Abstract
Melanotic neuroectodermal tumour of infancy (MNTI) is rare, rapidly growing, pigmented neoplasm of neural crest origin. It is generally accepted as a benign tumour despite of its rapid and locally destructive growth. It primarily affects the maxilla of infants during the first year of life. Surgical excision is considered as the treatment of choice. The recurrence rate varies between 10% and 15%, and malignant behaviour has been reported in 6.5% of cases. We report a case of MNTI, associated with an erupted primary tooth in a 5-month-old male child. We discuss the clinical, radiographic and histologic features of this rare tumour, as well as its surgical management and the follow-up.
Keywords: Melanotic, Neuroectoderm, Infancy, Tumour, Paediatric, Pigment
1. Introduction
Melanotic neuroectodermal tumour of infancy (MNTI) is a very rare benign neoplasm that occurs in early infancy. It was first described by Krompecher in 1918.1 The confusion regarding the tumour's origin is reflected in its variable nomenclature; such as congenital melanocarcinoma, retinal anlage tumour, pigmented congenital epulis or melanotic progonoma.2 In 1966, Borello and Gorlin suggested that the tumour originated from neural crest cells based on the fact that some of these tumours excreted large amounts of vanillylmandelic acid (VMA) which is associated with other neuroectodermal tumours as well.3, 4, 5 A total of 472 cases of this tumour had been reported between 1918 and 2013 as noted in the last extensive review published in 2015.5 We report a case of MNTI, associated with an erupted primary tooth in a 5-month-old male child. We discuss the clinical, radiographic and histologic features of this rare tumour, as well as its surgical management and the follow-up.
2. Case report
A 5-month-old male child presented with a mass in the anterior maxilla that had been growing since 2 months. The swelling was initially associated with an erupting tooth for which the parents reported to a private practitioner who extracted the tooth. The swelling then began to rapidly increase in size to the point that it obstructed the oral cavity and caused difficulties in feeding. The patient was referred to our Department of Oral and Maxillofacial Surgery where a provisional diagnosis of MNTI was made.
Physical examination showed prominent external facial deformity. A dome-shaped sessile mass was present in the right anterior maxillary alveolar ridge and hard palate region. The mass had multiple patches of bluish-black pigmentation that were suggestive of melanotic discolouration. Areas of ulceration were also present on account of trauma from the hands of the infant. The rapidly increasing size of the lesion caused difficulties in breathing and feeding and also increased the risk of aspiration. Urinary VMA levels were found to be normal (Fig. 1).
Fig. 1.

Dome shaped swelling in the anterior maxilla causing considerable external facial deformity, obstruction of oral cavity, difficulty in feeding and breathing.
Contrast enhanced computed tomography revealed an expansile osteolytic lesion [4 cm × 3 cm × 3 cm] present in the right maxillary alveolar ridge region and anterior part of hard palate and crossing the midline for a small extent. Well defined sclerotic borders were present along the superior aspect of the lesion in the region of the nasal cavity (Fig. 2).
Fig. 2.
Contrast enhanced computed tomography depicting an expansile osteolytic lesion present in the anterior maxillary region.
The specimen obtained during the biopsy revealed macroscopic bluish-black coloured gelatinous deposits. Histological examination was suggestive of MNTI. The cells were positive for immunohistochemical markers like HMB45 (Human Melanoma Black 45), Neuron Specifc Enolase (NSE), Pan CK (Cytokeratin) and Synaptophysin. Thus, a definitive diagnosis of MNTI was made.
The patient was taken under general anaesthesia. An incision was made in the mucosa along the boundaries of the tumour while maintaining a sufficient margin. Mucosa was reflected along the incision to expose healthy bony margins. The tumour was resected along with the embedded tooth germs (Fig. 3). Soft and hard tissue margins were further resected until found to be clear with the help of frozen sections. No oro-antral or oro-nasal communication was found to be present. On account of the large size of the surgical defect, primary closure could not be obtained and it was decided to allow healing by secondary granulation. The raw surface was covered with a layer of collagen membrane [reinforced with sofratoulle] by suturing it to the surrounding soft tissue margins (Fig. 4). The post-operative recovery was uneventful. At 3 months follow-up, the patient showed satisfactory healing and no signs of recurrence (Fig. 5).
Fig. 3.
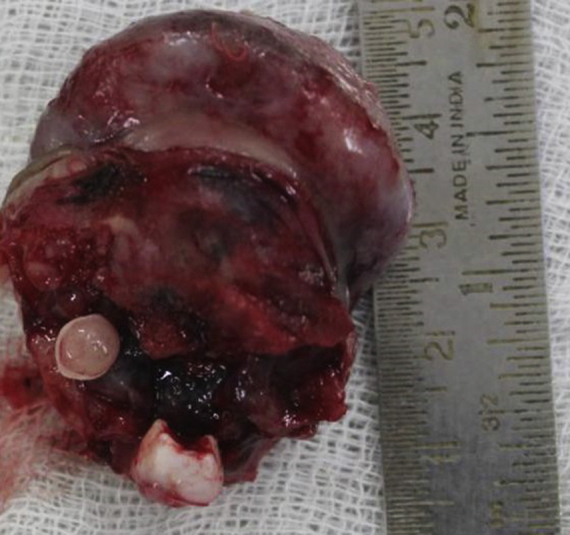
Resection of the tumour mass. Embedded tooth germs were also removed.
Fig. 4.
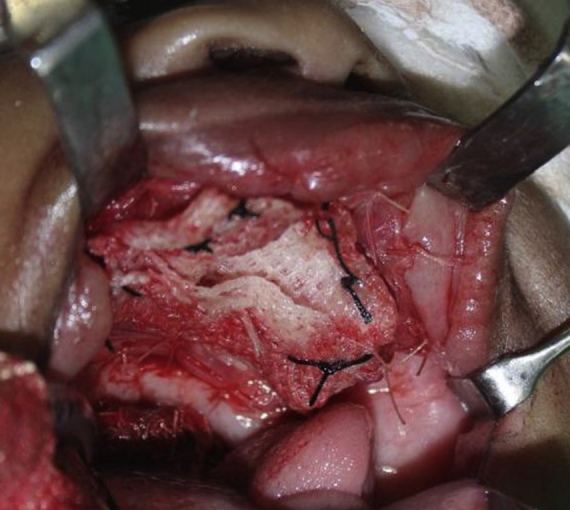
Surgical defect covered with a layer of collagen membrane [reinforced with sofratoulle].
Fig. 5.

Follow-up: intra oral view shows satisfactory healing with an erupting tooth and no signs of recurrence.
3. Discussion
3.1. Clinical presentation
The MNTI mainly arises in the craniofacial region with a predominance for the maxilla [62.2%] followed by the skull [15.6%], mandible [7.8%]. The other sites [14.3%] include the brain, epididymis, mediastinum, ovary, uterus and peripheral bones. There is a slight male predilection.5, 6
The MNTI is never congenital. It emerges within the 1st year of life [mostly below 6 months of age]. Infants present with a painless, non-ulcerative bluish black gingival mass that is often confused with an eruption cyst. It may appear to be malignant due to its rapid growth potential.7
The MNTI will usually carry one of the primary central incisors outward with it, if in the maxilla. It will not carry both central incisors because the tumour arises from one side of the midline.7
The lesion is destructive and radiographs show local irregular resorption of bone and displacement of tooth buds. The only radiopaque components present are those of the developing teeth buds.7
3.2. Differential diagnosis7
-
1.Neoplasm – on account of the rapid growth potential and their predilection for the head and neck region in infants.
-
a.Neuroblastomas.
-
b.Rhabdomyosarcomas.
-
c.Congenital granular cell tumour is present at birth as compared to MNTIs which are never congenital.
-
a.
-
2.
Non-neoplastic hemangiomas and lymphangiomas have bluish discolorations and a predilection for the head and neck region in children and tend to develop rapidly within a few months after birth.7
3.3. Diagnostic work-up7
-
1.
CT scans/occlusal radiograph – central incisor displaced to the periphery of a mass that itself shows destruction of the anterior maxilla.
-
2.
Incisional biopsy – confirmatory diagnosis.
-
3.
VMA [vanillylmandelic acid] level in a 24 h urine collection – elevated in only 10–15% of MNTIs – low diagnostic value.
3.4. Histopathology
The H&E stained section displayed a non-capsulated infiltrating tumour mass arranged in an alveolar pattern in a fibro cellular connective stroma. The alveolar spaces had a biphasic cell population. The peripheral irregular layer of cuboidal cells were large and contained melanin pigment. The central cells were small round and neuroblast like with hyper chromatic nuclei and scant cytoplasm. Based on these features, a histopathological diagnosis of MNTI was made.8, 9, 10
The peripheral cells were positive for immunohistochemical markers HMB45, Neuron Specifc Enolase, Pan CK and Synaptophysin. The central cells were positive for NSE and Synaptophysin (Fig. 6).8, 9, 10
Fig. 6.
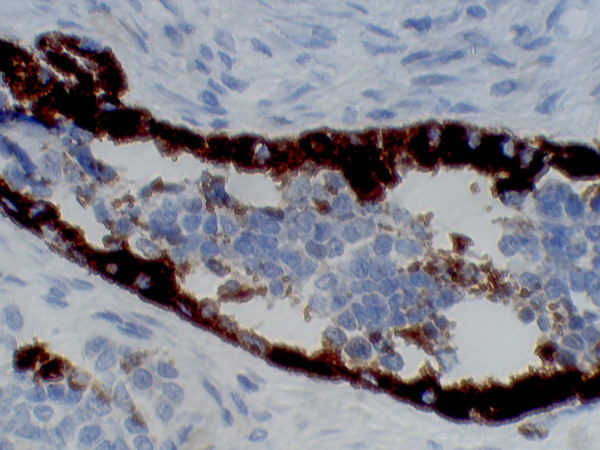
The peripheral and central cells were positive for Neuron Specific Enolase (NSE).
3.5. Management and prognosis
MNTIs have a significant destructive nature with high growth potential and are furthermore unencapsulated. Peripheral excisions with 2–5 mm margins were generally considered as ideal.8 However, a recent systematic review claims that no differences in recurrence rates were observed between curettage and resection. At 5 years after diagnosis, both sexes showed equal recurrence rates.5 The overall incidence of local recurrence was 10–15%.11
Currently, age at manifestation is a considered to be a strong prognostic indicator in MNTI. Infants who manifested within the first 2 months of birth were associated with a high risk of recurrence which generally occurred within 6 months from treatment. In contrast, manifestation from 2.5 to 4 months was associated with an intermediate risk and manifestation after 4.5 months of age had a minimal risk of recurrence.5
4. Conclusion
MNTI is usually a benign tumour arising from cells of neuroectodermal origin. Due to its rapid growth potential and locally destructive behaviour, early diagnosis is extremely important to limit local expansion and the extent of required tissue resection. However, the rarity of the tumour often leads to a delay in diagnosis, resulting in a less than desired outcome.
Conflicts of interest
The authors have none to declare.
Contributor Information
Neelam Noel Andrade, Email: drnnandrade@yahoo.co.in.
Paul C. Mathai, Email: paulmathai89@gmail.com.
Vyankatesh Sahu, Email: vvmsahu@gmail.com.
Neha Aggarwal, Email: agg.neha61@gmail.com.
Tanvi Andrade, Email: tanviandrade@gmail.com.
References
- 1.Krompecher E. Zur Histogenese und morphologie der adaman-tinome und sonstiger kiefergeschewulste. Beitr Pathol Anat. 1918;165 [Google Scholar]
- 2.Lurie H.I. Congenital melanocarcinoma, melanotic adamantinoma, retinal anlage tumor, progonoma, and pigmented epulis of infancy. Cancer. 1961;14:1090–1108. doi: 10.1002/1097-0142(196109/10)14:5<1090::aid-cncr2820140529>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Borello E., Gorlin R. Melanotic neuroectodermal tumor of infancy: a neoplasm of neural crest origin. Cancer. 1966;19:96. doi: 10.1002/1097-0142(196602)19:2<196::aid-cncr2820190210>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Hayward A.F., Fickling B.W., Lucas R.B. An electron microscope study of a pigmented tumour of the jaw of infants. Br J Cancer. 1969;23:702. doi: 10.1038/bjc.1969.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rachidi S., Sood A.J., Patel K.G. Melanotic neuroectodermal tumor of infancy: a systematic review. J Oral Maxillofac Surg. 2015;73(10):1946–1956. doi: 10.1016/j.joms.2015.03.061. [DOI] [PubMed] [Google Scholar]
- 6.Kruse-Lösler B., Gaertner C., Bürger H., Seper L., Joos U., Kleinheinz J. Melanotic neuroectodermal tumor of infancy: systematic review of the literature and presentation of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2006;102(2):204–216. doi: 10.1016/j.tripleo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Marx R.E., Stern D. 2nd ed. 2012. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. [Google Scholar]
- 8.Cutler L.S., Chaudhry A.P., Topazian R. Melanotic neuroectodermal tumor of infancy: an ultrastructural study, literature review, and reevaluation. Cancer. 1981;48(2):257–270. doi: 10.1002/1097-0142(19810715)48:2<257::aid-cncr2820480209>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Melissari M., Traani G., Gaetti L. Melanotic neuroectodermal tumor of infancy (MNTI): immunohistochemical and ultra-structural study of a case. J Craniomaxillofac Surg. 1988;16:330. doi: 10.1016/s1010-5182(88)80073-8. [DOI] [PubMed] [Google Scholar]
- 10.Stirling R.W., Powell G., Fletcher C.D.M. Pigmented neuroectodermal of infancy: an immunohistochemical study. Histopathology. 1988;12:425. doi: 10.1111/j.1365-2559.1988.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary A., Wakhlu A., Mittal N. Melanotic neuroectodermal tumor of infancy: 2 decades of clinical experience with 18 patients. J Oral Maxillofac Surg. 2009;67:47. doi: 10.1016/j.joms.2007.04.027. [DOI] [PubMed] [Google Scholar]



