Abstract
Calpains are a 15-member class of calcium activated nonlysosomal neutral proteases which are involved in a broad range of cellular function. Calpains are usually localized to the cytosol and within mitochondria. Calpastatin is an endogenous protein that specifically binds to and inhibits calpain. Overactivation of calpain has been implicated in a number of disease processes of the brain, eyes, heart, lungs, pancreas, kidneys, vascular system and skeletal muscle. Therefore, calpain may serve as a potential therapeutic target for a wide variety of disease processes. This review briefly outlines the current literature regarding the involvement of calpain overactivation in the pathogenesis of almost every organ in the body.
Keywords: Calpain, Pathogenesis, Human Disease
Introduction
Calpains are a class of calcium activated nonlysosomal neutral proteases which are involved in a broad range of cellular function. Overactivation of calpain has been implicated in a number of disease processes ranging from neurodegeneration to muscle atrophy. Inhibition of calpain overactivity has been found to be beneficial in a number of animal models. Therefore, calpain serves a potential medical target for a wide variety of disease processes. This review discusses the current literature regarding the effect of calpain overactivity on the pathogenesis of almost every organ in the body.
Background
Calpains are a 15-member class of calcium activated nonlysosomal neutral proteases which are localized to the cytosol and mitochondria. [1] Some calpains are ubiquitously expressed (1, 2, 4, 5, 7, 10) but others are thought to be localized in specific tissues as follows: 1+2: endothelial cells, 3: skeletal muscle, 6: placenta, 8: smooth muscle, 9: stomach, 11: testes, 12: skin, 13 testes and lung. Calpastatin is an endogenous protein that specifically binds to and inhibits calpain. Interestingly, calpastatin is thought to bind to calcium activated calpains suggesting that it does not interfere with basal calpain activity in the cell. [2]
Brain
Baseline calpain function is thought to be neuroprotective while over-expression of calpain activity is associated with neurologic dysfunction.[3] Calpain overactivation is linked to the synaptic plasticity and neurodegeneration associated with Alzheimer’s disease.[4,5] Calpain mediated proteolysis regulates the activity of important disease associated proteins including tau kinase, cylin dependent kinase 5 and glycogen kinase synthase 3.[5] Calpain inhibition has been shown to beneficially moderate the abnormal synaptic plasticity and memory produced by excess amyloid β in patients with Alzheimer’s disease.[6] Calpains are also thought to be mediators of neuronal damage after traumatic and ischemic neural injuries.[7] Most of the research regarding calpain and neurologic decline focuses on calpain 1 and 2. For example, mutations in calpain 1 have been shown to cause autosomal hereditary spastic paraplegia and spastic ataxia. [3] Excessive activation of calpain 2 has been linked to oligomer induced neuronal cell death leading to the dysfunction seen in patients with neurodegenerative diseases. [8] It is clear that overactivity of calpain is associated with neurological decline; more research is needed to determine the mechanism through which this dysfunction occurs.
Eyes
In situations of stress, overactivity of calpain in retinal cells is thought to cause retinal cellular dysfunction leading to vision loss. Ischemic injury in the retina leads to overactivity of calpain which causes the breakdown of α spectrin and results in cellular death in the hypoxic retinal cells. Inhibition of calpain activity in these cells is associated with decreased cell death. [9]
Overactivation of calpain is also thought to cause aberrant neogenesis which leads decreased blood flow and subsequent retinal dysfunction. In ischemic retinopathy, hypoxia leads to vascular endothelial growth factor-A (VEGF) induced abnormal neovascularization that in turn causes damage to the retina and results in blindness. This abnormal neovascularization is associated with increased calpain activity in the retinal endothelial cells and disrupts the actin cytoskeleton and microtubule strength leading to defective neo vessel formation. Calpain inhibition has been shown to reduce VEGF induced neovasculature architectural abnormalities, vascular leakage and retinal hypoxia. Calpain inhibitors are thought to work by improving the organization of the actin cytoskeleton in retinal endothelial cells and improving the stability of actin cables in new blood vessels. [10,11]
Interestingly, calpain has also been found to be upregulated in human lens epithelial cells in patients with diabetic retinopathy. Expression levels of calpain are highest in patients with 1) longer duration of diabetes mellitus 2) higher hemoglobin A1c levels and 3) a diagnosis of advanced diabetic retinopathy. [12]
Taken together, these data suggest that overactivation of calpain both in the retinal and lens epithelial cells and in the endothelial cells of the eye lead to reduced vision.
Heart
There is a large amount of research to suggest that calpain activity is overexpressed in myocardial tissue that is stressed (ie acute infarct, from high glucose levels, oxidative stress, right heart failure, chronic ischemia, sepsis and atrial fibrillation). [13]
Calpain activity is increased in myocardial cells after an acute myocardial infarct. This activation leads to post-myocardial infarction remodeling by disrupting N-cadherin and α-fodrin based cell adhesions. Inhibition of calpain attenuates this post-myocardial infarction remodeling. [14,15] Calpain inhibition after acute ischemia and reperfusion decreases myocardial infarct size and improves left ventricular contractility and myocardial hemodynamic function. [16,17]
High glucose levels induce apoptosis by both mitochondrial dependent and independent mechanisms. Both mechanisms result in calcium overload which leads to activation of calpain 1. Calpain 1 activation induces caspase 3 and poly ribose polymerase and cleaves apoptosis inducing factor. [1] This increased calpain activity results in increased cardiomyocyte oxidative stress and apoptosis. The final result is hypertrophy of the cardiomyocytes resulting in diabetic cardiomyopathy. [13,18] Calpain inhibition has been shown to attenuate the myocardial hypertrophy and fibrosis associated with diabetes. [19]
Right heart failure results from a number of disease processes which lead to acute right ventricular overload and contractility dysfunction. This ventricular contractile dysfunction has been associated with disruption of the focal adhesion proteins; talin, vinculin and α-actinin. Interestingly, calpain inhibition in the setting of right heart overload has been shown to preserve the organization of talin, vinculin and α-actinin resulting in the attenuated severity of right heart failure. [20]
Calpain inhibition has been shown to increase myocardial blood flow and vascular density in the setting of chronic myocardial ischemia.[21,22] GSK-3β is a known substrate of calpain which has been shown to play a dominant role in cardiomyocyte death.[23–25] Interestingly, GSK-3β inhibition in the same chronic ischemia model has been shown to have similar beneficial effects on myocardial blood flow.[26] Troponin T is an important sarcomeric protein that is involved regulation of cardiac muscle contraction. In chronically hypoxic cardiomyocytes calpain is thought to induce the breakdown of troponin T. [27,28]
Additionally, sepsis has been shown to cause overactivation of calpain in cardiomyocytes leading to increased caspase-3 activation and TNF-α expression. Inhibition of calpain activation in cardiomyocytes improves the myocardial dysfunction associated with endotoxaemia. [29,30]
Interestingly, calpain 2 protein levels have been found to be increased in the atrial tissue of patients with atrial fibrillation, valvular heart disease and diabetes. [31]
There is a large amount of research to suggest that calpain overactivity in stressed myocardial tissue leads to cardiac dysfunction and that inhibition of calpain activity is beneficial to cardiac function.
Lungs
Calpain is thought to play a critical role in the pathogenesis of the tissue remodeling associated with a number of pulmonary diseases. Calpain augments collagen-1 synthesis in patients with pulmonary fibrosis. [32] Inhibition of calpain has been found to ameliorate pulmonary fibrosis by decreasing expression of interleukin-6, transforming growth factor-β1, angiopoietin-1 and collagen-1 in lung tissue. [33]
Calpain overactivity has also been implicated in the pathogenesis of asthma through a similar mechanism. Calpain is thought to mediate cytokine induced collagen-1 proliferation by upregulating the mTOR/Akt signaling pathway. This results in the airway smooth muscle remodeling that occurs in patients with asthma. [34] Taken together, research suggests that calpain overactivity leads to pulmonary disease by upregulating collagen proliferation.
Pancreas
Calpain-10 gene expression has been found to be associated with the pancreatic insufficiency that occurs in patients with obesity and diabetes mellitus. [35–37] Beta cell dysfunction is a major cause of the pathogenesis of type 2 diabetes mellitus (T2DM). Calpain has been found to contribute to beta cell dysfunction and apoptosis in the setting of T2DM. [8]
One mechanism through which calpain may lead to beta cell dysfunction is thought to occur through the endoplasmic reticulum. Obesity and T2DM result in increased levels of plasma free fatty acids. These free fatty acids have been shown to lead to increased calcium ion expression and calpain activation that induces major endoplasmic reticulum stress markers in beta cells resulting in cellular apoptosis.[38]
Finally, current research suggests that variation of the calpain 10 gene impacts a variety of the clinical metabolic derangements related to type 2 diabetes mellitus.[39] However, the exact mechanism through which calpain works is currently unknown.
Kidney
Normal calpain activity is necessary for basic renal cell function. Calpain has been found to be localized to both the cytosol and mitochondria in renal proximal tubule cells. Interestingly, calpain 10 has been shown to be required for renal cell viability. Calpain 10 expression levels have been found to decrease with increasing age and renal dysfunction. [40]
In the setting of renal ischemia, calpains are released into the extracellular environment by tubular epithelial cells. This externalization of calpain increases epithelial cell mobility and helps with tubule repair. Calpains work by cleaving fibronectin and preventing its ability to bind to integrin. In a small animal model of ischemic acute renal failure, cytosolic calpain inhibition was associated with delayed tubule repair. [41]
Calpains are thought to mediate renal proximal tubule injury. [42,43] Calpain overactivation leads to cleavage of paxillin, talin and vinculin in renal proximal tubule cells. This cleavage of cytoskeletal proteins results in increased plasma membrane permeability and caspase activation resulting in renal cell death and necrosis. [1] Calpain inhibition preserves cytoskeletal protein levels and mediates plasma membrane permeability. [44]
In situations of stress, renal cells experience a loss of ATP. This ATP depletion leads to increased release of calcium from the endoplasmic reticulum. This increased calcium release leads to increased calpain activity. Calpain is thought to mediate late stage cell death by inducing a second extracellular calcium ion influx leading to cell swelling and loss of plasma membrane integrity. [45]
Calpain 10 cleaves electron transport chain proteins in the mitochondria of renal cells. This cleavage decreases mitochondrial respiration and leads to mitochondrial dysfunction which results in renal cell death. [1]
Taken together, these data suggest that basal levels of calpain activity play a protective role in the kidney but over-activation in the setting of stress leads to cell death.
Vascular System
Research shows that calpain overactivity results in endothelial dysfunction by a number of different mechanisms.
Endothelial dysfunction is a widely accepted cause of diabetic vasculopathy. Calpain activity has been shown to be increased in the microcirculation of rats with T2DM. Inhibition of calpain is associated with attenuated leukocyte-endothelium interactions, decreased expression of cell adhesion molecules and increased endothelia nitric oxide availability. [46]
In the setting of diabetes, calpain also plays a role in vascular endothelial reactive oxygen species (ROS) production. High glucose levels induce calpain activity leading to cellular apoptosis. Inhibition of calpain prevents high glucose-induced ROS production, mitochondrial superoxide generation and apoptosis in endothelial cells, and attenuates endothelium dependent aortic ring relaxation.[47] Interestingly inhibition of calpain in the setting of diabetes has also been found to prevent upregulation of leukocyte endothelial interactions by increasing the association of hsp90 with endothelial nitric oxide synthase and decreasing endothelial cell surface expression of proinflammatory adhesion molecules (ICAM and VCAM) during hyperglycemia.[48]
Angiongensin II is a critical mediator of cardiovascular remodeling in the setting of hypertension. Angiongensin II activates calpain which lead to vascular media hypertrophy, perivascular inflammation and fibrosis. Calpain inhibition blunts angiongensin II-induced medial hypertrophy, perivascular inflammation and fibrosis in the aorta and small kidney arteries. [49]
Over expression of calpain 1 by angiotensin II induces matrix metalloproteinase type 2 activity which has been linked to extracellular matrix remodeling by promoting collagen I and III production and inducing vascular calcification. Calpain 1 over expression also induces transforming growth factor-β1/Smad signaling, elastin degradation, alkaline phosphatase activation and increases total calcium content in cultured vascular smooth muscle cells and carotid artery rings. Interestingly, calpain 1 and collagen II protein levels increase with the age in human aortic intima. These same proteins have been found to be over expressed in atherosclerotic plaques. Taken together, this data suggests that calpain mediated extracellular matrix remodeling is associated with hypertension and atherosclerosis. [50]
Oxidized low density lipoprotein is known to contribute to atherosclerosis by inducing apoptosis of endothelial cells. Oxidized low density lipoprotein induces calpain dependent proteolysis of the cytoskeletal protein α-fodrin and anti-apoptotic protein Bcl-2 in endothelial cells. Inhibition of calpain mediates this effect. [51]
In endothelial cells, during situations of stress calcium ion overload leads to mitochondrial calpain 1 cleavage of the sodium/calcium exchanger which results in calcium accumulation in the mitochondria. Calpain 1 activity also cleaves Bid which induces cytochrome c release and leads to endothelial cell apoptosis. [1]
Taken together, research suggests that during stress calpains become overactivated leading to endothelial cell dysfunction and, ultimately, organ dysfunction.
Skeletal Muscle
Calpain activity has been found to be upregulated and associated with the muscle fiber atrophy seen in dermatomyositis. This calpain activity is localized to the perifascicular muscle fibers.[52] Calpain is known to cleave transcription factor YY1 which represses muscle restricted expression of sarcomeric α-actin genes.[53]
Conclusion
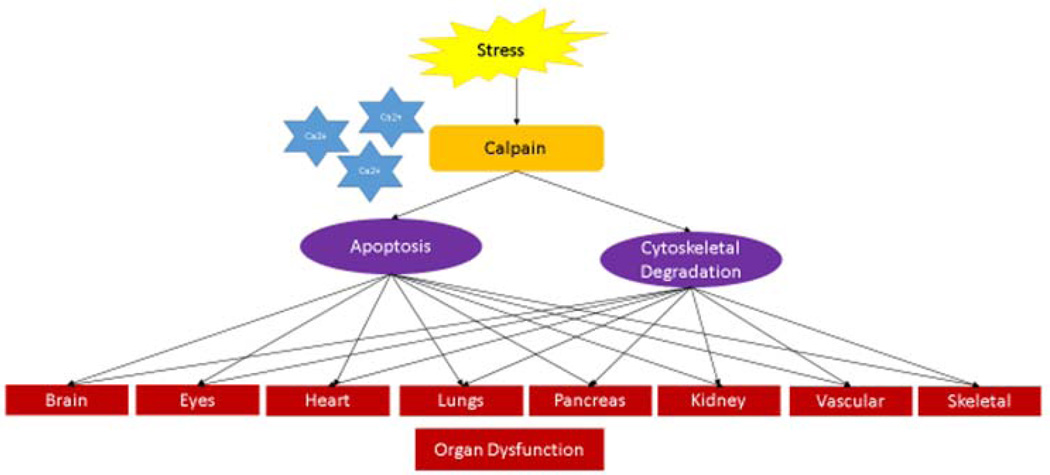
Calpain overactivity is involved in the pathophysiology of a large number of disease processes. Situations of stress lead to increased calcium ion release causing overactivation of calpain. Calpain then functions to promote cellular apoptosis and degrade cytoskeletal structure resulting in organ dysfunction. (Figure 1) Recent research has started to isolate which specific calpain family member is involved in each of the different disease processes. The next step will be to create a calpain inhibitor that is selective enough to inhibit a specific calpain in a cell- or tissue-specific manner to block the detrimental effects of calpain overactivation.
Figure 1. Calpain Activation in Situations of Stress Leads to Organ Dysfunction.
Situations of stress lead to increased calcium ion release causing over-activation of calpain. Calpain then acts to promote cellular apoptosis and degrade cytoskeletal structure resulting in organ dysfunction.
Acknowledgments
This work was supported in part by the National Heart, Lung, and Blood Institute (R01HL46716, RO1HL128831 Dr. Sellke); NIH/NIGMS GM1P20GM103652 (Project-3) and American Heart Association Grant-in-Aid 14GRNT20460291 (Dr. Abid); NIH/NIGMS Training Grant 2T32 GM065085 (Dr. Potz).
References
- 1.Smith MA, Schnellmann RG. Calpains, mitochondria, and apoptosis. Cardiovasc Res. 2012;96:32–37. doi: 10.1093/cvr/cvs163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potz BA, Sabe AA, Abid MR, Sellke FW. Calpains and Coronary Vascular Disease. Circ J. 2016;80:4–10. doi: 10.1253/circj.CJ-15-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gan-Or Z, Rouleau GA. Calpain 1 in neurodegeneration: a therapeutic target? Lancet Neurol. 2016;15:1118. doi: 10.1016/S1474-4422(16)30175-2. [DOI] [PubMed] [Google Scholar]
- 4.Baudry M, Chou MM, Bi X. Targeting calpain in synaptic plasticity. Expert Opin Ther Targets. 2013;17:579–592. doi: 10.1517/14728222.2013.766169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurbatskaya K, Phillips EC, Croft CL, Dentoni G, Hughes MM, Wade MA, et al. Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer’s disease brain. Acta Neuropathol Commun. 2016;4:34. doi: 10.1186/s40478-016-0299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fà M, Zhang H, Staniszewski A, Saeed F, Shen LW, Schiefer IT, et al. Novel Selective Calpain 1 Inhibitors as Potential Therapeutics in Alzheimer’s Disease. J Alzheimers Dis. 2015;49:707–721. doi: 10.3233/JAD-150618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar P, Choonara YE, Pillay V. In Silico Affinity Profiling of Neuroactive Polyphenols for Post-Traumatic Calpain Inactivation: A Molecular Docking and Atomistic Simulation Sensitivity Analysis. Molecules. 2015;20:135–168. doi: 10.3390/molecules20010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CJ, Gurlo T, Haataja L, Costes S, Daval M, Ryazantsev S, et al. Calcium-activated calpain-2 is a mediator of beta cell dysfunction and apoptosis in type 2 diabetes. J Biol Chem. 2010;285:339–348. doi: 10.1074/jbc.M109.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima E, Hammond KB, Rosales JL, Shearer TR, Azuma M. Calpain, not caspase, is the causative protease for hypoxic damage in cultured monkey retinal cells. Investig Ophthalmol Vis Sci. 2011;52:7059–7067. doi: 10.1167/iovs.11-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoang MV, Smith LESD. Calpain inhibitors reduce retinal hypoxia in ischemic retinopathy by improving neovascular architecture and functional perfusion. Biochim Biophys Acta. 2011;1812 doi: 10.1016/j.bbadis.2010.08.008. 997–1549–557003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoang MV, Nagy Ja, Fox JEB, Senger DR. Moderation of calpain activity promotes neovascular integration and lumen formation during VEGF-induced pathological angiogenesis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn YJ, Kim MS, Chung SK. Calpain and Caspase-12 Expression in Lens Epithelial Cells of Diabetic Cataracts. Am J Ophthalmol. 2016;167:31–37. doi: 10.1016/j.ajo.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Kain VSS. High glucose-induced Ca2+ overload and oxidative stress contribute to apoptosis of cardiac cells through mitochondrial dependent and independent pathways. Biochim Biophys Acta. 2012;1820:907–920. doi: 10.1016/j.bbagen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Kudo-Sakamoto Y, Akazawa H, Ito K, Takano J, Yano M, Yabumoto C, et al. Calpain-dependent Cleavage of N-cadherin Is Involved in the Progression of Post-myocardial Infarction Remodeling. J Biol Chem. 2014;289:19408–19419. doi: 10.1074/jbc.M114.567206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshikawa Y, Hagihara H, Ohga Y, Nakajima-Takenaka C, Murata KY, Taniguchi STM. Calpain inhibitor-1 protects the rat heart from ischemia-reperfusion injury: analysis by mechanical work and energetics. AJP Hear Circ Physiol. 2004;288:H1690–H1698. doi: 10.1152/ajpheart.00666.2004. [DOI] [PubMed] [Google Scholar]
- 16.Khalil PN, Neuhof C, Huss R, Pollhammer M, Khalil MN, Neuhof H, et al. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. Eur J Pharmacol. 2005;528:124–131. doi: 10.1016/j.ejphar.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Takeshita D, Tanaka M, Mitsuyama S, Yoshikawa Y, Zhang G-X, Obata K, et al. A new calpain inhibitor protects left ventricular dysfunction induced by mild ischemia-reperfusion in in situ rat hearts. J Physiol Sci. 2012:113–123. doi: 10.1007/s12576-012-0243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei M, Tang F, Lu M, He X, Wang H, Hou X, et al. Astragaloside IV attenuates apoptosis of hypertrophic cardiomyocyte through inhibiting oxidative stress and calpain-1 activation. Environ Toxicol Pharmacol. 2015;40:764–773. doi: 10.1016/j.etap.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Ma J, Zhu H, Singh M, Hill D, Greer PA, et al. Targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes. 2011;60:2985–2994. doi: 10.2337/db10-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad Ha, Lu L, Ye S, Schwartz GG, Greyson CR. Calpain inhibition preserves talin and attenuates right heart failure in acute pulmonary hypertension. Am J Respir Cell Mol Biol. 2012;47:379–386. doi: 10.1165/rcmb.2011-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabe AA, Potz BA, Elmadhun NY, Liu Y, Feng J, Abid MR, et al. Calpain Inhibition Improves Collateral Dependent Perfusion in a Hypercholesterolemic Swine Model of Chronic Myocardial Ischemia. J Thorac Cardiovasc Surg. 2016;151:245–252. doi: 10.1016/j.jtcvs.2015.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potz BA, Sabe AA, Elmadhun NY, Feng J, Liu Y, Mitchell H, et al. Calpain inhibition decreases myocardial apoptosis in a swine model of chronic myocardial ischemia. Surgery. 2015;158:445–452. doi: 10.1016/j.surg.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goñi-Oliver Paloma, Lucas José J, Avila Jesús, FH N-terminal Cleavage of GSK-3 by Calpain. J Biol Chem. 2007;282:22406–22413. doi: 10.1074/jbc.M702793200. [DOI] [PubMed] [Google Scholar]
- 24.Feng Ye, Xia Yiyuan, Yu Guang, Shu Xiji, Ge Haoliang, Zeng Kuan, JW, Xiaochuan W. Cleavage of GSK-3β by calpain counteracts the inhibitory effect of Ser9 phosphorylation on GSK-3β activity induced by H2O2. J Neurochem. 2013;126:234–242. doi: 10.1111/jnc.12285. [DOI] [PubMed] [Google Scholar]
- 25.Miura T, Miki T. GSK-3 β, a Therapeutic Target for Cardiomyocyte Protection. Circ J. 2009;73:1184–1192. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- 26.Potz Brittany A, Sabe Ashraf A, MD, Elmadhun Nassrene Y, MD, Feng Jun, MD, PhD, Clements Richard T, PhD, Abid M. Ruhul, MD, PhD, Sellke M Frank W. Glycogen Synthase Kinase 3B Inhibition Improves Myocardial Angiogenesis and Collateral-dependent Perfusion in a Swine Model of Metabolic Syndrome. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003694. pii: e003694. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kositprapa C, Zhang B, Berger S, Canty JM, Lee TC. Calpain-mediated proteolytic cleavage of troponin I induced by hypoxia or metabolic inhibition in cultured neonatal cardiomyocytes. Mol Cell Biochem. 2000;214:47–55. doi: 10.1023/a:1007160702275. [DOI] [PubMed] [Google Scholar]
- 28.Streng AS, de Boer D, van der Velden J, van Dieijen-Visser MP, Wodzig WKWH. Posttranslational modifications of cardiac troponin T: An overview. J Mol Cell Cardiol. 2013;63:47–56. doi: 10.1016/j.yjmcc.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Li Y, Shan L, Shen E, Chen R, Peng T. Over-expression of calpastatin inhibits calpain activation and attenuates myocardial dysfunction during endotoxaemia. Cardiovasc Res. 2009;83:72–79. doi: 10.1093/cvr/cvp100. [DOI] [PubMed] [Google Scholar]
- 30.Tissier S, Lancel S, Marechal X, Mordon S, Depontieu F, Scherpereel A, et al. Calpain inhibitors improve myocardial dysfunction and inflammation induced by endotoxin in rats. Shock. 2004;21:352–357. doi: 10.1097/00024382-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y, Cui G, Zhou N, Li C, Zhang Q, Sun H, et al. Calpain-Calcineurin-Nuclear Factor Signaling and the Development of Atrial Fibrillation in Patients with Valvular Heart Disease and Diabetes. J Diabetes Res. 2016;2016:1–7. doi: 10.1155/2016/4639654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li F-Z, Cai P-C, Song L-J, Zhou L-L, Zhang Q, Rao S-S, et al. Crosstalk between calpain activation and TGF-α1 augments collagen-I synthesis in pulmonary fibrosis. Biochim Biophys Acta. 2015;1852:1796–1804. doi: 10.1016/j.bbadis.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Tabata C, Tabata R, Nakano T. The calpain inhibitor calpeptin prevents bleomycin-induced pulmonary fibrosis in mice. Clin Exp Immunol. 2010;162:560–567. doi: 10.1111/j.1365-2249.2010.04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao S, Mu Q, Zeng Y, Cai P, Liu F, Yang J, et al. Calpain-activated mTORC2/Akt pathway mediates airway smooth muscle remodeling in asthma. Clin Exp Allergy. 2016 Sep 20; doi: 10.1111/cea.12805. [DOI] [PubMed] [Google Scholar]
- 35.Derbel S, Doumaguet C, Hubert D, Mosnier-Pudar H, Grabar S, Chelly J, et al. Calpain 10 and development of diabetes mellitus in cystic fibrosis. J Cyst Fibros. 2006;5:47–51. doi: 10.1016/j.jcf.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Cheverud JM, Fawcett GL, Jarvis JP, Norgard EA, Pavlicev M, Pletscher LS, Polonsky KS, Ye H, Bell GISC. Calpain-10 is a component of the obesity-related quantitative trait locus Adip1. J Lipid Res. 2010;51:907–913. doi: 10.1194/jlr.M900128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pablo P, Salazar AM, Burns AL, Ostrosky-Wegman P. Role of calpain-10 in the development of diabetes mellitus and its complications. Arch Med Res. 2014;45:103–115. doi: 10.1016/j.arcmed.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Cui W, Ma J, Wang X, Yang W, Zhang J, Ji Q. Free Fatty Acid Induces Endoplasmic Reticulum Stress and Apoptosis of β-cells by Ca2+/Calpain-2 Pathways. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang K, Fang Q, Zheng T, Jia W, Wang Y, Zhang R, et al. The impact of calpain-10 gene combined-SNP variation on type 2 diabetes mellitus and its related metabolic traits. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2001;18:426–430. [PubMed] [Google Scholar]
- 40.Covington Marisa D, Arrington David D, RGS Calpain 10 is required for cell viability and is decreased in the aging kidney. Am Physiol Ren Physiol. 2009;296:478–486. doi: 10.1152/ajprenal.90477.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frangie C, Zhang W, Perez J, Dubois YCX, Haymann JP, Baud L. Extracellular calpains increase tubular epithelial cell mobility: Implications for kidney repair after ischemia. J Biol Chem. 2006;281:26624–26632. doi: 10.1074/jbc.M603007200. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Rainey JJ, Harriman JAYF, Schnellmann RG, Rainey JJ, Harriman JF, et al. Calpains mediate acute renal cell death : role of autolysis and translocation. Am J Physiol Ren Phsiology. 2001;281:728–738. doi: 10.1152/ajprenal.2001.281.4.F728. [DOI] [PubMed] [Google Scholar]
- 43.Waters SL, Sarang SS, Wang KKW, Schnellmann RG. Calpains Mediate Calcium and Chloride Influx During the Late Phase of Cell Injury 2. J Pharmacol Exp Ther. 1997;283:1177–1184. [PubMed] [Google Scholar]
- 44.Liu X, Schnellmann RG. Calpain Mediates Progressive Plasma Membrane Permeability and Proteolysis of Cytoskeleton-Associated Paxillin, Talin, and Vinculin during Renal Cell Death. 2003;304:63–70. doi: 10.1124/jpet.102.043406. [DOI] [PubMed] [Google Scholar]
- 45.Harriman JF, XL, Aleo M, KM, Schnellmann RG. Endoplasmic reticulum Ca 2 + signaling and calpains mediate renal cell death. Cell Death. 2002;9:734–741. doi: 10.1038/sj.cdd.4401029. [DOI] [PubMed] [Google Scholar]
- 46.Stalker TJ, Gong YSR. The calcium-dependent protease calpain causes endothelial dysfunction in type 2 diabetes. Diabetes. 2005;54:1132–1140. doi: 10.2337/diabetes.54.4.1132. [DOI] [PubMed] [Google Scholar]
- 47.Chen B, Zhao Q, Ni R, Tang F, Shan L, Cepinskas I, et al. Inhibition of calpain reduces oxidative stress and attenuates endothelial dysfunction in diabetes. Cardiovasc Diabetol. 2014;13:1–12. doi: 10.1186/1475-2840-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stalker TJ, Skvarka CB, Scalia R. A novel role for calpains in the endothelial dysfunction of hyperglycemia. FASEB J. 2003;17:1511–1513. doi: 10.1096/fj.02-1213fje. [DOI] [PubMed] [Google Scholar]
- 49.Letavernier E, Perez J, Bellocq A, Mesnard L, De Castro Keller A, Haymann JP, et al. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 50.Jiang L, Zhang J, Monticone RE, Telljohann R, Wu J, Wang M, et al. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension. 2012;60:1192–1199. doi: 10.1161/HYPERTENSIONAHA.112.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MIP-Ä, Saido Takaomi C, TA, MPSA Oxidized low-density lipoprotein induces calpain-dependent cell death and ubiquitination of caspase 3 in HMEC-1 endothelial cells. Biochem J. 2003;411:403–411. doi: 10.1042/BJ20021955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallardo Eduard, PhD, De Andr’s Irene, PhD, Isabel Illa M. Cathepsins Are Upregulated by IFN- gamma / STAT1 in Human Muscle Culture : A Possible Active Factor in Dermatomyositis. J Neuropathol Exp Neurol. 2001;60:847–855. doi: 10.1093/jnen/60.9.847. [DOI] [PubMed] [Google Scholar]
- 53.Walowitz JL, Bradley ME, Chen S, Lee T. Proteolytic regulation of the zinc finger transcription factor YY1, a repressor of muscle-restricted gene expression. J Biol Chem. 1998;273:6656–6661. doi: 10.1074/jbc.273.12.6656. [DOI] [PubMed] [Google Scholar]