Abstract
Intravenous drug use (IDU) is one of the most important transmission routes for blood borne viruses, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). These infections alter the subset distributions of T cells; however, knowledge of such effects during HIV, HBV, and or HCV coinfection is limited. Therefore, we aimed to evaluate any associations between T cell distribution and the presence of HIV, HBV, and HCV coinfections among persons who inject drugs (PWID). Blood samples from 88 Caucasian PWID (mean age 30; 82% male) and 47 age-matched subjects negative for all three infections (mean age of 29; 83% male) were analyzed. The T cell markers CD3, CD4, CD8, CD45RA, CCR7, HLA-DR, and CCR5 were assessed using flow cytometry. Of the PWID, 40% were HIV+HBV+HCV+, 20% HBV+HCV+, 19% HCV+, and 13% negative for all three infections. The HIV+HBV+HCV+ PWID had lower percentages of CD4+ and higher percentages of CD8+ cells compared to triple negative PWID (p < 0.001 in all cases). The only difference between HBV+HCV+ with triple negative PWID was the lower CD4+ cell percentages among the former (52.1% and 58.6%, p = 0.021). Triple negative PWID had higher immune activation and number of CCR5+ cells compared to the controls. We suggest that the altered T cell subset distribution among PWID is mainly triggered by HIV infection and or IDU, while HBV and or HCV seropositivity has minimal additional effects on CD4+ cell distribution.
Introduction
Human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) are causing health problems around the world. Owing to these viruses sharing similar transmission routes, coinfections are common, especially among persons who inject drugs (PWID) (7,30,34). These viruses affect the immune system by increasing immune activation and causing changes to T cell subset distribution (7,8,12,14,27). Our previous research (14) and that of others (5,7,9,11,27) have shown the importance of CD4+ and CD8+ T cell responses in the susceptibility to HIV and in the progression of HIV, HBV, and HCV mono-infections. Furthermore, compared to mono-infections, HIV/HCV coinfection is characterized by relatively higher immune activation, acceleration of liver diseases, and higher HIV viral loads, while HIV/HBV coinfected individuals have a higher risk of developing chronic HBV (10,12,20,23).
To describe the effect of changes to T cell distribution, multiple classifications based on the cell surface marker expressions have been used. One of the most common classifications uses the expression of CD45RA and CCR7, to divide both CD4+ and CD8+ T cells into four groups of naive and memory cells: naive (TN; CD45RA+ CCR7+); central memory (TCM; CD45RA−CCR7+); effector memory (TEM; CD45RA−CCR7−); and terminally differentiated (TTEMRA; CD45RA+ CCR7−) (16). Each of these cell types has distinct immunological functions and a progenitor–product relationship, whereby they evolve from one cell type to another. TN cells progress into lymph-node homing TCM cells without cytotoxic functions and then into anti-inflammatory TEM cells (6). TTEMRA cells are terminally differentiated having low functional and proliferative capacities (22). In addition, both CD4+ and CD8+ T cells are able to express markers of immune activation, as defined by the presence of CD38+ and/or HLA-DR, and the HIV main coreceptor CCR5.
Despite the large number of studies that have investigated T cell distribution among HIV-positive individuals or those with chronic HBV or HCV, little is known about cellular immune responses among PWID, who are often seropositive for multiple viruses. Similar to other Eastern European PWID groups, the intravenous drug user (IDU) population in Estonia has high rate of HIV, HBV, and or HCV coinfections and a high percentage of opioid users (14,15,29,31). We have shown that HIV exposure increases CD45RA+ RO+ T cell percentages and immune activation among an Estonian PWID population (14); however, how the seropositivity of individuals with all three infections (HIV, HBV, and HCV) affects T cell distribution has thus far been seldom studied. Therefore, we aimed to investigate the effects of HIV, HBV, and HCV seropositivity and IDU itself upon T cell subset distribution, immune activation, and CCR5 expression among Caucasian PWID.
Materials and Methods
Study population
We recruited 88 PWID and 47 healthy volunteers (controls) of Caucasian origin. The PWID were recruited using a respondent driven sampling method (17), from individuals who were using the syringe exchange program in Tallinn (Estonia) during 2011 (13,14). The control group was recruited during 2012–2013 in Tartu (Estonia) and matched for age and gender to the HIV-negative PWID participants.
From each subject, 16 mL of venous blood was collected through venipuncture into EDTA tubes (VACUETTE®; Greiner Bio-One GmbH, Frickenhausen, Germany) and transported to the laboratory of the University of Tartu's Department of Microbiology. After 24 h, peripheral blood mononuclear cells (PBMCs) were separated using a Ficoll gradient. The samples were then stored in a medium consisting of 90% FBS and 10% DMSO at −80°C.
HIV-1 (genus Lentivirus, family Retroviridae) serostatus was assessed at the Estonian Central HIV Reference Laboratory of Western-Tallinn Central Hospital using a fourth generation enzyme-linked immunoassay (Vironostika HIV Uniform II Ag/Ab; BioMerieux, Marcy l'Etoile, France) and immunoblotting (INNO LIA HIV I/II Score Western blot; Microgen Bioproducts Ltd., Surrey, United Kingdom). HCV (anti HCV Ab) and HBV (HBsAg, anti-HBc) testing were performed at the Institute for Health Development using ETI-MAK-4 HBsAg, ETI-AB-COREK, and ETI-AB-HCVK-3 Kits (DiaSorin, Vercelli, Italy).
T cell immunophenotype analysis
T cell subpopulations were determined using multicolor flow cytometry, as described by Kallas et al. (14). Thawed PBMCs were diluted using 1× DPBS (Sigma Life Science, St. Louis, MO), stained with fluorochrome-conjugated monoclonal antibodies to CD3, CD4, CD8, CD45RA, CCR7, HLA-DR (BioLegend, San Diego, CA), and CCR5 (Becton Dickinson, Franklin Lakes, NJ), washed twice with 1× DPBS, and then fixed with a 1% paraformaldehyde solution. The flow cytometry was conducted within 2 h of fixation using a LSRFortessa cytometer and FACSDiva version 6.2 software (Becton Dickinson). All flow cytometry results were analyzed in a blinded manner and fluorescence minus one controls were used for the analyses of CCR5 and HLA-DR. From each participant, ∼10,000 CD3+ cells were analyzed. The results are presented as the percentage of cells from the parent cell population.
Statistical analysis
Any statistical differences in T cell subset percentages between groups were determined using Mann–Whitney-Wilcoxon's test with Holm–Bonferroni correction with R version 2.13 software. Corrected p < 0.05 was considered statistically significant.
Ethical considerations
This study was approved by the Ethics Committee of the University of Tartu. All study participants signed an informed consent form.
Results
Characteristics of the study population
The median age of the 88 PWID was 30 years old (IQR 25–33) and for controls 29 years old (IQR 25–33). Of the PWID and controls, 72 (82%) and 39 (83%), respectively, were male. The median duration of IDU was 11 years (IQR 7–14) among all PWID. In total, 41 (47%) of the PWID were HIV, 75 (85%) HCV, and 55 (63%) HBV seropositive. Three study subjects were HBsAg seropositive (two HIV+HBV+HCV+ and one HIV−HBV+HCV+). Of the PWID, 35 (40%) had triple infection (HIV+HBV+HCV+), and 11 PWID (8%) were seronegative for all three virus antibodies (Table 1). All triple negative subjects reported receptive syringe sharing during the last 6 months. Of the PWID and controls, 11 (13%) and 23 (49%), respectively, were immunized against HBV.
Table 1.
HIV, HCV, and HBV Serostatus Among PWID
| HIV+ (n = 41), n (%) | HIV− (n = 47), n (%) | |
|---|---|---|
| HBV− HCV− | 0 (0) | 11 (13) |
| HBV− HCV+ | 5 (6) | 17 (19) |
| HBV+ HCV− | 1 (1) | 1 (1) |
| HBV+ HCV+ | 35 (40) | 18 (20) |
HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; PWID, persons who inject drugs.
T cell subsets and HIV, HCV, and HBV infection
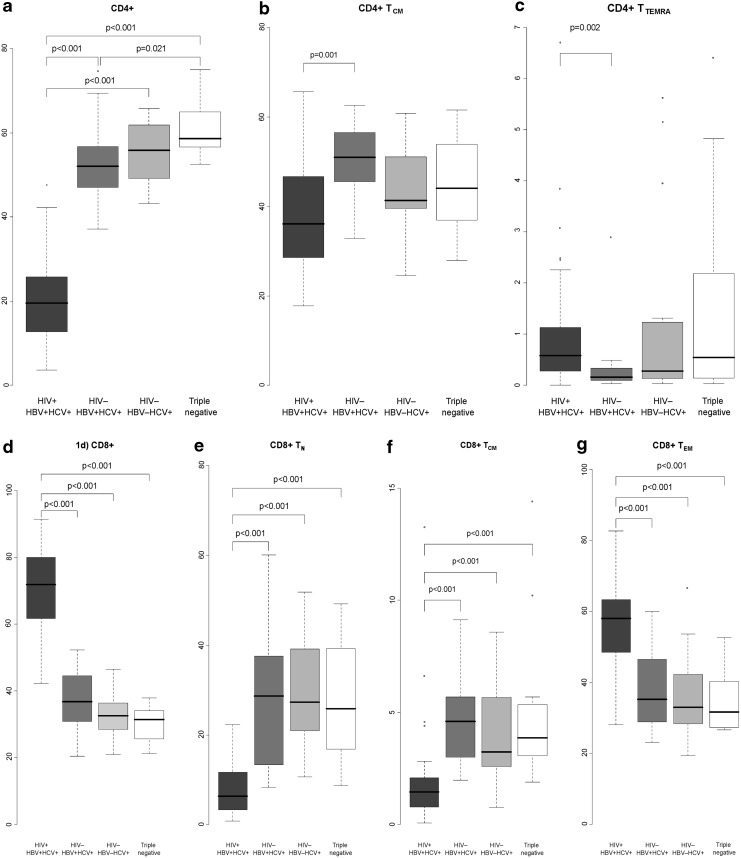
Lymphocyte subsets of triple-seropositive, HBV/HCV dual-seropositive, HCV mono-seropositive, and triple negative PWID are presented in Figure 1, Table 2, and Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/vim). Multiple statistically significant differences were found when comparing triple positive PWID with individuals without HIV infection (HBV/HCV dual infection, HCV mono-infection, or triple negative PWID). Triple positive individuals had lower percentages of CD4+ and higher percentages of CD8+ cells than subjects with HBV/HCV dual- or HCV mono-infections (p < 0.001 in all cases, Fig. 1a, d). Triple positives had lower percentages of CD4+ TCM and higher percentages of CD4+ TTEMRA cells than HCV+ HBV+ individuals (36.1% and 50.9%, p = 0.001 and 0.6% and 0.2%, p = 0.002, respectively, Fig. 1b, c). Regarding CD8+ cells, triple infected PWID had lower percentages of TN and TCM cells and higher percentages of TEM cells than all other PWID groups (p < 0.001 in all cases, Fig. 1e–g). In terms of immune activation and the presence of the HIV coreceptor CCR5, triple infected PWID had significantly higher percentages of HLA-DR+, CD8+ TN CCR5+, and CD8+ TCM CCR5+cells than PWID with HBV/HCV dual- or HCV mono-infection (Table 2).
FIG. 1.
The ratio (median%) of CD4+ lymphocyte subsets from the parent cell population among HIV+HBV+HCV+PWID (dark gray), HIV−HBV+HCV+ PWID (medium gray), HIV−HBV−HCV+ PWID (light gray), and triple negative PWID (white). Panel (a) indicates CD4+, (b) CD4+ TCM, (c) CD4+ TTEMRA, (d) CD8+, (e) CD8+ TN, (f) CD8+ TCM, and (g) CD8+ TCM cells. Holm–Bonferroni corrected p < 0.05 was considered statistically significant and is presented on the figure. HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; PWID, persons who inject drugs.
Table 2.
The Ratio (Median %) of HLA-DR+ and CCR5+ Lymphocyte Subsets to the Parent Cell Population Among PWID
| Cell populations | HIV+HBV+HCV+ (n = 35) | HIV−HBV+HCV+ (n = 18) | HIV−HBV−HCV+ (n = 17) | Triple negative (n = 11) | pa | pb | pc |
|---|---|---|---|---|---|---|---|
| HLA-DR+ lymphocyte subsets | |||||||
| CD4+ HLA-DR+ | 12.0 (6.6–19.2) | 4.9 (3.6–5.6) | 5.5 (4.2–6.5) | 5.4 (4.1–8.7) | <0.001 | <0.001 | 0.011 |
| TCM HLA-DR+ | 13.0 (8.9–19.5) | 4.0 (3.5–4.7) | 5.2 (3.8–5.5) | 4.9 (3.7–6.0) | <0.001 | <0.001 | <0.001 |
| TEM HLA-DR+ | 38.6 (30.2–50.5) | 18.0 (13.2–20.6) | 17.5 (14.9–23.5) | 23.2 (21.0–25.9) | <0.001 | <0.001 | 0.050 |
| CD8+ HLA-DR+ | 45.8 (37.4–53.1) | 13.6 (8.5–18.5) | 15.6 (13.0–22.0) | 23.9 (15.2–27.9) | <0.001 | <0.001 | <0.001 |
| TN HLA-DR+ | 4.0 (2.9–7.6) | 2.3 (1.8–3.7) | 2.3 (1.7–3.9) | 2.8 (1.1–3.8) | 0.017 | 0.017 | 0.076 |
| TCM HLA-DR+ | 33.9 (19.7–47.2) | 7.6 (6.4–8.9) | 10.3 (6.9–13.6) | 9.5 (5.3–12.9) | <0.001 | <0.001 | <0.001 |
| TEM HLA-DR+ | 54.1 (47.6–62.2) | 23.0 (12.6–31.1) | 29.1 (20.3–36.2) | 31.3 (23.5–38.6) | <0.001 | <0.001 | <0.001 |
| TTEMRA HLA-DR+ | 37.2 (27.2–46.0) | 21.2 (9.2–26.1) | 20.9 (14.7–26.1) | 30.8 (18.9–42.3) | <0.001 | <0.001 | 0.718 |
| CCR5+ lymphocyte subsets | |||||||
| CD4+ TEM CCR5+ | 7.0 (2.3–9.3) | 7.6 (4.1–10.0) | 9.6 (6.0–12.0) | 10.9 (8.2–12.6) | 0.987 | 0.239 | 0.026 |
| CD8+ TN CCR5+ | 1.5 (0.5–3.2) | 0.5 (0.3–1.4) | 0.3 (0.1–0.4) | 0.7 (0.1–1.0) | 0.055 | <0.001 | 0.162 |
| CD8+ TCM CCR5+ | 12.8 (5.4–25.8) | 2.8 (1.9–7.2) | 6.3 (3.9–8.7) | 3.2 (2.3–8.7) | 0.002 | 0.049 | 0.037 |
Only values with significant differences between cell populations are presented.
No differences were found between HIV−HBV+HCV+ and HIV−HBV−HCV+ subjects or HIV−HBV−HCV+ and triple negative subjects. Holm–Bonferroni corrected p < 0.05 is considered statistically significant and is marked in bold.
HIV+ HBV+ HCV+ compared to HIV−HBV+ HCV+.
HIV+ HBV+ HCV+ compared to HIV−HBV−HCV+.
HIV+ HBV+ HCV+ compared to triple negative.
TN, naive; TCM, central memory; TEM, effector memory; TTEMRA, terminally differentiated cells.
No differences in T cell subset distribution were found when HBV+ HCV+ coinfected PWID were compared to HCV mono-infected PWID or triple negative PWID to HCV mono-infected PWID. HBV+ HCV+ coinfected PWID had significantly lower percentages of CD4+ cells compared to triple negative PWID (52.1% and 58.6%, respectively, p = 0.021, Fig. 1a).
T cell subset distribution among noninfected PWID compared to controls
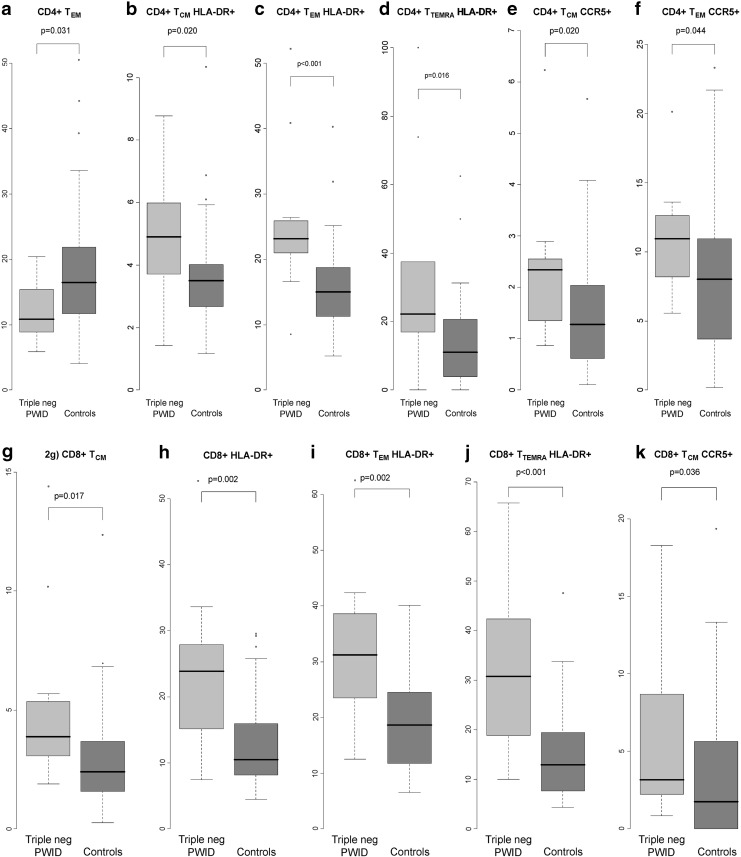
To analyze the effects of IDU, triple negative PWID were compared to controls. The results are presented in Supplementary Table S2 and in Figure 2. Triple negative PWID had lower percentages of CD4+ TEM cells and higher immune activation (based on HLA-DR+ cells) on multiple CD4+ cell types than controls (Fig. 2a–d). In addition, triple negative PWID had higher percentages of CD4+ CCR5+ TCM and TEM cells (Fig. 2e, f) compared to controls. In terms of CD8+ cells, triple negative PWID had higher percentages of TCM cells and immune activated cells (Fig. 2g–j) than controls. In addition, triple negative PWID had higher numbers of CD8+ TCM CCR5+ cells compared to controls (Fig. 2k).
FIG. 2.
The ratio (median%) of lymphocyte subsets from the parent cell population among HIV, HCV, and HBV seronegative PWID (triple negative PWID) (light gray) and controls (unexposed seronegative subjects) (medium gray). Panel (a) indicates TEM, (b) TCM HLA-DR+, (c) TEM HLA-DR+, (d) TTEMRA HLA-DR+, (e) TCM CCR5+, and (f) TEM CCR5+ cells (g) indicate TCM, (h) HLA-DR+, (i) TEM HLA-DR+, (j) TTEMRA HLA-DR+, and (k) TCM CCR5+ cells. p < 0.05 was considered statistically significant and is presented on the figure.
Discussion
Numerous studies have investigated potential correlations between T cell distribution and HIV, HBV, and HCV mono-infection, mostly among sexually infected individuals (2,9,21,25). The present study investigated PWID, a population characterized by high occurrences of blood-borne infections, exposure to multiple immunomodulatory substances, including opioids, and altered T cell distribution, and increased immune activation even when HIV seronegative (14,26). By analyzing the effects of coinfections and IDU on T cell distribution, we demonstrated in the present study that: (a) HIV/HBV/HCV triple infected individuals had lower percentages of CD4+ T cells and higher percentages of CD8+ TEM cells and immune activation compared to HIV negative PWID (HBV+HCV+, HCV+, and triple negative PWID) and controls; (b) HBV+HCV+ but HIV negative PWID had lower percentages of CD4+ cells than triple negative PWID and controls; and (c) triple negative PWID had higher percentages of CD8+ TCM cells, lower percentages of CD4+ TEM cells, increased immune activation markers, and higher percentages of CCR5-positive cells, than controls.
The first main finding was the significantly different T cell subset distribution between HIV+HBV+HCV+ triple infected PWID and the three HIV-negative PWID groups (HBV+ HCV+, HCV+, and triple negative PWID). More specifically, triple infected PWID had lower percentages of CD4+ cells and higher percentages of CD8+ cells and immune activation markers than HIV negative PWID groups, which was a finding similar to that for hetero- and homosexually infected HIV-positive individuals without known coinfections (21,25,35). Although we were unable to assess the distinct effects of HIV due to a lack of mono-infected subjects among the PWID, the changes in the T cell distribution among the HIV-positive PWID coinfected with HBV and or HCV seemed to be driven by the HIV infection, as HCV and HBV seropositivity did not seem to have any additional effects on the immunological characteristics evaluated.
The second main finding was the lower percentage of overall CD4+ T cells among the HIV negative but HBV+HCV+ PWID compared to the triple negative PWID. In previous studies of hepatitis mono-infected populations, an increase in the CD8+ TCM and TEM CD8+ cell populations through the acute to chronic phase of the illness has mainly been noted (1,5,28). In addition, an overall decrease in the number of CD4+ T cells has been found among HBV and HCV mono-infected chimpanzees, together with inadequate CD8+ T cell responses and subsequent persistent viral infections (2,9). One should also bear in mind that with hepatitis, the majority of T cell changes are localized in the liver, and thus, even if they exist, may not be detectable in peripheral blood (1,11,27). The interpretation of our results is even more complex, as PWID are more likely than sexually infected individuals to have been exposed to HIV without being infected. HIV exposure itself may lead to a lower percentage of CD4+ T cells, as shown in sexually HIV exposed but seronegative individuals (35), as well as in the triple negative PWID individuals in the present study. It seems likely that the studied PWID population is enriched by syringe sharing risk behavior and had increased viral exposure. Therefore, we propose that the mild decrease in the total CD4+ T cell population among HIV-negative HBV/HCV seropositive PWID subjects may have been caused either by HBV/HCV seropositivity and or exposure to HIV through syringe sharing.
The third main finding demonstrated the impact of IDU on T cell distribution and immune activation among triple negative PWID. To the best of our knowledge, this is the first study to demonstrate altered T cell subset distribution (higher percentages of CD8+ TCM cells, lower percentages of CD4+ TEM cells) and higher percentages of CD4+ and CD8+ CCR5+ and HLA-DR+ cells among triple negative PWID compared to controls. Of note such population is difficult to find due to a small number of HIV/HBV/HCV triple negatives among PWID with long histories of IDU. Studies of rhesus macaques have demonstrated CD4+ T cell and macrophage shifts in response to opioid exposure (3,33) and in vitro studies have found opioids to have additional effects that lead to an increase in the expression of the HIV coreceptor CCR5 (19,24,32,33). The increased CD38 and or HLA-DR, together with the differences in the multiple cell populations of the PWID found in the present study, could be explained by various factors, such as the abiotic substances injected, to an individual's systemic circulation, an unproductive infection with HIV, or the two hepatitis viruses (4,21,25,26). Overall, the changes seen in the triple negative PWID may have been caused by opioids or viral exposure; nevertheless, the immune system is already altered as a consequence of IDU, even when individuals remain seronegative to HIV, HBV, and HCV.
Some limitations to the present study should be noted. Investigating several infections simultaneously creates a significant amount of data, which increases the risk of misinterpretation. To account for the effects of multiple testing, we used Holm–Bonferroni correction. The lack of clinical and laboratory data did not allow us to distinguish between acute, chronic, and self-limiting hepatitis and, therefore, assess the relative effects of disease stage on T cell subset distribution and immune activation. In addition, as the study population consisted of PWID, HCV acute reinfections may be common and have influenced the studied T cell characteristics (9,18). The low statistical power (owing to the small sample size) of the analysis means that the results should be viewed with caution. Regardless of these limitations, we believe that our results showed clear patterns of T cell changes among PWID.
In conclusion, the T cell subset differences among triple infected individuals were probably triggered by the HIV infection, whereas the HBV and or HCV coinfections played a minimal (if any) role in these changes. In HIV-negative subjects, HBV/HCV coinfection and IDU appeared to influence T cell distribution in a way that could potentially increase the risk of HIV infection. This might help explain the extremely rapid spread of HIV among PWID populations, as has occurred in Estonia and many other locations.
Supplementary Material
Acknowledgments
The authors thank all the study participants, the team from the nongovernmental organization “Convictus,” Kristina Marsh for coordinating the study, and Karolin Toompere for suggestions regarding the statistical analysis. Financial support: This work was supported by the European Union through the European Regional Development Fund, Estonian Science Foundation (grants 8415 and 8856); Basic Financing and Target Financing of the Estonian Ministry of Education and Research (SF0180004s12) through institutional research grant No. IUT34-17; and grant R01 DA003574 from the US National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Appay V, Dunbar PR, Callan M, et al. . Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 2002;8:379–385 [DOI] [PubMed] [Google Scholar]
- 2.Asabe S, Wieland SF, Chattopadhyay PK, et al. . The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol 2009;83:9652–9662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer BM, Daussin S, Hernandez M, et al. . Morphine inhibition of lymphocyte activity is mediated by an opioid dependent mechanism. Neuropharmacology 1990;29:369–374 [DOI] [PubMed] [Google Scholar]
- 4.Biasin M, Caputo SL, Speciale L, et al. . Mucosal and systemic immune activation is present in human immunodeficiency virus-exposed seronegative women. J Infect Dis 2000;182:1365–1374 [DOI] [PubMed] [Google Scholar]
- 5.Carotenuto P, Artsen A, Osterhaus AD, et al. . Reciprocal changes of naïve and effector/memory CD8+ T lymphocytes in chronic hepatitis B virus infection. Viral Immunol 2011;24:27–33 [DOI] [PubMed] [Google Scholar]
- 6.Champagne P, Ogg GS, King AS, et al. . Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 2001;410:106. [DOI] [PubMed] [Google Scholar]
- 7.Chang JJ, Wightman F, Bartholorneusz A, et al. . Reduced hepatitis B virus (HBV)-specific CD4(+) T-Cell responses in human immunodeficiency virus type 1-HBV-Coinfected individuals receiving HBV-active antiretroviral therapy. J Virol 2005;79:3038–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dichamp I, Abbas W, Kumar A, et al. . Cellular activation and intracellular HCV load in peripheral blood monocytes isolated from HCV monoinfected and HIV-HCV coinfected patients. PLoS One 2014;9:e96907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grakoui A, Shoukry NH, Woollard DJ, et al. . HCV persistence and immune evasion in the absence of memory T cell help. Science 2003;302:659–662 [DOI] [PubMed] [Google Scholar]
- 10.Gras L, de Wolf F, Smit C, et al. . Changes in HIV RNA and CD4 cell count after acute HCV infection in chronically HIV-infected individuals. J Acquir Immune Defic Syndr 2015;68:536–542 [DOI] [PubMed] [Google Scholar]
- 11.He XS, Rehermann B, López-Labrador FX, et al. . Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci U S A 1999;96:5692–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodowanec AC, Brady KE, Gao W, et al. . Characterization of CD4+ T-cell immune activation and interleukin 10 levels among HIV, hepatitis C virus, and HIV/HCV-coinfected patients. J Acquir Immune Defic Syndr 2013;64:232–240 [DOI] [PubMed] [Google Scholar]
- 13.Huik K, Avi R, Pauskar M, et al. . Association between TLR3 rs3775291 and resistance to HIV among highly exposed Caucasian intravenous drug users. Infect Genet Evol 2013;20:78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallas E, Huik K, Türk S, et al. . Differences in T cell distribution and CCR5 expression in HIV-positive and HIV-exposed seronegative persons who inject drugs. Med Microbiol Immunol 2016;205:231–239 [DOI] [PubMed] [Google Scholar]
- 15.Laisaar KT, Avi R, DeHovitz J, et al. . Estonia at the threshold of the fourth decade of the AIDS era in Europe. AIDS Res Hum Retroviruses 2011;27:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahnke YD, Brodie TM, Sallusto F, et al. . The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol 2013;43:2797–2809 [DOI] [PubMed] [Google Scholar]
- 17.Malekinejad M, Johnston LG, Kendall C, et al. . Using respondent-driven sampling methodology for HIV biological and behavioral surveillance in international settings: a systematic review. AIDS Behav 2008;12:S105–S130 [DOI] [PubMed] [Google Scholar]
- 18.Osburn WO, Fisher BE, Dowd KA, et al. . Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 2010;138:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platt EJ, Wehrly K, Kuhmann SE, et al. . Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 1998;72:2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rallón NI, Soriano V, Restrepo C, et al. . HCV-specific T-cell responses in HIV/hepatitis C virus-coinfected patients on highly active antiretroviral therapy are comparable to those observed in hepatitis C virus-monoinfected individuals. J Acquir Immune Defic Syndr 2011;57:1–8 [DOI] [PubMed] [Google Scholar]
- 21.Restrepo C, Rallón NI, del Romero J, et al. . Low-level exposure to HIV induces virus-specific T cell responses and immune activation in exposed HIV-seronegative individuals. J Immunol 2010;185:982–989 [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Lenig D, Förster R, et al. . Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–712 [DOI] [PubMed] [Google Scholar]
- 23.Sandberg JK, Falconer K, and Gonzalez VD. Chronic immune activation in the T cell compartment of HCV/HIV-1 co-infected patients. Virulence 2010;1:177–179 [DOI] [PubMed] [Google Scholar]
- 24.Steele AD, Henderson EE, and Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology 2003;309:99–107 [DOI] [PubMed] [Google Scholar]
- 25.Suy A, Castro P, Nomdedeu M, et al. . Immunological profile of heterosexual highly HIV-exposed uninfected individuals: predominant role of CD4 and CD8 T-cell activation. J Infect Dis 2007;196:1191–1201 [DOI] [PubMed] [Google Scholar]
- 26.Tran HK, Chartier L, Troung LX, et al. . Systemic immune activation in HIV-1-exposed uninfected Vietnamese intravascular drug users. AIDS Res Hum Retroviruses 2006;22:255–261 [DOI] [PubMed] [Google Scholar]
- 27.Urbani S, Boni C, Amadei B, et al. . Acute phase HBV-specific T cell responses associated with HBV persistence after HBV/HCV coinfection. Hepatology 2005;41:826–831 [DOI] [PubMed] [Google Scholar]
- 28.Urbani S, Boni C, Missale G, et al. . Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J Virol 2002;76:12423–12434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uuskula A, Heimer R, Dehovitz J, et al. . Surveillance of HIV, hepatitis B virus, and hepatitis C virus in an estonian injection drug-using population: sensitivity and specificity of testing syringes for public health surveillance. J Infect Dis 2006;193:455–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vickerman P, Martin NK, Roy A, et al. . Is the HCV–HIV co-infection prevalence amongst injecting drug users a marker for the level of sexual and injection related HIV transmission? Drug Alcohol Depend 2013;132:172–181 [DOI] [PubMed] [Google Scholar]
- 31.Vorobjov S, Uusküla A, Des Jarlais DC, et al. . Multiple routes of drug administration and HIV risk among injecting drug users. J Subst Abuse Treat 2012;42:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Crawford K, Yuan M, et al. . Regulation of CC chemokine receptor 5 and CD4 expression and human immunodeficiency virus type 1 replication in human macrophages and microglia by T helper type 2 cytokines. J Infect Dis 2002;185:885–897 [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Zhang T, and Ho WZ. Opioids and HIV/HCV infection. J Neuroimmune Pharmacol 2011;6:477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiessing L, Ferri M, Grady B, et al. . Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One 2014;9:e103345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang OO, Boscardin WJ, Matud J, et al. . Immunologic profile of highly exposed yet HIV type 1-seronegative men. AIDS Res Hum Retroviruses 2002;18:1051–1065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.