Abstract
Activated microglia and macrophages exert dual beneficial and detrimental roles after central nervous system injury, which are thought to be due to their polarization along a continuum from a classical pro-inflammatory M1-like state to an alternative anti-inflammatory M2-like state. The goal of the present study was to analyze the temporal dynamics of microglia/macrophage polarization within the lesion micro-environment following traumatic brain injury (TBI) using a moderate-level controlled cortical impact (CCI) model in mice. We performed a detailed phenotypic analysis of M1- and M2-like polarized microglia/macrophages, as well as nicotinamide adenine dinucleotide phosphate oxidase (NOX2) expression, through 7 days post-injury using real-time polymerase chain reaction (qPCR), flow cytometry and image analyses. We demonstrated that microglia/macrophages express both M1- and M2-like phenotypic markers early after TBI, but the transient up-regulation of the M2-like phenotype was replaced by a predominant M1- or mixed transitional (Mtran) phenotype that expressed high levels of NOX2 at 7 days post-injury. The shift towards the M1-like and Mtran phenotype was associated with increased cortical and hippocampal neurodegeneration. In a follow up study, we administered a selective NOX2 inhibitor, gp91ds-tat, to CCI mice starting at 24 h post-injury to investigate the relationship between NOX2 and M1-like/Mtran phenotypes. Delayed gp91ds-tat treatment altered M1-/M2-like balance in favor of the anti-inflammatory M2-like phenotype, and significantly reduced oxidative damage in neurons at 7 days post-injury. Therefore, our data suggest that despite M1-like and M2-like polarized microglia/macrophages being activated after TBI, the early M2-like response becomes dysfunctional over time, resulting in development of pathological M1-like and Mtran phenotypes driven by increased NOX2 activity.
Keywords: : M1-like; M2-like; microglia/macrophage; NOX2, polarization; traumatic brain injury
Introduction
Normal tissue repair proceeds through overlapping phases of inflammation, proliferation, and remolding1; tissue macrophages are present throughout this progression, orchestrating the transitions within and among these critical tissue repair phases.2 Macrophages undergo phenotypic and functional changes in each phase, and this “functional plasticity” leads to efficient wound healing and tissue repair after injury.2 However, dysregulated macrophage activation can lead to impaired healing responses and chronic inflammation in several disease states.2
Microglia are the primary immune cells in the brain, and despite their distinct origin,3 they share many phenotypic and functional properties with macrophages.4 For example, microglia respond to pro-inflammatory molecules, such as lipopolysaccharide (LPS) or interferon-γ, to adopt a “classical” M1-like phenotype, which produces high levels of pro-inflammatory cytokines and oxidative metabolites that are essential for host defense and phagocytic activity.5 Microglia also respond to anti-inflammatory cytokines, such as interleukin (IL)-4 and IL-13, to induce an “alternative” M2a-like activation state, which is potently anti-inflammatory and contributes to tissue remodeling and matrix deposition.5 In addition, microglia adopt a “deactivated” M2c-like phenotype in response to IL-10, glucocorticoids, or transforming growth factor-β (TGFβ), which regulates resolution of inflammation and also is involved in tissue remodeling.5
Finally, microglia also can adopt an intermediate M2b-like phenotype in response to immune complex exposure and stimulation of toll-like receptors (TLRs).5 Theoretically, M1-like and M2-like polarized microglia should work in concert to fine-tune inflammatory responses, scavenge debris, and promote remodeling and repair after traumatic brain injury (TBI), thereby contributing to successful wound healing. However, experimental and clinical studies demonstrate a chronic and persistent M1-like phenotype for months to years after a single moderate-level TBI or repeated mild TBI,6–14 with limited capacity for tissue repair, particularly after moderate-to-severe TBI. The lesion micro-environment would appear to be a critical determinant for the polarization and development of microglia/macrophage phenotypes, with signals within the lesion biochemical milieu determining functional cellular responses after TBI. Further, oxidative stress and generation of reactive oxygen and nitrogen species (ROS and RNS, respectively) represent important secondary injury responses after TBI,15 and local redox signaling is known to regulate macrophage polarization and function.16
We recently demonstrated persistent oxidative damage in peri-lesional cortex and chronic expression of ROS-producing nicotinamide adenine dinucleotide phosphate oxidase (NOX2) in reactive microglia up to 12 months after a single moderate-level controlled cortical impact (CCI) that was associated with progressive neurodegeneration and chronic neurological deficits in TBI mice.9 There was a transient increase in expression of M2-like microglia in the acute phase after CCI that was lost at chronic time-points, when the persistent M1-like microglial phenotype predominated. Our studies support prior data in spinal cord injury (SCI) and TBI models, which demonstrate chronic expression of M1-like microglia/macrophages, and significantly diminished expression of the pro-repair M2-like phenotype at delayed times post-injury.17,18 Thus, microglia/macrophage phenotypes and tissue repair mechanisms appear to be dysregulated after central nervous system (CNS) injury, preventing efficient wound healing responses and leading to persistent neuroinflammation and related tissue damage.
From a therapeutic perspective, there may be an opportunity to harness the endogenous potential of microglia/macrophages to facilitate active repair following TBI, but the kinetics, interplay, and functional activities of distinct microglial/macrophage polarization states is currently poorly understood. Therefore, it is important to better delineate the kinetics and dynamics of various microglial/macrophage phenotypes after TBI, as well as to establish a role for the tissue micro-environment in controlling the polarizing state of these functionally plastic cells. Here, we used real-time polymerase chain reaction (qPCR), flow cytometry, and in situ imaging analyses to examine microglia/macrophage polarization dynamics during the acute phase after CCI, and investigated the relationship between NOX2 and M1-/M2-like polarization by inhibiting NOX2 activity after CCI using the selective NOX2 pharmacological inhibitor, gp91ds-tat.19
Methods
Animals
Studies were performed using young adult male C57Bl/6 mice (3 months old, 22–26 g), which were housed in the animal care facility at the University of Maryland School of Medicine under a 12 h light-dark cycle, with ad libitum access to food and water. All surgical procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine.
CCI
Our custom-designed CCI injury device consists of a microprocessor-controlled pneumatic impactor with a 3.5 mm diameter tip. C57Bl/6 mice were anesthetized with isoflurane evaporated in a gas mixture containing 70% N2O and 30% O2 and administered through a nose mask (induction at 4% and maintenance at 2%). Depth of anesthesia was assessed by monitoring respiration rate and pedal withdrawal reflexes. Mice were placed on a heated pad, and core body temperature was maintained at 37°C. The head was mounted in a stereotaxic frame, and the surgical site was clipped and cleaned with Povodine-Iodine Prep pads (Professional Disposables International, Inc., Orangeburg, NY) and alcohol pads (Dukal Corporation, Ronkonkoma, NY). A 10-mm midline incision was made over the skull, the skin and fascia were reflected, and a 4-mm craniotomy was made on the central aspect of the left parietal bone. The impounder tip of the injury device was then extended to its full stroke distance (44 mm), positioned to the surface of the exposed dura, and reset to impact the cortical surface. Moderate-level CCI was induced using an impactor velocity of 6 m/sec and deformation depth of 2 mm as previously described.20 After injury, the incision was closed with interrupted 6-0 silk sutures, anesthesia was terminated, and the animal was placed into a heated cage to maintain normal core temperature for 45 min post-injury. Sham animals underwent the same procedure as CCI mice except for the impact.
Study 1
Sham-injured (n = 7) and CCI (n = 5/time-point) mice were anesthetized (100 mg/kg sodium pentobarbital, intraperitoneally [i.p.]) at 1 h, 6 h, 24 h, 72 h, and 7 d post-injury and transcardially perfused with ice-cold 0.9% saline (100 mL). Ipsilateral cortical tissue was rapidly dissected and snap-frozen on liquid nitrogen for RNA extraction.
Study 2
Sham-injured and CCI mice were anesthetized (100 mg/kg sodium pentobarbital, i.p.) at 24 h, 72 h, and 7d post-injury and transcardially perfused with ice-cold 0.9% saline (100 mL). Ipsilateral cortical tissue was rapidly dissected and processed for CD11b positive selection and flow cytometry analysis. Three independent experiments were performed with n = 3 per group per time-point.
Study 3
Sham-injured and CCI mice (n = 5/time-point) were anesthetized (100 mg/kg sodium pentobarbital, i.p.) at 24 h, 72 h, and 7 d post-injury and transcardially perfused with ice-cold 0.9% saline (100 mL), followed by 300 mL of 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde overnight, and cryoprotected in 30% sucrose for histological analysis.
Study 4
Starting at 24 h post-injury, gp91ds-tat (5 mg/kg) or equal concentration of ds-tat scrambled peptide (AnaSpec Inc. Fremont, CA) was delivered by intraperitoneal injection to moderate-level CCI mice (n = 8/group), with repeated intraperitoneal administrations at 48 h and 72 h post-injury. Dosing of gp91ds-tat was based on reported therapeutic effects in mice.21 The ds-tat scrambled- and gp91ds-tat-treated CCI mice were anesthetized (100 mg/kg sodium pentobarbital, i.p.) at 7 d post-injury and transcardially perfused with ice-cold 0.9% saline (100 mL), followed by 300 mL of 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde overnight, and cryoprotected in 30% sucrose for histological analysis.
Real-time PCR analysis
Total RNA was extracted from snap-frozen sham and TBI cortical tissue using a RNeasy isolation kit (Qiagen, Valencia, CA) with on-column DNase treatment (Qiagen). Complementary DNA (cDNA) synthesis was performed on 1 μg of total RNA using a Verso cDNA RT kit (Thermo Scientific, Pittsburg, PA); the protocols used were according to the manufacturer's instructions. Real-time PCR was performed using TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA) on an ABI 7900 HT FAST Real Time PCR machine (Applied Biosystems). TaqMan assay IDs were as follows: inducible nitric oxide synthase (iNOS), Mm00440502_mL; tumor necrosis factor (TNF)-α, Mm00443258_mL; arginase 1, Mm00475988_mL; Ym1, Mm00657889_mH; Fizz1, Mm00445109_mL; CD206, Mm00485148_mL; TGFβ, Mm00441724_m1; SOCS3, Mm00545913_s1; IL12, Mm00434174_m1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), Mm99999915_g1. A total of 20 μL volume was added to each well (9 μL cDNA, 1 μL primer, and 10 μL Taqman® Universal PCR Master Mix; Applied Biosystems). Samples were assayed in duplicate in one run (40 cycles), which was composed of three stages, 50°C for 2 min, 95°C for 10 sec for each cycle (denaturation), and finally the transcription step at 60°C for 1 min. Gene expression was calculated relative to the endogenous control sample (GAPDH) to determine relative expression values (2−ΔΔCt, where Ct is the threshold cycle).
Isolation of CD11b-positive cells
A magnetic-bead conjugated anti-CD11b antibody was used to isolated microglia/macrophages cells from ipsilateral brain tissue using MACS® separation technology (Miltenyi Biotec, Auburn, CA). Ipsilateral cortical and hippocampal tissue from sham-injured and CCI mice was rapidly micro-dissected and a single-cell suspension was prepared using enzymatic digestion (Neural Tissue Dissociation Kit; Miltenyi Biotec) in combination with the gentle MACS dissociator. Tissue was processed immediately to remove myelin (Myelin Removal Beads II and LS column; Miltenyi Biotec), and CD11b-positive selection was performed using magnetic microbeads separation (CD11b Microbeads and MS column; Miltenyi Biotec). Briefly, tissue was incubated with CD11b microbeads and loaded onto a MS column placed in the magnetic field of a MACS separator. The negative fraction (flow through) was collected, the column washed three times with MACS buffer (Miltenyi Biotec), and CD11b-positive cells were eluted by removing the magnetic field. This procedure resulted in the isolation of approximately 93% viable CD11b-positve cells from sham-injured and TBI mice.
Cell staining and flow cytometry
Staining of cell surface markers was performed as follows: CD11b-positive cells were resuspended in MACS buffer (Miltenyi Biotec), and stained using anti-mouse CD11b-APC (1:10; Miltenyi Biotec), CD45-FITC (1:10; Miltenyi Biotec), or anti-mouse CD206-APC (1:50; BioLegend, San Diego, CA) for 30 min at 4°C in the dark. Control staining with appropriate matched isotype control antibodies (Biolegend) was performed. Cells were washed three times in 1 mL of MACS buffer and fixed in 1% paraformaldehyde, and then flow cytometry analysis was performed as detailed below.
For intracellular staining CD11b-positive cells were incubated with protein transport inhibitor (BD Golgi Stop; BD Biosciences, San Diego, CA) and fixation and permeabilization solutions (Cytofix/Cytoperm Kit; BD Biosciences). Briefly, CD11b-positive cells were resuspended in Golgi Stop solution and incubated for 1 h at 37°C, washed three times in 1× phosphate-buffered saline (PBS), resuspended in Cytofix/Cytoperm solution and incubated for 20 min on ice. Cells were washed in permeabilization and wash buffer (Perm/Wash Solution, BD Biosciences), and blocked with 10% goat serum and 20% fetal bovine serum (in perm/wash) for 30 min at room temperature. Cells were then washed with perm/wash solution and resuspended in perm/wash solution containing anti-mouse iNOS (1:50; BD Transduction Laboratories, San Jose, CA), anti-mouse IL-12 (1:50; R&D Systems, MN), anti-mouse arginase 1 (1:50, BD Transduction Laboratories), anti-mouse Ym1 (1:50; Stem Cell Technologies, Vancouver, British Columbia), or anti-mouse TGFβ (1:50, Biolegend), and incubated for 1.5 h at room temperature. Cells were washed with perm/wash solution, and incubated in perm/wash solution containing Alexa Fluor conjugated (488) secondary antibodies (Life Technologies, Grand Island, NY) for 20 min at room temperature. Finally, cells were washed two times with 1 mL of perm/wash solution, and fixed in 1% paraformaldehyde for flow cytometry analysis.
Flow cytometry was performed using a BD FACSCanto II cytometer (BD Bioscience), and 20,000 events were recorded. Data was analyzed using FlowJo Software (v.X; TreeStar, Inc., Ashland, OR), and gating was determined based on appropriate negative isotype controls. SSC(A) and FSC(A) was used to identify mononuclear cell populations, and doublets/triplets were removed by SSC(A) and SSC(W) to yield single cell populations for subsequent analysis of M1/M2-like protein expression (MFI). In addition, cell populations were analyzed using SSC(W) (y axis) and M1/M2-like staining (x axis) to discriminate low versus high cell populations based on defined gates for sham-injured control samples.
Immunofluorescence analysis
For semi-quantitative analysis, 20-μm coronal brain sections series from −1.70 mm bregma position were selected and standard immunostaining techniques were employed. Briefly, 20-μm brain sections were washed three times with 1× PBS, blocked for 1 h in goat serum containing 0.4% Triton X-100, and incubated overnight at 4°C with a combination of primary antibodies, including rat anti-CD16/32 (1:1000, BD Biosciences), rabbit anti-TGFβ (1:500; Torrey Pines Biolabs Inc., Secaucus, NJ), rabbit anti-iNOS (1:400; Calbiochem, San Diego, CA), goat anti-arginase 1 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-gp91phox (1:200; BD Transduction Laboratories), rat anti-CD68 (1:200; AbD Serotec, Raleigh, NC), rabbit anti-Iba1 (1:200; Wako Chemicals, Richmond, VA), rabbit anti-H2AX (1:2000; Novus Biologicals, Littleton, CO). Sections were washed three times with 1× PBS and incubated with appropriate Alexa Fluor conjugated secondary antibodies (Life Technologies) for 2 h at room temperature. Sections were washed three times with 1× PBS, counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/mL; Sigma, Dorset, UK), and mounted with glass coverslips using Hydromount solution (National Diagnostics, Atlanta, GA). Images were acquired using a fluorescent Nikon Ti-E inverted microscope (Nikon Instrument Inc., Melville, NY), at 10× (Plan Apo 10X NA 0.45;) or 20× (Plan APO 20X NA 0.75) magnification. Exposure times were kept constant for all sections in each experiment. All images were quantified using Nikon ND-Elements Software (AR 4.20.01), and co-localization of M1- and M2-like markers, as well as with gp91phox expression was performed by binary operation intersection followed by thresholding. 6,000–10,000 positive cell body areas were quantified per mouse per experiment (n = 4), and expression levels were expressed as binary area per region of interest (ROI) ×106.
Immunohistochemistry
Immunohistochemistry was performed on 60 μm coronal sections and standard immunostaining techniques were employed. Sections were incubated with anti-Iba-1 (1:1000; Wako Chemicals), overnight, washed in 1× PBS (three times), and incubated with biotinylated anti-rabbit IgG antibody (Vector Laboratories, Burlingame, CA) for 2 h at room temperature. Sections were incubated in avidin-biotin-horseradish peroxidase solution (Vectastain elite ABC kit; Vector Laboratories) for 1 h and then reacted with 3,3′-diaminobenzidine (Vector Laboratories) for color development and mounted for immunohistochemical analysis using a Leica DM4000B microscope (Leica Microsystems; Exton, PA).
Unbiased stereological assessment of neuronal survival
Cresyl violet–stained 60 μm coronal sections were used for neuronal loss analysis. For TBI-induced cortex and hippocampal neuronal cell loss, the optical fractionator method of stereology was used as previously described.24 Briefly, every fourth 60 μm section between −1.22 and −2.54 mm from bregma was analyzed beginning from a random start point (i.e., the section where different hippocampal subregions were distinctly visible). A total of five sections were analyzed. The optical dissector had a size of 50 × 50 μm in the x-axis and the y-axis, respectively, with a height of 10 μm and a guard zone of 4 μm from the top of the section. The sampled region for cortex and each hippocampal subregion was demarcated in the injured hemisphere and cresyl violet–stained neuronal cell bodies were counted using Stereoinvestigator Software (MBF Biosciences, Williston, VT). For the cornu ammonis 1 (CA1) and CA3 subregions grid spacings of 75 μm in the x-axis and 100 μm in the y-axis were used, resulting in an area fraction of 1/12. For the dentate gyrus subregion, a grid spacing of 175 μm in the x-axis and 100 μm in the y-axis was used, resulting in an area fraction of 1/28. The volume of the cortex and hippocampal subfields was measured using the cavalieri estimator method with a grid spacing of 50 μm. The estimated number of surviving neurons in each field was divided by the volume of the region of interest to obtain the cellular density expressed in counts/mm3. According to best stereologic practice, all stereologic probes were optimized to obtain a Gunderson coefficient of error (m = 1) value of less than 0.10 for the TBI tissue.
Statistical analysis
Real-time PCR, flow cytometry, image analysis, and stereological data were analyzed by one-way analysis of variance followed by post hoc adjustments using Student-Newman-Keuls test, and were expressed as mean ± standard error of the mean (SEM). Statistical tests were performed using GraphPad Prism Program, Version 3.02 for Windows (GraphPad Software, San Diego, CA). A p < 0.05 was considered statistically significant.
Results
Controlled cortical impact results in robust microglial/macrophage activation through 7 days post-injury
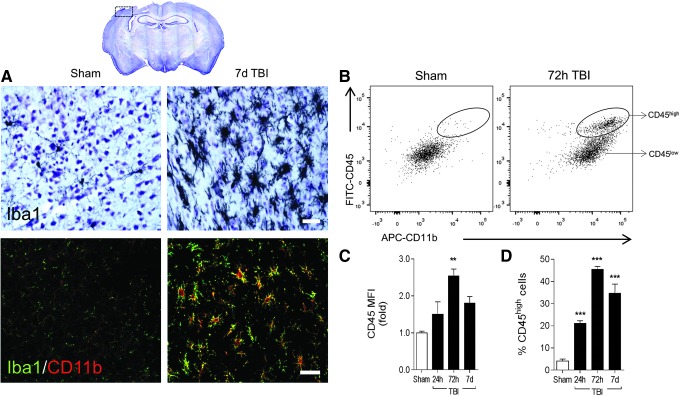
Immunohistochemical analysis of the microglial/macrophage marker, ionized calcium binding adapter molecule 1 (Iba1), demonstrated robust activation of microglia/macrophages in the ipsilateral cortex at 7 days post-injury, compared with sham-injured controls (Fig. 1A). Microglia in sham-injured controls had small cell bodies and thin, long, and highly branched processes indicative of a surveillant status. However, following CCI, microglia transformed to a highly activated state; their branched processes were withdrawn to form thick bundles around highly enlarged cell bodies and there was significant proliferation at the site of injury. Further, immunofluorescence imaging revealed that there was increased protein expression of Iba1/CD11b in highly activated microglia/macrophages at 7 days post-injury (Fig. 1A).
FIG. 1.
Microglial/macrophage activation dynamics after moderate-level controlled cortical impact (CCI) in C57Bl/6 mice. (A) Representative images at −2.06 mm from bregma of sham and CCI mice at 7 days post-injury. Immunohistochemistry (upper panels) for Iba1 and immunocytochemistry (lower panels) for Iba1 (green) and CD11b (red) at 7 days post-injury. Scale bar = 50μm. (B-D) Flow cytometry analysis of CD11b+ cells isolated from sham and CCI cortex and hippocampus using CD11b microbeads and MACS technology. Cells were labeled with CD11b and CD45 antibodies to differentiate peripheral myeloid cells (macrophages; CD11b+/CD45high) from resident microglia (CD11b+/CD45low) based on CD45 expression. (B) Representative bivariate dot plots of CD11b/CD45 labeling at 72 h post-injury. Bars represent CD45 protein expression (C; mean fluorescence intensity [MFI]) and percent CD45high cell number (D). Bars represent mean ± standard error of the mean. Statistical analysis by one-way analysis of variance, followed by post hoc adjustments using Student Newman Keuls multiple comparison test (**p < 0.01 and ***p < 0.001 vs. sham). Color image is available online at www.liebertpub.com/neu
Next, using a positive selection method (CD11b microbeads and MACS technology), we isolated microglia/macrophages (93% pure and viable; data not shown) from the ipsilateral cortex and hippocampus at 24 h, 72 h, and 7 days post-injury to assess activation dynamics by flow cytometry. CD11b-positive cells were labeled with CD11b and CD45 antibodies to differentiate peripheral myeloid cells (macrophages; CD11b+/CD45high) from resident microglia (CD11b+/CD45low) based on CD45 expression.22 Compared with sham-injured levels CD45 protein expression was significantly increased, with peak expression at 72 h post-injury (p < 0.01 vs. sham; Fig. 1B, 1C). Gating on CD45high populations revealed a rapid and robust infiltration of peripheral macrophages into the CCI brain, with a fivefold increase in the number of CD45high cells at 24 h post-injury, compared with sham-injured levels (p < 0.001; Fig. 1B, 1D). Peak infiltration of CD11b+/CD45high cells occurred at 72 h post injury (11-fold increase, p < 0.001 vs. sham), before a minor fall off in infiltration at 7 days post-injury (eightfold increase, p < 0.001 vs. sham).
M1- and M2-like polarized microglia/macrophages are activated during the acute phase response following CCI
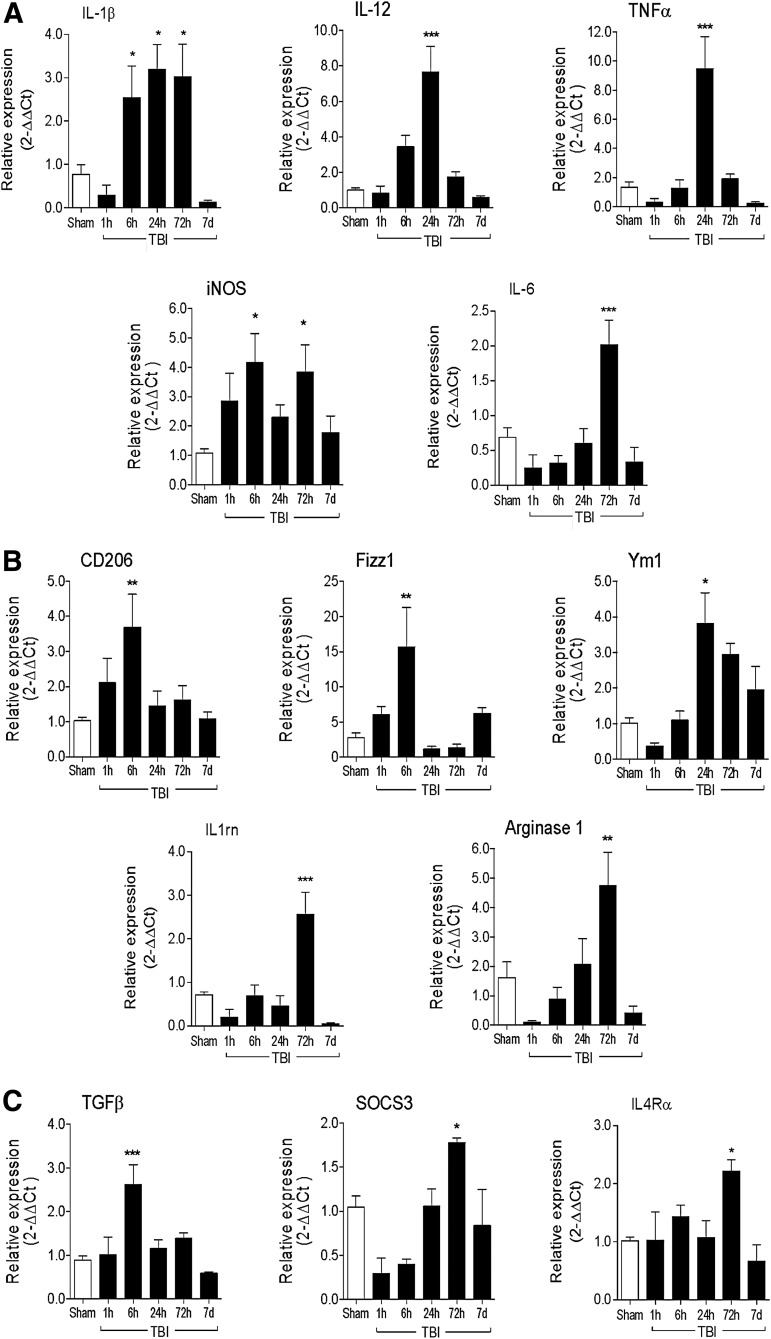
To assess microglial/macrophage polarization dynamics, we collected peri-lesional cortical tissue from CCI mice at 1 h, 6 h, 24 h, 72 h, and 7 days post-injury for gene expression analysis of markers of M1- and M2-like polarized microglia/macrophages. M1-like markers included IL-12, IL-1β, iNOS, TNFα, and IL-6 messenger RNAs (mRNAs), and M2-like markers included CD206, Fizz1, Ym1, IL-1rn, arginase 1, TGFβ, SOCS 3, and IL-4Rα mRNAs. Compared with sham-injured control levels, there was robust induction of M1-like genes following CCI with early and sustained IL-1β expression (2.5-fold increase; p < 0.05) starting at 6 h post-injury, followed by peak expression of IL-12 (sevenfold increase, p < 0.001) and TNFα (ninefold increase; p < 0.001) at 24 h post-injury, and peak IL-6 expression at 72 h post-injury (twofold increase; p < 0.001; Fig. 2A). Inducible nitric oxide synthase gene expression had a biphasic response following CCI, with an initial peak at 6 h post-injury (fourfold increase; p < 0.05 vs. sham) and a secondary peak at 72 h post-injury (fourfold increase; p < 0.05 vs. sham).
FIG. 2.
M1-like and M2-like microglial/macrophage activation genes are activated in the ipsilateral cortex after controlled cortical impact (CCI). Real-time polymerase chain reaction (PCR) was used to assess the expression levels of M1- and M2-like microglial/macrophage activation genes in cortex of sham, 1 h, 6 h, 24 h, 72 h, and 7 days CCI mice. (A) M1-like genes included interleukin (IL)-1β, IL-12, tumor necrosis factor (TNF) α, inducible nitric oxide synthase (iNOS), and IL-6. (B) M2a-like genes included CD206 (mannose receptor), Fizz1, Ym1, (chitinase3-like 3), IL-1rn, and arginase 1. (C) M2c-like genes included transforming growth factor (TGF) β, SOCS3 and IL4Rα. Bars represent mean ± standard error of the mean. Statistical analysis by one-way analysis of variance, followed by post hoc adjustments using Student Newman Keuls multiple comparison test (*p < 0.05, **p < 0.01 and ***p < 0.001 vs. sham).
For M2-like genes, there was an early induction of CD206, Fizz1, and TGFβ mRNA, with peak expression at 6 h post-injury (CD206 = 3.5-fold increase, p < 0.01; Fizz1 = 15-fold increase, p < 0.01; TGFβ = 2.5-fold increase, p < 0.001 vs. sham; Fig. 2B, 2C). CCI also resulted in a non-significant reduction in Ym1, IL-1rn, arginase 1 and SOCS3 mRNA at 1 h post-injury, and a delayed induction of gene expression, which peaked at 24 to 72 h post-injury (Ym1 = 3.5-fold increase [24 h], p < 0.05; IL-1rn = 2.5-fold increase [72 h], p < 0.001; arginase 1 = 4.5-fold increase [72 h], p < 0.01; SOCS3 = 1.75-fold increase [72 h], p < 0.05 vs. sham). Expression of IL-4Rα was stable early after CCI, peaked at 72 h post-injury (IL-4Rα = 2.3-fold-increase, p < 0.05 vs. sham), and returned to sham-injured levels at 7 days post-injury.
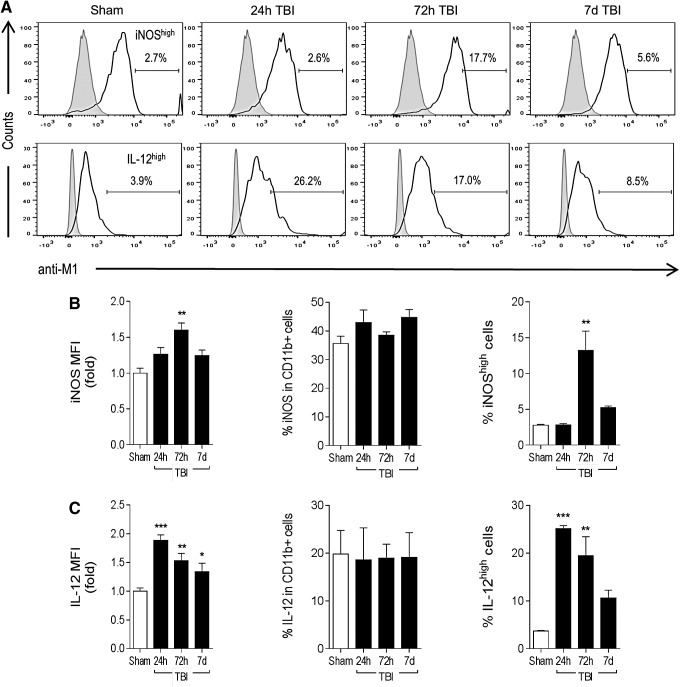
We then expanded our analysis to microglia/macrophages isolated from the CCI brain using MACS technology, and performed flow cytometry for selected M1- and M2-like polarization markers 4 using optimized cell surface and internalization staining protocols. Compared with sham-injured levels, there was a significant increase in iNOS protein expression in isolated microglia/macrophages after CCI, which peaked at 72 h post-injury (1.6-fold increase, p < 0.01; Fig. 3A, 3B). There was no change in percent of total iNOS-positive cells after CCI. When we gated on the most highly activated cells with respect to sham-injured control levels (iNOShigh population) there was a 4.5-fold increase in numbers of iNOShigh-positive microglia/macrophages at 72 h post-injury (p < 0.01 vs. sham). In addition, IL-12 protein expression was rapidly and robustly up-regulated in isolated microglia/macrophages at 24 h post-injury (twofold increase, p < 0.001 vs. sham; Fig. 3A, 3C). There was no change in percent of total IL-12-positive cells after CCI, but further analysis revealed a sevenfold increase in the number of IL-12high-positive microglia/macrophages at 24 h post-injury (p < 0.001 vs. sham), followed by fivefold (p < 0.01 vs. sham) and 2.5-fold increases in IL-12high-positive cells at 72 h and 7 days post-injury, respectively.
FIG. 3.
M1-like polarization of microglia/macrophages after controlled cortical impact (CCI). C57Bl/6 mice were subjected to sham-surgery or CCI, and CD11b+ cells were isolated at 24 h, 72 h, and 7 days post-injury to assess M1 protein expression by flow cytometry. (A) Representative histograms for M1 protein markers inducible nitric oxide synthase (iNOS) and interleukin (IL)-12, include gated iNOShigh and IL-12high populations at each time-point. (B) Mean fluorescence intensity (MFI) for iNOS, % of total iNOS-positive cells, and number of iNOShigh cells. (C) MFI for IL12, % of total IL-12-positive cells and number of IL-12high cells. Bars represent mean ± standard error of the mean. Statistical analysis by one-way analysis of variance, followed by post hoc adjustments using Student Newman Keuls multiple comparison test (*p < 0.05, **p < 0.01 and ***p < 0.001 vs. sham).
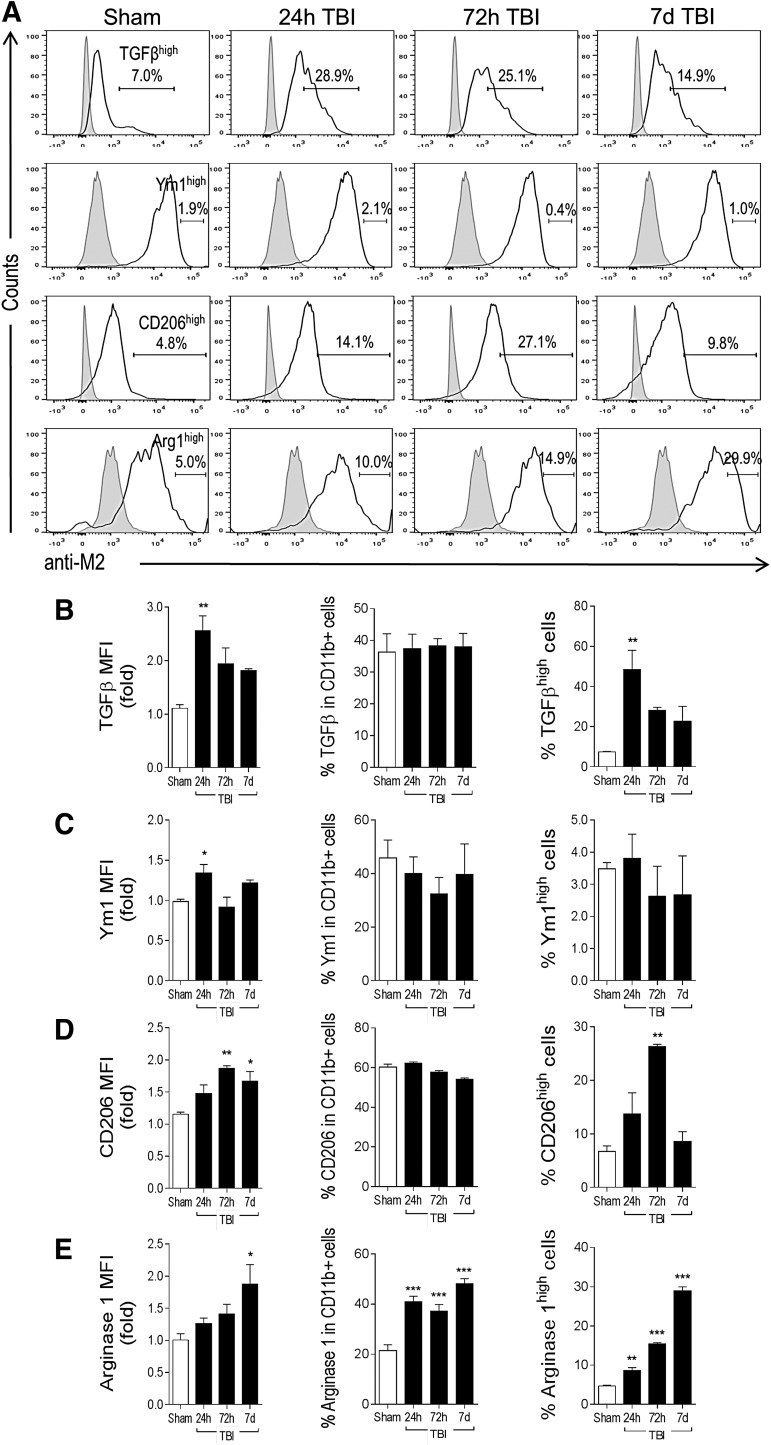
Markers of M2-like polarization also were up-regulated after CCI. TGFβ protein expression was rapidly and robustly up-regulated in isolated microglia/macrophages at 24 h post-injury (2.5-fold increase, p < 0.01 vs. sham; Fig. 4A, 4B). There was no change in percent of total TGFβ-positive cells after CCI. However, there was a sevenfold increase in the number of TGFβhigh-positive microglia/macrophages at 24 h post-injury (p < 0.01 vs. sham), which diminished to a threefold increase at 7 days post-injury. There was a modest, albeit significant, increase in Ym1 protein expression in isolated microglia/macrophages at 24 h post-injury (1.3-fold increase, p < 0.05 vs. sham; Fig. 4A, 4C), but no change in the number of Ym1high-positve cells at each time-point after CCI. There was a peak in CD206 protein expression at 72 h post-injury (1.8-fold increase, p < 0.01 vs. sham; Fig. 4A, 4D), which was sustained through 7 days post-injury. There was no change in percent of total CD206-positive cells after CCI, but analysis of CD206high-positive cells revealed a significant increase in this population at 72 h post-injury (fourfold increase, p < 0.01 vs. sham), which returned to sham-injured control levels by 7 days post-injury.
FIG. 4.
M2-like polarization of microglia/macrophages after controlled cortical impact (CCI). C57Bl/6 mice were subjected to sham-surgery or CCI, and CD11b+ cells were isolated at 24 h, 72 h, and 7 days post-injury to assess M2-like protein expression by flow cytometry. (A) Representative histograms for M2-like markers transforming growth factor (TGF) β, Ym1, CD206 and arginase 1, include TGFβhigh, Ym1high, CD206high, and arginase 1high populations at each time-point. (B) Mean fluorescence intensity (MFI) for TGFβ, % of total TGFβ-positive cells and number of TGFβhigh cells. (C) MFI for Ym1, % of total Ym1-positive cells and number of Ym1high cells. (D) MFI for CD206, % of total CD206-positive cells and number of CD206high cells. (E) MFI for arginase 1, % of total arginase 1-positive cells and number of arginase 1high cells. Bars represent mean ± standard error of the mean. Statistical analysis by one-way analysis of variance, followed by post hoc adjustments using Student Newman Keuls multiple comparison test (*p < 0.05, **p < 0.01 and ***p < 0.001 vs. sham).
Finally, arginase 1 protein expression was up-regulated at 7 days post-injury (1.8-fold increase, p < 0.05 vs. sham; Fig. 4A, 4E). Notably, there was a significant increase in percent of total arginase 1-positive microglia/macrophages within 24 h post-injury that was sustained through 7 days post-injury. Further analysis of arginase 1high populations revealed that there were significant increases in arginase 1high-positive cells starting at 24 h post-injury (twofold increase, p < 0.01 vs. sham), and further increases at 72 h (threefold increase, p < 0.01 vs. sham), and at 7 days post-injury (sixfold increase, p < 0.001 vs. sham).
M1-like and/or mixed transitional microglia/macrophages are highly expressed in peri-lesional cortex at 7 days post-injury
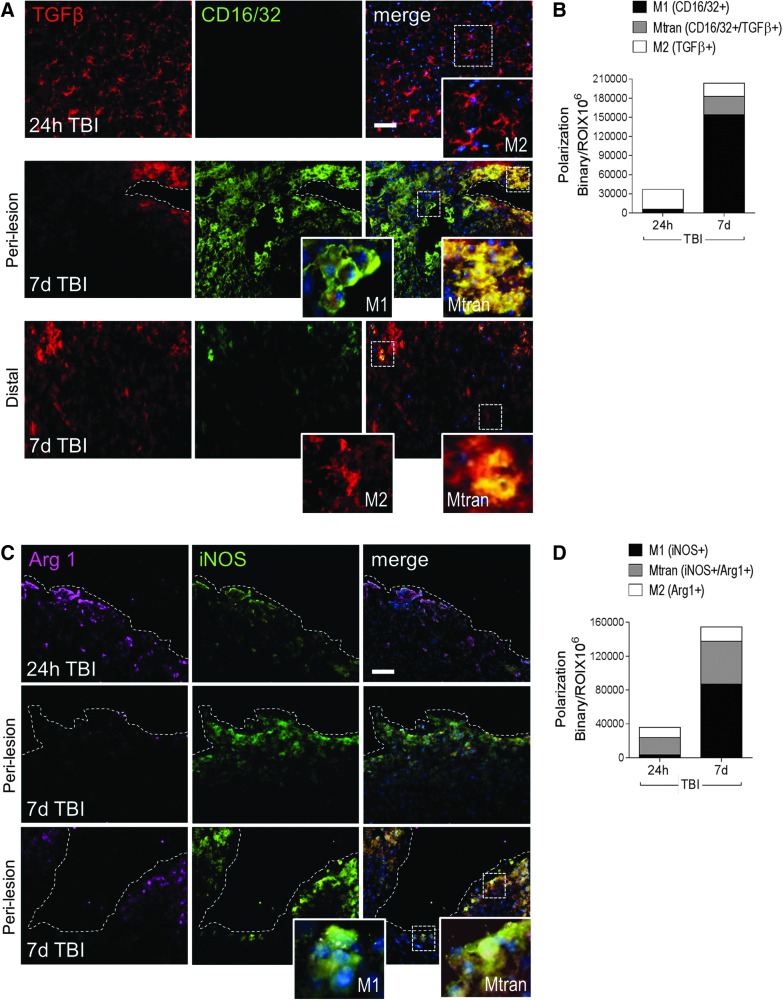
Next, we analyzed the expression of M1- and M2-like polarized microglia/macrophages in situ at 24 h and 7 days post-injury. Immunocytochemical analysis of CD16/32 and iNOS was performed for M1-like polarized cells, and TGFβ and arginase 1 for M2-like polarized cells. At 24 h post-injury, TGFβ was highly expressed in microglia/macrophages, displaying morphological features of surveillant cells (Fig. 5A, upper panel inset). By 7 days post-injury, there was robust CD16/32 expression throughout the peri-lesional cortex, and TGFβ expression was limited to cells displaying morphological features of amoeboid cells. Further, a significant proportion of TGFβ-positive cells at 7 days post-injury also co-expressed CD16/32, indicating a mixed transitional (Mtran) phenotype (Fig. 5A, middle panel insets), and there were relatively few single TGFβ-positive cells displaying ramified morphologies distal to the lesion (Fig. 5A, lower panel insets).
FIG. 5.
M1-like and mixed transitional (Mtran) microglial/macrophages predominate the peri-lesional cortex at 7 days post-injury. Representative images and analysis of M1- and M2-like polarized microglia/macrophages after controlled cortical impact (CCI). (A) Transforming growth factor (TGF) β+ (red) and CD16/32+ (green) microglia/macrophages in the peri-lesional and distal cortex at 24 h and 7 days post-injury. Insets display M1-like (CD16/32+), M2-like (TGFβ+), and Mtran (CD16/32+/TGFβ+) cells at each time-point. Scale bar = 50 μm. (B) Quantification of CD16/32+, TGFβ+, and CD16/32+/TGFβ+ cells at 24 h and 7 days post-injury. (C) Arginase 1+ (magenta) and inducible nitric oxide synthase (iNOS)+ (green) microglia/macrophages in the peri-lesional and distal cortex at 24 h and 7 days post-injury. Insets display M1-like (iNOS+) and Mtran (iNOS+/Arg1+) cells at 7 days post-injury. Scale bar = 50 μm (D) Quantification of iNOS+, Arg1+, and iNOS+/Arg1+ cells at 24 h and 7 days post-injury. Protein expression levels determined by binary area per region of interest (ROI) (mm2). n = 4 per time-point. Scale bar = 50 μm. Color image is available online at www.liebertpub.com/neu
With respect to iNOS and arginase 1 staining, there was modest expression of both proteins at 24 h post-injury, with some overlapping co-expression in amoeboid-like cells within the peri-lesional cortex (Mtran phenotype; Fig. 5B). By 7 days post-injury, there was a significant increase in expression of both proteins. There were relatively few arginase 1 single-positive cells at this time-point, but increased numbers of double-positive (arginase 1+/iNOS+; Mtran phenotype) and iNOS single-positive cells (Fig. 5B, lower panel insets) in the peri-lesional cortex. Quantification of protein expression of M1-like, M2-like, and Mtran cells revealed that there was a shift from single-positive M2-like cell populations at 24 h post-injury to predominant M1-like or Mtran microglial/macrophage populations within the injured cortex at 7 days post-injury (Fig. 5C, 7 days post-injury: M1-like [CD16/32+] = 76%, Mtran [CD16/32+/TGFβ+] = 14%, M2-like [TGFβ+] = 10%; Fig. 5D, 7 days post-injury: M1-like [iNOS+] = 56%, Mtran [iNOS+/Arg1+] = 33%, M2-like [Arg1+] = 11%).
NOX2 is associated with the M1-like and Mtran microglial/macrophage phenotypes and ongoing neurodegeneration after CCI
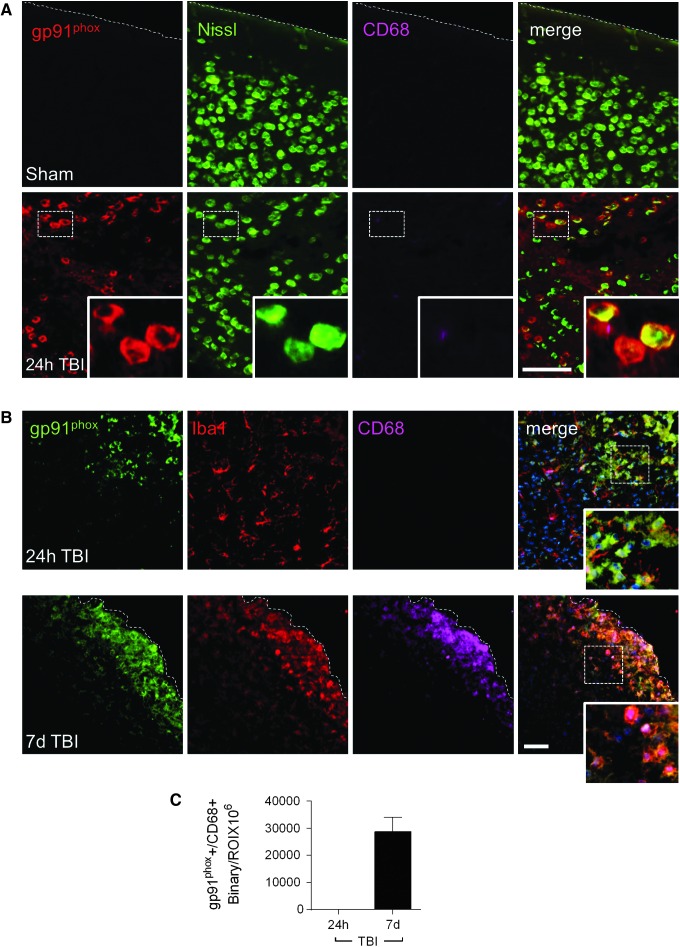
Previously, we reported that NOX2 was highly expressed in reactive microglia surrounding the lesion and in the ipsilateral thalamus at 12 months post-injury.9 Here, we sought to investigate the relationship between NOX2 expression and microglial/macrophage polarization during the acute phase after CCI. Immunocytochemical analysis of gp91phox (NOX2) expression in neurons and microglia/macrophages was performed at 24 h and 7 days post-injury. Compared with sham-injured levels, there was robust up-regulation of gp91phox in nissl-positive neurons in the peri-lesional cortex (Fig. 6A), as previously reported.23 There was minimal overlap in expression of gp91phox and Iba1-positive microglia/macrophages at 24 h post-injury (Fig. 6B). However, by 7 days post-injury, there was robust up-regulation of gp91phox in reactive microglia/macrophages (CD68+; Fig. 6B, 6C).
FIG. 6.
NADPH oxidase (NOX2) is expressed in reactive microglia/macrophages in the peri-lesional cortex at 7 days post-injury. Representative images of NOX2 in sham and controlled cortical impact cortex. (A) gp91phox+ (red), Nissil+ (green), and CD68+ (magenta) cells in the ipsilateral cortex of sham and at 24 h post-injury. (B) gp91phox+ (green), Iba1+ (red), CD68+ (magenta) cells in the ipsilateral cortex at 24 h and 7 days post-injury. (C) Quantification of gp91phox+/CD68+ expression at 24 h and 7 days post-injury. Protein expression levels determined by binary area per region of interest (ROI; mm2). n = 4 per time-point. Scale bar = 50 μm. Color image is available online at www.liebertpub.com/neu
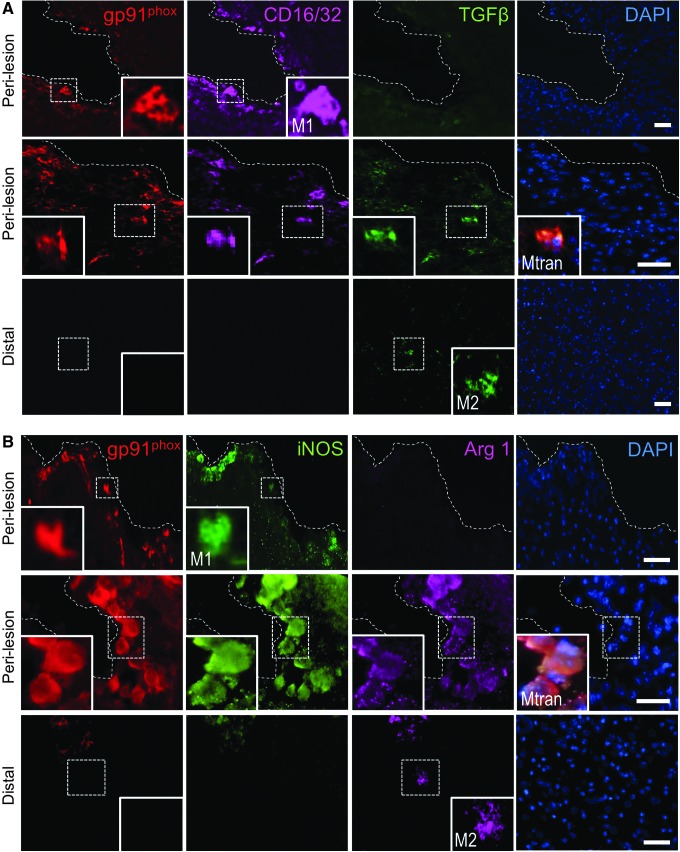
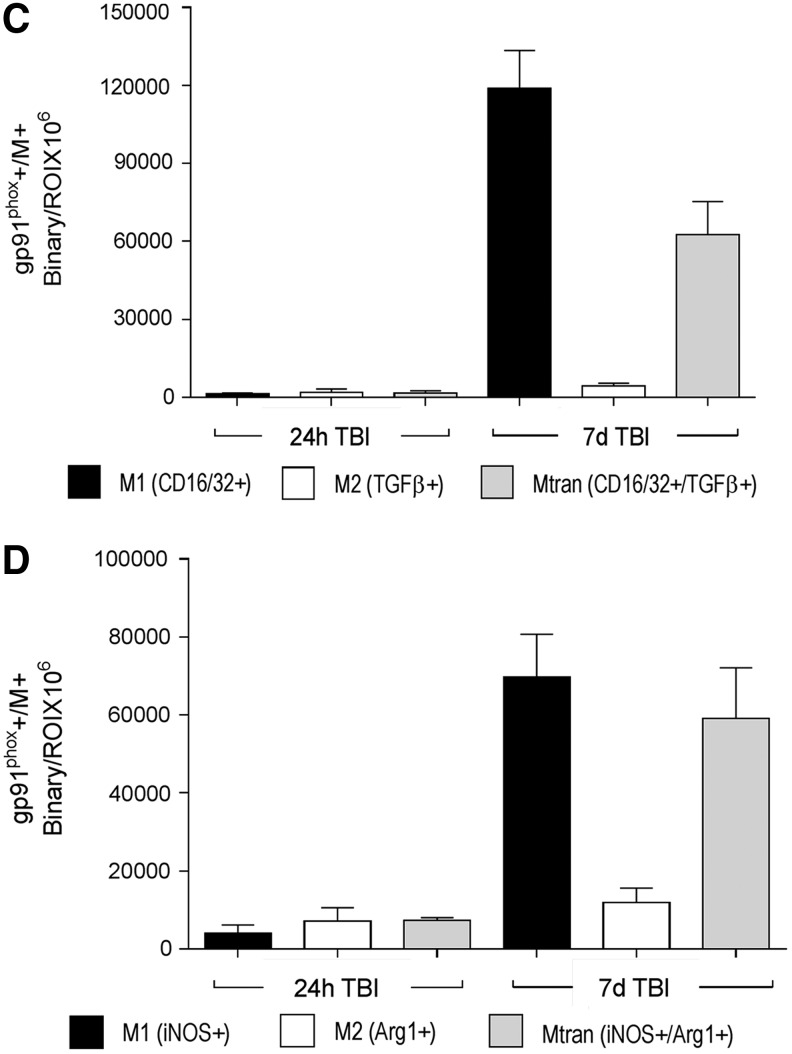
Next, we analyzed gp91phox expression in combination with markers of M1- (CD16/32 and iNOS) and M2-like (TGFβ and arginase 1) polarized cells. There was no overlapping expression of gp91phox with markers of M1- and M2-like polarized cells at 24 h post-injury (Fig. 7C, 7D). In contrast, by 7 days post-injury, there was significant overlap in expression of gp91phox with M1- and M2-like cells in the peri-lesional cortex, but not at distant sites from the lesion (Fig. 7C, 7D). Specifically, at 7 days post-injury, gp91phox co-localized with M1-like cells (gp91phox+/CD16/32+ = 64%; gp91phox+/iNOS+ = 50%; Fig. 7A, 7B, upper panel insets) and Mtran cells (gp91phox+/CD16/32+/TGFβ = 34%; gp91phox+/iNOS+/Arg1+ = 42%; Fig. 7A, 7B, middle panel insets), and there was negligible co-localization of gp91phox with M2-like cells (gp91phox+/TGBβ+ = 2%; gp91phox+/Arg1+ = 8%). Single-positive M2-like cells (TGFβ+ or Arg1+) were observed at distant sites from the lesion, and were negative for gp91phox (Fig. 7A, 7B, lower panel insets).
FIG. 7.
NADPH oxidase (NOX2) co-localizes with M1-like and mixed transitional (Mtran) microglia/macrophages at 7 days post-injury. Representative images of NOX2 expression with M1-like, M2-like, and Mtran microglia/macrophages after controlled cortical impact (CCI). (A) gp91phox+ (red), CD16/32+ (magenta), and transforming growth factor (TGF) β+ (green) cells in the peri-lesional cortex (upper panels) and at distant subcortical sites (lower panel). Insets display co-localization of gp91phox with CD16/32+ and CD16/32+/TGFβ+ cells in peri-lesional regions, and lack of gp91phox expression in TGFβ+ only cells in distal regions. (B) gp91phox+ (red), iNOS+ (green), and Arg1+ (magenta) cells in the peri-lesional cortex (upper panels) and at distant subcortical sites (lower panel). Insets display co-localization of gp91phox with inducible nitric oxide synthase (iNOS)+ and iNOS+/Arg1+ cells in peri-lesional regions, and lack of gp91phox expression in Arg1+ only cells in distal regions. Scale bar = 50 μm. (C) Quantification of co-localization of gp91phox+ with CD16/32+, TGFβ+, and CD16/32+/TGFβ+ in the peri-lesional and distal cortex at 24 h and 7 days post-injury. (D) Quantification of co-localization of gp91phox+ with iNOS+, Arg1+, and iNOS+/Arg1+ in the peri-lesional and distal cortex at 24 h and 7 days post-injury. Protein expression levels determined by binary area per region of interest (ROI; mm2). n = 4 per time-point. Color image is available online at www.liebertpub.com/neu
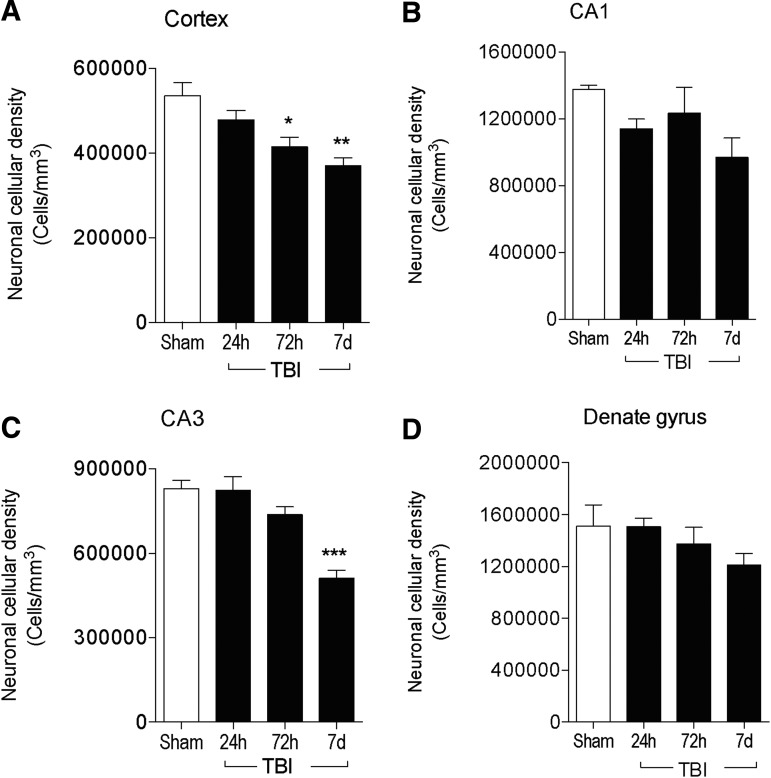
We then quantified post-traumatic neurodegeneration in the ipsilateral cortex and hippocampus at 24 h, 72 h, and 7 days post-injury. We performed stereological analysis of neuronal loss in the cortex, CA1, CA3, and dentate gyrus sub-regions of the hippocampus as previously described.24 Compared with sham-injured levels, CCI resulted in a significant loss of cortical neurons at 72 h (p < 0.05) and 7 days (p < 0.01; Fig. 8A). Similarly, there was ongoing neurodegeneration in the hippocampus, with increased neuronal loss in the CA1 (Fig. 8B) and dentate gyrus (Fig. 8D) sub-regions of the hippocampus, but cell loss failed to reach statistical significance in both sub-regions. However, there was a significant loss of neurons within the CA3 sub-region of the hippocampus at 7 days post-injury (p < 0.001 vs. sham; Fig. 8C).
FIG. 8.
Progressive neurodegeneration in the cortex and hippocampus through 7 days post-injury. Stereological assessment of surviving neurons in the ipsilateral cortex (A), CA1 (B), CA3 (C), and dentate gyrus (D) of the hippocampus at 24 h, 72 h, and 7 days post-injury. n = 4 per time-point. Statistical analysis by one-way analysis of variance, followed by post hoc adjustments using Student Newman Keuls multiple comparison test (*p < 0.05, **p < 0.01 and ***p < 0.001 vs. sham).
Systemic administration of a NOX2 inhibitor, gp91ds-tat, alters microglial/macrophage phenotypes and reduces oxidative stress-induced DNA damage after CCI
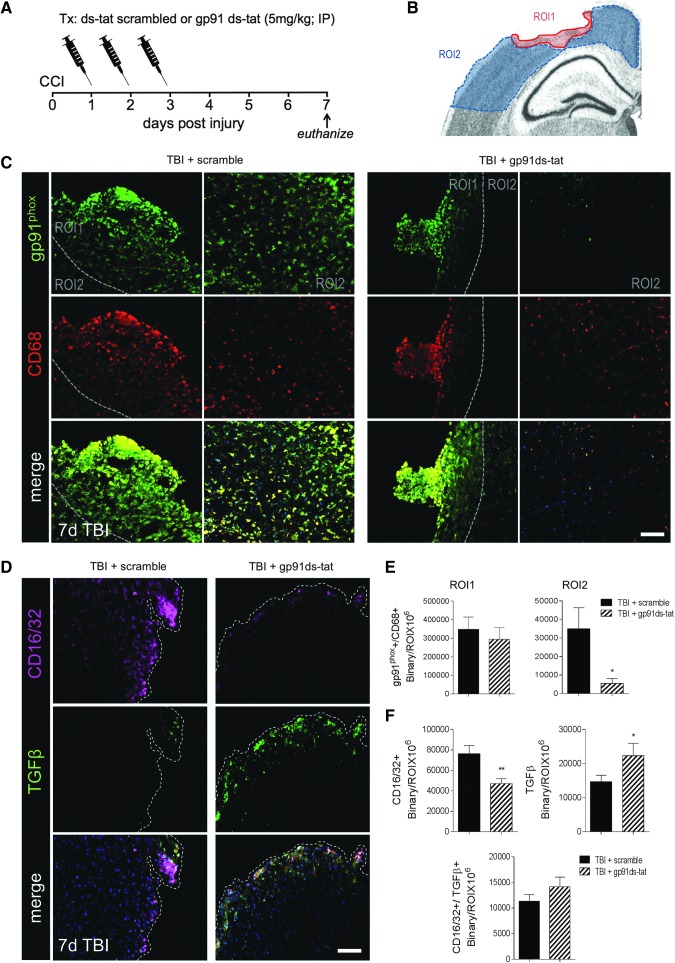
Given that NOX2 (gp91phox) is highly expressed in microglia/macrophages displaying the M1-like and Mtran phenotypes, we then sought to inhibit NOX2 activity using a selective peptide inhibitor, gp91ds-tat,19 in order to assess the effect of NOX2 inhibition on M1-/M2-like polarization after CCI. The gp91ds-tat (5 mg/kg) or equal concentration of ds-tat scrambled peptide was delivered by intraperitoneal injection (IP) to moderate-level CCI mice starting at 24 h post-injury, with repeated IP administrations at 48 and 72 h post-injury (Fig. 9A). The ds-tat scrambled and gp91ds-tat peptide-treated CCI mice were euthanized at 7 days post-injury for histological assessments. We analyzed the expression of gp91phox in reactive microglia/macrophages (CD68+) in two ROIs that included peri-lesional cortex (ROI1) and the surrounding cortex distal to the lesion (ROI2; Fig. 9B). Although no differences between treatment groups were observed in the peri-lesional cortex (ROI1), there was a significant reduction in gp91phox+/CD68+ expression in the surrounding cortex (ROI2) in gp91ds-tat-treated CCI mice (p < 0.01 vs. TBI + scramble; Fig. 9C, 9E). These data confirm inhibition of NOX2 in microglia/macrophages after CCI with gp91ds-tat treatment.
FIG. 9.
Dinucleotide phosphate oxidase (NOX2) inhibition alters microglial/macrophage polarization after controlled cortical impact (CCI). (A) Experimental design for gp91ds-tat intervention study. (B) Image analysis was performed in two regions of interest (ROIs) that included peri-lesional cortex (ROI1) and the surrounding cortex distal to the lesion (ROI2). (C) Representative images of gp91phox and CD68 expression in ds-tat scrambled peptide control-treated and gp91ds-tat-treated CCI mice at 7 days post-injury. gp91phox+ (green) and CD68+ (red) cells in ROI1 and ROI2. Scale bar = 50 μm. (D) Representative images of CD16/32 and transforming growth factor (TGF) β expression in ds-tat scrambled peptide control-treated and gp91ds-tat-treated CCI mice at 7 days post-injury. CD16/32+ (magenta) and TGFβ+ (green) cells in the ipsilateral cortex. Scale bar = 50 μm. (E) Quantification of expression of gp91phox and CD68 in ROI1 and ROI2 of the ipsilateral cortex at 7 days post-injury. (F) Quantification of expression of CD16/32+, TGFβ+, and CD16/32+/TGFβ+ in the peri-lesional cortex at 7 days post-injury. Protein expression levels determined by binary area per ROI (mm2). n = 4 per time-point. Color image is available online at www.liebertpub.com/neu
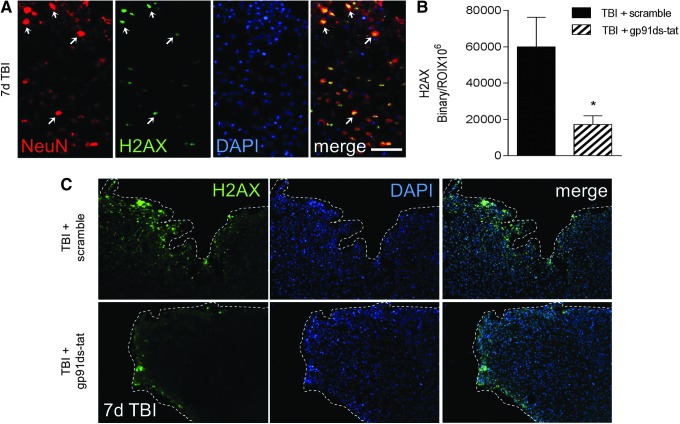
Next, we assessed microglial/macrophage polarization in each group using CD16/32 and TGFβ as markers of M1- and M2-like polarized cells, respectively. gp91ds-tat treatment significantly reduced CD16/32 expression (p < 0.01 vs. TBI + scramble; Fig. 9D, 9F) in the peri-lesional cortex. It had no effect on the Mtran phenotype (double-positive CD16/32+/TGFβ+ cells; Fig. 9F), but it significantly increased TGFβ expression (p < 0.05 vs. TBI + scramble) in the peri-lesional cortex (Fig. 8D, 8F), indicating that systemic treatment with gp91ds-tat alters microglial/macrophage polarization resulting in reduced M1-like and increased M2-like marker expression after CCI. Finally, to determine the consequence of altered microglial/macrophage polarization following NOX2 inhibition, we assessed H2AX expression in the peri-lesional cortex, which is a marker of oxidative stress-induced DNA damage.25 At 7 days post-injury, H2AX co-localizes with NeuN in the peri-lesional cortex, indicating that neurons are undergoing oxidative stress-induced DNA damage at this time-point (Fig. 10A). Notably, gp91ds-tat treatment significantly reduced H2AX expression (p < 0.05 vs. TBI + scramble; Fig. 10B, 10C) in the peri-lesional cortex at 7 days post-injury, indicating that NOX2 inhibition can reduce oxidative stress-induced neuronal damage following CCI.
FIG. 10.
Dinucleotide phosphate oxidase (NOX2) inhibition reduces H2AX expression after controlled cortical impact (CCI). (A) Representative images of H2AX expression in NeuN+ neurons in the peri-lesional cortex at 7 days post-injury. (B) Quantification of expression of H2AX+ cells in the peri-lesional cortex at 7 days post-injury. Protein expression levels determined by binary area per region of interest (ROI; mm2). n = 4 per time-point. (C) Representative images of H2AX expression in ds-tat scrambled peptide control-treated and gp91ds-tat-treated CCI mice at 7 days post-injury. H2AX+ (green) and 4′,6-diamidino-2-phenylindole (DAPI)+ (blue) cells in the ipsilateral cortex. Statistical analysis by Student's t-test (*p < 0.05 vs. TBI + scramble). Scale bar = 50 μm. Color image is available online at www.liebertpub.com/neu
Discussion
The goal of the present study was to analyze the temporal dynamics of microglia/macrophage polarization within the lesion micro-environment following moderate-level CCI, a well-established murine model of focal TBI. We performed a detailed phenotypic analysis of M1- and M2-like polarized microglia/macrophages and NOX2 expression up to 7 days post-injury using qPCR, flow cytometry and image analyses, and set these data within the context of ongoing neurodegeneration in the TBI cortex and hippocampus that is evident in this model.9,26 We found that microglia/macrophages express M1- and M2-like phenotypic markers early after TBI, but that the transient up-regulation of the M2-like phenotype is replaced by a predominant M1-like or Mtran (mixed) phenotype that expressed high levels of NOX2 at the site of injury at 7 days post-injury, and this was accompanied by ongoing cortical and hippocampal neurodegeneration. Further, when we inhibit NOX2 activity in microglia/macrophages after TBI, we can alter M1-/M2-like balance and repolarize microglia/macrophages towards an M2-like phenotype, and this is associated with reduced oxidative damage in neurons at 7 days post-injury.
To evaluate dynamic changes in M1-/M2-like polarization after TBI, we used mRNA and protein analysis of known M1- and M2-like phenotype makers; M1-like (IL-12, TNFα, IL-1β, IL-6, iNOS, CD16/32) and M2-like (CD206, Fizz1, IL-1rn, Arg1, Ym1, TGFβ, SOCS3, IL-4Rα).4,24 In general, there was excellent concordance between mRNA and protein expression levels for each M1- and M2-like polarization marker in our time course analyses, and the kinetics of mRNA expression for selected M1- and M2-like markers were consistent with recent published reports.18,27 For the mRNA analysis, we used cortical tissue samples from sham and CCI mice, and it is possible that RNA transcripts for M1-/M2-like markers could be made by other cells such as neurons, astrocytes, or oligodendrocytes within the contused cortex. To demonstrate the selective up-regulation of M1-/M2-like markers in microglia/macrophage, we isolated these cells from the sham-injured and TBI brain using a CD11b-positive selection method and analyzed selected M1-/M2-like proteins by flow cytometry.
Our analysis revealed that the M1-like proteins iNOS and IL-12 were robustly expressed after TBI, and despite no change in the total number of iNOS+ and IL-12+ cells after TBI, there was a significant increase in number of iNOShigh and IL-12high cells at 24–72 h post-injury. For the M2-like phenotype, the peak expression and number of TGFβhigh+ cells occurred at 24 h post-injury, while peak expression and number of CD206high+ cells occurred at 72 h, and peak expression and number of arginase 1high+ cells occurred at 7 days post-injury. In situ analysis of co-localized M1-like (CD16/32, iNOS) and M2-like (TGFβ, arginase 1) markers in peri-lesional cortex and at distant sites demonstrated that while M2-like markers were expressed at 24 h post-injury, there was a shift towards a predominant M1-like or Mtran phenotype at 7 days post-injury, with relatively few single-positive TGFβ or arginase 1 cells observed at distant sites from the lesion at this time-point. Combined, these data reveal a complex and dynamic pattern of microglia/macrophage activation following TBI.
Broadly, our mRNA analysis indicates activation of M1-like (IL-1β, IL-12, TNFα, iNOS) and M2a-like (CD206, Fizz1, Arg1, Ym1) phenotypic markers early within 24 h of TBI, followed by delayed activation of M2c-like markers (SOCS3, IL-4Rα) that peak at 72 h post-injury. However, outliers in this polarization model include robust early increases of the M2c-like marker, TGFβ, that drop off significantly within 48–72 h of TBI, and sustained expression of the M2a-like marker, arginase 1, that last through 7 days post-injury. Flow cytometry analysis of isolated microglia/macrophages from the TBI brain support the molecular activation patterns, and image analysis demonstrate that up to 42% of arginase 1-positive cells co-express the M1-like marker iNOS, indicative of a significant mixed population of microglia/macrophages (Mtran) at 7 days post-injury.
In an effort to gain insight into the functional impact of this polarization pattern, it is noticeable that there are similarities to M1-/M2-like dynamic responses of wound-healing macrophages outside the CNS, where a pro-inflammatory response (M1-like) is robustly increased after injury, followed by macrophages that promote wound-healing and anti-inflammatory responses (M2a-like), and finally a immunosuppressive and remodeling responses (M2c-like).28 However, in our TBI model, the anti-inflammatory and immunosuppressive M2-like phenotypes decrease over time while pathological M1-like phenotypes (iNOS+ or CD16/32+) or Mtran phenotypes remain elevated; this polarization profile is similar to macrophage activation patterns following contusion SCI.17,29 Thus, our study, and others17,18,29 indicate the activation of a dysfunctional wound healing response after TBI or SCI, and development of a persistent inflammatory response that contributes to neurodegeneration and long-term neurological impairments.
In our flow cytometry analysis, we were able to discriminate between infiltrating macrophages and resident microglia based on CD45 expression22 (macrophages = CD11b+/CD45high; microglia = CD11b+/CD45low), and there was a rapid and robust infiltration of peripheral macrophages within 24 h, with peak infiltration of CD11b+/CD45high cells occurring at 72 h post-injury. These data support other studies that evaluated macrophage and immune cell infiltration dynamics in TBI models.30,31 Due to technical limitations when combining internalization staining protocols for M1-/M2-like markers with CD11b/CD45 cell surface markers, it was not possible to determine whether the infiltrating macrophages after were more M1- or M2-like polarized, but the peak in infiltration corresponded with high expression levels of both M1- and M2-like protein markers in this study.
In a recent study Hsieh and colleagues used a reporter mouse model for M2-polarized macrophages (eYFP arginase 1) to investigate the role of infiltrating brain macrophage subsets after CCI, and demonstrated robust infiltration of arginase 1 (Arg1+)-positive macrophages that differentiated into at least two distinct subpopulations with unique chemokine expression patterns.30 Expression profiling of Arg1+ and Arg1- macrophage subpopulations that infiltrated after TBI revealed that they do not separate into true M1- or M2-like transcriptional profiles, but rather adopt mixed molecular phenotypes, and the ratio of macrophage subsets change over time after TBI.30 Interestingly, blockade of macrophage infiltration by inhibiting CCR2 either by genetic or pharmacological means improves outcomes after TBI. Compared with wild-type mice, CCR2 knockout mice subjected to CCI have reduced lesion volumes, decreased pro-inflammatory gene expression, decreased macrophage infiltration in the injured cortex and improved cognitive function recovery after TBI.32,33
Morganti and colleagues recently evaluated a CCR2 antagonist (CCX872) in CX3CR1GFP/+CCR2RFP/+ reporter mice using the CCI model, and demonstrated that CCX872 pre-treatment resulted in reduced macrophage infiltration acutely after TBI.34 CCR2 antagonism also improved cognitive function recovery after TBI, as demonstrated by increased performance of CCX872-treated mice in a radial arm water maze test at 28 days post-injury.34 They also showed that CCR2-positive infiltrating macrophages in the brain expressed both M1- and M2-like markers acutely after TBI, with sequential activation of M1-, followed by M2a-, and finally M2c- marker expression.34 However, the accumulation of CCR2-positive macrophages into the TBI brain ultimately resulted in up-regulation of pro-inflammatory M1-like cells with neurotoxic potential, and CCR2 antagonism robustly reduced this pro-inflammatory profile and ameliorated the long-term cognitive dysfunction induced by TBI.34 These studies highlight a significant contribution of infiltrating macrophage to polarization after TBI, and further research is needed to understand mechanisms driving the trafficking of macrophages subsets to the TBI brain, how they interact with and modulate resident glial cells (microglia and astrocytes), and their role in chronic neurodegeneration following TBI.
In this study, we also assessed NOX2 expression in M1-/M2-like polarized cells after TBI. NOX2 is a common and essential mechanism of microglial-mediated neurotoxicity in neurodegenerative disease.35 Activation of NOX2 in microglia is neurotoxic, both through the production of extracellular ROS that damage neighboring neurons36 and through initiation of redox signaling in microglia that amplifies the pro-inflammatory response.37,38 NOX2 activation contributes to oxidative stress and neuroinflammation after ischemic brain injury39,40 and TBI,9,23,41,42 and inhibition of this enzyme, either directly or indirectly, is highly neuroprotective in TBI models.23,41–45 Redox signaling is an essential component of M1-like polarization of macrophages,16 and reports also have confirmed ROS regulation of microglial M2-like activation.46 Notably, attenuation of microglial ROS through genetic or pharmacological inhibition of NOX2 increases microglial M2-like phenotype markers in response to LPS.47
Recently, we demonstrated an inverse relationship between increased NOX2 expression in a highly reactive tissue micro-environment and dysregulated/suppressed M2-like gene expression in the aged TBI brain.24 Further, NOX2 also is chronically expressed in reactive microglia in the peri-lesional cortex at 12 months post-injury when markers of M2-like polarized microglia are absent.9 Here, our analysis of NOX2 expression (gp91phox-positive staining) in the acute phase after TBI confirmed previous reports of transient NOX2 expression in neurons in the peri-lesional cortex at 24 h post-injury.23 This was followed by a robust increase of microglial NOX2 expression in the peri-lesional cortex at 7 days post-injury; the latter cells displayed morphological and phenotypic features of reactive microglia (high CD68 expression and amoeboid shape). NOX2 was highly expressed in M1-like (CD16/32- or iNOS-positive) cells or Mtran (CD16/32/TGFβ- or iNOS/arginase 1-positive) cells at 7 days post-injury, whereas it did not co-localize with single-positive M2-like cells at distant sites from the lesion. Therefore, in our model, NOX2 expression is associated with the M1-like and Mtran phenotypes. This profile is similar to NOX2 expression in neurotoxic M1-like microglia during end-state disease in an amyotrophic lateral sclerosis mouse model.46
In order to interrogate the relationship between NOX2 and M1-like and Mtran phenotypes, we performed an intervention study using gp91ds-tat to inhibit NOX2 activity after CCI. We delivered the selective NOX2 inhibitor, gp91ds-tat,19 or control scrambled peptides by intraperitoneal injection to CCI mice starting at 24 h post-injury, with repeat dosing at 48 and 72 h post-injury in order to inhibit NOX2 as resident microglia become activated and peripheral macrophages infiltrate the TBI brain. The gp91ds-tat treatment significantly reduced NOX2 expression in microglia/macrophages after TBI and also significantly reduced the expression of CD16/32 (M1-like marker) at 7 days post-injury. NOX2 inhibition had no effect on the Mtran phenotype (CD16/32+/TGFβ+), but it repolarized microglia/macrophages towards an M2-like phenotype, as demonstrated by significant increased expression TGFβ (M2-like marker). In addition, we demonstrated that gp91ds-tat treatment significantly reduced H2AX protein expression in damaged cortex, a markers of oxidative stress-induced DNA damage,25 at 7 days post-injury. Taken together, these data demonstrate that a delayed systemic intervention starting at 24 h post-injury that targets NOX2 activity in microglia/macrophages can alter M1-/M2-like balance in favor of the anti-inflammatory and neurorestorative M2-like phenotype, and this results in reduced oxidative damage in neurons at 7 days post-injury. These data are consistent with studies in mouse models of LPS- and amyloid-β (Aβ) -mediated neuroinflammation in which NOX2 inhibition results in increased microglial M2-like marker expression,47 and suggests that NOX2 acts as a balance switch between M1- and M2-like phenotypes after TBI, serving to activate the former and suppress the latter.
An intriguing finding in our study was the development of the Mtran phenotype (CD16/32+/TGFβ and iNOS+/arginase 1+ populations) at 7 days post-injury. Further detailed investigation into the functional role of Mtran phenotypes after TBI is warranted, but the co-expression of NOX2 in these cells suggests a potential role in microglial-mediated neurotoxicity. In SCI models, mixed M1- and M2-like phenotypes have been described,48 and mixed CD16/32+/arginase 1+ expressing macrophages accumulate intracellular iron and drive TNF-dependent M1-like polarization that is detrimental to recovery.49 In addition, there is a transition from an M1-like to a mixed M2a- and M2c-like neuroinflammatory phenotype that is associated with increased burden in Alzheimer's disease (AD) mouse models (APP/PS1 transgenic mice).50 In humans, patients with early AD are polarized towards an M1 or an M2a-like phenotype, but by end-stage AD, patients have combined M1-, M2a, and M2c- phenotypes,51 suggesting that a transition to a mixed phenotype is associated with disease progression and increased amyloid burden. However, a neuroprotective arginase 1+/IL-1β+ mixed population also has been reported; these cells promote neurite outgrowth ex vivo, and enhance recruitment of peripheral IL-4Rα+ myeloid cells to the injured tissue to facilitate repair and functional recovery after SCI.52 Thus, an important area of future research is to investigate the effects of Mtran phenotype after TBI to determine whether these populations provide neuroprotection and promote neurorestoration, or contribute to ongoing neurodegeneration following TBI.
In summary, we performed a detailed phenotypic analysis of M1- and M2-like polarized microglia/macrophages during the acute phase after CCI, and demonstrated that both phenotypes are activated early after TBI, but that the M2-like phenotype is replaced by M1-like and Mtran phenotypes that express high levels of NOX2 at 7 days post-injury. This shift towards M1-like and Mtran phenotypes is associated with increased neurodegeneration, and when we inhibit NOX2 activity in microglia/macrophages, we alter M1-/M2-like balance in favor of the anti-inflammatory M2-like phenotype, and significantly reduce oxidative damage in the injured cortex. These studies suggest that despite activation of both M1-like and M2-like microglia/macrophages after TBI, early M2-like responses becomes dysfunctional over time, resulting in the development of pathological M1-like and Mtran phenotypes that are driven by NOX2.
Acknowledgments
We thank Titilola Akintola for expert technical assistance and Kari Ann Shirey, PhD, for helpful discussion regarding flow cytometry protocol optimization. This work was supported by National Institutes of Health grant R01NS082308 (D.J. Loane), the National Institute on Aging Claude D. Pepper Older Americans Independence Center P30-AG028747 (D.J. Loane), and a Consejo Nacional de Ciencia y Tecnología Scholarship 249772/389071 (DM Alvarez-Croda).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gurtner G.C., Werner S., Barrandon Y., and Longaker M.T. (2008). Wound repair and regeneration. Nature 453, 314–321 [DOI] [PubMed] [Google Scholar]
- 2.Novak M.L. and Koh T.J. (2013). Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 183, 1352–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., Samokhvalov I.M., and Merad M. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colton C.A. (2009). Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 4, 399–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherry J.D., Olschowka J.A., and O'Banion M.K. (2014). Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation 11, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acosta S.A., Tajiri N., Shinozuka K., Ishikawa H., Grimmig B., Diamond D., Sanberg P.R., Bickford P.C., Kaneko Y., and Borlongan C.V. (2013). Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS One 8, e53376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aungst S.L., Kabadi S.V., Thompson S.M., Stoica B.A., and Faden A.I. (2014). Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J. Cereb. Blood Flow Metab. 34, 1223–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., and Stewart W. (2013). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loane D.J., Kumar A., Stoica B.A., Cabatbat R., and Faden A.I. (2014). Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 73, 14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouzon B.C., Bachmeier C., Ferro A., Ojo J.O., Crynen G., Acker C.M., Davies P., Mullan M., Stewart W., and Crawford F. (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 75, 241–254 [DOI] [PubMed] [Google Scholar]
- 11.Nagamoto-Combs K., McNeal D.W., Morecraft R.J., and Combs C.K. (2007). Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J. Neurotrauma 24, 1719–1742 [DOI] [PubMed] [Google Scholar]
- 12.Nonaka M., Chen X.H., Pierce J.E., Leoni M.J., McIntosh T.K., Wolf J.A., and Smith D.H. (1999). Prolonged activation of NF-kappaB following traumatic brain injury in rats. J. Neurotrauma 16, 1023–1034 [DOI] [PubMed] [Google Scholar]
- 13.Ramlackhansingh A.F., Brooks D.J., Greenwood R.J., Bose S.K., Turkheimer F.E., Kinnunen K.M., Gentleman S., Heckemann R.A., Gunanayagam K., Gelosa G., and Sharp D.J. (2011). Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 70, 374–383 [DOI] [PubMed] [Google Scholar]
- 14.Smith C., Gentleman S.M., Leclercq P.D., Murray L.S., Griffin W.S., Graham D.I., and Nicoll J.A. (2013). The neuroinflammatory response in humans after traumatic brain injury. Neuropathol. Appl. Neurobiol. 39, 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall E.D., Wang J.A., and Miller D.M. (2012). Relationship of nitric oxide synthase induction to peroxynitrite-mediated oxidative damage during the first week after experimental traumatic brain injury. Exp. Neurol. 238, 176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brune B., Dehne N., Grossmann N., Jung M., Namgaladze D., Schmid T., von Knethen A., and Weigert A. (2013). Redox control of inflammation in macrophages. Antioxid. Redox Signal. 19, 595–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., and Popovich P.G. (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G., Zhang J., Hu X., Zhang L., Mao L., Jiang X., Liou A.K., Leak R.K., Gao Y., and Chen J. (2013). Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J. Cereb. Blood Flow Metab. 33, 1864–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rey F.E., Cifuentes M.E., Kiarash A., Quinn M.T., and Pagano P.J. (2001). Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ. Res. 89, 408–414 [DOI] [PubMed] [Google Scholar]
- 20.Loane D.J., Pocivavsek A., Moussa C.E., Thompson R., Matsuoka Y., Faden A.I., Rebeck G.W., and Burns M.P. (2009). Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat. Med. 15, 377–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abais J.M., Zhang C., Xia M., Liu Q., Gehr T.W., Boini K.M., and Li P.L. (2013). NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid. Redox Signal. 18, 1537–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stirling D.P. and Yong V.W. (2008). Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. J. Neurosci. Res. 86, 1944–1958 [DOI] [PubMed] [Google Scholar]
- 23.Dohi K., Ohtaki H., Nakamachi T., Yofu S., Satoh K., Miyamoto K., Song D., Tsunawaki S., Shioda S., and Aruga T. (2010). Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J. Neuroinflammation 7, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A., Stoica B.A., Sabirzhanov B., Burns M.P., Faden A.I., and Loane D.J. (2013). Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging 34, 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva A.R., Santos A.C., Farfel J.M., Grinberg L.T., Ferretti R.E., Campos A.H., Cunha I.W., Begnami M.D., Rocha R.M., Carraro D.M., de Braganca Pereira C.A., Jacob-Filho W., and Brentani H. (2014). Repair of oxidative DNA damage, cell-cycle regulation and neuronal death may influence the clinical manifestation of Alzheimer's disease. PLoS One 9, e99897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabadi S.V., Stoica B.A., Byrnes K.R., Hanscom M., Loane D.J., and Faden A.I. (2012). Selective CDK inhibitor limits neuroinflammation and progressive neurodegeneration after brain trauma. J. Cereb. Blood Flow Metab. 32, 137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turtzo L.C., Lescher J., Janes L., Dean D.D., Budde M.D., and Frank J.A. (2014). Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J. Neuroinflammation 11, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deonarine K., Panelli M.C., Stashower M.E., Jin P., Smith K., Slade H.B., Norwood C., Wang E., Marincola F.M., and Stroncek D.F. (2007). Gene expression profiling of cutaneous wound healing. J. Transl. Med. 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gensel J.C. and Zhang B. (2015). Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619, 1–11 [DOI] [PubMed] [Google Scholar]
- 30.Hsieh C.L., Kim C.C., Ryba B.E., Niemi E.C., Bando J.K., Locksley R.M., Liu J., Nakamura M.C., and Seaman W.E. (2013). Traumatic brain injury induces macrophage subsets in the brain. Eur. J. Immunol. 43, 2010–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin X., Ishii H., Bai Z., Itokazu T., and Yamashita T. (2012). Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS One 7, e41892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh C.L., Niemi E.C., Wang S.H., Lee C.C., Bingham D., Zhang J., Cozen M.L., Charo I., Huang E.J., Liu J., and Nakamura M.C. (2014). CCRZ deficiency impairs macrophage infiltration and improves cognitive function after traumatic brain injury. J. Neurotrauma. 31, 1677–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Israelsson C., Kylberg A., Bengtsson H., Hillered L., Ebendal T. (2014). Interacting chemokine signals regulate dendritic cells in acute brain injury. PLoS One 9, e104754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morganti J.M., Jopson T.D., Liu S., Riparip L.K., Guandique C.K., Gupta N., Ferguson A.R., and Rosi S. (2015). CCR2 Antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J. Neurosci. 35, 748–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lull M.E. and Block M.L. (2010). Microglial activation and chronic neurodegeneration. Neurotherapeutics 7, 354–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin L., Liu Y., Wang T., Wei S.J., Block M.L., Wilson B., Liu B., and Hong J.S. (2004). NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J. Biol. Chem. 279, 1415–1421 [DOI] [PubMed] [Google Scholar]
- 37.Mander P. and Brown G.C. (2005). Activation of microglial NADPH oxidase is synergistic with glial iNOS expression in inducing neuronal death: a dual-key mechanism of inflammatory neurodegeneration. J. Neuroinflammation 2, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pawate S., Shen Q., Fan F., and Bhat N.R. (2004). Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J. Neurosci. Res. 77, 540–551 [DOI] [PubMed] [Google Scholar]
- 39.Chen H., Kim G.S., Okami N., Narasimhan P., and Chan P.H. (2011). NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol. Dis. 42, 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H., Song Y.S., and Chan P.H. (2009). Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow Metab. 29, 1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrnes K.R., Loane D.J., Stoica B.A., Zhang J., and Faden A.I. (2012). Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J. Neuroinflammation 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q.G., Laird M.D., Han D., Nguyen K., Scott E., Dong Y., Dhandapani K.M., and Brann D.W. (2012). Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One 7, e34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi B.Y., Jang B.G., Kim J.H., Lee B.E., Sohn M., Song H.K., and Suh S.W. (2012). Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res. 1481, 49–58 [DOI] [PubMed] [Google Scholar]
- 44.Ferreira A.P., Rodrigues F.S., Della-Pace I.D., Mota B.C., Oliveira S.M., Velho Gewehr C.D., Bobinski F., de Oliveira C.V., Brum J.S., Oliveira M.S., Furian A.F., de Barros C.S., Ferreira J., Santos A.R., Fighera M.R., and Royes L.F. (2013). The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: Role of inflammatory and oxidative brain damage. Neurochem. Int. 63, 583–593 [DOI] [PubMed] [Google Scholar]
- 45.Loane D.J., Stoica B.A., Byrnes K.R., Jeong W., and Faden A.I. (2013). Activation of mGluR5 and inhibition of NADPH oxidase improves functional recovery after traumatic brain injury. J. Neurotrauma 30, 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao B., Zhao W., Beers D.R., Henkel J.S., and Appel S.H. (2012). Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp. Neurol. 237, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi S.H., Aid S., Kim H.W., Jackson S.H., and Bosetti F. (2012). Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. J. Neurochem. 120, 292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David S. and Kroner A. (2011). Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. 12, 388–399 [DOI] [PubMed] [Google Scholar]
- 49.Kroner A., Greenhalgh A.D., Zarruk J.G., Passos Dos Santos R., Gaestel M., and David S. (2014). TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83, 1098–1116 [DOI] [PubMed] [Google Scholar]
- 50.Weekman E.M., Sudduth T.L., Abner E.L., Popa G.J., Mendenhall M.D., Brothers H.M., Braun K., Greenstein A., and Wilcock D.M. (2014). Transition from an M1 to a mixed neuroinflammatory phenotype increases amyloid deposition in APP/PS1 transgenic mice. J. Neuroinflammation 11, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sudduth T.L., Schmitt F.A., Nelson P.T., and Wilcock D.M. (2013). Neuroinflammatory phenotype in early Alzheimer's disease. Neurobiol. Aging 34, 1051–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fenn A.M., Hall J.C., Gensel J.C., Popovich P.G., and Godbout J.P. (2014). IL-4 signaling drives a unique arginase+/IL-1beta+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Ralpha after traumatic spinal cord injury. J. Neurosci. 34, 8904–8917 [DOI] [PMC free article] [PubMed] [Google Scholar]