Abstract
An adult free-ranged female maned wolf was rescued from a periurban area subject to anthropogenic disturbances in the Minas Gerais, Brazil. The animal presented poor body condition and anemia. The clinical condition rapidly deteriorated culminating in dead and a necropsy was performed. The main gross lesions were marked anemia and blood content in the intestines accompanied by many types of parasites. The protozoa Rangelia vitalii was identified by histopathological analysis predominantly within the cytoplasm of endothelial cells of capillaries of the small intestine. The lymph nodes, spleen, bone marrow, dermis, lungs and kidney had similar protozoal forms but with mild or moderate intensity. Rangelia vitalii was confirmed by molecular assays. Hepatozoon sp., Leishmania sp., and Entamoeba spp., apparently not related to the clinical signs were also detected. The myriad parasites found in the intestines included nematodes (Ancylostoma caninum, A. braziliensis,, Molineus sp., Pterygodermatites sp., and Trichuris sp.), cestodes (Spirometra sp.) and (acanthocephalans. To our knowledge, R. vitalii was identified in C. brachyurus for the first time. These findings emphasize the fragility of Brazilian ecosystems, especially in disturbed areas, reinforcing the necessity of efforts to preserve these areas and wild carnivores, some of which are threatened with extinction, such as the maned wolf.
Keywords: Chrysocyon brachyurus, Anthropization, Wild carnivores, Multiparasitism, Rangelia vitalii
Graphical abstract

Highlights
-
•
Rangelia vitalii was found in Chrysocyon brachyurus, especially in small intestine.
-
•
Co-infections with other parasites were found, including some with zoonotic potential.
-
•
Anemia was likely linked to R. vitalii, Ancylostoma spp and perhaps Spirometra.
-
•
Health status of the near threatened maned wolf reflects anthropogenic disturbed of habitat.
1. Introduction
The maned wolf (Chrysocyon brachyurus) is the largest canid of South America and inhabits the Cerrado biome, some areas in the Pantanal, and in the southeast region of Brazil (de Paula and Gambarini, 2013). The animal also is found in some provinces of Argentina, Paraguay, Peru, Bolivia and Uruguay (possibly extinct). Currently, the species is listed as near threatened in the vulnerable categories of the Red List of the International Union for Conservation of Nature (IUCN) (de Paula and de Matteo, 2015).
Significant threats to maned wolf populations include the drastic reduction of its habitats, especially due to the advancement of agriculture and livestock, the disorderly growth of urban areas, the increased contact in disturbed environments, the abundance of roads in proximity and the close contact with humans and domestic animals (Mangini et al., 2006, Lopes et al., 2008, de Paula and Gambarini, 2013). In free ranging wolves, mortalities due to diseases assume an important role, emerging recently as the major threat for carnivore conservation (Funk et al., 2001).
Studies about pathogens in free-ranging maned wolves are scarce. Despite that, parasites and other pathogens represent an elementary component of ecosystems and their biodiversity, their presence even in any natural hosts can be a threat in areas disturbed by habitat degradation and reduction (Mangini et al., 2006).
Rangelia vitalii, a member of the protozoan phylum Apicomplexa, order Piroplasmorida, causes a tick-borne disease in dogs referred to as “nambiuvú” (=blood dribbling down from the ear margins) or “peste de sangue” (=bleeding plague) (Krauspenhar et al., 2003). Currently, canine rangeliosis is confirmed to occur in Brazil (Krauspenhar et al., 2003), Argentina (Eiras et al., 2014), and Uruguay (Carvalho et al., 2015). Recently, in south and southeast Brazil, infection by R. vitalii was reported in wild Pampas fox (Lycalopex gymnocercus) (de Quadros et al., 2015) and crab-eating fox (Cerdocyon thous) (Soares et al., 2014, de Quadros et al., 2015, Fredo et al., 2015). However, there are no reports of infection and/or disease related to this protozoan in C. brachyurus. Due to the importance of conservation of C. brachyurus in Brazil, this investigation was performed to check for parasitism caused by R. vitalii and co-infections in one free-ranging maned female wolf from a periurban area subject to anthropogenic disturbances.
2. Material and methods
2.1. History
In November 2013, one adult maned wolf female weighting 18 kg was rescued from a periurban house located in the Rio Acima municipality (20°5′44.732″ S and 43°47′24.263″ W) in the state of Minas Gerais (MG), southeast of Brazil. The animal had physical debilitation and pain in the right foreleg. After its rescue by the Brazilian Federal Environmental Agency (IBAMA), it was transported to a veterinary clinic. Clinical evaluation showed right elbow luxation and tick infestation. Fipronil spray 0.25% was administered. Blood sample were collected in an ethylene diamine tetraacetic acid (EDTA) tube for further molecular analyses and for preparation of blood smears. Eleven days later, the animal died and necropsy was performed at the Pathology Sector, School of Veterinary Medicine, Universidade Federal de Minas Gerais (UFMG).
The study was approved by Ethics Committee for Animal Research of the UFMG under protocol 332/2013 and by the Brazilian Institute for Environment and Natural Renewable Resources (IBAMA, Belo Horizonte, MG, Brazil) under license 34633-5.
2.2. Necropsy and sampling
Necropsy was performed and the animal was grossly evaluated. Samples of brain, lung, trachea, heart, esophagus, stomach, intestine, kidney, bone marrow, spleen, and lymph nodes were collected in duplicate: one portion of each tissue was fixed in 10% neutral-buffered formalin for histopathology; and the other portion of the same tissue was frozen at −20 °C and stored for subsequent DNA extraction. For search for the parasites in the intestines, anatomical segments were separated by double ligatures, the contents removed and the mucosae scraped. The collected material was washed in strainers and the solid portion was fixed and preserved in acetic formaldehyde. For histopathological analysis, tissues were dehydrated using a series of increasing ethanol concentrations, cleared in xylene, and embedded in paraffin. Sections of 4 μm were obtained and stained with hematoxylin and eosin (HE) for routine histological evaluation under light microscopy (Luna, 1968). Parasite identification in histological sections was performed according to Gardiner and Poynton (1999).
2.3. Parasitological analysis
Blood and organ samples (spleen, mesenteric lymph node and intestines) were evaluated for hemoparasites in Laboratory of Veterinary Protozoology, Biology Institute, UFMG. Blood smears were subjected to quick Romanowsky staining (Panótico Rápido; Laborclin, Pinhais, PR, Brazil) and examined under immersion oil. Helminths recovered were examined at the Laboratory of Veterinary Helminthology, Biology Institute, UFMG. Images of the parasites were made with a digital camera Carl Zeiss (Oberkochen, Germany) and the measures of the parasites were performed using AxioVision 4.8 (Carl Zeiss). All collected specimens were identified according to Yamaguti, 1961, Travassos et al., 1969, Vicente et al., 1997, and Anderson et al. (2009). Samples of cestodes were stored in tagged vials containing 70% ethanol for DNA extraction.
2.4. DNA extraction and PCR amplification
DNA was extracted from helminths, organs and blood samples using Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA), according to the manufacturer's instructions for animal tissue and whole blood, respectively.
In all PCR assays, the reaction mixture in the first round contained 7.5 μL of GoTaq®Green Master Mix (Promega, Madison, WI, USA), 0.6 μL of a solution containing the mixed primers (10 mM) and 5.4 μL of nuclease free water. A 1.5 μL of total DNA was added to the reaction mixture to obtain a final volume of 15 μL. The reaction mixtures in the second round assays were similar, except the templates were the products from the first round PCR reactions (1.5 μL). Sets of primers used to detect the parasite species and amplifications were performed using a touchdown PCR programmed as previously (Gasser et al., 1996, Noyes et al., 1999, Soares et al., 2011, Spolidorio et al., 2011) (Table 1). Positive and negative controls were used for all PCR assays. The PCR amplicons were separated by electrophoresis on 1% agarose gels (40 min, 100 V), stained with GelRed™ (Biotium, Hayward, CA, USA), and visualized under ultraviolet light.
Table 1.
Specific primers used for the detection of parasites.
| Specificity | Primer sequence (5′- 3′) | Target | Name | Product size (bp) | Reference |
|---|---|---|---|---|---|
| Piroplasmida | CATGAAGCACTGGCCHTTCAA | hsp70 | hsp 70 F1 | 740 | Soares et al., 2011 |
| 1st reaction | GCNCKGCTGATGGTGGTGTTGTA | hsp 70 R1 | |||
| 2nd reaction | GGATCAACAAYGGMAAGAAC | hsp70 | hsp 70 F2 | 720 | Soares et al., 2011 |
| GBAGGTTGTTGTCCTTVGTCAT | hsp 70 R2 | ||||
| Hepatozoon spp. | GGTAATTCTAGAGCTAATA | 18SrRNA | HEP144-169 | 574 | Spolidorio et al., 2011 |
| ACAATAAAGTAAAAAACA | HEP743-718 | ||||
| Kinetoplastida | CAGAAACGAAACACGGGAG | ssrRNA gene | TRY816F | 1500–900 | Noyes et al., 1999 |
| 1st reaction | CCTACTGGGCAGCTTGGA | TRY816R | |||
| 2nd reaction | TGGGATAACAAAGGAGCA | ssrRNA | SSU450F | 450 | Noyes et al., 1999 |
| CTGAGACTGTAACCTCAAAGC | gene | SSU450R | |||
| Helminths | GTAGGTGAACCTGCGGAAGGATCATT | ITS1, 5.8 | NC5 | – | Gasser et al., 1996 |
| TTAGTTTCTTTTCCTCCGCT | S, ITS2 | NC2 |
For sequencing, positive products from PCR reactions were purified using a QIAquick PCR Purification Kit (Qiagen Biotecnologia Brasil, São Paulo, Brazil) according to manufacturer's instructions. The purified amplicons were sequencing using an Applied Biosystems model ABI3130 Genetic Analyzer (Life Technologies, Carlsbad, CA, USA) and the Applied Biosystems BigDye® Direct Cycle Sequencing Kit (v. 3.1), with the POP-7™ polymer as the separating matrix, using primers employed in the PCR reaction. Sequences obtained were aligned, edited, and analyzed using MEGA 6.0 software at the URL http://asparagin.cenargen.embrapa.br/phph (Tamura et al., 2013). The identity of each sequence was confirmed using alignment, via homology to sequences available at GenBank using BLAST software (Altschul et al., 1990).
The phylogenetic tree was constructed by analyzing the ITS gene from Diphyllobothriidae strains deposited in GenBank. Nucleotide sequences were aligned with MUSCLE from de MEGA 6.0 package (Tamura et al., 2013). Each alignment was analyzed using the Maximum Likelihood in MEGA 6.0 software. Internal branch confidence was assessed by the bootstrapping method using 1000 bootstrap replicates.
3. Results
3.1. Clinical history
Clinical examination revealed weakness, poor body condition, and anemia when the animal was admitted. Immobilization for traumatic right elbow luxation was performed. The clinical condition progressively worsened and ten days later, the animal died. Blood smear analysis revealed intracellular gamonts in the cytoplasm of neutrophils, suggesting Hepatozoon infection.
3.2. Gross and histopathology
The maned wolf had poor corporal condition and, surrounding the perineal area, there was dark-red material consistent with digested blood. There was also mild subcutaneous edema, and the peripheral lymph nodes were brown with multifocal to coalescing white foci. The musculature and parenchymatous organs were markedly pale red, indicating anemia. The mucosa of the middle and distal parts of the trachea and primary bronchi had 0.3 cm whitish nodules, with intralesional parasites suggestive of Oslerus sp. The spleen had moderate enlargement of the red pulp. The lumen of small and large intestines were filled with red hemorrhagic content. In addition, there were many acanthocephala and cestodes with characteristics of the order Pseudophyllidea, as well as nematodes (especially Ancylostomatidae) in the small intestine. The mucosa was thickened and with many petechial hemorrhages, especially in the small intestine. The mesenteric lymph nodes had drainage hemorrhages.
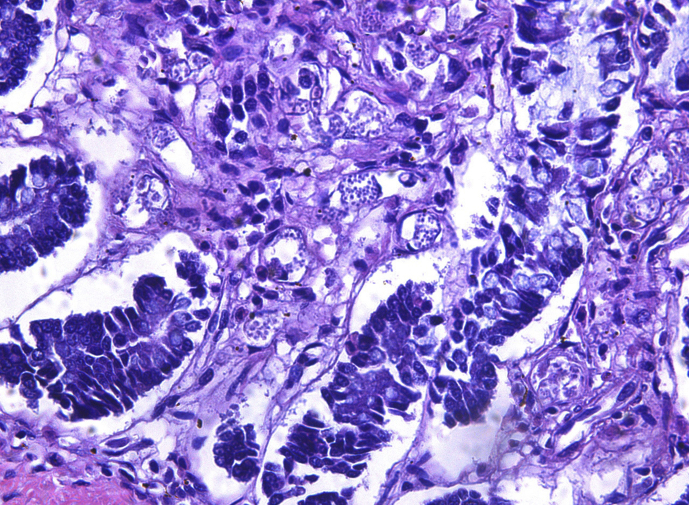
Histologically, there were multifocal lymphoplasmacytic tracheitis and bronchitis associated with O. osleri infection (Avelar et al., 2013). The small intestine had mild lymphoplasmacytic infiltration in the mucosa. Within the cytoplasm of endothelial cells of capillaries and venules, there were numerous round 3 μm protozoa with pale cytoplasm and basophilic nucleus, compatible with R. vitalii. This infection was particularly intense in the lamina propria and submucosa of the small intestine (Fig. 1). Similar protozoa but with moderate intensity were found in the lymph nodes, spleen, and bone marrow of femur. In spleen, bone marrow and lymph nodes, there were moderate plasmacytosis, erythrophagocytosis and hemosiderosis. In the dermis, lungs, heart, and kidneys, protozoa in small vessels were found without inflammatory reaction. Multiple foci of several round to ovoid 30–50 μm parasitic organisms were found in the cytoplasm of intestinal epithelial cells lying in direct proximity to the lamina propria (Fig. 2). Morphologically, these organisms were characterized by a basophilic eccentric nucleus with a large karyosome, vacuolated, granular and slight basophilic cytoplasm with smooth, thin and eosinophilic wall compatible with an amoeba form.
Fig. 1.
Small intestine of C. brachyurus. There are numerous round protozoa within cytoplasm of endothelial cells of the lamina propria compatible with Rangelia vitalli. H.E. 40X.
Fig. 2.
Small intestine of C. brachyurus. There are several round to ovoid parasitic organisms measuring of 30–50 μm of diameter in the cytoplasm of intestinal epithelial cells and in the lamina propria. H.E. 40X.
Intestinal lumen had many cestodes. These parasites did not have a body cavity, the organs were surrounded by parenchymatous matrix, and were enclosed by tegument. The digestive tube was not present but many calcareous bodies were seen. The testis and uterus were located centrally and the eggs were operculated and embryonated. Kidneys had mild membranous glomerulonephritis, multifocal interstitial lymphoplasmacytic nephritis, and mild tubular protein casts.
3.3. Parasitology
Even though initial veterinary report stated tick infestation, the identity of the tick species could not be ascertained due to subsequent administration of fipronil by the veterinarian. A total of 33 O. osleri (21 females and 16 males) were found in the trachea and bronchi. Ancylostomatidae were detected in the small intestine (503 females and 316 males). In addition, there were nematodes of genus Molineus (209 females and 198 males) and Pterygodermatites sp. (36 females and 26 males), 12 acantocephalus and 34 cestodes (Order Pseudophyllidea). Length of Pseudophyllidea specimens ranged from 12 cm to 48 cm, and morphological and morphometrical characteristics were compatible with the Family Diphyllobothriidae, according to Yamaguti (1961). Trichuris specimens (2 females and 4 males) were detected in the large intestine.
3.4. Molecular assays
All the positive results of PCR assays were confirmed for nucleotide sequences analysis by Blastn. Infection by R. vitalii was confirmed by PCR using DNA from mesenteric lymph node. A Blastn sequence analysis revealed that the sample was 99% identical to R. vitalii sequences obtained from a dog in Brazil (GenBank JF279603.1). Whole blood was PCR positive for Hepatozoon and sequencing showed 98% identity to H. americanum isolated from Amblyomma maculatum in USA (GenBank AF176836.1) and Hepatozoon spp. isolated from C. thous in Brazil (GenBank AY461377.2). Blood sample was also PCR positive for Kinetoplastid protozoa and sequencing showed 98% sequence identity with Leishmania infantum (GenBank XR_001203206.1) and L. donovani (GenBank FR799614.1; GQ332356.1). Molecular assays and analysis also revealed that sequences obtained from a gene of diphyllobothriid of this maned wolf were 88% identical to Spirometra erinaceieuropaei from dogs (GenBank FJ886746.1) and cats (GenBank KC561781.1). This proximity was reinforced by phylogenetic analysis, which revealed the formation of a cluster with a high percentage of internal branches among diphyllobothriid of this study (C129), Spirometra spp., and S. erinaceieuropaei (Fig. 3). The nucleotide sequences amplified from parasites were deposited in GenBank under the following accession numbers: KU500888 (Leishmania sp.); KU500889 (Spirometra sp.); KU507416 (Hepatozoon spp.); KU507417 (R. vitalii).
Fig. 3.
Maximum Likelihood tree based on ITS genes of helminths. Dipylidium caninum sample were used as outgroup. Scale bar represents the nucleotide substitutions per position. Branch lengths represent the amount of genetic distance change between the strains. * Spirometra - Chrysocyon brachyurus MG, Brazil denotes the sequences obtained from this study. The accession numbers of the publicly available reference sequences are indicated.
4. Discussion
The maned wolf of this study was intensely infected with multiple species of parasites. This multiparasitism resulted in a set of systemic changes, especially severe anemia. R. vitalii was detected in endothelial cells of various organs, but myriad organisms were observed especially in small intestine. Intense infection by R. vitalii, Ancylostomatidae, and perhaps the pseudophyllids, acted together resulting in anemia.
There has been an gradual increase in the occurrence of R. vitalii infections in Brazilian wild canids in the recent years, with the first reported infection in C. thous from the southeast region (São Paulo) and from the south (Rio Grande do Sul) in the absence of clinical signs (Soares et al., 2014). Subsequently, the protozoan was reported in pampas fox (L. gymnocercus) in another state in South (Santa Catarina). This animal developed a fatal and classical form of this disease with anemia and jaundice (de Quadros et al., 2015). In C. thous and L. gymnocercus, infections by R. vitalii concomitant to tick infestation by A. aureolatum and with anemia have been reported (Fredo et al., 2015). To the best of the authors' knowledge, this is the first report of infection by R. vitalii in a maned wolf, with severe anemia without jaundice and intense parasitism predominantly within the cytoplasm of endothelial cells of the small intestine. These findings differ from the lesions often found in domestic dogs (Krauspenhar et al., 2003, da Silva et al., 2011). In dogs, jaundice and persistent hemorrhages through the external surface of the ears and nose are the most common lesions. In these cases, R. vitalii were most numerous in the cytoplasm of endothelial cells of peripheral lymph nodes, bone marrow, kidneys, and choroid plexus (Loretti and Barros, 2005). In dogs, the anemia is regenerative and probably caused by extravascular immune-mediated hemolysis (França et al., 2013). Amblyoma aureolatum and Rhipicephalus sanguineus were suggested as vectors of R. vitalii in rural domestic dogs (Loretti and Barros, 2005, Soares et al., 2011) and probably the primary hosts of R. vitalii are the South American wild canids and their endemic ticks. Soares et al. (2014) detected this protozoa in wild canids without clinical signs of rangeliosis and suggested that C. thous is a natural reservoir of R. vitalii in Brazil.
Molecular analyzes revealed that the Hepatozoon found in this maned wolf was closely related to H. americanum, as found in leukocytes of whole blood smears from native C. thous in Brazil (Criado-Fornelio et al., 2006, André et al., 2010, Almeida et al., 2013). Hepatozoon species closely related to H. canis were detected in a maned wolf in captivity in São Paulo (André et al., 2010).
Leishmania closely related to the species responsible for visceral leishmaniasis (VL) was molecularly detected in whole blood of the C. brachyurus analyzed in this study. Canine visceral leishmaniasis is an endemic zoonosis in the metropolitan region of Belo Horizonte (Silva et al., 2001). Antibodies to this parasite have been detected in several Brazilian wild canids, including free maned wolf of the state of Minas Gerais (Curi et al., 2012) and a maned wolf kept in captivity in the Zoo of Belo Horizonte (Luppi et al., 2008). In the present study, there were no infected macrophages with intracytoplasmic amastigotes; however, the animal had membranous glomerulonephritis, which is frequently associated with Leishmania spp. infections (Luppi et al., 2008).
Based on fecal antigen ELISA, Alam et al. (2015), showed a relatively low prevalence rate of E. histolytica infection in dogs from Pakistan without clinical signs. In the present study, the histological characteristics of the parasite in the small intestine suggested trophozoites of Entamoeba sp. (Stedmen et al., 2003), but the infection was not related with mucosal necrosis, an unusual finding, both with respect to the anatomical location of infection as well as the infected species. There are no published reports of Entamoeba infections in wild canids.
A wide variety of helminths including hookworm, Molineus, Pterygodermatites, Trichuris, Spirometra and acanthocephalans were found in intestines. Hookworms cause blood spoliation and hemorrhages in the intestinal mucosa, leading to blood loss and anemia. Ancylostomatidae found in this study are common in domestic carnivores (Freitas, 1977), which reinforces the contact between this maned wolf with domestic species and their pathogens in periurban areas.
The organisms of the genus Molineus are known to parasitize carnivores worldwide (Durette-Desset and Chabaud, 1981). Molineus brachiurus was reported in maned wolf (Vieira et al., 2008). Pterygodermatites pluripectinata and P. affinis were found in high prevalence in C. thous of northeast region (Lima et al., 2013) but not in maned wolf.
Cestodes of Spirometra spp. and S. mansonoides were reported in C. thous (Ruas et al., 2008, Lima et al., 2013) and in L. gymnocercus (Ruas et al., 2008). Report of Spirometra infection in maned-wolf was not found. According to molecular analysis, specimens found in the present study were closely related to S. erinaceieuropaei. The definitive hosts are carnivores and their plerocercoid larvae are responsible for the sparganosis zoonosis (Dybing et al., 2013). Infections in humans in Brazil by Spirometra sp. have been described (Gomes et al., 1996, Mentz et al., 2011). Massive adult infections by Pseudophyllidea in the small intestine of humans can lead to megaloblastic anemia due to the deficiency caused by vitamin B12 (Scholz et al., 2009) and folic acid consumption by the parasite (Jimenez et al., 2012).
The maned wolf evaluated in this study was found in a periurban area. Anthropogenic interventions are considered primary factors for the emergence of infectious agents (Aguirre and Tabor, 2008). Parasites that affect wild and domestic carnivores can circulate between sympatric populations of animals, facilitating distribution of infections to humans (Polley, 2005). Wild carnivores require large territorial areas for maintenance (Pastoret and Brochier, 1999) which also facilitates the contact with other species of wild animals, human beings, domestic dogs, and their pathogens.
According to our knowledge, this is the first report in a maned wolf that includes parasites of all phyla (Arthropoda, Protozoa, Platyhelminthes, Nematoda and Acanthocephala), including some with zoonotic potential. These include Leishmania, Entamoeba and Spirometra. These findings emphasize the fragility of Brazilian ecosystems, especially in disturbed areas, reinforcing the necessity for efforts to preserve these areas and wild carnivores, some of which are threatened with extinction, including the maned wolf.
5. Conclusion
Myriads of Rangelia vitalii were identified in C. brachyurus for the first time, allowing us to relate the clinical pathological findings to the host's susceptibility to this protozoan. The multiparasitism indicated also the susceptibility of the animal to pathogens that cause disease in dogs. Probably, the infectious agents are acquired when wild animals access periurban zones. The parasites with zoonotic character found in this animal reveal the importance of the maned wolf for public health.
Conflicts of interest
There are no conflicts of interest in this work.
Acknowledgements
Scholarships were provided by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais). We thank Dr. Élida Mara Leite Rabelo for assistance with the laboratory studies, veterinary Marcos Motta Mourão for referring the animal for necropsy. Also, we are thank Brazilian agencies members of the Centro Nacional de Pesquisas para Conservação dos Predadores Naturais (CENAP), the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) and the Instituto Estadual de Florestas (IEF) for providing licences, samples and supporting every step of our conservation efforts.
References
- Aguirre A.A., Tabor G.M. Global factors driving emerging infectious diseases: impact on wildlife populations. Animal Biodivers. Emerg. Dis. Ann. Ny. Acad. Sci. 2008;1149:1–3. doi: 10.1196/annals.1428.052. http://www.ncbi.nlm.nih.gov/pubmed/19120161 Downloaded 16 December 2015. [DOI] [PubMed] [Google Scholar]
- Alam M.A., Maqbool A., Nazir M.M., Lateef M., Khan M.S., Lindsay D.S. Entamoeba infections in different populations of dogs in an endemic area of Lahore. Pak. Vet. Parasitol. 2015;30:216–219. doi: 10.1016/j.vetpar.2014.12.001. http://www.sciencedirect.com/science/article/pii/S0304401714006220 Downloaded 29 November 2015. [DOI] [PubMed] [Google Scholar]
- Almeida A.P., Souza T.D., Marcili A., Labruna M.B. Novel Ehrlichia and Hepatozoon agents infecting the crab-eating fox (Cerdocyon thous) in southeastern Brazil. J. Med. Entomol. 2013;50:640–646. doi: 10.1603/me12272. http://www.bioone.org/doi/abs/10.1603/ME12272 Downloaded 10 December 2015. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. http://www.ncbi.nlm.nih.gov/pubmed/2231712 Downloaded 20 December 2015. [DOI] [PubMed] [Google Scholar]
- Anderson R.C., Chabaud A.G., Willmott S. British Library; London, UK: 2009. Keys to the Nematode Parasites of Vertebrates; p. 480. [Google Scholar]
- André M.R., Adania C.H., Teixeira R.H., Vargas G.H., Falcade M., Sousa L., Salles A.R., Allegretti S.M., Felippe P.A., Machado R.Z. Molecular detection of Hepatozoon spp. in Brazilian and exotic wild carnivores. Vet. Parasitol. 2010;173:134–138. doi: 10.1016/j.vetpar.2010.06.014. http://www.ncbi.nlm.nih.gov/pubmed/20630658 Downloaded 10 December 2015. [DOI] [PubMed] [Google Scholar]
- Avelar I.O., Almeida L.R.A., D'Elia M.L., dos Santos H.A., Soares D.F.M., Pereira P.L.L., Lima W.S., Ecco R. Pathological and parasitological findings in a Brazilian hoary fox (Lycalopex vetulus, Lund, 1842) infected by Oslerus osleri (Cobbold, 1876) (Nematoda: Filaroididae) Braz. J. Vet. Pathol. 2013;6:111–115. http://bjvp.org.br/wp-content/uploads/2015/07/DOWNLOAD-FULL-ARTICLE-27-20881_2013_12_3_1_14.pdf Downloaded 24 January 2015. [Google Scholar]
- Carvalho L., Maya L., Dutra F., Venzal J.M., Labruna M.B. Molecular detection of Rangelia vitalii in domestic dogs from Uruguay. Vet. Parasitol. 2015;210:98–101. doi: 10.1016/j.vetpar.2015.03.013. http://www.ncbi.nlm.nih.gov/pubmed/25843009 Downloaded 16 December 2015. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A., Ruas J.L., Casado N., Farias N.A.R., Soare M.P., Muller G., Brum J.G.W., Berne M.E.A., Buling-Saraña A., Barba-Carretero J.C. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J. Parasitol. 2006;92:93–99. doi: 10.1645/GE-464R.1. http://www.bioone.org/doi/pdf/10.1645/GE-464R.1 Downloaded 10 December 2015. [DOI] [PubMed] [Google Scholar]
- Curi N.H.A., Coelho C.M., Malta M.C.C., Magni E.M., Sábato M.A., Araújo A.S., Lobato Z.I., Santos J.L., Santos H.A., Ragozo A.A., de Souza S.L. Pathogens of wild maned wolves (Chrysocyon brachyurus) in Brazil. J. Wildl. Dis. 2012;48:1052–1056. doi: 10.7589/2011-10-304. http://www.bioone.org/doi/pdf/10.7589/2011-10-304 Downloaded 22 November 2015. doi: 10.7589/2011-10-304. [DOI] [PubMed] [Google Scholar]
- da Silva A.S., França R.T., Costa M.M., Paim C.B., Paim F.C., Dornelles G.L., Soares J.F., Labruna M.B., Mazzanti C.M., Monteiro S.G., Lopes S.T. Experimental infection with Rangelia vitalii in dogs: acute phase, parasitemia, biological cycle, clinical-pathological aspects and treatment. Exp. Parasitol. 2011;128:347–352. doi: 10.1016/j.exppara.2011.04.010. http://www.sciencedirect.com/science/article/pii/S0014489411001494 Downloaded 11 December 2015. [DOI] [PubMed] [Google Scholar]
- de Paula R.C., Gambarini A. Avis Brasilis Editora; Vinhedo, SP: 2013. Stories of a Golden Wolf; p. 264. [Google Scholar]
- de Paula R.C., De Matteo K. 2015. Chrysocyon brachyurus. The IUCN Red List of Threatened Species 2015: e.T4819A82316878.http://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T4819A82316878.en Downloaded on 16 January 2016. [Google Scholar]
- de Quadros R.M., Soares J.F., Xavier J.S., Pilati C., da Costa J.L., Miotto B.A., Miletti L.C., Labruna M.B. Natural infection of the wild canid Lycalopex gymnocercus by the Protozoan Rangelia vitalii, the agent of canine rangeliosis. J. Wildl. Dis. 2015;51:787–789. doi: 10.7589/2014-08-194. http://www.bioone.org/doi/pdf/10.7589/2014-08-194 Downloaded 18 December 2015. doi: 10.7589/2014-08-194. [DOI] [PubMed] [Google Scholar]
- Durette-Desset M.C., Chabaud A.G. Sur les Molineinae parasites de Mammiferes. Ann. de Parasitol. Humaine Comparée. 1981;56:297–312. [PubMed] [Google Scholar]
- Dybing N.A., Fleming P.A., Adams P.J. Environmental conditions predict helminth prevalence in red foxes in Western Australia. Int. J. Parasitol. Parasites Wildl. 2013;13:165–172. doi: 10.1016/j.ijppaw.2013.04.004. http://www.sciencedirect.com/science/article/pii/S2213224413000151 Downloaded 27 August 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiras D.F., Craviotto M.B., Baneth G., Moré G. First report of Rangelia vitalii infection (canine rangeliosis) in Argentina. Parasitol. Int. 2014;63:729–734. doi: 10.1016/j.parint.2014.06.003. http://www.ncbi.nlm.nih.gov/pubmed/24970768 Downloaded 11 December 2015. [DOI] [PubMed] [Google Scholar]
- França R.T., Da Silva A.S., Costa M.M., Paim F.C., Soares J.F., Labruna M.B., Mazzanti C.M., Lopes S.T. Hematologic and bone marrow changes in dogs experimentally infected with Rangelia vitalii. Vet. Clin. Pathol. 2013;42:31–39. doi: 10.1111/vcp.12023. [DOI] [PubMed] [Google Scholar]
- Fredo G., Bianchi M.V., de Andrade C.P., de Souza S.O., Leite-Filho R.V., Bandinelli M.B., Amorim D.B., Driemeier D., Sonne L. Natural infection of wild canids (Cerdocyon thous and Lycalopex gymnocercus) with the intraendothelial piroplasm Rangelia vitalii in southern Brazil. J. Wildl. Dis. 2015;51:880–884. doi: 10.7589/2014-12-283. http://www.bioone.org/doi/pdf/10.7589/2014-12-283 Downloaded 11 December 2015. http://dx.doi.org/10.7589/2014-12-283. [DOI] [PubMed] [Google Scholar]
- Freitas M.G. Copiadora e Editora Rabelo & Brasil Ltda.; Belo Horizonte: 1977. Helmintologia Veterinária. [Google Scholar]
- Funk S.M., Fiorella C.V., Cleaveland S., Gompper M.E. Symposia of the Zoological Society of London. Cambridge University Press; Cambridge: 2001. The importance of disease in carnivore conservation; pp. 443–446. [Google Scholar]
- Gardiner C.H., Poynton S.L. Armed Forces Institute of Pathology; Washington: 1999. An Atlas of Metazoan Parasites in Animal Tissues; p. 64. [Google Scholar]
- Gasser R.B., Stewart L.E., Speare R. Genetic markers in ribosomal DNA for hookworm identification. Acta Trop. 1996;62:15–21. doi: 10.1016/s0001-706x(96)00015-0. http://www.sciencedirect.com/science/article/pii/S0001706X96000150 Downloaded 24 November 2015. [DOI] [PubMed] [Google Scholar]
- Gomes A.H.S., Cacciarro E.N., Mangini A.C.S., Dias R.M.D.S., Lapola S.R., César N.P.A., Corrêa M.O.A. Esparganose humana. Relato de um novo caso no estado de São Paulo. Rev. Inst. Adolfo Lutz. 1996;56:13–15. [Google Scholar]
- Jimenez J.A., Rodriguez S., Gamboa R., Rodriguez L., Garcia H.H., Cysticercosis Working Group in Peru Diphyllobothrium pacificum infection is seldom associated with megaloblastic anemia. Am. J. Trop. Med. Hyg. 2012;87:897–901. doi: 10.4269/ajtmh.2012.12-0067. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3516266/pdf/tropmed-87-897.pdf Downloaded 31 November 2015. doi: 10.4269/ajtmh.2012.12-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauspenhar C., Fighera R.A., Graça D.L. Anemia hemolítica em cães associada a protozoários. Medvep Rev. Cient. Med. Vet. Pequenos. Anim. Anim. Estim. 2003;1:273–281. http://medvep1.hospedagemdesites.ws/wp-content/uploads/2015/07/Mv004-05.pdf Downloaded 21 January 2016. [Google Scholar]
- Lima R.C., Hoppe E.G.L., Tebaldi J.H., Cruz B.C., Gomes A.A.B., Nascimento A.A. Helmintos gastrintestinais de Cerdocyon thous (Linnaeus, 1766) Smith, 1839 provenientes da área de caatinga do Estado da Paraíba, Brasil. Ciências Agrárias. 2013;34:2879–2888. http://repositorio.unesp.br/bitstream/handle/11449/111546/WOS000328275300030.pdf/sequence=1 Downloaded 02 November 2015. DOI: 10.5433/1679-0359.2013v34n6p2879. [Google Scholar]
- Lopes M.A., Ferrari S.F., Veiga L.M., Silva Júnior J.S. Chrysocyon brachyurus illiger, 1815. In: Machado A.B.M., Drummond G.M., Paglia A.P., editors. Livro vermelho da fauna brasileira ameaçada de extinção. Fundação Biodiversitas; Brasília, DF: MMA; Belo Horizonte, MG: 2008. pp. 780–782. 1420p. [Google Scholar]
- Loretti A.P., Barros S.S. Hemorrhagic disease in dogs infected with an unclassified intraendothelial piroplasm in southern Brazil. Vet. Parasitol. 2005;134:193–213. doi: 10.1016/j.vetpar.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Luna L.G. McGraw-Hill; New York: 1968. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. [Google Scholar]
- Luppi M.M., Malta M.C.C., Silva T.M., Silva F.L., Motta R.O., Miranda I., Ecco R., Santos R.L. Visceral leishmaniasis in captive wild canids in Brazil. Vet. Parasitol. 2008;155:146–151. doi: 10.1016/j.vetpar.2008.04.024. http://www.sciencedirect.com/science/article/pii/S0304401708002434 Downloaded 20 November 2015. [DOI] [PubMed] [Google Scholar]
- Mangini P.R., Vidolin G.P., Velastin G.O. Capítulo 18 – Pesquisa de macroparasitos em carnívoros selvagens: uma ferramenta para a conservação. In: Morato R.G., Rodrigues F.H.G., Eizirik E., Mangini P.R., de Azevedo F.C.C., Marinho-Filho J., editors. Manejo e conservação de carnívoros neotropicais: I workshop de pesquisa para a conservação. São Paulo: Ibama, 2006. 2006. p. 396. [Org.] 307-323. [Google Scholar]
- Mentz M.B., Procianoy F., Maestri M.K., Rott M.B. Human ocular sparganosis in southern Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2011;53:51–53. doi: 10.1590/s0036-46652011000100009. http://www.scielo.br/pdf/rimtsp/v53n1/v53n1a09.pdf Downloaded 22 November 2015. [DOI] [PubMed] [Google Scholar]
- Noyes H.A., Stevens J.R., Teixeira M., Phelan J., Holz P. A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. Int. J. Parasitol. 1999;29:331–339. doi: 10.1016/s0020-7519(98)00167-2. http://www.sciencedirect.com/science/article/pii/S0020751998001672 Erratum in: Int. J. Parasitol. 2000, 30, 228. Downloaded 10 January 2016. [DOI] [PubMed] [Google Scholar]
- Pastoret P.P., Brochier B. Epidemiology and control of fox rabies in Europe. Vaccine. 1999;17:1750–1754. doi: 10.1016/s0264-410x(98)00446-0. http://www.sciencedirect.com/science/article/pii/S0264410X98004460 Downloaded 19 December 2015. [DOI] [PubMed] [Google Scholar]
- Polley L. Navigating parasite webs and parasite flow: emerging and re-emerging parasitic zoonoses of wildlife origin. Int. J. Parasitol. 2005;35:1279–1294. doi: 10.1016/j.ijpara.2005.07.003. http://www.stoppinginvasives.org/dotAsset/010b5b4e-09e6-4c3d-b40c-2cc19c7952f0.pdf Downloaded 09 January 2015. [DOI] [PubMed] [Google Scholar]
- Ruas J.L., Muller G., Farias N.A.R., Gallina T., Lucas A.S., Pappen F.G., Sinkoc A.L., Brum J.G.W. Helminths of pampas fox Pseudalopex gymnocercus (Fischer, 1814) and of crab-eating fox Cerdocyon thous (Linnaeus, 1766) in the southern of the state of Rio Grande do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2008;17:87–92. doi: 10.1590/s1984-29612008000200005. http://www.scielo.br/pdf/rbpv/v17n2/05.pdf Downloaded 21 January 2015. [DOI] [PubMed] [Google Scholar]
- Scholz T., Garcia H.H., Kuchta R., Wicht B. Update on the human broad tapeworm (Genus Diphyllobothrium), including clinical relevance. Clin. Microbiol. Rev. 2009;22:146–160. doi: 10.1128/CMR.00033-08. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620636/pdf/0033-08.pdf Downloaded 08 November 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E.S., Gontijo C.M.F., Pacheco R.S., Fiuza V.O.P., Brazil R.P. Visceral leishmaniasis in metropolitan region of Belo Horizonte state of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz. 2001;96:285–291. doi: 10.1590/s0074-02762001000300002. http://www.scielo.br/pdf/mioc/v96n3/4111.pdf Downloaded 08 November 2015. [DOI] [PubMed] [Google Scholar]
- Soares J.F., Girotto A., Brandão P.E., da Silva A.S., França R.T., Lopes S.T., Labruna M.B. Detection and molecular characterization of a canine piroplasm from Brazil. Vet. Parasitol. 2011;180:203–208. doi: 10.1016/j.vetpar.2011.03.024. http://www.sciencedirect.com/science/article/pii/S0304401711001932 Downloaded 01 November 2015. [DOI] [PubMed] [Google Scholar]
- Soares J.F., Dall'Agnol B., Costa F.B., Krawczak F.S., Comerlato A.T., Rossato B.C., Linck C.M., Sigahi E.K., Teixeira R.H., Sonne L., Hagiwara M.K., Gregori F., Vieira M.I., Martins J.R., Reck J., Labruna M.B. Natural infection of the wild canid, Cerdocyon thous, with the piroplasmid Rangelia vitalii in Brazil. Vet. Parasitol. 2014;202:156–163. doi: 10.1016/j.vetpar.2014.02.058. http://www.sciencedirect.com/science/article/pii/S0304401714001575 DownloadeCd 22 November 2015. [DOI] [PubMed] [Google Scholar]
- Spolidorio M.G., Torres M.M., Campos W.N., Melo A.L., Igarashi M., Amude A.M., Labruna M.B., Aguiar D.M. Molecular detection of Hepatozoon canis and Babesia canis vogeli in domestic dogs from Cuiabá, Brazil. Rev. Bras. Parasitol. Vet. 2011;20:253–255. doi: 10.1590/s1984-29612011000300015. http://www.scielo.br/pdf/rbpv/v20n3/v20n3a15.pdf Downloaded 22 November 2015. [DOI] [PubMed] [Google Scholar]
- Stedmen N.L., Munday J.S., Esbeck R., Visvesvara G.S. Gastric amebiasis due to Entamoeba histolytica in a Dama Wallabay (Macropus eugenii) Vet. Pathol. 2003;40:340–342. doi: 10.1354/vp.40-3-340. http://vet.sagepub.com/content/40/3/340.full.pdf+html Downloaded 15 January 2016. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travassos L., Freitas T., Konh A. Trematódeos do Brasil. Mem. Inst. Oswaldo Cruz. 1969;67:1–886. http://mbe.oxfordjournals.org/content/30/12/2725.short Downloaded 21 January 2015. [PubMed] [Google Scholar]
- Vicente J.J., Rodrigues H.O., Gomes D.C., Pinto R.M. Nematóides do Brasil. Parte V: nematóides de Mamíferos. Rev. Bras. Zool. 1997;14:1–452. [Google Scholar]
- Vieira F.M., Luque J.L., Muniz-Pereira L.C. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa. 2008;1721:1–23. http://www.ufrrj.br/laboratorio/parasitologia/arquivos/publicacao/3_LIVRO.pdf Donwloaded 18 December 2015. [Google Scholar]
- Yamaguti S. vol. II. Interscience Publishers; New York & London: 1961. (Systema Helminthum, Nematodes). Part I. [Google Scholar]