Abstract
Purpose of review
Loss of ‘health-promoting’ microbes and overgrowth of pathogenic bacteria (dysbiosis) in ICU is believed to contribute to nosocomial infections, sepsis, and organ failure (multiple organ dysfunction syndrome). This review discusses new understanding of ICU dysbiosis, new data for probiotics and fecal transplantation in ICU, and new data characterizing the ICU microbiome.
Recent findings
ICU dysbiosis results from many factors, including ubiquitous antibiotic use and overuse. Despite advances in antibiotic therapy, infections and mortality from often multidrug-resistant organisms (i.e., Clostridium difficile) are increasing. This raises the question of whether restoration of a healthy microbiome via probiotics or other ‘dysbiosis therapies’ would be an optimal alternative, or parallel treatment option, to antibiotics. Recent clinical data demonstrate probiotics can reduce ICU infections and probiotics or fecal microbial transplant (FMT) can treat Clostridium difficile. This contributes to recommendations that probiotics should be considered to prevent infection in ICU. Unfortunately, significant clinical variability limits the strength of current recommendations and further large clinical trials of probiotics and FMT are needed. Before larger trials of ‘dysbiosis therapy’ can be thoughtfully undertaken, further characterization of ICU dysbiosis is needed. To addressing this, we conducted an initial analysis demonstrating a rapid and marked change from a ‘healthy’ microbiome to an often pathogen-dominant microbiota (dysbiosis) in a broad ICU population.
Summary
A growing body of evidence suggests critical illness and ubiquitous antibiotic use leads to ICU dysbiosis that is associated with increased ICU infection, sepsis, and multiple organ dysfunction syndrome. Probiotics and FMT show promise as ICU therapies for infection. We hope future-targeted therapies using microbiome signatures can be developed to correct ‘illness-promoting’ dysbiosis to restore a healthy microbiome post-ICU to improve patient outcomes.
Keywords: antibiotics, bacteria, critical care, fecal transplant, infection
INTRODUCTION: ROLE OF THE MICROBIOME IN HEALTH AND ILLNESS
One of the most exciting scientific advances in recent years has been the realization that commensal microorganisms (our microbiome) play key roles in our physiology, including protection against infection, in drug metabolism, vitamin synthesis, nutrition, as well as in response to disease. A surprising finding is that disruption of the homeostasis of the microbiota, known as ‘dysbiosis,’ may be as vital as host genetics in the development of a range of diseases, such as inflammatory bowel disease, obesity, diabetes, and cardiovascular disease. This suggests that it may be possible to monitor, prevent, or even cure human disease through regulating the human microbiota. Recent advances in culture-independent microbiome DNA sequencing methods, even in just the last few years, have resulted in an unprecedented growth in our understanding of this vital and dynamic organ. The medical community has put a large emphasis in the eradication of microbial life, and in many cases for good reason. But, perhaps, we should instead consider how to preserve or reestablish a ‘health-promoting’ microbiome during and after critical illness through targeted interventions, such as probiotics, prebiotics, fecal transplants, and or even synthetic ‘stool pills’ to improve outcome in critical illness.
Box 1.

no caption available
IS OUR CURRENT APPROACH TO INFECTION WORKING?
A great deal of time and effort is spent in eradicating bacteria and other microbial, fungal, and viral species in the ICU. The US Centers for Disease Control reveal 55% of all hospitalized patients receive an antibiotic during their stay, and in the ICU, this number increases to ∼70% of patients. This observation was confirmed by a recent multinational ICU study of more than 14 000 patients in over 1200 ICUs which found that 51% of patients were considered to be infected on day of survey and a striking 71% were receiving antibiotics [1]. The hospital mortality rate of infected patients was found to be more than twice that of noninfected patients (33 vs. 15%). Clearly, infection and sepsis remain a major driver of morbidity and mortality despite advances in hospital care and widespread ICU use antimicrobial therapy [1]. As recently described by Singer and Gynne [2], it is likely that this antibiotic use has in part contributed to an impressive 22-fold fall in crude mortality rates for infectious diseases in the United States between 1900 and 1980. Yet, it is troubling that mortality rates from infectious disease (up to 1996) increased – by 50% – with the septicemia rate nearly doubling [2]. It remains unclear if the earlier reductions in mortality and increased life expectancy were due primarily to antibiotics innovations, or more likely, because of improved public health and education.
The massive global reliance on antibiotic use comes at great financial expense with antibiotics accounting for up to 30% of a hospital's drug budget [3]. Unfortunately, the potential risk for patients far exceeds the financial costs. Evidence suggests that as many as 37% of antibiotic regimens are unnecessary or not compliant with guidelines [4▪▪]. This inappropriate antibiotic use leads to the emergence of multidrug resistant bacterial infections; the incidence of these infections is rising rapidly both in the United States and worldwide [5▪▪]. A recent New England Journal of Medicine article estimates antibiotic-resistant Clostridium difficile occurs now in more than 450 000 patients per year in the United States alone [5▪▪]. Additionally, these multidrug-resistant infections are also becoming increasingly lethal. For example, C. difficile is estimated to contribute to ∼30 000 deaths/year in the United States [5▪▪,6]. Further, the US Centers for Disease Control indicates ‘death rates from sepsis following infections (like C. difficile) have increased at a rate greater than any other common cause of mortality in the last year for which data were available’ [7]. And as stated, this is punctuated by mortality rates from infectious disease in general (up to 1996) increasing by 50%, again with the septicemia rate nearly doubling [2]. Thus, more advanced antibiotics do not appear to be translating to increased survival from infectious disease, but instead to increasingly aggressive resistant organisms and emergence of increasingly lethal pathogens like C. difficile. ‘Is it possible we need to rethink our strategy toward microbial therapy in the ICU?’
ANTIBIOTICS KILL MORE THEN JUST PATHOGENS
These concerns around antibiotics are compounded by the fact that antibiotics currently used to attempt to treat infection not only kill pathogens but also ‘health-promoting’ microbes. These adverse effects include the hypothesized loss of commensal gastrointestinal microbiota, which enables overgrowth of unwanted organisms (dysbiosis). This may have significant implications for organs far outside the gastrointestinal tract as well. The gut has long been described as the ‘motor’ of systemic inflammatory response syndrome and of organ failure regardless of the location of the initial infection [8▪▪]. Thus, the effect of alterations in the gut microbiota and gut barrier homeostasis are thought to be transmitted to and propagated by downstream organs, such as the spleen and lung where large immune cell populations are harbored [8▪▪,9,10], leading to inflammation-induced organ failure in the ICU.
At the cellular level, organ failure that ultimately leads to death in the ICU has long been attributed to mitochondrial failure. It has long been known that mitochondria trace their evolution from bacteria that produce energy for our cells. Recent literature not surprisingly reveals that mitochondria are known to be damaged by many of the antibiotics we commonly administer in the ICU [2]. Thus, we and others hypothesize that antibiotics may be contributing to organ failure by not only leading to dysbiosis but also by damaging the very core of our cells’ energy production [2].
IS THERE ANOTHER WAY TO PREVENT AND TREAT INFECTIONS?
‘Is it possible we should be giving “friendly” microbes to our patients, not eradicating them to improve outcome?’ For young Kaitlin Hunter, microbes were the best friends she could ever have. As described by national news reports (http://www.cnn.com/2012/09/26/health/fecal-transplant/) in 2011, when Kaitlin was only 20 years old, she was critically injured in a motor vehicle accident. She recovered from her injuries after just a month of hospitalization, but then as she neared returning home she was stricken with severe, life-threatening C. difficile colitis. Nine courses of antibiotics failed to treat the aggressive infection. In desperation, her physicians then turned to a novel idea: rather than treating the infection with more antibiotics why not give more bacteria in the form of a stool transplant from her mother. Almost immediately, Kaitlin's C. difficile was cured and never returned. Was this a miracle or should we have known this would be successful from the start? We know now that this was far from an uncommon miracle as recent data indicate that fecal transplantation has a more than 90% cure rate in even the most resistant C. difficile colitis cases [11▪]. How is it possible that using stool as therapy, what most would consider unsanitary at best (unethical at worst), is so effective?
The answer to Kaitlin's ‘miracle cure’ may lie in new insights from the study of the microbiome, which is beginning to call into question what it means to be ‘human.’ Perhaps, our success as a species is reliant on the trillions of bacterial symbiotes we harbor and depend on. It is first important to recognize that we live in a world dominated by bacteria [12▪]. Multicellular lineages like humans are rare with most of the molecular diversity of life residing in microbes. Further, what is it that truly makes us human? Many of us believe it is our cells that make us human; however, as shown in Fig. 1, new data show we are made up of as many bacterial cells as human cells (about a 1 : 1 ratio) [13▪]. Many would believe it is our genes that make us human; however, there are ∼20 000 human genes and an estimated 2–20 million microbial genes, making us about 1% human and 99% bacterial based on our genes alone. Again, this raises the key question that to fight infection organ failure should we be increasingly focused on treating dysbiosis by restoring a normal, healthy microbiome in our ICU patients, as it makes up as much or more of our bodies in health than our own cells or DNA?
FIGURE 1.
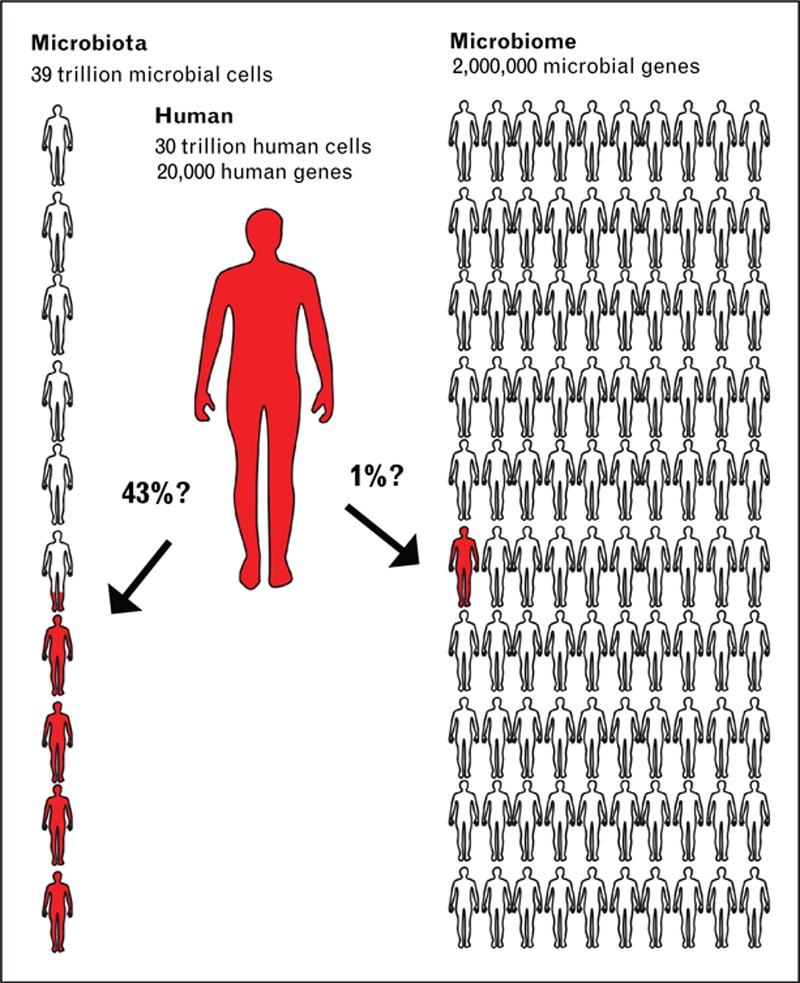
Ratio of microbial to human cells and genes in human body.
THE EVOLVING ROLE FOR PROBIOTICS AND FECAL TRANSPLANTATION TO ‘RESTORE HEALTH’ IN ILLNESS
More objectively, (as suggested by Loupazone et al.[14]), perhaps we need to ‘resod’ the lawn that is blighted by critical illness and antibiotics with probiotics, fecal transplants, and even ‘stool pills’ (see Fig. 2)? This concept is supported at a mechanistic level as described in a number of very recent and comprehensive review articles [8▪▪,15▪▪]. Alterations in intestinal homeostasis and gut microbiota in critical illness have been associated with increased inflammatory cytokine production, gut barrier dysfunction, and increased cellular apoptosis all of which can contribute to multiple organ failure. To modulate this ‘motor’ of systemic inflammation, it has been hypothesized that repletion of health-promoting bacteria via probiotics, prebiotics, stool transplantation, or combination therapies may be a promising intervention to maintain gut integrity and prevent pathologic alterations in the gut (and other body sites) microbiota or ‘dysbiosis’ [16▪,17▪,18▪▪]. Beneficial effects of probiotic interventions have been shown to include induction of host cell antimicrobial peptides, release of antimicrobial factors, suppression of the immune cell proliferation, stimulation of mucus and IgA production, antioxidative activity, inhibition of epithelial cell nuclear factor κ-B activation, prevention of gut apoptosis, and other epithelial barrier protective effects [8▪▪,19▪,20]. As a result, a growing number of clinical trials utilizing probiotics, and even stool transplantation, in critical illness are being conducted.
FIGURE 2.

Role of ‘dybiosis therapy’ to restore ‘health-promoting’ microbiome in critical illness.
REVIEW OF CURRENT DATA FOR PROBIOTIC USE IN CRITICAL ILLNESS
Probiotic use in critical care has recently been the subject of a number of meta-analyses focused on a range of outcomes after critical illness. A role for probiotics in reducing the risk of ICU infection was initially described by Petrof et al.[21] in 2012. Many clinical trials and meta-analysis efforts have focused on the role of reducing ventilator-associated pneumonia (VAP). In spite of promising data for probiotic use in reducing overall infections, the role of probiotics as a strategy to preventing VAP has been controversial. In 2010, Siempos et al.[22] aggregated five probiotic trials demonstrating a reduction in the incidence of VAP and subsequently, Barraud et al.[23] also showed a beneficial effect on ICU-acquired pneumonia. However, in 2012, Petrof et al.[21] and Wang et al.[24] were unable to demonstrate a significant effect of probiotics therapy on VAP. More recently, a Cochrane review of probiotic therapy specifically for VAP [25▪], found with a low quality of evidence that probiotic therapy is associated with a reduction in the incidence of VAP. In 2015, an updated meta-analysis from the Canadian Clinical Practice Guidelines Committee became available on www.criticalcarenutriton.com. This analysis evaluated 28 studies of probiotic therapy in critical care published up to late 2014. The new results indicate that probiotics continue to show a significant reduction in infections following critical illness [relative risk 0.82, 95% confidence interval (CI) 0.69, 0.97, P = 0.02, heterogeneity I2 = 41%] (see Fig. 3) and a trend to reduction in VAP (relative risk 0.74, 95% CI 0.55, 1.01, P = 0.06, heterogeneity I2 = 45%). A trend toward a decrease in ICU length of stay when results of 14 trials were pooled (weighted mean difference −3.26, 95% CI −7.82, 1.31, P = 0.16, heterogeneity I2 = 93%) although significant statistical heterogeneity was present in these data. No significant effect on mortality, hospital length of stay, or other outcomes was noted. The group noted that these estimates were found to be sensitive to the quality of the primary trials. This reduction in infections disappeared when only high-quality studies were considered. Further, the potential for statistical and clinical heterogeneity indicated that further trials were needed. Based on this analysis, the Canadian Clinical Practice Guidelines Committee concluded that ‘the use of probiotics should be considered in the critically ill patients.’ As a wide range of probiotic species and doses were utilized in these trials, no recommendation could be made for the dose or a particular type of probiotic, with the exception of Saccharomyces boulardii, which should not be used as it is considered unsafe in ICU patients. ‘However, further large and well designed clinical trials are needed to strengthen the recommendation of probiotic use and to confirm these benefits in critical illness’.
FIGURE 3.
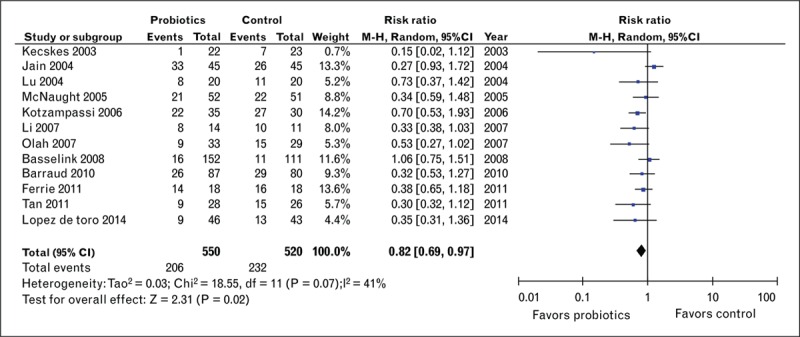
Effect of probiotic therapy on infection in ICU.
Very recently, a number of new trials of probiotic therapy in critical care have been published [26▪,27▪▪] not included in the recent unpublished Canadian Guidelines Meta-Analysis. These include a randomized, controlled multicenter trial by Zeng et al.[27▪▪] involving 235 critically ill adult patients expected to receive mechanical ventilation for at least 48 h. Patients were randomized to receive either a probiotics capsule containing live Bacillus subtilis and Enterococcus faecalis (Medilac-S), 0.5 g three times daily through a nasogastric feeding tube plus standard preventive strategies or standard preventive strategies alone, for a maximum of 14 days. The results revealed the incidence of microbiologically confirmed VAP in the probiotics group was significantly lower than in control patients (36.4 vs. 50.4%, respectively; P = 0.031). Further, the mean time to develop VAP was significantly longer in the probiotics group vs. control (10.4 vs. 7.5 days, respectively; P = 0.022). Future comprehensive meta-analysis systematic reviews including these newer trials are needed.
Focusing on antibiotic-associated diarrhea, a recent meta-analysis in JAMA showed in 63 studies and more than 11 800 patients that probiotics could reduce antibiotic-associated diarrhea by 40% [28]. Finally, a recent Cochrane meta-analysis of probiotic use in C. difficile colitis and diarrhea demonstrated that probiotics could reduce C. difficile-associated diarrhea by 64% in patients taking antibiotics (23 studies, n = 4213) [29▪▪]. Probiotics also reduced the risk of side-effects associated with antibiotic use in this analysis.
In addition to probiotic use, fecal transplantation has shown a more than 90% effectiveness in inducing a cure against C. difficile colitis [11▪,30] and ‘stool pills’ also may soon show promise for treating this increasingly aggressive infection. Finally, a recent case report of successful treatment of refractory severe sepsis and diarrhea with fecal transplant has been described [20]. Clinicaltrials.gov currently lists 157 clinical trials that are planned/completed or underway utilizing fecal transplantation (clinicaltrials.gov). Thus, much more data will soon be available regarding the efficacy of this therapy in multiple conditions.
CAN WE DEFINE THE ICU MICROBIOME (DYSBIOSIS) AND BETTER TARGET OUR THERAPY?
As stated, critical illness has been hypothesized to associate with loss of normal, ‘health-promoting’ commensal bacteria (dysbiosis), which might lead to a high susceptibility to hospital-acquired infections. Thus, a trial with prospective monitoring of the ICU microbiome with more comprehensive, culture-independent techniques to confirm and characterize this dysbiosis is urgently needed. Characterization of ICU microbiome changes may provide first steps in development of diagnostic and therapeutic interventions using microbiome signatures. Two recent pilot trials have begun to examine this question. Zaborin et al.[31▪▪] described ICU microbiome collection in 14 patients. The analysis focused on four patients with a prolonged length of ICU stay who showed significant disruption of the microbial community and the gut microbiota was shown to consist of ultra-low-diversity communities of multidrug-resistant pathogenic microbes. A second recent 12 patient trial by Ojima et al.[32▪] demonstrated changes in Bacteroidetes and Firmicutes may be related to mortality in ICU patients undergoing fecal microbiome analysis. These initial findings have led experts and major funding bodies in the field to conclude that there is urgent need for larger, more generalizable, prospective studies that characterize the microbiome in a larger critical care population to confirm and characterize this potential dysbiosis and move toward therapeutic interventions using microbiome signatures [33▪▪].
THE ICU MICROBIOME PROJECT: CHARACTERIZING THE DYSBIOSIS OF CRITICAL ILLNESS
To this end our research group (Wischmeyer et al.) have begun a collaboration with the Rob Knight Lab and have recently completed enrollment in an initial multicenter clinical trial of sequential microbiome sampling from ICU patients. To characterize the ICU microbiome, we collected fecal, oral, and skin samples from 115 mixed ICU patients across four centers in the United States and Canada. Samples were collected at two time points: within 48 h of ICU admission, and ICU discharge or ICU day 10. Sample collection and processing were performed under the Earth Microbiome Project protocols. We are utilizing a large control group of previously collected healthy controls and environmental surfaces including the American Gut Project, mammalian corpse decomposition samples, childhood (Global Gut), and house surfaces. In brief, the primary control group, the American Gut Project, is the largest crowd funded citizen science project, and includes more than 5000 participants, who range broadly in dietary habits, BMI, activity levels, medications, and age. For the purposes of our ICU Microbiome trial, we included healthy controls free of chronic disease and without recent antibiotic use.
CONCLUSION
Our initial results demonstrate that, when compared with healthy American gut study participants, critical illness shows rapid and distinct changes from a ‘healthy’ fecal and oral microbiome (Fig. 4). Fecal ICU samples tend to have a lower relative abundance of Firmicutes and increased relative abundance of Proteobacteria [34▪]. Large depletions were observed in organisms shown to confer anti-inflammatory benefits such as Faecalibacterium[35], which specifically is known to produce short-chain fatty acids that are vital to the gut. Conversely, many of the taxa which increased contain well recognized pathogens such as Enterobacter and Staphylococcus. Ongoing analysis will assess source composition of ICU samples and examine potential relationship of changes in the ICU microbiome to clinical outcome. In summary, our initial data from the ICU Microbiome Project confirm that severe dysbiosis occurs in a broad, larger population of critically ill study participants. These data may help guide creation of targeted microbial therapies, focused on correcting potentially ‘illness-promoting’ dysbiosis using specific probiotics or targeted, multimicrobe ‘stool pills’ to restore a healthy microbiome and improve outcomes in critical illness. And in the end, perhaps this is the beginning of a road to a better way to treat and prevent infection than the ubiquitous antibiotics universally given to most all patients in hospitals and ICUs today!
FIGURE 4.

Significant alteration in microbiome in critical illness vs. health study participants. Microbiome of fecal, oral, skin begins to converge in critical illness showing potential loss of diversity and possible indication of loss of barrier function.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323–2329. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Glynne P. Treating critical illness: the importance of first doing no harm. PLoS Med 2005; 2:e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruttimann S, Keck B, Hartmeier C, et al. Long-term antibiotic cost savings from a comprehensive intervention program in a medical department of a university-affiliated teaching hospital. Clin Infect Dis 2004; 38:348–356. [DOI] [PubMed] [Google Scholar]
- 4▪▪.Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]; The key report evaluates antibiotic prescribing practice in 183 US hospitals and shows antibiotic prescribing could be improved in 37.2% of the most common prescription scenarios reviewed. Further, they estimate if a 30% reduction in use of broad-spectrum antibiotics (would reduce overall antibiotic use by only 5%) could be achieved this would prevent 26% of C. difficile infections related to inpatient antibiotic use. Overall, evidence of incorrect prescribing and observed variability in current usage patterns suggest that improvements are needed and will benefit patients.
- 5▪▪.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]; Key report of active population and laboratory-based surveillance across 10 geographic areas in the United States in 2011 to identify cases of C. difficile infection. Results showed that C. difficile was responsible for almost half a million infections and was associated with approximately 29 000 deaths in 2011. (Funded by the Centers for Disease Control and Prevention.)
- 6.Rello J, Quintana E, Ausina V, et al. A three-year study of severe community-acquired pneumonia with emphasis on outcome. Chest 1993; 103:232–235. [DOI] [PubMed] [Google Scholar]
- 7.Milbrandt EB, Kersten A, Rahim MT, et al. Growth of intensive care unit resource use and its estimated cost in Medicare. Crit Care Med 2008; 36:2504–2510. [DOI] [PubMed] [Google Scholar]
- 8▪▪.Klingensmith NJ, Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Crit Care Clin 2016; 32:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent updated review of the role of the gut and gastrointestinal flora in multiorgan failure and critical illness.
- 9.Broquet A, Roquilly A, Jacqueline C, et al. Depletion of natural killer cells increases mice susceptibility in a Pseudomonas aeruginosa pneumonia model. Crit Care Med 2014; 42:e441–e450. [DOI] [PubMed] [Google Scholar]
- 10.Khailova L, Baird CH, Rush AA, et al. Lactobacillus rhamnosus GG improves outcome in experimental Pseudomonas aeruginosa pneumonia: potential role of regulatory T cells. Shock 2013; 40:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪.Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol 2016; 9:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review article of the use of fecal microbiome transplantation in a range of illnesses.
- 12▪.Locey KJ, Lennon JT. Scaling laws predict global microbial diversity. Proc Natl Acad Sci U S A 2016; 113:5970–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a global-scale compilation of ∼35 000 sites and ∼5.6×106 species, including the largest ever inventory of high-throughput molecular data and one of the largest compilations of plant and animal community data, similar rates of scaling in commonness and rarity across microorganisms and macroscopic plants and animals is demonstrated. Predicts Earth is home to upward of 1 trillion (1012) microbial species. Microbial biodiversity seems greater than ever anticipated yet predictable from the smallest to the largest microbiome.
- 13▪.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016; 164:337–340. [DOI] [PubMed] [Google Scholar]; Recent article carefully evaluating actual ratio of microbial cells to human cells in human body.
- 14.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature 2012; 489:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪▪.Dickson RP. The microbiome and critical illness. Lancet Respir Med 2016; 4:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive review and overview of role of microbiome in critical illness and known ecological effects of critical illness and clinical interventions on host microbiome. The study also has a major focus on lung and oral microbiome in ICU.
- 16▪.Marini JJ, Gattinoni L, Ince C, et al. A few of our favorite unconfirmed ideas. Crit Care 2015; 19 Suppl 3:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Proposals from leading experts in different critical care fields on future innovations in improving care of ICU patients that should undergo further evaluation and implementation. One area of future innovation discussed is use of microbiome to target probiotic, fecal transplant, and targeted stool pills to restore healthy microbiome in critically ill patients.
- 17▪.Andrade ME, Araujo RS, de Barros PA, et al. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin Nutr 2015; 34:1080–1087. [DOI] [PubMed] [Google Scholar]; Reviews the critical roles of immunomodulatory nutrients in supporting gut barrier integrity and function. Specifically, immunomodulators such as amino acids (glutamine), fatty acids (short-chain and omega-3 fatty acids and conjugated linoleic acids), and probiotics (Bifidobacterium and Lactobacillus) have been reported in the literature and are discussed here.
- 18▪▪.Krezalek MA, DeFazio J, Zaborina O, et al. The shift of an intestinal “microbiome” to a “pathobiome” governs the course and outcome of sepsis following surgical injury. Shock 2016; 45:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]; Key manuscript discussing novel approaches to integrating the molecular, ecological, and evolutionary dynamics of the evolving gut microbiome/pathobiome during critical illness are needed to understand and prevent the late onset sepsis that develops following prolonged critical illness.
- 19▪.Khailova L, Petrie B, Baird CH, et al. Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis. PLoS One 2014; 9:e97861. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study describing ability of probiotics to reduce lung injury in laboratory model of sepsis.
- 20.Shimizu K, Ogura H, Asahara T, et al. Probiotic/synbiotic therapy for treating critically ill patients from a gut microbiota perspective. Dig Dis Sci 2013; 58:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrof EO, Dhaliwal R, Manzanares W, et al. Probiotics in the critically ill: a systematic review of the randomized trial evidence. Crit Care Med 2012; 40:3290–3302. [DOI] [PubMed] [Google Scholar]
- 22.Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med 2010; 38:954–962. [DOI] [PubMed] [Google Scholar]
- 23.Barraud D, Bollaert PE, Gibot S. Impact of the administration of probiotics on mortality in critically ill adult patients: a meta-analysis of randomized controlled trials. Chest 2013; 143:646–655. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Liu KX, Ariani F, et al. Probiotics for preventing ventilator-associated pneumonia: a systematic review and meta-analysis of high-quality randomized controlled trials. PLoS One 2013; 8:e83934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25▪.Bo L, Li J, Tao T, et al. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev 2014; 10:CD009066. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent systematic analysis (meta-analysis) in eight RCTs, with 1083 critically ill patients showing use of probiotics decreased the incidence of VAP (odds ratio 0.70, 95% CI 0.52–0.95), although a reasonably low quality of evidence was found to exist in trials. Authors indicate given lower quality evidence no firm conclusions could be drawn for use of probiotics in VAP and larger trials are needed.
- 26▪.Rongrungruang Y, Krajangwittaya D, Pholtawornkulchai K, et al. Randomized controlled study of probiotics containing Lactobacillus casei (Shirota strain) for prevention of ventilator-associated pneumonia. J Med Assoc Thai 2015; 98:253–259. [PubMed] [Google Scholar]; Recent clinical trial showing administration of probiotics showing nonsignificant effect of Lactobacillus casei (Shirota strain) to reduce the incidence of VAP.
- 27▪▪.Zeng J, Wang CT, Zhang FS, et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med 2016; 42:1018–1028. [DOI] [PubMed] [Google Scholar]; Randomized controlled trial in 235 ICU patients of probiotics capsule containing live Bacillus subtilis and Enterococcus faecalis (Medilac-S), 0.5 g three times daily vs. standard of care showing incidence of microbiologically confirmed VAP in the probiotics group was significantly lower than in control patients (36.4 vs. 50.4%, respectively; P = 0.031). Further, the mean time to develop VAP was significantly longer in the probiotics group vs. control (10.4 vs. 7.5 days, respectively; P = 0.022).
- 28.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 2012; 307:1959–1969. [DOI] [PubMed] [Google Scholar]
- 29▪▪.Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013; 5:CD006095. [DOI] [PubMed] [Google Scholar]; Meta-analysis of 23 RCTs in 4213 patients (both pediatric and adult) showing probiotics can reduce risk of C. difficile diarrhea by 64%. Benefits were shown in both pediatric and adult patients, and benefits were observed regardless of probiotic strain used, dose, and in both higher and lower quality studies.
- 30.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 2013; 108:500–508. [DOI] [PubMed] [Google Scholar]
- 31▪▪.Zaborin A, Smith D, Garfield K, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio 2014; 5:e01361–e1414. [DOI] [PMC free article] [PubMed] [Google Scholar]; Key pilot trial of fecal ICU microbiome analysis in 14 patients. Analysis focused on four patients with a prolonged length of ICU stay who showed significant disruption of the microbial community and the gut microflora was shown to consist of ultra-low-diversity communities of multidrug-resistant pathogenic microbes.
- 32▪.Ojima M, Motooka D, Shimizu K, et al. Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig Dis Sci 2016; 61:1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent 12 patient pilot trial of fecal microbiome analysis showing changes in Bacteroidetes and Firmicutes may be related to mortality in ICU patients.
- 33▪▪.Lyons JD, Ford ML, Coopersmith CM. The microbiome in critical illness: firm conclusions or Bact to square one? Dig Dis Sci 2016; 61:1420–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]; Editorial published with [30] above and discussion of understanding of current state of ICU microbiome characterization. Authors indicate it is difficult to know how best to target the microbiome therapeutically when the understanding of this complex ecosystem is still in a relatively nascent state. Authors indicate need for larger, more generalizable, prospective studies that characterize the microbiome in a larger critical care population to confirm and characterize potential dysbiosis with the hope to move toward focused therapeutic interventions using microbiome data.
- 34▪.Wischmeyer P, McDonald D, Heyland DK, et al. Abstract 10: ICU microbiome project: critical illness is associated with loss of microbial diversity and major alterations in bacterial flora. JPEN J Parenter Enteral Nutr 2016; 40:123. [Google Scholar]; Abstract at Clinical Nutrition Week (ASPEN Congress) describing preliminary results from ICU Microbiome Project characterization of microbiome in ICU. Shows loss of microbial diversity and dybisosis in ICU patients as compared with healthy study participants’ microbiome.
- 35.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 2008; 105:16731–16736. [DOI] [PMC free article] [PubMed] [Google Scholar]


