lipid transfer out of HDL6–9). These CETP inhibitors increased HDL plasma levels by inhibiting cholesteryl ester transfer activity10–12), but success could not yet be achieved in phase III clinical trials9).
Plasma CETP modulates lipoprotein metabolism, and some lipoproteins can affect blood coagulation reactions with either procoagulant or anticoagulant effects13). Further, CETP itself might act as a procoagulant in plasma by enhancing prothrombinase activity14). Therefore, CETP is hypothesized to have roles in the pathogenesis of thrombotic diseases via lipoprotein regulation and/or its procoagulant activity. The association of CETP nucleotide polymorphisms has been reported for both VTE15) and cardiovascular diseases16–23). However, the direct measurement of CETP activity has led to contradicting results for the risk of coronary artery disease24–27). Since studies on the association of CETP lipid transfer activity with VTE have not been reported, we measured CETP lipid transfer activity in plasmas of VTE patients and matched controls to test the hypothesis that CETP activity is linked to VTE.
Aim
This study was performed to test the hypotheses that plasma CETP lipid transfer activity is linked to plasma blood clotting assays and to VTE and that the CETP Arg451Gln mutation affects CETP activity and venous thrombosis risk. The mechanisms for CETP procoagulant activity were also addressed using purified prothrombinase system studies and Surface plasmon resonance (SPR) binding assays for factors Xa and IXa.
Methods
Study Population and Blood Collection
The Scripps Venous Thrombosis Registry is an ongoing case-control study of risk factors for VTE as previously described5, 15, 28). Inclusion criteria for this study included age at thrombosis, i.e., < 55 years at the time of first episode of thrombosis, > 3 months since diagnosis of acute thrombosis, a life expectancy of at least 3 years, and no lipid lowering medications or cancer. Age- and sex-matched (± 2 years) healthy controls were recruited through the General Clinical Research Center's blood donation program. The protocol was approved by the Institutional Review Board and subjects provided written informed consent. In this study, VTE patients (49 males) and age- and sex-matched male controls were analyzed. Clinical characteristics and the frequency of identified risk factors are shown in Table 1. Of the 49 VTE patients, 40 (81.6%) presented with idiopathic VTE, defined as events that did not occur within 90 days of surgery, trauma, or major immobilization. Eighty-four percent of the VTE patients were taking warfarin when blood was donated. Blood was collected after a 12-hour fast. Ethylenediaminetetraacetic acid (EDTA)-plasma was stored at −80°C. The samples used for CETP measurement were collected between 2002 and 2003, and plasmas were stored at −80°C as aliquots. The samples which had never been previously defrosted were analyzed for CETP activity and antigen in 2009. Since all of the samples were carefully stored under the same conditions, we have full confidence in assay results.
Table 1. Study population.
| Variables | Control N = 49 | VTE N = 49 | p value |
|---|---|---|---|
| Age, yr (SD) | 46.3 (8.2) | 46.7 (8.6) | |
| Ethnic Group, % | |||
| Non-Hispanic White | 91.8 | 91.8 | |
| Body-mass Index, kg m−2 (SD) | 27.2 (4.3) | 30.7 (5.9) | 0.003 |
| Risk factors, number of subjects (%) | |||
| Family History for VTE | 4 (8.1) | 18 (37) | 0.006 |
| Factor V Leiden | 2 (4.1) | 13 (26.5) | 0.01 |
| Prothrombin 20210A | 2 (4.1) | 7 (14.3) | 0.18 |
| Unprovoked | 40 (81.6) | ||
| >one episode of thrombosis (%) | 18 (37) | ||
| pulmonary embolism (%) | 17 (35) | ||
| Diabetes (%) | 0 | 1 (2.0) | 1.0 |
| Hypertension (%) | 0 | 3 (6.1) | 0.25 |
| Prior smoking history (%) | 8 (16.3) | 6 (12.2) | 0.79 |
| Current smoking (%) | 5 (10.2) | 4 (8.2) | 1.0 |
| Total cholesterol, mg/dL (SD) | 206 (41) | 215 (45) | 0.3 |
| Triglyceride, mg/dL (SD) | 143 (84) | 159 (98) | 0.44 |
| HDL-C, mg/dL (SD) | 53.6 (15.0) | 46.3 (17.8) | 0.03 |
| LDL-C, mg/dL (SD) | 122 (33) | 135 (41) | 0.11 |
Normal citrated plasma samples for clotting assays were taken from 29 healthy adult volunteers after a 12-hour fast (14 males, 15 females) (35.8 ± 7.1 years, range 19–56 years) from the Scripps General Clinical Research Center's blood donation program. These healthy blood donors were not taking oral contraceptives or estrogen replacement therapy or any medications, and they did not have the thrombophilic risk factors of factor V Leiden or prothrombin nt20210A. Blood was collected after a 12-hour fast.
An additional 17-patient cross-over cohort (9 males, 8 females) (58.1 ± 18.6 years, range 27–83 years) was identified through the Scripps Anticoagulation Service. They were administered warfarin for clinical indications (prophylaxis of VTE, treatment of VTE, or atrial fibrillation)29). The crossover design was on-warfarin treatment and off-warfarin treatment. Blood draw was performed 4–6 weeks after initiation of warfarin with a therapeutic international normalized ratio (INR) value (ON-warfarin). The average INR at the time of blood draw was 2.50 ± 0.65. Blood draw was performed approximately 10 days after discontinuation of the clinically determined course of warfarin (OFF-warfarin). Blood was collected after 12-hour fast, and EDTA plasma or citrated plasma was stored at −80°C. All of the protocols for the Scripps Venous Thrombosis Registry, healthy donors, and warfarin study were approved by the Institutional Review Board and subjects provided written informed consent.
Clinical Analytes
Plasma CETP antigen was measured using a CETP enzyme-linked immunosorbent assay (ELISA) kit (Wako Pure Chemical Industries, Ltd, Osaka, Japan). A hundred percent CETP antigen level was defined as the average value of control subjects who did not carry the Pro373 and/or Gln451 mutation (n = 48, 1.97 µg/mL). CETP lipid transfer activity was determined by an ex vivo CETP Activity Assay kit (Roar Biomedical, Inc., New York, NY) measuring plasma derived CETP activity using 100 µL plasma and 5 µL reagent. A hundred percent CETP activity level was defined as the average value of control subjects27). The specific activity of CETP was defined as CETP activity level divided by CETP antigen level. Other lipid parameters were measured as described previously5).
Materials
cDNAs for recombinant (r) wt-human CETP, Ala373Pro-CETP, and Arg451Gln-CETP were kindly provided by Dr. Lloyd and Dr. Bamberger (Pfizer). rCETPs were expressed in HEK239 cells as previously described23). Prothrombin was from Enzyme Research Laboratories (South Bend, IN), and bovine serum albumin (BSA) was from Calbiochem-Novabiochem Corp (San Diego, CA). Factor Va, factor Xa, biotinylated (B)-EGR-factor Xa, Gla-domainless factor Xa (DG-factor Xa), BEGR-DG-factor Xa, factor IXa, and BEGR-chloromethylketone were purchased from Hematologic Technologies Inc. (Essex Junction, VT). BEGR-factor IXa was prepared as described28). Streptavidin chips (Sensor Chip SA) were obtained from GE Healthcare Bio-Sciences (Pittsburgh, PA).
Clotting Assay
Activated partial thromboplastin time (APTT) assays (Platelin LS, Trinity Biotec USA, Berkeley Heights, NJ) were performed for plasma from the cohort of healthy adult subjects following manufacturer's instructions to test the coagulability of individual plasma.
Statistical Analysis
Statistical analyses, including Mann–Whitney u-test, two-tailed Pearson test, and McNemar's test were used to evaluate the difference in proportions between matched pairs for categorical variables (e.g., VTE-positive family history, smoking status). Fisher's exact test were performed using Prism™ 4.0 software (Graph Pad Software Inc., San Diego, CA). Odds ratio (OR) for VTE were determined with a log–binomial regression model. The difference was considered significant when p was < 0.05.
Prothrombin Activation Assays
Prothrombin (0.76 µM final) activation by purified factors Xa (0.125 nM final) and Va (6.25 nM final) plus or minus varying concentrations of rCETP preparations was assayed in the absence of exogenously added phospholipids. Reactants were mixed and incubated at room temperature for 5 min to allow prothrombin activation before the reaction was quenched by EDTA, and then the amidolytic activity of thrombin was quantified using Pefachrome TH thrombin (IIa) chromogenic substrate. Prothrombin (0.76 µM final) was also activated by purified factors Xa (0.125 nM final) plus or minus varying concentrations of rCETP preparations for 120 min and was assayed in the absence of factor Va and phospholipid.
Sensorgrams for CETP Interaction with Biotinylated Proteins Coupled to Streptavidin Sensor Chips
Coupling of BEGR-factor Xa, BEGR-DG-factor Xa, or BEGR-factor IXa to the gold surface of the sensor chip for SPR studies was achieved by flowing 100 µg/mL of biotin-labeled proteins in 10 mM Hepes buffer, 300 mM NaCl, and 5 mM CaCl2 (pH 7.4) over a streptavidin sensor chip28). Coupling was carried out at 300 mM NaCl in order to collapse the dextran matrix and thus, minimize artifacts that might occur during the experiments. A control surface was prepared by flowing free biotin (0.003 mg/mL) in the same buffer over a second channel of the streptavidin sensor chip, and this blank channel provided background values that were subtracted from the sample data.
Sensorgrams were collected for different CETP concentrations that flowed over a sensor chip containing biotin-labeled proteins or biotin alone (present in the control flow cell) in 10 mM Hepes buffer, 150 mM NaCl, 5 mM CaCl2, and 0.1% BSA (pH 7.4). Preliminary experiments revealed a flow rate dependence on the dissociation rate constant (koff) in which flow rates less than 50 µL/min showed increasing kd values with increasing flow rates. All experiments were, therefore, carried out at the maximum flow rate of 100 µL/min and at a sampling rate of 1 Hz on a Biacore 3000. Surface regeneration was carried out using 2 M NaCl at 100 µL/min flow rate for 2 min, following which 50 mM NaOH was injected at 50 µL/min flow rate to prepare the surface for the next run. Rate constants for association (kon) and dissociation (koff) were obtained by globally fitting the data from five to six injections of CETP by using the BIAe-valuation software version 3.2 and the 1:1 Langmuir binding model (global fitting).
Results
Correlation of Plasma CETP Lipid Transfer Activity with Coagulability
A shortened APTT is associated with the risk of VTE30). When we assessed the correlation of plasma CETP lipid transfer activity with APTT, we found a significant correlation showing that thehigher the plasma CETP activity, theshorter the clotting time (r = −0.47, p = 0.01) (Fig. 1).
Fig. 1.
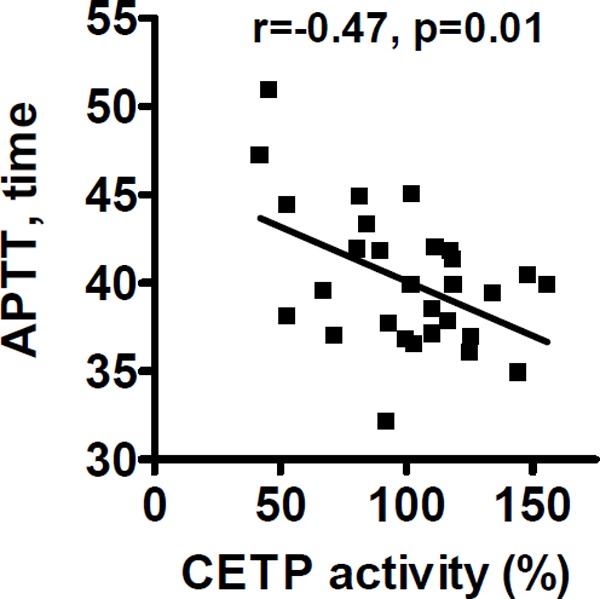
CETP lipid transfer activity and procoagulant activity
Correlation between plasma CETP activities and APTT in healthy blood donors is shown. Coagulability of fasting citrated plasma samples from 29 healthy donors (14 males, 15 females who gave informed consent) was assayed using APTT. Pearson correlation coefficient (r = −0.47) and the p value are noted.
Plasma CETP Activity and Antigen in VTE
Plasma CETP lipid transfer and antigen levels were measured for male VTE patients (n = 49) and matched controls (n = 49). Patients with VTE had significantly higher plasma levels of CETP activity than matched controls without VTE [median 115%; interquartile ranges (IQRs), 94.6% – 145% vs median 97.6%; IQRs, 78.4% – 117% (p = 0.0008)] (Fig. 2). There was no statistically significant difference in CETP antigen levels between VTE cases and controls [median 96.6%; IQRs, 86.7% – 121% vs median 97.6%; IQRs, 77.6% – 118%) (p = 0.49)]. An elevated level of CETP activity (> 90th percentile of control) was significantly associated with VTE, with an OR = 5.1 (95%CI, 1.7 – 15). Although some VTE patients were under warfarin treatment, we found that plasma CETP lipid transfer activity levels were not significantly altered by warfarin based on samples from our warfarin cross-over study (n = 16 subjects)29) [(median 98.6%; interquartile ranges (IQRs), 98.5% – 105% vs median 101%; IQRs, 97.3% – 106%) (p = 0.87)].
Fig. 2.
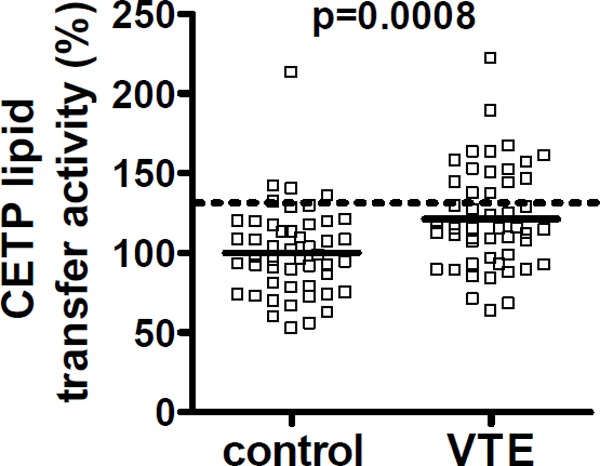
Plasma CETP lipid transfer activity for VTE subjects and matched controls
Plasma CETP activities were measured for 49 male VTE d and 49 matched male controls. Solid, thick lines indicate median values and the dotted line indicates the value for the 90th percentile of the control values.
VTE patients carrying Ala373Pro and Arg-451Gln mutations showed significantly higher CETP activity and antigen levels in plasma compared with non-carriers of those mutations (Fig. 3A and 3B) for CETP activity [median 155% (IQRs 123% – 166%) vs median 115% (IQRs 94.9% – 113%), (p = 0.006)], and for CETP antigen levels [median 137% (IQRs 84.7% – 175%) vs median 96.2% (IQRs 84.0% – 113%), (p = 0.02)]. Among subjects with normal genotypes, i.e., those not carrying either the Ala373Pro or Arg451Gln mutations, the specific activity of CETP was higher for VTE cases than for controls [median 1.08 (IQRs 0.97 – 1.37) vs median 0.97 (IQRs 0.65 – 1.32), (p = 0.01) (Fig. 3C). In contrast, there was no difference in the specific activity between VTE patients with normal genotypes and VTE patients carrying the Ala373Pro and Arg451Gln mutations (p = 0.90) (Fig. 3C). This indicates that the specific activity difference between cases and controls was unrelated to the 373 and 451 mutations, respectively.
Fig. 3.
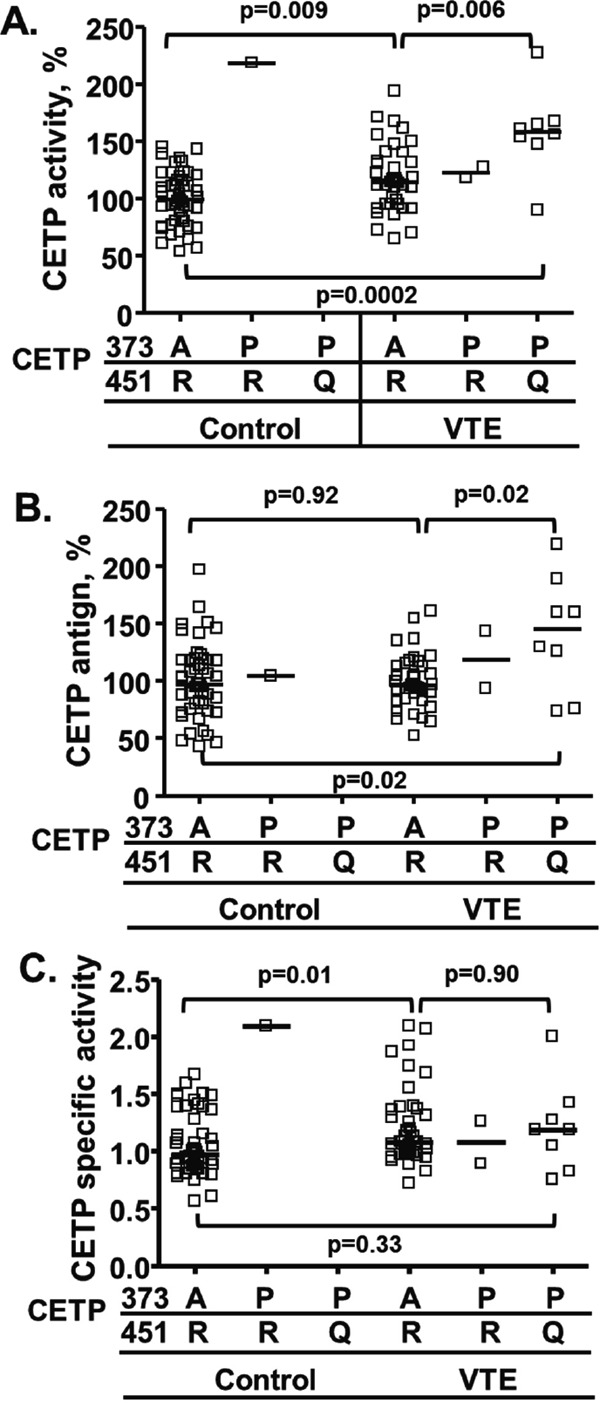
CETP activity and antigen in VTE cases and matched controls with normal genotype or with Ala373 or Arg451 variations
Plasma CETP lipid transfer activity (A), antigen (B) and specific activity (C) (ratio of activity per antigen) for subgroups of cases (n = 49) and controls (n = 49): VTE cases with normal CETP A373 and R451 genotype; subjects carrying one uncommon CETP variant, P373 and normal R451 (n = 1 control and n = 2 VTE cases); and subjects carrying two uncommon CETP variants, P373 and Q451 (n = 0 controls and n = 8 VTE cases). The solid lines indicate median values.
CETP Binds to BEGR-Factor Xa and BEGR-DG-Factor Xa
SPR analyses were performed to study the binding of rCETP to BEGR-factor Xa. rCETP bound to factor Xa in a dose-dependent fashion (Fig. 4A). kd of binding of CETP to BEGR-factor Xa was 15 nM with a fast-on and slow-off rate binding pattern (kon = 4.0 × 105/Ms,koff = 6.0 × 10−3/s, Kd = 15 nM). When using BEGR-DG-factor Xa as immobilized protein instead of factor Xa, Kd of binding of CETP to BEGR-factor Xa was 59 nM with a fast-on and slow-off rate binding pattern (kon = 2.6 × 104/Ms, koff = 1.5 × 10−3/s) using regular kinetic binding models (Fig. 4B). rCETP preparations contained BSA as stabilizer; controls showed that BSA (1 mg/mL) did not affect the signal on the factor Xa binding (Fig. 4A dotted line). In a control to show specificity for factor Xa, no binding of rCETP to BEGR-factor IXa was detected by SPR (data not shown). Other controls for the SPR methodology showed that phospholipid vesicles (80%PC/20%PS) bound immobilized factor Xa but des – Gla – factor Xa, as one would predict (data not shown).
Fig. 4.
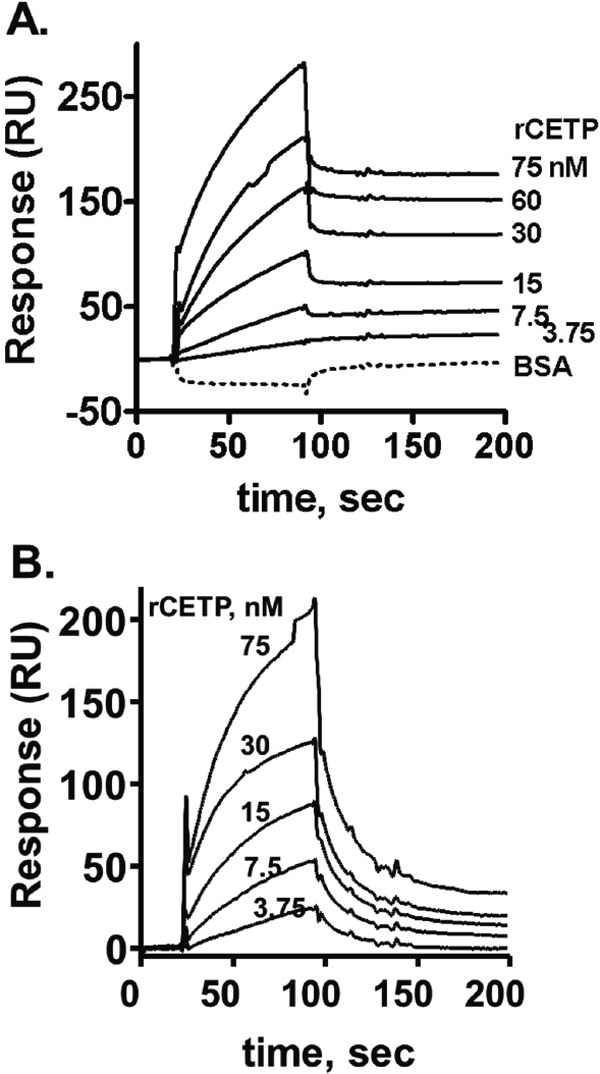
Binding of CETP to factor Xa or DG-factor Xa using SPR
Surface Plasmon Resonance (SPR) was used to monitor binding of CETP (3.75 – 75 nM) either (A) to BEGR-factor Xa (solid lines) or 10 mg/mL BSA alone (dotted line) or (B) to BEGR-(DG)-factor Xa. Sensorgrams depict the kinetics for binding and dissociation of rCETP at different indicated rCETP concentrations.
CETP Promotes Prothrombin Activation by Factor Xa/Factor Va but not by DG-Factor Xa/Factor Va
CETP enhanced thrombin generation by factor Xa and factor Va in a dose-dependent fashion (Fig. 5A). When DG-factor Xa, lacking the N-terminal Gla domain of the enzyme, was employed as an enzyme for prothrombin activation, CETP did not enhance thrombin generation even in the presence of factor Va. In the absence of factor Va, rCETP did not enhance prothrombin activation (Fig. 5B). This indicates that both the Gla domain of factor Xa and factor Va were required for the procoagulant activity of CETP
Fig. 5.
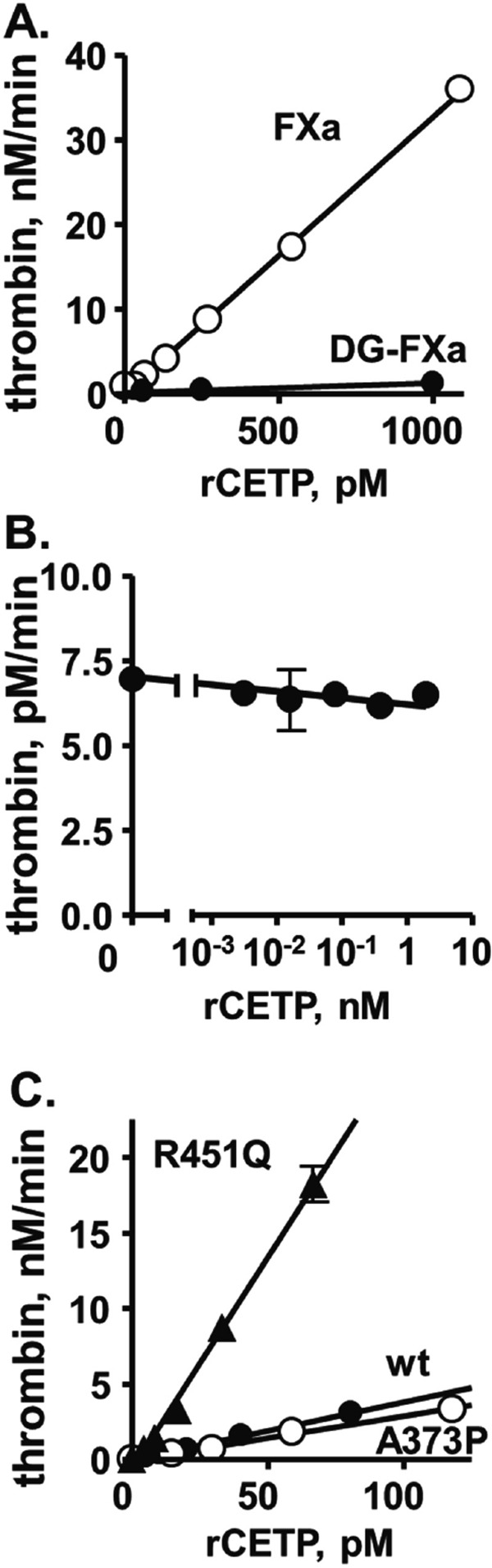
The effect of rCETP on prothrombinase activity in purified prothrombin activation assays.
(A) The influence of exogenously added rCETP on purified prothrombin activation by factor Xa (○) or DG-factor Xa (●) in the presence of factor Va. The rate of thrombin generation is shown as nM thrombin formed per minute. (B) The influence of exogenously added rCETP on prothrombin activation by factor Xa in the absence of factor Va. (C) The ability of recombinant wt-CETP, Pro373-CETP, and Gln451-CETP to enhance activation of purified prothrombin by factor Xa in the presence of factor Va. Reagent concentrations were 0.76 µM prothrombin, 0.125 nM factor Xa or DG-factor Xa, 6.25 nM factor Va (panels A. and B.), and 5 mM calcium ions.
Effect of Ala373 and Arg451 Mutations on CETP Procoagulant Activity
Recombinant wt-CETP promotes blood clotting reactions when it is added to plasma or to purified prothrombinase assay mixtures (Fig. 5A)14). For new studies, recombinant wt and mutant CETPs were made in a stable HEK293 cell expression system, and the ability of recombinant wt-rCETP, Pro373-rCETP, and Gln451-rCETP to enhance thrombin generation in reaction mixtures containing purified factor Xa, factor Va, and prothrombin was measured14) (Fig. 5C). Whereas the Pro373-CETP mutant had the same activity as wt-CETP, the Gln451 mutation increased the ability of recombinant Gln451-CETP by five-fold to enhance thrombin generation.
Discussion
CETP is an important lipid transfer factor in plasma that enhances prothrombinase activity in purified reaction mixtures14). Notably, a negative trend for APTT, a classical clotting time assay, may be associated with the risk of VTE30), and APTT was shown to be inversely correlated with CETP activity in this study. We suggest that CETP activity contributes to plasma coagulability which, in turn, may contribute to the risk of VTE. Indeed, when plasma CETP activity was measured in male VTE patients and matched controls, significantly higher plasma levels of CETP activity were found in VTE cases than matched controls.
The association of CETP nucleotide polymorphisms with the risk of cardiovascular diseases has been reported16–23). We previously reported that the CETP TaqI B1 allele was also more common in VTE cases compared with controls15) and that the two relatively rare CETP genetic variations, Ala373Pro (rs5880) and Arg451Gln (rs1800777), which cause decreased levels of HDL, are more common in male VTE patients compared with male controls,5, 15). The Ala373Pro polymorphism is in high-linkage disequilibrium with the Arg451Gln polymorphism, so for VTE studies, these individuals were subdivided into groups with Ala373Pro in combination with Arg451Gln and with Ala373Pro alone. We found that patients carrying the Ala373Pro and/or Arg451Gln mutations had significantly higher plasma CETP activity and antigen levels than the non-carriers of these mutations. Some studies on wt-CETP and these two CETP mutants imply that CETP higher activity and antigen is due to the longer half-lives of Pro373-CETP and Gln451-CETP22, 23). In contrast, there was no difference in CETP antigen levels for the VTE patients not carrying the P373/Q451 mutation. The discrepancy between association of CETP antigen and CETP activity has been reported for coronary artery diseases26). When analyzing the specific activity of CETP lipid transfer activity, the specific activity was higher in the VTE subjects than in controls (Fig. 3C). This might suggest the lower activity of natural plasma CETP inhibitors in the VTE group because the CETP activity assay used here is sensitive to the plasma CETP inhibitors. However, CETP inhibitors were not measured in our studies.
Generally, the APTT assay is not sensitive to endogenous plasma lipids and lipoproteins due to the use of large amounts of exogenously added negatively charged phospholipid reagents. HDL-C did not correlate with APTT (Spearman's r = −0.24, p = 0.22). Thus, the significant correlation between CETP activity and APTT may be due to CETP – coagulation factor interactions rather than CETP's influence on endogenous lipid/lipoprotein modulation. Consistent with this idea, we noted that CETP enhances the activity of the purified prothrombinase complex (Fig. 5)14), and thus, we hypothesized that there are direct interactions of CETP with prothrombinase components. Consequently, we tested the kinetics of binding of rCETP to factor Xa using SPR analyses. Indeed, rCETP bound to factor Xa, and the kinetics of the binding showed fast association and slow dissociation with a tight binding affinity (Kd = 15 nM). Factor Xa lacking the Gla domain still bound to rCETP affinity (Kd = 59 nM) suggesting that CETP binds to factor Xa outside of its Gla domain. Nonetheless, in contrast to the binding assay, rCETP did not enhance prothrombin activation by factor Xa lacking the Gla-domain. rCETP also required factor Va to enhance prothrombinase activity, and the Gla domain likely contributed to factor Xa – factor Va interactions.
Based on the above findings, we hypothesize that CETP bound to factor Xa provides or transfers lipids to the Gla-domain of factor Xa and factor Va to stabilize factor Xa – factor Va complex. CETP binds both neutral and charged lipids (e.g., cholesteryl ester, triglyceride, cholesterol, and phospholipids) and transfers each type of lipid from donors to acceptors. Mechanisms for lipid exchange by CETP may involve pure shuttle and exchange mechanisms or, alternatively, bridging mechanisms with donor and acceptor simultaneously bound to CETP to facilitate lipid transfer. CETP may conceivably use both of these mechanisms. The x-ray crystal structure of CETP shows four bound lipids31), consistent with the notion that CETP may act as a lipid shuttle. In the CETP structure, phospholipid binding sites are located between the Ala373 and Arg451 residues. Thus, the CETP structure is consistent with the possibility that the Ala373Pro and/or Arg451Gln mutation could cause transfer of procoagulant lipids to factor Xa better than wt-CETP and thereby increase the coagulability to increase the risk for VTE. This raises the obvious question of whether these mutations result in higher procoagulant activity. Thus, recombinant wt and CETP mutants were tested for their procoagulant activity. Remarkably, the Gln451-CETP mutant had five-fold higher procoagulant activity in the prothrombin activation assay than wt-CETP or Pro373-CETP. We confirmed the previous report22) that the lipid transfer activity of wt-CETP was not different from that of Pro373-CETP or Gln451-CETP (data not shown). Since the procoagulant activity of Pro373-CETP was the same as wt-CETP, we did not study CETP carrying both mutations because we wanted to identify which amino acid mutation altered CETP's activities.
This study has the limitation of having relatively small numbers of subjects and only male subjects. However, over 80% of these male VTE subjects had VTE of unprovoked etiology and are considered likely to enable discovery of novel risk factors. The findings in this study require replication in further studies, which should include larger numbers of subjects and females, especially more VTE cases with unprovoked thrombosis. Nonetheless, the value of this retrospective case-control pilot study is based on novel functional data related to CETP's procoagulant properties, including the discovery that the Gln451-CETP mutant, which was found at higher frequency in male VTE cases, has five-fold elevated procoagulant activity.
Conclusions
Increased CETP lipid transfer activity correlates with shorter APTT values and with increased risk for venous thrombosis. CETP directly binds to factor Xa outside of Gla domain, but CETP requires factor Xa's Gla domain and factor Va to enhance prothrombinase activity. The uncommon mutation, R451Q, in CETP causes five-fold increased procoagulant activity and is associated with increased risk for venous thrombosis.
Acknowledgements
We are grateful to Patricia M. Averell for assistance with the Scripps Venous Thrombosis Registry and to Drs. David Lloyd and Mark Bamberger (Pfizer) for providing cDNAs for CETP constructs. This work was supported by the National Institutes of Health grants HL021544, HL031950 and HL052246 (J.H.G.).
Abbreviations
- CETP
cholesteryl ester transfer protein
- VTE
venous thrombosis
- OR
odds ratio
- APTT
activated partial thromboplastin time
- IQR
interquartile ranges
- DVT
deep venous thrombosis
Conflict of Interest Disclosure
The authors declare no competing financial interests.
References
- 1). Hesler CB, Swenson TL, Tall AR: Purification and characterization of a human plasma cholesteryl ester transfer protein. J Biol Chem, 1987; 262: 2275-2282 [PubMed] [Google Scholar]
- 2). Le GW, Guerin M, Chapman MJ: Pharmacological modulation of cholesteryl ester transfer protein, a new therapeutic target in atherogenic dyslipidemia. Pharmacol Ther, 2004; 101: 17-38 [DOI] [PubMed] [Google Scholar]
- 3). Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA: Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol, 1998; 18: 1046-1053 [DOI] [PubMed] [Google Scholar]
- 4). Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA: Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am J Cardiol, 2002; 90: 71i-76i [DOI] [PubMed] [Google Scholar]
- 5). Deguchi H, Pecheniuk NM, Elias DJ, Averell PM, Griffin JH: High-density lipoprotein deficiency and dyslipoproteinemia associated with venous thrombosis in men Circulation, 2005; 112: 893-899 [DOI] [PubMed] [Google Scholar]
- 6). Barter PJ, Brewer HB, Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR: Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol, 2003; 23: 160-167 [DOI] [PubMed] [Google Scholar]
- 7). Brewer HB., Jr Increasing HDL Cholesterol Levels. N Engl J Med, 2004; 350: 1491-1494 [DOI] [PubMed] [Google Scholar]
- 8). Brousseau ME, Diffenderfer MR, Millar JS, Nartsupha C, Asztalos BF, Welty FK, Wolfe ML, Rudling M, Björkhem I, Angelin B, Mancuso JP, Digenio AG, Rader DJ, Schaefer EJ: Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler Thromb Vasc Biol, 2005; 25: 1057-1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Sheridan C. CETP inhibitors boost ‘good’ cholesterol to no avail. Nat Biotechnol. 2016; 34: 5-6 [DOI] [PubMed] [Google Scholar]
- 10). Tall AR: CETP inhibitors to increase HDL cholesterol levels N Engl J Med, 2007; 356: 1364-1366 [DOI] [PubMed] [Google Scholar]
- 11). Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B; ILLUMINATE Investigators: Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med, 2007; 357: 2109-2122 [DOI] [PubMed] [Google Scholar]
- 12). de Grooth GJ, Kuivenhoven JA, Stalenhoef AF, de Graaf J, Zwinderman AH, Posma JL, van Tol A, Kastelein JJ: Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase II dose-response study. Circulation, 2002; 105: 2159-2165 [DOI] [PubMed] [Google Scholar]
- 13). Griffin JH, Fernandez JA, Deguchi H: Plasma lipoproteins, hemostasis and thrombosis. Thromb Haemost, 2001; 86: 386-394 [PubMed] [Google Scholar]
- 14). Deguchi H, Fernandez JA, Griffin JH: Plasma cholesteryl ester transfer protein and blood coagulability Thromb Haemost, 2007; 98: 1160-1164 [PubMed] [Google Scholar]
- 15). Pecheniuk NM, Deguchi H, Elias DJ, Xu X, Griffin JH: Cholesteryl ester transfer protein genotypes associated with venous thrombosis and dyslipoproteinemia in males. J Thromb Haemost, 2006; 4: 2080-2082 [DOI] [PubMed] [Google Scholar]
- 16). Agerholm-Larsen B, Tybjaerg-Hansen A, Schnohr P, Steffensen R, Nordestgaard BG: Common cholesteryl ester transfer protein mutations, decreased HDL cholesterol, and possible decreased risk of ischemic heart disease: The Copenhagen City Heart Study. Circulation, 2000; 102: 2197-2203 [DOI] [PubMed] [Google Scholar]
- 17). Thompson A, Di Angelantonio E, Sarwar N, Erqou S, Saleheen D, Dullaart RP, Keavney B, Ye Z, Danesh J: Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA, 2008; 299: 2777-2788 [DOI] [PubMed] [Google Scholar]
- 18). Boekholdt SM, Thompson JF. Natural genetic variation as a tool in understanding the role of CETP in lipid levels and disease J Lipid Res, 2003; 44: 1080-1093 [DOI] [PubMed] [Google Scholar]
- 19). Kakko S, Tamminen M, Paivansalo M, Kauma H, Rantala AO, Lilja M, Reunanen A, Kesaniemi YA, Savolainen MJ: Cholesteryl ester transfer protein gene polymorphisms are associated with carotid atherosclerosis in men. Eur J Clin Invest, 2000; 30: 18-25 [DOI] [PubMed] [Google Scholar]
- 20). Kakko S, Tamminen M, Paivansalo M, Kauma H, Rantala AO, Lilja M, Reunanen A, Kesaniemi YA, Savolainen MJ. Variation at the cholesteryl ester transfer protein gene in relation to plasma high density lipoproteins cholesterol levels and carotid intima-media thickness. Eur J Clin Invest, 2000; 31: 593-602 [DOI] [PubMed] [Google Scholar]
- 21). Klerkx AH, Tanck MW, Kastelein JJ, Molhuizen HO, Jukema JW, Zwinderman AH, Kuivenhoven JA: Haplotype analysis of the CETP gene: not TaqIB, but the closely linked −629C--<A polymorphism and a novel promoter variant are independently associated with CETP concentration. Hum Mol Genet, 2003; 12: 111-123 [DOI] [PubMed] [Google Scholar]
- 22). Thompson JF, Durham LK, Lira ME, Shear C, Milos PM: CETP polymorphisms associated with HDL cholesterol may differ from those associated with cardiovascular disease. Atherosclerosis, 2005; 181: 45-53 [DOI] [PubMed] [Google Scholar]
- 23). Lloyd DB, Lira ME, Wood LS, Durham LK, Freeman TB, Preston GM, Qiu X, Sugarman E, Bonnette P, Lanzetti A, Milos PM, Thompson JF: Cholesteryl ester transfer protein variants have differential stability but uniform inhibition by torcetrapib. J Biol Chem, 2005; 15: 280, 14918-14922 [DOI] [PubMed] [Google Scholar]
- 24). Vasan RS, Pencina MJ, Robins SJ, Zachariah JP, Kaur G, D'Agostino RB, Ordovas JM: Association of circulating cholesteryl ester transfer protein activity with incidence of cardiovascular disease in the community. Circulation, 2009; 120: 2414-2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Duwensee K, Breitling LP, Tancevski I, Rothenbacher D, Demetz E, Patsch JR, Ritsch A, Eller P, Brenner H: Cholesteryl ester transfer protein in patients with coronary heart disease. Eur J Clin Invest, 2010; 40: 616-622 [DOI] [PubMed] [Google Scholar]
- 26). Kappelle PJ, Perton F, Hillege HL, Dallinga-Thie GM, Dullaart RP: High plasma cholesteryl ester transfer but not CETP mass predicts incident cardiovascular disease: A nested case-control study. Atherosclerosis, 2011; 217: 249-252 [DOI] [PubMed] [Google Scholar]
- 27). Robins SJ, Lyass A, Brocia RW, Massaro JM, Vasan RS: Plasma lipid transfer proteins and cardiovascular disease. The Framingham Heart Study. Atherosclerosis, 2013; 228: 230-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Deguchi H, Banerjee Y, Trauger S, Siuzdak G, Kalisiak E, Fernández JA, Hoang L, Tran M, Yegneswaran S, Elias DJ, Griffin JH: Acylcarnitines are anticoagulants that inhibit factor Xa and are reduced in venous thrombosis based on metabolomics data. Blood, 2015; 24; 126: 1595-1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Deguchi H, Elias DJ, Trauger S, Zhang HM, Kalisiak E, Siuzdak G, Griffin JH: Warfarin untargeted metabolomics study identifies novel procoagulant ethanolamide plasma lipids. Br J Haematol, 2014; 165: 409-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Tripodi A, Chantarangkul V, Martinelli I, Bucciarelli P, Mannucci PM: A shortened activated partial thromboplastin time is associated with the risk of venous thromboembolism. Blood, 2004; 104: 3631-3634 [DOI] [PubMed] [Google Scholar]
- 31). Qiu X, Mistry A, Ammirati MJ, Chrunyk BA, Clark RW, Cong Y, Culp JS, Danley DE, Freeman TB, Geoghegan KF, Griffor MC, Hawrylik SJ, Hayward CM, Hensley P, Hoth LR, Karam GA, Lira ME, Lloyd DB, McGrath KM, Stutzman-Engwall KJ, Subashi AK, Subashi TA, Thompson JF, Wang IK, Zhao H, Seddon AP: Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat Struct Mol Biol, 2007; 14: 106-113 [DOI] [PubMed] [Google Scholar]


