Abstract
Angiogenesis is considered to be an important biological process in disease progression and tumorigenesis. Among the various factors associated with angiogenesis, vascular endothelial growth factor (VEGF) is a leading candidate. VEGF induces the proliferation, differentiation, and migration of vascular endothelial cells, increases capillary permeability, and enhances endothelial cell survival by preventing apoptosis. This article reviews and highlights the role of VEGF in health, and various oral diseases.
Keywords: Oral diseases, Oral cancer, Angiogenesis, Vascular endothelial growth factor (VEGF), Oral squamous cell carcinoma (OSCC)
1. Introduction
The cardiovascular system is the primary organ system to develop in our body. The preliminary steps consist of “vasculogenesis,” which is the precursor process of endothelial cell. The juvenile vascular system evolves from the primary capillary plexus by subsequent pruning and reorganization of endothelial cells in a process called “angiogenesis.”1, 2
Vascular endothelial growth factors (VEGFs) are vital regulators of vascular development during vasculogenesis and angiogenesis.2
VEGF is a type of glycoprotein, which has both angiogenic and mitogenic factors, as well as vascular permeability factor (VPF), thus enhancing activity in endothelial cells. The VEGF family includes VEGF-A, B, C and D, as well as placenta growth factor. In several human cancer, a positive association among VEGF-C and VEGFR-3 expressions was seen in the primary tumor, which has both lymphatic invasion, as well as lymph node metastasis.1
2. History
Virchow is known as the founder of pathological anatomy. He drew awareness to the vast number of blood vessels in a growth mass in about 1865.1 In 1980s, there was the discovery of the first molecules that provoked angiogenesis. Heparin-affinity chromatography was used to purify basic fibroblast growth factor and VEGF. Later on, Senger et al. described the fractional purification of a tumor product, which promoted better vascular permeability in guinea pig skin and had the potency of about 50,000 times than histamine.3, 4 VEGF/VPF is shown in both in vitro and in vivo by various tumor cell lines.1, 2, 3
3. Types of VEGF (VEGF isoforms)
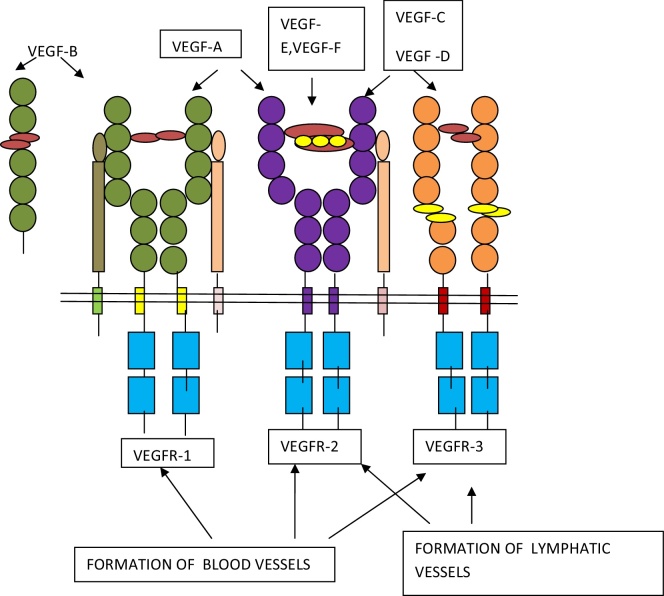
The human VEGF-A gene is structured in eight exons, which is divided by seven introns, and is restricted to chromosome 6p21.3. The exon splicing results in the production of four different isoforms, having 121, 165, 189, and 206 amino acids, VEGF121, VEGF165, VEGF189, VEGF206, and VEGF165. The major isoform lacks the residues encoded by exon 6, whereas VEGF121 lacks exons 6 and 7. Other types include VEGF145, VEGF183, VEGF16, and VEGF165b1, 5 (Fig. 1).6
Fig. 1.
Schematic representation of ligands of VEGF family and their receptors. VEGF-1, 2, and 3 regulate formation of blood vessels, whereas VEGF-2 and 3 regulate the formation of lymphatic vessels.
4. Mode of action of VEGF (VEGF isoforms)
4.1. VEGFR-1 regulates blood vessel morphogenesis
VEGFR-1 is a 180-kDa glycoprotein expressed in many hematopoietic cells. The receptor is required for normal blood vessel development during embryogenesis, since homozygous deletion of VEGFR-1 is lethal in mice at embryonic day E8.5 due to severe malformation of the vasculature.7 More recent data indicate that the kinase activity of VEGFR-1 plays an essential role during pathological angiogenesis and in wound healing, by potentiating VEGFR-2 signaling.8 The interaction of binding sites of VEGFR-1 (A) and VEGFR-2 (B) is shown (Fig. 2). The two VEGF monomers are shown to be in a head-to-tail orientation, which are indicated by arrows, and held together by disulfide bonds, which are in orange. The main cluster of VEGF amino acids that bind VEGFR-1 is located at one end of the VEGF monomer (A); the main cluster of VEGF amino acids that bind VEGFR-2 is located at the opposite pole of the VEGF monomer (B). Although the VEGF domains that bind VEGFR-1 and VEGFR-2 overlap, the main VEGF-binding domain of the VEGFR-1 and VEGFR-2 receptors is located in immunoglobulin-like loop 2, but loop 3 also participates in the binding. For the initiation of signal transduction, the two VEGFR-1 receptors form a dimer that undergoes autophosphorylation on tyrosine residues located in the cytoplasmic part of the VEGFR-1 receptors (P), leading to. The dimer is held together by the interaction of each VEGFR-1 with a common VEGF dimer and is further stabilized by interactions between amino acids located at the loop 4 dimerization domain (C).
Fig. 2.
The interaction of VEGF with its signaling tyrosine kinase receptors.
4.2. VEGFR-2 regulates vessel permeability
VEGF also regulates vascular permeability, so also known as VPF.9 Increase in vascular permeability is seen after VEGF administration, which leads to formation of vesicular-vacuolar organelles10 and fenestrae.11 In mature vessels, VEGF regulates vascular permeability by loosening the junctions between endothelial cells, giving rise to the formation of transcellular gaps. Phosphorylation of major components of tight, adherens, and gap junctions, such as VE-cadherin,12 β-catenin,13 occluding and zonula occluden 1,14, 15and connexin,16 have been reported in response to VEGF.
4.3. VEGFR-3 regulates lymphangiogenesis
VEGFR-3 is synthesized as a 195-kDa-precursor protein consisting of seven extracellular Ig-like domains. Paracrine expression of VEGF-C at sites of lymphatic sprouting further supports a role for VEGFR-3 in the development of lymphatic vessels.17
5. Functions of VEGF in healthy condition
5.1. Blood vessels formation and endothelial survival
An accepted in vitro action of VEGF is the ability to promote growth of vascular endothelial cells derived from arteries, veins, and lymphatics.2 The endothelial cells are the prime objective of VEGF. Various studies have reported mitogenic activity on certain nonendothelial cell types, such as retinal pigment epithelial cells,18 lung epithelial cells,19 and colorectal cells.20
5.2. Relation of NO and VEGF
Angiogenesis, diameter of vessel, rate of blood flow, and vascular permeability were found to be comparative to nitric oxide levels, and it plays an essential or major role in angiogenesis, as well as vascular permeability along with VEGF.1, 2
5.3. Role of VEGF in endochondral bone formation
VEGF mRNA is articulated by hypertrophic chondrocytes in the epiphyseal growth plate, signifying that a VEGF gradient is required for directional growth.1 After VEGF blockade with a soluble VEGFR-1 chimeric protein or an anti-VEGF monoclonal antibody, blood vessel invasion is almost completely suppressed, concomitant with impaired bone formation.21
6. VEGF expression in oral lesions
6.1. Expression of VEGF in premalignant oral lesions and oral squamous cell carcinoma (OSCC)
Salem et al. investigated angiogenesis through cluster of differentiation 34 (CD34) and VEGF expression in oral lichen planus and evaluated the relation of these markers with the degree of inflammation and found a significantly higher rate of positive VEGF expression in lichen planus patients compared with controls.22 Tao et al. studied microvessel density and VEGF level in lichen planus subjects by using immunohistochemistry and ELISA and suggested new insights into the pathogenesis and treatment strategy of lichen planus.23 VEGF expression was studied by Cheng et al. in normal oral mucosa and OSCC with positive lymph node metastasis and found that VEGF were identified as independent unfavorable prognosis factors for OSCC patients.24
The potent biomarkers for carcinogenesis, such as VEGF, matrix metalloproteinases 2 (MMP2), and MMP9, were analyzed by Mukherjee et al. in oral submucous fibrosis and leukoplakia, as well as OSCC in comparison to normal. They found a strong positivity of VEGF, MMP2, and MMP9 in both precancerous and cancerous tissue sections.25
Lalla et al. studied VEGF receptors on tumor cells in head and neck squamous cell carcinoma (HNSCC), and concluded that HNSCC tumor cells expressed VEGFR-1, VEGFR-2, VEGFR-3 in all specimens evaluated and indicated that VEGF may be an autocrine regulator of tumor cell activity in addition to angiogenic effect on endothelial cells.26
6.2. Expression of VEGF in salivary gland tumors
Faur et al. studied 45 cases of salivary gland lesions including 8 pleomorphic adenomas, 7 Warthin tumors, 5 basal cell adenomas, 6 carcinomas ex-pleomorphic adenoma, 6 mucoepidermoid carcinomas, 5 acinic cell carcinomas, 4 adenoid cystic carcinomas, and 4 adenocarcinomas not otherwise specified and concluded that VEGF expression is significantly more important (p = 0.001) in malignant salivary tumors, as compared with benign ones. The VEGF expression and the microvascularization in salivary gland tumors are important elements to be considered for making a diagnosis and assessing case evolutions in patients with such tumors.27
6.3. Expression of VEGF in inflammatory and reactive lesions
VEGF expression was detected in healthy gingival, periodontitis and pyogenic granuloma by Yuan et al. to clarify the pathogenesis of pyogenic granuloma. They found that pyogenic granuloma expressed significantly more VEGF and the positive staining was mostly localized in the cytoplasm of macrophages and fibroblasts.28
6.4. Expression of VEGF in odontogenic cysts and tumors
Rubini et al. established a significant different expression of VEGF in keratocysts compared to follicular cysts. Both parakeratotic odontogenic keratocysts and orthokeratotic odontogenic keratocysts showed more than 50% VEGF-positive epithelial cells, whereas the majority of epithelial cells of follicular cysts were either negative or showed less than 10% positive cells. Similarly, both parakeratotic and orthokeratotic keratocysts showed more than 50% positive endothelial cells, whereas the follicular cysts were either negative or showed less than 10% VEGF-positive endothelial cells. There were no statistically significant differences observed between parakeratotic and orthokeratotic keratocysts in VEGF expression in the epithelial and endothelial cells. Their results supported the hypothesis that angiogenesis is an active mechanism in the invasive growth of the odontogenic keratocyst or keratocystic odontogenic tumor.29
VEGF was studied in radicular and residual cysts by Ruitz et al., and its expression in epithelial lining of cysts might be important for the enlargement of these lesions and VEGF might play important roles in the angiogenesis in residual cysts and radicular cysts.30
Mitrou et al. studied VEGF in odontogenic cysts, which was expressed in the epithelium of odontogenic keratocysts, dentigerous cysts, and radicular cysts in varying intensity, and concluded that VEGF expression by the odontogenic epithelium is not induced solely by inflammation.31 Leonardi et al. studied detection of VEGF/VPF in periapical lesions, using anti-VEGF antibody and observed that, in periapical granulomas without epithelium, almost all of the inflammatory cells were immunoreactive to anti-VEGF/VPF antibody. On the other hand, epithelial cells always were stained by VEGF/VPF antibody, both in periapical lesions with epithelium and in radicular cysts. They concluded that VEGF/VPF expression plays an important role in the pathogenesis and enlargement of radicular cysts by several mechanisms.32
6.5. Expression of VEGF in ameloblastoma
Chen et al. determined the correlation between the expression of inducible nitric oxide synthase, VEGF, and CD34 in ameloblastoma and examined the relationships of this expression to angiogenesis with the clinical and biological behaviors of the tumor. They concluded that inducible nitric oxide synthase expression and VEGF expression may be closely related to the angiogenesis and invasive biological behavior of ameloblastomas.33
The expression of VEGF and microvessel density by using anti-CD31 antibody was assessed by Nouaem and concluded that the VEGF acts as an important factor of angiogenesis in the epithelial odontogenic tumors.34 Kumamoto et al. evaluated that immunoreactivity for VEGF was detected in both normal and neoplastic odontogenic epithelial cells, and weakly in microvessels near odontogenic epithelial cells, suggesting that this angiogenic factor acts on endothelial cells via a paracrine mechanism in odontogenic tissues.35
6.6. Expression of VEGF in other lesions
Amura et al. established VEGF, both in epithelial cells and also cystic fluid of liver cysts. They also showed that cystic epithelial cells also express VEGF receptors. They showed that VEGF can promote cholangiocyte proliferation, which is inhibited by VEGF receptor inhibitor.36
In a study done by Sato et al. on VEGF in cyst fluid of enlarging and recurrent thyroid nodules, it was noticed that there is increased concentration of VEGF in cyst fluid, which is gradually increasing in size. This suggests that VEGF is at least involved in the pathogenesis of cyst enlargement.37
6.7. Expression of VEGF in vascular diseases
6.7.1. Hemangioma
Dyduch studied the expression of GLUT-1, VEGF, Ki-67, and apoptotic index in cases of infantile capillary hemangioma, lobular capillary hemangioma, and epithelioid hemangioma and showed that the proliferative index and VEGF expression were highest in lobular capillary hemangioma.38 According to Mahajan et al., high VEGF expression leads to increased angiogenic activity in endothelial cells of cavernous hemangioma.39
6.7.2. Lymphangioma
Lymphangioma on a mouse model was studied by Mancardi et al. and found that in addition to VEGFR-1 and VEGFR-3, the newly formed tumor endothelium also expresses intracellular adhesion molecule-1.40
6.7.3. Kaposi's sarcoma
VEGF expression was found to be strongly expressed by the blood vessels surrounding Kaposi's sarcoma but not the spindle-shaped cells, as evaluated by Skobe et al.41
7. Conclusion
In recent years, VEGF occupies a central position in the process of angiogenesis, and advances in the understanding of their contribution to the vascular homeostasis have been made. The identification of different isoforms has raised the possibility of more detailed diagnostic and prognostic profiling and follow-up of various diseases. Increased levels of this angiogenic factor could enhance tumor growth by promoting neovascularization. It may also become an important target for cancer therapy. Hence, VEGF expression may be a useful marker in the assessment of angiogenesis in developing precancerous lesions and invasive OSCCs. Thus, the examination of VEGF expression before treatment may be useful in assessing the biologic behavior of tumors and thereby suggesting antiangiogenic therapies, thus leading to early detection and reduced mortality and morbidity in cases of oral cancer. The development of new agents capable of modifying the function of these endothelial molecules has led to the use of novel treatments in both malignant and nonmalignant diseases. More insight is needed into the exact role of various growth mediators, the pathways they initiate, and the changes, which may occur by inhibiting its receptors, and thus it may act as a prognostic marker for the malignant lesions.
Conflicts of interest
The authors have none to declare.
References
- 1.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N., Davis S.T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 3.Olsson A.K., Dimberg A., Kreuger J., Welsh L.C. VEGF receptor signalling in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 4.Senger D.R., Connolly D.T., Peruzzi C.A. Purification of vascular permeability factor (VPF) from tumor cell conditioned medium. Fed Proc. 1987;46:2102. [Google Scholar]
- 5.Ribatti D., Vacca A., Dammacco F. The role of the vascular phase in solid tumor growth: a historical review. Neoplasia. 1999;1(4):293–302. doi: 10.1038/sj.neo.7900038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suarez S.C., Fjällman A.Z., Hofer K.B. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci. 2006;63:601–615. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong G.H., Rossant J., Gertsenstein M., Breitman M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 8.Autiero M., Waltenberger J., Communi D. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 9.Senger D.R., Galli S.J., Dvorak A.M., Perruzzi C.A., Harvey V.S., Dvorak H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 10.Dvorak A.M., Feng D. The vesiculo-vacuolar organelle (VVO): a new endothelial cell permeability organelle. J Histochem Cytochem. 2001;49:419–432. doi: 10.1177/002215540104900401. [DOI] [PubMed] [Google Scholar]
- 11.Roberts W.G., Palade G.E. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57:765–772. [PubMed] [Google Scholar]
- 12.Esser S., Lampugnani M.G., Corada M., DeJana E., Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111:1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 13.Cohen A.W., Carbajal J.M., Schaeffer R.C., Jr. VEGF stimulates tyrosine phosphorylation of beta-catenin and small-pore endothelial barrier dysfunction. Am J Physiol. 1999;277:2038–2049. doi: 10.1152/ajpheart.1999.277.5.H2038. [DOI] [PubMed] [Google Scholar]
- 14.Kevil C.G., Payne D.K., Mire E., Alexander J.S. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem. 1998;273:15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- 15.Antonetti D.A., Barber A.J., Hollinger L.A., Wolpert E.B., Gardner T.W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1: a potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 16.Suarez S., Ballmer-Hofer K. VEGF transiently disrupts gap junctional communication in endothelial cells. J Cell Sci. 2001;114:1229–1235. doi: 10.1242/jcs.114.6.1229. [DOI] [PubMed] [Google Scholar]
- 17.Kukk E., Lymboussaki A., Taira S., Kaipainen A., Jeltsch M., Joukov V. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 18.Oshima Y., Oshima S., Nambu H. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J Cell Physiol. 2004;201(3):343–400. doi: 10.1002/jcp.20110. [DOI] [PubMed] [Google Scholar]
- 19.Brown K.R., England K.M., Goss K.L., Snyder J.M., Acarregui M.J. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol. 2001;281(October (4)):L1001–L1010. doi: 10.1152/ajplung.2001.281.4.L1001. [DOI] [PubMed] [Google Scholar]
- 20.Simiartonari N. Epithelial expression of VEGF receptors in colorectal carcinomas. Anticancer Res. 2007;27:3245–3250. [PubMed] [Google Scholar]
- 21.Kumar V., Abbas A.K., Fausto N. 7th ed. Elsevier Saunders; Philadelphia: 2005. Robbins and Cotran Pathologic Basis of Disease. [Google Scholar]
- 22.Salem S.A., Aly D.G., Youssef N.S. Immunohistochemical assessment of angiogenesis and vascular endothelial growth factor expression in cutaneous lichen planus: relation to the degree of inflammation. Eur J Dermatol. 2011;21(2):197–202. doi: 10.1684/ejd.2011.1221. [DOI] [PubMed] [Google Scholar]
- 23.Tao X., Huang Y., Li R. Assessment of local angiogenesis and vascular endothelial growth factor in the patients with atrophic-erosive and reticular oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(5):661–669. doi: 10.1016/j.tripleo.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Cheng S.J., Lee J.J., Kok S.H. Expression of vascular endothelial growth factor is significantly associated with progression and prognosis of oral squamous cell carcinomas in Taiwan. J Formos Med Assoc. 2011;110(1):50–57. doi: 10.1016/S0929-6646(11)60008-9. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee S., Ray J.G., Chaudhuri K. Microscopic analysis of histological and immunohistochemical sections to differentiate normal, precancer and cancerous oral squamous epithelial tissues. In: Méndez-Vilas A., Díaz J., editors. Microscopy: Science, Technology, Applications and Education. ©FORMATEX; 2010. [Google Scholar]
- 26.Lalla R.V., Boisoneau D.S., Spiro J.D., Kreutzer D.L. Expression of VEGF receptors on tumor cells in HNSCC. Arch Otolaryngol Head Neck Surg. 2003;129:882–888. doi: 10.1001/archotol.129.8.882. [DOI] [PubMed] [Google Scholar]
- 27.Faur A.C., Lazar E., Cornianu M. Vascular endothelial growth factor (VEGF) expression and microvascular density in salivary gland tumours. APMIS. 2014;122(5):418–426. doi: 10.1111/apm.12160. [DOI] [PubMed] [Google Scholar]
- 28.Yuan K., Jin Y.T., Lin M.T. The detection and comparison of angiogenesis-associated factors in pyogenic granuloma by immunohistochemistry. J Periodontol. 2000;71(5):701–709. doi: 10.1902/jop.2000.71.5.701. [DOI] [PubMed] [Google Scholar]
- 29.Rubini C., Artese L., Zizzi A. Immunohistochemical expression of vascular endothelial growth factor (VEGF) in different types of odontogenic cysts. Clin Oral Investig. 2011;15(5):757–761. doi: 10.1007/s00784-010-0433-7. [DOI] [PubMed] [Google Scholar]
- 30.Ruitz P.A., Toledo O.A., Pinto L.P. Immunohistochemical expression of vascular endothelial growth factor and matrix metalloproteinase-9 in radicular and residual radicular cysts. Appl Oral Sci. 2010;18(6):613–620. doi: 10.1590/S1678-77572010000600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitrou G.K., Tosios K.I., Kyroudi A., Sklavounou A. Odontogenic keratocyst expresses vascular endothelial growth factor: an immunohistochemical study. J Oral Pathol Med. 2009;38:470–475. doi: 10.1111/j.1600-0714.2009.00755.x. [DOI] [PubMed] [Google Scholar]
- 32.Leonardi R., Caltabiano M., Pagano M., Pezzuto V., Loret C., Palestro G. Detection of vascular endothelial growth factor/vascular permeability factor in periapical lesions. J Endod. 2003;29(3):180–183. doi: 10.1097/00004770-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Chen W.L., Ouyang K.X., Li H.G., Huang Z.Q., Li J.S., Wang J.G. Expression of inducible nitric oxide synthase and vascular endothelial growth factor in ameloblastoma. IOFAC Surg. 2009;20(1):171–175. doi: 10.1097/SCS.0b013e31818435cd. [DOI] [PubMed] [Google Scholar]
- 34.Nouaem M.I.E. Association between vascular endothelial growth factor (VEGF) expression and tumor angiogenesis in benign and malignant ameloblastomas. Egypt Dent J. 2005;51:177–186. [Google Scholar]
- 35.Kumamoto H., Ohki K., Ooya K. Association between vascular endothelial growth factor (VEGF) expression and tumor angiogenesis in ameloblastomas. J Oral Pathol Med. 2002;31:28–34. doi: 10.1046/j.0904-2512.2001.10061.x. [DOI] [PubMed] [Google Scholar]
- 36.Amura C.R., Brodsky K.S., Gattone V.H., Voelkel N.F., Doctor R.B. VEGF receptor inhibition blocks liver cysts growth in pkd2(WS25/−) mice. Am J Physiol. 2007;293:419–428. doi: 10.1152/ajpcell.00038.2007. [DOI] [PubMed] [Google Scholar]
- 37.Sato K., Miyakawa M., Onoda N. Increased concentration of vascular endothelial growth factor/vascular permeability factor in cyst fluid of enlarging and recurrent thyroid nodules. J Clin Endocrinol Metab. 1997;82(6):1968–1973. doi: 10.1210/jcem.82.6.3989. [DOI] [PubMed] [Google Scholar]
- 38.Dyduch G., Okon K., Mierzyn W. Benign vascular proliferations – an immunohistochemical and comparative study. Pol J Pathol. 2004;55(2):59–64. PL ISSN 1233-9687. [PubMed] [Google Scholar]
- 39.Mahajan D., Miller C., Hirose K., McCullough A., Yerian L. Incidental reduction in the size of liver hemangioma following use of VEGF inhibitor bevacizumabq. J Hepatol. 2008;49:867–870. doi: 10.1016/j.jhep.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 40.Mancardi S., Stanta G., Dusetti N. Lymphatic endothelial tumors induced by intraperitoneal injection of incomplete Freund's adjuvant. Exp Cell Res. 1999;246:368–375. doi: 10.1006/excr.1998.4270. [DOI] [PubMed] [Google Scholar]
- 41.Skobe M., Brown L.F., Tognazzi K. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi's sarcoma. J Investig Dermatol. 1999;113:1047–1053. doi: 10.1046/j.1523-1747.1999.00798.x. [DOI] [PubMed] [Google Scholar]