Abstract
Background
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are immunotoxic in laboratory studies. Humans studies of immune effects are inconsistent. Using the U.S. National Health and Nutrition Examination Survey (NHANES) we examined PFAS serum concentration and indicators of prevalent immune function among 12 to 19 year old children.
Methods
In this cross-sectional study we examined PFAS serum concentration in relation to measles, mumps, and rubella antibody concentrations in NHANES 1999 – 2000 and 2003 – 2004 (n=1,191) and to allergic conditions and allergic sensitization in NHANES 2005 – 2006 (n=640).
Results
In adjusted, survey-weighted models, a doubling of perfluorooctane sulfonate (PFOS) concentration among seropositive children was associated with a 13.3% (95% CI −19.9, −6.2) decrease in rubella antibody concentration and a 5.9% decrease in mumps antibody concentration (95% CI −9.9, −1.6). We observed no adverse association between exposure and current allergic conditions, including asthma. Children with higher PFOS concentration were less likely to be sensitized to any allergen (OR 0.74, 95% CI 0.58, 0.95).
Conclusion
Increased exposure to several PFAS was associated with lower levels to mumps and rubella antibody concentrations, especially among seropositive individuals. These lower antibody concentrations may indicate a less robust response to vaccination or greater waning of vaccine-derived immunity over time.
INTRODUCTION
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) have been widely used since the 1950s as surfactants, surface treatment chemicals, and processing aids for many products, including oil, stain, grease, and water repellent coatings on carpet, textiles, leather, and paper (1). Human exposure typically occurs through transfer from food packaging and preparation materials, bioaccumulation in the food chain, and household dust (2). Some PFAS are persistent organic pollutants (3) and detected worldwide in wildlife and humans (4).
The U.S. National Health and Nutrition Examination Survey (NHANES) began PFAS biomonitoring in the 1999 – 2000 survey (5). The two most commonly studied PFAS – perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) – were detected in all serum samples with geometric mean concentrations of 5.21 ng/mL and 30.4 ng/mL, respectively (5). Measurable levels of PFAS also have been found in amniotic fluid (6, 7), maternal and umbilical cord blood (8, 9), and breast milk (10, 11). While efforts to reduce U.S. production and use of these compounds (12, 13) have led to declining serum concentrations, compounds such as perfluorononanoate (PFNA) have been increasing in both percent of the population with detectable levels and average serum concentration (14). Many PFAS have long biological half-lives. The serum elimination half-life is estimated at 2 to 4 years for PFOA (15, 16), 5 years for PFOS (16), and 8.5 years for perfluorohexane sulfonate (PFHxS) (16). Given their long half-lives and worldwide dispersion, exposure even to the legacy compounds will likely persist for some time.
Toxicological studies underscore the immunotoxic potential of PFAS. PFOS and PFOA alter inflammatory responses, cytokine expression, and adaptive and innate immune responses in rodent, avian, and reptilian models as well as in mammalian and non-mammalian wildlife (17). Immune effects have been observed in laboratory models at serum concentrations close to those in highly exposed humans and wildlife (17). These immune effects appear to work through numerous pathways including activation of peroxisome proliferator-activated receptor-alpha (PPAR-α), which can be anti-inflammatory (17) and activation of nuclear factor-kappa B (NF-KB), which can suppress cytokine secretion by immune cells (18). Mice exposed to PFOA exhibited increased IgE response after exposure to environmental allergens (19). In a separate murine model, neither PFOA nor PFOS appeared to be risk factors for allergic asthma-like symptoms even though PFOA induced airway inflammation and altered airway function (20). In human cells, PFOA triggered mast cell allergic reactions by histamine release and expression of pro-inflammatory cytokines (21). A recent study of human cord blood gene expression provided support for immune effects mediated through PPAR and NF-KB (22).
Epidemiological evidence of PFAS exposure and immune perturbation is mixed. Several studies examined associations between PFAS concentrations and various markers and measures of immune function from birth through 10 years of age. BraMat (n=99), a sub-cohort of the Norwegian Mother and Child Study, reported inverse associations between prenatal PFAS levels and serum antibody concentrations against rubella at age 3 (23). A Faroe Islands birth cohort (n=587) reported inverse associations between prenatal PFAS concentration and tetanus and diphtheria toxoids at ages 5 and 7 (24). A separate Faroese cohort (n=38) observed a negative association between prenatal PFOS and the autoantibody anti-actin IgG at age 7, although the clinical meaning of anti-actin IgG is unknown (25).
Three studies reported divergent associations between PFAS and serum IgE levels. In Japan (n=343), prenatal PFOA was negatively associated with cord blood IgE among female infants (26). In Taiwan (n=244), cord blood PFOS and PFOA were positively associated with cord blood IgE among male infants (27). A Taiwanese case-control study (n=456) reported positive associations between higher PFAS exposure and serum IgE among 10 – 15 year old asthmatics; non-asthmatic controls were not tested (28).
In examinations of clinical disease, the Japanese study found no relation between exposures and allergic disease or otitis media at age 18 months despite the elevated IgE levels at birth (26). BraMat reported that higher maternal PFAS levels at delivery were associated with increased risk of common cold (PFOA, PFNA) and gastroenteritis (PFOA, PFHxS) in children up to age 3; no associations were found with allergy or asthma-related outcomes (23). A subset of the Danish National Birth Cohort (n=1400) observed no clear pattern between prenatal PFOS and PFOA exposure and risk for infectious disease hospitalizations in childhood (29). INUENDO reported a reduced risk of wheeze in unadjusted models among 5 – 9 year old Ukrainian children (n=492) with higher prenatal PFOS exposure (30). The Taiwanese case-control study reported increased risk of asthma among those with the highest PFAS serum concentrations, ranging from an odds ratio (OR) of 1.81 (95% confidence interval (CI) 1.02 – 3.23) for perfluorododecanoic acid (PFDoA) to an OR of 4.05 (95% CI 2.21 – 7.42) for PFOA (28).
Recently, Humblet et al, using four waves of NHANES for children aged 12 – 19 years (n=1,877), also found a positive association between PFOA serum concentration and report of ever being diagnosed with asthma (31). In this cross-sectional study a doubling of PFOA serum concentration yielded an OR of 1.18 (95% CI 1.01 – 1.39). When the model accounted for NHANES survey weights, however, the magnitude of effect was diminished (OR 1.11, 95% CI 0.87 – 1.42). The measure of ever receiving an asthma diagnosis was selected because of the long half-life of many PFAS (31), but the validity of this measure in a cross-sectional study of 12 – 19 year olds depends on the hypothesized window of susceptibility.
To further explore possible links between PFAS exposure and perturbations of the immune system, we undertook a comprehensive examination of PFAS serum concentration and several indicators of immune function among children aged 12 to 19 years using multiple waves of NHANES. We examined PFAS serum concentration in relation to measles, mumps, and rubella antibody concentration levels in NHANES 1999 – 2000 and 2003 – 2004 and to allergic conditions and allergic sensitization in NHANES 2005 – 2006. In this cross-sectional study we focused exclusively on prevalent outcomes, which is the time period most relevant to prevalent exposure measures.
RESULTS
Vaccine Study
The Vaccine Study included 1,191 children with PFAS serum concentrations ranging from geometric mean 0.76 ng/mL (95% CI 0.65, 0.90) for PFNA to 20.8 ng/mL (95% CI 19.1, 22.7) for PFOS (Table 1). Less than 1% of the PFAS were below the LOD (PFOS 0%, PFOA 0.01%, PFHxS 0.14%, PFNA 0.17%). Pairwise correlations among PFAS were moderate to high (Table S1) except for PFHxS and PFNA (r=0.14, p<0.001). In adjusted models, there was no association between any of the PFAS and measles antibody levels (Table 2). A doubling of PFOS was associated with a 7.4% (95% CI −12.8, −1.7) decrease in mumps antibodies. This association was similar when restricted to seropositive children (−5.9%, 95% CI −9.9, −1.6). In the seropositive model there was also a 6.6% (95% CI −11.7, −1.5) decrease in mumps antibodies with a doubling of PFOA. The largest decrease in antibody concentrations was for rubella, although only among the seropositive subset. A doubling of PFOS was associated with a 13.3% (95%CI −19.9, −6.2) decrease in rubella antibodies. Decreases in rubella antibodies were also present for PFOA and PFHxS. Similar associations were observed in models with categorical treatment of PFAS (results not shown). In adjusted models, age was associated with antibody concentration level only for measles. In age-stratified models, the associations between PFAS and antibody concentration levels were not substantively different between 12 – 15 year olds and 16 – 19 year olds (results not shown).
Table 1.
Geometric Mean (95% Confidence Interval) Polyfluorinated Compound Concentrations (ng/mL) by Vaccine Antibody Study Characteristics among Children Aged 12 – 19 Years, 1999 – 2000 and 2003 – 2004 National Health and Nutrition Examination Surveys, n=1191
| PFOS ng/mL | PFOA ng/mL | PFHxS ng/mL | PFNA ng/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | GeoMean | 95% CI | GeoMean | 95% CI | GeoMean | 95% CI | GeoMean | 95% CI | |
| Overall | 1191 | 20.8 | (19.1, 22.7) | 4.13 | (3.76, 4.53) | 2.47 | (2.15, 2.85) | 0.765 | (0.648, 0.903) |
| Survey Year | |||||||||
| 1999 – 2000 | 551 | 29.0 | (26.3, 31.8) | 5.41 | (5.00, 5.85) | 2.64 | (2.14, 3.26) | 0.471 | (0.411, 0.54) |
| 2003 – 2004 | 640 | 19.3 | (17.6, 21.3) | 3.89 | (3.49, 4.33) | 2.44 | (2.06, 2.88) | 0.852 | (0.703, 1.03) |
| Age, years | |||||||||
| 12 – 15 | 587 | 20.9 | (18.8, 23.3) | 4.08 | (3.69, 4.51) | 2.64 | (2.21, 3.16) | 0.734 | (0.620, 0.868) |
| 16 – 19 | 604 | 20.7 | (18.9, 22.6) | 4.18 | (3.77, 4.63) | 2.32 | (1.98, 2.71) | 0.798 | (0.668, 0.953) |
| Sex | |||||||||
| Male | 626 | 22.4 | (20.2, 24.7) | 4.69 | (4.26, 5.16) | 2.71 | (2.25, 3.27) | 0.852 | (0.711, 1.02) |
| Female | 565 | 19.3 | (17.6, 21.0) | 3.60 | (3.24, 4.00) | 2.24 | (1.94, 2.60) | 0.682 | (0.583, 0.799) |
| Race/ethnicity | |||||||||
| Non-Hispanic white | 278 | 22.0 | (19.6, 24.7) | 4.32 | (3.88, 4.81) | 2.77 | (2.29, 3.35) | 0.812 | (0.703, 0.938) |
| Non-Hispanic black | 364 | 21.0 | (18.3, 24.1) | 4.14 | (3.75, 4.57) | 2.61 | (2.12, 3.21) | 0.886 | (0.652, 1.21) |
| Mexican American | 447 | 16.5 | (15.2, 18.0) | 3.55 | (3.28, 3.84) | 1.73 | (1.54, 1.95) | 0.542 | (0.477, 0.617) |
| Other | 102 | 19.4 | (16.2, 23.2) | 3.79 | (3.06, 4.69) | 1.86 | (1.48, 2.33) | 0.668 | (0.465, 0.959) |
| Measles seroconversion | |||||||||
| Positive | 1152 | 20.8 | (19.1, 22.7) | 4.15 | (3.77, 4.56) | 2.46 | (2.13, 2.84) | 0.768 | (0.649, 0.909) |
| Negative | 38 | 20.2 | (15.6, 26.2) | 3.56 | (2.84, 4.46) | 2.78 | (1.55, 4.99) | 0.674 | (0.465, 0.977) |
| Mumps seroconversion | |||||||||
| Positive | 1101 | 20.7 | (18.9, 22.6) | 4.13 | (3.73, 4.56) | 2.46 | (2.12, 2.86) | 0.765 | (0.644, 0.909) |
| Negative | 85 | 22.1 | (18.8, 25.9) | 4.15 | (3.79, 4.54) | 2.59 | (1.81, 3.70) | 0.781 | (0.646, 0.944) |
| Rubella seroconversion | |||||||||
| Positive | 1148 | 20.9 | (19.2, 22.8) | 4.16 | (3.78, 4.57) | 2.48 | (2.16, 2.84) | 0.768 | (0.647, 0.912) |
| Negative | 42 | 18.6 | (14.0, 24.8) | 3.64 | (3.01, 4.40) | 2.36 | (1.32, 4.21) | 0.701 | (0.608, 0.807) |
Table 2.
Adjusted Percent Change (95% Confidence Interval) in Measles, Mumps, and Rubella Antibody Titer with a Doubling in Polyfluorinated Compound Serum Concentration among Children Aged 12 – 19 Years, National Health and Nutrition Examination Survey, 1999 – 2000 and 2003 – 2004
| Measles | Mumps | Rubella | ||||
|---|---|---|---|---|---|---|
| All (n=1188) | Seropositive (n=1152) | All (n=1186) | Seropositive (n=1101) | All (n=1190) | Seropositive (n=1148) | |
| Polyfluorinated Compound | ||||||
| PFOS | −3.5 (−18.3, 14.0) | −2.9 (−17.3, 13.9) | −7.4 (−12.8, −1.7) | −5.9 (−9.9, −1.6) | −8.4 (−17.9, 2.1) | −13.3 (−19.9, −6.2) |
| PFOA | −0.1 (−13.8, 15.6) | −3.4 (−16.7, 11.9) | −6.0 (−12.4, 0.9) | −6.6 (−11.7, −1.5) | −2.5 (−9.1, 5.3) | −8.9 (−14.6, −2.9) |
| PFHxS | −3.8 (−11.5, 4.6) | −2.8 (−10.1, 5.21) | −2.6 (−6.7, 1.7) | −2.3 (−5.5, 0.9) | −5.0 (−10.8, 1.2) | −6.0 (−9.6, −2.2) |
| PFNA | 1.8 (−11.4, 17.1) | 1.1 (−11.8, 15.9) | −2.7 (−7.2, 2.0) | −2.7 (−8.4, 3.4) | 3.2 (−5.3, 12.6) | 0.6 (−6.7, 8.5) |
Adjusted for age (continuous), sex, race/ethnicity, survey year
Allergy Study
The Allergy Study included 640 children with serum concentrations ranging from geometric mean 0.93 ng/mL (95% CI 0.78, 1.1) for PFNA to 15.0 ng/mL (95% CI 14.3, 15.7) for PFOS (Table 3). Overall 11.3% of children reported current asthma, 20.4% reported current allergies, and 45.9% were sensitized to at least one of 19 measured allergens. Less than 1% of the PFAS were below the LOD (PFOS 0%, PFOA 0.01%, PFHxS 0.51%, PFNA 0.12%). Pairwise correlations among PFAS were moderate to high except for PFHxS and PFNA (Table S2; r=0.15, p<0.0001). In adjusted models, the pattern of association between PFAS exposures and current allergic conditions appeared null for both continuous (Table 4) and categorical (results not shown) treatment of PFAS. The strongest finding, as well as the only statistically significant finding, was for increased PFOA and prevalent rhinitis (IQR OR 1.35, 95% CI 1.10, 1.66).
Table 3.
Geometric Mean (95% Confidence Interval) Polyfluorinated Compound Concentrations (ng/mL) by Allergic Conditions and Allergic Sensitization Study Characteristics among Children Aged 12 – 19 Years, 2005 – 2006 National Health and Nutrition Examination Survey, n=640
| PFOS ng/mL | PFOA ng/mL | PFHxS ng/mL | PFNA ng/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | GeoMean | 95% CI | GeoMean | 95% CI | GeoMean | 95% CI | GeoMean | 95% CI | |
| Overall | 640 | 15.0 | (14.3, 15.7) | 3.59 | (3.26, 3.96) | 2.09 | (1.74, 2.52) | 0.929 | (0.782, 1.1) |
| Age, years | |||||||||
| 12 – 15 | 313 | 13.7 | (12.9, 14.6) | 3.42 | (3.10, 3.78) | 1.85 | (1.45, 2.37) | 0.917 | (0.740, 1.14) |
| 16 – 19 | 321 | 16.4 | (15.2, 17.6) | 3.77 | (3.36, 4.22) | 2.36 | (1.81, 3.08) | 0.941 | (0.774, 1.14) |
| Sex | |||||||||
| Male | 328 | 16.5 | (15.2, 17.9) | 3.95 | (3.57, 4.37) | 2.15 | (1.78, 2.61) | 0.985 | (0.847, 1.15) |
| Female | 312 | 13.6 | (12.1, 15.3) | 3.26 | (2.81, 3.77) | 2.03 | (1.54, 2.68) | 0.875 | (0.699, 1.10) |
| Race/ethnicity | |||||||||
| Non-Hispanic white | 160 | 16.4 | (15.0, 18.1) | 3.85 | (3.44, 4.3) | 2.39 | (1.96, 2.91) | 0.937 | (0.765, 1.15) |
| Non-Hispanic black | 220 | 14.3 | (12.8, 16.1) | 3.23 | (2.64, 3.94) | 1.79 | (1.25, 2.56) | 0.966 | (0.828, 1.13) |
| Mexican American | 212 | 10.6 | (9.54, 11.8) | 2.95 | (2.65, 3.27) | 1.57 | (1.10, 2.23) | 0.820 | (0.685, 0.981) |
| Other | 48 | 13.3 | (11.5, 15.4) | 3.44 | (2.96, 4.00) | 1.6 | (1.04, 2.47) | 0.958 | (0.687, 1.33) |
| Weight Status | |||||||||
| Under or target weight | 394 | 15.8 | (14.7, 17.0) | 3.7 | (3.33, 4.09) | 2.26 | (1.83, 2.78) | 0.947 | (0.803, 1.12) |
| Overweight | 93 | 13.2 | (11.1, 15.8) | 3.23 | (2.60, 4.01) | 1.99 | (1.32, 2.99) | 0.876 | (0.690, 1.11) |
| Obese | 150 | 13.5 | (11.5, 15.9) | 3.48 | (3.07, 3.94) | 1.68 | (1.05, 2.69) | 0.902 | (0.693, 1.18) |
| Serum Cotinine (ng/mL) | |||||||||
| Below LOD (<0.015) | 93 | 13.3 | (11.6, 15.3) | 3.49 | (3.00, 4.05) | 1.56 | (1.09, 2.22) | 0.926 | (0.724, 1.18) |
| Low (0.015 – 10) | 460 | 14.6 | (13.8, 15.5) | 3.41 | (3.05, 3.83) | 2.08 | (1.73, 2.50) | 0.891 | (0.747, 1.06) |
| High (>10) | 87 | 18.5 | (16.1, 21.3) | 4.54 | (3.79, 5.43) | 2.84 | (1.83, 4.40) | 1.10 | (0.891, 1.36) |
| Current Allergic Condition | |||||||||
| Asthma | |||||||||
| No | 570 | 14.9 | (14.2, 15.7) | 3.56 | (3.23, 3.92) | 2.11 | (1.72, 2.59) | 0.917 | (0.768, 1.10) |
| Yes | 70 | 15.5 | (13.4, 17.9) | 3.86 | (3.26, 4.56) | 1.95 | (0.883, 4.29) | 1.03 | (0.832, 1.26) |
| Wheeze | |||||||||
| No | 568 | 15.1 | (14.2, 16.0) | 3.58 | (3.25, 3.95) | 2.08 | (1.68, 2.58) | 0.927 | (0.789, 1.09) |
| Yes | 72 | 14.2 | (11.1, 18.1) | 3.66 | (2.77, 4.83) | 2.21 | (1.70, 2.88) | 0.944 | (0.665, 1.34) |
| Allergy | |||||||||
| No | 537 | 14.8 | (13.9, 15.8) | 3.55 | (3.22, 3.90) | 2.11 | (1.73, 2.57) | 0.918 | (0.758, 1.11) |
| Yes | 102 | 15.5 | (14.3, 16.8) | 3.75 | (3.24, 4.34) | 1.99 | (1.34, 2.96) | 0.969 | (0.846, 1.11) |
| Rhinitis | |||||||||
| No | 476 | 14.6 | (13.7, 15.5) | 3.45 | (3.09, 3.85) | 2.16 | (1.79, 2.61) | 0.897 | (0.736, 1.09) |
| Yes | 164 | 16.0 | (14.4, 17.8) | 3.95 | (3.60, 4.33) | 1.94 | (1.34, 2.81) | 1.01 | (0.881, 1.15) |
| Allergic Sensitization | |||||||||
| Any | |||||||||
| No | 297 | 15.8 | (14.7, 17.0) | 3.64 | (3.23, 4.11) | 2.20 | (1.68, 2.87) | 0.917 | (0.733, 1.15) |
| Yes | 343 | 14.0 | (13.1, 15.1) | 3.53 | (3.21, 3.88) | 1.97 | (1.53, 2.54) | 0.943 | (0.818, 1.09) |
| Plants | |||||||||
| No | 413 | 15.6 | (14.6, 16.7) | 3.66 | (3.29, 4.07) | 2.16 | (1.73, 2.71) | 0.936 | (0.76, 1.15) |
| Yes | 227 | 13.6 | (12.4, 15.0) | 3.44 | (3.09, 3.83) | 1.94 | (1.34, 2.79) | 0.913 | (0.811, 1.03) |
| Dust mites | |||||||||
| No | 462 | 15.0 | (14.1, 15.9) | 3.61 | (3.29, 3.95) | 2.10 | (1.72, 2.56) | 0.922 | (0.765, 1.11) |
| Yes | 178 | 15.0 | (13.5, 16.8) | 3.54 | (3.09, 4.05) | 2.08 | (1.58, 2.74) | 0.952 | (0.774, 1.17) |
| Pets | |||||||||
| No | 491 | 15.1 | (14.3, 16.0) | 3.52 | (3.16, 3.93) | 2.11 | (1.73, 2.56) | 0.902 | (0.73, 1.11) |
| Yes | 149 | 14.6 | (12.7, 16.6) | 3.84 | (3.41, 4.32) | 2.05 | (1.46, 2.86) | 1.03 | (0.899, 1.18) |
| Cockroach or shrimp | |||||||||
| No | 514 | 15.4 | (14.5, 16.4) | 3.65 | (3.29, 4.05) | 2.23 | (1.86, 2.67) | 0.937 | (0.784, 1.12) |
| Yes | 126 | 12.5 | (11.0, 14.2) | 3.25 | (2.86, 3.70) | 1.44 | (0.924, 2.25) | 0.881 | (0.698, 1.11) |
| Rodents | |||||||||
| No | 612 | 15.0 | (14.2, 15.9) | 3.56 | (3.22, 3.95) | 2.11 | (1.75, 2.55) | 0.915 | (0.762, 1.1) |
| Yes | 28 | 14.3 | (8.43, 24.4) | 4.34 | (3.08, 6.10) | 1.65 | (0.966, 2.82) | 1.35 | (0.94, 1.95) |
| Mold | |||||||||
| No | 535 | 14.6 | (13.9, 15.4) | 3.54 | (3.20, 3.93) | 2.09 | (1.69, 2.58) | 0.91 | (0.745, 1.11) |
| Yes | 105 | 17.0 | (15.4, 18.8) | 3.86 | (3.33, 4.47) | 2.11 | (1.44, 3.11) | 1.04 | (0.863, 1.25) |
| Food | |||||||||
| No | 583 | 15.1 | (14.3, 16.0) | 3.58 | (3.23, 3.97) | 2.09 | (1.73, 2.53) | 0.929 | (0.777, 1.11) |
| Yes | 57 | 13.4 | (10.2, 17.6) | 3.67 | (3.00, 4.50) | 2.10 | (1.49, 2.95) | 0.923 | (0.708, 1.2) |
Table 4.
Adjusted Associations [OR (95% CI)] between Polyfluorinated Compound Serum Concentration and Current Asthma and Allergic Conditions among Children Aged 12 – 19 Years, National Health and Nutrition Examination Survey, 2005 – 2006, n=638
| Polyfluorinated Compound | Asthma | Wheeze | Allergy | Rhinitis |
|---|---|---|---|---|
| PFOS | 1.20 (0.88, 1.63) | 0.76 (0.45, 1.29) | 1.05 (0.80, 1.37) | 1.16 (0.90, 1.50) |
| PFOA | 1.28 (0.81, 2.04) | 0.94 (0.51, 1.73) | 1.12 (0.85, 1.47) | 1.35 (1.10, 1.66) |
| PFHxS | 0.98 (0.51, 1.87) | 0.99 (0.68, 1.44) | 0.83 (0.59, 1.17) | 0.81 (0.57, 1.16) |
| PFNA | 1.26 (0.79, 2.01) | 0.99 (0.58, 1.68) | 1.12 (0.85, 1.47) | 1.24 (0.97, 1.60) |
Adjusted for age (continuous), gender, race, weight status, serum cotinine
Effect estimates represent the odds ratio for the outcome with a shift from the 25th percentile to the 75th percentile in PFC serum levels (IQR [lnPFOS] = 0.76; IQR [lnPFOA] =0.78; IQR [lnPFHxS] = 1.46; IQR [lnPFHxS] =0.84)
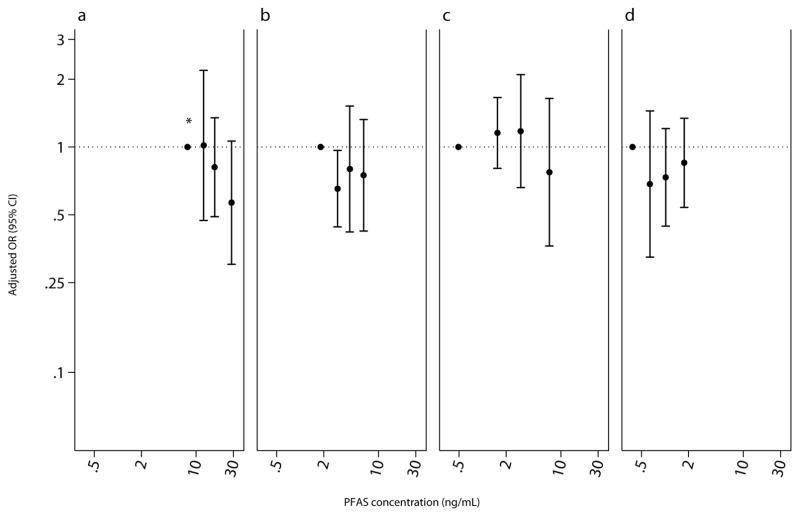
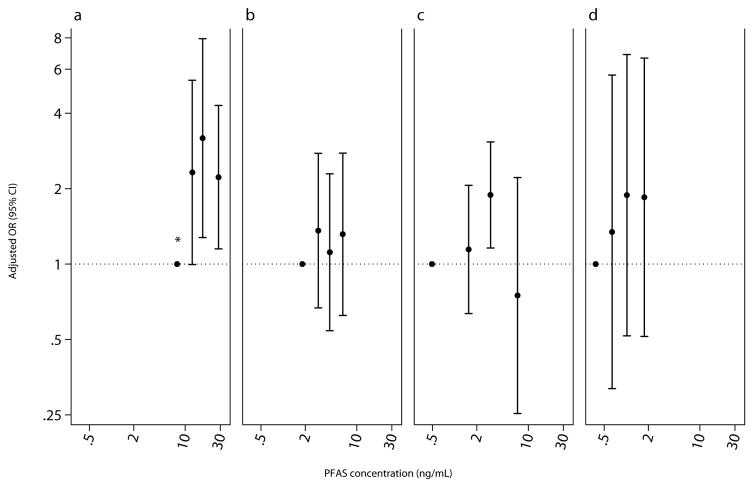
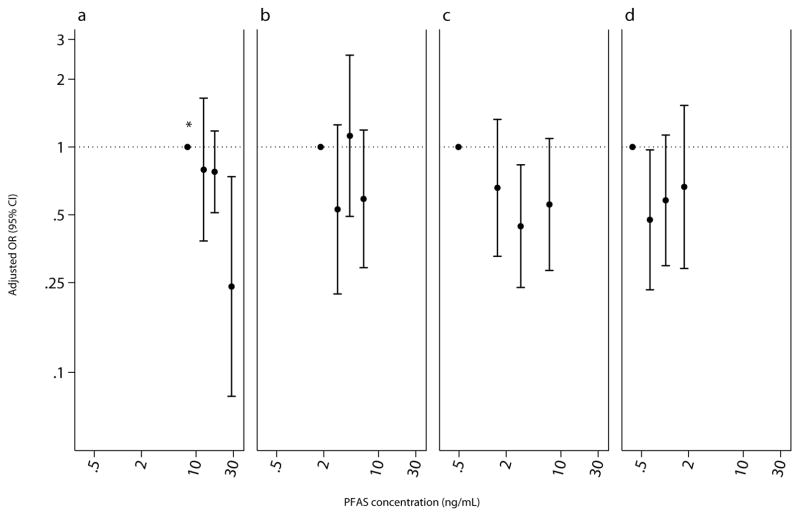
Children with higher PFOS serum concentration were less likely to be sensitized to any allergen (IQR OR 0.74, 95% CI 0.58, 0.95; Table 5). Specifically, children with higher levels to some PFAS were less likely to be sensitized to plants (PFOS IQR OR 0.71, 0.53, 0.97) and cockroach or shrimp (PFOS IQR OR 0.67, 95% CI 0.48, 0.93; PFHxS IQR OR 0.72, 95% CI 0.56, 0.93). Children with higher PFOS levels, however, were more likely to be sensitized to mold (IQR OR 1.33 95% CI 1.06, 1.69). Categorical treatment of PFAS endorsed the observed associations for any (PFOS p-trend=0.02), cockroach or shrimp (PFOS p-trend=0.003), and mold sensitizations (PFOS p-trend=0.04; Figures 1 – 3). The association with mold appears to be driven by Alternaria; a doubling of PFOS was associated with a 7.5% (95% CI 0.5, 15.1) increase in Alternaria IgE antibodies. Despite no other meaningful associations with sensitization to any allergen-specific antibodies, children with higher PFOA and PFNA levels had higher total IgE antibodies (Table 6).
Table 5.
Adjusted Associations [OR (95% CI)] between Polyfluorinated Compound Serum Concentration and Allergic Sensitization (sIgE ≥ 0.35 kU/L) among Children Aged 12 – 19 Years, National Health and Nutrition Examination Survey, 2005 – 2006, n=638
| Polyfluorinated Compound | Any | Plants | Dust mites | Pets | Cockroach, shrimp | Rodents | Mold | Food |
|---|---|---|---|---|---|---|---|---|
| PFOS | 0.74 (0.58, 0.95) | 0.71 (0.53, 0.97) | 1.00 (0.73, 1.38) | 0.83 (0.56, 1.22) | 0.67 (0.48, 0.93) | 0.85 (0.29, 2.45) | 1.33 (1.06, 1.69) | 0.74 (0.39, 1.40) |
| PFOA | 0.93 (0.72, 1.21) | 0.88 (0.67, 1.15) | 0.93 (0.75, 1.16) | 1.17 (0.81, 1.68) | 0.79 (0.55, 1.13) | 1.65 (0.59, 4.60) | 1.21 (0.85, 1.72) | 1.02 (0.60, 1.73) |
| PFHxS | 0.92 (0.66, 1.28) | 0.93 (0.62, 1.39) | 1.01 (0.84, 1.22) | 0.96 (0.71, 1.30) | 0.72 (0.56, 0.93) | 0.81 (0.54, 1.21) | 0.98 (0.65, 1.47) | 1.03 (0.74, 1.42) |
| PFNA | 1.04 (0.80, 1.35) | 0.96 (0.74, 1.23) | 1.05 (0.78, 1.41) | 1.26 (0.85, 1.87) | 0.86 (0.60, 1.24) | 2.25 (0.83, 6.10) | 1.31 (0.83, 2.06) | 0.91 (0.55, 1.50) |
Adjusted for age (continuous), gender, race, weight status, serum cotinine
Effect estimates represent the odds ratio for the outcome with a shift from the 25th percentile to the 75th percentile in PFC serum levels (IQR [lnPFOS] = 0.76; IQR [lnPFOA] =0.78; IQR [lnPFHxS] = 1.46; IQR [lnPFHxS] =0.84)
Figure 1.
Adjusted odds ratio (95% CI) for any IgE sensitization (IgE ≥0.35 kU/L) among children aged 12 – 19 years, National Health and Nutrition Examination Survey, 2005 – 2006 (n=638). PFAS biomarker concentrations plotted at the median by quartile of exposure. Adjusted for age, sex, race/ethnicity, BMI, cotinine, and survey weights.(a) PFOS, P for trend<0.05. (b) PFOA. (c) PFHxS. (d) PFNA
Figure 3.
Adjusted odds ratio (95% CI) for mold (Alternaria and Aspergillus species) IgE sensitization (IgE ≥0.35 kU/L) among children aged 12 – 19 years, National Health and Nutrition Examination Survey, 2005 – 2006 (n=638). PFAS biomarker concentrations plotted at the median by quartile of exposure. Adjusted for age, sex, race/ethnicity, BMI, cotinine, and survey weights. (a) PFOS, P for trend<0.05. (b) PFOA. (c) PFHxS. (d) PFNA
Table 6.
Adjusted Percent Change (95% Confidence Interval) in Serum IgE Antibody Titer with a Doubling in Polyfluorinated Compound Serum Concentration among Children Aged 12 – 19 Years, National Health and Nutrition Examination Survey, 2005 – 2006, n=634
| IgE | |
|---|---|
| Polyfluorinated Compound | |
| PFOS | 5.4 (−15.4, 31.4) |
| PFOA | 10.5 (0.17, 22) |
| PFHxS | 1.1 (−8.4, 11.7) |
| PFNA | 19.4 (8.3, 31.7) |
Adjusted for age (continuous), gender, race, weight status, serum cotinine
DISCUSSION
We observed that PFAS serum concentration was related to allergen-specific IgE sensitization. To our knowledge this is the first such report. Children with higher PFAS exposure were less likely to have IgE sensitization to any of 19 allergens (PFOS) and specifically to plants (PFOS) and cockroaches or shrimp (PFOS, PFHxS). Children with higher PFOS exposure, however, were more likely to be sensitized to mold. Curiously, although PFOA and PFNA were not related to most measured allergen-specific IgEs or to report of current asthma or allergies, a doubling of these exposures was associated with 10% and 19% increases in total IgE, respectively. This association between higher exposure and higher total IgE, but not between higher exposure and clinical disease, is interesting because higher total IgE levels are typically indicative of asthma and allergies (32). This seemingly paradoxical observation may reflect our reliance on self-reported allergic conditions, be due to unmeasured confounding, or be an artifact of our study’s cross-sectional design. For instance, an analysis of the longitudinal Multi-centre Allergy Study showed that an increase in total IgE during childhood was temporally related to onset of respiratory allergies (33). In this cross-sectional study of serum PFAS concentrations and indicators of immune function among children aged 12 to 19 we observed no adverse association between exposure and current allergic conditions, including asthma.
In relation to vaccine antibody concentration levels, higher PFAS exposure was associated with lower antibody concentrations to mumps and rubella, but not measles. The validity of this finding was strengthened by the observation that the association between PFAS concentration and antibody concentration was comparable among 12 – 15 and 16 – 19 year olds. Lower antibody concentration levels, especially among seropositive individuals, may indicate a less robust response to vaccination or greater waning of vaccine-derived immunity over time.
Five studies have examined allergic conditions in relation to PFAS exposures, with results divided between protective/null (23, 26, 30) and adverse (28, 31) effects. In a Taiwanese case-control study of children aged 10 – 15 years Dong et al reported elevated ORs for asthma at the highest compared to lowest quartile of exposure for PFOS, PFOA, PFHxS, and PFNA (28). In this population PFOS levels were higher and PFOA levels were lower as compared to our population. Additionally, asthma cases were recruited from hospitals and controls had no personal or family history of asthma. This recruitment strategy likely resulted in less outcome misclassification than the questionnaire data available for our study. Humblet et al, however, reported a small, adverse association between PFOA concentration and lifetime asthma diagnosis in NHANES, which became null when adjusting for NHANES complex survey design (31). Only lifetime asthma diagnosis – not current asthma – was statistically significant. In a cross-sectional study such as NHANES it is uncertain that a contemporary measure of PFAS relates to a past diagnosis with asthma.
The Tawainese case-control study also reported adverse associations between PFOS and PFOA and serum IgE among asthma cases (28), which suggests that PFAS may be related to allergic inflammation among asthmatic children. Two studies looked at the associations between maternal prenatal (26) and cord blood (27) PFAS concentrations and cord blood IgE levels. One study observed a protective association, but only among female neonates (26). The other study observed an adverse association, but only among male neonates (27). Divergent study populations, exposure levels, and analytical methods make comparing the magnitude of effects difficult. For instance, the former study examined asthmatic children and the latter studies examined disease-naïve neonates.
Higher PFOS levels appear to be associated with decreased IgE sensitization to plants and cockroaches or shrimp and increased IgE sensitization to mold. An interquartile increase in serum PFOS concentration was associated with a 26% reduction in odds of IgE sensitization to any of 19 specific measured allergens. These primarily inverse associations appear counter to toxicological studies showing increased IgE response in PFOA-dosed mice after exposure to environmental allergens (19), although PFOS and PFOA may act through different mechanisms.
Two birth cohorts reported adverse associations between prenatal PFAS concentration and vaccine antibody study also observed an adverse association with rubella and no association with measles. Our study is the first to report associations with mumps antibody concentration levels.
The clinical relevance of lower vaccine antibody concentrations at the levels we report – a 13% decrease for rubella with a doubling of PFOS concentration – is uncertain, particularly because this finding was among a subset of children who had antibody levels high enough to be considered protected against disease. Rubella and mumps are strong immunogens and antibodies for these viral vaccines typically last decades (34). In comparison, diptheria and tetanus need regular boosting to keep antibodies high enough for disease protection. It is noteworthy, then, that the greatest observed reduction was for rubella. Vaccination information was not available in NHANES, although beginning in 1990 most states have required a measles-mumps-rubella booster before starting school. Approximately 60% of the children included in our analyses are young enough to have entered school since 1990. While we don’t know which children were vaccinated or on what schedule, our restriction to the seropostive population serves as a proxy for vaccination (35). If we see a 13% reduction in antibodies after what may be fewer than 15 years since vaccination it is possible that with longer follow-up time antibodies would continue to decline and eventually convert to seronegative.
The limitations of this study are typical of a cross-sectional study of existing data. We can make no claims of causality and were reliant on self-report for designation of allergic conditions. NHANES, however, does ensure a representative population, which is particularly important as serum concentrations of PFOS and PFOA decline and PFHxS and PFNA increase over time. There may also be less exposure misclassification than in a typical cross-sectional study because these compounds have long serum half lives. Additionally, NHANES 2005 – 2006 included specific IgE allergen testing, which is a unique feature of our investigation. NHANES also allowed us to examine several indicators of immune function, although lack of information on receipt and/or timing of measles-mumps-rubella vaccination is a weakness of the survey. With four PFAS and numerous outcomes, however, we subjected the data to multiple testing. Nonetheless, the observed strong, consistent association between increasing levels of PFOS and decreasing levels of rubella antibody concentration is concerning and deserves further investigation to better elucidate the relation and understand the mechanism of action.
Although the United States eliminated production and use of PFOS(12) and PFOA(13), these chemicals remain detectable in human sera and biomonitoring studies indicate that replacement chemicals are increasingly detectable (14). These replacement chemicals may have similar toxicological effects so our study of older (PFOS, PFOA) and newer (PFHxS, PFNA) compounds is relevant. Several findings may be important to public health. We observed that higher PFOS concentration was associated with decreased mumps and rubella antibody concentrations and that higher PFOA and PFNA concentrations were associated with increased total serum IgE. Overall the relationship between PFAS and allergic disease appears complicated with both increases and decreases in allergen specific IgE with greater PFAS exposures. Our results suggest a complex interaction between PFAS and the human immune system that may influence vaccine-induced immunity as well as susceptibility to allergic disease. Additional investigation into the role of PFAS and response to vaccination is warranted.
METHODS
Study Population
NHANES is a nationally representative survey conducted by the U.S. Centers for Disease Control and Prevention designed to monitor the health and nutrition status of the non-institutionalized, civilian population (36). The survey includes interviews, examinations, and laboratory tests on approximately 5,000 persons each year; data are released in 2-year cycles. This investigation was conducted as two separate analyses because the information collected varies across NHANES cycles. We used NHANES 1999 – 2000 and 2003 – 2004 for examination of vaccine antibody concentrations (Vaccine Study) and NHANES 2005 – 2006 for examination of allergic conditions and allergic sensitization (Allergy Study). We included children aged 12 – 19 years (36) with serum PFAS measurements and data on the vaccine or allergy outcomes relevant to each study. NHANES is publicly available data and its use is not considered human subject research.
Exposure Assessment
PFAS were measured in sera from eligible participants aged 12 years and older. In 1999 – 2000 11 PFAS were measured in surplus sera. In 2003 – 2004 and 2005 – 2006 12 PFAS were measured in a one-third sub-sample of participants (36). Detailed descriptions of the analytic methods have been previously published (5, 14). In brief, PFAS were detected and quantified in serum using a modification of online solid-phase extraction coupled to reversed-phase high-performance liquid chromatography–tandem mass spectrometry. PFAS concentrations below the limit of detection (LOD) were substituted with LOD divided by the square root of two. We restricted our investigation to four highly detected PFAS (PFOS, PFOA, PFHxS, PFNA). PFAS were natural-log transformed to correct for non-normal distribution and also ranked into quartiles. We obtained p-trend for quartiles by using the quartiles as ordinal variables.
Outcome Assessment
IgG antibodies to measles and rubella were detected using standard protocols for the California Viral and Rickettsial Disease Laboratory enzyme immunoassay (37). Optical density values were determined by enzyme-linked immunosorbent assay. For measles, optical density values ≥ 1.0 were considered seropositive. For rubella, International Unit values ≥ 10 were considered seropositive. IgG antibodies to mumps were measured using standard procedures for the Wampole IgG ELISA II test (38). For mumps optical density values ≥ 1.1 were considered seropositive. Antibody concentrations were natural log transformed to correct for non-normal distributions.
Allergy Study outcomes were derived from questionnaire data for conditions specific to the past 12 months. Participants responding that they had ever been told by a doctor that they had asthma and either still had asthma or had an asthma attack in the past year were considered to have current asthma. Participants reporting wheezing or whistling in their chest in the past year were considered to have current wheeze. Participants reporting sneezing or a runny or blocked nose without a cold in the past year were considered to have current rhinitis. Participants responding Yes to the questions “Has a doctor or other health professional ever told you that you have allergies” and “During the past 12 months have you had any allergy symptoms or an allergy attack?” were considered to have current allergies.
Additionally, allergen-specific and total IgE antibodies were measured in serum using the Pharmacia Diagnostics ImmunoCAP 1000 System (Kalamazoo, MI) (39). IgE levels were measured against 19 specific allergens. Following Salo et al’s work to group these allergens measured in NHANES into clusters sharing similar biological and statistical properties (40) we focused on seven IgE clusters: plants (Bermuda grass, rye grass, birch, oak, ragweed, Russian thistle, and peanut-specific IgE); dust mites (D farina and D pteronyssinus); pets (dog and cat); cockroach and shrimp; rodents (mouse and rat); molds (Alternaria and Aspergillus species); and foods (egg white and cow’s milk). All children with peanut allergies also had allergies to at least one other allergen in the plant cluster. Participants with allergen-specific IgE ≥ 0.35 kU/L were considered sensitized to that specific allergen and were considered sensitized to an IgE cluster if they were sensitized to any of the allergens within the cluster. We also examined a measure indicating sensitization to any of the 19 allergens and continuous, natural-log transformed measures of total and allergen-specific IgE antibodies.
Statistical Analysis
To account for NHANES study design, recommended sampling weights and design variables were included in all analyses and analyses were performed using SAS survey procedures (36). Descriptive statistics calculated geometric means (95% CI) for untransformed PFAS concentration by study characteristics and Pearson correlation coefficients for natural log transformed values. For both studies we ran unadjusted and adjusted regression models. Adjusted regression models included age (continuous), sex, and race/ethnicity (non-Hispanic white; non-Hispanic black; Mexican American; other) as a priori factors. Next we identified a set of potential confounders based on the literature and then tested to determine whether these factors were associated with both exposure and outcome, necessary criteria for confounders . For the Vaccine Study no covariate met the criteria so the only addition to the adjusted model beyond the a priori covariates was a term for survey year. For the Allergy Study serum cotinine (below LOD: <0.015 ng/mL; low: 0.015 – 10 ng/mL; high: >10 ng/mL) and weight status based on CDC guidelines for age- and sex-specific body mass index (BMI) percentiles (<85th percentile; 85th – <95th percentile; ≥ 95th percentile) met the criteria and were included in all adjusted models.
For the Vaccine Study we used linear regression to estimate the percent change (95% CI) in serum antibody concentration level for a doubling of serum PFAS concentration. Both PFAS and antibody concentrations were natural log-transformed so we applied the formula ((2^beta)-1)*100 to calculate percent change. We also used linear regression to estimate the change in natural log-transformed serum antibody concentration level (95% CI) by quartile of PFAS concentration. All models were run twice: once for the full population and once restricted to the seropositive population with the assumption that seropositive children have been exposed to the vaccine strain of these viruses (35). Receipt and/or timing of measles-mumps-rubella vaccination are not available in NHANES. Additionally, to explore the potential for residual confounding by age we ran age-stratified (12 – 15 years; 16 – 19 years) models.
For the Allergy Study we used logistic regression to estimate ORs (95% CI) for a shift from the 25th to 75th percentile (ln-interquartile range [IQR]) of PFAS serum concentration and by quartile of PFAS concentration. We used linear regression to estimate the percent change (95% CI) in total and allergen-specific IgE antibodies for a doubling of serum PFAS concentration. We also used linear regression to estimate the change in natural log-transformed total IgE antibody level (95% CI) by quartile of PFAS concentration.
Supplementary Material
Figure 2.
Adjusted odds ratio (95% CI) for cockroach or shrimp IgE sensitization (IgE ≥0.35 kU/L) among children aged 12 – 19 years, National Health and Nutrition Examination Survey, 2005 – 2006 (n=638). PFAS biomarker concentrations plotted at the median by quartile of exposure. Adjusted for age, sex, race/ethnicity, BMI, cotinine, and survey weights..(a) PFOS, P for trend<0.05. (b) PFOA. (c) PFHxS. (d) PFNA.
Acknowledgments
Statement of Financial Support
Cheryl Stein and Mary Wolff were supported by the National Institute of Environmental Health Sciences (Research Triangle Park, NC; K01 ES019156, P30ES023515). Paul Maglione was supported by a fellowship from the Jeffrey Modell Foundation (New York, NY). We report no financial ties to products in the study or potentially/perceived conflicts of interest.
References
- 1.Perfluorooctanoic Acid (PFOA) U.S. Environmental Protection Agency; 2009. [Accessed March 1, 2013]. at http://www.epa.gov/oppt/pfoa/index.html. [Google Scholar]
- 2.D’Eon JC, Mabury SA. Is Indirect Exposure a Significant Contributor to the Burden of Perfluorinated Acids Observed in Humans? Environ Sci Technol. 2011;45:7974–84. doi: 10.1021/es200171y. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed March 1, 2013];The new POPs under the Stockholm Convention. 2008 at http://chm.pops.int/Implementation/NewPOPs/TheNewPOPs/tabid/672/Default.aspx.
- 4.Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: A review. Environ Sci Technol. 2006;40:3463–73. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- 5.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the u.s. population: data from the national health and nutrition examination survey (NHANES) Environ Sci Technol. 2007;41:2237–42. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MS, Norgaard-Pedersen B, Toft G, et al. Phthalates and Perfluorooctanesulfonic Acid in Human Amniotic Fluid: Temporal Trends and Timing of Amniocentesis in Pregnancy. Environ Health Perspect. 2012;120:897–903. doi: 10.1289/ehp.1104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein CR, Wolff MS, Calafat AM, Kato K, Engel SM. Comparison of polyfluoroalkyl compound concentrations in maternal serum and amniotic fluid: a pilot study. Reprod Toxicol. 2012;34:312–6. doi: 10.1016/j.reprotox.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue K, Okada F, Ito R, et al. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect. 2004;112:1204–7. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midasch O, Drexler H, Hart N, Beckmann MW, Angerer J. Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: a pilot study. Int Arch Occup Environ Health. 2007;80:643–8. doi: 10.1007/s00420-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 10.Kuklenyik Z, Reich JA, Tully JS, Needham LL, Calafat AM. Automated solid-phase extraction and measurement of perfluorinated organic acids and amides in human serum and milk. Environ Sci Technol. 2004;38:3698–704. doi: 10.1021/es040332u. [DOI] [PubMed] [Google Scholar]
- 11.So MK, Yamashita N, Taniyasu S, et al. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ Sci Technol. 2006;40:2924–9. doi: 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Environmental Protection Agency. EPA and 3M ANNOUNCE PHASE OUT OF PFOS. 2000. [Google Scholar]
- 13. [Accessed March 1, 2013];2010/2015 PFOA Stewardship Program. at http://www.epa.gov/oppt/pfoa/pubs/stewardship/index.html.
- 14.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in Exposure to Polyfluoroalkyl Chemicals in the U.S. Population: 1999–2008. Environ Sci Technol. 2011;45:8037–45. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 15.Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect. 2010;118:222–8. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicologic pathology. 2012;40:300–11. doi: 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- 18.Corsini E, Sangiovanni E, Avogadro A, et al. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs) Toxicol Appl Pharmacol. 2012;258:248–55. doi: 10.1016/j.taap.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Fairley KJ, Purdy R, Kearns S, Anderson SE, Meade B. Exposure to the immunosuppressant, perfluorooctanoic acid, enhances the murine IgE and airway hyperreactivity response to ovalbumin. Toxicol Sci. 2007;97:375–83. doi: 10.1093/toxsci/kfm053. [DOI] [PubMed] [Google Scholar]
- 20.Ryu MH, Jha A, Ojo OO, et al. Chronic exposure to perfluorinated compounds: impact on airway hyperresponsiveness and inflammation. American journal of physiology Lung cellular and molecular physiology. 2014;307:L765–74. doi: 10.1152/ajplung.00100.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh TS, Lee S, Kim HH, Choi JK, Kim SH. Perfluorooctanoic acid induces mast cell-mediated allergic inflammation by the release of histamine and inflammatory mediators. Toxicol Lett. 2012;210:64–70. doi: 10.1016/j.toxlet.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Pennings JL, Jennen DG, Nygaard UC, et al. Cord blood gene expression supports that prenatal exposure to perfluoroalkyl substances causes depressed immune functionality in early childhood. Journal of immunotoxicology. 2015:1–8. doi: 10.3109/1547691X.2015.1029147. [DOI] [PubMed] [Google Scholar]
- 23.Granum B, Haug LS, Namork E, et al. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. Journal of immunotoxicology. 2013;10:373–9. doi: 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- 24.Grandjean P, Andersen EW, Budtz-Jorgensen E, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–7. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osuna CE, Grandjean P, Weihe P, El-Fawal HA. Autoantibodies Associated with Prenatal and Childhood Exposure to Environmental Chemicals in Faroese Children. Toxicol Sci. 2014;114:158–66. doi: 10.1093/toxsci/kfu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada E, Sasaki S, Saijo Y, et al. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ Res. 2012;112:118–25. doi: 10.1016/j.envres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Wang IJ, Hsieh WS, Chen CY, et al. The effect of prenatal perfluorinated chemicals exposures on pediatric atopy. Environ Res. 2011;111:785–91. doi: 10.1016/j.envres.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Dong GH, Tung KY, Tsai CH, et al. Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case-control study of Taiwanese children. Environ Health Perspect. 2013;121:507–13. 13e1–8. doi: 10.1289/ehp.1205351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood. Environ Res. 2010;110:773–7. doi: 10.1016/j.envres.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Smit LA, Lenters V, Hoyer BB, et al. Prenatal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy. 2015;70:653–60. doi: 10.1111/all.12605. [DOI] [PubMed] [Google Scholar]
- 31.Humblet O, Diaz-Ramirez LG, Balmes JR, Pinney SM, Hiatt RA. Perfluoroalkyl Chemicals and Asthma among Children 12–19 Years of Age: NHANES (1999–2008) Environ Health Perspect. 2014;122:1129–33. doi: 10.1289/ehp.1306606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson SG. Raised levels of a new immunoglobulin class (IgND) in asthma. Lancet. 1967;2:951–3. doi: 10.1016/s0140-6736(67)90792-1. [DOI] [PubMed] [Google Scholar]
- 33.Matricardi PM, Bockelbrink A, Gruber C, et al. Longitudinal trends of total and allergen-specific IgE throughout childhood. Allergy. 2009;64:1093–8. doi: 10.1111/j.1398-9995.2009.02055.x. [DOI] [PubMed] [Google Scholar]
- 34.Kakoulidou M, Forsgren M, Lewensohn-Fuchs I, Johansen K. Serum levels of rubella-specific antibodies in Swedish women following three decades of vaccination programmes. Vaccine. 2010;28:1002–7. doi: 10.1016/j.vaccine.2009.10.130. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher CM, Smith DM, Meliker JR. Total blood mercury and serum measles antibodies in US children, NHANES 2003–2004. Sci Total Environ. 2011;410–411:65–71. doi: 10.1016/j.scitotenv.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 36.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital and health statistics Series 2, Data evaluation and methods research. 2013:1–24. [PubMed] [Google Scholar]
- 37.U.S. Centers for Disease Control and Prevention. Laboratory Procedures Manual. Measles, Rubella, and Varicella-Zoster Antibodies in Serum NHANES 1999–2000. [Google Scholar]
- 38. [Accessed March 10, 2015, 2015];Antibody to Mumps Virus (Surplus Sera) (SSMUMP_A) 2009 at http://wwwn.cdc.gov/nchs/nhanes/1999-2000/SSMUMP_A.htm.
- 39.U.S. Centers for Disease Control and Prevention. Laboratory Procedures Manual 2006. Specific IgE/Total IgE Allergens in Serum NHANES 2005–2006. [Google Scholar]
- 40.Salo PM, Calatroni A, Gergen PJ, et al. Allergy-related outcomes in relation to serum IgE: results from the National Health and Nutrition Examination Survey 2005–2006. The Journal of allergy and clinical immunology. 2011;127:1226–35. e7. doi: 10.1016/j.jaci.2010.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.