Abstract
Microbial biofilms and most eukaryotic tissues consist of cells embedded in a three-dimensional extracellular matrix. This matrix serves as a scaffold for cell adhesion and a dynamic milieu that provides varying chemical and physical signals to the cells. Besides a vast array of specific molecular components, an extracellular matrix can provide locally heterogeneous microenvironments differing in porosity/diffusion, stiffness, pH, oxygen and metabolites or nutrient levels. Mechanisms of matrix formation, mechanosensing, matrix remodeling, and modulation of cell-cell or cell-matrix interactions and dispersal are being revealed. This perspective article aims to identify such concepts from the fields of biofilm or eukaryotic matrix biology relevant to the other field to help stimulate new questions, approaches, and insights.
Biofilms, tissues, and organs are three-dimensional structures that share a common property: the presence of an extracellular matrix. The matrix surrounds and cements cells together, and in addition it organizes them into cohesive and functional 3D multicellular biological structures (Fig.1). Thus, extracellular matrix provides a scaffold for cellular organization and signaling critical for maintaining tissue and biofilm architecture. Concomitantly, the matrix provides dynamic, heterogeneous 3D microenvironments, where cells interact bi-directionally with constantly changing chemical and physical signals.
Figure 1. Cells in tissue and in biofilm adhere to, and are surrounded by, extracellular matrix.

Microscopic images of biofilm and eukaryotic cells within extracellular matrix. Confocal fluorescence images of (a) developing mouse salivary gland showing epithelial cells (magenta) and fibronectin in ECM (green); (b) eukaryotic cell (fibroblast, orange) within a 3D collagen matrix (green); inset shows fibroblast-assembled ECM (multiple colors). (c) The EPS matrix (red) creates compartmentalized bacterial microcolonies (green) and heterogeneous microenvironments; (d) bacteria (streptococci) embedded within EPS matrix. Inset shows SEM images of EPS surrounding bacteria and forming a mesh-like structure; the image was pseudo-colored using Adobe Photoshop software for visualization purposes.
Extensive research in cell and developmental biology established that cells sense both physical and chemical cues in their extracellular environment, which triggers cellular responses that regulate cellular functions including remodeling their surrounding 3D matrix. This reciprocal, bidirectional, and highly dynamic interaction between cells and matrix affects all facets of cell biology and pathology, by modulating tissue and organ morphogenesis, homeostasis, and tumorigenesis [1,2]. Similar dynamic cell-matrix interactions occur in biofilms, where microbial cells such as bacteria and fungi adhere and generate a surrounding matrix comprised of extracellular polymeric substances (EPS) (Fig 2). The EPS-matrix is critical for the existence of biofilm. It modulates biofilm assembly/disassembly, and enables the biofilm lifestyle of microbial pathogens, affecting the microenvironment and the pathogenesis of many infectious diseases [3–6].
Figure 2. EPS matrix and the dynamics of biofilm development.
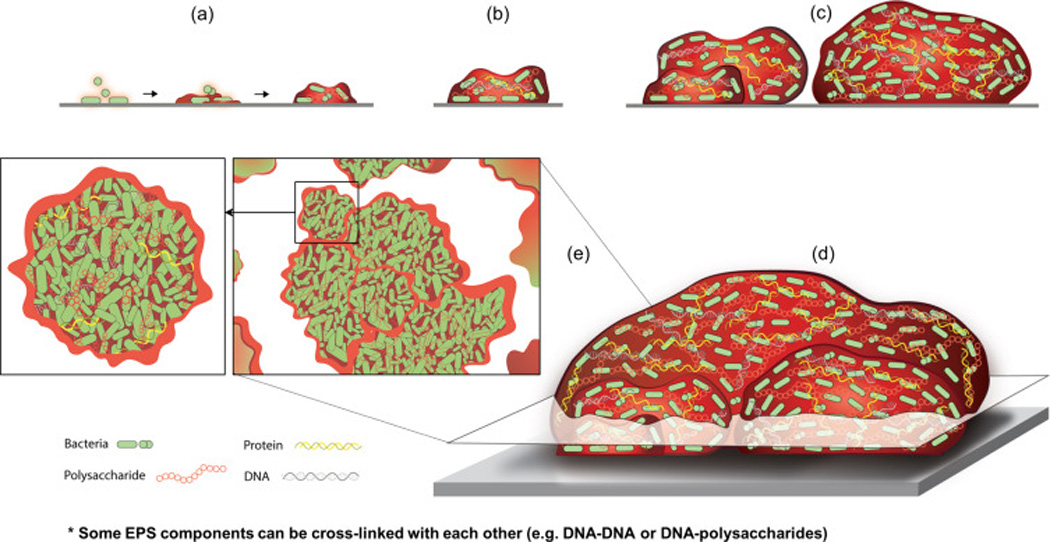
(a) EPS can be deposited directly on a surface for attachment or on microbial cell surfaces to promote the initial adhesion and local accumulation of microorganisms. (b) As the biofilms develops, the EPS produced locally (as well as acquired from the host environment) enmeshes the microorganisms into a diffusion-limiting polymeric matrix. The matrix help microbes to form highly organized cell clusters or aggregates known as microcolonies, which can vary in shape and size. (c,d) In some cases, these clusters can also serve as building blocks to form larger biofilm superstructures. (e) The diagram depicts a cross-section (top-view) of the complex structure of multi-microcolony biofilm architecture found in some biofilms. Close-up view of the microcolony diagram showing densely packed cells tightly associated with matrix components, such as polysaccharides, protein, and eDNA. It is noteworthy that polymer cross-linkers, such as ionic or nucleic acid-binding proteins, can help assemble DNA-DNA or DNA-polysaccharides complexes. The composition and structure of EPS matrix is complex and varies with time, type of microorganisms, and availability of substrates. The matrix helps to create chemical and physical heterogeneity as well as compartmentalized microenvironments. It influences local cellular activity/behavior and competitive or synergistic inter-species (or even inter-cluster) interactions. At later stages (not shown), production of matrix-degrading molecules by a sub-population of microbial cells and localized mechanical instability trigger biofilm breakdown and cell dispersal.
This perspective article aims to incorporate relevant concepts concerning eukaryotic cell-matrix interactions into biofilm biology and vice versa, hoping that knowledge from one field will help advance the other. We acknowledge that there can be some fundamental differences in the biology and matrix architecture of prokaryotic and eukaryotic cells. Nevertheless, both fields have increasingly recognized the importance of understanding the composition and functions of the extracellular matrix. Many review articles explore in detail the biofilm matrix or cell-matrix interactions in eukaryotic systems [1–9]. Here, we focus primarily on how concepts of matrix and cell-matrix interactions in each field can generate new questions and hypotheses for innovative research advances in biofilm or cell and developmental biology.
Molecular and functional diversity of extracellular matrix
The extracellular matrix in eukaryotic cell systems (abbreviated ECM) is a complex, crosslinked meshwork of proteins, proteoglycans, glycoproteins, and polysaccharides with diverse physical and biochemical properties [1,2,7]. The eukaryotic ECM also serves as a reservoir for growth factors and cytokines, as well as ECM-remodeling enzymes, which cooperate to assemble and remodel extracellular matrices. This entire ensemble of molecules is termed the ‘Matrisome.’ The mammalian matrisome consists of: (1) core ECM proteins (collagens, proteoglycans, and glycoproteins) comprising the basic backbone of the matrix, and (2) ECM-associated components. ECM-associated components include: i) ECM-bound growth and secreted factors, ii) modifiers of ECM structure and function, iii) cellular receptors for ECM, and iv) other ECM-affiliated proteins (Table 1). They provide an interface between the extracellular chemical and physical environment and the cellular scaffolding and signaling machinery, which varies substantially depending on cell and tissue type [1,2,8]. Databases cataloguing the expression and distribution of ECM components include the Matrixome Project, Human Protein Atlas, and MatrixDB.*
Table 1.
Extracellular matrix constituents and function in biofilm and eukaryotic systems
| Matrix Components | Function | Microbial species* |
||
|---|---|---|---|---|
| Name | Type | Location | ||
| TasA and TapB BslA eps operon-encoded exopolysaccharide PGA (poly-γ-glutamate) eDNA Flagellum |
Protein Protein Polysaccharide Polysaccharide Nucleic Acids Protein |
Extracellular Extracellular Extracellular Extracellular Extracellular Cell-associated |
Scaffolding, cell-to-cell binding Protection Adhesion, Scaffolding Adhesion, scaffolding Adhesion, cohesion, scaffolding Adhesion |
B. subtilis |
| β-1,3/β-1,6 glucan Mannan β-N-acetyl glucosamine - amino sugars Chitin eDNA Glycerolipids, sphingolipids Als and Hwp (adhesins) |
Polysaccharide Polysaccharide Polysaccharide/protein Polysaccharide Nucleic Acids Lipids Protein |
Cell-wall/Extracellular Cell-wall/Extracellular Extracellular Cell-wall/Extracellular Extracellular Cell-wall/Extracellular Cell-wall |
Scaffolding, protection Protection Cell-to-cell binding, scaffolding Unknown Scaffolding, protection Unknown Adhesion, cell-to-cell binding |
C. albicans |
| Curli (CsgA/CsgB) Flagellin (flagellum) Fimbrin (fimbriae) Ag43 (adhesins) AidA and TibA (adhesins) Cellulose Colonic acid Poly-β-1,6-N-acetyl-glucosamine (PGA) Lipopolysaccharides eDNA |
Protein Protein Protein Protein Protein Polysaccharide Polysaccharide Polysaccharide Polysaccharide Nucleic acids |
Cell-associated Cell-associated Cell-associated Cell-associated Extracellular Extracellular Cell-assoc./extracellular Extracellular Cell-assoc./extracellular Extracellular |
Adhesion, scaffolding, cell-to-cell binding, protection Adhesion Adhesion Adhesion, cell-to-cell binding Adhesion, cell-to-cell binding Protection, scaffolding, stability Scaffolding Adhesion, cell-to-cell binding Unknown Scaffolding |
E. coli |
| Alginate Psl Pel Cellulose Type IV pili LecA and LecB eDNA |
Polysaccharide Polysaccharide Polysaccharide Polysaccharide Protein Protein Nucleic acids |
Extracellular Cell-associated Cell-associated Extracellular Extracellular Cell-assoc./extracellular Extracellular |
Scaffolding, protection Adhesion, scaffolding, cell-to-cell binding, stability Scaffolding Scaffolding, protection Adhesion, scaffolding, stability Cell-to-cell binding, adhesion Adhesion, scaffolding, stability |
P. aeruginosa |
| α-1,3/α-1,6 linked glucans β-2,1 linked fructans Glucosyltransferases/fructosyltransferases Dextranase P1 (adhesin) GbpB/GbpC eDNA |
Polysaccharide Protein Protein Protein Protein Nucleic acid |
Extracellular Cell-assoc./extracellular Extracellular Cell-associated Extracellular Extracellular |
Adhesion, cohesion, scaffolding, stability, protection, cell-to-cell binding, nutrient EPS production EPS degradation/remodeling Adhesion Adhesion, scaffolding, stability Adhesion, scaffolding, stability |
S. mutans |
| Poly-N-acetyl glucosamine (PNAG) or Polysaccharide intercellular adhesin (PIA) Fibronectin-binding proteins (FnBPA and FnBPB), protein A, SasG Biofilm associated protein (BAP) Phenol-soluble modulins (PSMs) Teichoic and lipoteichoic acids eDNA |
Polysaccharides Protein Protein Protein Lipids/polysaccharides Nucleic acids |
Extracellular Cell-assoc./extracellular Cell-assoc./extracellular Extracellular Cell-assoc./extracellular Extracellular |
Adhesion, cohesion, scaffolding, stability, protection Adhesion, cell-to-cell binding Cell-to-cell binding, scaffolding, stability Protection Adhesion, cohesion Cell-to-cell binding, scaffolding, stability |
S.aureus |
| VPS (Vibrio polysaccharide) Bap1 RbmA RbmC MSHA (mannose-sensitive haemagglutinin) pili Flagellum eDNA |
Polysaccharides Protein Protein Protein Protein Protein Nucleic Acids |
Cell-assoc./extracellular Cell-assoc./extracellular Cell-assoc./extracellular Cell-assoc./extracellular Cell-associated Cell-associated Extracellular |
Adhesion, Cohesion, Scaffolding, Stability Adhesion, protection Cell-to-cell binding, scaffolding, stability, protection Scaffolding Adhesion Adhesion Scaffolding, stability, nutrient |
V. cholerae |
| Eukaryotic cells** |
||||
| Collagens1 | Glycoproteins | Cell-assoc./extracellular | Scaffolding/Adhesion/Signaling1 | Wide distribution2 |
| Laminins2 | Glycoproteins | Extracellular | Scaffolding/Adhesion/Signaling2 | Wide distribution2 |
| Fibronectin | Glycoprotein | Extracellular | Scaffolding/Adhesion/Signaling | Wide distribution2 |
| Proteoglycans3 | Proteoglycans | Cell-assoc./extracellular intracellular |
Scaffolding/Adhesion/Signaling3 | Wide distribution2 |
| Hyaluronan (hyaluronic acid) | Polysaccharide | Extracellular | Space-filling/Cell migration | Wide distribution2 |
| Integrins (24 heterodimers in humans) | Glycoproteins | Transmembrane | Adhesion/Remodeling/Signaling | Wide distribution2 |
| Other matrisome components4 | Proteins, glycoproteins, glycosaminoglycans, lipids |
Cell-assoc./extracellular | Scaffolding/Adhesion/Signaling | Wide distribution2 |
Additional details about the EPS components can be found in the following references [1,2,3,11,14,41,68]
There are at least 28 collagens with widely varying locations and functions (see review by Ricard-Blum, 2011; [69])
These ECM constituents are synthesized by a wide variety of eukaryotic cells, but the isoforms or protein types can be either cell-type specific or more commonly secreted by a varying range of cell types.
There are at least 16 laminins with varying functions (see review by Domogatskaya et al., 2012; [70])
There are at least 43 proteoglycan genes that produce an exceptionally wide diversity of proteoglycans that can be classified into four major classes; proteoglycans can be extracellular, pericellular, transmembrane, GPI-anchored, and intracellular proteoglycans (see review by Iozzo and Schaefer, 2015; [71]).
There are numerous additional protein components of the ECM with a vast range of functions (see reference [8] and matrix websites listed in this review).
In biofilms, the matrix is similarly comprised of extracellular polymeric substances (EPS). The composition and structure of EPS can vary greatly depending on the type of microorganism, local shear forces, availability of substrates, and the host environment [3,4,6,9]. Biofilm matrix can contain exopolysaccharides, proteins, lipids, nucleic acids, lipoteichoic acids, and even lipopolysaccharides, which can be also cross-linked [6,10,11]. EPS components are: (1) secreted extracellularly or (2) microbial surface-associated (Table 1). Bacterial-derived exoenzymes acting in concert can help to produce or degrade the EPS matrix, while extracellular bacterial structures (appendages) such as flagella, amyloid-like fibers, and fimbriae affect the properties and structural integrity of the biofilm. Further in-depth characterizations are needed of the molecular, structural, and functional diversity of biofilm matrix components, i.e., a full inventory of EPS and the functional interactions between its many constituents [3–6,11–13]. The availability of complete genome sequences coupled with recent advances in analytical, imaging, and spectrometry methods [14] will facilitate this detailed characterization of the ‘Biofilm Matrisome’ necessary to elucidate its roles in biofilm physiology and pathogenicity. For example, function-composition analyses of in vitro and in vivo biofilm matrix revealed clinical relevance of certain matrix components in limiting antifungal [15] and antibiotic [16] penetration, contributing to drug resistance. Additionally, host ECM components can mediate interactions between microbes and eukaryotic cells in initiating biofilm-associated infections (see later). Thus, elucidating the changing molecular composition of interfaces between EPS and ECM will enhance our understanding of microbial-host interactions.
*http://www.matrixome.com/bm/Home/home/home.asp; http://www.proteinatlas.org; http://matrixdb.ibcp.fr
Matrix scaffolding for cell-matrix adhesion and mechanical stability
The diverse roles of the ECM in eukaryotic physiology (or pathogenesis) are based on its complex but well-characterized physical, biochemical, and biomechanical properties. The ECM provides binding sites for cell attachment via cell-matrix adhesions, serving a physical/structural (scaffolding) role essential for tissue and organ morphogenesis, and its dysregulation can promote tumorigenesis or metastasis [1]. Microorganisms also express membrane-associated proteins that, analogous to eukaryotic cells, can recognize and bind specific polymeric components of the matrix [3–6]. The production of EPS by microorganisms enhances cell adhesion to solid surfaces and cohesion between organisms to increase microbial accumulation, forming microcolonies of varying shapes and sizes [5,9] (Fig. 2). For example, the oral pathogen Streptococcus mutans secretes EPS-producing exoenzymes termed glucosyltransferases (Gtfs) that can bind to both tooth and microbial surfaces, including fungi (Candida albicans) [17]. Intriguingly, these surface-bound Gtfs remain highly active despite major conformational changes [18], producing large amounts of EPS to provide bacterial binding sites that promote mixed-species (and even cross-kingdom) biofilm formation [19]. Motile bacteria (Bacillus subtilis and Vibrio cholera) appear to use flagella as mechanosensors to scan surfaces and initiate cell adhesion [5,20]. The production of EPS components, whether by secretion or lysis, is a tightly regulated process involving the biofilm cell population (or sub-population) [5,6]. EPS then accumulates to form a complex polymeric 3D matrix scaffold in which microorganisms become enmeshed and assemble a highly organized and compartmentalized 3D biofilm architecture [3–6,13] (Fig. 2).
Biophysical analyses of biofilms and EPS are clarifying their physical contributions to local microenvironments and resistance to mechanical removal or antimicrobials [9]. Well-established biofilms are mechanically difficult to remove from surfaces and often display viscoelastic properties, which can help them persist by partially yielding rather than detaching when subjected to external (fluid) shear stresses [9,21]. EPS deposition on surfaces and development into polymeric matrix affect the mechanical properties of biofilms, such as increasing adhesive strength to surfaces and cohesiveness [9]. Matrix stiffness appears to increase as the biofilm matures [21]. However, it remains to be determined how EPS components and bacterial interactions modulate local adhesive and cohesive forces. Highly heterogeneous viscoelastic properties were identified throughout 3D biofilms using magnetic tweezers [22], where the local mechanical profile depended on cell-surface appendages, such as pili or curli. Likewise, analyses of ECM can identify global and local matrix physical properties directly affecting cellular behavior and tissue/organ development. For example, ultrasound and atomic force microscopy provide details about ECM architecture, deposition, and function [23–25]. Local stiffening of the ECM with collagen fiber alignment is associated with solid tumors [25–27]. Enhanced understanding of the biomechanical properties of EPS matrix may lead to innovative approaches to remove or disassemble pathogenic biofilms, while understanding local ECM changes may provide new approaches for tissue engineering and cancer biology.
The eukaryotic ECM is initially constructed but also remodeled by cell-to-matrix adhesions, as reviewed extensively (e.g. [1,2]). ECM remodeling is crucial during morphogenesis (the development of form in embryos). For example, local remodeling to generate hundreds of microscopic holes in basement membranes surrounding developing organs permits controlled expansion of tissues without tissue mixing [28]. Conversely, larger basement membrane holes permit invasion of tumor cells into normal tissues [29]. During tumor metastasis, cancer cells can invade or disperse to distant sites through complex processes of local matrix or target-tissue microenvironmental remodeling [30]. Likewise, EPS matrix remodeling may affect biofilm development by altering the mechanical properties and/or providing new binding sites to additional microorganisms that were unavailable during initiation. Furthermore, at later stages, mature biofilms release small aggregates or even individual cells, often through matrix degradation, to seed uncolonized sites and reinitiate the biofilm life cycle [31]. Understanding how bacterial EPS is produced locally or deposited on abiotic and biotic surfaces dynamically, and how microbial binding sites and cell-matrix adhesive interactions affect bacterial cell function/virulence, microcolony shape, and assembly/disassembly of surrounding 3D microenvironments need to be determined in detail.
Although ECM formation by eukaryotic cells has been studied by fluorescence time-lapse microscopy, recent advances in biofilm research provide exciting opportunities for both fields. Super-resolution microscopy and novel solid-state NMR methods have provided impressive details about EPS secretion and matrix organization by V. cholerae at the single-cell level with single-polymer/protein-labeling precision [5,32]. These approaches reveal the spatio-temporal order of deposition of four essential matrix constituents (a polysaccharide and three proteins). These extracellular materials accumulate at different locations on the cell surface, each with complementary roles in biofilm development: mediating cell-cell adhesion, formation of cell clusters and adherence to a surface, and forming dynamic, flexible, and ordered envelopes that encase cell clusters [5]. Recently, 3D-structured illumination super-resolution microscopy revealed a coordinated ‘explosive cell lysis’ by sub-population of Pseudomonas aeruginonas cells, releasing eDNA and other biofilm matrix constituents that are critical for microcolony development [33]. How bacteria spatially segregate matrix material within the biofilm, and how the matrix stretches and expands to accommodate cell growth or promote dispersion remain unknown. Similar approaches applied to mammalian cells should provide new insights into local ECM assembly and remodeling for tissue expansion.
Matrix modulation of microenvironmental heterogeneity
The 3D assembly of biofilm matrix can create highly heterogeneous and compartmentalized microenvironments, a phenomenon also prominent in tumor ECM. The formation of chemical and nutritional gradients within most biofilms appears to involve a balance between matrix acting as a physical barrier affecting diffusion of substances in and out of the biofilms and local microbial consumption/metabolism, resulting in numerous niches with varying concentrations of pH, O2, signaling molecules and other solutes [34]. During biofilm matrix synthesis by an oral pathogen (S. mutans), a fluorescent pH indicator probe was directly immobilized on the 3D matrix scaffold [35]. This in situ 3D pH mapping within intact biofilms revealed a fascinating distribution of matrix-delimited regions of low pH values (4.5–5.5) at sites throughout the biofilm architecture. How acids accumulate in these local niches requires elucidation, potentially via diffusion-limitation combined with local metabolic activity [35,36]. Likewise, bacterial aggregates confined within “microtraps” using gelatin-based 3D microprinting create oxygen micro-gradients and induce phenotypic changes in a subpopulation of cells within the aggregate [37]. The development of additional probes for incorporating (or cross-linking) into the matrix and other microfabrication technologies should elucidate the assembly dynamics of spatially heterogeneous biofilm microenvironments in 3D.
Local eukaryotic tissue microenvironments also differ substantially in molecular composition, density, porosity, stiffness, and other features. Besides increased stiffness in scars and fibrosis, connective tissue ECMs can become strikingly dense (“desmoplastic”) in many advanced tumors; such physical properties can have major effects on cellular functions including invasiveness [25,38,39]. Within tumors, there are often local regions of hypoxia, acidic pH, tumor cell death, and release of proteases and other cellular constituents; new genetically encoded or chemical sensors for hypoxia or pH should provide detailed information about local oxygenation or pH [40,41]. More generally, the eukaryotic ECM has well-known functions as a chemical reservoir of growth factors, cytokines, proteases, and other extracellular factors, and ECM can limit the diffusive range, accessibility, and concentrations of signaling ligands.
Conceptually similar mechanisms may exist during biofilm development. The biofilm matrix can provide both protection of embedded bacteria and tailored niches. For example, the positioning of microorganisms and their stress response mechanisms may correlate with their susceptibility to, or affinity for, low pH or hypoxic environments and availability of specific ligands, nutrients or metabolites. Alkali-generating or bacteriocin/toxin-producing bacteria within biofilms can influence interspecies interactions, local pH, and microenvironment dynamics [42–44]. Combining matrix and environment analyses with new methods of multi-bacterial species labeling [45] should help characterize the location and functional roles of niches within biofilms.
A heterogeneous milieu can modulate gene expression locally and influence the metabolic exchange and intercellular signaling among different species or between different clusters of cells distributed within the greater biofilm architecture, orchestrating their communal ‘social behavior’, spatial localization and/or physiological heterogeneity [5,44,46–48]. For example, exopolysaccharides can actively sequester quorum sensing molecules within the biofilm, while quorum sensing signaling in P. aureginosa can be switched off or on by the production of EPS matrix [49]. The matrix itself can also serve as local nutrient reservoir, e.g., by providing fermentable carbohydrates [13]. In addition, the biofilm matrix may act as an external digestion system by immobilizing exoenzymes, allowing them to metabolize substrates in close proximity to cells while also participating in matrix remodeling [3,13,31,50].
Cell-matrix interactions
Eukaryotic cell-ECM interactions provide spatial, chemical, and mechanical cues to activate intracellular signaling cascades. For example, matrix glycoproteins and proteoglycans can enhance signaling and adhesive functions, while growth factors and cytokines incorporated within ECM can locally stimulate adherent cells [1,51]. Similarly, the biofilm matrix provides highly structured yet spatially and chemically heterogeneous environments that locally affect cellular physiology, transcriptional activity, and survival [3,4,34].
The mechanical characteristics of ECM, such as rigidity, porosity, and cross-linking can be sensed by eukaryotic cells through mechanotransduction, affecting proliferation and gene expression locally [52–54]. Matrix stiffness and architecture can also alter differentiation, signaling, and tumor metastasis [26,27,55–58]. Whether the ECM is linearly versus non-linearly elastic can regulate use of a novel ‘nuclear piston’ mode of 3D cell migration [59]. Analogously, mapping local viscoelastic properties of intact biofilms revealed mechanical micro-niches modulated through bacterial surface appendages [22]. Mechano-sensing via membrane channels gated with osmotic pressure has been described in microbes [60,61], but the physical sensing and responses to environmental rigidity observed in eukaryotic cells have not yet been identified in bacteria. Intriguingly, a recent study demonstrated that bacteria (Bacillus subtilis) can create internal forces within biofilms; such force, associated with growth-induced pressure, helps bacteria to shape biofilm structure [62]. Clearly, further studies on microbial bi-directional interactions affecting local mechanical properties of the matrix are needed.
By offering a variety of chemical and physical milieus, the biofilm matrix may locally modulate complex interspecies competition, synergism, and/or mutualism in regions undergoing active matrix synthesis, remodeling, and metabolism. Recent characterizations of pathways regulating biofilm matrix gene expression have identified several extra- and intra-cellular signaling molecules, plus non-signaling mechanisms for activation of matrix production [5,44]. Nevertheless, it is unclear how matrix triggers cell heterogeneity and influences chemical or mechanical sensing/signaling, localized acid/alkali, or even antimicrobial production within biofilms. How bacterial binding to specific EPS-matrix ligands activates intracellular nucleotide-based signaling also needs further elucidation. Opportunities to create structured physical and chemical microenvironments within microfluidic devices [63] or using 3D printing to create EPS-delineated compartments [64] may help elucidate how the matrix modulates niche biology. Similarly, microfluidic organ/tissue-on-chip technologies can provide insights into eukaryotic tissue function and pathologies [65].
Eukaryotic ECM-biofilm interactions
Laminins, collagens, and fibronectin are abundant ECM components of basement membranes and interstitial spaces/connective tissue. These ECM constituents are often targeted by microorganisms for adhesion and biofilm formation, causing chronic, localized infection as well as facilitating microbial invasion to “metastasize” to other sites in the human body. The attachment of microbial pathogens to the ECM of host tissues is mediated by multiple surface proteins known as adhesins, which vary in size and structure (with single or multiple binding domains). Collagen-, fibronectin-, and laminin-binding adhesins have been identified in a wide range of microbes; adhesins recognize specific epitopes/domains (protein-protein interactions) or carbohydrate side chains of these ECM constituents [66]. S. aureus is a common, effective colonizer of host tissues, expressing a myriad of adhesins targeting a wide spectrum of ECM ligands [66]. For example, several cell-wall anchored proteins termed MSCRAMMs* such as ClfA and FnbpA/B can bind to fibrinogen and fibronectin, while CNA is a collagen-binding adhesin. Interestingly, several secreted proteins not only help assemble biofilm matrix, but also bind to ECM components (e.g., Emp and Eap adhesins). Thus, a myriad of dynamic biofilm matrix-ECM interactions occur during microbial infection, which may directly modulate both bacterial and host cell responses. Details of these bi-directional interactions remain to be elucidated.
*Microbial Surface Components Recognizing Surface Adhesive Matrix Molecules
Once microorganisms attach and accumulate in biofilms, they can degrade eukaryotic ECM directly via production/secretion of proteolytic enzymes, such as metallo- and serine-proteases, but also indirectly through activation of host inflammatory responses, induction of host matrix metalloproteinases (MMPs), recruitment of neutrophils, or even ‘hijacking’ host proteases such as plasminogen [66]. Likewise, biofilms formed on adjacent surfaces, including teeth at the gingival interface, bone, or indwelling medical devices such catheters, can also alter the local ECM of host tissues. Interestingly, periodontal pathogens such as Porphyromonas gingivalis can manipulate host immune responses by subverting complement function to evade neutrophil-mediating killing [67]. Persistence of inflammatory environments is advantageous to inflammophilic bacteria because degraded ECM components, such as collagen peptides released from tissue breakdown, serve as nutrients for these organisms. Furthermore, microbial colonization and biofilm formation can modify the local environment by releasing metabolites and other byproducts, as well as altering pH and oxygen levels, which could further enhance degradation and exposure of ECM components. This process enhances the adherence and persistence of pathogens and promotes inflammation, leading to localized chronic infections such as chronic wounds, cystic fibrosis, or periodontal diseases. Degradation of ECM can also pave the way for bacterial invasion deeper into host tissues, breaching cellular barriers and leading to infection dissemination.
Some microorganisms can exploit cell-ECM interactions to internalize into host cells [66]. For example, Listeria monocytogenes can directly bind to E-cadherin via surface proteins (interlins), causing actin rearrangement and membrane phospholipid changes that allow pathogen entry. Because microbes can use ECM of host tissues for attachment, immune evasion, invasion and even internalization, intriguing questions include: What is the binding affinity and specificity of specific microbes to the various ECM isoforms found in a particular tissue or organ? Are products released during ECM degradation incorporated into local biofilm matrix to change its properties and alter microbial physiology and pathogenicity? Finally, can biofilm-ECM interactions influence cancer pathogenesis?
Figure 3. Schematic summary of dynamic cell-matrix interactions in biofilms and eukaryotic systems.
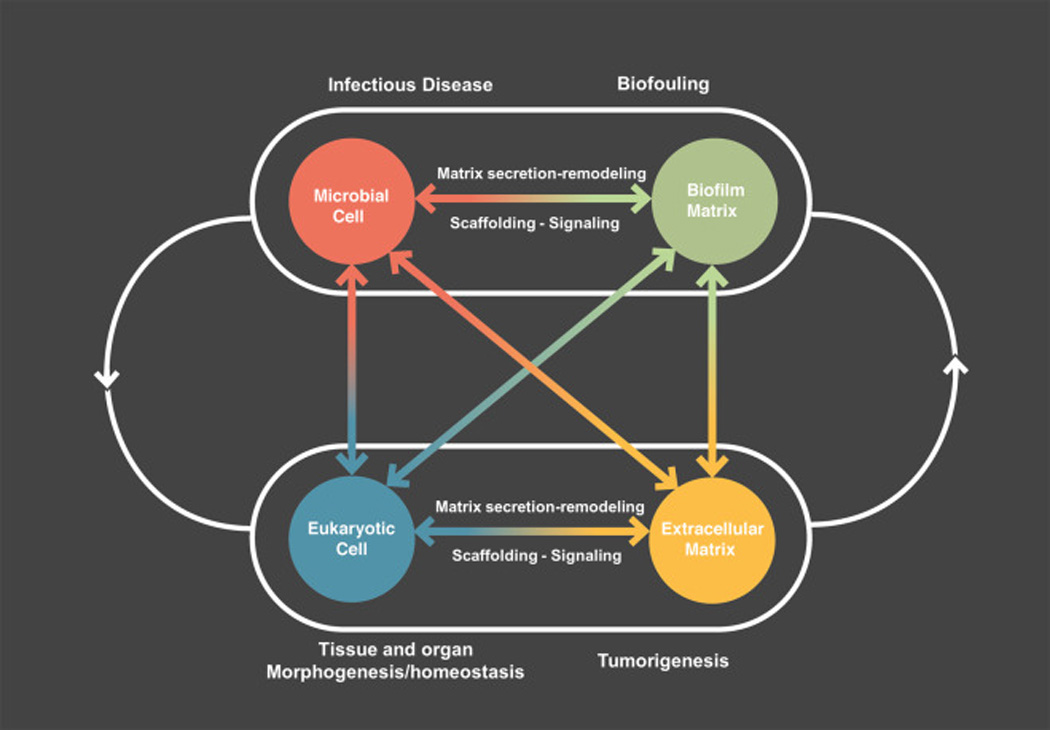
Microbial and eukaryotic cells can secrete and remodel the extracellular matrix. The matrix not only surrounds and cements cell together but also organizes cells into a cohesive and functional 3D polymeric network that provides a scaffold for multicellular biological structures and signaling. It provides a dynamic 3D microenvironment where cells can interact bi-directionally with constantly changing chemical and physical cues that modulate morphogenesis, homeostasis, and pathogenesis.
Summary and future perspectives.
The extracellular matrix of both biofilms and tissues provides structural scaffolds, and also governs cellular activity either directly or through generation of local microenvironments or niches. How such diffusion-limiting barriers and compartmentalization modulate microenvironmental heterogeneity and niche biology over time, and how they locally affect cell physiology, tumor progression, or bacterial survival, need further elucidation.
The composition, structure, and function of extracellular matrix components in both biofilms and in eukaryotic tissues are incredibly diverse. Further detailed characterizations are needed of the molecular and functional diversity of the matrix or “Matrisome,’ particularly in biofilms.
Eukaryotic cell-matrix adhesion provides an interactive interface between extracellular and intracellular milieus via sensing and signaling, resulting in cellular responses to chemical and physical matrix cues. Whether bacterial cells interact reciprocally with the biofilm matrix via mechanotransduction, chemical signaling, or binding/release of extracellular signaling molecules in a similar manner requires further exploration.
Both bacterial and eukaryotic cells constantly remodel the matrix to dynamically restructure the microenvironment. Further investigation into the regulation of matrix remodeling and how the altered matrix composition/structural organization affects cell functions may lead to exciting discoveries concerning the mechanisms of bacterial dispersal from biofilms, tissue morphogenesis, and tumor progression/metastasis.
Matrix-mediated changes in the biofilm and tissue microenvironment can also modify cell-cell interactions, specifically between different cell types or microbial species. In turn, dynamic reciprocity between cells and matrix, or cell-cell interactions and matrix, may provide a complex, interconnected molecular network governing cellular functionality at both single-cell and multicellular levels.
Eukaryotic ECM-biofilm interactions are critical for localized infection and inflammation, and may be also involved in cancer development.
Unveiling and comparing the detailed mechanisms by which spatial, chemical, and mechanical cues and heterogeneity of the matrix impact intra- and inter-cellular responses locally will advance our understanding of biofilm and tissue/organ homeostasis and pathogenesis (Fig. 3).
Acknowledgments
The authors’ research is supported by the National Institute for Dental and Craniofacial Research (NIDCR), NIH Intramural Research Program (KMY) and by NIDCR grants DE16139, DE18023, and DE25220 (HK). We thank Dr. Marlise Klein (State University of Sao Paulo, Brazil) and Dr. William H. Bowen (University of Rochester Medical Center, Rochester, NY, USA) for helpful suggestions and comments. We are also grateful to Jill Harunaga, Ryan Petrie, and Roumen Pankov for the fluorescence images. The diagrams and schematics were designed by Long Do and Mirae Jang (The University of Arts, Philadelphia, PA, USA; Instructor: Joel Katz). The authors regret that many studies could only be cited indirectly owing to space and reference number limitation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO, Yamada KM, editors. Extracellular matrix biology. Cold Spring Harbor Laboratory Press; 2012. [Google Scholar]
- 2.Mecham RP, editor. The Extracellular Matrix: an Overview. Springer Science & Business Media; 2011. [Google Scholar]
- 3.Flemming H, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 4.Mann EE, Wozniak DJ. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev. 2012;36:893–916. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teschler JK, Zamorano-Sánchez D, Utada AS, Warner CJ, Wong GC, Linington RG, Yildiz FH. Living in the matrix: assembly and control of Vibrio cholerae biofilms. Nat Rev Microbiol. 2015;13:255–268. doi: 10.1038/nrmicro3433. • This article reviews recent advances concerning the functional roles of various matrix components in biofilm formation and the regulatory network controlling matrix production
- 6. Hobley L, Harkins C, MacPhee CE, Stanley-Wall NR. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol Rev. 2015;39:649–669. doi: 10.1093/femsre/fuv015. • This article provides a comprehensive review of the role of EPS matrix from four prototypical biofilm-forming bacteria in the architectural integrity and functionality of biofilms.
- 7.Hynes RO, Naba A. Overview of the matrisome-an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: Tools and insights for the "omics" era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. • This review describes proteomic and other research tools for characterization of the matrisome and comparisons between tissues and tumors.
- 9. Peterson BW, He Y, Ren Y, Zerdoum A, Libera MR, Sharma PK, van Winkelhoff AJ, Neut D, Stoodley P, van der Mei HC, Busscher HJ. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol Rev. 2015;39:234–245. doi: 10.1093/femsre/fuu008. • This article discusses the importance of EPS and viscoelastic properties of biofilms to their resistance to antimicrobials and mechanical removal.
- 10.Brockson ME, Novotny LA, Mokrzan EM, Malhotra S, Jurcisek JA, Akbar R, Devaraj A, Goodman SD, Bakaletz LO. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Mol Microbiol. 2014;93:1246–1258. doi: 10.1111/mmi.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jennings LK, Storek KM, Ledvina HE, Coulon C, Marmont LS, Sadovskaya I, Secor PR, Tseng BS, Scian M, Filloux A, et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc Natl Acad Sci USA. 2015;112:11353–11358. doi: 10.1073/pnas.1503058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein MI, Hwang G, Santos PH, Campanella OH, Koo H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol. 2015;5:10. doi: 10.3389/fcimb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen WH, Koo H. Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neu TR, Lawrence JR. Innovative techniques, sensors, and approaches for imaging biofilms at different scales. Trends Microbiol. 2015;23:233–242. doi: 10.1016/j.tim.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 15. Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, Lounes-Hadj Sahraoui A, Fontaine J, Sanchez H, Hatfield RD, et al. Novel entries in a fungal biofilm matrix encyclopedia. MBio. 2014;5:e01333–e01314. doi: 10.1128/mBio.01333-14. • This article provides a comprehensive composition analysis of the Candida albicans biofilm matrix in vitro and in vivo, and demonstrates the importance of matrix components in antifungal drug resistance
- 16.Doroshenko N, Tseng BS, Howlin RP, Deacon J, Wharton JA, Thurner PJ, Gilmore BF, Parsek MR, Stoodley P. Extracellular DNA impedes the transport of vancomycin in Staphylococcus epidermidis biofilms preexposed to subinhibitory concentrations of vancomycin. Antimicrob Agents Chemother. 2014;58:7273–7282. doi: 10.1128/AAC.03132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang G, Marsh G, Gao L, Waugh R, Koo H. Binding Force Dynamics of Streptococcus mutans-glucosyltransferase B to Candida albicans. J Dent Res. 2015;94:1310–1317. doi: 10.1177/0022034515592859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fears KP, Gonzalez-Begne M, Love CT, Day DE, Koo H. Surface-induced changes in the conformation and glucan production of glucosyltransferase adsorbed on saliva-coated hydroxyapatite. Langmuir. 2015;31:4654–4662. doi: 10.1021/la504461h. [DOI] [PubMed] [Google Scholar]
- 19. Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai C, Gonzalez M, Watson G, Krysan DJ, Bowen WH, Koo H. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes the virulence of plaque-biofilms in vivo. Infect Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. • This article shows that bacterially-derived EPS formed directly on the fungal (C. albicans) cell surface promotes cross-kingdom interactions to synergistically enhance biofilm matrix assembly and augment biofilm virulence in vivo
- 20.Cairns LS, Marlow VL, Bissett E, Ostrowski A, Stanley-Wall NR. A mechanical signal transmitted by the flagellum controls signaling in Bacillus subtilis. Mol Microbiol. 2013;90:6–21. doi: 10.1111/mmi.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang G, Klein MI, Koo H. Analysis of the mechanical stability and surface detachment of mature Streptococcus mutans biofilms by applying a range of external shear forces. Biofouling. 2014;30:1079–1091. doi: 10.1080/08927014.2014.969249. [DOI] [PubMed] [Google Scholar]
- 22.Galy O, Latour-Lambert P, Zrelli K, Ghigo J, Beloin C, Henry N. Mapping of bacterial biofilm local mechanics by magnetic microparticle actuation. Biophys J. 2012;103:1400–1408. doi: 10.1016/j.bpj.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng CX, Hong X, Stegemann JP. Ultrasound imaging techniques for spatiotemporal characterization of composition, microstructure, and mechanical properties in tissue engineering. Tissue Eng Part B Rev. 2016 doi: 10.1089/ten.teb.2015.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doyle AD, Carvajal N, Jin A, Matsumoto K, Yamada KM. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat Commun. 2015;6:8720. doi: 10.1038/ncomms9720. • This article describes the regulation of 3D cell adhesion dynamics by 3D collagen gels of varying matrix microarchitectures and stiffness, reporting that a local balancing of contractility with ECM stiffness stabilizes cell adhesions and promotes cell migration.
- 25. Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES, Weaver VM. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol. 2015;7:1120–1134. doi: 10.1039/c5ib00040h. • In this study, the authors establish a link between human breast cancer progression/aggressiveness and increased local deposition of collagen that is linearly aligned, along with enhanced local tissue stiffness and inflammation.
- 26.Du J, Zu Y, Li J, Du S, Xu Y, Zhang L, Jiang L, Wang Z, Chien S, Yang C. Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Sci Rep. 2016;6:20395. doi: 10.1038/srep20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riching KM, Cox BL, Salick MR, Pehlke C, Riching AS, Ponik SM, Bass BR, Crone WC, Jiang Y, Weaver AM, Eliceiri KW, Keely PJ. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys J. 2014;107:2546–2558. doi: 10.1016/j.bpj.2014.10.035. • In this study, the authors provide evidence that aligned ECM topography rather than stiffness is importance for enhanced tumor cell 3D migration by restricting cell protrusions along aligned collagen fibers.
- 28. Harunaga JS, Doyle AD, Yamada KM. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev Biol. 2014;394:197–205. doi: 10.1016/j.ydbio.2014.08.014. • This study characterizes the dynamics of embryonic organ formation to show that local protease remodeling, microscopic cell protrusions, and global translocation of the basement membrane cooperate during tissue morphogenesis.
- 29.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol. 2012;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- 32.Berk V, Fong JC, Dempsey GT, Develioglu ON, Zhuang X, Liphardt J, Yildiz FH, Chu S. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science. 2012;337:236–239. doi: 10.1126/science.1222981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnbull L, Toyofuku M, Hynen AL, Kurosawa M, Pessi G, Petty NK, Osvath SR, Cárcamo-Oyarce G, Gloag ES, Shimoni R. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun. 2016;7:11220. doi: 10.1038/ncomms11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008;6:199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 35.Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JRI, Heydorn A, Koo H. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8:e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo L, Hu W, He X, Lux R, McLean J, Shi W. Investigating acid production by Streptococcus mutans with a surface-displayed pH-sensitive green fluorescent protein. PLoS One. 2013;8:e57182. doi: 10.1371/journal.pone.0057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wessel AK, Arshad TA, Fitzpatrick M, Connell JL, Bonnecaze RT, Shear JB, Whiteley M. Oxygen limitation within a bacterial aggregate. MBio. 2014;5:e00992–e00914. doi: 10.1128/mBio.00992-14. • This article shows how 3D printing technologies can create artificial microenvironments that mimic the formation of bacterial aggregates and oxygen gradients typically found in biofilms, and to study their role in bacterial metabolism, social behavior, and drug resistance.
- 38.Artym VV, Swatkoski S, Matsumoto K, Campbell CB, Petrie RJ, Dimitriadis EK, Li X, Mueller SC, Bugge TH, Gucek M, Yamada KM. Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. J Cell Biol. 2015;208:331–350. doi: 10.1083/jcb.201405099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, Yang J. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. • This article describes a novel mechanism of mechanotransduction in which a TWIST1-activated pathway stimulates epithelial-mesenchymal transition (EMT) in response to increased ECM stiffness to promote tumor cell invasion.
- 40.Erapaneedi R, Belousov VV, Schafers M, Kiefer F. A novel family of fluorescent hypoxia sensors reveal strong heterogeneity in tumor hypoxia at the cellular level. EMBO J. 2016;35:102–113. doi: 10.15252/embj.201592775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilson RC, Tang R, Som A, Klajer C, Sarder P, Sudlow GP, Akers WJ, Achilefu S. Protonation and trapping of a small pH-sensitive near-infrared fluorescent molecule in the acidic tumor environment delineate diverse tumors in vivo. Mol Pharm. 2015;12:4237–4246. doi: 10.1021/acs.molpharmaceut.5b00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merritt J, Qi F. The mutacins of Streptococcus mutans: regulation and ecology. Mol Oral Microbiol. 2012;27:57–69. doi: 10.1111/j.2041-1014.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nascimento MM, Browngardt C, Xiaohui X, Klepac-Ceraj V, Paster BJ, Burne RA. The effect of arginine on oral biofilm communities. Mol Oral Microbiol. 2014;29:45–54. doi: 10.1111/omi.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol. 2013;11:157–168. doi: 10.1038/nrmicro2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci USA. 2016;113:E791–E800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serra DO, Richter AM, Klauck G, Mika F, Hengge R. Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. MBio. 2013;4:e00103–e00113. doi: 10.1128/mBio.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Prindle A, Humphries J, Gabalda-Sagarra M, Asally M, Dong-yeon DL, Ly S, Garcia-Ojalvo J, Süel GM. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature. 2015;523:550–554. doi: 10.1038/nature14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell KF, Zarnowski R, Sanchez H, Edward JA, Reinicke EL, Nett JE, Mitchell AP, Andes DR. Community participation in biofilm matrix assembly and function. Proc Natl Acad Sci USA. 2015;112:4092–4097. doi: 10.1073/pnas.1421437112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irie Y, Borlee BR, O'Connor JR, Hill PJ, Harwood CS, Wozniak DJ, Parsek MR. Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2012;109:20632–20636. doi: 10.1073/pnas.1217993109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan JB. Biofilm Matrix-Degrading Enzymes. In: Donelli G, editor. Microbial Biofilms: Methods and Protocols. New York: Springer; 2014. pp. 203–213. [DOI] [PubMed] [Google Scholar]
- 51.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 53.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 56.Sack KD, Teran M, Nugent MA. Extracellular matrix stiffness controls VEGF signaling and processing in Endothelial cells. J Cell Physiol. 2016 doi: 10.1002/jcp.25312. [DOI] [PubMed] [Google Scholar]
- 57.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parekh A, Weaver AM. Regulation of invadopodia by mechanical signaling. Exp Cell Res. 2015 doi: 10.1016/j.yexcr.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petrie RJ, Koo H, Yamada KM. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science. 2014;345:1062–1065. doi: 10.1126/science.1256965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinac B. Bacterial mechanosensitive channels as a paradigm for mechanosensory transduction. Cell Physiol Biochem. 2011;28:1051–1060. doi: 10.1159/000335842. [DOI] [PubMed] [Google Scholar]
- 61.Rubinstein SM, Kolodkin-Gal I, Mcloon A, Chai L, Kolter R, Losick R, Weitz DA. Osmotic pressure can regulate matrix gene expression in Bacillus subtilis. Mol Microbiol. 2012;86:426–436. doi: 10.1111/j.1365-2958.2012.08201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Douarche C, Allain J, Raspaud E. Bacillus subtilis bacteria generate an internal mechanical force within a biofilm. Biophys J. 2015;109:2195–2202. doi: 10.1016/j.bpj.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wessel AK, Hmelo L, Parsek MR, Whiteley M. Going local: technologies for exploring bacterial microenvironments. Nat Rev Microbiol. 2013;11:337–348. doi: 10.1038/nrmicro3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Connell JL, Kim J, Shear JB, Bard AJ, Whiteley M. Real-time monitoring of quorum sensing in 3D-printed bacterial aggregates using scanning electrochemical microscopy. Proc Natl Acad Sci USA. 2014;111:18255–18260. doi: 10.1073/pnas.1421211111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh B, Fleury C, Jalalvand F, Riesbeck K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol Rev. 2012;36:1122–1180. doi: 10.1111/j.1574-6976.2012.00340.x. [DOI] [PubMed] [Google Scholar]
- 67.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng G, Vad BS, Dueholm MS, Christiansen G, Nilsson M, Tolker-Nielsen T, Nielsen PH, Meyer RL, Otzen DE. Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front Microbiol. 2015;6:1099. doi: 10.3389/fmicb.2015.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol. 2012;28:523–553. doi: 10.1146/annurev-cellbio-101011-155750. [DOI] [PubMed] [Google Scholar]
- 71.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


