Abstract
Objectives
Acute kidney injury (AKI) is common in very low birth weight infants (VLBW) and is associated with increased mortality. Serum creatinine (SCr) based AKI definitions have many limitations. Non-invasive urinary biomarkers may improve early identification, differentiate etiology, and predict outcomes with AKI.
Study Design
We performed 2 nested case-control studies to compare the ability of six urine biomarkers to predict AKI (rise in SCr of at least 0.3 mg/dl) and mortality (death before 36 weeks post-menstrual age).
Results
Compared to non-AKI subjects (N=21), those with AKI (N=9) had higher maximum neutrophil gelatinase-associated lipocalin (NGAL) [OR = 1.2 (1.0, 1.6) p< 0.01; ROC AUC = 0.80] and higher maxiumum osteopontin (OPN) [OR = 3.2 (1.5, 9.9); p< 0.01; ROC AUC = 0.83]. Compared to survivors (N=100), non-survivors (N=23) had higher maximum kidney injury molecule – 1 (KIM-1) [OR = 1.1 (1.0, 1.2); p<0.02; ROC AUC = 0.64]and higher maximum OPN [OR = 1.8 (1.2, 2.7); p<0.001; AUC of ROC = 0.78]. Combination of biomarkers improved predictability for both AKI and mortality. Controlling for gestational age and, birth weight did not considerably affect results.
Conclusions
Urinary biomarkers can predict AKI and mortality in VLBW infants independent of gestational age and birth weight.
Keywords: Kidney injury molecule, Neutrophil Gelatinase Associated Lipocalin, Osteopontin, Premature, Acute renal failure, KIM-1, NGAL, Neonate, OPN
INTRODUCTION
Despite advancements in the care of the premature infant, morbidity and mortality remain high(1, 2). Acute kidney injury (AKI), previously referred to acute renal failure, is common premature infants(3, 4). AKI is an independent predictor of mortality in critically ill neonates(4, 5), children(6, 7), and adults(8–12) even after controlling for co-morbidities, interventions and demographics. AKI not only impairs fluid and electrolyte homeostasis but may hamper systemic inflammatory auto-regulation (13) which support the concept that kidneys are not just innocent bystanders but may play a key role in the systemic derangement present during multi-organ failure. Therapies known to prevent or ameliorate AKI in animal models have not shown reduction in clinical AKI studies. One of the possible reasons for these negative results is that these interventions were instituted only after a rise in serum creatinine (SCr) was seen. As it usually take days after renal injury for a rise in SCr to occur (14), the search for early AKI biomarkers has taken a prominent role in advancement of AKI research.
Currently, SCr-based definitions are used to diagnose AKI(15, 16). However, SCr-based definitions are not ideal for the following reasons(3): 1) SCr measures function, not injury, 2) SCr may not change until 25–50% of the kidney function has already been lost 3) SCr overestimates renal function due to tubular secretion of creatinine at lower GFR, 4) SCr varies by muscle mass, hydration status, sex, age, gender medications and endogenous substances like bilirubin 5) SCr can be non-specific for AKI, especially with pre-renal azotemia (a transient, reversible decrease in GFR), and 6) SCr cannot be used to assess kidney function while patients receive dialysis. Additional problems with using SCr as a measure of AKI specific to neonates include the facts that SCr in the first few days of life reflects mother’s and not the infant’s kidney function, and there is a very wide distribution of normal serum creatinine values which change over time, dependent on level of prematurity(17) (18).
In order to determine if urine biomarkers can be used to detect AKI in very low birth weight (VLBW) infants, we evaluated six previously identified candidate urinary biomarkers: neutrophil gelatinase associated lipocalin, (NGAL), interleukin – 18 (IL-18), kidney injury molecule -1 (KIM - 1), osteopontin (OPN), beta-2 microglobulin (B2mG)(19, 20) and cystatin-C (Cys-C). We explored the individual and combined ability of these biomarkers to predict AKI, as well as their ability to predict mortality. Because some of these biomarkers may vary by gestational age and birth weight(21–23), we performed regression analysis to control for these potential confounders.
METHODS
We conducted 2 separate nested case-control studies to determine the ability of 6 urine biomarkers to predict AKI and mortality. In a nested case-control study, data is collected prospectively. Using this data, cases who have the disease (AKI in this manuscript) that occur in the defined cohort are identified. Then, controls [those who do not have the disease (No AKI group in this study)] are selected from the prospective cohort. The advantage of this design is that a nested case-control design potentially offers reductions in costs and efforts of data collection compared with the full cohort approach, with relatively minor loss in statistical efficiency. It is particularly advantageous for studies of biologic precursors of disease such as those described in this manuscript. (24)
The original cohort comprised of VLBW infants (birth weight range of 500–1500g) admitted to the regional quaternary care neonatal intensive care units of the University of Alabama at Birmingham (UAB) and the Children’s Hospital of Alabama between February 2008 and July 2009. Infants were excluded if they did not survive to 48 hours of life or if they had any significant known congenital abnormality of the kidney. The incidence of AKI and association with outcomes in this cohort has been recently described(4). Parental consent was obtained and the Institutional Review Board at UAB approved the study.
Populations used to evaluate the ability of biomarkers to detect AKI
Cases include VLBW infants who experienced AKI, defined as an acute rise in SCr of at least 0.3 mg/dl within 48 hours (Stage 1 of the Acute Kidney Injury Network (AKIN) definition) (15) Controls consisted of VLBW infants who did not have AKI but had had ample blood samples to confirm negative AKI status around the time of urine sample collection, Serum was analyzed from remnant serum samples when available and from the patient’s medical records performed as part of routine hospital care. Table 1 compared infant and maternal characteristics between those with and without AKI.
Table I.
Demographic differences between infants with and without AKI
| No AKI (n = 21) | AKI (n = 9) | P value | |
|---|---|---|---|
| Infant Characteristics | |||
| Birth weight (g) | 958 ± 283 | 691 ± 200 | <.01 |
| Gestational age (wk) | 27.7 ± 2.7 | 25.9 ± 2.8 | .11 |
| Sex | |||
| Male | 10 (47.6) | 4 (44.4) | 1.0 |
| Female | 11 (52.4) | 5 (55.6) | |
| Race | |||
| White | 9 (42.9) | 7 (77.8) | .12 |
| Black | 11 (47.8) | 1 (11.1) | |
| Hispanic | 1 (4.8) | 1 (11.1) | |
| 1-minute Apgar score* | 5 (2, 7) | 3 (1, 5) | .32 |
| 5-minute Apgar score* | 7 (5, 8) | 7 (5, 7) | .78 |
| Vancomycin | 14 (66.7) | 8 (88.9) | .37 |
| Aminoglycocide | 20 (95.2) | 8 (100) | 1.0 |
| Indomethacin | 11 (52.4) | 4 (44.4) | 1.0 |
| Umbilical catheter | 11 (52.4) | 7 (77.8) | .25 |
| Maternal Characteristics | |||
| Age (years) | 28.5 ± 7.1 | 26.4 ± 5.5 | .41 |
| Hypertension | 10 (47.6) | 4 (44.4) | 1.0 |
Continuous: mean ± SD (except * in which median [25%, 75% IQR]).
Categorical: n (%).
Population used to evaluate the ability of biomarkers to predict mortality
Cases (non-survivors) were those infants who did not survive to 36 weeks post-menstrual age (PMA). Controls (survivors) were defined as those who were discharged home or survived to 36 weeks PMA. Hospital discharge or survival to 36 weeks post menstrual age was used to define survival as most preterm infants’ mortality occurs within the first few weeks of life, and mortality subsequent to this time point is very unlikely(25, 26).Table 2 compares infant and maternal demographic characteristics between survivors and non-survivors.
Table II.
Demographic differences between survivors and nonsurvivors
| Survivors (n = 100) | Nonsurvivors (n = 23) | P value | |
|---|---|---|---|
| Infant characteristics | |||
| Birth weight (g) | 952.2 ± 280.2 | 675.9 ± 230.01 | <.0001 |
| Gestational age (wks) | 27.5 ± 0.25 | 25.3 ± 0.21 | <.001 |
| Sex | |||
| Male | 42 (75%) | 14 (25%) | .10 |
| Female | 58 (86.6%) | 9 (13.4%) | |
| Race | |||
| White | 39 (39.0%) | 13 (56.5%) | .27 |
| Black | 53 (53.0%) | 8 (37.8%) | |
| Hispanic | 8 (8.0%) | 2 (8.7%) | |
| 1 minute Apgar Score* | 4 (2, 7) | 3 (1, 5) | .07 |
| 5 minute Apgar Score* | 7 (6, 8) | 6 (4, 7) 26% | <.03 |
| Vancomycin | 66 (66.0%) | 21 (91.3%) | <.02 |
| Aminoglycocide | 95 (95%) | 22 (96%) | .89 |
| Indomethacin | 50 (50%) | 9 (39%) | .35 |
| Umbilical Catheter | 51 (51%) | 16 (69.7%) | .10 |
| AKI present | 12 (16.2%) | 15 (78.9%) | <.0001 |
| Maternal Characteristics | |||
| Age | 26.1 ± 6.3 | 25.4 ± 5.1 | .62 |
| Hypertension | 43 (43.0%) | 5 (21.7%) | .06 |
Continuous: mean ± SD (except * in which median [25%, 75% IQR]).
Categorical: n (%).
Biomarker analysis
Urine was collected during the first 6 days of life using cotton balls placed at the perineum. Urine was extracted, centrifuged for 10 minutes to remove any cotton fibers or cellular elements, and then frozen at −70°C until sample evaluation. Urine biomarker analysis was performed by Core A of the NIH P30 O’Brien Core Center for AKI research (www.obrienaki.org) using Meso Scale Discovery (Gaithersburg, MD).
NGAL, Cys-C, OPN and B2mG were measured in urine using a prototype four value multiplex (4-plex) assay. IL-18 and KIM-1 were measured with a prototype duplex (2-plex) assays developed for this study. The intra and inter assay precisions were <3% and <5% respectively for both 4-plex and 2-plex assays. Standard back-calculated recoveries were 90–110%. Calibrators and sample duplicate correlation variability ranged from 0.12% – 7.9%.
For the evaluation of urine biomarkers as predictors of AKI, we calculated the maximum value of the biomarkers from the urines obtained around the time of AKI (or confirmation of no AKI). Due to sample design, not all subjects had daily biomarker measurements every single day. The median number of specimens for the AKI and no AKI group was 4 (range 1–6) and 4 (range 1–5), respectively. For the evaluation of urine biomarkers as predictors of mortality, we calculated the maximum value of the biomarkers from the urines obtained during the first week of life. The median number of specimens for the survivors and non-survivors was 4 (range 1–7) and 4 (range 1–6), respectively. Table 3 and 4 provide comparison of these biomarker values categorized by AKI status and survival respectively.
Table III.
Urine biomarkers according to acute kidney injury status
| No AKI (n = 21) | AKI (n = 9) | P value | ROC AUC | |
|---|---|---|---|---|
| NGAL* (ng/mL) | 458 (210, 587) | 985 (452, 1398) | .01 | 0.80 |
| KIM-1* (pg/mL) | 835 (311, 775) | 867 (252, 1145) | .90 | 0.50 |
| IL-18* (pg/mL) | 307 (73, 399) | 754 (90, 975) | .43 | 0.60 |
| OPN* (ng/mL) | 217 (115, 280) | 468 (247, 655) | <.01 | 0.83 |
| Cys-C* (ng/mL) | 2150 (219, 3930) | 3889 (2130, 5790) | <.06 | 0.73 |
| B2mG (μg/mL) | 2.1 (0.9, 2.7) | 1.5 (1.2, 1.7) | <.06 | 0.66 |
Median (25%,75%); AUC for AKI.
For non-normal distribution, we used the Mann-Whitney U-test (Wilcoxon 2-sample test).
Table IV.
Predictive models for acute kidney injury**
| Model | OR (95% CI) | P value | ROC AUC |
|---|---|---|---|
| NGAL (every 100 ng/mL) | 1.2 (1.0, 1.6) | <.01 | 0.80 |
| OPN (every 100 ng/mL) | 3.2 (1.5, 9.9) | <.01 | 0.83 |
| NGAL (every 100 ng/mL) | 1.3 (0.9, 1.8) | <.01 | 0.90 |
| OPN (every 100 ng/mL) | 3.3 (1.4, 11.9) | ||
| NGAL (every 100 ng/mL) | 1.3 (1.0–2.1) | <.01 | 0.88 |
| OPN (every 100 ng/mL) | 3.6 (1.3–17.1) | ||
| Gestational age (every week) | 1.2 (0.7–2.1) | ||
| Birth weight (every 100 grams) | 1.0 (1.0–1.0) |
No major changes were seen with the addition of other biomarkers.
Statistical Methods
Descriptive statistics were performed to determine differences between groups. The Shapiro–Wilk test and normal probability plot were used to test for normality of data. Normally distributed continuous variables were compared using Student’s t-test or Fisher’s exact test when appropriate. Non-normal distributed continuous variables were analyzed using Mann-Whitney U-test. For all descriptive statistics, an alpha value of 0.05 was used.
Univariate analysis was performed to determine the association between exposure variables (biomarkers and demographics) and outcomes (AKI or survival). Multiple logistic regression analysis was performed to determine how multiple biomarkers performed together and to control for potential confounders. SAS 9.2 (SAS Institute Inc., Cary, NC, USA) was used for the all statistical analysis.
RESULTS
Baseline Characteristics
Acute Kidney Injury
Nine infants were classified as having AKI, and twenty-one infants were classified as no AKI and used as a control group. Table 1 describes differences in the patient characteristics and co-morbidities between those with and without AKI. Infants with AKI had lower birth weight than those without (Mean±SD; 691 ± 200 vs. 958 ± 283 grams; P<0.01). Infants with AKI tended to have lower gestational age than those without AKI [25.9 ± 2.8 weeks. vs. 27.7 ± 2.7); P=0.11)]. Otherwise, no major differences were detected between groups.
Mortality
One hundred infants were classified as survivors and 23 infants were classified as non-survivors. Table 2 describes differences in the patient characteristics and co-morbidities between the two survival groups. Non-survivors had lower birth weight than survivors (676 ± 230 g vs 952 ± 280; p<0.0001). Similarly, gestational age was lower in non-survivors than survivors (25 ± 0.2 vs. 27 ± 0.3 weeks, p<0.001). AKI was more common in non-survivors than survivors [(15 / 23 (79%) vs. 12 / 100 (16.%) p<0.0001)]. Other variables more common in non-survivors included lower median 5 minute Apgar score (p=0.03), receipt of vancomycin (p<0.02), and maternal hypertension (p = 0.06).
Biomarker values
Acute Kidney Injury
Differences in biomarkers by AKI status are shown in Table 3. Maximum NGAL levels were significantly higher in those with AKI compared to controls [985 ng/mL (95% CI = 452, 1398) vs. 458 (210, 587); P <0.01). Similarly, maximum OPN levels of patients with AKI were significantly elevated compared to controls [468 ng/mL (95% CI 247, 655) vs. 217 (115, 280) ; P<0.01). The difference in maximum B2mG, Cys-c, KIM-1 and IL-18 concentrations was not statistically significant between those with and without AKI.
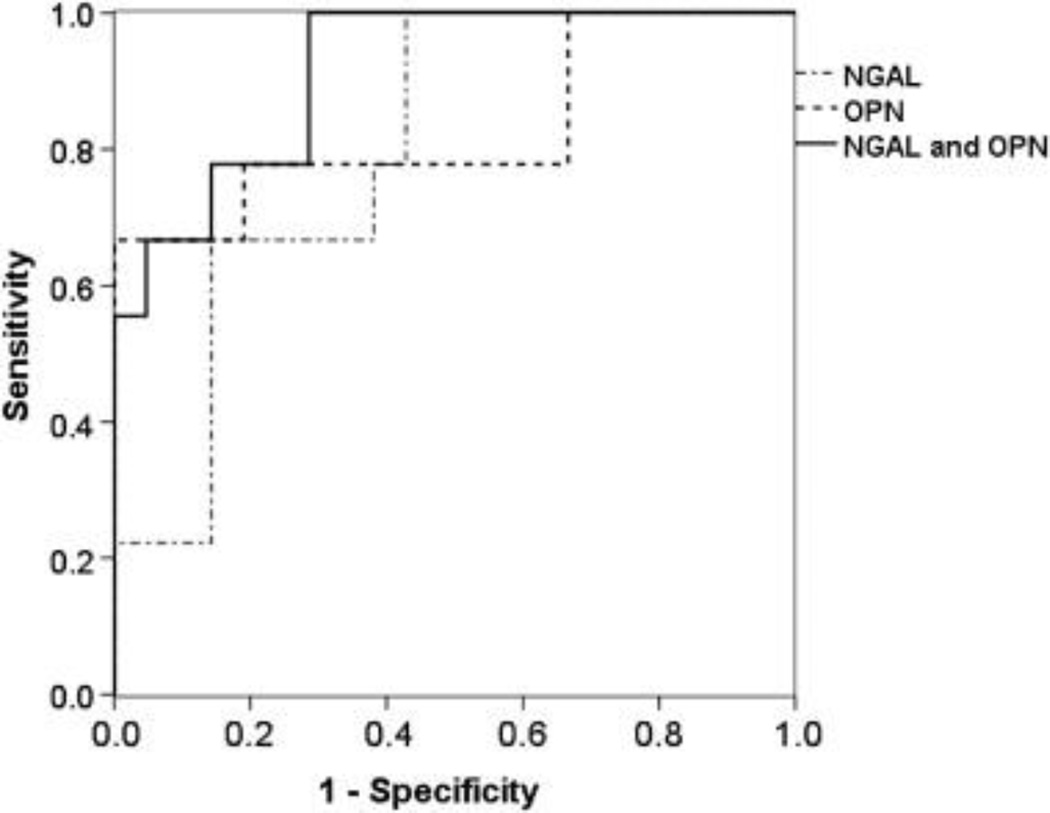
For every 100 ng/mL rise in OPN, the odds of having AKI increased by 220% [(OR = 3.2 (95% CI, 1.5, 9.9); P<0.01]. For every 100 ng/mL rise in NGAL, the odds of having AKI increased by 20% [OR = 1.2 (95%CI, 1.0, 1.6) P<0.01] (Table 4). These maximum value of these biomarkers showed excellent ability to predict AKI with area under the curve (AUC) receiver operator characteristic (ROC) of 0.80 for NGAL and 0.83 for OPN. Combining NGAL and OPN in the models improved the ability to detect AKI (ROC = 0.90) (Figure 1).
Figure 1.
ROC AUC for NGAL, OPN, and both NGAL and OPN: their predictive abilities to detect AKI in very-low-birth-weight premature infants.
Mortality
Differences in biomarkers by survivor status are shown in Table 5. Maximum OPN levels were significantly higher in non-survivors compared to survivors [482 ng/mL (95% CI = 281, 631) vs. 230 (95% CI = 112, 371); p < 0.01]. Similarly, maximum KIM-1 levels were higher in non-survivors than survivors [385 pg/mL (95% CI 231, 1028) vs. 264 (95% CI = 147, 549); p=0.03]. The difference in B2mG, Cys-c, NGAL and IL-18 concentrations was not statistically significant between survivors and non-survivors (Table 5).
Table V.
Urine biomarkers according to survival status
| Survivors (n = 100) | Nonsurvivors (n = 23) | P value | ROC AUC | |
|---|---|---|---|---|
| NGAL* (ng/mL) | 395 (189, 662) | 493 (283, 1484) | .11 | 0.61 |
| KIM-1* (pg/mL) | 263.9 (147, 549) | 385 (231, 1028) | <.03 | 0.65 |
| IL-18* (pg/mL) | 162 (55, 435) | 158 (84, 450) | .64 | 0.47 |
| (n = 39) | (n = 10) | |||
| OPN* (ng/mL) | 230 (112, 371) | 482 (281, 631) | <.01 | 0.78 |
| Cys-C* (ng/mL) | 2030 (717, 4459) | 1884 (400, 4589) | .87 | 0.51 |
| B2mG (μg/mL) | 1.8 (1.1, 2.5) | 1.7 (0.9, 3.0) | 1.0 | 0.50 |
Median (25%, 75%); AUC for mortality.
For non-normal distribution, the Mann-Whitney U-test (Wilcoxon 2-sample test) was used.
For every 100 ng/mL rise in OPN, there was an 80% higher odds of death [OR = 1.8 (95%CI, 1.2, 2.7) p < 0.001) (Table 6). For every 100 pg/mL rise in KIM-1 there was a 10% higher odds of death [(OR = 1.1 (95% CI, 1.0, 1.2); P <0.02]. The ROC AUC were 0.78 and 0.64 for OPN and KIM-1, respectively. Combining OPN and KIM-1 improved the ability to predict mortality (AUC of ROC = 0.83) (Figure 2).
Table VI.
Predictive models of neonatal mortality *
| Model | OR (95% CI) | P value | ROC AUC |
|---|---|---|---|
| OPN (every 100 ng/mL) | 1.8 (1.2, 2.7) | <.001 | 0.78 |
| KIM-1 (every 100 pg/mL) | 1.1 (1.0, 1.2) | <.02 | 0.64 |
| OPN (every 100 ng/mL) | 1.8 (1.2, 2.9) | .01 | 0.83 |
| KIM-1 (every 100 pg/mL) | 1.1 (0.95, 1.2) | .09 | |
| OPN (every 100 ng/mL) | 2.1 (1.2, 3.7) | <.01 | 0.88 |
| KIM-1 (every 100 pg/mL) | 1.1 (0.99, 1.15) | .12 | |
| Gestational age (every week) | 0.4 (0.2, 1.0) | .06 | |
| Birth weight (every 100 grams) | 1.6 (0.9, 3.3) | .17 |
No major changes were seen with the addition of other biomarkers.
Figure 2.
ROC AUC for KIM-1, OPN, and both KIM-1 and OPN: their predictive abilities to detect mortality in very-low-birth-weight premature infants.
As more immature infants of lower gestational age and birth weight may possibly have higher baseline value of some urinary AKI biomarkers, we incorporated these variables into predictive models to control for these potential confounders. In both the AKI models (Table 5) and the Survival Models (Table 6), the addition of gestational age and birth weight somewhat improved the models but the biomarkers continued to be important to the predictive models.
DISCUSSION
This is the first study to evaluate the association between six known urine AKI biomarkers with AKI and mortality in VLBW premature infants. We found that maximum concentrations of urine NGAL and OPN can predict AKI. In addition, we showed that urine KIM-1 and OPN obtained during the first week of life can predict mortality. Combining biomarkers improved the ability to predict both AKI and mortality. This data provides new insights about the potential role of urine AKI biomarkers in premature infants, and is the first study to test OPN as an AKI biomarker in humans.
Several studies have shown that some urinary biomarker concentrations depend on gestational age, and birth weight(21–23) This may be secondary to the inability of immature tubules to reabsorb these proteins in underdeveloped kidneys. Controlling for these important confounders are necessary to assure that the associations between urine biomarkers and the stated outcomes (AKI or mortality) are not simply just a reflection of prematurity. Therefore, we performed regression analysis to control for these important potential confounders. When these were incorporated into the AKI and survival models, the association between biomarkers and outcomes persisted suggesting that these biomarkers can predict both AKI and mortality, independent of birth weight/ gestational age.
Many studies in children and adults show that urine NGAL, KIM-1 and other urine biomarkers, can predict a rise in SCr in critically ill neonatal, pediatric and adult populations(27, 28). Our data provide important information about potential role for this and other urine biomarkers to predict AKI in this very vulnerable population. OPN (a cytokine which is broadly expressed and up-regulated during inflammation) has been shown to predict requirement of renal replacement therapy and mortality in critically ill adults with AKI(29). To our knowledge, OPN has not been studied as a biomarker to detect AKI. Our findings that OPN can predict AKI should encourage other investigators to explore its role as an AKI biomarker in other populations.
Urinary biomarkers have also been found to be predictors of mortality in children and adults (30, 31). As we move to incorporate these biomarkers into the definition of neonatal AKI, it is important to assure that biomarkers not only predict an elevation in SCr, but also forecast hard clinical endpoints such as mortality. A recent multicenter pooled analysis of prospective studies shows that NGAL can predict mortality even in patients who do not have a rise in their serum creatinine(32) In premature infants, urine NGAL has been shown to predict sepsis(33). Our results support these biomarkers as prognostic indicators in this unique population with a high incidence of poor outcomes after AKI(4).
The strengths of this study are the evaluation of six urinary biomarkers individually and jointly as markers. We showed that these biomarkers are associated with AKI and mortality independent of gestational age and birth weight. Despite the important information the current study provides, some potential research limitations have been identified. As we did not have daily urine samples on every individual, and the number of infants with AKI was low., the precise day in which these biomarkers will be most useful or whether these biomarkers are better than our current gold-standard (rise in SCr) cannot be deciphered from this data. Future studies in larger cohorts of premature infants with daily urine collection will be needed to determine the best biomarkers and the most useful time point(s).
In addition, although we controlled for several potential confounders, due to limitations of sample size, we were not able to control for other known and unknown potential confounders that predict mortality in our analysis. Future studies will be needed confirm these findings in larger populations. We acknowledge that SCr-based definitions have significant shortcomings. Nonetheless, we have used the most accepted classification definition, which has been accepted by both critical care and nephrology communities. Future definitions will likely need to incorporate injury markers such as the ones described in this manuscript. Evaluations of these biomarkers against hard clinical endpoints (such as mortality, length of stay, requirement of renal replacement therapy) will be needed to incorporate these biomarkers into the definition of AKI.
In conclusion, peak NGAL and OPN concentrations predict AKI. Peak values of urine OPN and KIM-1 obtained in the first week of life predict mortality in premature infants. These markers perform well individually, and better collectively. These findings suggest that these biomarkers could be used to define AKI which could significantly improve our ability to prognosticate outcomes, prescribe fluids, medications, and renal support therapy. In addition, having biomarkers to define AKI early in the disease process can help researchers find preventive and therapeutic interventions to improve the outcomes of these vulnerable infants. Future studies with larger cohorts are greatly needed to confirm these findings, control for important potential confounders and delineate the optimal timing of assessment of these biomarkers.
Acknowledgments
We would like to thank Dr. Stuart Goldstein and Dr. Ravi Mehta for assistance in conducting this analysis. We are indebted to Amy Logue RN and the many nurses in the nephrology and neonatology divisions at the University of Alabama at Birmingham for assisting in obtaining laboratory samples.
Funding source: This study was supported by grants provided to Dr. Askenazi by the National Kidney Foundation Young Investigators Award, the Kaul Pediatric Research Institute and a pilot and feasibility grant from the NIH-sponsored O’Brien Center for Acute Kidney Injury research (P30DK079337) (www.obrienaki.org).
P.D is co-inventor on patents relating to the use of neutrophil gelatinase associated lipocalin as a biomarker of acute kidney injury, and is a consultant for Abbott Diagnostics and Biosite Inc/Inverness Medical.
LIST OF ABBREVIATIONS
- AKI
Acute kidney injury
- AKIN
Acute Kidney Injury Network
- B2mG
Beta-2 microglobulin
- Cys-C
Cystatin-C
- IL-18
Interleukin–18
- KIM-1
Kidney injury molecule-1
- NGAL
Neutrophil gelatinase associated lipocalin
- OPN
Osteopontin
- PMA
Post-menstrual age
- SCr
Serum creatinine
- VLBW
Very low birth weight infants
- UAB
University of Alabama at Birmingham
Footnotes
Conflict of interest statement
None of the other authors have any conflicts of interest to declare for this study.
REFERENCES
- 1.Behrman . Nelson Textbook of Pediatrics. 17th. Philadelphia: Saunders; 2004. [Google Scholar]
- 2.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005 Mar 5–11;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol. 2009 Feb;24(2):265–274. doi: 10.1007/s00467-008-1060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D. Acute Kidney Injury Reduces Survival in Very Low Birth Weight Infant. Pediatric Research. 2010 doi: 10.1203/PDR.0b013e31820b95ca. [DOI] [PubMed] [Google Scholar]
- 5.Askenazi DJ, Griffin R, McGwin G, Carlo W, Ambalavanan N. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol. 2009 May;24(5):991–997. doi: 10.1007/s00467-009-1133-x. [DOI] [PubMed] [Google Scholar]
- 6.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007 May;71(10):1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 7.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008 Jul;3(4):948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008 Mar;73(5):538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 9.Cuhaci B. More data on epidemiology and outcome of acute kidney injury with AKIN criteria: benefits of standardized definitions, AKIN and RIFLE classifications. Crit Care Med. 2009 Sep;37(9):2659–2661. doi: 10.1097/CCM.0b013e3181ad76c2. [DOI] [PubMed] [Google Scholar]
- 10.Uchino S. Outcome prediction for patients with acute kidney injury. Nephron Clin Pract. 2008;109(4):c217–c223. doi: 10.1159/000142931. [DOI] [PubMed] [Google Scholar]
- 11.Macedo E, Castro I, Yu L, Abdulkader RR, Vieira JM., Jr Impact of mild acute kidney injury (AKI) on outcome after open repair of aortic aneurysms. Ren Fail. 2008;30(3):287–296. doi: 10.1080/08860220701857522. [DOI] [PubMed] [Google Scholar]
- 12.Bagshaw SM, George C, Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11(3):R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elapavaluru S, Kellum JA. Why do patients die of acute kidney injury? Acta Clin Belg Suppl. 2007;(2):326–331. doi: 10.1179/acb.2007.074. [DOI] [PubMed] [Google Scholar]
- 14.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007 Mar;2(2):356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network (AKIN): report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007 Mar 1;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoste EA, Kellum JA. RIFLE criteria provide robust assessment of kidney dysfunction and correlate with hospital mortality. Crit Care Med. 2006 Jul;34(7):2016–2017. doi: 10.1097/01.CCM.0000219374.43963.B5. [DOI] [PubMed] [Google Scholar]
- 17.Gallini F, Maggio L, Romagnoli C, Marrocco G, Tortorolo G. Progression of renal function in preterm neonates with gestational age < or = 32 weeks. Pediatr Nephrol. 2000 Nov;15(1–2):119–124. doi: 10.1007/s004670000356. [DOI] [PubMed] [Google Scholar]
- 18.Brion LP, Fleischman AR, McCarton C, Schwartz GJ. A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J Pediatr. 1986 Oct;109(4):698–707. doi: 10.1016/s0022-3476(86)80245-1. [DOI] [PubMed] [Google Scholar]
- 19.Heise D, Rentsch K, Braeuer A, Friedrich M, Quintel M. Comparison of urinary neutrophil glucosaminidase-associated lipocalin, cystatin C, and alpha(1)-microglobulin for early detection of acute renal injury after cardiac surgery. Eur J Cardiothorac Surg. 2010 Jul 20; doi: 10.1016/j.ejcts.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Herrero-Morin JD, Malaga S, Fernandez N, Rey C, Dieguez MA, Solis G, et al. Cystatin C and beta2-microglobulin: markers of glomerular filtration in critically ill children. Crit Care. 2007;11(3):R59. doi: 10.1186/cc5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavery AP, Meinzen-Derr JK, Anderson E, Ma Q, Bennett MR, Devarajan P, et al. Urinary NGAL in premature infants. Pediatr Res. 2008 Oct;64(4):423–428. doi: 10.1203/PDR.0b013e318181b3b2. [DOI] [PubMed] [Google Scholar]
- 22.Huynh TK, Bateman DA, Parravicini E, Lorenz JM, Nemerofsky SL, Sise ME, et al. Reference values of urinary neutrophil gelatinase-associated lipocalin in very low birth weight infants. Pediatr Res. 2009 Nov;66(5):528–532. doi: 10.1203/PDR.0b013e3181baa3dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Askenazi D, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, Mehta R, Ambalavanan N. Baseline Values of Candidate Urine Acute Kidney Injury (AKI) Biomarkers Vary by Gestational Age in Premature Infants. Pediatric Research. 2011;2011 doi: 10.1203/PDR.0b013e3182275164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernster VL. Nested case-control studies. Prev Med. 1994 Sep;23(5):587–590. doi: 10.1006/pmed.1994.1093. [DOI] [PubMed] [Google Scholar]
- 25.Ambalavanan N, Carlo WA, Bobashev G, Mathias E, Liu B, Poole K, et al. Prediction of death for extremely low birth weight neonates. Pediatrics. 2005 Dec;116(6):1367–1373. doi: 10.1542/peds.2004-2099. [DOI] [PubMed] [Google Scholar]
- 26.Meadow W, Reimshisel T, Lantos J. Birth weight-specific mortality for extremely low birth weight infants vanishes by four days of life: epidemiology and ethics in the neonatal intensive care unit. Pediatrics. 1996 May;97(5):636–643. [PubMed] [Google Scholar]
- 27.Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010 Jun;15(4):419–428. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 28.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010 Apr;4(2):265–280. doi: 10.2217/bmm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzen JM, Hafer C, Faulhaber-Walter R, Kumpers P, Kielstein JT, Haller H, et al. Osteopontin predicts survival in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2010 Aug 23; doi: 10.1093/ndt/gfq498. [DOI] [PubMed] [Google Scholar]
- 30.Al-Ismaili Z, Palijan A, Zappitelli M. Biomarkers of acute kidney injury in children: discovery, evaluation, and clinical application. Pediatr Nephrol. 2010 Jul 10; doi: 10.1007/s00467-010-1576-0. [DOI] [PubMed] [Google Scholar]
- 31.Lisowska-Myjak B. Serum and urinary biomarkers of acute kidney injury. Blood Purif. 2010;29(4):357–365. doi: 10.1159/000309421. [DOI] [PubMed] [Google Scholar]
- 32.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-assocaiated lipocalin positve subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011 doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parravicini E, Nemerofsky SL, Michelson KA, Huynh TK, Sise ME, Bateman DA, et al. Urinary neutrophil gelatinase-associated lipocalin is a promising biomarker for late onset culture-positive sepsis in very low birth weight infants. Pediatr Res. 2010 Jun;67(6):636–640. doi: 10.1203/PDR.0b013e3181da75c1. [DOI] [PMC free article] [PubMed] [Google Scholar]