Abstract
Aim
This study was designed to assess the reliability of blood glucose level estimation in gingival crevicular blood(GCB) for screening diabetes mellitus.
Materials and method
70 patients were included in study. A randomized, double-blind clinical trial was performed. Among these, 39 patients were diabetic (including 4 patients who were diagnosed during the study) and rest 31 patients were non-diabetic. GCB obtained during routine periodontal examination was analyzed by glucometer to know blood glucose level. The same patient underwent for finger stick blood (FSB) glucose level estimation with glucometer and venous blood (VB) glucose level with standardized laboratory method as per American Diabetes Association Guidelines.1 All the three blood glucose levels were compared. Periodontal parameters were also recorded including gingival index (GI) and probing pocket depth (PPD).
Results
A strong positive correlation (r) was observed between glucose levels of GCB with FSB and VB with the values of 0.986 and 0.972 in diabetic group and 0.820 and 0.721 in non-diabetic group. As well, the mean values of GI and PPD were more in diabetic group than non-diabetic group with the statistically significant difference (p < 0.005).
Conclusion
GCB can be reliably used to measure the blood glucose level as the values were closest to glucose levels estimated by VB. The technique is safe, easy to perform and non-invasive to the patient and can increase the frequency of diagnosing diabetes during routine periodontal therapy.
Keywords: Diabetes mellitus, Glucometer, Gingival crevicular blood, Finger stick blood, Venous blood
1. Introduction
Advances in science and technology, over the last century, have greatly expanded our knowledge about the relationship of periodontitis with systemic diseases. Periodontal diseases and diabetes mellitus (DM) are closely associated and are highly prevalent chronic diseases with many similarities in pathobiology.2
DM is a complex disease of multiple conditions and syndromes which have glucose intolerance in common.3 DM is associated with a wide range of complications, such as retinopathy, nephropathy, micro and macro vascular diseases, altered wound healing and periodontitis.4 DM is the one of the most frequent metabolic disorders with estimated prevalence of 7% in industrialized countries of which nearly half of cases are undiagnosed. India has nearly 33 million diabetic subjects today with an overall prevalence rate of 4.3%.5
Type 2 DM i.e. non-insulin dependent diabetes mellitus (NIDDM) constitutes nearly 90% of diabetic population in any country, with a prevalence of 2.4% in rural population and 11.6% in urban population.6 The current classification of periodontal disease and conditions lists DM associated gingivitis under dental plaque induced gingival diseases modified by systemic factors.7
The level of diabetic control is a more important aspect than plaque control in relation to the severity of gingival inflammation. Periodontitis has been proposed as a sixth complication of DM.8 The early diagnosis of diabetes, however, might help to prevent its long-term complications that are responsible for the high morbidity and mortality of diabetic patients.9
Routine probing during a periodontal examination is more familiar to the practitioner and less traumatic. It is possible that gingival crevicular blood (GCB) from probing may be an excellent source of blood glucometric analysis using the technology of portable glucose monitors and therefore no extra procedure, e.g. finger puncture with sharp lancet, is necessary to obtain blood for glucometric analysis.
Even in the case of very low gingival crevicular bleeding, a glucose measurement is possible with the self-monitoring device. Also, the sampling procedure is much easier to perform and less time-consuming. The present study was planned therefore to assess the GCB for estimation of blood glucose level, and to compare this glucose level with that of finger stick blood (FSB) and venous blood (VB)in diabetic and non-diabetic subjects.
2. Materials and methods
The study population comprised of patients visiting the Department of Periodontics, Government Dental College and Hospital, Ahmedabad, Gujarat, India. Ethical clearance for study was taken from institutional ethical committee. All the patients, underwent the study had been fully informed and given written consent for the study procedure as per Declaration of Helsinki. A randomized, double-blind clinical trial was performed over 70 adult patients comprising 28 males and 42 females. Initially, among these 35 patients were known diabetic (diabetic group) and rest 35 patients were unaware of their glycemic status (non-diabetic group). The inclusion criteria for the study were: subjects in the age group of 20–70 years, subjects having at least twenty remaining teeth and patients with moderate to advanced periodontitis.
The exclusion criteria were: subjects with intake of supplemental ascorbic acid [vitamin C], which could interfere with the glucose test strip oxidation reaction, subjects with history of prolonged usage drugs that interfere with the coagulation system, e.g. coumarin derivatives, non-steroidal anti-inflammatory drugs (NSAID),subjects previously diagnosed with polycythemia, severe anemia or those undergoing renal dialysis or subjects with history of severe cardiovascular, hepatic, immunologic, renal, hematological, or other organ impairment, pregnant woman and nursing mothers, subjects with any history of periodontal treatment during past 6 months, requirement for antibiotic premedication or need for any medication, tooth with suppuration or any disorder that can cause abnormally low or high hematocrit value.
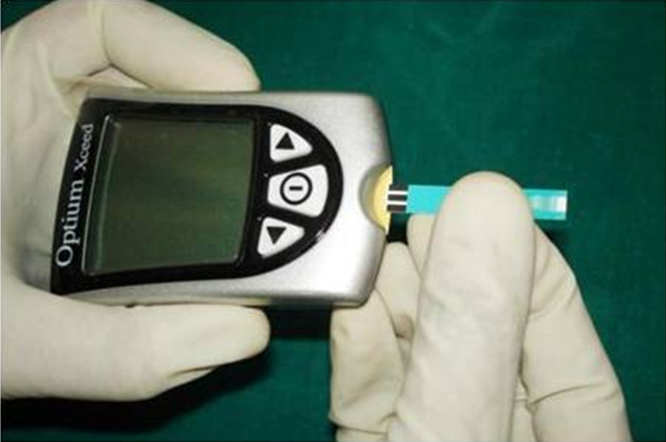
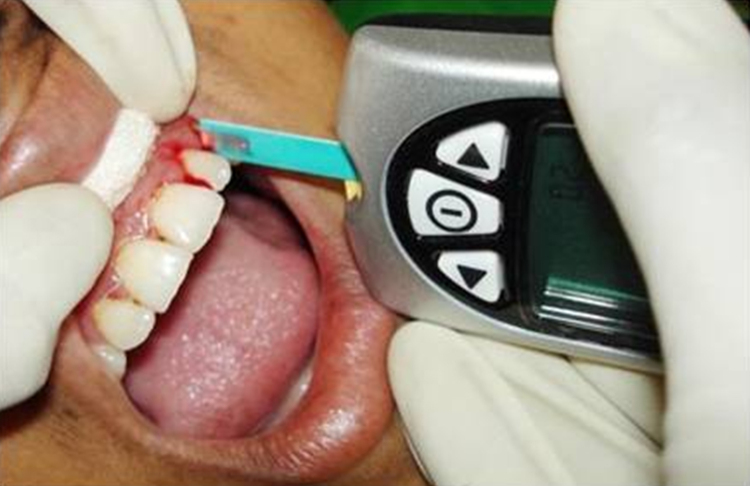
Blood from three regions was assessed for blood glucose estimation: gingival crevice, finger bed and anterior cubital vein. For the estimation of blood glucose level using GCB, the test site was isolated with cotton roll and air-dried. The glucometer(Optium Xceed glucometer-IInd generation) was turned on by insertion of the test strip in the provided slot(Fig. 1). UNC-15 probe was gently passed along the gingival sulcus. A blood drop was allowed to touch into the test area of the strip (Fig. 2). The result of the test was displayed on the screen of the glucometer, after around 20 s. The value was recorded.
Fig. 1.
Insertion of glucometer test strip.
Fig. 2.
Estimation of blood glucose using gingival crevicular blood (GCB) with glucometer.
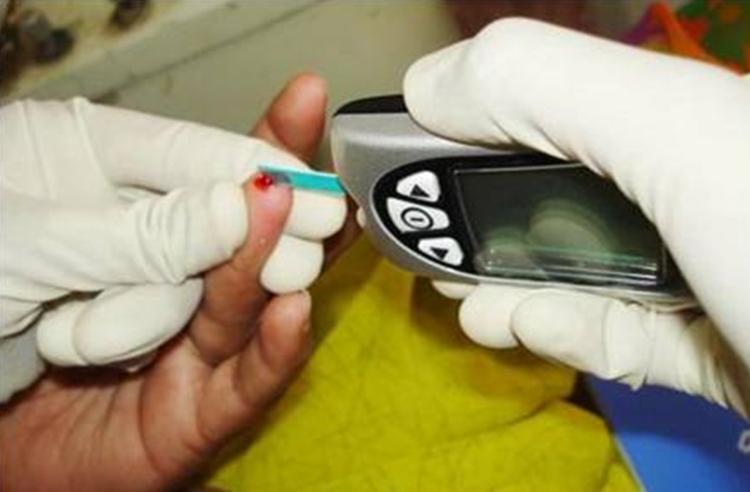
For the estimation of blood glucose level using FSB, the soft surface of the fingertip was wiped with surgical spirit and the spirit was allowed to evaporate. Inserting the test strip into its slot turned on the glucometer. The surface of the finger was then punctured with a sterile lancet and the drop of blood oozing was allowed to be drawn into the test area of the strip (Fig. 3). After the test time around 20 s, the result of the test was displayed on the screen of the glucometer. The value was recorded.
Fig. 3.
Estimation of blood glucose using finger stick blood (FSB) with glucometer.
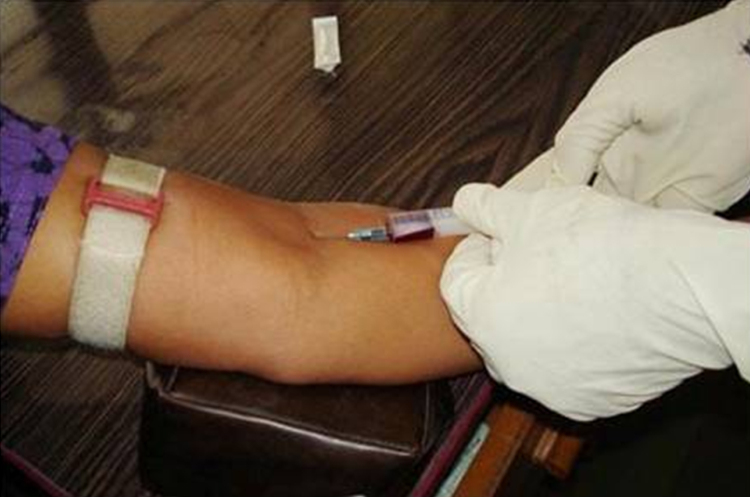
Immediately after these two tests, the estimation of blood glucose level using VB was carried out. Blood was obtained by venepuncture from the anterior cubital vein, using a sterile syringe and needle (Fig. 4). 2 ml of blood was collected in a plane bulb. With the help of automated chemistry analyzer the venous blood glucose level was recorded.
Fig. 4.
Collection of venous blood (VB) sample for the estimation of blood glucose.
Recording of periodontal parameters was also done including gingival index (GI) and probing pocket depth (PPD), taken by UNC-15 periodontal probe.
The data was tabulated and subjected to statistical analysis which included mean and standard deviations of all the parameters, Karl Pearson correlation coefficient, unpaired ‘t’ test and associated ‘p’ values.
3. Statistics and results
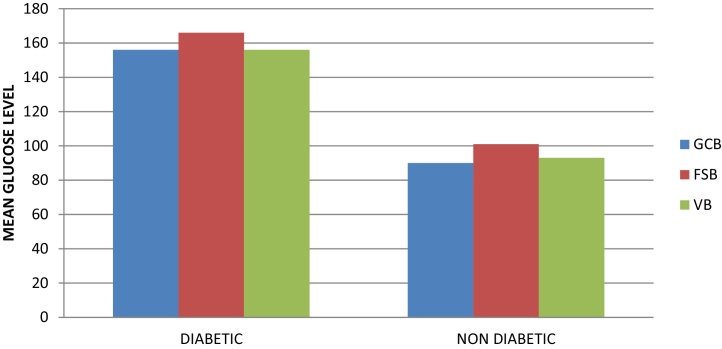
The GCB glucose level estimation in a total of 70 subjects showed that among the 35 subjects with unknown glycemic status, 4 subjects were diabetic (new diabetic patients). So finally, the study comprised 39 diabetic patients (diabetic group) and 31 non-diabetic subjects (non-diabetic group). For the diabetic group, the blood glucose levels were in the range of 92–262 mg/dl, with a mean of 156.07 ± 49.23 mg/dl. For the non-diabetic group, blood glucose levels were in the range of 76–122 mg/dl, with mean of 90.80 ± 11.07 mg/dl (Table 1, Graph 1).
Table 1.
Blood glucose estimation from gingival crevicular blood (GCB).
| Number of subjects | Range of blood glucose level (mg/dl) | Mean | SD |
|---|---|---|---|
| 39 (Diabetic group) | 92–262 | 156.07 | ±49.23 |
| 31 (Non-diabetic group) | 76–122 | 90.80 | ±11.07 |
Graph 1.
Mean glucose level at different sites in two groups.
The comparison of blood glucose levels of GCB with FSB and VB showed that for diabetic group, the mean GCB, FSB and VB glucose level were 156.07 ± 49.23 mg/dl, 166.61 ± 52.18 mg/dl and 156.12 ± 49.89 mg/dl respectively. Similarly, for the non-diabetic group, the mean GCB, FSB and VB glucose level were 90.80 ± 11.07 mg/dl, 101.35 ± 13.05 mg/dl and 93.41 ± 9.30 mg/dl respectively. The results were statistically highly significant (p < 0.005) in both the groups for GCB with FSB while non-significant (p > 0.005) for GCB with VB in both the groups (Table 2).
Table 2.
Comparison and correlation (r) of blood glucose levels of gingival crevicular blood (GCB) with finger stick blood (FSB)and venous blood (VB).
| Group | Variable | Mean ± SD (mg/dl) | ‘p’ value and correlation (r) |
|---|---|---|---|
| Diabetic group | GCB | 156.07 ± 49.23 | |
| FSB | 166.61 ± 52.18 |
p < 0.005, HS r = 0.986 |
|
| VB | 156.12 ± 49.89 |
p > 0.005, NS r = 0.972 |
|
| Non-diabetic group | GCB | 90.80 ± 11.07 | |
| FSB | 101.35 ± 13.05 |
p < 0.005, HS r = 0.820 |
|
| VB | 93.41 ± 9.30 |
p > 0.005, NS r = 0.721 |
|
p < 0.005, significant; p > 0.005, not significant.
1 > r > −1, significant correlation; r = 0, no correlation.
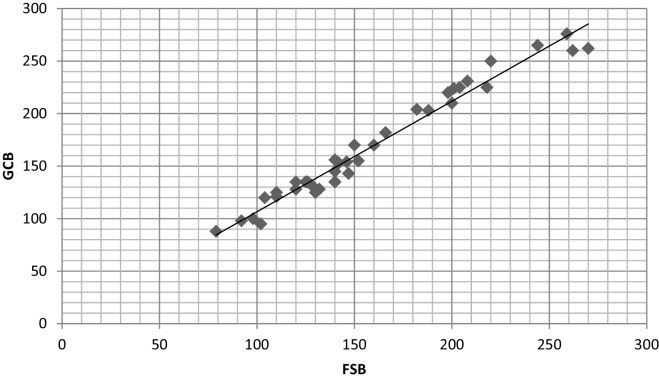
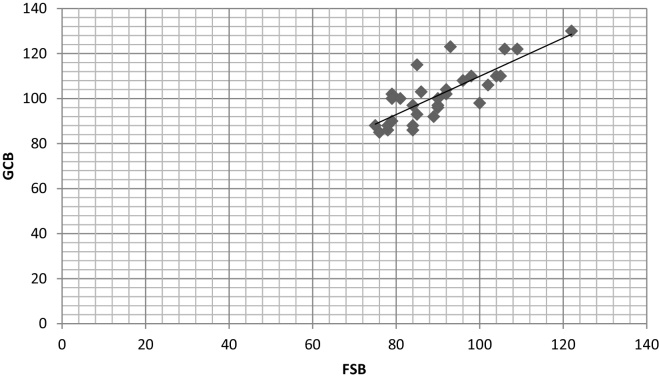
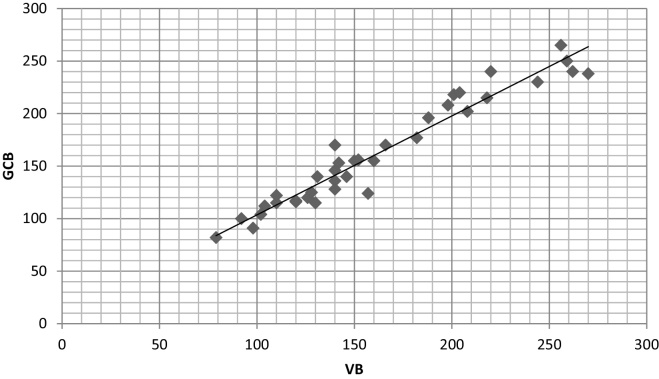
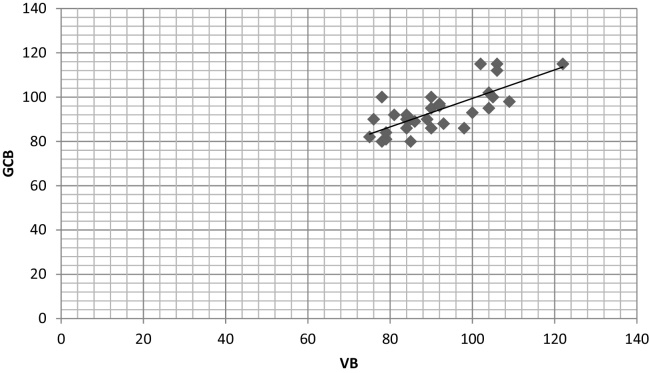
The correlation coefficient (r) in both the groups showed a strong positive correlation between glucose levels of GCB with FSB and VB with the values of 0.986 and 0.972 in diabetic group and 0.820 and 0.721 in non-diabetic group respectively (Table 2, Scatter Graph 4, Graph 5, Graph 6, Graph 7).
Graph 4.
Scatter graph showing correlation between GCB and FSB in diabetic group.
Graph 5.
Scatter graph showing correlation between GCB and FSB in non-diabetic group.
Graph 6.
Scatter graph showing correlation between GCB and VB in diabetic group.
Graph 7.
Scatter graph showing correlation between GCB and VB in non-diabetic group.
The correlation between the glucose levels of FSB and VB showed a coefficient (r) value of 0.972 and 0.808 in both diabetic and non-diabetic groups and represents a strong positive relationship (Table 3).
Table 3.
Correlation of blood glucose levels of finger stick blood (FSB) with venous blood (VB).
| Group | Correlation (r) |
|---|---|
| Diabetic | 0.972 |
| Non-diabetic | 0.808 |
1 > r > −1, significant correlation; r = 0, no correlation.
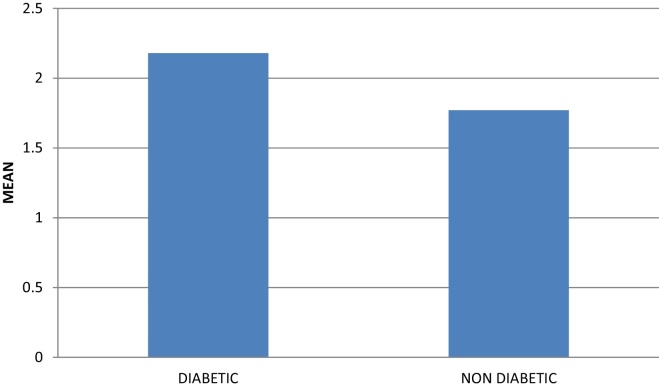
On comparing the GI between the diabetic and non-diabetic groups, a statistically significant difference (p < 0.005) was obtained with mean gingival index score of 2.18 ± 0.39 mm for diabetic group and 1.77 ± 0.28 mm for non-diabetic group (Table 4, Graph 2).
Table 4.
Gingival index (in mm) and probing pocket depth (in mm).
| Group | Mean | Standard deviation | ‘p’ value |
|---|---|---|---|
| Gingival index | |||
| Diabetic | 2.18 | 0.39 | <0.0005 |
| Non-diabetic | 1.77 | 0.28 | <0.0005 |
| Probing pocket depth | |||
| Diabetic | 4.43 | 0.97 | <0.0005 |
| Non-diabetic | 3.96 | 0.75 | <0.031 |
p < 0.005, significant; p > 0.005, not significant.
Graph 2.
Mean of gingival index in diabetic and non-diabetic groups.
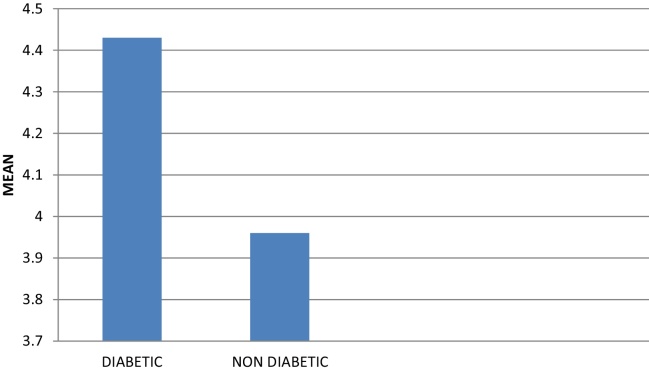
Similarly, on comparison of PPD between the diabetic and non-diabetic groups, a mean depth score obtained for diabetic group was 4.43 ± 0.97 mm and 3.96 ± 0.75 mm for non-diabetic group which was also statistically significant (p < 0.005) (Table 4, Graph 3).
Graph 3.
Mean of probing pocket depth in diabetic and non-diabetic groups.
4. Discussion
There is a two-way relationship between DM and periodontitis. On one hand, poorly controlled DM increases the risk for developing destructive periodontitis and impairs treatment outcome. On the other hand, chronic inflammatory periodontal disease considerably complicates diabetic control.10
Due to this close interrelationship between diabetes and periodontitis, it can be assumed that the dental practitioners especially periodontist are extremely likely to encounter an increasing number of undiagnosed diabetes patients with periodontitis.8
With regard to the development of painless and non-invasive methods to measure blood glucose, considerable efforts have been made in the past few years.11 However, until now, none are in the routine clinical practice.12 Since periodontal inflammation with or without complication factor of DM is known to produce ample extravasate of blood during diagnostic periodontal examination, it can be used for the routine random blood glucose level estimation.13 Strauss et al.14 also reported that GCB samples were suitable in persons with sufficient bleeding on probing, to obtain a sample without touching the tooth or the gingival margin (i.e., in patients having the basic clinical signs of gingivitis or periodontal disease). Moreover, the technique is more familiar and less traumatic to the patient than a finger puncture.
In the present study, GCB was used for blood glucose estimation in all 70 subjects. Out of 35 subjects with unknown glycemic status, 4 subjects were diagnosed as new diabetic patients, so that the study comprised 39 diabetic patients and 31 non-diabetic subjects. Newly diagnosed diabetic patients were than referred to physician and he confirmed the results with fasting blood sugar (FBS) and post-prandial blood sugar (PPBS) estimation.
Tsutsui et al.,3 Parker et al.2 and Beikler et al.15 have used GCB with a glucometer for the determination of blood glucose levels in diabetic as well as non-diabetic patients and found it reliable.2, 3, 15
The present study also supports the results of previous researches as the estimation of glucose level by GCB showed that for the diabetic group, the blood glucose levels were in the range of 92–262 mg/dl, with a mean of 156.07 ± 49.23 mg/dl and for the non-diabetic group, blood glucose levels were in the range of 76–122 mg/dl, with mean of 90.80 ± 11.07 mg/dl.
However, the glucometer which was used in the present study [Optium Xceed (IInd generation)] offers the advantage over the first generation glucometer, used in the studies of Parker et al.2 that requires a larger blood sample i.e. about 10–15 μl, which is much more than the quantity required by the glucometer in present study and used by Tsutsui et al.3 in which the blood sample placed on the test strips, had to be wiped off by the user after a certain time interval, thus giving a reading by color matching.2, 3 In addition, the IInd generation glucometer also has an edge over the IIIrd generation glucometer.
The comparison of blood glucose levels of GCB with FSB and VB in our study showed that for diabetic group, the mean GCB, FSB and VB glucose level were 156.07 ± 49.23 mg/dl, 166.61 ± 52.18 mg/dl and 156.12 ± 49.89 mg/dl respectively. Similarly, for the non-diabetic group, the mean GCB, FSB and VB glucose level were 90.80 ± 11.07 mg/dl, 101.35 ± 13.05 mg/dl and 93.41 ± 9.30 mg/dl respectively.
The results were highly significant (p < 0.005) in both the groups for GCB with FSB while non-significant (p > 0.005) for GCB with VB in both the groups. The correlation coefficient (r) in both the groups showed a strong positive correlation between glucose levels of GCB with FSB and VB with the values of 0.986 and 0.972 in diabetic group and 0.820 and 0.721 in non-diabetic group respectively (Table 2).
Similar correlation was obtained by Parker et al.2 (r = 0.98, p < 0.0001),and Beikler et al.15 (r = 0.9814, p < 0.0001) in their studies. However, Tsutsui et al.3 found a slightly lower correlation between the glucose values of GCB and FSB (r = 0.782). It may be due to the difference in the instrument and methodology used, as in their study, blood was transferred on the test strip which used to be manually timed and wiped off before measuring with the glucometer. There might be a non-uniform rubbing of the blood on the strip, which might have damaged the test strip surface. Moreover, manual timing of the test strip reaction also could have been possible source of error in the study.
In the present study, these drawbacks have been tried to be overcome by using a glucometer, which is self-timing, requires only 1 μl of blood, and used test strips, which have an inherent capillary action for drawing blood into the test area.
It was also observed that among all the three values, the GCB glucose values were lower than both FSB and VB values in both diabetic and non-diabetic groups. This can be due to minor contamination of GCB by gingival crevicular fluid which dilutes the glucose concentration producing lower measurements.16 However, it can be highlighted that GCB glucose levels were closer to glucose levels estimated by VB rather than FSB glucose values (Graph 1).
The correlation between the glucose levels of FSB and VB showed a coefficient (r) value of 0.972 and 0.808 in both diabetic and non-diabetic groups and represents a strong positive relationship (Table 3).
Though, a strong correlation has been seen in the study between GCB, FSB and VB glucose measurements, it is not the correlation of the whole group but instead the predictability of a single measurements on one patient. This is important because even a perfect correlation can have poor clinical significance for individual measurements. However, the precision must be considered to better weigh the values of individual measurements.
On comparing the GI between the diabetic and non-diabetic groups in this study, a statistically significant difference (p < 0.005) was obtained with mean gingival index score of 2.18 ± 0.39 mm for diabetic group and 1.77 ± 0.28 mm for non-diabetic group. The results were consistent with the studies of Beneveniste et al.,17 Ervasti et al.,13 De-Pommereaun et al.,18 Firatli et al.,19 Aren et al.20 and Campus et al.21 who found similar observations.
Likewise, on comparison of PPD between the diabetic and non-diabetic groups, a mean depth score obtained for diabetic group was 4.43 ± 0.97 mm and 3.96 ± 0.75 mm for non-diabetic group which was also statistically significant (p < 0.005). Other studies also had similar observations such as Beneveniste et al.,17 Schlossman et al.,22 Pinson et al.23 and Arrieta-Blanco et al.24
The significant difference in means of GI and PPD of diabetic and non-diabetic groups in our study could be because of multifactorial etiology of periodontal disease and complex nature of diabetes. Though, there are patients who develop complications after a short duration of diabetes, even with a reasonable level of metabolic control, there are some patients who never have periodontal complications even with long standing poorly controlled disease and poor oral hygiene. This is probably due to a variation in individual susceptibility assumed to be related to differences in genetic background.25
American Diabetic Association found that the prediction error of blood glucose monitoring devices falls within 15% of the laboratory standard.26 Also, dental practitioners find intraoral sampling technique more convenient for the screening of DM, as the sample can be obtained through routine scaling and the strip system provides a more objective indicator for referral to physicians rather than traditionally used medical history review and observation of symptoms.
5. Conclusion
It can be established that GCB collected during diagnostic periodontal examination may be an excellent source of blood for glucometric analysis. The technique is safe, easier to perform and comfortable for the patients and therefore, helps to increase the frequency of diagnosing the potentially diabetic patients during routine periodontal therapy. Dental surgeons may thus increase their importance as a member of the health team by participating in the search for undiagnosed asymptomatic DM cases.
Conflicts of interest
The authors have none to declare.
References
- 1.Sacks D.B., Arnold M., Bakris G.L. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34:e61–e99. doi: 10.2337/dc11-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker R.C., Rapley J.W., Isley W., Spencer P., Killoy W.J. Gingival crevicular blood for assessment of blood glucose in diabetic patient. J Periodontol. 1993;64:666–672. doi: 10.1902/jop.1993.64.7.666. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsui P., Rich S.K., Schonfeld S.E. Reliability of intraoral blood for diabetes screening. J Oral Med. 1985;40:62–66. [PubMed] [Google Scholar]
- 4.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran A. Epidemiology of diabetes in India: three decades of research. J Assoc Physicians India. 2005;53:34–38. [PubMed] [Google Scholar]
- 6.Ramachandran A. Epidemiology of type-2 diabetes in Indians. J Indian Med Assoc. 2002;100:425–427. [PubMed] [Google Scholar]
- 7.Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Loe H. Periodontal disease: the 6th complication of diabetes. Diabetes Care. 1993;16:329–334. [PubMed] [Google Scholar]
- 9.Harris M.I., Eastman R.C. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000;1:230–236. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Grossi S.G., Genco R.J. Periodontal disease and diabetes mellitus: a two way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 11.Kost J., Mitragotri S., Gabbay R.A., Pishko M., Langer R. Transdermal monitoring of glucose and other analytes using ultrasound. Nat Med. 2000;6:347–350. doi: 10.1038/73213. [DOI] [PubMed] [Google Scholar]
- 12.Klonoff D.G. Non-invasive blood glucose monitoring. Diabetes Care. 1997;20:433–437. doi: 10.2337/diacare.20.3.433. [DOI] [PubMed] [Google Scholar]
- 13.Ervasti T., knuuttila M., Pohjamo L., Haukipuro K. Relation between control of diabetes and gingival bleeding. J Periodontol. 1985;56:151–157. doi: 10.1902/jop.1985.56.3.154. [DOI] [PubMed] [Google Scholar]
- 14.Strauss S.M., Wheeler A.J., Russell S.L. The potential use of gingival crevicular blood for measuring glucose to screen for diabetes: an examination based on characteristics of the blood collection site. J Periodontol. 2009;80:907–914. doi: 10.1902/jop.2009.080542. [DOI] [PubMed] [Google Scholar]
- 15.Beikler T., Kuczek A., Petersilka G., Flemmig T.F. In dental-office screening for diabetes mellitus using gingival crevicular blood. J Clin Periodontol. 2002;29:216–218. doi: 10.1034/j.1600-051x.2002.290306.x. [DOI] [PubMed] [Google Scholar]
- 16.Muller H.P., Behbehani E. Screening of elevated glucose levels in gingival crevice blood using a novel, sensitive self-monitoring device. Med Princ Pract. 2004;13:361–365. doi: 10.1159/000080474. [DOI] [PubMed] [Google Scholar]
- 17.Beneviste R., Bixler D., Conneally P.M. Periodontal disease in diabetics. J Periodontol. 1967;38:271–279. doi: 10.1902/jop.1967.38.4.271. [DOI] [PubMed] [Google Scholar]
- 18.De-Pommereaun V., Dargent-Pare C., Robert J.J. Periodontal status in insulin dependent diabetic adolescents. J Clin Periodontol. 1992;19:628–632. doi: 10.1111/j.1600-051x.1992.tb01710.x. [DOI] [PubMed] [Google Scholar]
- 19.Firatli E., Yilmaz O., Onan U. The relationship between clinical attachment loss and the duration of insulin dependent diabetes mellitus (IDDM) in children and adolescents. J Clin Periodontol. 1996;23:362–366. doi: 10.1111/j.1600-051x.1996.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 20.Aren G., Sepet E., Ozdemir D., Dinççağ N., Güvener B., Firatli E. Periodontal health, salivary status and metabolic control in children with type 1 diabetes mellitus. J Periodontol. 2003;74:1789–1795. doi: 10.1902/jop.2003.74.12.1789. [DOI] [PubMed] [Google Scholar]
- 21.Campus G., Salem A., Uzzau S., Baldoni E., Tonolo G. Diabetes and periodontal disease. A case–control study. J Periodontol. 2005;76:418–425. doi: 10.1902/jop.2005.76.3.418. [DOI] [PubMed] [Google Scholar]
- 22.Schlossman M., Knower W.C., Pettit D.J., Genco R.J. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc. 1990;121:532–536. doi: 10.14219/jada.archive.1990.0211. [DOI] [PubMed] [Google Scholar]
- 23.Pinson M., Hoffman W.H., Garnick J.J., Litaker M.S. Periodontal disease and type 1 diabetes mellitus in children and adolescents. J Clin Periodontol. 1995;22:118–123. doi: 10.1111/j.1600-051x.1995.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 24.Arrieta-Blanco J.J., Bartolome-Villar B., Jimenz-Martinez E., Saavedra-Vallejo P., Arrieta-Blanco F.J. Dental problems in patients with diabetes mellitus (II): gingival index and periodontal disease. Med Oral. 2003;8:233–247. [PubMed] [Google Scholar]
- 25.Rosenthal I.M., Abrams H., Kopczyk R.A. The relationship of inflammatory periodontal disease to diabetic status in insulin dependent diabetes mellitus patients. J Clin Periodontol. 1988;15:425–429. doi: 10.1111/j.1600-051x.1988.tb01596.x. [DOI] [PubMed] [Google Scholar]
- 26.Consensus statement on self-monitoring of blood glucose. Diabetes Care. 1987;10:95–99. [PubMed] [Google Scholar]