Abstract
Cerebral microdialysis has enabled the clinical characterization of excitotoxicity (glutamate >10 μM) and non-ischemic metabolic crisis (lactate/pyruvate ratio [LPR] >40) as important components of secondary damage in severe traumatic brain injury (TBI). Spreading depolarizations (SD) are pathological waves that occur in many patients in the days following TBI and, in animal models, cause elevations in extracellular glutamate, increased anaerobic metabolism, and energy substrate depletion. Here, we examined the association of SD with changes in cerebral neurochemistry by placing a microdialysis probe alongside a subdural electrode strip in peri-lesional cortex of 16 TBI patients requiring neurosurgery. In 107 h (median; range: 76–117 h) of monitoring, 135 SDs were recorded in six patients. Glutamate (50 μmol/L) and lactate (3.7 mmol/L) were significantly elevated on day 0 in patients with SD compared with subsequent days and with patients without SD, whereas pyruvate was decreased in the latter group on days 0 and 1 (two-way analysis of variance [ANOVA], p values <0.05). In patients with SD, both glutamate and LPR increased in a dose-dependent manner with the number of SDs in the microdialysis sampling period (0, 1, ≥2 SD) [glutamate: 2.1→7.0→52.3 μmol/L; LPR: 27.8→29.9→45.0, p values <0.05]. In these patients, there was a 10% probability of SD occurring when glutamate and LPR were in normal ranges, but a 60% probability when both variables were abnormal (>10 μmol/L and >40 μmol/L, respectively). Taken together with previous studies, these preliminary clinical results suggest SDs are a key pathophysiological process of secondary brain injury associated with non-ischemic glutamate excitotoxicity and severe metabolic crisis in severe TBI patients.
Keywords: : electrocorticography, multi-modal monitoring, spreading depression
Introduction
Since Persson and Hillered's initial clinical study,1 cerebral microdialysis has been used to gain insight into secondary injury processes of traumatic brain injury (TBI). Continuous sampling of the brain's extracellular fluid permits analysis of energy-related metabolites (glucose, lactate, and pyruvate) to detect impaired metabolism and the excitatory amino acids (glutamate) as a possible marker of excitotoxic injury processes. In TBI, increases in extracellular lactate/pyruvate ratio ([LPR] >25)2–4 and glutamate (>10 μM)2,4–6 are established markers of secondary brain injury associated with the initial injury severity, and ultimately poor outcomes. Increases in the LPR, an established marker of metabolic crisis that signals a switch from aerobic to anaerobic metabolism due to ischemia and/or mitochondrial dysfunction,7,8 were initially thought to reflect secondary hypoxic/ischemic events.5,6,8–12 However, recent reports have shown these perturbations are more enigmatic, as severe metabolic crisis (LPR >40) is observed even after adequate resuscitation,13 in the absence of frank ischemia,14 and independent of cerebral perfusion pressures.15 Vespa and colleagues have shown that non-convulsive seizures are one pathophysiological source of such a non-ischemic “metabolic crisis.”16
Rapid-sample microdialysis in cats and humans suggest that spreading depolarizations (SD) may also be a major mechanism triggering increases in the LPR.17–19 SDs are a class of pathological waves that propagate through cerebral gray matter at 1 to 8 mm/min and are characterized by sustained (∼2 min) depolarization of neurons and astrocytes and consequent suppression of synaptic activity (spreading depression).20,21 SD occurs in ∼55% of severe TBI patients, often in a repetitive pattern lasting hours to days,22,23 and is independently associated with worse outcomes.24 SD triggers a complete breakdown of transmembrane ionic gradients25,26 with an intracellular calcium surge up to 25 μM, and the release of extracellular glutamate and other neurotransmitters in pathological quantities.27–29 Thus, recovery from SD is highly energy-demanding, as adenosine triphosphate (ATP) is consumed to operate pumps and transporters that restore equilibrium.30,31 Thus, in animal models, SDs trigger increases in extracellular lactate and decreases in glucose in both normal and ischemic cortex,18,32–34 and these findings are confirmed clinically in TBI patients.17,19
Here, we hypothesized that the occurrence of SD in cerebral cortex may be an important mechanism associated with elevations in glutamate and LPR in some TBI patients. To examine associations between these phenomena, a microdialysis probe was placed within 10 mm of a subdural electrode strip in peri-lesional cortex of patients requiring neurosurgical intervention. Although overall differences in extracellular neurochemistry between patients with and without SD were minimal, we found that both glutamate and LPR were elevated in a dose-dependent manner when SDs occurred. Taken together with pre-clinical studies, results of this pilot study suggest that prevention of SD may offer an important neuroprotective strategy to forestall derangements of cerebral neurochemistry.
Methods
Patients
Sixteen patients with acute TBI were prospectively enrolled in the Co-Operative Studies on Brain Injury Depolarizations (COSBID) at Virginia Commonwealth University. Inclusion criteria were the clinical decision for neurological surgery for lesion evacuation and/or decompression and age ≥18 years. Patients with fixed, dilated pupils were excluded. Research protocols were approved by institutional review boards, surrogate informed consent was obtained for all patients, and research was conducted in accordance with the Declaration of Helsinki. Data from patient 12 have been reported previously.35
Procedures
For electrocorticographical monitoring of SD, a single electrode strip was placed on the surface of the brain in the operating room following lesion evacuation and hemostasis. The strip consisted of six platinum electrodes (4.2 mm2 exposed area) spaced at 10 mm (Wyler, Ad-Tech Medical, Racine, WI). The strip was targeted to viable peri-lesional cortex judged to be at highest risk of secondary injury and was placed along a single gyrus when possible.24,36–38 A microdialysis catheter (CMA70 Brain Microdialysis Catheter, 10 cm flexible shaft, 10 mm membrane length, 20 kDa cutoff, and an external diameter of 0.6 mm, CMA, Stockholm, Sweden) was inserted 1.5–2.0 cm into the parenchyma within 1 cm adjacent to the electrode strip to monitor the same tissue. The microdialysis catheter was connected to a portable, battery-driven syringe pump (CMA 107 Microdialysis Pump, CMA Microdialysis) that perfused sterile normal saline at the rate of 2 μL/min. As the perfusion flow rate is inversely related to relative recovery, at the given perfusion rate of 2 μL/min, the expected recovery of the analytes glucose, lactate, pyruvate, and glutamate is some 40% of the true extracellular concentration.39,40 Microdialysis samples were collected in 60-min intervals in all patients except patients 1–3 and 6 (90 min) and immediately frozen (–20°C) in air-tight vials.
The subdural electrode strip was connected to two AC-coupled Dual Bioamp amplifiers (high pass cutoff: 0.02 Hz; ADInstruments, New South Wales, Australia) in a sequential bipolar fashion and ground was provided by a self-adhesive Ag/AgCl patch electrode on the shoulder. Electrocorticography data were digitized and recorded at 200 Hz sampling with Powerlab 16/SP and Chart 5 software (ADInstruments, Inc.). During monitoring, patients were sedated, ventilated, and pharmacologically immobilized as required. Sedation was maintained with propofol or midazolam and analgesia was provided with fentanyl or morphine. Phenytoin was administered for seizure control or prophylaxis in all patients. Intracranial pressure (ICP) was monitored in all patients through a ventricular drainage catheter or intraparenchymal transducer (Codman, Raynham, MA). Neurocritical care followed the Brain Trauma Foundation guidelines41 and aimed to maintain ICP <20 mm Hg and cerebral perfusion pressure (CPP) >60 mm Hg. Neuromonitoring was terminated and devices were removed at the patient's bedside when invasive neuromonitoring was no longer clinically required or after a maximum of 7 days. Clinical outcome was assessed at 6 months during telephone interview or at the clinical visit according to the Glasgow Outcome Score-Extended (GOS-E).
Data analysis
Frozen microdialysis samples (n = 1214) were analyzed in a batch fashion each week using standard reagents on the CMA microdialysis analyzer (CMA 600, Solna, Sweden) for concentrations of glucose, lactate, pyruvate, and glutamate.40 The absolute concentrations of the neurochemicals should not be affected by this freeze/thaw procedure as prior work has shown this did not significantly alter the concentrations.42 The bedside nurse and the research team maintained a detailed patient event log to identify important events and to record times of vial sampling. To allow for stabilization of the probe after placement, the first sample was excluded from analysis. Samples were classified as “abnormal” based on established thresholds2: glucose <1.0 mmol/L, lactate >4 mmol/L, pyruvate <50 μmol/L, glutamate >10 μmol/L, and LPR >40. “Normal” values for human microdialysis have been reported previously.3 Electrocorticography recordings were analyzed for SD according to methods described previously.24,37,38 Briefly, SDs were identified by (1) the occurrence of slow-potential changes, reflecting high-pass filtering at 0.02 Hz of the 5–15 mV negative shift in DC potential,43 (2) amplitude depression of high-frequency spontaneous activity (0.5–50 Hz) lasting at least several minutes, with onset synchronous with the slow-potential change in the same channel, and (3) the sequential occurrence of slow-potential changes and depressions on adjacent channels, demonstrating spread across the cortex at 1–8 mm/min. The time of the first slow-potential change in any channel was used as the time stamp for that SD. Day 0 was defined as the first 24 h after injury.
Statistical analysis
Data were sorted and analyzed with custom programs written in MATLAB (The MathWorks, Natick, MA). Data are presented as medians (interquartile range) and statistical significance was defined as p < 0.05.
Results
Patient summary
Patient injury characteristics are summarized in Table 1. The majority of patients were male and median age was 30 years (range: 20–40 years). All patients had severe TBI (Glasgow Comas Scale [GCS] score <9) at hospital admission, except for patient 5, and underwent neurosurgery for treatment of hemorrhagic contusions or acute subdural hematomas. Lateralized decompressive craniectomies were performed in 14 cases, one patient underwent bifrontal craniectomy, and another had craniotomy with bone flap replacement. Microdialysis and electrocorticography were performed simultaneously in the intensive care unit for 107 h (median; range: 76–117 h). During monitoring, CPP ranged 66–95 mm Hg and plasma glucose ranged 106–186 mg/dL, generally above the level of tight glycemic control (<110 mg/dL) (Table 1). At 6 months, 13 patients (81%) had a favorable outcome (GOS-E 5–8).
Table 1.
Patient and Recording Characteristics
| Patient | Age | Sex | Cause of TBI | ADM GCS | Primary lesion | Midline shift (mm) | Hours of monitoring | Plasma glucose (mg/dL) | CPP (mm Hg) | Number of SDs | GOS-E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | M | GSW | 7 | Contusions | 0 | 50 | 115 ± 13 | 82 ± 14 | 0 | 5 |
| 2 | 47 | M | Fall | 3T | Contusions | 4 | 61 | 106 ± 7 | 95 ± 11 | 4 | 5 |
| 3 | 27 | M | ATV | 8 | Contusions | 0 | 118 | 148 ± 27 | 78 ± 9 | 0 | 1 |
| 4 | 40 | M | MVA-P | 6 | Contusions | 7 | 111 | 179 ± 27 | 72 ± 9 | 14 | 7 |
| 5 | 38 | M | Assault | 12 | Contusions | 4 | 137 | 127 ± 30 | 88 ± 10 | 6 | 7 |
| 6 | 32 | F | Bicycle | 3T | Contusions | 2 | 115 | 185 ± 37 | 81 ± 9 | 0 | 8 |
| 7 | 41 | M | Motocycle | 3T | Contusions | 0 | 84 | 186 ± 33 | 95 ± 13 | 0 | 5 |
| 8 | 60 | F | MVA | 3T | Unk | Unk | 88 | 124 ± 24 | 81 ± 9 | 0 | 8 |
| 9 | 18 | M | Motorcycle | 3 | SDH | 15 | 73 | 113 ± 7 | 67 ± 6 | 0 | 6 |
| 10 | 35 | M | Motorcycle | 3T | SAH/SDH | 3 | 119 | 127 ± 20 | 77 ± 10 | 0 | 6 |
| 11 | 20 | F | Bicycle | 4 | SDH | 9 | 114 | 118 ± 8 | 71 ± 7 | 0 | 7 |
| 12 | 20 | M | Fall | 8 | SDH | 5 | 67 | 147 ± 31 | 66 ± 9 | 34 | 8 |
| 13 | 22 | M | Fall | 5 | SDH | 13 | 85 | 132 ± 18 | 71 ± 11 | 2 | 4 |
| 14 | 20 | F | MVA | 8T | SDH | 7 | 114 | 139 ± 19 | 78 ± 8 | 0 | 5 |
| 15 | 20 | M | MVA | 7T | Contusions | 4 | 120 | 128 ± 20 | 66 ± 7 | 0 | 6 |
| 16 | 40 | M | Bicycle | 7T | Contusions | 0 | 102 | 160 ± 27 | 74 ± 8 | 75 | 1 |
ADM GCS, Admission Glasgow Coma Scale; CPP, cerebral perfusion pressure; GOS-E, Glasgow Outcome Score-Extended; GSW, gunshot wound; MVA, motor vehicle accident; MVA-P, pedestrian involved in motor vehicle accident; SAH, subarachnoid hemorrhage; SD, spreading depolarization; SDH, subdural hematoma; Unk, unknown.
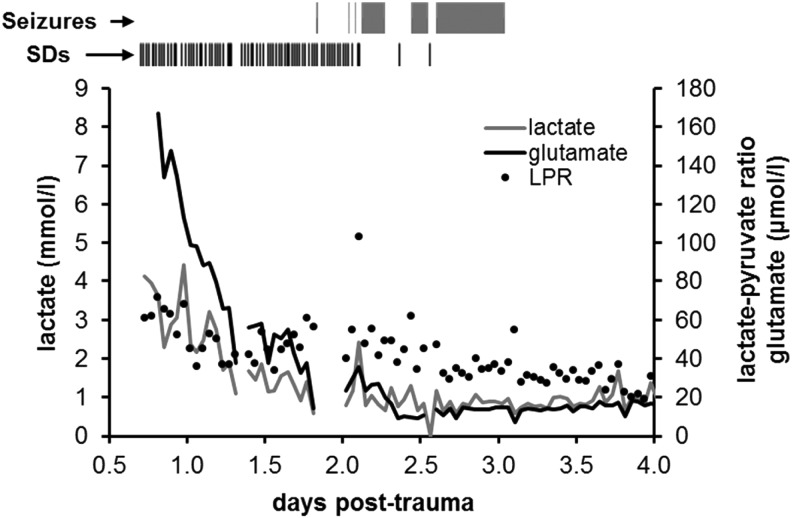
Data were analyzed to determine whether SDs were associated with particular derangements of cerebral neurochemistry. Overall, 19% of microdialysate glutamate values were elevated (>10 μmol/L), 83% of glucose values were low (<1 mmol/L), and 61% of pyruvate values were low (<50 μmol/L), mainly accounting for elevated LPR (>40) in 30% of samples; lactate was elevated (>4 mmol/L) in only 0.7% of samples. A total of 135 SDs were observed in 6 of 16 (37.5%) patients, with most occurring in patients 12 and 16. Electrocorticographic seizures occurred in these same two patients only, as described previously by Fabricius and colleagues as patients 7 and 6, respectively.44 Patient 12 had a single seizure lasting <4 min, whereas patient 16 had seizure-like activity for 27 h. Figure 1 shows that this episode of status epilepticus developed 2 days post-trauma, following termination of repetitive continuous SD in the initial 30 h of monitoring. We note that the lower incidence of SD and better outcomes of patients in the present study compared with previous reports is likely attributable to the common use of large decompressive craniectomies for surgical TBI management.24,45
FIG. 1.
Time course of microdialysis and electrocorticographical abnormalities in severe TBI patient. For patient 16, the timings of seizures and SDs are shown above with the same time axis as the microdialysis graph. Individual SDs are shown as vertical tick marks and bars show the durations of continuous seizure-like activity. SDs were observed continuously from the beginning of monitoring and then were progressively replaced at 2 days post-trauma by seizure-like activity, consisting of high-amplitude spike discharges repeating at 2- to 5-s intervals. SD, spreading depolarization; TBI, traumatic brain injury.
Overall trends
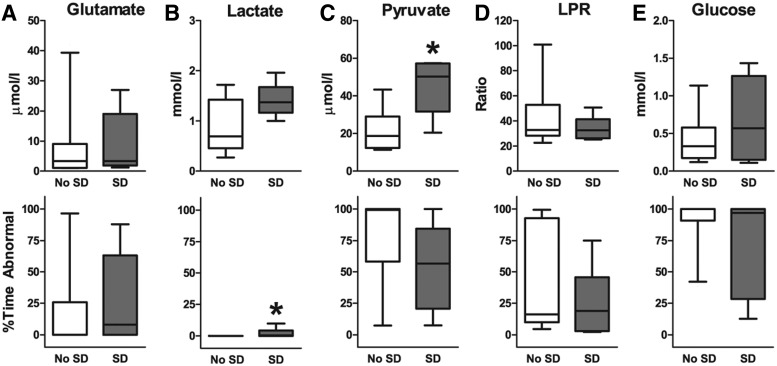
We first examined whether patients with and without SDs differed overall in cerebral microdialysate measures. For each patient, we calculated the median value and the percentage of samples that deviated from a normal range over the entire monitoring period (Fig. 2). Differences between patients with and without SD were minimal. Only extracellular pyruvate was significantly higher in patients with SDs (51.2 vs.16.6 μmol/L; Mann-Whitney U test p = 0.008), whereas the percentage of abnormal values (SD 57% vs. no SD 99%) did not reach significance (Fig. 2C). Extracellular lactate also trended toward higher values in patients with SD (1.3 vs. 0.7 mmol/L; Mann-Whitney U test p = 0.056).
FIG. 2.
Comparison of microdialysis values in patients without and with SD over entire recording period. Graphs (median; interquartile range, and 10–90% values) display concentrations and percent times of abnormal values for (A) extracellular glutamate (μmol/L), (B) lactate (mmol/L), (C) pyruvate (μmol/L), (D) lactate/pyruvate ratio (LPR), and (E) glucose (mmol/L). Only pyruvate concentrations and percent time of abnormal lactate were significantly different between patients with and without SDs (*, p < 0.05, Mann-Whitney U tests). SD, spreading depolarization.
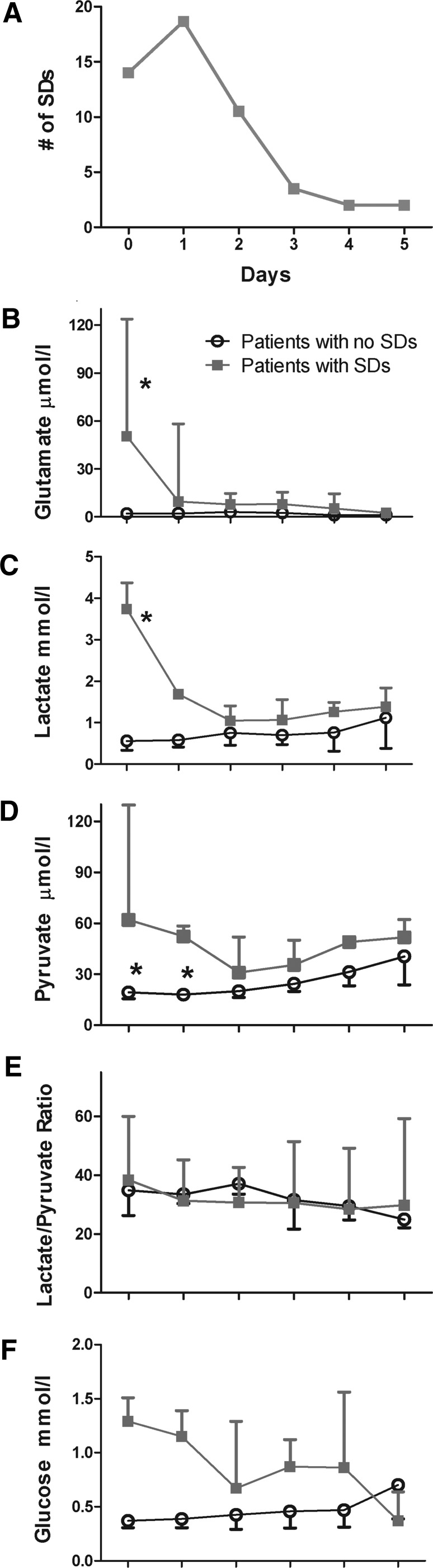
More substantial differences between the groups are evident when examining daily values (Fig. 3). Patients with SD had significant elevations in both glutamate (two-way ANOVA, F[5,52] = 3.15, p = 0.028; Fig. 3B) and lactate (two-way ANOVA, F[5,52] = 5.42, p < 0.001; Fig. 3C) on day 0 compared with subsequent days and also with patients without SD. Pyruvate was significantly decreased in patients without SDs on days 0–1 (two-way ANOVA, F[5,52] = 2.84, p = 0.024; Fig. 3D). There were no differences in glucose or LPR (Fig. 3E,F). The time course of changes in microdialysate values matched the temporal profile of SD activity, which was highest on days 0–1 and subsided to a constant low level on days 3–5 (Fig. 3A).
FIG. 3.
Time course of SDs and microdialysate values. (A) Daily totals of SDs show the highest incidence on days 0 and 1. Median and interquartile range of daily concentrations of (B) extracellular glutamate, (C) lactate, (D) pyruvate, (E) LPR, (F) and glucose. Patients with SD had significant increases in extracellular glutamate and lactate in the first day of recording and significant increases in pyruvate for the first two days (*, p values <0.05, two-way ANOVA with Bonferroni post hoc test). ANOVA, analysis of variance; LPR, lactate/pyruvate ratio; SD, spreading depolarization.
Analysis of samples by SD activity
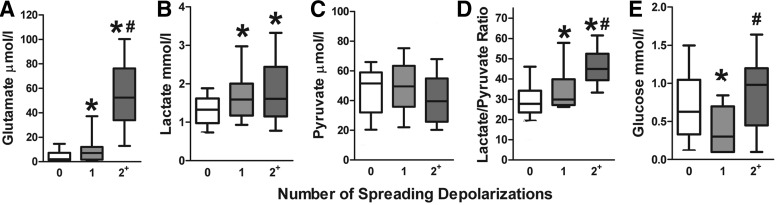
To examine the association between SD and cerebral neurochemistry in greater detail, microdialysis samples were grouped based on the number of SDs that occurred during the time of each sample collection for the six patients with SDs. The box and whisker plots in Figure 4 show that glutamate was significantly elevated in a dose-dependent fashion when SDs occurred: the presence of multiple SDs in the sample period was associated with glutamate concentrations of 52.3 μmol/L, compared with 7.0 μmol/L when only a single SD occurred and only 2.1 μmol/L when no SDs were present (Kruskal-Wallis test = 79.80, p < 0.001, Dunn's multiple comparison test, p < 0.05; Fig. 4A). A similar effect was observed for the LPR, which exceeded 40 when multiple SDs occurred but was near 30 when either a single or no SD was present (Kruskal-Wallis test = 44.83, p < 0.001, Dunn's multiple comparison test, p < 0.05; Fig. 4D). This was due to changes in both lactate and pyruvate concentrations, although only differences in lactate, and not pyruvate, were significant (Kruskal-Wallis test = 17.16, p < 0.001, Dunn's multiple comparison, p < 0.05; Fig. 4B,C).
FIG. 4.
Glutamate and LPR are elevated during SDs. In patients with SDs, microdialysis samples were grouped according to the number of SDs (0, 1, or ≥2) that occurred during sample collection. Graphs show median concentrations, interquartile ranges (box), and 10–90% ranges (whiskers) of (A) extracellular glutamate, (B) lactate, (C) pyruvate, (D) LPR, and (E) glucose. Extracellular glutamate and LPR progressively increased with SDs, exceeding the pathological thresholds for excitotoxicity (glutamate >50 μmol/L) and metabolic crisis (LPR >40) during multiple SDs (* vs. 0 SD; # vs. 1 SD; p values <0.05, Kruskal-Wallis with Dunn's multiple comparison). LPR, lactate/pyruvate ratio; SD, spreading depolarization.
Probability of SD in comparison to MD values
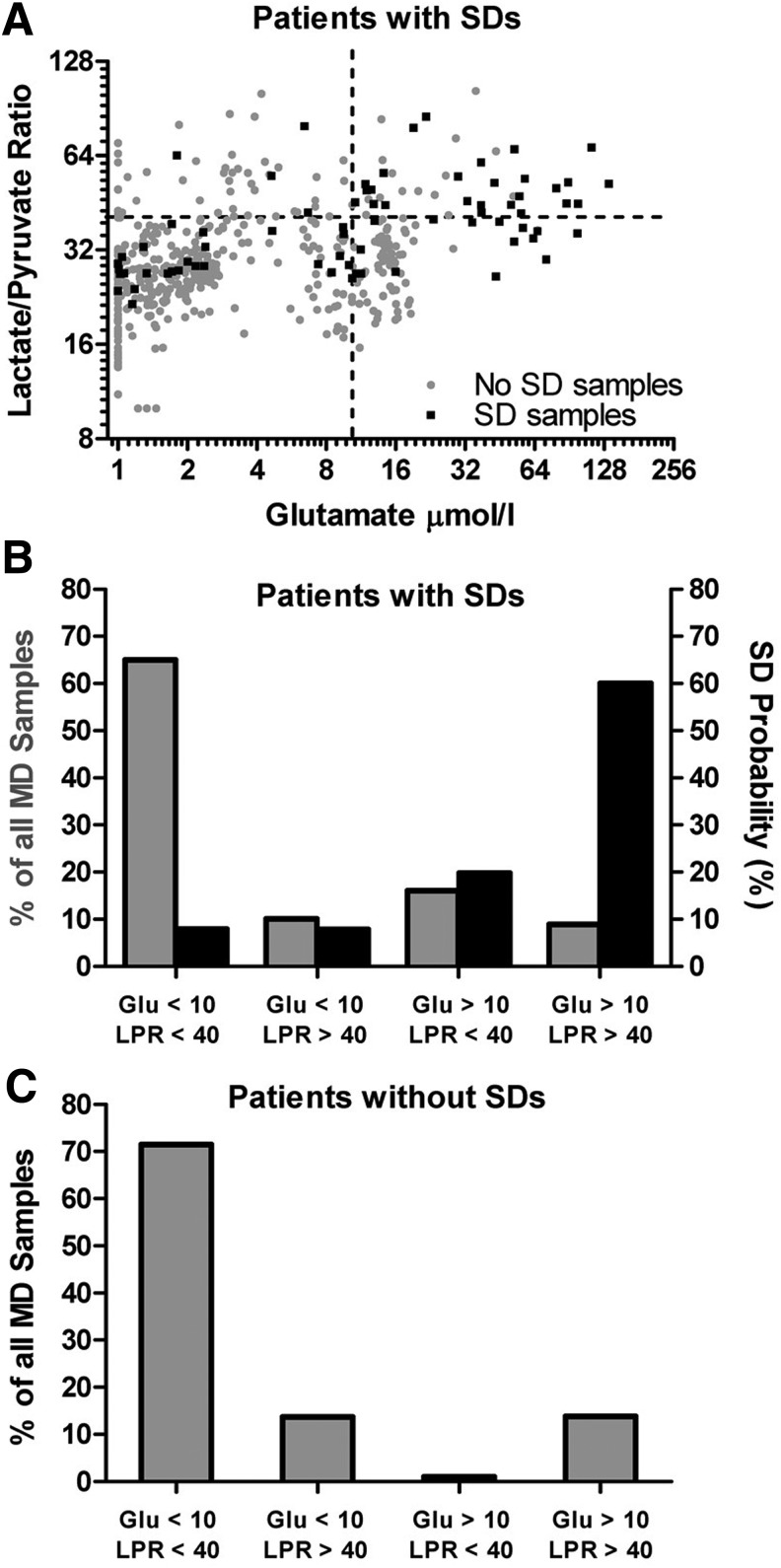
Because glutamate concentrations and LPR were elevated when SDs occurred, we conversely examined whether microdialysate values are predictive of SD occurrence. Figure 5A plots the LPR and glutamate concentrations for all the microdialysis samples of the six patients with SD. Four quadrants were defined by the abnormal thresholds for each variable (glutamate >10 μmol/L and LPR >40) and samples grouped based on whether SD occurred in the sampling period. For the majority of time (65%), glutamate and LPR values were within the normal range (lower left quadrant, Fig. 5A; gray bar, Fig. 5B) and only 8% of these samples were associated with SDs (black bar, Fig. 5B). By contrast, 9% of samples had elevated glutamate concentrations and LPRs (upper right quadrant, Fig. 5A; gray bar, Fig. 5B) but these were associated with a 60% incidence of SD (black bar, Fig. 5B). Patients without SDs exhibited a similar proportion of samples with normal (72%) and abnormal (14%) levels of glutamate and LPR (Fig. 5C).
FIG. 5.
High probability of SD during elevated glutamate and LPR. (A) Plot of all the microdialysis samples in six patients with SDs depicting the LPR and extracellular glutamate concentration on logarithmic scales. The samples are grouped based on whether an SD occurred (black square) or not (gray circle) in the sampling period and sorted into four quadrants using the pathological thresholds for glutamate (>50 μmol/L) and LPR (>40 μmol/L). (B) The relative occurrence of microdialysis values within the four quadrants in patients with SDs (gray bars) and the probability of SD occurring (black bars) when microdialysis values fall within the quadrants. In 65% of the samples, glutamate and LPR were within their physiological range and the probability of SD was 8%. The combination of elevated glutamate and LPR was rare (9%) but carried the highest probability of SD (60%). (C) The relative occurrence of microdialysis values within the four quadrants in patients without SDs. LPR, lactate/pyruvate ratio; SD, spreading depolarization.
Discussion
Here we examined the association of SDs with perturbations in cerebral neurochemistry by multimodal monitoring of electrocorticography and microdialysis in peri-lesional cortex of TBI patients who required neurosurgery. Microdialysate values in patients with SDs (6/16) were generally similar to patients without SDs. However, the former group had significantly elevated glutamate and lactate in the first 24 h of monitoring, and hourly analysis showed dose-dependent increases in glutamate, lactate, and LPR when SDs occurred. Normal glutamate and LPR were associated with only an 8% probability of SD occurrence, but pathological elevations of both measures were associated with a 60% SD probability. Although the sample size of this pilot study is small, results suggest that SD is an important pathophysiological mechanism associated with glutamate excitotoxicity and severe metabolic crisis in human brain injury. Prior studies in animals and humans suggest that SD is causal in inducing changes in neurochemistry, but also that these factors may have reciprocal cause-effect relationships in a cycle of worsening pathology.
Glutamate excitotoxicity
Since the discovery of elevated extracellular glutamate after experimental TBI,46,47 numerous clinical studies have documented similar changes. Similar to our data, results from other studies found that glutamate is typically most elevated at the start of monitoring, declining to normal or steady, elevated values after 24–48 h.4,6 Compromise of the blood–brain barrier and cellular membranes from the primary mechanical forces is a key contributor to this large (>30 μmol/L) initial glutamate surge and also likely contributes to the initiation and propagation of SDs.6,48,49 Thus, SDs exhibit a temporal profile similar to glutamate elevation after brain injury, with peak rates in the first 36 h followed by a decline, suggesting a possible association.50
Here, we confirmed the similar time courses of these pathologies by monitoring both variables in the same population. Although extracellular glutamate levels showed a sharp decline after the initial 24 h post-injury, they remained considerably above the normal limit of 10 μmol/L for several days (Figs. 1 and 3A,B), consistent with 1) the more prolonged time course of SDs and 2) the levels of extracellular glutamate provoked by SD.27,29 Such extracellular glutamate increases were not observed in patients without SD. We further found that the occurrence of SD is associated with elevated glutamate levels in a dose-dependent manner, reaching >50 μmol/L when multiple SDs occurred in the microdialysis sample period (Fig. 4A). Previously, elevations in glutamate have been associated with increases in ICP, decreases in brain tissue oxygenation, cerebral ischemia,5,51 and overall worse outcomes.4–6,12 Until now, the only neuronal pathophysiological process clearly associated with increases in extracellular glutamate has been seizures.12,52 However, seizures are less common than SD24 and did not contribute to glutamate elevations in this patient series. It is noteworthy, in fact, that glutamate decreased to a normal range (>20 to ∼10 μmol/L) in one patient (Fig. 1) shortly after SDs ceased and were replaced by continuous seizure activity.
Does elevated glutamate trigger SD, or the reverse? Prior studies have shown that SDs evoked experimentally in the healthy brain cause elevations in extracellular glutamate,27–29 making it possible that SD contributes to sustained glutamate elevations in the human brain. However, the accumulation and diffusion of extracellular glutamate is also likely a critical mechanism of SD initiation and propagation, as originally proposed by Van Harreveld.49 Recent ex vivo experiments have provided direct support for his hypothesis by showing that SDs can be initiated by regenerative glutamate release and that N-methyl-D-aspartate (NMDA) receptor signaling is essential for propagation and sustainment of the mass depolarization.53,54 Thus, although it is tempting to attribute a unidirectional causality to the association of SDs and elevated glutamate levels, most likely these mechanisms are mutually reinforcing, and to a large extent, different facets of the same process.
This view may also shed light on the condundrum of what extracellular glutamate level constitutes an excitotoxic threshold. The glutamate concentrations required to induce excitotoxicity in vitro (30 μmol/L for 30 min55) are substantially lower than those required in vivo (reverse microdialysis of 0.1 mol/L for 30 min56). However, acute excitotoxicity may only occur in vivo in connection with SD. In focal cerebral ischemia, we found that transient and terminal SDs associated with lesion growth caused synchronous changes in extracellular glutamate, but that glutamate never increased in the absence of SD either in the ischemic core or penumbra.29 Although glutamate levels never exceeded 20 μmol/L in these experiments, they may contribute to excitotoxic processes during SD because cells are depolarized, Mg2+ block on NMDA receptors is released, and the Na+ gradients that drive excitatory amino acid re-uptake are disrupted.29
Metabolic crisis
Increased LPR is regarded as the most reliable marker of a switch from aerobic to anaerobic metabolism due to mitochondrial dysfunction from inadequate supply of oxygen and glucose.2,3,57,58 The importance of this measure was demonstrated in the largest microdialysis study of severe TBI, where Timofeev and associates2 found that elevated LPR was an independent predictor of mortality and metabolic crisis (LPR >25) was associated with unfavorable outcomes. Until recently, ischemia was presumed to be solely responsible for this phenomenon, because LPR has been shown to be CPP-dependent59 with higher ratios in peri-contusional compared with normal tissue.2,15 However, the discovery of severe metabolic crisis (LPR >40) in non-ischemic peri-contusional cortex suggests that mitochondrial dysfunction can occur independent of cerebral ischemia.14,15 Here we found that LPR increased in a dose-dependent manner in association with SDs, exceeding the threshold for severe metabolic crisis (LPR >40) during multiple events (Fig. 4D). This result supports the hypothesis that SD is a pathological cause of non-ischemic metabolic crisis, a hypothesis strongly supported by SD's well-established metabolic profile.
The ionic fluxes associated with SDs, which are 5- to 10-fold greater than those produced by seizures, include an increase of extracellular [K+] from 3 to 60 mM and decrease of extracellular [Ca2+] from 1.3 to <0.1 mM.60 The restoration of ionic equilibrium and sequestration of cytosolic Ca2+ requires energy-dependent pumps,61 thereby greatly increasing metabolic demand. The Ca2+ influx is also associated with mitochondrial depolarization during SD,62 impairing oxidative phosphorylation and forcing reliance on cytosolic glycolysis for ATP production.31,61,63 Thus, with each SD wave, tissue ATP and glucose decline,19,30 and lactate accumulates. This metabolic response to SD has been well characterized in experimental18,32 and clinical17,19,64 studies that demonstrate the causal role of SD: stepwise accumulation of lactate and depletion of glucose follow, and do not precede, the passing of successive depolarization waves.
Here, we failed to detect decreases in extracellular glucose in association with SDs, which is likely attributable to the high prevalence of already abnormal low values in a majority of the severe TBI samples (Fig. 2E). Nor did our evidence support the suggestion that low baseline cerebral glucose is a risk factor for SD occurrence.18,65 In addition to the comparisons of patients (Fig. 2,3) and samples (Fig. 4) with and without SD, the overall SD incidence was low in this patient cohort despite the common finding of low cerebral glucose.
Study limitations
This study has several limitations. First, we did not assess cerebral blood flow or partial pressure of tissue oxygenation. Although CPP was maintained >60 mm Hg, it is possible that ischemic or hypoxic conditions in the monitored peri-lesional regions played a role in inducing SD or perturbations of microdialysate variables. Second, the absolute concentrations in microdialysis samples may not always reflect true extracellular values because recovery rates of microdialysis probes can vary depending on changes in the volume of the extracellular space, both within and across patients. Such variance may particularly occur during SDs, which induce cellular edema and transiently shrink the extracellular space by up to ∼70%.25,66,67 Because of this, ratios of compounds with similar structures, such as the LPR, have been preferred for comparisons within and across studies as these ratios are independent of probe recovery and equally affected by changes in extracellular diffusion.8,68 Third, microdialysis provides an averaged measure of dynamic changes in the extracellular fluid surrounding the probe during the sampling period. Because SDs are relatively short events (mean duration ∼2 min)22,69 associated with transient disruptions in cerebral metabolism (<20 min),19,64 their effects may be underestimated with microdialysis sampling periods of 60 min or more. If SDs occur frequently in temporal clusters, this dilution effect is diminished, because neurochemical changes may accumulate over the sampling period rather than dissipate within it.19 Such effect may partly contribute to the sharper increase in glutamate and LPR when ≥2 SDs occur. Finally, this is a small pilot study of only six patients who had SDs. Although significant results are consistent with the hypotheses that SD contributes to glutamate excitotoxicity and metabolic crisis, a larger and more comprehensive study is warranted to determine the overlap and causality of these phenomena.
Conclusions
The results of this pilot study support the hypothesis that SD is a key pathophysiological process of secondary brain injury associated with non-ischemic glutamate excitotoxicity and severe metabolic crisis in severe TBI patients. Although the overall neurochemical values were similarly disturbed in patients with and without SDs, the dose-dependent increases in extracellular glutamate and LPR in hourly samples suggests SDs are directly related to transient neurochemical perturbations. Overall, these results suggest that prevention of SD may offer an important neuroprotective strategy to forestall derangements of cerebral neurochemistry.
Acknowledgments
This work was funded by NINDS PO1 NS12587-27 (MRB) at Virginia Commonwealth University.
Authors Disclosure Statement
No competing financial interests exists.
References
- 1.Persson L., and Hillered L. (1992). Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J. Neurosurg. 76, 72–80 [DOI] [PubMed] [Google Scholar]
- 2.Timofeev I., Carpenter K.L., Nortje J., Al-Rawi P.G., O'Connell M.T., Czosnyka M., Smielewski P., Pickard J.D., Menon D.K., Kirkpatrick P.J., Gupta A.K., and Hutchinson P.J. (2011). Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain 134, 484–494 [DOI] [PubMed] [Google Scholar]
- 3.Reinstrup P., Stahl N., Mellergard P., Uski T., Ungerstedt U., and Nordstrom C.H. (2000). Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery 47, 701–709; discussion 709–710. [DOI] [PubMed] [Google Scholar]
- 4.Chamoun R., Suki D., Gopinath S.P., Goodman J.C., and Robertson C. (2010). Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 113, 564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zauner A., Bullock R., Kuta A.J., Woodward J., and Young H.F. (1996). Glutamate release and cerebral blood flow after severe human head injury. Acta Neurochir. Suppl. 67, 40–44 [DOI] [PubMed] [Google Scholar]
- 6.Bullock R., Zauner A., Woodward J.J., Myseros J., Choi S.C., Ward J.D., Marmarou A., and Young H.F. (1998). Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 89, 507–518 [DOI] [PubMed] [Google Scholar]
- 7.Bellander B.M., Cantais E., Enblad P., Hutchinson P., Nordstrom C.H., Robertson C., Sahuquillo J., Smith M., Stocchetti N., Ungerstedt U., Unterberg A., and Olsen N.V. (2004). Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 30, 2166–2169 [DOI] [PubMed] [Google Scholar]
- 8.Enblad P., Valtysson J., Andersson J., Lilja A., Valind S., Antoni G., Langstrom B., Hillered L., and Persson L. (1996). Simultaneous intracerebral microdialysis and positron emission tomography in the detection of ischemia in patients with subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 16, 637–644 [DOI] [PubMed] [Google Scholar]
- 9.Timofeev I., Czosnyka M., Carpenter K.L., Nortje J., Kirkpatrick P.J., Al-Rawi P.G., Menon D.K., Pickard J.D., Gupta A.K., and Hutchinson P.J. (2011). Interaction between brain chemistry and physiology after traumatic brain injury: impact of autoregulation and microdialysis catheter location. J. Neurotrauma 28, 849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timofeev I., Dahyot-Fizelier C., Keong N., Nortje J., Al-Rawi P.G., Czosnyka M., Menon D.K., Kirkpatrick P.J., Gupta A.K., and Hutchinson P.J. (2008). Ventriculostomy for control of raised ICP in acute traumatic brain injury. Acta Neurochir. Suppl. 102, 99–104 [DOI] [PubMed] [Google Scholar]
- 11.Timofeev I., Nortje J., Al-Rawi P.G., Hutchinson P.J., and Gupta A.K. (2013). Extracellular brain pH with or without hypoxia is a marker of profound metabolic derangement and increased mortality after traumatic brain injury. J. Cereb. Blood Flow Metab. 33, 422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vespa P., Prins M., Ronne-Engstrom E., Caron M., Shalmon E., Hovda D.A., Martin N.A., and Becker D.P. (1998). Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J. Neurosurg. 89, 971–982 [DOI] [PubMed] [Google Scholar]
- 13.Stein N.R., McArthur D.L., Etchepare M., and Vespa P.M. (2012). Early cerebral metabolic crisis after TBI influences outcome despite adequate hemodynamic resuscitation. Neurocrit. Care 17, 49–57 [DOI] [PubMed] [Google Scholar]
- 14.Vespa P., Bergsneider M., Hattori N., Wu H.M., Huang S.C., Martin N.A., Glenn T.C., McArthur D.L., and Hovda D.A. (2005). Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 25, 763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vespa P.M., O'Phelan K., McArthur D., Miller C., Eliseo M., Hirt D., Glenn T., and Hovda D.A. (2007). Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit. Care Med. 35, 1153–1160 [DOI] [PubMed] [Google Scholar]
- 16.Vespa P.M., Miller C., McArthur D., Eliseo M., Etchepare M., Hirt D., Glenn T.C., Martin N., and Hovda D. (2007). Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit. Care Med. 35, 2830–2836 [PMC free article] [PubMed] [Google Scholar]
- 17.Parkin M., Hopwood S., Jones D.A., Hashemi P., Landolt H., Fabricius M., Lauritzen M., Boutelle M.G., and Strong A.J. (2005). Dynamic changes in brain glucose and lactate in pericontusional areas of the human cerebral cortex, monitored with rapid sampling on-line microdialysis: relationship with depolarisation-like events. J. Cereb. Blood Flow Met. 25, 402–413 [DOI] [PubMed] [Google Scholar]
- 18.Hopwood S.E., Parkin M.C., Bezzina E.L., Boutelle M.G., and Strong A.J. (2005). Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J. Cereb. Blood Flow Metab. 25, 391–401 [DOI] [PubMed] [Google Scholar]
- 19.Feuerstein D., Manning A., Hashemi P., Bhatia R., Fabricius M., Tolias C., Pahl C., Ervine M., Strong A.J., and Boutelle M.G. (2010). Dynamic metabolic response to multiple spreading depolarizations in patients with acute brain injury: an online microdialysis study. J. Cereb. Blood Flow Metab. 30, 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somjen G.G. (2001). Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev. 81, 1065–1096 [DOI] [PubMed] [Google Scholar]
- 21.Dreier J.P. (2011). The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nature Med. 17, 439–447 [DOI] [PubMed] [Google Scholar]
- 22.Hartings J.A., Watanabe T., Bullock M.R., Okonkwo D.O., Fabricius M., Woitzik J., Dreier J.P., Puccio A., Shutter L.A., Pahl C., and Strong A.J. (2011). Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain 134, 1529–1540 [DOI] [PubMed] [Google Scholar]
- 23.Hartings J.A., Wilson J.A., Hinzman J.M., Pollandt S., Dreier J.P., DiNapoli V., Ficker D.M., Shutter L.A., and Andaluz N. (2014). Spreading depression in continuous electroencephalography of brain trauma. Ann. Neurol. 76, 681–694 [DOI] [PubMed] [Google Scholar]
- 24.Hartings J.A., Bullock M.R., Okonkwo D.O., Murray L.S., Murray G.D., Fabricius M., Maas A.I., Woitzik J., Sakowitz O., Mathern B., Roozenbeek B., Lingsma H., Dreier J.P., Puccio A.M., Shutter L.A., Pahl C., and Strong A.J. (2011). Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol. 10, 1058–1064 [DOI] [PubMed] [Google Scholar]
- 25.Kraig R.P., and Nicholson C. (1978). Extracellular ionic variations during spreading depression. Neuroscience 3, 1045–1059 [DOI] [PubMed] [Google Scholar]
- 26.Hansen A.J., and Zeuthen T. (1981). Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol. Scand. 113, 437–445 [DOI] [PubMed] [Google Scholar]
- 27.Fabricius M., Jensen L.H., and Lauritzen M. (1993). Microdialysis of interstitial amino acids during spreading depression and anoxic depolarization in rat neocortex. Brain Res. 612, 61–69 [DOI] [PubMed] [Google Scholar]
- 28.Iijima T., Shimase C., Iwao Y., and Sankawa H. (1998). Relationships between glutamate release, blood flow and spreading depression: real-time monitoring using an electroenzymatic dialysis electrode. Neurosci. Res. 32, 201–207 [DOI] [PubMed] [Google Scholar]
- 29.Hinzman J.M., DiNapoli V.A., Mahoney E.J., Gerhardt G.A., and Hartings J.A. (2015). Spreading depolarizations mediate excitotoxicity in the development of acute cortical lesions. Exp. Neurol. 267, 243–253 [DOI] [PubMed] [Google Scholar]
- 30.Mies G., and Paschen W. (1984). Regional changes of blood flow, glucose, and ATP content determined on brain sections during a single passage of spreading depression in rat brain cortex. Exp. Neurol. 84, 249–258 [DOI] [PubMed] [Google Scholar]
- 31.Shinohara M., Dollinger B., Brown G., Rapoport S., and Sokoloff L. (1979). Cerebral glucose utilization: local changes during and after recovery from spreading cortical depression. Science 203, 188–190 [DOI] [PubMed] [Google Scholar]
- 32.Hashemi P., Bhatia R., Nakamura H., Dreier J.P., Graf R., Strong A.J., and Boutelle M.G. (2008). Persisting depletion of brain glucose following cortical spreading depression, despite apparent hyperaemia: evidence for risk of an adverse effect of Leão's spreading depression. J. Cereb. Blood Flow Metab. 29, 166–175 [DOI] [PubMed] [Google Scholar]
- 33.Taylor D.L., Richards D.A., Obrenovitch T.P., and Symon L. (1994). Time course of changes in extracellular lactate evoked by transient K(+)-induced depolarisation in the rat striatum. J. Neurochem. 62, 2368–2374 [DOI] [PubMed] [Google Scholar]
- 34.Cruz N.F., Adachi K., and Dienel G.A. (1999). Rapid efflux of lactate from cerebral cortex during K+ -induced spreading cortical depression. J. Cereb. Blood Flow Metab. 19, 380–392 [DOI] [PubMed] [Google Scholar]
- 35.Hartings J.A., Gugliotta M., Gilman C., Strong A.J., Tortella F.C., and Bullock M.R. (2008). Repetitive cortical spreading depolarizations in a case of severe brain trauma. Neurol. Res. 30, 876–882 [DOI] [PubMed] [Google Scholar]
- 36.Strong A.J., Fabricius M., Boutelle M.G., Hibbins S.J., Hopwood S.E., Jones R., Parkin M.C., and Lauritzen M. (2002). Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke 33, 2738–2743 [DOI] [PubMed] [Google Scholar]
- 37.Fabricius M., Fuhr S., Bhatia R., Boutelle M., Hashemi P., Strong A.J., and Lauritzen M. (2006). Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain 129, 778–790 [DOI] [PubMed] [Google Scholar]
- 38.Hartings J.A., Strong A.J., Fabricius M., Manning A., Bhatia R., Dreier J.P., Mazzeo A.T., Tortella F.C., and Bullock M.R. (2009). Spreading depolarizations and late secondary insults after traumatic brain injury. J. Neurotrauma 26, 1857–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menzel M., Doppenberg E.M., Zauner A., Soukup J., Reinert M.M., and Bullock R. (1999). Increased inspired oxygen concentration as a factor in improved brain tissue oxygenation and tissue lactate levels after severe human head injury. J. Neurosurg. 91, 1–10 [DOI] [PubMed] [Google Scholar]
- 40.Mazzeo A.T., Alves O.L., Gilman C.B., Hayes R.L., Tolias C., Niki Kunene K., and Ross Bullock M. (2008). Brain metabolic and hemodynamic effects of cyclosporin A after human severe traumatic brain injury: a microdialysis study. Acta Neurochir. (Wien) 150, 1019–1031; discussion 1031 [DOI] [PubMed] [Google Scholar]
- 41.Bullock M.R., Chestnut R., and Ghajar J. (2000). Guidelines for the management of severe traumatic brain injury. J. Neurotrauma 17, 449–554 [Google Scholar]
- 42.Hutchinson P.J., O'Connell M.T., Al-Rawi P.G., Maskell L.B., Kett-White R., Gupta A.K., Richards H.K., Hutchinson D.B., Kirkpatrick P.J., and Pickard J.D. (2000). Clinical cerebral microdialysis: a methodological study. J. Neurosurg. 93, 37–43 [DOI] [PubMed] [Google Scholar]
- 43.Hartings J.A., Watanabe T., Dreier J.P., Major S., Vendelbo L., and Fabricius M. (2009). Recovery of slow potentials in AC-coupled electrocorticography: application to spreading depolarizations in rat and human cerebral cortex. J. Neurophysiol. 102, 2563–2575 [DOI] [PubMed] [Google Scholar]
- 44.Fabricius M., Fuhr S., Willumsen L., Dreier J.P., Bhatia R., Boutelle M.G., Hartings J.A., Bullock R., Strong A.J., and Lauritzen M. (2008). Association of seizures with cortical spreading depression and peri-infarct depolarisations in the acutely injured human brain. Clin. Neurophysiol. 119, 1973–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartings J.A., Vidgeon S., Strong A.J., Zacko C., Vagal A., Andaluz N., Ridder T., Stanger R., Fabricius M., Mathern B., Pahl C., Tolias C.M., and Bullock M.R. (2014). Surgical management of traumatic brain injury: a comparative-effectiveness study of 2 centers. J. Neurosurg. 120, 434–446 [DOI] [PubMed] [Google Scholar]
- 46.Nilsson P., Hillered L., Ponten U., and Ungerstedt U. (1990). Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J. Cereb. Blood Flow Metab. 10, 631–637 [DOI] [PubMed] [Google Scholar]
- 47.Faden A.I., Demediuk P., Panter S.S., and Vink R. (1989). The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244, 798–800 [DOI] [PubMed] [Google Scholar]
- 48.Schmidt R.H., and Grady M.S. (1993). Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J. Neurotrauma 10, 415–430 [DOI] [PubMed] [Google Scholar]
- 49.Van Harreveld A. (1959). Compounds in brain extracts causing spreading depression of cerebral cortical activity and contraction of crustacean muscle. J. Neurochem. 3, 300–315 [DOI] [PubMed] [Google Scholar]
- 50.Hartings J.A., Strong A.J., Okonkwo D.O., and Bullock M.R. (2012). Spreading depolarisations and traumatic brain injury: time course and mechanisms. Lancet Neurol. 11, 389–390(author reply). [DOI] [PubMed] [Google Scholar]
- 51.Hillered L., Persson L., Ponten U., and Ungerstedt U. (1990). Neurometabolic monitoring of the ischaemic human brain using microdialysis. Acta Neurochir. 02, 91–97 [DOI] [PubMed] [Google Scholar]
- 52.Wilson C.L., Maidment N.T., Shomer M.H., Behnke E.J., Ackerson L., Fried I., and Engel J., Jr. (1996). Comparison of seizure related amino acid release in human epileptic hippocampus versus a chronic, kainate rat model of hippocampal epilepsy. Epilepsy Res. 26, 245–254 [DOI] [PubMed] [Google Scholar]
- 53.Zhou N., Rungta R.L., Malik A., Han H., Wu D.C., and MacVicar B.A. (2013). Regenerative glutamate release by presynaptic NMDA receptors contributes to spreading depression. J. Cereb. Blood Flow Metab. 33, 1582–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aiba I., and Shuttleworth C.W. (2012). Sustained NMDA receptor activation by spreading depolarizations can initiate excitotoxic injury in metabolically compromised neurons. J. Physiol. 590, 5877–5893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garthwaite G., Williams G.D., and Garthwaite J. (1992). Glutamate toxicity: an experimental and theoretical analysis. Eur. J. Neurosci. 4, 353–360 [DOI] [PubMed] [Google Scholar]
- 56.Landolt H., Fujisawa H., Graham D.I., Maxwell W.L., and Bullock R. (1998). Reproducible peracute glutamate-induced focal lesions of the normal rat brain using microdialysis. J. Clin. Neurosci. 5, 193–202 [DOI] [PubMed] [Google Scholar]
- 57.Hlatky R., Valadka A.B., Goodman J.C., Contant C.F., and Robertson C.S. (2004). Patterns of energy substrates during ischemia measured in the brain by microdialysis. J. Neurotrauma 21, 894–906 [DOI] [PubMed] [Google Scholar]
- 58.Meierhans R., Bechir M., Ludwig S., Sommerfeld J., Brandi G., Haberthur C., Stocker R., and Stover J.F. (2010). Brain metabolism is significantly impaired at blood glucose below 6 mM and brain glucose below 1 mM in patients with severe traumatic brain injury. Crit. Care 14,R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Fazio M., Rammo R., O'Phelan K., and Bullock M.R. (2011). Alterations in cerebral oxidative metabolism following traumatic brain injury. Neurocrit. Care 14, 91–96 [DOI] [PubMed] [Google Scholar]
- 60.Hablitz J.J., and Heinemann U. (1989). Alterations in the microenvironment during spreading depression associated with epileptiform activity in the immature neocortex. Brain Res. Dev. Brain Res. 46, 243–252 [DOI] [PubMed] [Google Scholar]
- 61.Siesjo B.K., and Bengtsson F. (1989). Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J. Cereb. Blood Flow Metab. 9, 127–140 [DOI] [PubMed] [Google Scholar]
- 62.Bahar S., Fayuk D., Somjen G.G., Aitken P.G., and Turner D.A. (2000). Mitochondrial and intrinsic optical signals imaged during hypoxia and spreading depression in rat hippocampal slices. J. Neurophysiol. 84, 311–324 [DOI] [PubMed] [Google Scholar]
- 63.Camacho A., and Massieu L. (2006). Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch. Med. Res. 37, 11–18 [DOI] [PubMed] [Google Scholar]
- 64.Krajewski K.L., Orakcioglu B., Haux D., Hertle D.N., Santos E., Kiening K.L., Unterberg A.W., and Sakowitz O.W. (2011). Cerebral microdialysis in acutely brain-injured patients with spreading depolarizations. Acta Neurochir. Suppl. 110, 125–130 [DOI] [PubMed] [Google Scholar]
- 65.Strong A.J., Boutelle M.G., Vespa P.M., Bullock M.R., Bhatia R., and Hashemi P. (2005). Treatment of critical care patients with substantial acute ischemic or traumatic brain injury. Crit. Care Med. 33, 2147–2149; author reply 2149. [DOI] [PubMed] [Google Scholar]
- 66.Somjen G.G. (2004). Ions in the Brain: Normal Function, Seizures, and Stroke. Oxford University Press: Oxford; New York [Google Scholar]
- 67.Risher W.C., Croom D., and Kirov S.A. (2012). Persistent astroglial swelling accompanies rapid reversible dendritic injury during stroke-induced spreading depolarizations. Glia 60, 1709–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Persson L., Valtysson J., Enblad P., Warme P.E., Cesarini K., Lewen A., and Hillered L. (1996). Neurochemical monitoring using intracerebral microdialysis in patients with subarachnoid hemorrhage. J. Neurosurg. 84, 606–616 [DOI] [PubMed] [Google Scholar]
- 69.Hartings J.A., Wilson J.A., Look A.C., Vagal A., Shutter L.A., Dreier J.P., Ringer A., and Zuccarello M. (2013). Full-band electrocorticography of spreading depolarizations in patients with aneurysmal subarachnoid hemorrhage. Acta Neurochir. Suppl. 115, 131–141 [DOI] [PubMed] [Google Scholar]