Abstract
Background
Despite considerable effort, the neurobiological underpinnings of hyper-responsive threat processing specific to patients suffering from generalized anxiety disorder (GAD) remain poorly understood. The current functional magnetic resonance imaging (fMRI) study aims to delineate GAD-specific brain activity during immediate threat processing by comparing GAD patients to healthy controls (HC), to social anxiety disorder (SAD) and to panic disorder (PD) patients.
Method
Brain activation and functional connectivity patterns to threat vs. neutral pictures were investigated using event-related fMRI. The sample consisted of 21 GAD, 21 PD, 21 SAD and 21 HC.
Results
GAD-specific elevated activity to threat vs. neutral pictures was found in cingulate cortex, dorsal anterior insula/frontal operculum (daI/FO) and posterior dorsolateral prefrontal cortex (dlPFC). Defining these effects as seed regions, we detected GAD-specific increased functional connectivity to threat vs. neutral pictures between posterior dlPFC and ventrolateral prefrontal cortex, between cingulate cortex and amygdala, between cingulate cortex and anterior insula, as well as decreased functional connectivity between daI/FO and mid-dlPFC.
Conclusion
The findings present the first evidence for GAD-specific neural correlates of hyper-responsive threat processing, possibly reflecting exaggerated threat sensitivity, maladaptive appraisal and attention-allocation processes.
Keywords: Cingulate cortex, Prefrontal cortex, Anterior insula, Threat processing, RDoC
Highlights
-
•
Threat processing investigation across multiple anxiety disorders
-
•
First neural evidence of GAD-specific threat-related alterations
-
•
GAD-specific alterations primarily located in prefrontal cortex
-
•
Alterations are suggestive of exaggerated threat sensitivity
1. Introduction
Generalized anxiety disorder (GAD) is characterized by excessive and uncontrollable worry across a variety of domains (American Psychiatric Association, 2000, Duval et al., 2015). GAD is associated with increased health care costs (Lieb et al., 2005), often comorbid with other anxiety and mood disorders (Newman et al., 2013) and remains to have the lowest rate of remission after treatment in comparison to other anxiety disorders (Kinney et al., 2016). According to recent reviews and established cognitive models, GAD patients are suggested to be hypervigilant to threat: they are biased towards worry-related information in their environment, struggle with disengaging from that information, and tend to appraise information as threatening under ambiguous conditions (Behar et al., 2009, Hayes and Hirsch, 2007, Mennin et al., 2009, Newman et al., 2013). Favoring the processing of threat serves among other factors to maintain high levels of anxiety and initiates as well as maintains pathological worrying in GAD patients (Behar et al., 2009, Newman et al., 2013). Consequently, hyper-responsive threat processing seems to play a key role in the etiology and maintenance of GAD pathophysiology (Hayes and Hirsch, 2007). Gaining a comprehensive understanding of threat processing in GAD is of utmost importance for conceptualization and treatment development of the disorder.
Despite considerable effort, underlying neural circuits of immediate threat processing in GAD have remained elusive. Previous functional magnetic resonance imaging (fMRI) studies have primarily compared GAD patients to healthy controls (HC), yielding no consistent pattern of immediate threat-related blood oxygen level-dependent (BOLD) responses in GAD. Depending on the paradigm, GAD patients responded with increased, decreased or no differentiating activity in areas such as amygdala or prefrontal cortex (PFC) (Ball et al., 2013, Blair et al., 2012, Blair et al., 2008, Etkin et al., 2010, Etkin and Schatzberg, 2011, Fonzo et al., 2015, Fonzo et al., 2014, Hölzel et al., 2013, Moon and Jeong, 2015, Nitschke et al., 2009, Palm et al., 2011, Price et al., 2011, Whalen et al., 2008). To elucidate GAD-specific threat-related BOLD response and to identify shared neural circuits underlying threat processing in anxiety disorders in general, it may be promising to follow the line of the Research Domain Criteria project (RDoC) (Kozak and Cuthbert, 2016). RDoC provides a framework to study functions and deficits across a spectrum of psychopathology and across the health-illness dimension. To date, few fMRI studies investigating brain activity have followed this approach by comparing GAD patients not only to HC but also to other anxiety disorder patients (Ball et al., 2013, Blair et al., 2012, Blair et al., 2008, Fonzo et al., 2015). Two studies used affective facial stimuli and compared brain responses in GAD patients to HC and additionally to patients with social anxiety disorder (SAD) and/or panic disorder (PD) (Blair et al., 2008, Fonzo et al., 2015). One study detected a GAD-specific reduced amygdala activity in response to fearful facial stimuli comparing GAD patients relative to SAD patients and to HC (Blair et al., 2008). Yet, in another study no GAD-specific brain activity pattern was detected in response to facial stimuli relative to HC, PD and SAD patients (Fonzo et al., 2015). It appears that the relevance of affective facial stimuli might be limited to GAD patients and not sufficient to induce disorder-specific threat processing. Other studies used affective scene pictures presented in an explicit or implicit emotion-regulation context and compared GAD patients either to PD or SAD patients, and to HC (Ball et al., 2013, Blair et al., 2012). GAD patients displayed reduced activity of parietal/occipital cortex and subcortical structures to threat compared to PD patients or HC (Ball et al., 2013), while no differences emerged between GAD and SAD patients (Blair et al., 2012). Taken together, clear evidence of GAD-specific threat-related brain activity is lacking, at least under instructed and/or demanding picture presentation conditions (Ball et al., 2013, Blair et al., 2012).
Similarly, clear evidence of GAD-specific functional connectivity (FC) patterns in response to threat relative to HC and other psychiatric patients is lacking (Andreescu et al., 2015, Chen and Etkin, 2013, Etkin et al., 2010, Etkin and Schatzberg, 2011, Makovac et al., 2015): Worry-induction studies comparing GAD patients to HC showed altered FC in amygdala-PFC circuitry associated with monitoring the salience of interoceptive and external events (salience network [SN]) and with emotion regulation (executive control network [ECN]) (Andreescu et al., 2015, Makovac et al., 2015). Another study detected a negative correlation between activations in amygdala and anterior cingulate cortex (ACC) in GAD patients relative to HC in response to affective facial stimuli in an emotional conflict task (Etkin et al., 2010). Following that, aberrant engagement of the amygdala-PFC circuitry has been suggested as a key factor underlying GAD pathophysiology (Makovac et al., 2015). Yet, when comparing GAD patients not only to HC, but additionally to patients with major depressive disorder (MDD) or post-traumatic stress disorder (PTSD) patients, using the same emotional conflict task or a similar attention-demanding task, no GAD-specific FC was detected (Chen and Etkin, 2013, Etkin and Schatzberg, 2011). This highlights the importance of investigating FC across disorders to reveal specific and unspecific alterations in GAD. Overall, current findings render an investigation of threat-related brain activity and FC in GAD relative to SAD and PD patients necessary.
The aim of the present fMRI study was to identify neural circuits underlying immediate threat processing in GAD patients relative to HC and other anxiety disorder patients. In the present study brain responses of GAD patients were compared to SAD and PD patients, because the disorders are highly prevalent, often comorbid and are marked by widely generalized fear/anxiety (Fonzo et al., 2015). Threat-related brain activity was investigated by presenting threatening and neutral pictures in an event-related design. Threat relative to neutral pictures was expected to engage an emotion-processing network in all groups comprising amygdala, insula, prefrontal and occipital cortex, and thalamus. Due to the inconsistent picture regarding immediate threat processing in GAD (Ball et al., 2013, Blair et al., 2012, Blair et al., 2008, Etkin et al., 2010, Etkin and Schatzberg, 2011, Fonzo et al., 2015, Fonzo et al., 2014, Hölzel et al., 2013, Moon and Jeong, 2015, Nitschke et al., 2009, Palm et al., 2011, Price et al., 2011, Whalen et al., 2008), our hypotheses were based on worry induction studies. These studies revealed a rather consistent finding of elevated activity in medial PFC (mPFC) and ACC, suggesting both areas to play cardinal roles in GAD pathophysiology (Andreescu et al., 2011, Paulesu et al., 2010). Following the suggested important role of ACC/mPFC in GAD pathophysiology, GAD patients were expected to show altered ACC/mPFC activity to threat (Etkin et al., 2011, Paulesu et al., 2010). If observing GAD-specific threat-related brain activations, we were interested in whether these brain regions additionally showed GAD-specific FC to other areas. We expected positive correlations within SN, reflecting exaggerated threat processing, and reduced FC within ECN, and between SN and ECN, reflecting deficient emotion regulation (Mennin et al., 2009).
2. Methods and materials
2.1. Subjects
Sixty-three patients (GAD: n = 21, PD: n = 21, SAD: n = 21) and 21 HC were recruited through public advertisements and an outpatient clinic. Data of one GAD patient was excluded from analysis due to misunderstanding of task instructions. The final sample consisted of 20 GAD, 21 PD, 21 SAD patients and 21 HC matched for age, education and gender (see Supplement Table S3).
An experienced clinical psychologist diagnosed participants by means of the Structured Clinical Interview for DSM-IV axis-I Disorders (Wittchen et al., 1997). Patients met the criteria for the corresponding anxiety disorder as primary diagnosis and were excluded if they had a comorbid anxiety disorder from one of the other two disorder groups. Patients presented with the following axis I comorbidities: specific phobia (GAD: n = 1, PD: n = 1, SAD: n = 4), major depressive disorder (recurrent) (GAD: n = 1, PD: n = 3, SAD: n = 5), dysthymic disorder (PD: n = 1), obsessive-compulsive disorder (PD: n = 1, SAD: n = 2), eating disorder (GAD: n = 1, SAD: n = 1), posttraumatic stress disorder (GAD: n = 1), somatization disorder (PD: n = 1). HC were free of any current or past axis-I disorder. Disorder-related questionnaires given to the corresponding disorder group supported the diagnosis (GAD: Penn State Worry Questionnaire (Meyer et al., 1990) (M = 65.95, SD = 8.46); PD: Panic and Agoraphobia Scale (Bandelow, 1997) (M = 21.90, SD = 6.83); SAD: Liebowitz Social Anxiety Scale (Fresco et al., 2001) (M = 70.81, SD = 17.57)). Patient groups did not differ with regard to Beck Depression Inventory-II scores (BDI) (Beck et al., 1996) or Anxiety Sensitivity Index (ASI ) (Reiss et al., 1986) (see Supplement Table S3).
Six to seven patients per group took long-term medication (antidepressive medication, one GAD patient used Pregabalin) (see Supplement Table S3), and had been stabilized on such medication for at least four weeks prior to study participation. All subjects had normal or corrected-to-normal vision and gave written informed consent. Exclusion criteria were neurological disorders, presence or history of psychotic or bipolar disorder, current drug abuse or dependence, or fMRI contraindications. The study has been approved by the ethics committee of the University of Muenster and is in compliance with the latest declaration of Helsinki.
2.2. Stimuli
Fifty threat and 50 neutral pictures were chosen from the International Affective Picture System (Lang and Bradley, 2007) (threat pictures: n = 48, neutral pictures: n = 14) and the Emotional Picture Set (Wessa et al., 2010) (threat pictures: n = 2, neutral pictures: n = 36). Threat pictures showed for example motor vehicle accidents, violence, threatening animals or injuries. Neutral pictures showed for example animals or objects. Picture sets were matched for color scheme, luminance and complexity (see Supplement Table S4).
2.3. Experimental design
Scanning took 8 min and 32 s. Pictures were presented in pseudo-randomized order controlled by Presentation Software (v17.2, Neurobehavioral Systems, Albany, California, USA). Each picture was presented for 800 ms, followed by a fixation cross (with a jittered duration of 1280–18,960 ms, M = 3,890 ms; determined using the optseq algorithm [http://www.surfer.nmr.mgh.harvard.edu/optseq/]). To ensure sufficient attention to the pictures, participants were instructed to press a button in response to blurred pictures, which were presented five times during the run. After scanning, participants rated the stimuli on 9-point Likert scales for arousal (1 = not arousing at all, 9 = highly arousing), valence (1 = very negative, 5 = neutral, 9 = very positive), and anxiety induction (1 = not anxiety-inducing, 9 = highly anxiety-inducing). Participants received standardized instructions as well as training outside and inside the scanner. Training blocks consisted of seven trials comprising five positive and two blurred pictures.
2.4. Analysis of sociodemographic data, clinical questionnaire and rating data
Sociodemographic data, clinical questionnaires, and rating data were analyzed using IBM SPSS software (v22, Armonk, New York, USA). Rating data for anxiety, valence and arousal were subjected to separate 2 (picture valence: threat picture, neutral picture) by 4 (group: GAD, PD, SAD, HC) mixed model analysis of variance (ANOVA). A probability level of p ≤ 0.05 was considered statistically significant. Post-hoc pairwise Bonferroni-corrected comparisons resolved the main effects for group, and Bonferroni-corrected t-tests resolved interaction effects (corrected significance level p ≤ 0.008).
2.5. fMRI acquisition and analysis
Anatomical and functional data were collected with a 3 Tesla magnetic resonance scanner (“Magnetom PRISMA”, Siemens, Erlangen, Germany) using a 20 channel head-neck coil. After acquiring a high-resolution T1-weighted anatomical scan with 192 slices, functional data were recorded with a T2-weighted echo-planar sequence (TE = 30 ms, flip angle = 90°, matrix = 92 × 92 voxels, FOV = 208 mm2, TR = 2080 ms). 255 volumes consisting of 36 axial slices (thickness = 3 mm, 0.3 mm gap, in plane resolution = 2.26 mm × 2.26 mm) were acquired.
FMRI data were preprocessed and analyzed using BrainVoyager QX (BVQX, Version 2.8, Brain Innovation, Maastricht, Netherlands). To ensure steady-state tissue magnetization, the first 10 volumes were discarded. Data were corrected for slice time errors and controlled for excessive head movement artifacts (> 3 mm in any direction). Anatomical and functional data were co-registered and normalized to Talairach space (Talairach and Tournoux, 1988). Subsequently, data were smoothed spatially (6 mm full-width half maximum [FWHM] Gaussian kernel) and temporally (high pass filter: 10 cycles per run; low pass filter: 2.8 s; linear trend removal). Volumes were resampled to 2 × 2 × 2 mm voxel size.
Statistical analysis comprised a multistage approach: A canonical double-gamma hemodynamic response function (HRF) modeled the expected BOLD signal for each predictor. Predictors of interest were threat and neutral pictures, while blurred pictures were defined as predictors of no interest. First, predictor estimates based on z-standardized time course data were calculated, with adjustment for autocorrelation following a global AR(1) model. Second, a random-effects general linear model was computed and subjected to a 2 (picture valence: threat pictures, neutral pictures) by 4 (group: GAD, PD, SAD, HC) regions of interests (ROI, see below) ANOVA. In the next step, interactions were resolved by planned t-tests (see below). Disorder-specific brain activity to threat was defined as differential brain activity to threat vs neutral pictures relative to each other group.
In case of disorder-specific brain activity to threat vs. neutral pictures, we were also interested in disorder-specific FC patterns. As such, GAD-specific brain activity clusters derived from interaction effects of the 2 × 4 ROI ANOVA (see above) were used as seed regions in psychophysiological interaction (PPI) analyses. PPI analyses were conducted per seed region within ROI (see below) (Friston et al., 1997). An interaction regressor, which was the product of the HRF-convolved task regressor (psychological factor) and the seed region time course (physiological factor), was subjected to a one-way ANOVA with group (GAD, PD, SAD, HC) as between-subjects factor. We only report clusters for which planned comparisons revealed GAD-specific FC alterations (as compared to the other groups).
Analyses were performed for a priori defined ROIs. ROIs were based on the current literature on GAD pathophysiology (Ball et al., 2013, Blair et al., 2012, Blair et al., 2008, Etkin et al., 2010, Etkin and Schatzberg, 2011, Fonzo et al., 2015, Fonzo et al., 2014, Hölzel et al., 2013, Moon and Jeong, 2015, Nitschke et al., 2009, Palm et al., 2011, Price et al., 2011, Whalen et al., 2008) and pictorial stimuli processing (Sabatinelli et al., 2011): cingulate cortex (anterior, middle and posterior), insula, PFC (medial and lateral), thalamus, amygdala, occipital cortex (OCC) and fusiform gyrus (FG). ROIs were defined based on the Automated Anatomical Labeling (AAL) atlas (Maldjian et al., 2004, Maldjian et al., 2003, Tzourio-Mazoyer et al., 2002) and transformed into Talairach space (Lancaster et al., 2007) using ICBM2TAL in Matlab (v8.2, The MathWorks Inc., Natick, Massachusetts, USA). The anatomically driven subdivision for cingulate cortex was used (Vogt, 2014). The widely used and functionally defined term of dorsal anterior cingulate cortex (dACC) refers to the same region as anterior mid cingulate cortex (aMCC).
Statistical parametric maps resulting from voxel-wise analyses were considered significant for clusters that survived cluster-based correction for multiple comparisons. For ROI and PPI analyses, the voxel-level threshold was set to the voxel-level threshold of p < 0.005 in order to balance between Type I and II error rates (Lieberman and Cunningham, 2009).
Using the cluster-level statistical threshold estimator plugin for BVQX (Goebel et al., 2006), a mask consisting of all predefined ROIs (comprising cingulate cortex, insula, PFC, thalamus, amygdala, OCC, FG) was applied to the thresholded maps. ROI-specific correction criteria were based on the estimates of the maps' spatial smoothness and on an iterative procedure (Monte Carlo simulation) applied to estimate cluster-level false-positive rates (Forman et al., 1995). This procedure yielded after 1000 iterations a minimum cluster size to generate a map-wise corrected false positive rate of p < 0.05. To account for multiple testing in PPI analyses a Bonferroni-corrected threshold (p ≤ 0.01) was used. Analysis of covariance (ANCOVA) in ROI and PPI analyses tested whether results were maintained after inclusion of BDI level, ASI score, medication intake, and subjective ratings as covariates.
To facilitate interpretation of the ROI main (picture valence) and interaction effects (picture valence × group) and PPI effect (group), the average percent signal change across all voxels within each cluster was extracted for each regressor per subject and resolved through SPSS.
3. Results
3.1. Rating data
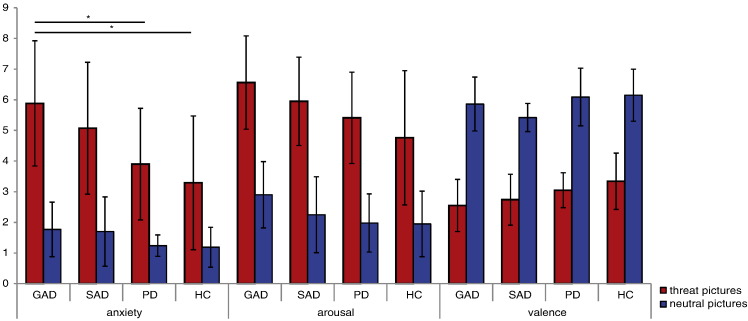
Rating data were available for 82 participants (see Supplement Fig. S4). Main effect group (arousal: F[3,78] = 4.69, p = 0.005; valence: F[3,78] = 6.55 p = 0.001; anxiety: F[3,78] = 6.78, p < 0.001) displayed that threat pictures were rated as more negative (M = 2.92, SD = 0.85), more arousing (M = 5.66, SD = 1.79) and more anxiety-inducing (M = 4.53, SD = 2.26) than neutral pictures (valence: M = 5.88, SD = 0.84; arousal: M = 2.27, SD = 1.14; anxiety: M = 1.48, SD = 0.84) by all participants. Main effect picture valence (arousal: F[1,78] = 456.74, p < 0.001; valence: F[1,78] = 522.47, p < 001; anxiety: F[1,78] = 226.05, p < 0.001) showed that all stimuli were rated as more negative by GAD patients vs. HC (p = 0.014), SAD patients vs. HC (p = 0.001) and SAD vs. PD patients (p = 0.036). Moreover, stimuli were rated as more arousing by GAD patients vs. HC (p = 0.004) and as more anxiety-inducing by GAD vs. PD patients (p = 0.015), GAD patients vs. HC (p = 0.001), and SAD patients vs. HC (p = 0.026). The significant interaction effect group by picture valence for anxiety ratings (F[3,78] = 4.56, p = 0.005) showed that threat pictures were rated as more anxiety-inducing by GAD patients vs. PD (t[38] = 3.24, p = 0.003) and vs. HC (t[39] = 3.92, p < 0.001). All other effects failed to reach significance (p > 0.05).
Supplement Fig. S4.
Mean ratings for arousal (1 = not arousing at all, 9 = highly arousing), valence (1 = very negative, 5 = neutral, 9 = very positive), and anxiety induction (1 = not anxiety-inducing, 9 = highly anxiety-inducing) for threat and neutral pictures per group. *p < 0.05. GAD, generalized anxiety disorder; SAD, social anxiety disorder; PD, panic disorder; HC, healthy controls.
3.2. ROI analysis
3.2.1. Main effects picture valence (across participants)
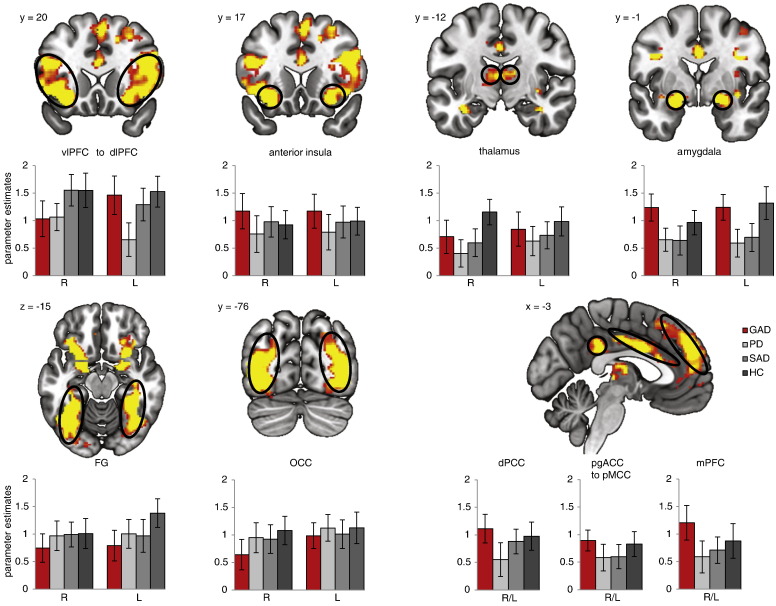
Threat vs. neutral pictures resulted in increased activity in amygdala, thalamus, OCC, ventrolateral PFC (vlPFC), dorsolateral PFC (dlPFC), mPFC, FG, anterior insula (aI), pregenual anterior cingulate cortex (pgACC), aMCC, posterior mid cingulate cortex (pMCC), and posterior cingulate cortex (PCC) (Table 1; Fig. 1). Two clusters in vlPFC and one in dlPFC showed decreased activity to threat vs. neutral pictures.
Table 1.
ROI analysis: Significant main and interaction effects of the picture valence (threat, neutral pictures) by group (GAD, PD, SAD, HC) ANOVA.
| Region | Lateralization | x | y | z | F | mm3 |
|---|---|---|---|---|---|---|
| Main effect: picture valence | ||||||
| Increased activity to threat > neutral pictures | ||||||
| pgACC/aMCC/pMCC | L/R | − 2 | 0 | 31 | 16.15 | 4920a |
| PCC | L/R | − 14 | − 39 | 36 | 14.69 | 3112a |
| Anterior insula ventral/dorsal | L | − 25 | 13 | − 10 | 19.19 | 2843a |
| R | 28 | 21 | − 6 | 16.54 | 3201a | |
| Amygdala | L | − 19 | − 5 | − 10 | 24.75 | 1560a |
| R | 16 | − 6 | − 11 | 23.35 | 1872a | |
| OCC | L | − 39 | − 55 | − 10 | 30.43 | 21302a |
| R | 37 | − 71 | − 6 | 21.87 | 16734a | |
| Thalamus | L | − 13 | − 29 | 0 | 13.04 | 2207a |
| R | 1 | − 13 | 8 | 12.57 | 1305a | |
| FG | L | − 39 | − 55 | − 11 | 29.17 | 6888a |
| R | 38 | − 47 | − 10 | 24.70 | 7345a | |
| L | − 36 | − 12 | − 21 | 13.88 | 648 | |
| vlPFC to dlPFC | L | − 47 | 29 | 10 | 20.53 | 20896a |
| R | 41 | 5 | 30 | 23.17 | 22171a | |
| dlPFC | R | 40 | − 2 | 52 | 9.49 | 160 |
| mPFC | R/L | − 3 | 49 | 34 | 14.91 | 13984a |
| Reduced activity to threat > neutral pictures | ||||||
| vlPFC | L | − 23 | 48 | 1 | 11.87 | 560 |
| R | 27 | 54 | 7 | 9.84 | 976 | |
| dlPFC | R | 28 | 14 | 56 | 11.38 | 2600 |
| Interaction effect: picture valence by group | ||||||
| aMCC | L | − 9 | 28 | 20 | 6.00 | 192b |
| pMCC | L | − 14 | − 13 | 43 | 6.22 | 184b |
| dPCC | R | 10 | − 42 | 34 | 5.29 | 88b |
| daI/FO | L | − 32 | 15 | 17 | 6.14 | 160b |
| posterior dlPFC | L | − 31 | 27 | 48 | 5.22 | 472b |
| vlPFC | L | − 38 | 33 | 6 | 4.92 | 56 |
| Dissolving the interaction effects (picture valence by group) in ROI analysis in response to threat versus neutral pictures | ||||||
|---|---|---|---|---|---|---|
| aMCC | pMCC | dPCC | daI/FO | posterior dlPFC | vlPFC | |
| GAD vs. SAD | t[39] = 5.03, p < 0.001 | t[39] = 5.08, p < 0.001 | t[39] = 3.12, p = 0.003 | t[39] = 4.95, p < 0.001 | t[39] = 3.05, p = 0.004 | t[39] = 2.52 p = 0.016 |
| GAD vs. PD | t[39] = 2.84, p = 0.007 | t[39] = 3.88, p < 0.001 | t[39] = 3.64, p = 0.001 | t[39] = 3.68, p = 0.001 | t[39] = 3.36, p = 0.002 | t[39] = 2.23 p = 0.031 |
| GAD vs. HC | t[39] = 3.53, p = 0.001 | t[39] = 2.71, p = 0.010 | t[39] = 2.94, p = 0.005 | t[39] = 2.95, p = 0.005 | t[39] = 2.42, p = 0.020 | n.s. |
| SAD vs PD | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| SAD vs. HC | n.s. | n.s. | n.s. | n.s. | n.s. | t[40] = 2.79, p = 0.008 |
| PD vs. HC | n.s. | n.s. | n.s. | n.s. | n.s. | t[40] = 2.52, p = 0.016 |
Note. ROI, region of interest; ANOVA, analysis of variance; GAD, generalized anxiety disorder; SAD, social anxiety disorder; PD, panic disorder; HC, healthy controls; pgACC, pregenual anterior cingulate cortex; aMCC, anterior mid cingulate cortex; pMCC, posterior mid cingulate cortex; PCC, posterior cingulate cortex; dPCC, dorsal posterior cingulate cortex; OCC, occipital cortex; aI, anterior insula; FG, fusiform gyrus; mPFC, medial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; daI/FO, dorsal anterior insula/frontal operculum L, left; R, right; x,y,z, Talairach coordinates of maximally activated voxel (activation threshold: p < 0.05 corrected).
Depicted in Fig. 1.
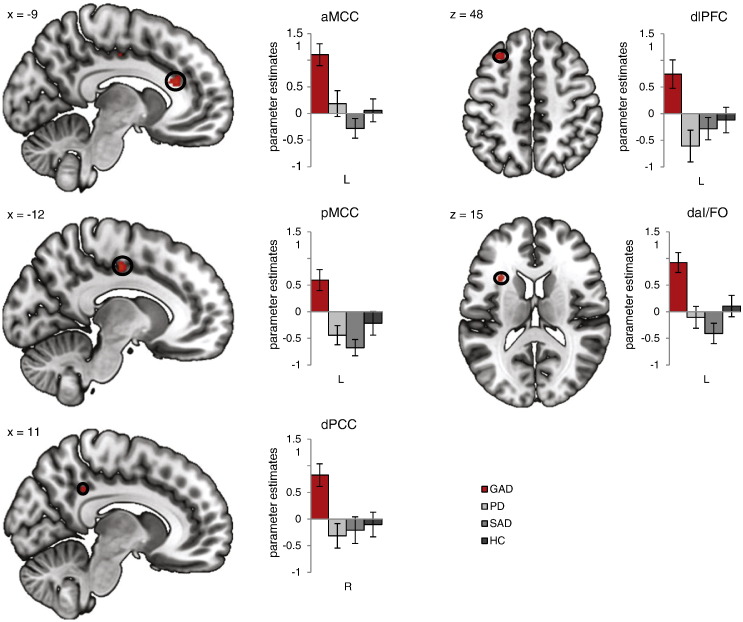
Depicted in Fig. 2.
Fig. 1.
Increased activation to threat vs. neutral pictures across all groups (GAD, generalized anxiety disorder; SAD, social anxiety disorder; PD, panic disorder; HC, healthy controls). The 2 (picture valence) by 4 (group) region of interest analysis of variance (ROI ANOVA) revealed significant brain activation clusters for the main effect picture valence in the following regions: amygdala, thalamus, pregenual anterior cingulate cortex (pgACC), anterior mid cingulate (aMCC), posterior mid cingulate cortex (pMCC), posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), ventrolateral prefrontal cortex (vlPFC), dorsolateral prefrontal cortex (dlPFC), occipital cortex (OCC), fusiform gyrus (FG) (Table 1). To visualize brain responses of each group within main effect clusters of picture valence, graphs display contrasts of parameter estimates (threat > neutral pictures [mean ± standard error for activation cluster]) per group. Statistical parametric maps are overlaid on an averaged T1 scan (radiological convention: left = right).
3.2.2. Interaction effects picture valence by group
ROI analysis revealed six brain regions in which there were significant interactions between picture valance by group: left aMCC, left pMCC, right dorsal PCC (dPCC), left posterior dlPFC, left vlPFC and left dorsal anterior insula/frontal operculum (daI/FO) (Table 1; Fig. 2). The influence of medication intake, subjective ratings, ASI score and BDI level were tested by means of ANCOVAs and revealed no significant effects of the covariates (all p > 0.05) and maintained group effects (all F-values ≥ 3.83, all p < 0.05).
Fig. 2.
Generalized anxiety disorder (GAD)-specific brain activations to threat. The 2 (picture valence) by 4 (group) region of interest analysis of variance (ROI ANOVA) revealed significant brain activations for the interaction effect picture valence × group in which GAD patients showed significant disorder-specific responding relative to each group (vs. panic disorder patients [PD], vs. social anxiety disorder patients [SAD], vs. healthy controls [HC]; all p < 0.05) in the following regions: anterior mid cingulate cortex (aMCC), posterior mid cingulate cortex (pMCC), dorsal posterior cingulate cortex (dPCC), dorsolateral prefrontal cortex (dlPFC), dorsal anterior insula/frontal operculum (daI/FO) (Table 1). Statistical parametric maps are overlaid on an averaged T1 scan (radiological convention: left = right). Graphs display contrasts of parameter estimates (threat > neutral pictures [mean ± standard error for activation cluster]) per group.
Interaction effects in aMCC, pMCC, dPCC, dlPFC and daI/FO derived from increased activity to threat vs. neutral pictures in GAD patients relative to SAD or PD patients or to HC (Table 1). As such GAD patients showed a GAD-specific brain activity pattern in these brain regions. Comparisons of SAD patients vs. HC, SAD patients vs. PD patients or PD patients vs. HC in aMCC, pMCC, dPCC, dlPFC and daI/FO revealed no significant effects, underlying that neither SAD or PD patients showed a disorder-specific brain activity responding in these regions in response to threat vs. neutral pictures. In addition, there was another interaction effect in one cluster in left vlPFC in which SAD and PD patients displayed reduced activity in response to threat vs. neutral pictures. Left vlPFC was the only brain region in which a significant deviating brain response was detected when comparing SAD patients vs. HC or PD patients vs. HC (Table 1).
3.3. PPI analyses
3.3.1. Main effects group
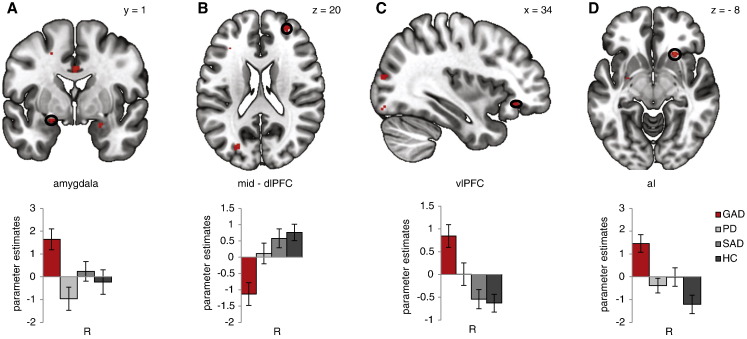
One-way ANOVAs per seed region (aMCC, pMCC, dPCC, dlPFC, daI/FO) revealed group effects in several regions. GAD patients showed increased FC for threat vs. neutral pictures relative to each group between left pMCC and right amygdala, between pMCC and right ventral aI and between left posterior dlPFC and right anterior vlPFC, as well as decreased FC between left daI/FO and right mid-dlPFC (Table 2; Fig. 3). All results remained highly significant after including medication intake, BDI level, ASI score and subjective ratings as covariates (all F-values ≥ 5.19, all p < 0.05), although the covariate itself was significant in three cases (for medication intake: FC between pMCC-amygdala, F[1,78] = 5.25, p = 0.025; for BDI level: FC between daI/FO-dlPFC, F[1,78] = 7.44, p = 0.008; and for ASI score: FC between daI/FO-dlPFC F[1,78] = 7.94, p = 0.006).
Table 2.
PPI analyses: Defining GAD-specific interaction effects of the ROI analysis as seed regions (daI/FO, pMCC, dlPFC) revealed significant FC differences between GAD patients and each other group in response to threat vs. neutral pictures.
| Seed region | Finding region | x | y | z | F | mm3 |
|---|---|---|---|---|---|---|
| Left daI/FO | Right mid-dlPFC | 24 | 49 | 16 | 5.58 | 296 |
| Left pMCC | Right amygdala | 22 | 1 | − 19 | 5.70 | 152 |
| Right ventral anterior insula | 23 | 20 | − 8 | 5.98 | 144 | |
| Left posterior dlPFC | Right vlPFC | 30 | 26 | − 13 | 6.30 | 144 |
| Planned comparisons revealed GAD-specific FC patterns in response to threat vs. neutral pictures (seed– finding region) | ||||
|---|---|---|---|---|
| pMCC - amygdala | pMCC - aI | daI/FO - mid-dlPFC | dlPFC - vlPFC | |
| GAD vs. SAD | t[39] = 2.25, p = 0.030 | t[39] = 2.64, p = 0.012 | t[39] = 3.78, p = 0.001 | t[39] = 4.28, p < 0.001 |
| GAD vs. PD | t[39] = 3.82, p < 0.001 | t[39] = 3.69, p = 0.001 | t[39] = 2.63, p = 0.012 | t[39] = 2.40, p = 0.021 |
| GAD vs. HC | t[39] = 4.05, p < 0.001 | t[39] = 4.80, p < 0.001 | t[39] = 4.41, p < 0.001 | t[39] = 4.67, p < 0.001 |
Note. PPI, psychophysiological interaction analyses; FC, functional connectivity; GAD, generalized anxiety disorder; SAD, social anxiety disorder; PD, panic disorder; HC, healthy controls; pMCC, posterior mid cingulate cortex; vlPFC, ventrolateral prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; daI/FO, dorsal anterior insula/frontal operculum; L, left; R, right; x, y, z, Talairach coordinates of maximally activated voxel (activation threshold: p < 0.05 corrected).
Fig. 3.
Generalized anxiety disorder (GAD)-specific effects in psychophysiological interaction analyses (PPI). One-way region of interest analyses of variances (ROI ANOVAs) per seed region revealed significant functional connectivity (FC) effects for the main effect group in which generalized anxiety disorder (GAD) patients showed disorder-specific responding to threat vs. neutral pictures relative to each group (vs. panic disorder patients [PD], vs. social anxiety disorder patients [SAD], vs. healthy controls [HC]; all p < 0.05) (Table 2). A: Seeding left posterior mid cingulate cortex (pMCC) revealed significantly increased FC with right amygdala for GAD patients relative to healthy controls (HC), social anxiety disorder (SAD) patients or panic disorder (PD) patients. B: Seeding left dorsal anterior insula/frontal operculum (daI/FO) revealed significantly decreased FC with right mid-dlPFC for GAD patients relative to HC, SAD or PD patients. C: Seeding left posterior dorsolateral prefrontal cortex (dlPFC) revealed significantly increased FC with right ventrolateral prefrontal cortex (vlPFC) for GAD patients relative to HC, SAD or PD patients. D: Seeding left posterior mid cingulate cortex (pMCC) revealed significantly increased FC with right ventral anterior insula (aI) for GAD patients relative to HC, SAD or PD patients. Statistical parametric maps are overlaid on an averaged T1 scan (radiological convention: left = right). Graphs display contrasts of parameter estimates threat > neutral pictures [(mean ± standard error for activation cluster)] per group.
4. Discussion
In order to identify GAD-specific neural circuits underlying immediate threat processing, threat and neutral pictures were presented in an event-related design and GAD brain activity was compared between GAD, SAD and PD patients as well as HC. Shared elevated brain responses to threat in GAD, SAD, PD and HC were found in amygdala, aI, thalamus, lateral and medial PFC, cingulate and visual cortex. GAD-specific threat-related increased brain responses emerged in aMCC, pMCC, dPCC, posterior dlPFC and daI/FO. In addition, GAD-specific enhanced activity to threat in pMCC, dlPFC and daI/FO showed a GAD-specific FC pattern to other regions, too: increased FC was found between posterior dlPFC and vlPFC, between pMCC and amygdala, between pMCC and aI, and decreased FC was found between daI/FO and mid-dlPFC. With regard to RDoC, we provide evidence of shared neural networks underlying threat processing across anxious patients and HC. Crucially, GAD patients were also marked by threat-related disorder-specific brain responses.
To our knowledge, the present study is the first to report GAD-specific cingulate cortex involvement during threat processing. Previous studies (Ball et al., 2013, Blair et al., 2012, Blair et al., 2008, Fonzo et al., 2015) failed to reveal such GAD-specificity, possibly due to experimental designs with instructed, demanding stimulus presentation or facial expressions as stimuli. In the present study, GAD patients shared elevated activity in pgACC/aMCC/pMCC and in PCC to threat with SAD and PD patients, as well as with HC. PgACC, aMCC, pMCC and PCC activities were shown to be associated with the elicitation of negative affect, threat appraisal, expression of learned fear/action selection and control of attentional focus, respectively (Etkin et al., 2011, Kalisch and Gerlicher, 2014, Leech and Sharp, 2014, Maier et al., 2012, Mechias et al., 2010, Stevens et al., 2011, Vogt, 2014). In addition to shared neural responses to threat, GAD patients showed increased activity in aMCC, pMCC and dPCC. Thus, GAD patients appeared particularly sensitive to threat, as they may have recruited additional appraisal, attention allocation and response preparation processes, possibly resulting in overestimation or over-interpretation of threat (Hirsch and Mathews, 2012). Previous studies suggested cingulate cortex to play a key role in the pathophysiology of GAD: Elevated aMCC activity was observed in GAD patients vs. HC, triggered by negative pictures (Blair and Blair, 2012, Blair et al., 2012) or in worry-induction tasks (Andreescu et al., 2011, Paulesu et al., 2010). In addition, reduced anterior cingulate cortex/ mid cingulate cortex activity was associated with medication-treatment response (Hoehn-Saric et al., 2004), which was even predictive of the magnitude of reduction (Nitschke et al., 2009, Whalen et al., 2008). Thus, GAD patients are marked by deviating aMCC recruitment in diverse contexts, possibly reflecting exaggerated negative appraisal. Concordantly, maladaptive appraisal is treated in cognitive behavioral therapy for GAD, to procure a more elaborate and adaptive threat appraisal (Kalisch and Gerlicher, 2014).
Furthermore, GAD patients displayed disorder-specific increased daI/FO activity and shared elevated aI activity with SAD, PD and HC in response to threat pictures. Shared aI activity was detected in both ventral and dorsal parts, replicating previous findings (Fonzo et al., 2015). AI is assumed to represent processing of interoceptive sensations and to signal information to brain areas critical for attention allocation and action execution, such as cingulate cortex (Lindquist and Barrett, 2012). Activity in daI/FO has been associated with focal attention regulation (Higo et al., 2011, Lindquist and Barrett, 2012, Nelson et al., 2010). Along these lines, all participants appeared to show similar neural representations of interoceptive sensations in response to threat. Yet, it seems that interoceptive sensations may have been more salient to GAD patients, indicated by the GAD-specific daI/FO activity (Higo et al., 2011, Lindquist and Barrett, 2012, Nelson et al., 2010).
Moreover, GAD patients displayed disorder-specific posterior dlPFC activity and shared PFC activity in medial and lateral PFC in response to threat. Activity in medial and lateral PFC regions is linked to evaluative and regulative aspects of emotions (Cohen et al., 2013, Phan et al., 2005). Posterior dlPFC is associated with attentional top-down processes underlying the processing of task-relevant information (Warren et al., 2013). GAD patients may have experienced more difficulty in shifting attention away from threat pictures (irrelevant task information), resulting in exaggerated attention regulation through posterior dlPFC. Hence, threat-related GAD-specific posterior dlPFC activity seems to corroborate GAD-specific exaggerated attention regulation processes in response to threat.
FC analyses displayed that brain areas with GAD-specific threat-related responding (aMCC, pMCC, daI/FO, dlPFC) showed a GAD-specific FC pattern to other emotion generating and attention regulating regions. In line with our expectation of increased FC within SN, pMCC activity was positively connected to amygdala and ventral aI activity. Amygdala activity is linked to faster threat processing and ventral aI activity to the representation of interoceptive sensations (Lindquist and Barrett, 2012). Following that, heightened threat sensitivity in GAD seems supported. In line with our expectation of reduced SN and ECN connectivity, activity in daI/FO was negatively connected to mid-dlPFC activity in GAD patients. Mid-dlPFC is suggested to be involved in attention shifting processes (Warren et al., 2013), Thus, GAD patients may have focused strongly on interoceptive sensations in response to threat together with deficient attention shifting away from threat (Warren et al., 2013). Contrary to the hypothesized reduced FC within ECN, we detected GAD-specific positive coupling of dlPFC with vlPFC activity. VlPFC is suggested to be involved in regulation of unpleasant emotions (Cohen et al., 2013, Phan et al., 2005). Increased dlPFC activity may reflect heightened effort to ignore task-irrelevant information and increased connectivity to vlPFC may point towards heightened attempts of downregulating unpleasant emotions in GAD patients (Cohen et al., 2013, Phan et al., 2005). The current FC results may indicate that GAD patients displayed exaggerated threat processing with heightened attempts to regulate attention and failure to resist distraction. However, other studies failed to detect GAD-specific FC alterations in amygdala-PFC circuitry relative to MDD or PTSD patients (Chen and Etkin, 2013, Etkin and Schatzberg, 2011). One explanation may be that our design presented a variety of very different threat pictures that are possibly more relevant to GAD patients, rather than facial expressions only and threat pictures were presented in a non-demanding task. Overall, FC results of the present study are in line with previous suggestions of alterations within amygdala-PFC circuitry and with reduced FC between insula and dlPFC (Andreescu et al., 2015, Etkin et al., 2010, Makovac et al., 2015) and support the suggestion of heightened threat sensitivity and altered attention regulation processes in GAD.
The authors of a recent review stated that there is currently no reason to believe that GAD patients show heightened threat processing (Blair et al., 2012). This tentative conclusion was derived based on studies using facial stimuli or sounds, as well as threat pictures presented in a cued task design (Blair et al., 2008, Grillon et al., 2009, Hoehn-Saric et al., 1989, Nitschke et al., 2009, Palm et al., 2011, Whalen et al., 2008). Possible explanations for the divergent results may be that our design presented a variety of threat pictures which likely were more relevant to GAD patients than facial expressions or sounds, and that these were presented in a non-demanding context. Cued designs might have induced preparation for upcoming picture exposure, an effect prevented in the present experimental design.
However, some limitations need to be addressed. First, FC analyses were restricted to seed regions based on GAD-specific findings. Future studies could use a comprehensive ROI approach. Second, six to seven patients per group were medicated. Unfortunately, studies with large samples always face possible confounds, and analyses showed that medication intake did not influence effects. Last, patients had comorbidities. Given that comorbidities frequently occur in GAD, the present sample is likely to be representative of the GAD population (Newman et al., 2013). Nevertheless, it would be beneficial to investigate patients without medication or comorbidities in future studies, possibly also accounting for other variables such as personality, genotypes, and intelligence and to include further control groups, such as patients suffering from MDD.
Overall, the present results show that GAD patients share common brain responses to threat with SAD and PD patients and HC. Yet, GAD patients also displayed disorder-specific brain activity and FC patterns during immediate threat processing, suggestive of hyper-responsive threat processing in GAD primarily in frontal cortex. These findings support a neurobiological model of immediate threat processing in GAD, with increased threat sensitivity paralleled by maladaptive appraisal and exaggerated attention allocation, presumably resulting in over-interpretation and overestimation of threat, which distinguishes GAD from other anxiety disorders (Hölzel et al., 2013, Olatunji et al., 2011). Therewith, findings of the present study are in line with established cognitive models of GAD postulating patients to be hyper-responsive to threat and provide first neural evidence (Behar et al., 2009, Hayes and Hirsch, 2007, Mennin et al., 2009, Newman et al., 2013). Nevertheless, further investigations on immediate threat processing in GAD and replication of current findings will aid to conceptualization and treatment development of the disorder.
The following are the supplementary data related to this article.
Sociodemographic and clinical data.
Stimuli characteristics.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG: SFB/TRR 58: C06, C07) and the Open Access Publication Fund of the University of Muenster.
References
- American Psychiatric Association . 4th ed., Text Revision. Author; Washington, DC: 2000. Diagnostic and statistical manual of mentaldisorders. [Google Scholar]
- Andreescu C., Gross J.J., Lenze E., Edelman K.D., Snyder S., Tanase C., Aizenstein H. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depress. Anxiety. 2011;28(3):202–209. doi: 10.1002/da.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C., Sheu L.K., Tudorascu D., Gross J.J., Walker S., Banihashemi L., Aizenstein H. Emotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment. Am. J. Geriatr. Psychiatry. 2015;23(2):200–214. doi: 10.1016/j.jagp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T.M., Ramsawh H.J., Campbell-Sills L., Paulus M.P., Stein M.B. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol. Med. 2013;43(7):1475–1486. doi: 10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B. Vol. iii. Hogrefe & Huber Publishers; Ashland, OH, US: 1997. Panic and Agoraphobia Scale (PAS) [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. 1996. Beck-Depression-Inventory-II. San Antonio. [Google Scholar]
- Behar E., DiMarco I.D., Hekler E.B., Mohlman J., Staples A.M. Current theoretical models of generalized anxiety disorder (GAD): conceptual review and treatment implications. J. Anxiety Disord. 2009;23(8):1011–1023. doi: 10.1016/j.janxdis.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Blair K., Blair R. A cognitive neuroscience approach to generalized anxiety disorder and social phobia. Emot. Rev. 2012;4(2):133–138. [Google Scholar]
- Blair K., Shaywitz J., Smith B.W., Rhodes R., Geraci M., Jones M.…Pine D.S. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am. J. Psychiatr. 2008;165(9):1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K., Geraci M., Smith B.W., Hollon N., DeVido J., Otero M.…Pine D.S. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol. Psychiatry. 2012;72(6):476–482. doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.C., Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38(10):1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.R., Berkman E.T., Lieberman M.D. Oxford University Press; 2013. Intentional and Incidental Self-control in Ventrolateral PFC. [Google Scholar]
- Duval E.R., Javanbakht A., Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther. Clin. Risk Manag. 2015;11:115–126. doi: 10.2147/TCRM.S48528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Schatzberg A.F. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am. J. Psychiatr. 2011;168(9):968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Hoeft F., Menon V., Schatzberg A.F. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am. J. Psychiatr. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo G.A., Ramsawh H.J., Flagan T.M., Sullivan S.G., Simmons A.N., Paulus M.P., Stein M.B. Cognitive-behavioral therapy for generalized anxiety disorder is associated with attenuation of limbic activation to threat-related facial emotions. J. Affect. Disord. 2014;169:76–85. doi: 10.1016/j.jad.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo G.A., Ramsawh H.J., Flagan T.M., Sullivan S.G., Letamendi A., Simmons A.N.…Stein M.B. Common and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders. Br. J. Psychiatry. 2015;114:149880. doi: 10.1192/bjp.bp.114.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fresco D.M., Coles M.E., Heimberg R.G., Liebowitz M.R., Hami S., Stein M.B., Goetz D. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol. Med. 2001;31(6):1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Friston K., Buechel C., Fink G., Morris J., Rolls E., Dolan R. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Goebel R., Esposito F., Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006;27(5):392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Pine D.S., Lissek S., Rabin S., Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol. Psychiatry. 2009;66(1):47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S., Hirsch C.R. Information processing biases in generalized anxiety disorder. Psychiatry. 2007;6(5):176–182. [Google Scholar]
- Higo T., Mars R.B., Boorman E.D., Buch E.R., Rushworth M.F.S. Distributed and causal influence of frontal operculum in task control. Proc. Natl. Acad. Sci. U. S. A. 2011;108(10):4230–4235. doi: 10.1073/pnas.1013361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch C.R., Mathews A. A cognitive model of pathological worry. Behav. Res. Ther. 2012;50(10):636–646. doi: 10.1016/j.brat.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn-Saric R., McLeod D.R., Zimmerli W.D. Somatic manifestations in women with generalized anxiety disorder: psychophysiological responses to psychological stress. Arch. Gen. Psychiatry. 1989;46(12):1113–1119. doi: 10.1001/archpsyc.1989.01810120055009. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R., Schlund M.W., Wong S.H. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res. 2004;131(1):11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Hölzel B.K., Hoge E.A., Greve D.N., Gard T., Creswell J.D., Brown K.W.…Lazar S.W. Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. NeuroImage: Clinical. 2013;2:448–458. doi: 10.1016/j.nicl.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R., Gerlicher A.M.V. Making a mountain out of a molehill: on the role of the rostral dorsal anterior cingulate and dorsomedial prefrontal cortex in conscious threat appraisal, catastrophizing, and worrying. Neurosci. Biobehav. Rev. 2014;42:1–8. doi: 10.1016/j.neubiorev.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Kinney K.L., Boffa J.W., Amir N. Gender difference in attentional bias toward negative and positive stimuli in generalized anxiety disorder. Behav. Ther. 2016 doi: 10.1016/j.beth.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Kozak M.J., Cuthbert B.N. The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology. 2016;53(3):286–297. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., Salinas F., Evans A., Zilles K.…Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P., Bradley M.M. vol. 29. Oxford University Press; USA: 2007. (The International Affective Picture System (IAPS) in the Study of Emotion and Attention). [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb R., Becker E., Altamura C. The epidemiology of generalized anxiety disorder in Europe. Eur. Neuropsychopharmacol. 2005;15(4):445–452. doi: 10.1016/j.euroneuro.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009 doi: 10.1093/scan/nsp052. nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Barrett L.F. A functional architecture of the human brain: emerging insights from the science of emotion. Trends Cogn. Sci. 2012;16(11):533–540. doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S., Szalkowski A., Kamphausen S., Perlov E., Feige B., Blechert J.…Tüscher O. Clarifying the role of the rostral dmPFC/dACC in fear/anxiety: learning, appraisal or expression? PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovac E., Meeten F., Watson D.R., Herman A., Garfinkel S.N., Critchley H.D., Ottaviani C. Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Mechias M.L., Etkin A., Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. NeuroImage. 2010;49(2):1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Mennin D.S., McLaughlin K.A., Flanagan T.J. Emotion regulation deficits in generalized anxiety disorder, social anxiety disorder, and their co-occurrence. J. Anxiety Disord. 2009;23(7):866–871. doi: 10.1016/j.janxdis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T.J., Miller M.L., Metzger R.L., Borkovec T.D. Development and validation of the Penn State Worry Questionnaire. Behav. Res. Ther. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Moon C.M., Jeong G.W. Functional neuroanatomy on the working memory under emotional distraction in patients with generalized anxiety disorder. Psychiatry Clin. Neurosci. 2015;69(10):609–619. doi: 10.1111/pcn.12295. [DOI] [PubMed] [Google Scholar]
- Nelson S.M., Dosenbach N.U., Cohen A.L., Wheeler M.E., Schlaggar B.L., Petersen S.E. Role of the anterior insula in task-level control and focal attention. Brain Struct. Funct. 2010;214(5–6):669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M.G., Llera S.J., Erickson T.M., Przeworski A., Castonguay L.G. Worry and generalized anxiety disorder: a review and theoretical synthesis of evidence on nature, etiology, mechanisms, and treatment. Annu. Rev. Clin. Psychol. 2013;9:275–297. doi: 10.1146/annurev-clinpsy-050212-185544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke J.B., Sarinopoulos I., Oathes D.J., Johnstone T., Whalen P.J., Davidson R.J., Kalin N.H. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am. J. Psychiatr. 2009;166(3):302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji B.O., Ciesielski B.G., Armstrong T., Zhao M., Zald D.H. Making something out of nothing: neutral content modulates attention in generalized anxiety disorder. Depress. Anxiety. 2011;28(5):427–434. doi: 10.1002/da.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm M.E., Elliott R., McKie S., Deakin J.F., Anderson I.M. Attenuated responses to emotional expressions in women with generalized anxiety disorder. Psychol. Med. 2011;41(5):1009–1018. doi: 10.1017/S0033291710001455. [DOI] [PubMed] [Google Scholar]
- Paulesu E., Sambugaro E., Torti T., Danelli L., Ferri F., Scialfa G.…Sassaroli S. Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychol. Med. 2010;40(1):117–124. doi: 10.1017/S0033291709005649. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Price R.B., Eldreth D.A., Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Transl. Psychiatry. 2011;1 doi: 10.1038/tp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S., Peterson R.A., Gursky D.M., McNally R.J. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav. Res. Ther. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Fortune E.E., Li Q., Siddiqui A., Krafft C., Oliver W.T.…Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. NeuroImage. 2011;54(3):2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Stevens F.L., Hurley R.A., Taber K.H. Anterior cingulate cortex: unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 2011;23(2):121–125. doi: 10.1176/jnp.23.2.jnp121. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme; New York: 1988. Co-Planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N.…Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vogt B.A. Submodalities of emotion in the context of cingulate subregions. Cortex. 2014;59:197–202. doi: 10.1016/j.cortex.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Warren S.L., Crocker L.D., Spielberg J.M., Engels A.S., Banich M.T., Sutton B.P.…Heller W. Cortical organization of inhibition-related functions and modulation by psychopathology. Front. Hum. Neurosci. 2013:7. doi: 10.3389/fnhum.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M., Kankse P., Neumeister P., Bode K., Heissel J., Schönfelder S. EmoPics: Subjektive und psychophysiologische Evaluationen neuen Bildmaterials für die klinisch-bio-psychologische Forschung. Z. Klin. Psychol. Psychother. Suppl. 2010;77 [Google Scholar]
- Whalen P.J., Johnstone T., Somerville L.H., Nitschke J.B., Polis S., Alexander A.L.…Kalin N.H. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol. Psychiatry. 2008;63(9):858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H.-U., Wunderlich U., Gruschwitz S., Zaudig M. SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I. Z. Klin. Psychol. Psychother. 1997;28:68–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sociodemographic and clinical data.
Stimuli characteristics.