Abstract
Nanomedicine of synergistic drug combinations has shown increasing significance in cancer therapy due to its promise in providing superior therapeutic benefits to the current drug combination therapy used in clinical practice. In this article, we will examine the rationale, principles, and advantages of applying nanocarriers to improve anticancer drug combination therapy, review the use of nanocarriers for delivery of a variety of combinations of different classes of anticancer agents including small molecule drugs and biologics, and discuss the challenges and future perspectives of the nanocarrier-based combination therapy. The goal of this review is to provide better understanding of this increasingly important new paradigm of cancer treatment and key considerations for rational design of nanomedicine of synergistic drug combinations for cancer therapy.
Keywords: Nanomedicine, synergistic drug combinations, cancer therapy, strategies, perspectives
Graphical abstract
1. Introduction
Globally over 14.1 million new cases of cancer were estimated to occur in just 2012 even excluding non-melanoma skin cancer [1]. Despite tremendous efforts to combat cancer for several decades and recent advances in new therapeutic agents and modalities, cancer remains the second leading cause of diseases-associated death in the United States [2] and rises to the first leading cause of death in Canada [3]. The disappointing clinical outcomes of cancer therapies are largely attributable to the heterogeneity and complexity of this devastating disease. Conventionally, surgery and radiotherapy are employed for treatment of localized disease, while hormone therapy, chemotherapy, immunotherapy, and targeted therapy are administered for systemic treatment alone or in combination with other modalities [4-7]. Chemotherapy is a standard treatment for primary and metastatic cancer for a long history; however, its clinical benefits are limited, in particular when a single agent (mono-therapy) is administered. The common issues associated with mono-therapy include drug resistance at cellular and tumor levels, harsh tumor microenvironment that hinders drug penetration and efficacy, tumor heterogeneity, and dose-limiting toxicity (Supplementary Material - Table S1). Therefore, combination chemotherapy regimens containing two or more classical anticancer drugs have been applied for decades in clinical practice to treat a variety of cancers (Supplementary Material - Table S2) [5, 8-11]. However, the outcomes are still unsatisfactory. With increasing understanding of tumor biology, molecular pathways, tumor microenvironment, and tumor-host interactions, new combination therapies have been developed. These new combination therapies include chemotherapy with immunotherapy, chemotherapy with targeted therapy, chemotherapy with gene (i.e. DNA- and RNA-based) therapy or epigenetic therapy, and immunotherapy with targeted therapy [12-20].
Theoretically, combination therapy with therapeutic agents that are demonstrated effective as mono-therapy in clinic should provide better therapeutic effects without additional toxicity. Nevertheless, clinical outcome of combination therapy is not always as good as anticipated; rather is often associated with higher toxicities, although non-overlapping toxicity is a major consideration for selecting drug combination candidates [21-23]. In addition to the unwanted escalation of toxicity, the success of combination drug therapy is hampered by inability of component drugs to achieve desired spatiotemporal distribution when they are delivered in free molecule form [24-26]. It is critical that the component drugs are delivered to the right place at the right timing; yet inherent differences in physicochemical and pharmacokinetic properties among drug components prevent this from happening unless an efficient drug carrier is utilized.
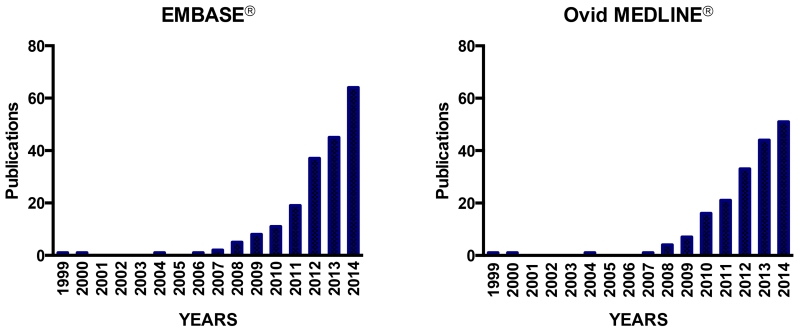
Having realized this inherent problem and necessity for synergistic drugs to arrive at the same cellular target for overcoming efflux transporter-mediated multidrug resistance (MDR), attempts have been made as early as in 1999 to deliver an anticancer drug with a chemosensitizer or a P-glycoprotein (P-gp) inhibitor, by the same carrier to P-gp overexpressing cancerous cells [27-30]. The strategy to co-deliver drug combinations using a carrier has been taken by an increasing number of researchers since then. The reports about co-delivering drug combinations using the same carrier have increased exponentially since 2007 (Figure 1) based on the publication search from two databases, EMBASE® and Ovid MEDLINE® from 1999 to 2014 (Supplementary Material – Table S3).
Figure 1.
EMBASE® and MEDLINE® search results on drug delivery systems for co-delivering combination chemotherapy over the past fifteen years. According to systematic review from both EMBASE® and MEDLINE®, the earliest research on co-loading anticancer drug and chemosensitizing drug within the same drug carrier to enhance drug toxicity against P-gp overexpressing multidrug resistant cells were first reported by Liu et al, 1999, followed by Soma et al., 2000, Liu et al., 2003, and Wong et al. 2004; 2006 [27-31].
In the past decade, the use of nanocarriers to improve drug delivery has been a flourishing strategy for cancer treatment. Several classes of nanocarriers have been studied for anticancer drug delivery, including liposomes, polymer nanoparticles, polymeric micelles, dendrimers, nanocapsules, solid lipid nanoparticles, polymer-lipid hybrid nanoparticles, inorganic nanoparticles, inorganic-organic hybrid nanoparticles, nanoemulsions, and nanogels. These nanodelivery systems can serve as the key platforms to implement a combined drug therapy of cancer in a more efficient, more effective and safer manner. A variety of nanocarrier systems have been investigated to deliver drug combinations, of which a few have reached clinical trial stages [32-37].
In this article, we will examine the rationale, principles, and advantages of applying nanocarriers to improve anticancer drug combination therapy, review the use of nanocarriers for delivery of a variety of combinations of different classes of anticancer drugs including small molecule and large molecule drugs, and discuss the challenges and future perspectives of the nanocarrier-based combination therapy. The goal of this review is to provide better understanding of this increasingly important new paradigm of cancer treatment and key considerations for rational design of nanomedicine of synergistic drug combinations for cancer therapy.
2. Rationales and strategies of nanocarrier-based combination therapy of cancer
2.1 General principles and concept of combination therapy
For decades, selection of combination chemotherapy in clinical practice has been based on the following general principles: to use drugs with 1) clinically proven activity as single agents, 2) no overlapping toxicities, 3) no cross-drug resistance; 4) different mechanisms of drug action, 5) target different cell cycles, and 6) generate anticancer synergy [23, 38, 39]. In clinic, drug combination therapy via sequential administration is recommended in guidelines as standard cancer chemotherapy, while simultaneous administration is not [40]. The reason behind this is that prominent adverse effects and absence of obvious benefits in the overall survival were found in clinical trials for simultaneously administered combination chemotherapy, despite a higher tumor response rate [41-43]. However, positive tumor response and low toxicity profiles were reported in recent Phase II clinical trials on low dose metronomic drug combinations [44, 45]. These mixed clinical results of drug combination therapy present an opportunity to nanocarrier-based approaches, which are able to mitigate the problems encountered by free drug combination therapies. Detailed discussion of issues with free drug combination therapy and advantages of nanocarrier-based drug combination therapies will be presented later.
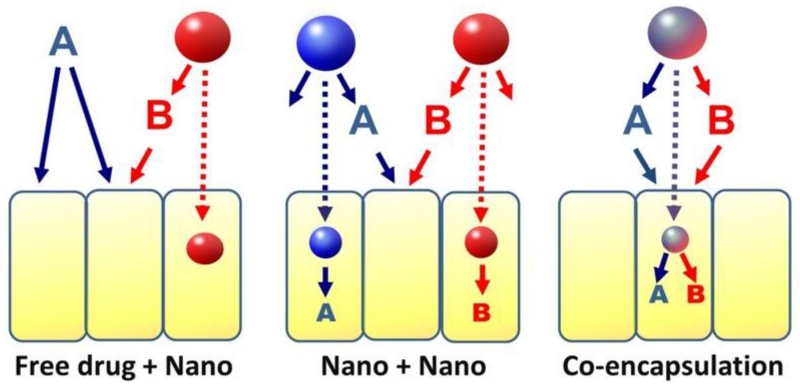
When combined with nanotechnology, there are several ways to deliver multiple drugs. For a two-drug combination, one drug can be delivered as free form and the other by a nanocarrier (Free drug + Nano), or both drugs delivered by separate nanocarriers (Nano + Nano), or both drugs are co-encapsulated in the same nanocarrier (co-encapsulation) (Figure 2). The first two approaches (Free drug + Nano & Nano + Nano) are often delivered in sequential manner, whereas co-encapsulation approach delivers drugs simultaneously, providing controlled temporal and spatial delivery of multiple drugs, thus, increasing intracellular drug concentration and coordinating drug synergy against cancer cells. Examples of various combinations therapies will be presented in Section 3.
Figure 2.
Scheme of different approaches to implement a two-drug combination involving nanocarriers.
The first approach is the closest to the current practice of using nanoparticle formulations for cancer treatment. The “Nano+Nano” approach may offer more flexibility in dosing and formulation than co-encapsulation; however, nanocarriers loading different drugs of a combination may not reach the same cells to achieve the optimal synergistic effects. The co-encapsulation approach may be most effective among three in coordination of drug actions at cellular levels.
Abraxane® (paclitaxel loaded albumin nanoparticles) or Doxil® (PEGylated liposomal doxorubicin) are often used with other drugs or together with other components given as free drug solution [46, 47]. In addition, this approach also allows multiple agents to be delivered in sequential instead of simultaneous manner. For example, by treating breast cancer with cis-platinum nanoparticles first, followed by PI828, an inhibitor of PI3K, the highest efficacy of the combination therapy was achieved [48].
The benefits of co-encapsulation of synergistic drug combinations in the same carrier have been demonstrated by various groups in vitro and in vivo [30, 34-39, 49-55]. An early proof-of-concept preclinical study by Liu et al. showed that even intratumoral co-administration of an anticancer drug doxorubicin (DOX) and a chemosensitizer of P-gp (i.e. verapamil) loaded in separate particle formulations could only generate modest effect on tumor growth delay while noticeable toxicity was observed in MDR breast tumor bearing murine model [28]. Similarly, a study of co-encapsulation of DOX and a P-gp inhibitor [30] or two anticancer drugs [55] in the same nanocarrier showed that co-encapsulation is therapeutically superior to the “Nano+Nano” approach and the free drug combination [30, 55, 56]. In both wild-type and resistant MDA-MB-435/LCC6 human breast cancer cells, DOX-mitomycin C (MMC) co-loaded hybrid polymer-lipid nanoparticles (DMPLN) was found significantly more effective against resistant cancer cells than the co-administration of single agent-loaded PLN (i.e. DOXPLN + MMCPLN), suggesting that co-encapsulation amplified the drug interaction synergistically [56].
Nonetheless, co-encapsulation of multiple drugs of various properties (e.g. water-solubility, polarity, and stability) in the same nanocarrier is very challenging. Normally the doses of the multiple drugs in the same carrier are fixed. Any substantial adjustments of their ratio often require careful re-formulation of the nanosystem to prevent undesirable changes of its physicochemical properties and stability. All in all, there are pros and cons for each approach and which one should be chosen probably depends on the specific situations (e.g. choice of drugs in the combination, mechanism of drug synergy, cancer type, cancer location).
2.2. Rational selection of drug combination candidates and treatment schedule
In addition to the fundamental principles described above, other factors have to be considered in the choice of drug combination candidates, including drug-drug interaction-induced alteration of metabolism, disposition, and elimination, optimal drug-to-drug ratio and administration scheduling. Although the ultimate goal of drug combination therapy is to achieve maximal therapeutic efficacy and minimal toxicity, the anticancer effect of a combination therapy may not always be synergistic, yet it can be additive, or even antagonistic, depending on the design and the manner in which a therapy is implemented. It has been found that drug-to-drug ratio and drug administration schedule are critical factors determining whether the drug interaction will generate synergistic effect or not [38, 49-51, 54, 56, 57]. In case the drug actions are required to occur on the same cell, the optimal drug ratio and scheduling can be determined in vitro prior to designing a suitable delivery system.
The effect of drug interaction is normally evaluated by using the widely applied median effect analysis [39, 52, 54, 58], such that a combination index (CI) is calculated to determine whether the effects were additive (CI=1), synergistic (CI<1), or antagonistic (CI>1) [58]. Additive interaction means that the combined effect of all drugs is equal to the sum of the effect of the drugs taken separately, while synergistic interaction and antagonistic interaction mean the combined effect is greater or smaller than the sum of the individual effect, respectively. In general, a “good” combination will lead to additive or synergistic therapeutic effect, and this is usually designed by combining agents with non-overlapping mechanisms of action and toxicity profiles [58]. When agents are properly combined, they may delay or overcome MDR and prevent patients treated with standard therapy from the relapse [39, 58-60].
Ensuring an optimal drug-to-drug ratio to be delivered into the tumor is critical for achieving increased efficacy. Mayer and co-workers reported that co-delivery of irinotecan and floxuridine at 5:1 synergistic molar ratios within the liposome increased anticancer activity in colorectal tumor xenograft whereas attenuated anticancer activity was observed when antagonistic drug combination ratio was delivered into the tumor [50]. Hasenstain et al also demonstrated that co-delivery of the synergistic drug combination of paclitaxel, rapamycin and 17-AAG at a 2:1:3 molar ratio, using micelles as a carrier, is important for enhanced therapeutic effectiveness in vivo [34].
The timing of administering drug combination is also important for synergistic effect to occur [57]. Cheung et al. found that for DOX and MMC at a fixed 1.7:1 molar ratio, the synergic anticancer effect against EMT6 breast cancer cells was the strongest when DOX and MMC were co-currently applied or when DOX was given before MMC treatment, but not when MMC was applied as pre-treatment [57]. Further study on mechanism of drug interaction showed that formaldehyde produced from MMC bio-reductive metabolism can facilitate intracellular DOX accumulation via binding to detoxifying enzyme glutathione (GSH). Together with bio-activated MMC cross-linking of DNA, DNA repair enzymes may be activated to a great extent to allow ample opportunity of topoisomerase II-α colliding with DOX in nucleus, leading to more DNA double strand breaks and higher cell kill [39].
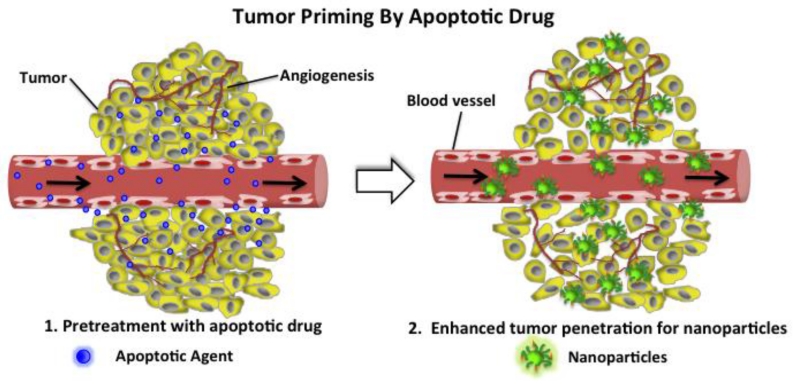
Tumor priming is another example of rational design of drug combination scheduling. Pretreating tumors with an apoptotic drug (i.e. paclitaxel) has been shown to make tumor more permeable to protein drugs or nanoparticles (Figure 3) [61, 62]. The tumor is pretreated with a conventional cytotoxic drug (e.g. paclitaxel) below its standard dose (Figure 3(1)). Drug-loaded nanoparticles or protein drugs are then administered within a time of optimized apoptosis window (e.g. 24 h −72 h). Therefore, the penetration of the nanoparticles or proteins in the solid tumor can be greatly enhanced via expansion of interstitial space (Figure 3(2)), resulting in potential synergistic anticancer effects by the sequential combination of two drugs.
Figure 3.
Illustration of tumor priming by pretreatment with an apoptotic drug (1) which generates more interstitial space for effective penetration of subsequent drugs or drug-loaded nanoparticles into tumor tissue (2).
Drug combination therapy may also be employed to target different cancer cell sub-populations. For instance, one can use standard cytotoxic chemotherapy drugs to kill the bulk of cancer cells in a tumor and a targeted therapy drug to specifically tackle the tumorigenic sub-population (sometimes known as cancer stem cells or tumor initiating cells), so that repopulation of the less tumorigenic cell sub-populations can be suppressed [26, 63].
In addition to chemotherapy using drug combinations of conventional anticancer drugs (Supplementary Material - Table S2), combinations of chemotherapy with P-gp inhibitors, immunotherapy, targeted therapy, gene (i.e. DNA- and RNA-based) therapy, or with epigenetic therapy, and immunotherapy with targeted therapy are useful combination therapies. These combinations are designed based on various mechanisms.
2.3. Common issues with free drug combinations
A key concern about combination drug therapy is “in what manner the multiple components of the therapy should be administered to achieve the optimal outcomes”. As summarized in Table 1, several obstacles could compromise the effects of combination therapy in free drug solutions, leading to undesirable antagonistic outcomes. It should be noted that these issues are commonly encountered in clinical cancer treatment. For instance, many of the chemotherapy drugs and the newer targeted drugs have poor water solubility and cannot be efficiently delivered to the tumor site [64]. Similar scenarios happen when one of the component drugs has permeation or stability issues, e.g. inclusion of small interference RNA (siRNA) in the combination therapy [65]. In many cases, component drugs have different tumor disposition and pharmacokinetic profiles. Thus not all component drugs of a combination can reach the tumor tissues and cells at the desirable ratio and duration, resulting in sub-optimal efficacy and increased non-specific toxicity of the therapy.
Table 1. Issues that may potentially compromise the effectiveness of combination therapy of cancer.
| Issue | Description | Example |
|---|---|---|
| Limited solubility | One of the drugs is poorly soluble and requires drastic measures to dissolve, compromising the therapy |
Paclitaxel has limited solubility and requires high surfactant concentration to solubilize the drug, increasing the risk of adverse drug effects |
| Limited permeation | One of the drugs has limited ability to permeate across cell membranes, so not all drugs of the combination can achieve sufficiently high intracellular levels |
In a combination of chemotherapy drugs with nucleic acid therapy, the nucleic acid molecules can efficiently reach the cytoplasm |
|
Inadequate tumor
delivery |
In a combination, the drugs that have high molecular weights and have high protein binding affinities tend to penetrate poorly into a solid tumor |
DOX does not penetrate far in a tightly packed epithelial tumor, leading to suboptimal DOX levels in portions of tumor |
|
Uneven drug
distribution in tumor |
Cells in the same tumor are not exposed to all drugs of a combination at their therapeutic levels |
For a combination of chemotherapy drugs and chemosensitizer, some cells are not adequately sensitized so they are not fully responsive to the chemo components |
|
Different drug
stabilities |
One or more of the therapeutic agents in a combination degrade much faster than the other drug(s) |
RNA and peptide agents tend to have short in vivo half-lives when compared to many lipophilic chemotherapy drugs, so may need to dose the different components at different frequencies |
|
Different drug half- lives and tumor accumulation |
One or more of the therapeutic agents in a combination is eliminated much faster than the other drug(s) |
DOX has an elimination half-life 7-10 h, while MMC 15-40 min; MMC accumulates more in tumor at short times, while disappears at 24 h |
|
Alteration of
pharmacokinetics and/or metabolism |
The component drugs share the same metabolic enzymes and/or elimination pathways |
P-gp inhibitors (e.g. valsodar, Elacridar) intended to reverse P-gp-mediated drug resistance could reduce anticancer drugs (e.g. DOX, paclitaxel, vincristine) elimination through the liver or kidneys |
|
Overlapping
mechanisms |
In a combination, two or more drugs target the same molecular pathways, leading to sub- optimal therapeutic effect and triggering chemoresistance |
Use of two taxanes (e.g. paclitaxel and docetaxel) or two anthracyclines (e.g. DOX and daunorubicin) |
|
Overlapping
toxicities |
In a combination, two or more drugs share similar tissue/ organ toxicity profiles, thus amplifying the adverse drug effects and making it difficult to use near-maximal doses |
Overlapping toxicity profiles of bolus 5- FU and irinotecan, which both result in high rates of severe diarrhea |
|
Poor timing or sub- optimal sequence |
Some combinations work better with all of the drugs administered simultaneously while some work more optimally if one of the drugs is administered earlier |
Apoptotic agent paclitaxel may not help other drugs to penetrate a solid tumor if given together or less than 24 h before other drugs |
2.4. Advantages and strategies of nanocarrier-based combination therapy of cancer
Nanocarrier systems exhibit many advantages, such as drug encapsulation and solubilization of poorly soluble drugs, drug protection, cellular uptake of nanoparticles via endocytosis, passive tumor accumulation via enhanced permeability and retention (EPR) effect, targeted delivery, and controlled/ sustained release kinetics. By taking these advantages, it is possible to efficiently deliver multiple anticancer agents and manipulate where and when the agents distribute in the body [66, 67]. In addition, nanocarriers have many other advantages, such as co-loading of both soluble and poorly soluble drugs, co-delivery of small molecule drugs and macromolecule agents, and stimulus-responsive drug release. These advantages of nanocarrier systems are all valuable for addressing the aforementioned issues of combination therapy of cancer associated with free drug formulations. By properly integrating nanotechnology into combination therapy, several positive outcomes can be achieved. Given the particular challenges for co-encapsulation of several drugs, e.g. for ratio-metric dosing, in the following text we will discuss mainly the advantages and strategies using nanocarrier systems for this application.
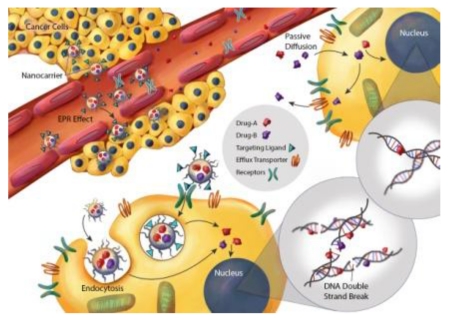
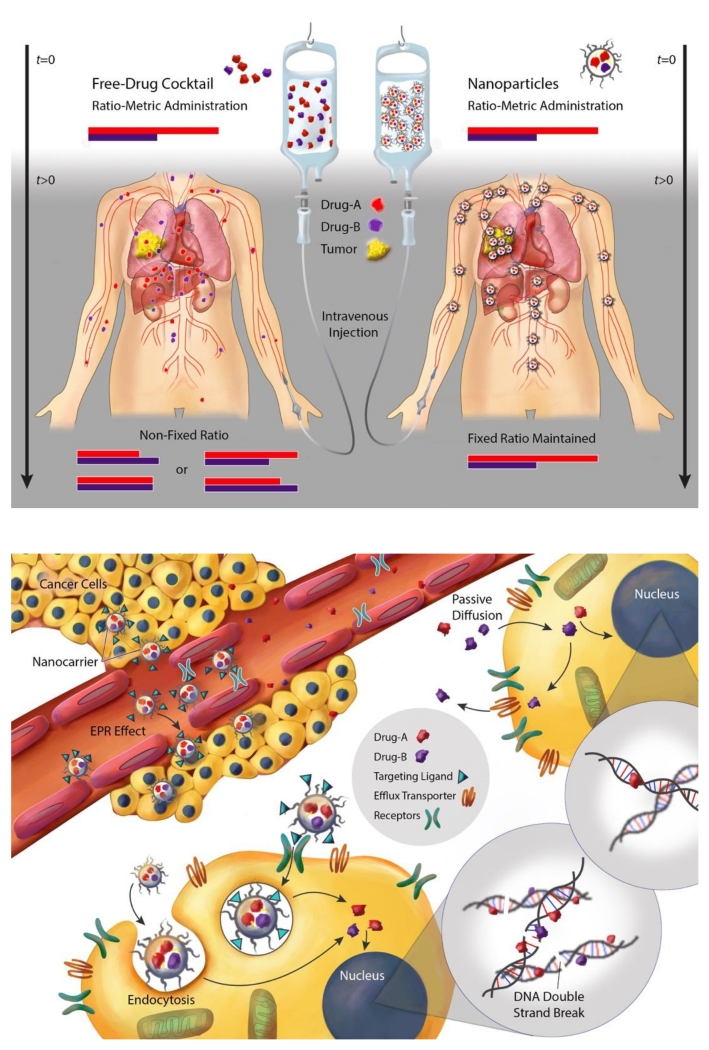
As aforementioned, a fixed ratio of two drugs delivered to cancer cells is required for certain drug combinations to generate synergistic anticancer effect; yet this is extremely difficult to achieve if a free solution of drug combination is administered. Owing to different pharmacokinetics, tissue disposition, and cell uptake of component drugs, the initial drug-to-drug ratio is no longer maintained in the circulation and in the tumor tissue after a short time (Figure 4A, left panel). Furthermore, drug-drug interactions as a result of shared metabolism and excretion pathways could result in higher tissue toxicity and altered drug ratio, as well as low drug accumulation of both drugs inside tumor. In contrast, when these drugs are co-encapsulated within one nanocarrier and released at a similar rate, their synergistic ratio (e.g. 2:1 for Drug A to Drug B) can be maintained at high concentration in the blood and in the tumor for a long period of time (Figure 4A, right panel)[38, 50, 51, 55]. The biodistribution, tumor accumulation, and local drug availability can be synchronized by the nanocarrier that accumulates in the tumor tissue via the EPR effect or receptor-mediated extravasation, followed by cell uptake via passive or receptor-mediated endocytosis (Figure 4B). The nanocarriers can then release the drugs inside cancer cells to generate synergistic effects in this case more DNA double strand breaks as demonstrated by Shuhendler et al. using DOX and MMC–co-loaded PLN (Figure 4B) [39, 56]. Thereby, therapeutic efficacy is significantly enhanced and off-target toxicity dramatically reduced [32, 33, 38, 53-55].
Figure 4.
Illustration of potential benefits of using nanocarriers for ratio-metric delivery of synergistic drug combination for cancer treatment in clinical applications. A) Alteration of pharmacokinetics and biodistribution of ratiometric drug combination delivered by free solution. Upon intravenous (i.v.) administration, traditional free drug combination fails to maintain desired drug ratio (e.g. 2:1) before reaching the tumor (left). B) Nanocarrier delivered synergistic drug combination into cancer cells against multidrug-resistance in cancer. The nanoparticles accumulate in the tumor by the ERP effect or receptor-mediated extravasation, bind with tumor receptors, enter cancer cells via endocytosis, and release drugs within cancer cells. The released drugs translocate into nucleus where the site of drug action occurs in this case, to exert synergistic action, resulting in enhanced DNA double strand breaks. The figures were illustrated by Caitlin Swanberg.
Co-encapsulating drugs within nanoparticles is also useful for delivering two or more drugs that act on different targets but require coordinated delivery at sub-cellular levels. One common strategy to enhance drug accumulation in multidrug resistant cancer cells is to use P-gp inhibitor. Wong et al., showed that co-loading DOX (P-gp substrate) and Elacridar (GG918) (P-gp inhibitor) within PLN is superior to co-administration of DOX and GG918 in separate PLN against MDR human breast cancer, indicating that co-localization of GG918 with DOX by the nanoparticles is important to enhance DOX cellular uptake, retention, and thereby higher cytotoxicity [30]. Triolimus (micelle co-loaded with triple drugs: paclitaxel, rapamycin and 17-AAG) is another example of co-encapsulation within one nanocarrier to achieve synergism against several cancer cell lines via targeting multiple cellular targets [34-36]. Paclitaxel is a mitotic inhibitor and its cytotoxicity can further be enhanced by rapamycin via inhibition of p70s6k phosphorylation, the mechanistic target of rapamycin (mTOR). Meanwhile 17-AAG can block the compensatory Akt pathway that can be activated upon treatment of rapamycin. Thus, such a triple drug combination requires coordinated delivery within the same nanoparticle to achieve such synergism via simultaneously block both p70s6k and Erk1/2 phosphorylation [34].
It should be noted that the term “small molecule anticancer drugs” actually encompasses molecules of highly diverse physicochemical properties, ranging from lipophilic, poorly water-soluble drugs (e.g. taxanes) to inorganic, water-soluble drugs (e.g. platinum drugs). For co-encapsulation of compounds with a large difference in lipophilicity into the same nano-system, two strategies are often used. The first strategy is to modify a drug into a pro-drug that has more suitable lipophilicity. The modification sometimes may also create new functional group for direct conjugation to the nano-system. For example, Xiao et al attempted to co-encapsulate daunomycin and oxaliplatin into polymeric nanocarriers [68]. To better encapsulate oxaliplatin, it was converted into a prodrug with an axial carboxyl group for conjugation to the polymer. The second strategy is the use of nanocarriers with both lipophilic and hydrophilic elements to interact with both types of compounds. For instance, Wong et al. developed a solid polymer-lipid nanoparticles to co-encapsulate lipophilic drugs such as Elacridar (GG918) and water-soluble DOX hydrochloride by inclusion of an ionic polymer to bind the oppositely charged drug [27, 30]. Shuhendler et al. also designed “stealth” PLN system for co-delivery of DOX hydrochloride and MMC of different polarity and water-solubility at a synergistic ratio [56].
3. Examples of nanocarrier-based combination therapies of cancer
Because small molecule drugs (i.e. cytotoxic anticancer drugs) and large molecule drugs (i.e. biologics, including proteins, DNA and RNA) tend to encounter distinctly different sets of problems in their delivery, we have reviewed them separately in the following sections. Moreover, we have only listed, in the tables, representative examples of nanoparticle co-delivered drug combinations that have been investigated in both in vitro and preclinical studies or in clinical trials. There are many examples studied in vitro only that are not listed due to space limitation.
3.1. Nanocarrier-based combinations of small molecule drugs only
Small molecule anticancer therapy generally includes chemotherapy and sometimes hormonal therapy when the cancer is hormone-dependent (e.g. breast cancer). Combining multiple small molecules may increase anticancer efficacy, thus is frequently used in clinical settings. The development of nanocarrier-based combination therapy of cancer follows a similar trend. The combination generally includes anticancer drugs with non-overlapping cytotoxic mechanisms with additional advantages of nanocarriers as discussed in Section 2.4.
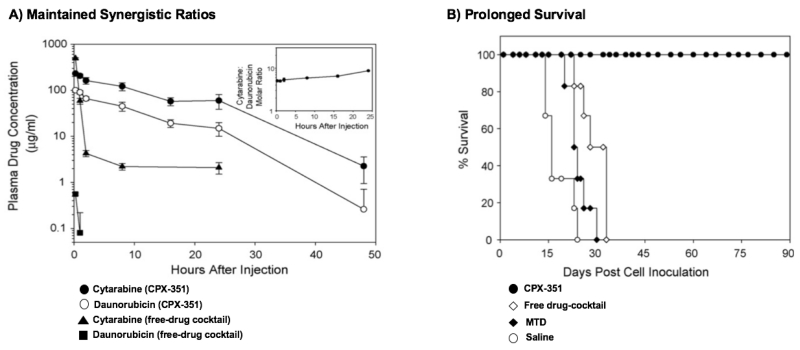
Table 2 presents selective examples of co-delivery of anticancer drug combinations using nanoparticles that have been investigated both in vitro and in vivo [32-36, 38, 39, 49-56, 69-80]. On the top of the list are liposome formulations co-loaded with irinotecan and floxuridine at 1:1 molar ratio (CPX-1) or cytarabine and daunorubicin at 5:1 molar ratios (CPX-351) developed by Celator® Pharmaceuticals. CPX-1 has been advanced to Phase II trial for the treatment of advanced colorectal cancer [69] and CPX-351 (VYXEOSTM) to Phase III clinical trial for refractory acute myeloid leukemia [33, 81, 82]. In various pre-clinical leukemia tumor models, the injectable liposomal formulation CPX-351 maintained synergistic ratios of cytarabine and daunorubicin combination during the blood circulation, resulting in significantly enhanced therapeutic efficacy and low toxicity compared to free drug combination at the equivalent dose ratio (Figure 5) [38]. In a recently completed Phase III randomized trial in patients with secondary acute leukemia, CPX-351 (VYXEOSTM) significantly improved overall survival, even free survival, and response without increased adverse effects compared to traditional “7+3” combination regimen of cytarabine and dauorubicin in free solution [82]. Another example is Triolimus, a micelle formulation of triple drugs (i.e. paclitaxel, rapamycin and 17-AAG at 2:1:3 molar ratio) which has been in a Phase I clinical trial for angiosarcoma [37]. The remaining studies on the list have demonstrated enhanced anticancer efficacy in preclinical tumor xenografts using dual agents co-loaded nanoparticle formulations.
Table 2. Examples of nanocarrier-based co-delivery of synergistic drug combination (small molecules) in cancer therapy. All studies were performed both in vitro and in vivo with *the nanoparticle formulation in clinical trials.
| Nanocarriers | Tumor Model | Results | Reference |
|---|---|---|---|
|
CPX-1 liposomes* (irinotecan: floxuridine) |
Patients with advanced colorectal cancer; Subcutaneous HT-29 human colon cancer |
Simultaneous release of drugs at synergistic ratios from the liposome and maintained the synergistic ratio up to 12 h; improved efficacy in clinical trial. |
[32, 49, 50, 69, 71] |
|
CPX-351 liposomes* (cytarabine:daunorubicin) |
Patients with refractory acute myeloid leukemia; i.v. inoculation of Murine P388, L1210 and WEHI- 3B leukemia; i.v. inoculation Human HL- 60B & CCRF-CEM human leukemia |
Maintaining an optimized drug ratio greater than 24 h in phase I dose- limiting studies; Significant improvement of patient survival, response and low adverse effects in phase III randomized trial; Drug ratio of antagonism in vitro correlates with low survival and maximum tolerated dose. |
[33, 82] |
|
Triolimus* (paclitaxel: rapamycin:17- AAG) |
In clinical trial for angiosarcoma. Subcutaneous A549 human lung cancer and MDA-MB-231 human breast cancer |
Triple-loaded polymeric micelle simultaneously targeting different cellular sites; strong synergistic anticancer effect against multiple human cancer cell lines; enhanced efficacy and low toxicity. |
[34-36] |
|
Prodrug-based nanocarriers (paclitaxel:baicalein) |
A549/PTX human lung cancer (inoculation method is unknown) |
Combination of dual targeting ligands (folate and hyaluronic acid) and dual loading drugs showed the most tumor regressions in MDR human lung cancer model; |
[79] |
|
Telodendrimer (cisplatin: paclitaxel) |
Subcutaneous SKOV-3 human ovarian cancer |
Co-encapsulating two drugs with distinct physical properties (i.e. hydrophobic paclitaxel and metallic cisplatin) at various ratios (2:1, 4:1 etc) maximized synergy by fine tuning the nanoparticles |
[80] |
|
Polymeric nanoparticles (oxaliplatin:gemcitabine) |
Subcutaneous AsPc-1 & BxPc-3 human pancreatic ductal adenocarcinoma |
Nanoparticles with dual-loaded drugs can inhibit two different types of tumor growth at very low dose versus high dose free drug combination exhibited severe adverse effect and low tumor response |
[76] |
|
Polymer-lipid hybrid nanoparticles (DOX:MMC) |
Orthotopic murine EMT6 (sensitive and resistant) and human MDR-MB-435 human breast tumor (sensitive and resistant) models |
Precise delivering synergistic ratio of DOX and MMC within nanoparticles to tumor with reduced formation of cardiotoxic DOX metabolite, doxorubicinol; reduced tumor growth and prolonged survival in MDR breast tumor with attenuated cardiotoxicity |
[39, 53-56] |
|
Polymeric nanoparticles (ABT-737:camptothecin) |
Subcutaneous MC38 human colon cancer |
Synergistically inducing cancer cell apoptosis in vitro and in vivo and synergy involves molecular regulation including activation of caspase 3/7/8/9, up-regulation of p53 and down-regulation of Bcl-2 |
[77] |
|
Polymer micelles (rapamycin:paclitaxel) |
Orthotopic MDA-MB-468 human breast cancer |
Maintained precise synergistic drug ratio within breast tumor for 48 hours; Mechanism involves suppression of feedback loop Akt phosphorylation resulting in increased cancer cell apoptosis, decreased oncogenic protein translation and cell cycle progression. |
[70] |
|
Polymeric micelles (DOX: disulfiram) |
Subcutaneous MCF- 7/ADR human breast cancer |
pH-sensitive release of two drugs at subcellular level and disulfiram (P-gp inhibitor and apoptosis inhibitor) released first before DOX to increased DOX cytotoxicity. |
[72] |
|
EGFR-polymer nanoparticles (lonidamine:paclitaxel) |
Orthotopic MDA-MB-231 hypoxic human breast cancer |
Targeted EGFR nanoparticles showed advantage of improved PK compared to non-targeted nanoparticles and improved tumor regression. |
[75] |
|
CPX-571 Liposomes (irinotecan:cisplatin) |
Subcutaneous H69 & NSCLC H1299 human lung cancer & HT29 human colon cancer & Capan-1 human pancreatic cancer |
In vitro screening showed the zone of antagonism of irinotecan/cisplatin between molar ratio of 1:2 and 1:4. Synergy of irinotecan/cisplatin between 5:1 and 10:1 was optimal. Superior antitumor activities were observed in multiple different tumor types in liposome co-loaded synergistic ratio of 7:1. |
[38, 51] |
Figure 5.
A) Pharmacokinetics of cytarabine and daunorubicin in the plasma after i.v. administration of CPX-351 (12:5.3 mg/kg) or free-drug cocktail (600:9 mg/kg) to CD-1 nude mice. The insert shows that CPX-351 maintained molar drug ratios of co-loaded cytarabine and daunorubicin between 5:1 and 9:1 over 24 h during the blood circulation. These ratios had been determined to be the most synergistic ratios in vitro; B) Survival curves of CD-1 nude mice bearing WEHI-3B monomyelocytic leukemia tumor treated with saline, maximum tolerated dose (MTD) of free cytarabine and daunorubicin cocktails (300:4.5 mg/kg), ratio-matched free cytarabine and daunorubicin cocktails (12:5.3 mg/kg) or CPX-351 (12: 5.3 mg/kg). The figures are permitted and reproduced from Tardi et al.2009 [38].
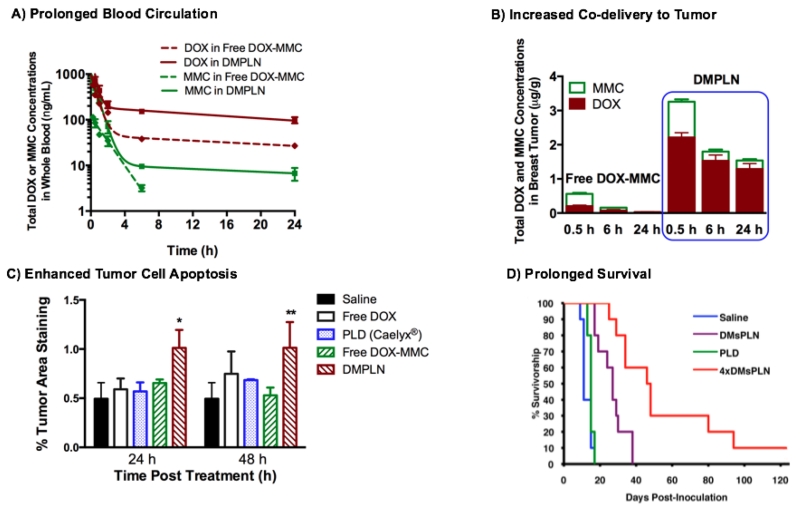
DMPLN is DOX and MMC co-encapsulated polymer-lipid hybrid nanoparticles (PLN) nanoparticle formulation which was developed by the Wu group based on thorough understanding of intracellular molecular mechanisms that led to increased DNA double strand breaks and cell kill [39, 54]. DMPLN demonstrated the synergistic anticancer effect with CI <1 against multiple human and murine breast cancer cells in vitro [52, 54, 56]. In orthotopic breast tumor murine models when, compared with free DOX-MMC combination, DMPLN prolonged the circulation of both DOX and MMC and selectively delivered drugs at the fixed effective ratios to the breast tumor, resulting in enhanced tumor cell apoptosis and prolonged survival of breast tumor-bearing mice (Figure 6) [55]. Compared to clinically used nanomedicine PLD (Caelyx®), DMPLN significantly delayed tumor growth in both sensitive and resistant breast tumors and attenuated cardiotoxicity by forming less toxic metabolites doxorubicinol in the heart compared to free DOX-MMC combination and PEGylated liposomal DOX (Caelyx®) [53-55].
Figure 6.
A) Whole blood analysis of pharmacokinetics of DOX and MMC co-delivered by DMPLN or in free drug combination; B) Breast tumor accumulation of DOX and MMC co-delivered by DMPLN or in free drug combination; C) Breast tumor cell apoptosis determined by percent activation of caspase-3 treated by DMPLN and other DOX formulations; D) Kaplan-Meier survival curves of breast tumor bearing mice treated with DMPLN (1× or 4× injections) or clinically used PLD (Caelyx®) (1× injection). An orthotopic EMT6/WT breast tumor model was used and treated with i.v. administration of various DOX and DOX-MMC formulations at equivalent drug doses (9.2 mg/kg DOX or in additional 2.9 mg/kg MMC). The figures are permitted and reproduced from Zhang et al. 2016 [55] and Shuhendler et al. 2014 [54].
In addition to the co-delivery of anticancer drugs, nanocarrier systems have been developed to deliver combinations of cytotoxic drugs and non-cytotoxic agents, e.g. rapamycin. Rapamycin was originally indicated as an antifungal agent, but was shown to block Akt signaling to lower cancer cell resistance to chemotherapy. A number of nanocarriers, therefore, have been designed to combine it with chemotherapy agents such as DOX and paclitaxel for treatment of chemoresistant cancer with reported synergistic cytotoxicity [83-85]. Ceramide and paclitaxel have been co-delivered by nanocarriers to MDR cancer cells [86]. Recently, researchers are drawn to less cytotoxic alternatives [87]. Existing drugs or herbal ingredients that demonstrate activities to inhibit cancer cell proliferation, reduce inflammation, or lower cancer drug resistance have been “repurposed” for cancer treatment [87]. Examples include all-trans-retinoic acid, rapamycin and curcumin [88]. Anti-inflammatory drugs and herbal medicine ingredients have also been considered as candidates for combination therapy with cytotoxic drugs [83, 89, 90]. Oral co-delivery of chemo-preventive agents, aspirin and curcumin within solid lipid nanoparticles in combination with sulforaphane was reported to reduce effective inhibitory dose by a factor of ten comparing to the free form combination in pancreatic cancer prevention [90].
3.2. Nanocarrier-based combinations including large molecule drugs
Large molecule drugs include proteins/peptides, antibodies, nucleic-acids (RNA and DNA). The delivery of these large molecule drugs is limited by their poor permeability, low stability and tendency to be eliminated by the reticuloendothelial system (RES) from systemic circulation. These limitations can be overcome by delivering them with suitable nanocarriers. As delivery of nucleic acids (i.e. DNA, miRNA, siRNA) involves different considerations from delivering antibodies, we will review and present them separately in Table 3 and Table 4, respectively.
Table 3. Examples of nanocarrier-based co-delivery for combined cancer gene and /or chemotherapeutic therapy.
| Nanocarriers | Nucleic Acid & Chemo-Drug |
Tumor Model | Results | Reference |
|---|---|---|---|---|
| Polymer |
anti-REV1/REV3L siRNA & cisplatin |
Subcutaneous LNCaP human prostate tumor |
Extended suppression of targeted genes for 3 days after transfection and induced chemosensitization of MDR cancer cells to platinum treatment |
[110] |
| Polymeric micelles |
anti-survivin siRNA & paclitaxel |
Subcutaneous SKOV3- tr paclitaxel resistant tumor |
Down-regulation of the protein survivin increased sensitization of MDR human ovarian cancer cells in response to paclitaxel |
[104, 105] |
| Polymer |
anti-survivin, Bcl-2 and P-gp siRNA & cisplatin |
Subcutaneous SKOV-3 cisplatin resistant ovarian tumor |
Effective multiple MDR gene silencing and enhanced cisplatin treatment in MDR ovarian cancer |
[102] |
| Nanoparticle | Tumor suppressor miRNA-34a & DOX |
subcutaneous triple negative MDA-MB- 231 human breast cancer |
Restoration of miRNA-34a inhibited NOTCH-1 signaling pathway of angiogenesis and suppressed non-pump resistance and increased antitumor activity of DOX |
[100] |
| Copolymer micelles |
miRNA-21 inhibitor & DOX |
subcutaneous LN229 glioma |
Increased anti-proliferative efficiency via Bcl-2 apoptosis of PI3K phosphorylation pathway |
[103] |
| Liposome | (hTRAIL) DNA & paclitaxel |
Intracranial U-87 MG human glioblastoma cells |
Increased efficacy by deep penetration into interior brain tumor Lower toxicity and longer survival than clinical used Temozolomide |
[106] |
| Dendrimers |
pORF-hTRAIL DNA & DOX |
orthotopic U8 MG human glioma tumor |
DOX synergized with gene hTRAIL to accumulate in brain glioma and to induce apoptosis (with combination index <1) and lower side effects |
[111] |
| Hydrogel |
anti-Akt1 shRNA & paclitaxel |
subcutaneous MDA- MB-231 human breast cancer tumor |
Co-delivery of paclitaxel and anti-Akt1 shRNA arrested cell cycle progression and inhibited angiogenesis |
[101] |
| Nanoparticle |
iMdr-2 shRNA & iSurvivin shRNA |
subcutaneous MCF- 7/ADR resistant human breast tumor |
Co-treatment of DOX and gene therapy combination increased DOX treatment sensitivity and uptake in MDR tumor and altered cancer cell cycle to sub G1 phase. |
[107] |
| Liposome |
anti-cMyc siRNA & apoptosis miRNA-34a |
i.v. injection of lung metastasis murine B16F10 melanoma |
First report of co-delivering RNA- based gene therapy in a single formulation and showed enhanced efficacy against metastasis lung cancer. |
[99] |
Table 4. Examples of nanocarrier-based delivery for combining monoclonal antibody and anticancer drugs.
| Nanoparticles | Antibody & Chemo- Drugs |
Tumor Model | Results | Reference |
|---|---|---|---|---|
| Nanocapsules |
anti-HER2 antibody & DOX & paclitaxel |
Subcutaneous SKBR human breast adenocarcinoma |
pH responsive and HER2 targeting nanoparticles co- encapsulating dual anticancer drugs; enhanced efficacy against human breast cancer |
[117] |
| C225-ILS-DOX (liposome) |
anti-EGFR (HER1) & DOX |
Phase I & II: various tumor overexpressing EGFRs |
Phase I: well tolerated and safe and promising clinical activity for warranting phase II clinical trial |
[122, 123] |
| MM-302 (liposome) |
anti-HER2 scFv & DOX | Subcutaneous BT- 474 M3C5 human breast tumor |
Phase I: safe and signs of clinical activity in metastasized breast cancer patients; Increased bioavailability of DOX in tumor and nucleus compared to non-targeted liposome; Superior antitumor activity and 6- fold greater intracellular uptake in cancer cells |
[121, 124, 128] |
3.2.1 Co-delivery of anti-cancer genes and chemotherapeutic agents
Traditional gene therapy uses a viral vector to deliver nucleic acids including DNA and RNA interference (RNAi) into cancer cells to allow cancer cells to kill themselves or arrest their own growth [91-93]. However, such delivery strategy could have potential risks to patients, such as severe immune system reaction, viral vector antigenicity, and potential virus infection [94]. In addition, fast clearance by the liver and limitation in packaging various genes also prevents cancer gene therapy from translating into an effective clinical treatment [92, 95].
Non-viral nanoparticles allow more nucleic acids packaging and high degree of surface modification which enables the high transfection efficiency for loaded gene via both passive and active targeting cancer cells [96, 97]. Yet, mono-delivery of gene therapy for cancer treatment exhibits only partial and transient antitumor effect. Co-delivery of gene and other anticancer drugs within one nanoparticle hold promise to overcome MDR, promote apoptosis, and inhibit angiogenesis by generating synergistic antitumor effect [98]. Table 3 lists some examples of multifunctional nanoparticle formulations to facilitate gene and anticancer drug combination [99-107].
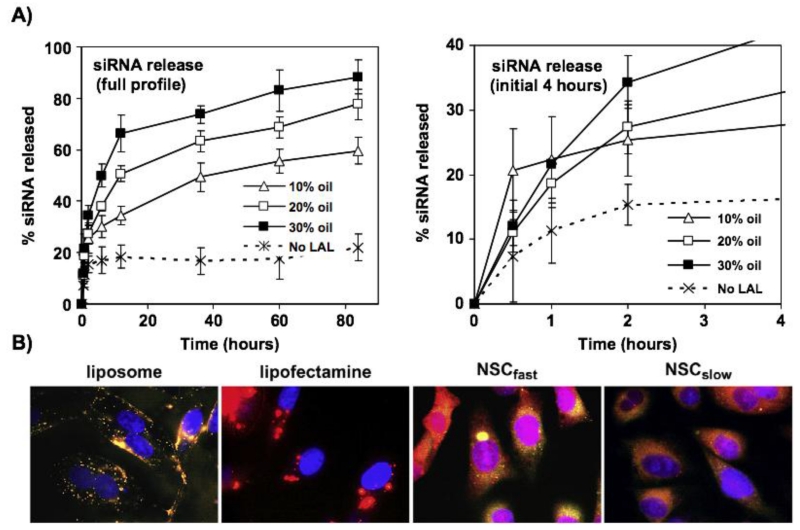
RNAi has been extensively studied in these two decades for anticancer purpose. The key RNAi mediators such as siRNA, short hairpin RNA (shRNA) and microRNA (miRNA) are able to effectively knock down an oncogenic or chemoresistance pathway in a fairly specific and potent manner [97]. Xue and Wong’s study provides an example of siRNA and chemotherapy combinations [108]. Using a lipid-based nanocarrier, it was shown that the chemosensitizing anti-survivin siRNA can be intracellular released in a sustained manner (Figure 7). The extended action of the siRNA made the cancer cells sensitive to anticancer drug docetaxel, leading to significantly improved in vitro and in vivo efficacy.
Figure 7.
Lipid-based nanostructured carriers (NLC) exhibited sustained intracellular release of anti-survivin siRNA in the human refractory prostate PC3 cancer cells. A) Lysosomal acid lipase (LAL) mediated lipid degradation for tailored release of siRNA by manipulating oil content (oleic acid) in NLC formulation; B) Intracellular siRNA and nanocarrier distribution 1 day after transfection in prostate cancer cells. Compared to liposome and lipofectamine, NLC distributed more in cytoplasm. Fluorescent rhodamine siRNA was in red, NBD-PE labeled lipid carriers was in green, and the co-localization of siRNA and nanocarrier appeared to be orange to yellow colors. The cell nuclei were stained with DRAQ5 in blue. The figures are permitted and reproduced from Xue et al. 2011 [108].
Combining miRNA with anticancer drugs has also been shown to be effective in promoting apoptosis, reverting metastasis, and down-regulating MDR-associated efflux transporters [109]. Comparing siRNA that is double stranded and made exogenously to bind precisely onto the targeted mRNA, miRNA is single strand and can non-specifically bind to various part of mRNA to inhibit the translation. Deregulation of miRNA is common in malignant cells. miRNA undergoes genetic alteration through amplification, deletion, and epigenetic changes [97]. For example, overexpression of miRNA-21 plays an important role in oncogenetic activity. Co-loading miRNA-21 inhibitor and DOX in micelles significantly increased antitumor activity in glioma mouse model. Blockage of miRNA-21 facilitated DOX induced apoptosis by suppression of anti-apoptotic Bcl-2 [100, 103].
3.2.2 Combining antibody and chemotherapeutic drugs
Monoclonal antibody (mAb) is a major biological medicine to treat cancer because of its targeting specificity on antigen expressed on the cancer cell surface as well as cytotoxicity via unique signaling pathways [112]. However, mono-treatment of mAb provides only modest therapeutic effect in clinics. Combination of mAb and chemotherapeutic drugs could provide synergistic effect against cancer and prolonged survival rate of patients. For example, combination of anti-HER2 antibody (known as Herceptin®) with anthracycline and cyclophosphamide increased tumor response rate and prolonged progression-free survival in phase III clinical trial [113].
To direct chemotherapeutic drugs to the tumor site and minimize the side effect, drugs can be directly conjugated to mAb, known as antibody-drug conjugates (ADC). ADC takes advantages of mAb specificity to deliver potent anticancer drugs into tumor cells overexpressing targeted surface antigen [114-116]. However, significant morbidity and mortality of ADC toxicity due to systemically delivered chemotherapeutic drugs still exist [112, 113, 115].
Antibody-targeted nanomedicine provides attachment site for specific binding to receptors uniquely expressed on certain cancer cells, thus enhances cytotoxicity of loaded anticancer drugs and circumvent toxicity and instability of chemotherapeutic drugs when exposing to the systemic circulation [117-126]. Yet, reported studies of using mAbs for their therapeutic effect on downstream signaling pathway are limited to anti-HER antibodies [127]. Table 4 shows some examples of nanocarriers that harness the therapeutic effects of both mAb and anticancer drugs. A typical example is MM-302, a trastuzumab conjugated liposomal DOX formulation which showed promising clinical activity and tolerable toxicity in heavily treated metastatic HER2 positive patients in phase I clinic trial [128]. Preclinical study also demonstrated that anti-HER2 antibody targeted nanoparticles can enhance dual loaded anticancer drugs, i.e. DOX and paclitaxel, into HER2 positive breast cancer cells and further enhanced tumor suppression compared to administering anticancer drug alone [117], suggesting the important role of nanoparticles in synergistic drug delivery via active targeted drug delivery.
3.3. Epigenetic therapy combined chemotherapy
In conjunction with accumulation of genetic mutations that permanently alter DNA sequences, epigenetic changes play a crucial and complementary role in gene deregulation in tumorigenesis [129-131]. Epigenetic modifications regulate gene expression patterns (DNA or miRNA) without changing primary nucleic acids sequences via DNA methylation and histone modification, among which DNA hypo- and hyper-methylation is the most stable epigenetic signature associated with MDR in cancer [131]. Hypomethylation of the gene MDR1 promoter in addition to acetylation of histone 3 induce overexpression of P-gp resulting in a broad spectrum of anticancer drug resistance [132]. Hypermethylation of the gene for enzyme sphingomyelinase alters lipid biosynthesis in cancer membrane and reduces the membrane fluidity preventing anticancer drugs from passive diffusion into cancer cells [133].
DNA methyltransferases (DNMT) inhibitors and histone deacetylases (HDAC) inhibitors are two classes of epigenetic drugs used in combination with other anticancer drug to increase sensitivity of MDR tumor in response to chemotherapy [134]. Decitabine (DAC) is a DNMT inhibitor, which exerts its antineoplastic effects via direct incorporation into DNA and inhibition of DNMT, resulting in demethylation of DNA. Epigenetic disruption has been suggested to involve in early stage of breast cancer cell transformation in multidrug resistance [133, 135]. Sequential treatment of DAC and DOX exhibited synergistic anticancer effect against resistant MCF-7/Adr cancer cells via enhanced DOX accumulation. Epigenetic therapy can serve as tumor priming role to facilitate both anticancer drugs and nanoparticle entry into the cancer cells [136, 137].
However, epidrugs are unstable in vivo and has transient antiproliferative effect [138, 139]. To safely and effectively deliver epidrugs, nanocarriers have been employed to extend dosing regimen and improve PK profiles. DAC loaded in 100~ 200 nm nanogel induced sustained depletion of DNMT1 and prolonged arrest of DOX resistant breast cancer cells and DAC-resistant melanoma cells in G2/M cell cycle, which in turn, enhanced antiproliferative effect [137]. Co-loading DAC and DOX into lipid-polymer nanoparticles significantly enhanced the sensitivity of cancer cells to DOX and increased the expression of tumor suppressor genes in MDA-MB-231 breast cancer cell lines [140]. In vivo, the combination of DAC nanoparticles and DOX nanoparticles demonstrated the enhanced apoptotic tumor cells and breast tumor inhibition effect [141]. All these studies show that nanotechnology has ability to improve therapeutic potential of the combination of epigenetic therapy with chemotherapeutic drugs.
4. Technical challenges and limitations
4.1. EPR effect-mediated passive targeting of nanomedicine
Like other non-targeted nanoparticles, drug combination nanomedicine will utilize the EPR effect to preferentially accumulate in tumor tissue [142, 143]. EPR effect-mediated passive targeting of nanoparticles has been demonstrated in numerous preclinical and clinical studies [142], however its extent may vary with the type, location, stage, and treatment history of the tumors. Some solid tumors (e.g. pancreatic cancer) are less vascular where EPR effect may be less significant than others (e.g. glioblastoma). On the other hand, for the same tumor, due to heterogeneous vascular distribution and function and presence of necrotic regions, administered nanoparticles may only reach limited locations. Moreover, tumor angiogenesis and inflammation, the main contributors to leaky blood vessels that allow nanoparticles to permeate into tumor mass, may change with the stage of the tumor. For example, newly developed tumor neovasculature in earlier stage of tumor growth may be leakier than the matured vasculature in later stage.
Poor lymphatic drainage is another factor which is believed to enhance the retention of nanoparticles in solid tumors, a necessary component of EPR effect. Nevertheless, lymphatic drainage may not always be reduced, owing to lymphangiogenesis, especially in aggressive and metastatic tumors. In such tumors, cancer cells can spread through lymph vessels, and so nanoparticles, with much smaller sizes than cells, would not be retained well. Shuhendler et al. revealed that the use of Matrigel, a mouse sarcoma-derived basement membrane protein mixture, in the inoculation of orthotopic human breast cancer xenografts in mice, significantly increased tumor perfusion and lymphatic flow rate and altered architecture of tumor blood and lymph vessels [144]. These changes were found to impact negatively on nanoparticle retention. This finding suggested that variations in lymphatic drainage in tumors could influence passive targeting and efficacy of nanomedicine. In addition, differences in angiogenesis and lymphangiogenesis between xenograft mouse models and human tumors [145] may contribute to unexpected results of nanomedicine in clinical trials.
Moreover, tumor angiogenesis and inflammation which the EPR effect stems from may be affected by treatment history. The emerging trend of combining anti-angiogenic or anti-inflammatory agents with chemotherapy could attenuate the EPR effect. Hence selection of an optimal window to administer nanomedicine is critical. Some cytotoxic agents also exhibit an anti-angiogenic effect. For example, Prasad et al. found that a single treatment with i.v. injection of DOX and MMC co-loaded PLN (DMsPLN) resulted in ~65% reduction in the microvessel density in orthotopic breast tumor xenografts [53]. This anti-angiogenic effect could prevent further tumor uptake of the nanoparticles thus leading to the lack of additional therapeutic effect of consecutive treatments with DMsPLN every four days [53]. The alteration of blood vessel morphology after initial treatment could be a limiting factor for success in applying nanomedicine to humans where multiple treatments are required. Employment of vessel dilating agent, such as nitroglycerin [146] or focused ultrasound [147] may be necessary to enhance nanoparticle extravasation in tumor.
4.2 Drug release kinetics, local bioavailability and pharmacological effect of nanomedicine
It is well known that nanocarriers can alter the pharmacokinetics and toxicity profile of the payload as they can change the circulation time, tissue distribution, metabolism, and elimination of the drug. For simultaneous co-delivery, synchronization of drug release kinetics and co-localization of the drugs in the tumor is critical. In addition to thorough understanding of PK/PB/PD and toxicity of single agent and drug combination, knowledge about in vivo drug release kinetics and local (microscopic and intracellular) bioavailability is important for designing a safe and effective synergistic drug combination nanomedicine. On the one hand, premature drug release in the circulation could reduce efficacy and increase toxicity to normal tissue due to synergistic toxicity or interference in elimination (e.g. DOX and MMC [55], transtuzumab and DOX [148, 149]). On the other hand, excessively slow drug release could diminish the pharmacological effect of the nanomedicine [55, 150]. As the pharmacological effect comes from the released drug in tumor, the total drug (released and unreleased drug) concentration in the tumor may not be predictive of local bioavailability. Therefore, accurate determination of drug release kinetics is critical.
However, measuring drug release kinetics in vivo is extremely challenging due to the difficulty in differentiating released drug from the drug that is still inside the nanoparticles. In special cases when the drug is fluorescent and displays differential intensity in the aggregated state from dissolved state, it is possible to determine in vivo drug release kinetics. Nevertheless, separation of released drug from nanoparticles in tissue samples is a daunting task. Hence, prediction of in vivo release kinetics largely relies on the in vitro measurement, however the method itself is inaccurate [151]. Therefore, it is urgently needed to establish a facile and efficient method to more accurately determine drug release kinetics of nanomedicine [152].
4.3. Side effects of drug combination therapies
One major obstacle of the currently used drug cocktail therapy is the high incidence of adverse effect as a result of drug-drug interactions. It is known that overexpression of the P-gp efflux transporter reduces intracellular drug concentration, thus is one of the main cellular mechanisms contributing to MDR in cancer. To overcome P-gp efflux associated MDR, P-gp inhibitors (i.e. valspodar, tariquidar, laniquidar) have been co-applied with anticancer drugs in many clinical trials. But so far, none of these P-gp inhibitors are approved by US Food and Drug Administration (FDA) because of non-specific toxicity on normal tissues [153]. Drug combination can also potentially enhance the adverse effect caused by the component drugs. For example, in clinic, combination of trastruzumab and DOX significantly increased the risks of cardiac dysfunction [148, 149]. A possible mechanism is that trastuzumab can inhibit dimerization of HER4 and HER2 and further activate angiotensin II pathway, leading to production of toxic reactive oxygen species in cardiomyocytes, which adds on top of DOX induced oxidative stress. Such high oxidative stress activates ASK-1 pathway, resulting in apoptosis of cardiomyocytes and heart failure [149]. To abolish drug-associated toxicity, development of nano-sized drug delivery system that is capable of co-encapsulating versatile drug combinations is needed for more targeting cancer therapy.
However, nanomedicine-based drug combination is not without its drawback. Surface conjugation of antibodies or proteins on the nanoparticles may enhance tumor recognition and binding, but it may increase chances of being removed from the circulation by the RES. For example, nanoparticles (liposome, SLN or nanoparticle coated with chitosan) conjugated with integrin (αvβ3) specific ligand cyclic Arg-Gly-Asp Peptide (cRGD) showed much greater hepatic uptake than non-targeted nanoparticles, resulting in lower tumor accumulation [154-156]. In addition to higher liver uptake of nanoparticles observed with targeting moieties than non-targeted nanoparticles, the difference in targeting receptor expressions between rodents for preclinical studies and humans. For examples, most animal tumor models are xenografts of human tumors grown in immunocompromised mice. So only the tumor expresses humanized receptors and thus humanized antibodies could only target to the tumor, not other tissues. In this case, the treatment results using targeted nanoparticle formulations may overestimate the selectivity. Once administered to human patients, off-target toxicity may amplify due to expression of the same receptors in normal tissues, such as HER2 in the human cardiomyocytes. Thus, potential normal tissue toxicity should be considered thoroughly when designing targeted drug combination nanoparticle formulations.
4.4. Precisely loading and releasing drug combinations at desired ratios
Despite intriguing anticancer effect of drug combination at optimized synergistic ratios, accurately loading drug combination into nanocarriers at desired ratios and releasing them at a synchronized rate within cancer cells are very challenging. However, through thorough understanding of interactions among excipients and drugs, drug distribution inside nanoparticles, and properties of nanoparticle components in relation to their biofate and drug release mechanism, it is possible to engineer nanocarrier to meet the goals. For example, Dicko et al studied the drug-drug and drug-excipient molecular interactions that drive the co-loading process and coordinated release of irinotecan and floxuridine from the liposomal formulation CPX-1 [71]. To co-encapsulate drugs at the desired ratio, irinotecan was loaded via the neutral active antiport by exchanging its proton with triethanolamine buffer across the liposomal membrane, whereas floxuridine was passively loaded into the liposome. Examinations using fluorescence, NMR and UV-Vis revealed that the interplay between copper gluconate buffer and drugs limited the aggregate formation of irinotecan, allowing for slow release of irinotecan together with floxuridine from the liposomes [71].
Polymer-lipid hybrid nanoparticles (PLN) is another example of nanocarrier platform with unique advantages of superior co-loading multiple drugs with different properties [157]. Ability of the precise loading combined drugs at specific ratios into PLN requires manipulation of the amount and types of lipids and polymers, the ratio of lipid and polymer, and initial drug ratio in the feed during PLN formulation design [157, 158]. The optimal formulation can be identified by mathematic modeling (e.g. artificial neural networks) [159]. As the release of the drugs from the PLN is governed by polymer dissolution and lipid erosion process for a given ionic strength, synchronized release rate of co-encapsulated drugs may be obtained [55, 56].
Lysosomal acid lipase (LAL) mediated lipid biodegradation has been utilized to control release of siRNA by tailoring the content and amount of the lipid [108]. The high oil content in the nanocarrier reduced the crystallinity of solid lipid matrix, allowing fast release of siRNA due to a higher rate of LAL mediated lipid degradation [108]. Conjugation of drugs onto the same polymer is an alternative approach to precisely controlling the molar ratio of different drugs and synchronizing their intracellular release via biodegradable polymers and cleavable linkers. For example, a series of xyloglucan (polysaccharide)-DOX/MMC conjugates were prepared at different molar ratios using tripeptide (Gly-Leu-Gly) as the spacer for drug binding. DOX-MMC were then simultaneously released at their conjugated ratios via cleavage of tripeptide linker by lysosomal degradation [160].
5. Conclusions and future perspectives
Cancer is a complex disease that involves multiple origins, molecular pathways, interactions of various factor systems, including gene mutations, metabolic alteration, immune suppression, and epigenetic modifications, in a progressive process. With increasing understanding of this complexity and underlying mechanisms, more rational drug combination therapies will be designed and nanomedicines for synergistic drug combination cocktail will be developed to target different dysfunctional areas in tumor microenvironment, cancer cells, and host. The final goal of nanomedicine for drug combination therapies is to enhance efficacy, reduce normal tissue toxicity, and improve host antitumor immunity. To achieve this goal, a more rational consideration of drug combinations is required at both macro- and microscopic levels. At macroscopic levels, better understanding and prediction of potential in vivo drug-drug interactions and their induced overlapping toxicity are needed, which may be attained by cancer system biology and PKPB modeling of nanoparticle co-loaded drug combination. At microscopic levels, effective intracellular delivery of drug combinations and enhanced local drug bioavailability in tumor ought to be considered in the design of nanomedicine. Finally, acceleration of regulatory approval of nanocarrier-based drug combination formulations is pivotal for scientific translations from bench to bed in treatment of cancer. With integrated knowledge of various fields and efforts of all relevant sectors, new horizon of effective and safe cancer therapies enabled by synergistic drug combination nanomedicine will emerge.
Supplementary Material
Acknowledgments
This research was supported in part by a grant from the Canadian Breast Cancer Foundation-Ontario Region to X.Y. Wu, the University of Toronto Open Scholarship to R.X. Zhang, and by a National Institute of Health/National Cancer Institute R01 grant (grant no. R01CA168917) to H.L. Wong.
Abbreviations
- ADC
Antibody-Drug Conjugate
- Akt
Serine/Threonine-Specific Protein Kinase, or known as Protein Kinase B
- BD
Biodistribution
- CI
Combination Index
- cRGD Peptide
cyclic Arg-Gly-Asp Peptide
- DAC
Decitabine
- DNA
Deoxyribonucleic Acid
- DOX
Doxorubicin
- DMPLN
Doxorubicin and Mitomycin C Co-loaded Polymer-Lipid Hybrid Nanoparticles
- DNMT
DNA Methyltransferases
- EGFR
Epidermal Growth Factor Receptor
- EPR
Enhanced Permeability and Retention Effect
- FDA
Food and Drug Administration
- GSH
Glutathione
- HDAC
Histone Deacetylases
- HER2
Human Epidermal Growth Factor Receptor 2
- LAL
Lysosomal acid lipase
- hTRAIL
Human Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand
- i.v. administration
intravenous administration
- MDR
Multidrug Resistance
- mAb
Monoclonal Antibody
- miRNA
Micro-RNA
- mTOR
Mechanistic Target of Rapamycin
- MMC
Mitomycin C
- P-gp
P-glycoprotein
- PD
Pharmacodynamics
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PK
Pharmacokinetics
- PLD
Pegylated liposomal doxorubicin
- PLN
Polymer-Lipid Hybrid Nanoparticles
- RES
Reticuloendothelial System
- RNA
Ribonucleic Acid
- siRNA
Small Interfering RNA
- shRNA
Small Hairpin RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].American Cancer Society . Global Cancer Facts & Figures. American Cancer Society; Atlanta: 2015. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-044738.pdf. [Google Scholar]
- [2].American Cancer Society . Cancer Facts & Figures 2016. American Cancer Society; Atlanta: 2016. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. [Google Scholar]
- [3].C.C. Statistics, Canadian Cancer Society’s Advisory Committee On Cancer Statistics. in, Canadian Cancer Society, Canadian Cancer Society; 2015. [Google Scholar]
- [4].Chabner BA, Thompson EC. Modalities of Cancer Therapy. Merck Mannual Professional Edition. http://www.merckmanuals.com/professional/hematology-and-oncology/principles-of-cancer-therapy/modalities-of-cancer-therapy.
- [5].Chu E., Jr. Physicians’ Cancer Chemotherapy Drug Mannual. Jones & Bartlett Learning; 2015. [Google Scholar]
- [6].Rini B. Future approaches in immunotherapy. Semin Oncol. 2014;41(Suppl 5):S30–40. doi: 10.1053/j.seminoncol.2014.09.005. [DOI] [PubMed] [Google Scholar]
- [7].Makkouk A, Weiner GJ. Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge. Cancer Res. 2015;75:5–10. doi: 10.1158/0008-5472.CAN-14-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Combination Chemotherapy Regimen by Type of Cancer . Facts and Comparisons. University of Utah Hospitals and Clinics; Salt Lake City, UT: 2001. [Google Scholar]
- [9].Anne DFH, Schott F, Dizon Don S. Systemic Treatment of Metastatic Breast Cancer in Women: Chemotherapy. UpToDate, Inc; 2015. [Google Scholar]
- [10].Carrick S, Parker S, Thornton CE, Ghersi D, Simes J, Wilcken N. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2009:CD003372. doi: 10.1002/14651858.CD003372.pub2. [DOI] [PubMed] [Google Scholar]
- [11].Pinto AC, Moreira JN, Simões R, InTech . Current Cancer Treatment - Novel Beyond Conventional Approaches. 2011. Combination chemotherapy in cancer: Principles, evaluation and drug delivery strategies. [Google Scholar]
- [12].Cowen RL, Garside EJ, Fitzpatrick B, Papadopoulou MV, Williams KJ. Gene therapy approaches to enhance bioreductive drug treatment. Br J Radiol. 2008;81(Spec No 1):S45–56. doi: 10.1259/bjr/55070206. [DOI] [PubMed] [Google Scholar]
- [13].Panowksi S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. MAbs. 2014;6:34–45. doi: 10.4161/mabs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang M, Garbuzenko OB, Reuhl KR, Rodriguez-Rodriguez L, Minko T. Two-in-one: combined targeted chemo and gene therapy for tumor suppression and prevention of metastases. Nanomedicine (Lond) 2012;7:185–197. doi: 10.2217/nnm.11.131. [DOI] [PubMed] [Google Scholar]
- [16].Drake CG. Combination immunotherapy approaches. Ann Oncol. 2012;23(Suppl 8):viii41–46. doi: 10.1093/annonc/mds262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hamilton GS. Antibody-drug conjugates for cancer therapy: The technological and regulatory challenges of developing drug-biologic hybrids. Biologicals. 2015 doi: 10.1016/j.biologicals.2015.05.006. [DOI] [PubMed] [Google Scholar]
- [18].Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- [19].Slovin S. Chemotherapy and immunotherapy combination in advanced prostate cancer. Clin Adv Hematol Oncol. 2012;10:90–100. [PubMed] [Google Scholar]
- [20].Wargo JA, Reuben A, Cooper ZA, Oh KS, Sullivan RJ. Immune Effects of Chemotherapy, Radiation, and Targeted Therapy and Opportunities for Combination With Immunotherapy. Semin Oncol. 2015;42:601–616. doi: 10.1053/j.seminoncol.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Frei EI, Eder J. Holland-Frei Cancer Medicine. 6th edition BC Decker; Hamilton (ON): 2003. [Google Scholar]
- [22].Lee JH, Nan A. Combination drug delivery approaches in metastatic breast cancer. J Drug Deliv. 2012;2012:915375. doi: 10.1155/2012/915375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Waterhouse DN, Gelmon KA, Klasa R, Chi K, Huntsman D, Ramsay E, Bally MB. Development and assessment of conventional and targeted drug combinations for use in the treatment of aggressive breast cancers. Curr Cancer Drug Targets. 2006;6:455–489. doi: 10.2174/156800906778194586. [DOI] [PubMed] [Google Scholar]
- [24].Yhee JY, Son S, Lee H, Kim K. Nanoparticle-Based Combination Therapy for Cancer Treatment. Curr Pharm Des. 2015;21:3158–3166. doi: 10.2174/1381612821666150531165059. [DOI] [PubMed] [Google Scholar]
- [25].Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- [26].Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol Med. 2015;21:223–232. doi: 10.1016/j.molmed.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wong HL, Bendayan R, Rauth AM, Wu XY. Development of solid lipid nanoparticles containing ionically complexed chemotherapeutic drugs and chemosensitizers. J Pharm Sci. 2004;93:1993–2008. doi: 10.1002/jps.20100. [DOI] [PubMed] [Google Scholar]
- [28].Liu Z, Ballinger JR, Rauth AM, Bendayan R, Wu XY. Delivery of an anticancer drug and a chemosensitizer to murine breast sarcoma by intratumoral injection of sulfopropyl dextran microspheres. J Pharm Pharmacol. 2003;55:1063–1073. doi: 10.1211/0022357021567. [DOI] [PubMed] [Google Scholar]
- [29].Liu Z, Wu XY, Bendayan R. In vitro investigation of ionic polysaccharide microspheres for simultaneous delivery of chemosensitizer and antineoplastic agent to multidrug-resistant cells. J Pharm Sci. 1999;88:412–418. doi: 10.1021/js9803353. [DOI] [PubMed] [Google Scholar]
- [30].Wong HL, Bendayan R, Rauth AM, Wu XY. Simultaneous delivery of doxorubicin and GG918 (Elacridar) by new polymer-lipid hybrid nanoparticles (PLN) for enhanced treatment of multidrug-resistant breast cancer. J Control Release. 2006;116:275–284. doi: 10.1016/j.jconrel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- [31].Soma CE, Dubernet C, Bentolila D, Benita S, Couvreur P. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin A in polyalkylcyanoacrylate nanoparticles. Biomaterials. 2000;21:1–7. doi: 10.1016/s0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- [32].Batist G, Gelmon KA, Chi KN, Miller WH, Jr., Chia SK, Mayer LD, Louie AC. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15:692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- [33].Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF, Louie AC. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hasenstein JR, Shin HC, Kasmerchak K, Buehler D, Kwon GS, Kozak KR. Antitumor activity of Triolimus: a novel multidrug-loaded micelle containing Paclitaxel, Rapamycin, and 17-AAG. Mol Cancer Ther. 2012;11:2233–2242. doi: 10.1158/1535-7163.MCT-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shin HC, Alani AW, Cho H, Bae Y, Kolesar JM, Kwon GS. A 3-in-1 polymeric micelle nanocontainer for poorly water-soluble drugs. Mol Pharm. 2011;8:1257–1265. doi: 10.1021/mp2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shin HC, Cho H, Lai TC, Kozak KR, Kolesar JM, Kwon GS. Pharmacokinetic study of 3-in-1 poly(ethylene glycol)-block-poly(D, L-lactic acid) micelles carrying paclitaxel, 17-allylamino-17-demethoxygeldanamycin, and rapamycin. J Control Release. 2012;163:93–99. doi: 10.1016/j.jconrel.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Therapeutics C-D. Co-D Therapeutics. 2013 http://co-drx.com/pipeline/
- [38].Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, Mayer L. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33:129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- [39].Shuhendler AJ, O’Brien PJ, Rauth AM, Wu XY. On the synergistic effect of doxorubicin and mitomycin C against breast cancer cells. Drug Metabol Drug Interact. 2007;22:201–233. doi: 10.1515/dmdi.2007.22.4.201. [DOI] [PubMed] [Google Scholar]
- [40].Cardoso F, Bedard PL, Winer EP, Pagani O, Senkus-Konefka E, Fallowfield LJ, Force E-MT. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101:1174–1181. doi: 10.1093/jnci/djp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Breast Cancer (Invasive) Treatment Regimens. Haymarket Media, Inc; 2015. [Google Scholar]
- [42].Alba E, Martin M, Ramos M, Adrover E, Balil A, Jara C, G. Spanish Breast Cancer Research Multicenter randomized trial comparing sequential with concomitant administration of doxorubicin and docetaxel as first-line treatment of metastatic breast cancer: a Spanish Breast Cancer Research Group (GEICAM-9903) phase III study. J Clin Oncol. 2004;22:2587–2593. doi: 10.1200/JCO.2004.08.125. [DOI] [PubMed] [Google Scholar]
- [43].Joensuu H, Holli K, Heikkinen M, Suonio E, Aro AR, Hietanen P, Huovinen R. Combination chemotherapy versus single-agent therapy as first- and second-line treatment in metastatic breast cancer: a prospective randomized trial. J Clin Oncol. 1998;16:3720–3730. doi: 10.1200/JCO.1998.16.12.3720. [DOI] [PubMed] [Google Scholar]
- [44].Cancello G, Bagnardi V, Sangalli C, Montagna E, Dellapasqua S, Sporchia A, Colleoni M. Phase II Study With Epirubicin, Cisplatin, and Infusional Fluorouracil Followed by Weekly Paclitaxel With Metronomic Cyclophosphamide as a Preoperative Treatment of Triple-Negative Breast Cancer. Clin Breast Cancer. 2015 doi: 10.1016/j.clbc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- [45].Masuda N, Higaki K, Takano T, Matsunami N, Morimoto T, Ohtani S, Toi M. A phase II study of metronomic paclitaxel/cyclophosphamide/capecitabine followed by 5-fluorouracil/epirubicin/cyclophosphamide as preoperative chemotherapy for triple-negative or low hormone receptor expressing/HER2-negative primary breast cancer. Cancer Chemother Pharmacol. 2014;74:229–238. doi: 10.1007/s00280-014-2492-y. [DOI] [PubMed] [Google Scholar]
- [46].Megerdichian C, Olimpiadi Y, Hurvitz SA. nab-Paclitaxel in combination with biologically targeted agents for early and metastatic breast cancer. Cancer Treat Rev. 2014;40:614–625. doi: 10.1016/j.ctrv.2014.02.001. [DOI] [PubMed] [Google Scholar]
- [47].Pignata S, Lauraine EP, du Bois A, Pisano C. Pegylated liposomal doxorubicin combined with carboplatin: a rational treatment choice for advanced ovarian cancer. Crit Rev Oncol Hematol. 2010;73:23–30. doi: 10.1016/j.critrevonc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [48].Pandey A, Kulkarni A, Roy B, Goldman A, Sarangi S, Sengupta P, Sengupta S. Sequential application of a cytotoxic nanoparticle and a PI3K inhibitor enhances antitumor efficacy. Cancer Res. 2014;74:675–685. doi: 10.1158/0008-5472.CAN-12-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Harasym TO, Tardi PG, Harasym NL, Harvie P, Johnstone SA, Mayer LD. Increased preclinical efficacy of irinotecan and floxuridine coencapsulated inside liposomes is associated with tumor delivery of synergistic drug ratios. Oncol Res. 2007;16:361–374. doi: 10.3727/000000006783980937. [DOI] [PubMed] [Google Scholar]
- [50].Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Janoff AS. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- [51].Tardi PG, Dos Santos N, Harasym TO, Johnstone SA, Zisman N, Tsang AW, Mayer LD. Drug ratio-dependent antitumor activity of irinotecan and cisplatin combinations in vitro and in vivo. Mol Cancer Ther. 2009;8:2266–2275. doi: 10.1158/1535-7163.MCT-09-0243. [DOI] [PubMed] [Google Scholar]
- [52].Prasad P, Cheng J, Shuhendler A, Rauth AM, Wu XY. A novel nanoparticle formulation overcomes multiple types of membrane efflux pumps in human breast cancer cells. Drug Deliv Transl Res. 2012;2:95–105. doi: 10.1007/s13346-011-0051-1. [DOI] [PubMed] [Google Scholar]
- [53].Prasad P, Shuhendler A, Cai P, Rauth AM, Wu XY. Doxorubicin and mitomycin C co-loaded polymer-lipid hybrid nanoparticles inhibit growth of sensitive and multidrug resistant human mammary tumor xenografts. Cancer Lett. 2013;334:263–273. doi: 10.1016/j.canlet.2012.08.008. [DOI] [PubMed] [Google Scholar]
- [54].Shuhendler AJ, Prasad P, Zhang RX, Amini MA, Sun M, Liu PP, Wu XY. Synergistic nanoparticulate drug combination overcomes multidrug resistance, increases efficacy, and reduces cardiotoxicity in a nonimmunocompromised breast tumor model. Mol Pharm. 2014;11:2659–2674. doi: 10.1021/mp500093c. [DOI] [PubMed] [Google Scholar]
- [55].Zhang RX, Cai P, Zhang T, Chen K, Li J, Cheng J, Wu XY. Polymer-Lipid Hybrid Nanoparticles Synchronize Pharmacokinetics of Co-encapsulated Doxorubicin-Mitomycin C and Enable Their Spatiotemporal Co-delivery and Local Bioavailability in Breast Tumor. Nanomedicine. 2016 doi: 10.1016/j.nano.2015.12.383. [DOI] [PubMed] [Google Scholar]
- [56].Shuhendler AJ, Cheung RY, Manias J, Connor A, Rauth AM, Wu XY. A novel doxorubicin-mitomycin C co-encapsulated nanoparticle formulation exhibits anti-cancer synergy in multidrug resistant human breast cancer cells. Breast Cancer Res Treat. 2010;119:255–269. doi: 10.1007/s10549-008-0271-3. [DOI] [PubMed] [Google Scholar]
- [57].Cheung RY. A study of alternative delivery strategies and systems to enhance the therapeutic effect and cytotoxic activity of anticancer agents. University of Toronto; 2005. PhD Desertation. [Google Scholar]
- [58].Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- [59].Jhaveri A, Deshpande P, Torchilin V. Stimuli-sensitive nanopreparations for combination cancer therapy. J Control Release. 2014;190:352–370. doi: 10.1016/j.jconrel.2014.05.002. [DOI] [PubMed] [Google Scholar]
- [60].Cheung RY, Rauth AM, Ronaldson PT, Bendayan R, Wu XY. In vitro toxicity to breast cancer cells of microsphere-delivered mitomycin C and its combination with doxorubicin. Eur J Pharm Biopharm. 2006;62:321–331. doi: 10.1016/j.ejpb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- [61].Wong HL, Shen Z, Lu Z, Wientjes MG, Au JL. Paclitaxel tumor-priming enhances siRNA delivery and transfection in 3-dimensional tumor cultures. Mol Pharm. 2011;8:833–840. doi: 10.1021/mp1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang J, Lu Z, Gao Y, Wientjes MG, Au JL. Improving delivery and efficacy of nanomedicines in solid tumors: role of tumor priming. Nanomedicine (Lond) 2011;6:1605–1620. doi: 10.2217/nnm.11.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang G, Park MA, Mitchell C, Hamed H, Rahmani M, Martin AP, Dent P. Vorinostat and sorafenib synergistically kill tumor cells via FLIP suppression and CD95 activation. Clin Cancer Res. 2008;14:5385–5399. doi: 10.1158/1078-0432.CCR-08-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Narvekar M, Xue HY, Eoh JY, Wong HL. Nanocarrier for poorly water-soluble anticancer drugs--barriers of translation and solutions. AAPS PharmSciTech. 2014;15:822–833. doi: 10.1208/s12249-014-0107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Xue HY, Guo P, Wen WC, Wong HL. Lipid-Based Nanocarriers for RNA Delivery. Curr Pharm Des. 2015;21:3140–3147. doi: 10.2174/1381612821666150531164540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kummar S, Chen HX, Wright J, Holbeck S, Millin MD, Tomaszewski J, Doroshow JH. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements. Nat Rev Drug Discov. 2010;9:843–856. doi: 10.1038/nrd3216. [DOI] [PubMed] [Google Scholar]
- [67].Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- [68].Xiao H, Li W, Qi R, Yan L, Wang R, Liu S, Jing X. Co-delivery of daunomycin and oxaliplatin by biodegradable polymers for safer and more efficacious combination therapy. J Control Release. 2012;163:304–314. doi: 10.1016/j.jconrel.2012.06.004. [DOI] [PubMed] [Google Scholar]
- [69].Batist G, Sawyer M, Gabrail N, Christiansen N, Marshall JL, Spigel DR, Louie A. A multicenter, phase II study of CPX-1 liposome injection in patients (pts) with advanced colorectal cancer (CRC) Journal of Clinical Oncology. 2008;26 [Google Scholar]
- [70].Blanco E, Sangai T, Wu S, Hsiao A, Ruiz-Esparza GU, Gonzalez-Delgado CA, Ferrari M. Colocalized delivery of rapamycin and paclitaxel to tumors enhances synergistic targeting of the PI3K/Akt/mTOR pathway. Mol Ther. 2014;22:1310–1319. doi: 10.1038/mt.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dicko A, Frazier AA, Liboiron BD, Hinderliter A, Ellena JF, Xie X, Mayer LD. Intra and inter-molecular interactions dictate the aggregation state of irinotecan co-encapsulated with floxuridine inside liposomes. Pharm Res. 2008;25:1702–1713. doi: 10.1007/s11095-008-9561-z. [DOI] [PubMed] [Google Scholar]
- [72].Duan X, Xiao J, Yin Q, Zhang Z, Yu H, Mao S, Li Y. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano. 2013;7:5858–5869. doi: 10.1021/nn4010796. [DOI] [PubMed] [Google Scholar]
- [73].Fang JH, Lai YH, Chiu TL, Chen YY, Hu SH, Chen SY. Magnetic core-shell nanocapsules with dual-targeting capabilities and co-delivery of multiple drugs to treat brain gliomas. Adv Healthc Mater. 2014;3:1250–1260. doi: 10.1002/adhm.201300598. [DOI] [PubMed] [Google Scholar]
- [74].Lammers T, Subr V, Ulbrich K, Peschke P, Huber PE, Hennink WE, Storm G. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials. 2009;30:3466–3475. doi: 10.1016/j.biomaterials.2009.02.040. [DOI] [PubMed] [Google Scholar]
- [75].Milane L, Duan ZF, Amiji M. Pharmacokinetics and biodistribution of lonidamine/paclitaxel loaded, EGFR-targeted nanoparticles in an orthotopic animal model of multi-drug resistant breast cancer. Nanomedicine. 2011;7:435–444. doi: 10.1016/j.nano.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Poon C, He C, Liu D, Lu K, Lin W. Self-assembled nanoscale coordination polymers carrying oxaliplatin and gemcitabine for synergistic combination therapy of pancreatic cancer. J Control Release. 2015;201:90–99. doi: 10.1016/j.jconrel.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Schmid D, Jarvis GE, Fay F, Small DM, Greene MK, Majkut J, Scott CJ. Nanoencapsulation of ABT-737 and camptothecin enhances their clinical potential through synergistic antitumor effects and reduction of systemic toxicity. Cell Death Dis. 2014;5:e1454. doi: 10.1038/cddis.2014.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- [79].Wang W, Xi M, Duan X, Wang Y, Kong F. Delivery of baicalein and paclitaxel using self-assembled nanoparticles: synergistic antitumor effect in vitro and in vivo. Int J Nanomedicine. 2015;10:3737–3750. doi: 10.2147/IJN.S80297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cai L, Xu G, Shi C, Guo D, Wang X, Luo J. Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: A synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials. 2015;37:456–468. doi: 10.1016/j.biomaterials.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Celator Pharmaceuticals 2012 ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01696084?term=CPX-351+Phase+3&rank=1, in, https://clinicaltrials.gov/ct2/show/NCT01696084?term=CPX-351+Phase+3&rank=1.
- [82].Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, Bixby DL. Final Results of a Phase III Randomized Trial of CPX-351 versus 7+3 in Older Patients with Newly Diagnosed High Risk (secondary) AML (abstract # 7000) American Society of Clinical Oncology (ASCO); 2016. [Google Scholar]
- [83].Dilnawaz F, Singh A, Mohanty C, Sahoo SK. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials. 2010;31:3694–3706. doi: 10.1016/j.biomaterials.2010.01.057. [DOI] [PubMed] [Google Scholar]
- [84].Liu Q, Zhang J, Sun W, Xie QR, Xia W, Gu H. Delivering hydrophilic and hydrophobic chemotherapeutics simultaneously by magnetic mesoporous silica nanoparticles to inhibit cancer cells. Int J Nanomedicine. 2012;7:999–1013. doi: 10.2147/IJN.S28088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Singh A, Dilnawaz F, Mewar S, Sharma U, Jagannathan NR, Sahoo SK. Composite polymeric magnetic nanoparticles for co-delivery of hydrophobic and hydrophilic anticancer drugs and MRI imaging for cancer therapy. ACS Appl Mater Interfaces. 2011;3:842–856. doi: 10.1021/am101196v. [DOI] [PubMed] [Google Scholar]
- [86].Milane L, Ganesh S, Shah S, Duan ZF, Amiji M. Multi-modal strategies for overcoming tumor drug resistance: hypoxia, the Warburg effect, stem cells, and multifunctional nanotechnology. J Control Release. 2011;155:237–247. doi: 10.1016/j.jconrel.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Huang Y, Cai T, Xia X, Cai Y, Wu XY. Research Advances in the Intervention of Inflammation and Cancer by Active Ingredients of Traditional Chinese Medicine. J Pharm Pharm Sci. 2016;19:114–126. doi: 10.18433/J3SG7K. [DOI] [PubMed] [Google Scholar]
- [88].Cragg GM, Grothaus PG, Newman DJ. New horizons for old drugs and drug leads. J Nat Prod. 2014;77:703–723. doi: 10.1021/np5000796. [DOI] [PubMed] [Google Scholar]
- [89].Tran TH, Bae BC, Lee YK, Na K, Huh KM. Heparin-folate-retinoic acid bioconjugates for targeted delivery of hydrophobic photosensitizers. Carbohydr Polym. 2013;92:1615–1624. doi: 10.1016/j.carbpol.2012.10.075. [DOI] [PubMed] [Google Scholar]
- [90].Yang CS, Wang H, Hu B. Combination of chemopreventive agents in nanoparticles for cancer prevention. Cancer Prev Res (Phila) 2013;6:1011–1014. doi: 10.1158/1940-6207.CAPR-13-0312. [DOI] [PubMed] [Google Scholar]
- [91].Kirtane AR, Panyam J. Polymer nanoparticles: Weighing up gene delivery. Nat Nanotechnol. 2013;8:805–806. doi: 10.1038/nnano.2013.234. [DOI] [PubMed] [Google Scholar]
- [92].McCormick F. Cancer gene therapy: fringe or cutting edge? Nat Rev Cancer. 2001;1:130–141. doi: 10.1038/35101008. [DOI] [PubMed] [Google Scholar]
- [93].Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mayo Clinic, Mayo Clinic Gene Therapy. 2015 http://www.mayoclinic.org/tests-procedures/gene-therapy/basics/risks/prc-20014778.
- [95].Lin G, Zhang H, Huang L. Smart polymeric nanoparticles for cancer gene delivery. Mol Pharm. 2015;12:314–321. doi: 10.1021/mp500656v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Choi YS, Lee MY, David AE. Nanoparticles for gene delivery: therapeutic and toxic effects. Mol Cell Toxicol. 2014;10:1–8. Y.S. [Google Scholar]
- [97].Iyer AK, Duan Z, Amiji MM. Nanodelivery systems for nucleic acid therapeutics in drug resistant tumors. Mol Pharm. 2014;11:2511–2526. doi: 10.1021/mp500024p. [DOI] [PubMed] [Google Scholar]
- [98].Li J, Wang Y, Zhu Y, Oupicky D. Recent advances in delivery of drug-nucleic acid combinations for cancer treatment. J Control Release. 2013;172:589–600. doi: 10.1016/j.jconrel.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Ther. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z, Zeng Y. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35:4333–4344. doi: 10.1016/j.biomaterials.2014.02.006. [DOI] [PubMed] [Google Scholar]
- [101].Guo DD, Hong SH, Jiang HL, Kim JH, Minai-Tehrani A, Kim JE, Cho MH. Synergistic effects of Akt1 shRNA and paclitaxel-incorporated conjugated linoleic acid-coupled poloxamer thermosensitive hydrogel on breast cancer. Biomaterials. 2012;33:2272–2281. doi: 10.1016/j.biomaterials.2011.12.011. [DOI] [PubMed] [Google Scholar]
- [102].He C, Liu D, Lin W. Self-assembled nanoscale coordination polymers carrying siRNAs and cisplatin for effective treatment of resistant ovarian cancer. Biomaterials. 2015;36:124–133. doi: 10.1016/j.biomaterials.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Qian X, Long L, Shi Z, Liu C, Qiu M, Sheng J, Kang C. Star-branched amphiphilic PLA-b-PDMAEMA copolymers for co-delivery of miR-21 inhibitor and doxorubicin to treat glioma. Biomaterials. 2014;35:2322–2335. doi: 10.1016/j.biomaterials.2013.11.039. [DOI] [PubMed] [Google Scholar]
- [104].Salzano G, Navarro G, Trivedi MS, De Rosa G, Torchilin VP. Multifunctional Polymeric Micelles Co-loaded with Anti-Survivin siRNA and Paclitaxel Overcome Drug Resistance in an Animal Model of Ovarian Cancer. Mol Cancer Ther. 2015;14:1075–1084. doi: 10.1158/1535-7163.MCT-14-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Salzano G, Riehle R, Navarro G, Perche F, De Rosa G, Torchilin VP. Polymeric micelles containing reversibly phospholipid-modified anti-survivin siRNA: a promising strategy to overcome drug resistance in cancer. Cancer Lett. 2014;343:224–231. doi: 10.1016/j.canlet.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Sun X, Pang Z, Ye H, Qiu B, Guo L, Li J, Jiang X. Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials. 2012;33:916–924. doi: 10.1016/j.biomaterials.2011.10.035. [DOI] [PubMed] [Google Scholar]
- [107].Yin Q, Shen J, Chen L, Zhang Z, Gu W, Li Y. Overcoming multidrug resistance by co-delivery of Mdr-1 and survivin-targeting RNA with reduction-responsible cationic poly(beta-amino esters) Biomaterials. 2012;33:6495–6506. doi: 10.1016/j.biomaterials.2012.05.039. [DOI] [PubMed] [Google Scholar]
- [108].Xue HY, Wong HL. Tailoring nanostructured solid-lipid carriers for time-controlled intracellular siRNA kinetics to sustain RNAi-mediated chemosensitization. Biomaterials. 2011;32:2662–2672. doi: 10.1016/j.biomaterials.2010.12.029. [DOI] [PubMed] [Google Scholar]
- [109].Dai X, Tan C. Combination of microRNA therapeutics with small-molecule anticancer drugs: mechanism of action and co-delivery nanocarriers. Adv Drug Deliv Rev. 2015;81:184–197. doi: 10.1016/j.addr.2014.09.010. [DOI] [PubMed] [Google Scholar]
- [110].Xu X, Xie K, Zhang XQ, Pridgen EM, Park GY, Cui DS, Farokhzad OC. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proc Natl Acad Sci U S A. 2013;110:18638–18643. doi: 10.1073/pnas.1303958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Liu S, Guo Y, Huang R, Li J, Huang S, Kuang Y, Jiang C. Gene and doxorubicin co-delivery system for targeting therapy of glioma. Biomaterials. 2012;33:4907–4916. doi: 10.1016/j.biomaterials.2012.03.031. [DOI] [PubMed] [Google Scholar]
- [112].Goodall S, Jones ML, Mahler S. Monoclonal antibody-targeted polymeric nanoparticles for cancer therapy - future prospects. J Chem Technol Biotechnol. 2015;90:1169–1176. [Google Scholar]
- [113].Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- [114].Mullard A. 2013 FDA drug approvals. Nat Rev Drug Discov. 2014;13:85–89. doi: 10.1038/nrd4239. [DOI] [PubMed] [Google Scholar]
- [115].Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, Borzilleri RM. Antibody-drug conjugates: current status and future directions. Drug Discov Today. 2014;19:869–881. doi: 10.1016/j.drudis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- [116].Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Chen R. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Chiang CS, Hu SH, Liao BJ, Chang YC, Chen SY. Enhancement of cancer therapy efficacy by trastuzumab-conjugated and pH-sensitive nanocapsules with the simultaneous encapsulation of hydrophilic and hydrophobic compounds. Nanomedicine. 2014;10:99–107. doi: 10.1016/j.nano.2013.07.009. [DOI] [PubMed] [Google Scholar]
- [118].ElBayoumi TA, Torchilin VP. Tumor-targeted nanomedicines: enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin Cancer Res. 2009;15:1973–1980. doi: 10.1158/1078-0432.CCR-08-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hatakeyama H, Akita H, Ishida E, Hashimoto K, Kobayashi H, Aoki T, Harashima H. Tumor targeting of doxorubicin by anti-MT1-MMP antibody-modified PEG liposomes. Int J Pharm. 2007;342:194–200. doi: 10.1016/j.ijpharm.2007.04.037. [DOI] [PubMed] [Google Scholar]
- [120].Jin C, Qian N, Zhao W, Yang W, Bai L, Wu H, Dou K. Improved therapeutic effect of DOX-PLGA-PEG micelles decorated with bivalent fragment HAb18 F(ab’)(2) for hepatocellular carcinoma. Biomacromolecules. 2010;11:2422–2431. doi: 10.1021/bm1005992. [DOI] [PubMed] [Google Scholar]
- [121].Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- [122].Mamot C, Ritschard R, Wicki A, Kung W, Schuller J, Herrmann R, Rochlitz C. Immunoliposomal delivery of doxorubicin can overcome multidrug resistance mechanisms in EGFR-overexpressing tumor cells. J Drug Target. 2012;20:422–432. doi: 10.3109/1061186X.2012.680960. [DOI] [PubMed] [Google Scholar]
- [123].Mamot C, Ritschard R, Wicki A, Stehle G, Dieterle T, Bubendorf L, Rochlitz C. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: a phase 1 dose-escalation study. Lancet Oncol. 2012;13:1234–1241. doi: 10.1016/S1470-2045(12)70476-X. [DOI] [PubMed] [Google Scholar]
- [124].Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Benz CC. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8:1172–1181. [PubMed] [Google Scholar]
- [125].Qiang J, Mitchell CE, Venturini M. Suppression of microbunching instability using bending magnets in free-electron-laser linacs. Phys Rev Lett. 2013;111:054801. doi: 10.1103/PhysRevLett.111.054801. [DOI] [PubMed] [Google Scholar]
- [126].Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci U S A. 2003;100:6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].van der Meel R, Vehmeijer LJ, Kok RJ, Storm G, van Gaal EV. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status. Adv Drug Deliv Rev. 2013;65:1284–1298. doi: 10.1016/j.addr.2013.08.012. [DOI] [PubMed] [Google Scholar]
- [128].AACR. American Association For Cancer Research . New Antibody-Drug Conjugate Shows Early Promise for Patients With Metastatic HER2-positive Breast Cancer. 2015. [Google Scholar]
- [129].Beyond the genome (Editorial Article) Nature. 2015;518:273. [Google Scholar]
- [130].Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- [131].Sandoval J, Esteller M. Cancer epigenomics: beyond genomics. Curr Opin Genet Dev. 2012;22:50–55. doi: 10.1016/j.gde.2012.02.008. [DOI] [PubMed] [Google Scholar]
- [132].Chen KG, Sikic BI. Molecular pathways: regulation and therapeutic implications of multidrug resistance. Clin Cancer Res. 2012;18:1863–1869. doi: 10.1158/1078-0432.CCR-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Vijayaraghavalu S, Peetla C, Lu S, Labhasetwar V. Epigenetic modulation of the biophysical properties of drug-resistant cell lipids to restore drug transport and endocytic functions. Mol Pharm. 2012;9:2730–2742. doi: 10.1021/mp300281t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hamm CA, Costa FF. Epigenomes as therapeutic targets. Pharmacol Ther. 2015;151:72–86. doi: 10.1016/j.pharmthera.2015.03.003. [DOI] [PubMed] [Google Scholar]
- [135].Lu S, Labhasetwar V. Drug Resistant Breast Cancer Cell Line Displays Cancer Stem Cell Phenotype and Responds Sensitively to Epigenetic Drug SAHA. Drug Deliv Transl Res. 2013;3:183–194. doi: 10.1007/s13346-012-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Stewart DJ, Nunez MI, Jelinek J, Hong D, Gupta S, Issa JP, Kurzrock R. Decitabine impact on the endocytosis regulator RhoA, the folate carriers RFC1 and FOLR1, and the glucose transporter GLUT4 in human tumors. Clin Epigenetics. 2014;6:2. doi: 10.1186/1868-7083-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Vijayaraghavalu S, Dermawan JK, Cheriyath V, Labhasetwar V. Highly synergistic effect of sequential treatment with epigenetic and anticancer drugs to overcome drug resistance in breast cancer cells is mediated via activation of p21 gene expression leading to G2/M cycle arrest. Mol Pharm. 2013;10:337–352. doi: 10.1021/mp3004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Cramer SA, Adjei IM, Labhasetwar V. Advancements in the delivery of epigenetic drugs. Expert Opin Drug Deliv. 2015;12:1501–1512. doi: 10.1517/17425247.2015.1021678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Ivanov M, Barragan I, Ingelman-Sundberg M. Epigenetic mechanisms of importance for drug treatment. Trends Pharmacol Sci. 2014;35:384–396. doi: 10.1016/j.tips.2014.05.004. [DOI] [PubMed] [Google Scholar]
- [140].Su X, Wang Z, Li L, Zheng M, Zheng C, Gong P, Cai L. Lipid-polymer nanoparticles encapsulating doxorubicin and 2′-deoxy-5-azacytidine enhance the sensitivity of cancer cells to chemical therapeutics. Mol Pharm. 2013;10:1901–1909. doi: 10.1021/mp300675c. [DOI] [PubMed] [Google Scholar]
- [141].Li SY, Sun R, Wang HX, Shen S, Liu Y, Du XJ, Jun W. Combination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cells. J Control Release. 2015;205:7–14. doi: 10.1016/j.jconrel.2014.11.011. [DOI] [PubMed] [Google Scholar]
- [142].Maeda H, Tsukigawa K, Fang J. A Retrospective 30 Years After Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy—Problems, Solutions, and Prospects. Microcirculation. 2015;00 doi: 10.1111/micc.12228. [DOI] [PubMed] [Google Scholar]
- [143].Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- [144].Shuhendler AJ, Prasad P, Cai P, Hui KK, Henderson JT, Rauth AM, Wu XY. Matrigel alters the pathophysiology of orthotopic human breast adenocarcinoma xenografts with implications for nanomedicine evaluation. Nanomedicine. 2013;9:795–805. doi: 10.1016/j.nano.2013.01.005. [DOI] [PubMed] [Google Scholar]
- [145].Eklunda L, Bryb M, Alitalob K. Mouse Models for studying angiogenesis and lymphangiogenesis in cancer. Mol Oncol. 2013;7:259–282. doi: 10.1016/j.molonc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- [147].Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol. 2012;38:1716–1725. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wadhwa D, Fallah-Rad N, Grenier D, Krahn M, Fang T, Ahmadie R, Jassal DS. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: a retrospective study. Breast Cancer Res Treat. 2009;117:357–364. doi: 10.1007/s10549-008-0260-6. [DOI] [PubMed] [Google Scholar]
- [149].Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab-induced cardiac dysfunction: A ‘dual-hit’. Exp Clin Cardiol. 2011;16:70–74. [PMC free article] [PubMed] [Google Scholar]
- [150].O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, C.B.C.S. Group Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- [151].Wu XY. Are we really measuring drug release from nanoparticles? - An overview of challenges and future perspectives, Challenges and Future Perspectives of Accurate Determination of Drug Release from Nanoparticles In Vitro and In Vivo. AAPS Roundtable Session; Washington DC: 2011. [Google Scholar]
- [152].Zhou Y, Abbasi AZ, He C, Ni J, Cai Y, Guo X, Wu XY. A simple and effective numerical method for quantifying drug release from nano-/microparticles inside dialysis tubing; 42nd Symposium of Controlled Release Society; 2015. [Google Scholar]
- [153].Binkhathlan Z, Lavasanifar A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: current status and future perspectives. Curr Cancer Drug Targets. 2013;13:326–346. doi: 10.2174/15680096113139990076. [DOI] [PubMed] [Google Scholar]
- [154].Hu G, Lijowski M, Zhang H, Partlow KC, Caruthers SD, Kiefer G, Lanza GM. Imaging of Vx-2 rabbit tumors with alpha(nu)beta3-integrin-targeted 111In nanoparticles. Int J Cancer. 2007;120:1951–1957. doi: 10.1002/ijc.22581. [DOI] [PubMed] [Google Scholar]
- [155].Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. Multivalent effects of RGD peptides obtained by nanoparticle display. J Med Chem. 2006;49:6087–6093. doi: 10.1021/jm060515m. [DOI] [PubMed] [Google Scholar]
- [156].Shuhendler AJ, Prasad P, Leung M, Rauth AM, Dacosta RS, Wu XY. A novel solid lipid nanoparticle formulation for active targeting to tumor alpha(v) beta(3) integrin receptors reveals cyclic RGD as a double-edged sword. Adv Healthc Mater. 2012;1:600–608. doi: 10.1002/adhm.201200006. [DOI] [PubMed] [Google Scholar]
- [157].Wu XY. Strategies for optimizing polymer-lipid hybrid nanoparticle-mediated drug delivery. Expert Opin Drug Deliv. 2016 doi: 10.1517/17425247.2016.1165662. [DOI] [PubMed] [Google Scholar]
- [158].Li Y, Taulier N, Rauth AM, Wu XY. Screening of lipid carriers and characterization of drug-polymer-lipid interactions for the rational design of polymer-lipid hybrid nanoparticles (PLN) Pharm Res. 2006;23:1877–1887. doi: 10.1007/s11095-006-9033-2. [DOI] [PubMed] [Google Scholar]
- [159].Li Y, Abbaspour MR, Grootendorst PV, Rauth AM, Wu XY. Optimization of controlled release nanoparticle formulation of verapamil hydrochloride using artificial neural networks with genetic algorithm and response surface methodology. Eur J Pharm Biopharm. 2015;94:170–179. doi: 10.1016/j.ejpb.2015.04.028. [DOI] [PubMed] [Google Scholar]
- [160].Luo S, Gu Y, Zhang Y, Guo P, Mukerabigwi JF, Liu M, Huang X. Precise Ratiometric Control of Dual Drugs through a Single Macromolecule for Combination Therapy. Mol Pharm. 2015;12:2318–2327. doi: 10.1021/mp500867g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.