Abstract
Objective
Although new treatments for rheumatoid arthritis (RA) are extremely effective in preventing disease progression, rates of total knee replacement (TKR) continue to rise. The ongoing need for TKR is problematic, especially as functional outcomes have been reported to be worse than in patients with osteoarthritis (OA). The purpose of this study is to assess pain, function, and quality of life 2 years after TKR in a contemporary RA patients, compared to patients with OA.
Methods
Primary TKR cases enrolled between 5/1/2007 and 7/1/2010 in a single institution TKR registry were eligible for this study. Validated RA cases were compared to OA at baseline and at 2-years.
Results
We identified 4,456 eligible TKRs, including 136 RA. Compared to OA, RA TKR had significantly worse pre-operative WOMAC pain (55.9 vs. 46.6; p-value<0.0001) and function (58.7 vs. 47.3; p-value<0.0001) , however there were no differences at 2 years. Within RA, there was no difference for patients who used biologic DMARDs vs. those who did not in pain (p-value= 0.41) or function (p-value= 0.39) at 2 years. In a multivariate regression, controlling for multiple potential confounders, there was no independent association of RA with 2-year pain (p-value=0.18) or function (p-value=0.71).Satisfaction was high for both RA and OA.
Conclusion
RA patients undergoing primary TKR have excellent 2-year outcomes, comparable to OA, in spite of worse pre-operative pain and function. In this contemporary cohort, RA is not an independent risk factor for poor outcomes.
Keywords: Rheumatoid Arthritis, Arthroplasty, Disease-Modifying Antirheumatic Drugs, Osteoarthritis
Introduction
Rheumatoid arthritis is a form of inflammatory arthritis which can destroy cartilage and erode joints, leading to significant pain and functional impairment. Historically, over 50% of patients with RA have undergone orthopedic surgery, most commonly arthroplasty, over the course of their illness (1-3). Although most reports describe significant pain relief for RA patients undertaking total knee replacement (TKR) (1, 4), others have reported less successful outcomes (5). Moreover, demonstration of improvement in function and other quality of life measures has not been consistent (1-3) and, importantly, improvement in function has not been equivalent to OA (4). For patients with OA, end-stage knee damage is often a localized problem, which can be effectively treated with TKR. However, for RA, knee destruction is only one component of a systemic disease, which may explain why replacement of a single joint may not leads to the same degree of functional improvement compared with OA.
The increased use of DMARDs and biologics has resulted in tremendous improvements in function and quality of life, while decreasing articular destruction in contemporary RA patients (6). Not surprisingly, rates of many types of orthopedic surgery in RA, such as soft tissue procedures, have significantly decreased (7, 8). However, while the proportion of arthroplasty performed for RA has decreased compared to OA, total knee replacement (TKR) rates among RA patients continue to increase(9) (10, 11). It appears that TKR will remain an important treatment option for RA patients with advanced knee damage.
The purpose of this study is to evaluate the pain, function and quality of life after primary TKR in a contemporary cohort of patients with RA compared to OA. Our hypothesis is that among RA TKR patients with high DMARD and biologic use, outcomes after TKR will be comparable to OA.
Materials and Methods
This study was performed in a high volume center which performs over 4,300 TKRs annually. Most surgeons were experienced in performing arthroplasty in RA patients; only 10% of the RA cases were performed by surgeons with fewer than 10 RA cases during the study period. All primary TKR patients enrolled in a prospective joint replacement registry between May 1, 2007, and July 1, 2010, who were alive 2 years after surgery, and had baseline data were eligible for the study.
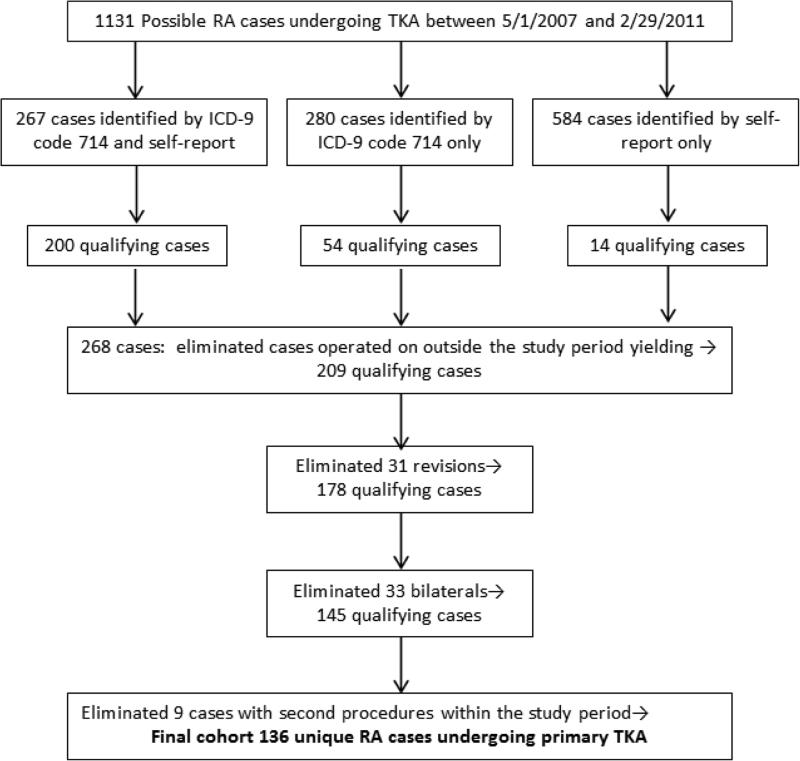
(Supplemental Figure 1). We initially identified 1131 patients identified by ICD-9 code or self report as RA, who were entered into the arthroplasty registry for all knee procedures (primary, revision, bilateral)between May 1, 2007, and February 29, 2011. Of 267 cases identified by ICD-9 code and self-report, 200 met pre-determined criteria for RA. Of 280 identified by ICD-9 code only, 54 met our criteria, and of 584 cases identified by self report only, 14 cases met criteria, yielding a total of 268 cases. From this cohort of 268 cases identified and validated between May 1, 2007, and February 29, 2011, we then excluded the cases enrolled outside of our study cohort dates of May 1, 2007 and July 1, 2010, selected to permit 2-year follow-up, leaving 209 cases. We then excluded revision cases (31) and bilateral cases (33), and cases with second procedures during the study period (9), leaving our study cohort of 136. There were no deaths among the RA cases during this time period. Patients with ICD-9 codes for fracture, avascular necrosis, or other inflammatory diseases besides RA, as well as patients undergoing a revision or bilateral primary TKR, were excluded. Patients who had two eligible procedures only contributed data from the second procedure. Approximately 80% of patients undergoing TKR consent to enroll in the registry.
RA cases were identified by self-report or ICD-9 code 714.0, and the diagnosis was validated by physician (BKJ) review of medical records(12),. As investigators did not have access to rheumatology-specific clinical records to ascertain ACR criteria for RA, the diagnosis was validated by meeting pre-determined criteria: when pre-operative evaluation by a rheumatologist confirmed the diagnosis of RA or a pre-operative evaluation by an internist confirmed the diagnosis of RA and the patient was receiving a disease modifying drug (DMARD) or biologic agent (excluding steroids). The addition of a rheumatologist's diagnosis of RA and documented use of DMARDs significantly increases the accuracy of RA diagnosis for cases identified by ICD-9 code (13, 14).
Additional RA-specific information about medication use was obtained by a questionnaire sent 6 months to 3.5 years after the TKR. Information regarding RA medication use was also obtained from the admission history. Self-report outcome measures were gathered systematically pre-operatively and at 2 years, including the Knee Osteoarthritis Outcome Scale (KOOS), from which the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) is derived (15), and the Short Form12 (SF-12) (16). We additionally utilized our hospital administrative database to obtain the American Society of Anesthesia (ASA) scores and the ICD-9 based Deyo Co-morbidities (excluding RA) (17),
Pain, function and quality of life were assessed using the WOMAC and SF-12 questionnaires. The WOMAC is a widely used self-report instrument which is specific for the lower extremity. Lower extremity pain, stiffness, and function are assessed using three subscales, on which a higher score indicates worse status. A difference of 10-15 points is clinically significant, and a score >40 indicates significant pain and poor function(18) (19)
The SF-12 is a generic measure of general health and wellbeing. The 12 item scale contains 2 subscales, the physical component scale (PCS) and the mental component scale (MCS) scored 1-100. Higher scores on the SF-12 indicate better status. A change of 5 points is clinically significant (16). Satisfaction was assessed at 2 years. Patients are asked about their satisfaction with the surgery in four Specific areas 1- relief of pain, 2-improving ability to do recreational activities, and 3-overall satisfaction with the results of the surgery. Satisfaction scores are assessed in each area using a five point Likert scale. A global satisfactions question asks, “How much did the surgery improve the quality of your life?” with answers ranging from “more improvement than I ever dreamed possible” to “the quality of my life is worse”. Expectations were assessed using the validated HSS Knee Expectations Survey, which covers areas specific to recovery from knee surgery(20).
Administrative data included the Deyo comorbidity index, which is based on ICD-9 codes and is used to assess co-morbid conditions which contribute to overall health. For the patients at our institution undergoing almost exclusively elective TKR, the scores rarely exceed 3, although the total score possible is 26 (17). Due to this lack of variability, we evaluated the number of Deyo-Charlson comorbidities rather than calculating the index. The American Society of Anesthesiology (ASA) score is a ranking used to quantify surgical risk and ranges from 0-6, with a score of 0 indicating excellent health and a score of 6 indicating an organ transplant donor (21).
Descriptive statistics were performed using Student's t-test, Chi-Square or Fisher's exact test as appropriate. Significant characteristics of interest were identified in the univariate analysis and included in the multivariable model. The primary outcome measures, WOMAC pain and function, were analyzed as both continuous and dichotomized variables. In the dichotomized analysis, WOMAC pain and function> 40 was defined as a poor outcome. Multivariate linear and logistic regression analyses were then performed controlling for potentially significant confounding variables together with variables of clinical interest, even if not -statistically significant, to evaluate the independent association of RA with 2-year pain or function. Collinearity was tested and was not observed during the model building process.
This study was approved by our Institutional Review Board.
Results
We identified 9,830 primary TKR cases between May 1, 2007 and December 31, 2010, After exclusions, 4,456 cases remained eligible for this analysis: 4320 OA cases and 136 validated RA cases.
For the OA cases, 2 year data was available on 94.7% (Table 6). For OA, there were minimal differences between those who completed the 2 year follow-up survey (n=4220) and those who did not (n=236). For RA, 108 (79%) of patients had 2-year data. There was no significant difference between patients who responded to the 2-year questionnaire and those who did not for age (63.0 vs. 65.4; p-value=0.24), BMI (28.4 vs. 29.0; p-value=0.75), gender (female 90% vs. 93%; p-value= 1.00), or race (Caucasian 74% vs. 79%; p-value=0.62). However, those without 2 year data had less educational achievement.: 96% of those without 2 year data had no college education compared to 56% of those with 2 year data (p-value <0.0001). There was no significant difference in baseline WOMAC pain (with 2-year response 44.6 (SD18.1) vs without 39.5 (SD14.6) , p-value =0.3473) or WOMAC function (with 2-year response 41.1 (SD19.7) vs. without 43.0 (SD14.0) p-value=0.8244). We included all patients with RA, regardless of 2-year data, in order to maximize the size of the RA cohort.
Table 6.
Patient Characteristics for patients w/o 2- year outcomes
| All patients (N=4456) | |||
|---|---|---|---|
| With 2-year outcome (n = 4220; 94.7%) | Without 2-year outcome (n = 236; 5.3%) | P value | |
| Age | 67.1 (10.3) | 66.9 (8.9) | 0.7143 |
| Female, n (%) | 2454 (59%) | 150 (64%) | 0.1008 |
| BMI | 30.6 (6.2) | 31.8 (7.1) | 0.0140 |
| Education status at baseline, n (%) | 0.0045 | ||
| College or up | 2522 (60%) | 119 (50%) | |
| No College | 1698 (40%) | 117 (50%) | |
| Race, n (%) | 0.6579 | ||
| White | 3672 (87%) | 203 (86%) | |
| Non-White | 548 (13%) | 33 (14%) | |
| RA yes, n (%) | 108 (3%) | 28 (12%) | |
| Baseline Deyo-Charlson Comorbidities, n (%) | 0.3892 | ||
| 0 comorbidities | 2952 (71%) | 159 (68%) | |
| 1+comorbidities | 1230 (29%) | 75 (32%) | |
| ASA Class, n (%) | 0.2396 | ||
| Class 1 | 154 (4%) | 5 (2%) | |
| Class 2 | 3054 (72%) | 162 (69%) | |
| Class 3+ | 1009 (24%) | 68 (29%) | |
|
RA patients (N=136) | |||
| With 2-year outcome (n = 108; 79%) | Without 2-year outcome (n = 28; 21%) | P value | |
| Age | 63.0 (12.1) | 65.4 (8.6) | 0.2376 |
| Female, n (%) | 96 (90%) | 26 (93%) | 1.0000 |
| BMI | 28.4 (6.7) | 29.0 (9.3) | 0.7455 |
| Education status at baseline, n (%) | <0.0001 | ||
| College or up | 47 (44%) | 1 (4%) | |
| No College | 61 (56%) | 27 (96%) | |
| Race, n (%) | 0.6243 | ||
| White | 80 (74%) | 22 (79%) | |
| Non-White | 28 (26%) | 6 (21%) | |
| Baseline Deyo-Charlson Comorbidities, n (%) | 0.4354 | ||
| 0 comorbidities | 41 (38%) | 13 (46%) | |
| 1+comorbidities | 66 (62%) | 15 (54%) | |
| ASA Class, n (%) | 0.0815 | ||
| Class 1 | 0 (0%) | 0 (0%) | |
| Class 2 | 69 (64%) | 13 (46%) | |
| Class 3+ | 38 (36%) | 15 (54%) | |
Baseline characteristics (Table 1)
Table 1.
Patient Characteristics
| OA (n = 4320) | RA (n = 136) | P value | |
|---|---|---|---|
| Age | 67.2 (10.1) | 63.5 (11.4) | 0.0002 |
| Female, n (%) | 2482 (58%) | 122 (90%) | <0.0001 |
| BMI | 30.7 (6.2) | 28.5 (7.3) | <0.0001 |
| Length of stay (days) | 5.1 (1.6) | 5.5 (1.6) | 0.0002 |
| Education status at baseline, n (%) | 0.0147 | ||
| No College | 820(19%) | 32 (21%) | |
| Some college or above | 3292 (78%) | 68 (65%) | |
| Other | 130 (3%) | 5 (5%) | |
| Race, n (%) | 0.0017 | ||
| White | 3672 (86%) | 104 (77%) | |
| Asian | 25 (0.6%) | 3 (2%) | |
| Black or African American | 240 (6%) | 16 (12%) | |
| Hispanic | 139 (3%) | 9 (7%) | |
| Other/Mixed | 186 (4%) | 3 (2%) | |
| Baseline Deyo-Charlson Comorbidities, n (%) | <0.0001 | ||
| 0 comorbidities | 3057 (71%) | 54 (40%) | |
| 1-2 comorbidities | 1108 (26%) | 75 (56%) | |
| 3+ comorbidities | 116 (3%) | 6 (4%) | |
| ASA Class, n (%) | <0.0001 | ||
| Class 1-2 | 3293(74%) | 82 (61%) | |
| Class 3-4 | 1024 (24%) | 53 (39%) | |
| Presence of back pain at baseline, n (%) | 1606 (42%) | 41 (42%) | 0.9403 |
| HSS Expectation Score at baseline | 78.6 (17.9) | 68.9 (23.2) | 0.0006 |
| Underwent prior knee replacement, n (%) | 793 (21%) | 31 (32%) | 0.0083 |
RA patients were younger (63.5 years vs. 67.2 years; p-value=0.0002) and more likely to be female, (90% vs. 58% ) (p-value <0.0001). BMI was significantly lower for RA (28.5 vs. 30.7; p-value<0.0001). There was a significant difference between RA and OA in terms of educational achievement; 68% of RA had some college education or above, compared with 78% of OA (p-value=0.015). Fewer RA were Caucasian (77% vs. 86%) and more RA were African American (12% vs. 6%; p-value=0.002). RA patients had significantly more co-morbidities: 40% of RA had no Deyo comorbidities, while 71% of OA had no Deyo comorbidities (p-value<0.0001). RA cases also had worse ASA scores (ASA 1 or 2: 61% vs. OA 77%; p-value<0.0001). Length of stay was significantly longer for RA (5.5 days vs. 5.1 days; p-value=0.0002). 13% of the RA patients were on no DMARDs, 36% were on non-biologic DMARDs only, 38.5% were on TNF inhibitors (TNFi), 7.4 % were on non TNFi biologics, and 5.2% were on corticosteroids alone (Table 2). RA patients had significantly lower expectations of outcome than OA patients, with a total HSS Expectations score of 68.9 vs. 78.6, (p-value=0.0006; 100= highest expectations) and a significantly higher proportion of RA patients had undergone a prior contralateral TKR, (32% vs. 21%; p-value=0.008). There was no significant difference in the presence of back pain reported at the time of TKR, 42% for both RA and OA patients, (p-value=0.94).
Table 2.
RA Medications. Patients could be taking more than one medication
| Medicine | Frequency | Percent | Cumulative Frequency | Cumulative Percent |
|---|---|---|---|---|
| DMARDs | 48 | 35.56 | 48 | 35.56 |
| Non TNF biologics | 10 | 7.41 | 58 | 42.96 |
| Steroid | 7 | 5.19 | 65 | 48.15 |
| TNF | 52 | 38.52 | 117 | 86.67 |
| none | 18 | 13.33 | 135 | 100.00 |
WOMAC pain was significantly worse at baseline for RA patients undergoing TKR (55.9 vs. 46.6; p-value<0.0001) compared to OA (Table 3). However, WOMAC scores at 2 years were equivalent, with excellent pain scores (13.3 vs. 12.7; p-value=0.65) for both groups. Almost all RA and OA cases achieved a clinically meaningful (ΔWOMAC>10) improvement in pain (89% in both, p-value =0.83). There was no difference in the percent of patients with RA or OA who had poor outcomes (WOMAC >40) for pain (10% vs. 7%; p-value=0.44). Among RA, there was no association between use of biologic DMARDs and a poor outcome for pain (WOMAC >40) (p-value=0.85).
Table 3.
Pre-Operative and Two-Year Pain and Function
| OA (n = 4320) | RA (n = 136) | P value | |
|---|---|---|---|
| WOMAC BaselinePain | 46.6 (18.0) | 55.9 (17.8) | <0.0001 |
| WOMAC 2-year Pain | 12.7 (16.1) | 13.3 (15.7) | 0.6506 |
| WOMAC Baseline Function | 47.3 (18.3) | 58.7 (19.1) | <0.0001 |
| WOMAC 2-year Function | 14.7 (14.1) | 17.4 (13.0) | 0.6038 |
| Δ WOMAC > 10, Pain, n (%) | 2470 (89%) | 59 (89%) | 0.8276 |
| Δ WOMAC > 10, Function, n (%) | 2030 (87%) | 50 (93%) | 0.2088 |
| Poor outcome at 2 years, WOMAC Pain >40, n (%) | 215 (7%) | 7 (10%) | 0.4374 |
| Poor outcome at 2 years, WOMAC Function >40, n (%) | 276 (9%) | 12 (16%) | 0.0527 |
| SF-12 PCS Baseline | 33.9 (8.2) | 28.7 (7.9) | <0.0001 |
| SF-12 PCS at 2 years | 45.7 (10.1) | 40.5 (10.5) | <0.0001 |
| SF-12 MCS Baseline | 50.8 (12.2) | 46.4 (13.2) | 0.0009 |
| SF-12 MCS at 2 years | 53.8 (9.4) | 48.9 (11.8) | 0.0003 |
RA patients undergoing TKR had clinically and statistically significantly worse baseline WOMAC function (58.7 vs. 47.3; p-value<0.0001) compared to OA. However, WOMAC scores at 2 years were equivalent, with excellent function scores (17.4 vs. 14.7; p-value=0.60) for both groups. Almost all RA and OA cases achieved a clinically meaningful change (ΔWOMAC>10) in function (93% vs. 87%; p-value=0.21). In the dichotomized analysis, the difference in the percent of patients with RA or OA who had poor outcomes (WOMAC >40) for function was on the borderline of statistical significance (16% vs. 9%; p value=0.053). Among RA, there was no difference in outcome for poor function (WOMAC >40) for patients using biologic and synthetic DMARDs (p-value= 0.25). ).
Quality of Life and Satisfaction
Pre-operative SF-12 PCS was clinically and statistically significantly worse for RA (28.7 vs. 33.9; p-value<0.0001) and remained so at two years (40.5 vs. 45.7; p-value<0.0001). SF-12 MCS was statistically but not clinically significantly worse for RA both pre-operatively (RA 46.4 vs. OA 50.8; p-value=0.0009) and at 2 years (48.9 vs. 53.8: p-value=0.0003). Satisfaction was high for both RA and OA for TKR (Supplementary Table 1). Both RA and OA report that they were very satisfied with pain relief (RA 81% vs. OA 77%; p-value=0.89). There was no significant difference in satisfaction reported for RA or OA in the ability to perform recreational activities (very satisfied 54% vs. 58%; p-value=0.44), or overall satisfaction (very satisfied 72% vs. 74%; p-value=0.78). There was no significant difference in satisfaction with the improved quality of life, with 74% of RA patients reporting “more improvement than I ever dreamed possible” and “great improvement” compared to 75% of OA (p-value=0.24).
Predictors of Poor Post-operative Pain (Table 4 and 5)
Table 4.
Univariate Analysis for Pain or Function Two Years After Surgery*
| WOMAC Pain at 2 years Estimated Coefficient (Standard Error) | WOMAC Function at 2 years Estimated Coefficient (Standard Error) | |
|---|---|---|
| Age | −0.12 (−0.19, −0.05)*** | 0.03 (−0.04, 0.10)*** |
| Female vs. Male | 2.98 (1.63, 4.33)** | 3.29 (1.92, 4.67)** |
| RA vs. OA | −0.22 (−4.59, 4.14)*** | 1.85 (−2.57, 6.26)*** |
| ASA Class 2 vs.ASA Class 1 | 2.07 (−1.31, 5.45) | 3.51 (0.06, 6.96) |
| ASA Class 3+vs. ASA Class 1 | 3.32 (−0.26, 6.90) | 6.85 (3.19, 10.52) |
| ≥1 Deyo Comorbidities vs. 0 Deyo Comorbidities | 0.64 (−0.85, 2.13) | 1.76 (0.23, 3.28) |
| BMI | 0.09 (−0.02, 0.20) | 0.19 (0.08, 0.30) |
| ≥College education vs. <College | −3.70 (−5.09, −2.31)** | −3.94 (−5.36, −2.52)** |
| Caucasian vs. Non-Caucasian | −4.68 (−6.91, −2.45)** | −4.93 (−7.20, −2.66)** |
| Pre-Op WOMAC Pain | 0.21 (0.17, 0.24)** | 0.18 (0.15, 0.22)** |
| Pre-Op WOMAC Function | 0.21 (0.17, 0.25)** | 0.26 (0.22, 0.30)** |
| Pre-Op PCS | −0.32 (−0.41, −0.24)** | −0.43 (−0.52, −0.35)** |
| Pre-Op MCS | −0.27 (−0.33, −0.21)** | −0.34 (−0.39, −0.28)** |
| Previous Replacement vs. No Prev Replacement | −0.41 (−2.03, 1.22) | 0.82 (−0.85, 2.48) |
| Back Pain vs. No Back Pain | 4.55 (3.20, 5.89)** | 4.41 (3.04, 5.79)** |
| Expectation Score | −0.03 (−0.07, 0.01) | −0.05 (−0.09, −0.002) |
Univariate linear regression controlling for each individual predictor as listed in the table
Variables with p-value<0.05 in the univariate analysis and included in the multivariate regression
Variables with p-value>0.05 in the univariate analysis; included in the multivariate regression based on the research of interest
Bolding indicates a significant value
Table 5.
MultivariateAnalysis for Pain or Function Two Years After Surgery*
| WOMAC Pain at 2 years Estimated Coefficient (95% CI) | WOMAC Function at 2 years Estimated Coefficient (95% CI) | |
|---|---|---|
| Age | −0.10 (−0.17, −0.02)*** | 0.04 (−0.03, 0.12)*** |
| Female vs. Male | 1.47 (−0.02, 2.96) | 0.83 (−0.60, 2.26)** |
| RA vs. OA | −3.32 (−8.20, 1.56)*** | −0.61 (−5.18, 3.96)*** |
| ≥College education vs. <College | −1.56 (−3.09, −0.04) | −1.36 (−2.81, 0.08)** |
| Caucasian vs. Non-Caucasian | −3.49 (−5.90, −1.07) | −3.93 (−6.21, −1.65)** |
| Pre-Op WOMAC Pain | 0.09 (0.02, 0.16)** | −0.04 (−0.11, 0.02) |
| Pre-Op WOMAC Function | 0.04 (−0.04, 0.11) | 0.18 (0.11, 0.26)** |
| Pre-Op PCS | −0.13 (−0.24, −0.01) | −0.24 (−0.35, −0.14)** |
| Pre-Op MCS | −0.16 (−0.23, −0.10)** | −0.24 (−0.31, −0.18)** |
| Back Pain vs. No Back Pain | 2.83 (1.35, 4.31) | 1.78 (0.38, 3.19) |
Multivariate linear regression controlling for age, gender, diagnosis, education, race, pre-operative WOMAC pain score, pre-operative WOMAC function score, pre-operative PCS, pre-operative MCS and previous back pain
Variables with p-value<0.05 in the multivariate regression
Variables with p-value >0.05 in the univariate analysis; Included in the multivariate regression based on the research interest
Bolding indicates a significant value
A multivariate linear regression analysis was performed to identify predictors of poor pain (WOMAC>40) at 2 years (Table 5). This analysis, controlling for age, sex, diagnosis, education, race, pre-operative WOMAC pain, pre-operative WOMAC function, pre-operative MCS and PCS, and back pain showed that RA was not an independent risk factor for poor post-operative pain (Estimated coefficient −3.32 (95% CI −8.2, 1.56, p-value=0.18). ). Age, sex, education, race, pre-operative function, and pre-operative PCS were not associated with pain at 2 years.. Higher pre-operative WOMAC pain scores significantly increased the likelihood of a poor pain outcome (Estimated coefficient 0.09 (95% CI 0.02, 0.16) Higher pre-operative MCS strongly decreased the likelihood of a poor pain outcome (Estimated coefficient −0.16 (95% CI −0.23, −0.10). Similar results were obtained when WOMAC pain was analyzed as a dichotomous variable in a logistic regression controlling for age, sex, education, race, baseline WOMAC pain, baseline WOMAC function, SF-12 PCS, SF-12 MCS, and presence of back pain (Supplemental Table 1). RA was not a significant risk factor for a poor outcome (OR 1.11; 95% CI 0.40-3.06).
Predictors of Poor post-Operative Function (Tables 4 and 5)
A multivariate linear regression was performed to identify predictors of function at 2 years (Table 5). This analysis, controlling for age, gender, diagnosis, education, race, pre-operative WOMAC pain and function, pre-operative MCS and PCS, and back pain showed that RA was not an independent risk factor for poor function (Estimate −0.6, 95% CI −5.18-3.95, p-value=0.79) Higher (worse) pre-operative WOMAC function score was a significant predictor of poor functional outcome (Estimated coefficient 0.18 (95% CI 0.11, 0.26)Higher pre-operative PCS(Estimated coefficient −0.20 (95% CI-0.32, −0.08)higher MCS (Estimated coefficient −0.21 (95% CI −0.28, −0.14)) and being Caucasian (Estimated coefficient −2.73 (95% CI −5.23, −0.24) were protective against poor function. When WOMAC function was analyzed as a dichotomous variable in a logistic regression controlling for age, sex, education, race, baseline WOMAC pain, baseline WOMAC function, SF-12 PCS, SF-12 MCS, and presence of back pain, RA was not a significant risk factor for poor outcome (WOMAC>40)at 2 years (OR 1.08; 95% CI 0.43-2.70). (Supplemental Table 2).
Discussion
RA patients in a contemporary cohort achieved excellent pain and function outcomes after primary TKR, and no longer lag behind OA patients, despite having significantly worse pre-operative pain and function. While equivalent outcomes for pain have been described for patients with RA (4), equivalent outcomes in function have not been described (5). Poor baseline pain and function were significant risk factors for poor outcomes for patients with RA, similar to descriptions for patients with OA (22, 23). In addition, the excellent outcomes for RA patients after TKR occurred in spite of having more comorbidities, another known risk factor for poor outcomes in OA patients after TKR (24). It is tempting to speculate that patients with RA, a chronic painful musculoskeletal disease, are better able to cope with the demands of a painful post-operative physical therapy regimen, which is particularly important for achieving good outcomes after TKR.
Contemporary RA have better overall status when compared to RA patients several decades ago (6), however, pain and function at the time our subjects elected TKR was significantly worse in RA compared with OA patients. Therefore, improved TKR outcomes do not simply reflect this overall improved status. We recently demonstrated that RA patients undergoing THR during the same time period in the same institution were significantly more likely to have a poor outcome for WOMAC function than patients with OA (25). However, there were differences between the RA patients undergoing TKR described here compared to the RA patients undergoing THR. Compared to the TKR group, the THR group had a higher co-morbidity burden, and fewer had a college education. Prior TKR series have demonstrated that education is strongly predictive of a good outcome for the patients undergoing TKR (29). Other recent TKR series also demonstrated an improvement in function and quality of life for RA (26) while older TKR series described worse functional outcomes and similar pain after TKR for RA compared to OA. In this older study, all primary TKR cases were eligible, whereas only the second TKR was included in our study if 2 procedures were performed (4). As RA was associated with poor 2-year pain scores only in those undergoing their first THR (29) which might also apply to the TKR patients. High RA specific surgical volume has been associated with less likelihood of complications after arthroplasty, and might contribute to improved functional outcomes in our cohort as well (27). Additionally, the inclusion of older cases during a time of significant change in medical, anesthetic, and orthopedic care may explain the difference in these results.
The improved overall quality of life for RA has been attributed to the widespread use of potent DMARDs and biologic agents such as the TNFi among RA patients (28). In our cohort, 86.7% of RA patients undergoing primary TKR were on DMARDs, biologics, and corticosteroids with no difference in outcomes compared to those not on synthetic DMARDs or biologics... Although better function is reported after knee arthroplasty in high volume centers (19), this would not affect the strength of our comparison of RA patients to OA TKR performed in the same high volume center.
To our knowledge, this is the first large study of TKR in contemporary RA patients utilizing prospectively gathered data with carefully validated diagnosis, demonstrating equivalent outcomes for RA and OA patients undergoing TKR. The diagnosis of RA in our study was validated by chart review with an algorithm utilizing both DMARD use and a rheumatologist's diagnosis. While administrative databases alone are well validated for accurate identification of total hip or total knee arthroplasty cases (29, 30), the diagnosis of RA by administrative data alone may not be accurate (14). However, the addition of DMARD therapy increases the accuracy from a positive predictive value (PPV) of 30% to 60%, and addition of a rheumatologist's diagnosis further increases the PPV to 88-91% (13, 14). Importantly, we utilized patient-reported outcomes such as the WOMAC to assess pain and function, as this lower-extremity specific survey has been found to be more responsive to change after TKR than generic patient reported outcomes such as the SF-36 or the Health Assessment Questionnaire (HAQ) (31, 32).
A weakness of this study is that all surgery was performed at a specialized high volume tertiary referral center, with TKR performed by surgeons with high RA specific volume, so our results may not be generalizable. While the difference between OA and RA patients in the proportion with poor post-operative function approached statistical significance, we may be underpowered to detect a significant difference. However, as there was no difference in baseline WOMAC function between those with and without 2 year responses, it is unlikely that we systematically excluded those at higher risk of doing poorly. In addition, we did not have direct access to treating rheumatologist's medical records to validate RA cases using ACR criteria. We had no information about RA disease activity at the time of the surgery, which might have an impact on post-operative course. Although there was little difference in the 79% of patients with 2 year data compared to those without in multiple parameters including baseline pain and function, bias could be introduced if there was selective non-response by those with poorer outcomes. In summary, RA patients undergoing TKR in a contemporary cohort with high prevalence of DMARD and biologic therapy have excellent outcomes and report improvements which are as good as the outcomes of OA patients for both pain and function after undergoing primary TKR. Our study demonstrates that RA is no longer an independent risk factor for poor TKR outcomes for either pain or function. As RA patients continue to undergo TKR at increasing rates (33), it is important to have an accurate assessment of TKR outcomes so patients can be given appropriate expectations of TKR.
Supplementary Material
Figure 1.
Flow diagram demonstrating the case selection process from 1131 cases identified as RA by ICD-9 code 714 or self- report, and validated as RA by pre-established criteria.
Acknowledgments
Funding for this project was obtained by the Weill Cornell Clinical Translational Science Center (CTSC) (UL1-TR000457-06), AHRQ CERT GRANT: U18 HS016075, and the Block Family Foundation
Footnotes
This work should be attributed to the Division of Rheumatology, Weill Cornell Medical School, and Departments of Medicine, Orthopedic Surgery, and Biostatistics Core at the Hospital for Special Surgery.
Contributor Information
Susan M. Goodman, Associate Professor of Clinical Medicine, Weill Cornell Medicine College, Associate Attending Physician, Rheumatology Hospital for Special Surgery.
Beverly Johnson, Assistant Professor of Medicine, Albert Einstein College of Medicine, Director of Rheumatology, Jacobi Medical Center & North Central Bronx Hospital.
Meng Zhang, Biostatistician, Hospital for Special Surgery.
Wei-Ti Huang, Biostatistician, Hospital for Special Surgery.
Rebecca Zhu, Research Assistant, Research and Rheumatology, Hospital for Special Surgery.
Mark Figgie, Professor of Orthopedic Surgery, Weill Cornell College of Medicine, Attending Orthopedic Surgeon, Chief of Surgical Arthritis Service, Hospital for Special Surgery.
Michael Alexiades, Associate Professor of Orthopedic Surgery, Weill Cornell Medicine College, Associate Attending, Hospital for Special Surgery.
Lisa A Mandl, Assistant Professor of Research Medicine, Assistant Professor of Public Health, Weill Cornell Medicine College, Assistant Attending Physician, Rheumatology, Hospital for Special Surgery.
References
- 1.Osnes-Ringen H, Kvien TK, Henriksen JE, Mowinckel P, Dagfinrud H. Orthopaedic surgery in 255 patients with inflammatory arthropathies: longitudinal effects on pain, physical function and health-related quality of life. Ann Rheum Dis. 2009;68:1596–601. doi: 10.1136/ard.2008.096362. [DOI] [PubMed] [Google Scholar]
- 2.Kapetanovic MC, Lindqvist E, Saxne T, Eberhardt K. Orthopaedic surgery in patients with rheumatoid arthritis over 20 years: prevalence and predictive factors of large joint replacement. Ann Rheum Dis. 2008;67:1412–6. doi: 10.1136/ard.2007.086710. [DOI] [PubMed] [Google Scholar]
- 3.Massardo L, Gabriel SE, Crowson CS, O'Fallon WM, Matteson EL. A population based assessment of the use of orthopedic surgery in patients with rheumatoid arthritis. J Rheumatol. 2002;29:52–6. [PubMed] [Google Scholar]
- 4.Singh JA, Lewallen DG. Better functional and similar pain outcomes in osteoarthritis compared to rheumatoid arthritis after primary total knee arthroplasty: A cohort study. Arthritis Care Res (Hoboken) 2013;65:1936–41. doi: 10.1002/acr.22090. [DOI] [PubMed] [Google Scholar]
- 5.Kirwan JR, Currey HL, Freeman MA, Snow S, Young PJ. Overall long-term impact of total hip and knee joint replacement surgery on patients with osteoarthritis and rheumatoid arthritis. Br J Rheumatol. 1994;33:357–60. doi: 10.1093/rheumatology/33.4.357. [DOI] [PubMed] [Google Scholar]
- 6.Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum. 2005;52:1009–19. doi: 10.1002/art.20941. [DOI] [PubMed] [Google Scholar]
- 7.Hekmat K, Jacobsson L, Nilsson JA, Petersson IF, Robertsson O, Garellick G, et al. Decrease in the incidence of total hip arthroplasties in patients with rheumatoid arthritis - results from a well defined population in south Sweden. Arthritis Res Ther. 2011;13:R67. doi: 10.1186/ar3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss RJ, Stark A, Wick MC, Ehlin A, Palmblad K, Wretenberg P. Orthopaedic surgery of the lower limbs in 49,802 rheumatoid arthritis patients: results from the Swedish National Inpatient Registry during 1987 to 2001. Ann Rheum Dis. 2006;65:335–41. doi: 10.1136/ard.2005.039420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66:1432–9. doi: 10.1002/art.38384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mont MA, Jacobs JJ. AAOS clinical practice guideline: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg. 2011;19:777–8. doi: 10.5435/00124635-201112000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–97. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 12.Johnson BK, Goodman SM, Alexiades MM, Figgie MP, Demmer RT, Mandl LA. Patterns and associated risk of perioperative use of anti-tumor necrosis factor in patients with rheumatoid arthritis undergoing total knee replacement. J Rheumatol. 2013;40:617–23. doi: 10.3899/jrheum.121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng B, Aslam F, Petersen NJ, Yu HJ, Suarez-Almazor ME. Identification of rheumatoid arthritis patients using an administrative database: a Veterans Affairs study. Arthritis Care Res (Hoboken) 2012;64:1490–6. doi: 10.1002/acr.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellamy N. Instruments to assess osteoarthritis--current status and future needs. Ann Rheum Dis. 1995;54:692–3. doi: 10.1136/ard.54.9.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busija L, Osborne RH, Nilsdotter A, Buchbinder R, Roos EM. Magnitude and meaningfulness of change in SF-36 scores in four types of orthopedic surgery. Health Qual Life Outcomes. 2008;6:55. doi: 10.1186/1477-7525-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Escobar A, Garcia Perez L, Herrera-Espineira C, Aizpuru F, Sarasqueta C, Gonzalez Saenz de Tejada M, et al. Total knee replacement; minimal clinically important differences and responders. Osteoarthritis Cartilage. 2013;21:2006–12. doi: 10.1016/j.joca.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Katz JN, Mahomed NN, Baron JA, Barrett JA, Fossel AH, Creel AH, et al. Association of hospital and surgeon procedure volume with patient-centered outcomes of total knee replacement in a population-based cohort of patients age 65 years and older. Arthritis Rheum. 2007;56:568–74. doi: 10.1002/art.22333. [DOI] [PubMed] [Google Scholar]
- 20.Mancuso CA, Sculco TP, Wickiewicz TL, Jones EC, Robbins L, Warren RF, et al. Patients' expectations of knee surgery. J Bone Joint Surg Am. 2001;83-A:1005–12. doi: 10.2106/00004623-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 21. http://www.asahq.org/clinical/physicalstatus.htm.
- 22.Fortin PR, Clarke AE, Joseph L, Liang MH, Tanzer M, Ferland D, et al. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42:1722–8. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 23.Dempsey KE, Collins JE, Ghazinouri R, Alcantara L, Thornhill TS, Katz JN. Associations between preoperative functional status and functional outcomes of total joint replacement in the Dominican Republic. Rheumatology (Oxford) 2013;52:1802–8. doi: 10.1093/rheumatology/ket180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh JA, Lewallen DG. Medical and psychological comorbidity predicts poor pain outcomes after total knee arthroplasty. Rheumatology (Oxford) 2013;52:916–23. doi: 10.1093/rheumatology/kes402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman SM, Ramsden-Stein DN, Huang WT, Zhu R, Figgie MP, Alexiades MM, et al. Patients with Rheumatoid Arthritis Are More Likely to Have Pain and Poor Function After Total Hip Replacements than Patients with Osteoarthritis. J Rheumatol. 2014;41:1774–80. doi: 10.3899/jrheum.140011. [DOI] [PubMed] [Google Scholar]
- 26.Benoni AC, Bremander A, Nilsdotter A. Patient-reported outcome after rheumatoid arthritis-related surgery in the lower extremities. Acta Orthop. 2012;83:179–84. doi: 10.3109/17453674.2011.645193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravi B, Croxford R, Austin PC, Hollands S, Paterson JM, Bogoch E, et al. Increased surgeon experience with rheumatoid arthritis reduces the risk of complications following total joint arthroplasty. Arthritis Rheumatol. 2014;66:488–96. doi: 10.1002/art.38205. [DOI] [PubMed] [Google Scholar]
- 28.Strand V, Sharp V, Koenig AS, Park G, Shi Y, Wang B, et al. Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis and psoriasis and effects of etanercept treatment. Ann Rheum Dis. 2012;71:1143–50. doi: 10.1136/annrheumdis-2011-200387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozic KJ, Chiu VW, Takemoto SK, Greenbaum JN, Smith TM, Jerabek SA, et al. The validity of using administrative claims data in total joint arthroplasty outcomes research. J Arthroplasty. 2010;25:58–61. doi: 10.1016/j.arth.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Daneshvar P, Forster AJ, Dervin GF. Accuracy of administrative coding in identifying hip and knee primary replacements and revisions. J Eval Clin Pract. 2012;18:555–9. doi: 10.1111/j.1365-2753.2010.01622.x. [DOI] [PubMed] [Google Scholar]
- 31.Bachmeier CJ, March LM, Cross MJ, Lapsley HM, Tribe KL, Courtenay BG, et al. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cartilage. 2001;9:137–46. doi: 10.1053/joca.2000.0369. [DOI] [PubMed] [Google Scholar]
- 32.March LM, Cross MJ, Lapsley H, Brnabic AJ, Tribe KL, Bachmeier CJ, et al. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients' quality of life before and after surgery with age-related population norms. Med J Aust. 1999;171:235–8. [PubMed] [Google Scholar]
- 33.Mertelsmann-Voss Trends in US Arthroplasty Rates 1991-2005: Patients with Inflammatory Arthritis Continue to Require Joint Replacement. Arthritis and Rheumatism. 2012;64:S1122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.