Abstract
Placebo-controlled pharmacotherapy trials for alcohol use disorder (AUD) require an active behavioral platform to avoid putting participants at risk for untreated AUD and to better assess the effectiveness of the medication. Therapist-delivered platforms (TDP) can be costly and present a risk to study design because of the variability in therapist fidelity. Take Control is a novel computer-delivered behavioral platform developed for use in pharmacotherapy trials sponsored by the National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG). This behavioral platform was developed with the goal of reducing trial implementation costs and limiting potential bias introduced by therapists providing TDP. This exploratory study is the first to compare Take Control with TDP on measures related to placebo response rate, medication adherence, and participant retention. Data were drawn from the placebo arms of four multisite, double-blind, randomized controlled trials (RCT) for AUD conducted by NCIG from 2007 to 2015. Data were compared from subjects receiving TDP (N=156) in two RCTs and Take Control (N=155) in another two RCTs. Placebo response rate, as represented by weekly percentage of heavy drinking days, was similar between groups. Subjects who received Take Control had a higher rate of medication adherence than those who received TDP. Subject retention was not significantly different between groups. The findings suggest that Take Control is comparable to TDP on measures of retention, medication adherence, and placebo response. Additional research is needed to evaluate Take Control directly against TDPs in a randomized trial.
Keywords: Behavioral platform, Alcohol use disorder, Randomized controlled trial, Computer-based intervention, Take Control
1. Introduction
Effective evaluation of medications for the treatment of alcohol use disorder (AUD) hinges on a strong placebo-controlled design. Studies that provide only a placebo without standard care, however, place subjects at an unacceptable level of risk of morbidity and mortality [1–3] from an otherwise preventable disease with a range of effective treatment options [4]. Industry guidelines encourage the use of an active control, which provides an established treatment when testing medications for a serious illness, when satisfactory treatments are available [5].
Although the use of an active control provides the needed platform to ethically evaluate medications in addiction trials, this design can create challenges in early phase II pharmacotherapy research. In fact, the active control can potentially obscure medication effects [6]. Furthermore, when the active control is a psychosocial intervention, there often is considerable variability in how the intervention is delivered by therapists within the same site or across multiple sites. Even with rigorous training measures in place and careful supervision, therapists often inadvertently drift from manualized approaches [7,8]. For example, some therapists may deliver a greater intensity treatment, provide additional attention, or give unequal treatment to subjects who fail to respond [9]. Ensuring treatment fidelity has become a cornerstone in the evaluation and dissemination of evidence-based treatments [10] but the standards for ensuring fidelity can be substantial and costly. Within addictions research, the current treatment fidelity standards for psychosocial interventions include developing a manualized approach, providing a standards-based training, developing minimum proficiency criteria for certification, monitoring performance using a validated rating scale, and providing corrective feedback or supervision [10]. Even with high levels of fidelity, it is still possible to have significant variation in treatment effects as a result of clinician characteristics (e.g. empathy) which are not readily controlled through experimental design. These characteristics also may differ across sites and are a potential cause of small treatment effect sizes in multisite addiction trials [11].
A number of psychosocial platforms have been used as active controls in medication trials for addiction [6] including Brief Behavioral Compliance Enhancement Treatment (12), the BRENDA Approach (13), and Medical Management (14). These platforms are used with both the medication and placebo treatment arms in placebo-controlled randomized clinical trials (RCTs). Some of these have been effective, especially in trials where the effects of the investigational medication were clinically meaningful and statistically significant [15,16]. Still, it is possible that the treatment effect sizes have been diminished because therapist effects have increased the placebo response. It is also possible that some trials deemed unsuccessful were simply the result of problems with treatment fidelity and the conclusions of “no benefit from medication” were erroneous type-2 errors [17]. Clearly it is a challenge to design a study that ensures high levels of treatment fidelity, provides consistent care, and which can be compared easily across sites and at a reasonable cost.
1.1 Specific Aims
Take Control is a novel computerized bibliotherapy platform, developed by Megan Ryan and Eric Devine and funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). It is derived from NIAAA’s web-based, interactive self-help approach, Rethinking Drinking [18]. Take Control was developed as a minimal intensity behavioral platform for use in RCTs for alcohol use disorder. To date, Take Control has been used in two completed multisite clinical trials for alcohol dependence funded by the NIAAA Clinical Investigators Group (NCIG) [19,20]. The purpose of the current study is to compare the Take Control platform used in these trials with the therapist-delivered behavioral platforms used in two other NCIG trials [21,22] on measures of placebo response, medication adherence, and two related measures of participant retention.
2. Hypotheses
In this study, the following hypotheses were tested:
Given that Take Control is a computerized platform, the nonspecific effects of human interaction should be minimized and, consequently, studies that used Take Control should have a lower (or at least similar) placebo response compared with studies that delivered the behavioral platform in person.
Given that Take Control highlights the importance of medication adherence and participation, the rates for adherence and retention should be similar to other therapist driven interventions.
Given that it is not therapist delivered, studies that used Take Control should show reduced (or at least similar) within-site and between-site variability on all key outcomes of interest.
3. Methods
Data were obtained from 4 multisite, double-blind, randomized controlled trials (RCT) conducted by NCIG between 2007 and 2015 [19–22]. These 4 trials were selected for the present study because of the high degree of similarity in study design, study management staff, performance site personnel, and subject characteristics (see Table 1 for an overview of the key study characteristics, subject characteristics, and entry criteria across these four studies. The study methods and trial results have been extensively described in the main outcome papers, and are briefly summarized here.
Table 1.
study characteristics, subject characteristics, and entry criteria
| Trial Characteristics | Quetiapine | Leviteracitam | Varenicline | ABT-436 |
|---|---|---|---|---|
| Behavioral platform | Medical Management | BBCET | Take Control | Take Control |
| Number of sessions administered | 9 | 11 | 6 | 7 |
| Years Partipant Recruitment | 2007–2009 | 2009–2010 | 2011–2012 | 2013–2014 |
| Subjects assigned to Placebo | 113 | 66 | 101 | 71 |
| Drug exposure (weeks) | 13 | 16 | 13 | 12 |
| Maintenance period (study weeks) | 3–11 | 5–14 | 2–13 | 2–12 |
| Target Dose (per day) | 400 mg (2 tablets qd) | 2,000 mg (4 tablets qd) | 2 mg (2 tablets bid) | 800 mg (2 tablets bid) |
| Primary outcome | Percent Heavy Drinking Days | |||
| Subject Baseline Characteristics (placebo group) | Quetiapine (n=113) | Leviteracitam (n=66) | Varenicline (n=101) | ABT-436 (n=71) |
| Age | 45.5 (9.8) | 47.0 (11.5) | 45.0 (12.3) | 45.5 (11.6) |
| Male | 77.0% | 78.8% | 68.3% | 64.80% |
| Employed | 74.3% | 69.7% | 76.2% | 76.10% |
| Married | 46.9% | 43.9% | 37.6% | 45.10% |
| White | 77.9% | 71.2% | 70.3% | 73.20% |
| Black | 15.9% | 25.8% | 26.7% | 21.10% |
| Drinks per day | 12.9 (4.5) | 13.7 (7.1) | 12.5 (8.9) | 10.1 (5.4) |
| Drinks per drinking day | 14.4 (5.0) | 15.8 (9.6) | 13.6 (9.0) | 11.7 (5.9) |
| Percent Heavy drinking days | 86.9 (16.1) | 88.3 (16.2) | 87.2 (16.4) | 80.1 (20.5) |
| Age of onset regular drinking | 21.2 (8.2) | 18.6 (4.3) | 19.3 (5.5) | 18.8 (6.4) |
| Key Entry Criteria | Quetiapine | Leviteracitam | Varenicline | ABT-436 |
| Alcohol Dependence (DSM-IV) | Yes | Yes | Yes | Yes |
| Age | 18–65 | 18+ | 18+ | 18–65 |
| Alcohol Consumption | 8+/10+ drinks per drinking day (women/men) for at least 40% of any 60 days during days 31–60 of a 3-month pre-screening period AND at least 1 day 8+/10+ drinks per drinking day during the 14-days before randomization | 8+/10+ drinks per drinking day (women/men) for at least 40% of any 60 days during 3-month pre-screening period AND at least 1 day 4+/5+ drinks per drinking day during the 14-days before randomization | 28+/35+ drinks per week (women/men) during the 28-day prescreen period AND during the 7-days before randomization | 28+/35+ drinks per week (women/men) during the 28-day prescreen period |
| Psychiatric Comorbidity | No psychotic, major depressive, panic, and eating disorders | |||
| Substance Comorbidity | No dependence on any substance (except alcohol and nicotine) | No abuse or dependence on any substance (except alcohol and nicotine); however, cannabis abuse is acceptable | ||
3.1 Data
The first RCT, NCIG-001, assessed the efficacy of quetiapine fumarate extended-release in a 12-week trial of 224 alcohol-dependent patients. Patients were recruited from December 2007 to May 2009 across 6 academic clinical sites and used the behavioral platform Medical Management [14].
The second RCT, NCIG-002, assessed the efficacy of levetiracetam extended-release in a 14-week trial of 130 alcohol-dependent patients. Patients were recruited from November 2009 to May 2010 across the same 6 clinical sites as NCIG-001, with the substitution of Johns Hopkins University for Brown University. This trial used the Brief Behavioral Compliance Enhancement Treatment (BBCET) behavioral platform [12]
The third RCT, NCIG-003, assessed the efficacy of varenicline tartrate in a 13-week trial of 200 alcohol-dependent patients. Patients were recruited from February 2011 to February 2012 across the same 6 clinical sites as NCIG-002 and used the Take Control behavioral platform.
The final RCT, NCIG-004, assessed the efficacy of ABT-436 a novel, non-FDA approved, potent, selective arginine vasopressin (AVP) type 1B receptor (V1B) antagonist manufactured and provided by AbbVie, Inc. ABT-436 was evaluated in a 12-week trial of 148 alcohol dependent patients. Patients were recruited from February 2013 to October 2014 across 4 of the same clinical sites as NCIG-002 (i.e., minus University of Virginia-Richmond and Dartmouth University) and used the Take Control behavioral platform.
Each of the four NCIG trials included the same core clinical sites and had similar entry criteria and similar participant baseline characteristics (see Table 1). In all trials, study activities were conducted under the review of Institutional Review Boards and written informed consent was obtained from all subjects.
3.2 Behavioral Platforms
Medical Management (MM)
MM is a psychosocial, medically based, minimally intensive intervention developed and used in the COMBINE study [14]. MM was designed to assess side effects, educate the participant about excessive drinking, provide advice to maintain abstinence, encourage adherence to the study medication regimen, provide support for recovery, and encourage the use of mutual self-help groups such as Alcoholics Anonymous (AA). The first session was delivered at the randomization visit; with subsequent sessions occurring at each in-person clinic visit thereafter, for a total of 9 sessions. MM was used in the NCIG-001 Quetiapine trial. MM administrators were trained and certified prior to the start of the study and monitored for compliance throughout the length of the study.
Brief Behavioral Adherence Enhancement Treatment (BBCET)
BBCET is a brief (15 to 30 minutes per session) standardized treatment platform used in conjunction with a pharmacological intervention for the treatment of alcohol dependence [12]. BBCET was designed to enhance adherence with the medication and with other aspects of the treatment regimen. BBCET sessions address patient issues related to personal barriers of adherence, focusing on how medication can assist the patient in achieving his or her drinking goals, and, if necessary, addressing the management of adverse events. BBCET was used in the NCIG-002 Levetiracetam trial. The first session was delivered at the randomization visit, with subsequent sessions occurring at each in-person clinic visit thereafter for a total of 11 sessions. BBCET administrators were trained and certified prior to the start of the study. All BBCET sessions were audiotaped and random samples were monitored for adherence to the BBCET guidelines throughout the study.
Take Control
Take Control is a novel computerized bibliotherapy platform derived from the NIAAA’s self-help approach, Rethinking Drinking [18]. Rethinking Drinking provides evidence-based alcohol education to help problem drinkers cut back or quit. The published workbook and accompanying website (http://rethinkingdrinking.niaaa.nih.gov/) show drinkers how to estimate their consumption in standard drink units, describe the health risks of alcohol and the signs and symptoms of dependence, encourage drinkers to consider the pros and cons of change, and provide practical tips and strategies for cutting back or quitting drinking. Using flash-based animation tools, Rethinking Drinking was translated into a seven–session therapy platform with didactic information, animated graphics, and professional therapist voiceover. The Take Control platform addresses the following topics: 1) thinking about change? 2) how much is too much? 3) tips and strategies, 4) meeting goals, 5) obstacles to change, 6) module review, and 7) treatment options (see Appendix A for a detailed description of the session content for each module). Although not part of Rethinking Drinking, the Take Control platform provides a brief medication adherence component at the end of each module. The medication adherence component emphasizes the importance of remembering to take the study medication, adhering to the medication schedule, and encourages subjects to discuss issues involving the medication with the prescribing study physician. Take Control was produced in a modular format and a single module was viewed by all patients at each clinic visit. Take Control was used in the NCIG-003 Varenicline trial and the NCIG-004 ABT-436 trial. For the NCIG-003 trial, modules 2 and 3 were combined to yield 6 modules across the 6 clinic visits. For the NCIG-004 trial, all 7 modules were used across the 7 clinic visits.
3.3 Outcomes
The primary objective of this study is to determine if the Take Control behavioral platform has equivalent performance to human-delivered behavioral platforms commonly used in RCTs for alcohol use disorder. We chose placebo response, medication adherence, and retention as outcomes because the risks of dropout, poor medication adherence, or an exceptionally strong placebo intervention all pose a significant threat to the design of an RCT. All study outcomes were collected at the level of the individual participant, during study Weeks 5–11 (the overlapping maintenance period shared by all four trials). Outcomes were assessed only among participants assigned to the placebo arm in each trial. Subjects assigned to the active medication arms in each study were not included in the present study as differing medication effects in each trial would have a differential impact on the outcomes. The following three outcomes were evaluated:
Placebo Response
The placebo response was determined by the weekly percentage of heavy drinking days (PHDD) during each trial. PHDD was calculated by dividing the number of heavy drinking days each week by the number of days in the week with non-missing drinking data and multiplying by 100. A heavy drinking day was defined as 4 or more drinks per day for women and 5 or more drinks per day for men. PHDD was selected to represent the placebo effect because it was the primary outcome in each of the 4 RCTs. The higher the PHDD value the greater the amount of drinking. Thus, a higher PHDD finding indicates a relatively lower placebo response. In all trials, daily alcohol consumption was captured via the Time-Line Follow-back (TLFB) method and Form 90 interview [23,24]. One standard drink was defined as 0.5 ounces of absolute alcohol, equivalent to 10 ounces of beer (5% ABV), 4 ounces of wine (12% ABV), or 1.25 ounces of liquor (40% ABV).
Medication Adherence
Medication adherence was determined as the percentage of medication taken as prescribed and was calculated by the total number of pills taken divided by the total number of pills prescribed, and multiplied by 100. In all trials, data for the calculation of medication adherence were verified by comparing the subjects’ self-reported daily dose taken to the pill count. Because medication adherence was calculated only for the portion of the trial during which medication was prescribed for a given participant, this measure is thus unrelated to the amount of time the participant was in the trial. Participants who dropped out of the trial prior to taking any medication were excluded from analysis.
Participant Retention
Participant retention was measured using two related outcomes:
Complete TLFB Data
The first measure of participant retention was determined by the percentage of participants with complete TLFB data and was calculated as the number of participants with complete drinking data divided by the total number of participants, and multiplied by 100.
Visit Participation
The second measure of retention was determined by the percentage of trial visits attended (including both telephone calls and in-clinic visits) and was calculated as the number of visits attended divided by the number of possible visits that could be attended, and multiplied by 100.
3.4 Statistical Analysis
Outcome analyses were performed on a modified intention-to-treat (mITT) population that included all randomized patients who took at least one dose of placebo medication and provided valid outcome data during study Weeks 5–11. For all statistical tests, p<0.05 (two-tailed) was considered statistically significant. Data were analyzed with SAS 9.3 (SAS Institute, Inc., Cary, NC) and Stata 14 (StataCorp LP, College Station, TX).
Participant-level data were combined for the two trials that used the Take Control platform (Varenicline and ABT-436, now referred to as Take Control) vs. the other two trials that used therapist-delivered platforms (Quetiapine and Levetiracetam, now referred to as TDP). All subsequent outcome analyses compared the two Take Control trials with the two TDP trials to determine the independent variable of interest (i.e., Behavioral Platform).
The continuous outcome, PHDD (representing the placebo response), was analyzed using a repeated-measures mixed effects model (PROC MIXED, SAS), with factors (clinical site, week, and baseline PHDD) treated as fixed effects and study participants treated as the random effect. Baseline PHDD was used as a covariate to adjust the outcome for any baseline differences on the Behavioral Platform. A Toeplitz covariance matrix was used to model the correlations between repeated measures among patients. Least-square means (LSMEANs), standard errors (SEs), 95% confidence intervals (CIs), and standard deviations (SDs) were determined for the Behavioral Platform and for each clinical site (within Behavioral Platform), and averaged across the maintenance period. LSMEANS for the Behavioral Platform were tested for significance using the variance-weighted least squares test. The between-site variances of the LSMEANS for the Behavioral Platform were tested for significance using Levene’s robust test.
For two other continuous outcomes, medication adherence and visit participation, means were computed for the Behavioral Platform and for each clinical site (within the Behavioral Platform). Means for the Behavioral Platform were skewed and thus tested for significance using the Kruskal-Wallis equality-of-populations rank test. The between-site variances for the Behavioral Platform were tested for significance using Levene’s robust test.
For the dichotomous outcome, participants with complete drinking data, prevalence rates were computed for the Behavioral Platform and for each clinical site (within the Behavioral Platform). Prevalence rates for the Behavioral Platform were tested for significance using the Wald test within logistic regression model with no covariates. The between-site variances for the Behavioral Platform were tested for significance using Levene’s robust test.
Power was calculated using Proc Power in SAS. Averaging across clinical site, the study had approximately 80% power to detect a 12% mean difference between the Take Control and TDP groups on the percent heavy drinking days outcome, a 5% mean difference on the medication adherence outcome, a 10% difference on the complete drinking data outcome, and an 8% mean difference on the visit participation outcome.
4.0 Results
4.1 Placebo Response (PHDD outcome)
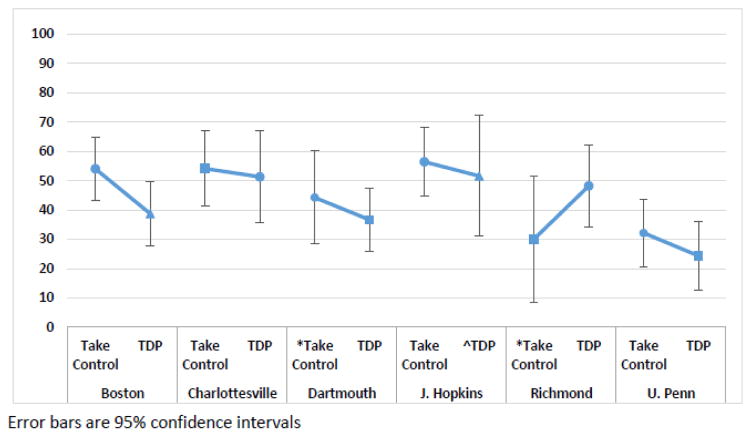
When averaged across clinical sites, the two studies using Take Control had a placebo response (PHDD) that was statistically similar to the two studies using TDP (LSMEAN [95% CI]): Take Control = 45.1% (39.1%–51.2%) vs. TDP = 41.8% (36.0%–47.6%); (p = .425). The groups also were similar when evaluated by clinical site. In all sites except one (Richmond), the combined Take Control studies had similar or slightly lower placebo response (i.e., higher PHDD) than the TDP group (Table 2, Figure 1). The groups also had similar within-site variability across all sites (Table 2). For example, within the Boston site the SDs were similar in both groups (33.9% and 34.5%, respectively). Finally, the groups had statistically similar between-site variability (SD: Take Control = 11.7% vs. TDP = 10.7%; p= .705).
Table 2.
Placebo Response Rate (Percent Heavy Drinking Days outcome) - Take Control vs Therapist-Delivered Platform
| Boston | Take Control | 38 | 54.0 | 33.9 | 5.5 | 43.2 | – | 64.9 |
| TDP | 38 | 38.7 | 34.5 | 5.6 | 27.7 | – | 49.8 | |
| Charlottesville | Take Control | 27 | 54.1 | 33.9 | 6.5 | 41.2 | – | 67.0 |
| TDP | 17 | 51.3 | 32.9 | 8.0 | 35.5 | – | 67.1 | |
| Dartmouth | *Take Control | 17 | 44.2 | 33.8 | 8.2 | 28.0 | – | 60.5 |
| TDP | 39 | 36.6 | 33.6 | 5.4 | 26.0 | – | 47.3 | |
| J. Hopkins | Take Control | 34 | 56.4 | 34.3 | 5.9 | 44.8 | – | 68.0 |
| ^TDP | 13 | 51.6 | 35.8 | 9.9 | 32.0 | – | 71.2 | |
| Richmond | *Take Control | 9 | 30.0 | 33.7 | 11.2 | 7.8 | – | 52.2 |
| TDP | 23 | 48.2 | 34.3 | 7.1 | 34.1 | – | 62.3 | |
| U. Penn | Take Control | 34 | 32.1 | 34.0 | 5.8 | 20.6 | – | 43.7 |
| TDP | 34 | 24.3 | 34.7 | 6.0 | 12.5 | – | 36.1 |
Note: Mixed model results; covariates = week, site, baseline PHDD
Figure 1.
Placebo Response Rate (Percent Heavy Drinking Days outcome) - Take Control vs Therapist Delivered Platform
4.2 Medication Adherence
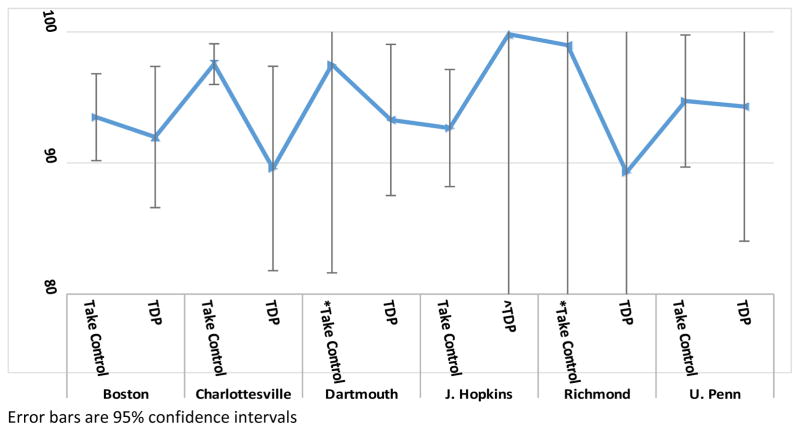
When averaged across clinical sites, the Take Control group had a medication adherence rate that was statistically greater than the TDP group (mean [95% CI]): Take Control = 95.1% (93.4%–96.8%) vs. TDP = 92.7% (89.3%–96.0%); p = .042). The groups were also similar when evaluated by clinical site. In all sites except one (Johns Hopkins), the combined Take Control group had a similar or somewhat greater medication adherence rate than the TDP group (Table 3, Figure 2). In all sites except one (Johns Hopkins), the within-site variability was smaller in the combined Take Control group than in the TDP group (Table 3). For example, within the Boston site the SD was smaller in the Take Control group than in the TDP group (10.3% and 16.1%, respectively). Finally, the groups had statistically similar between-site variability (SD: Take Control = 2.5% vs. TDP = 3.9%; p=.597).
Table 3.
Medication Adherence Rate - Take Control vs Therapist-Delivered Platform
| Boston | Take Control | 38 | 93.5 | 10.3 | 1.7 | 90.2 | – | 96.8 |
| TDP | 35 | 92.0 | 16.1 | 2.7 | 86.6 | – | 97.4 | |
| Charlottesville | Take Control | 27 | 97.5 | 4.1 | 0.8 | 96.0 | – | 99.1 |
| TDP | 17 | 89.6 | 16.3 | 3.9 | 81.8 | – | 97.4 | |
| Dartmouth | *Take Control | 17 | 97.5 | 5.0 | 1.2 | 95.1 | – | 99.9 |
| TDP | 38 | 93.3 | 18.0 | 2.9 | 87.5 | – | 99.0 | |
| J. Hopkins | Take Control | 30 | 92.7 | 12.4 | 2.3 | 88.2 | – | 97.1 |
| ^TDP | 11 | 99.8 | 0.6 | 0.2 | 99.4 | – | 100.2 | |
| Richmond | *Take Control | 9 | 99.0 | 2.3 | 0.8 | 97.4 | – | 100.5 |
| TDP | 23 | 89.3 | 28.5 | 5.9 | 77.5 | – | 101.0 | |
| U. Penn | Take Control | 34 | 94.7 | 14.9 | 2.5 | 89.7 | – | 99.8 |
| TDP | 32 | 94.3 | 29.4 | 5.2 | 84.0 | – | 104.6 |
Figure 2.
Medication Adherence Rate - Take Control vs vs Therapist-Delivered Platform
4.3 Participant Retention
Complete Drinking Data
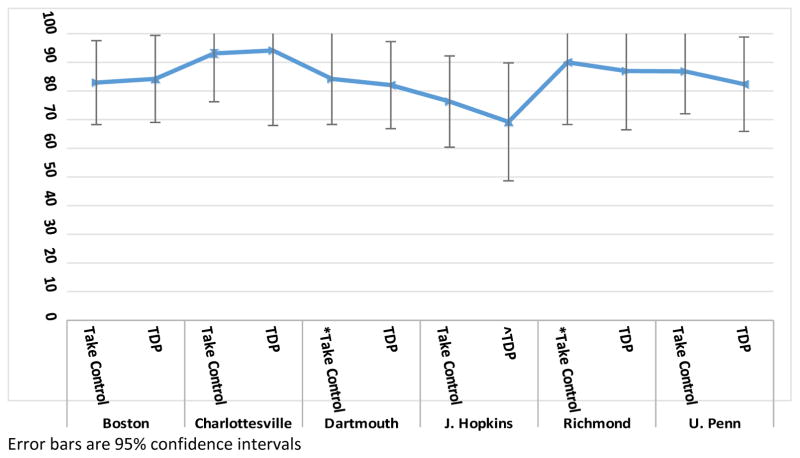
When averaged across clinical sites, the Take Control and TDP groups had statistically similar rates of complete drinking data (mean [95% CI]): Take Control = 84.6% (78.4–%89.2%) vs. TDP = 83.5% (77.1%–88.5%); p=.795). The groups were also similar when evaluated by clinical site. In all sites, the Take Control group had medication adherence rates equivalent to the TDP group (Table 4, Figure 3). The groups also had similar within-site variability in all sites (Table 4). For example, within the Boston site, the SDs were similar in the Take Control and TDP groups (37.6% and 36.5%, respectively). Finally, the groups had statistically similar between-site variability (SD: Take Control = 5.9% vs. TDP = 8.1%; p=.757).
Table 4.
Prevalence of Complete Drinking Data - Take Control vs Therapist-Delivered Platform
| Boston | Take Control | 41 | 82.9 | 37.6 | 5.9 | 68.3 | – | 91.6 |
| TDP | 38 | 84.2 | 36.5 | 5.9 | 69.0 | – | 92.7 | |
| Charlottesville | Take Control | 29 | 93.1 | 25.3 | 4.7 | 76.2 | – | 98.3 |
| TDP | 17 | 94.1 | 23.5 | 5.7 | 68.0 | – | 99.2 | |
| Dartmouth | *Take Control | 19 | 84.2 | 36.5 | 8.4 | 60.8 | – | 94.8 |
| TDP | 39 | 82.1 | 38.4 | 6.1 | 66.9 | – | 91.2 | |
| J. Hopkins | Take Control | 38 | 76.3 | 42.5 | 6.9 | 60.4 | – | 87.2 |
| ^TDP | 13 | 69.2 | 46.2 | 12.8 | 40.9 | – | 88.0 | |
| Richmond | *Take Control | 10 | 90.0 | 30.0 | 9.5 | 53.3 | – | 98.6 |
| TDP | 23 | 87.0 | 33.7 | 7.0 | 66.5 | – | 95.7 | |
| U. Penn | Take Control | 38 | 86.8 | 33.8 | 5.5 | 72.0 | – | 94.4 |
| TDP | 34 | 82.4 | 38.1 | 6.5 | 65.9 | – | 91.9 |
Figure 3.
Prevalence of Complete Drinking Data - Take Control vsvs Therapist-Delivered Platform
Visit Participation
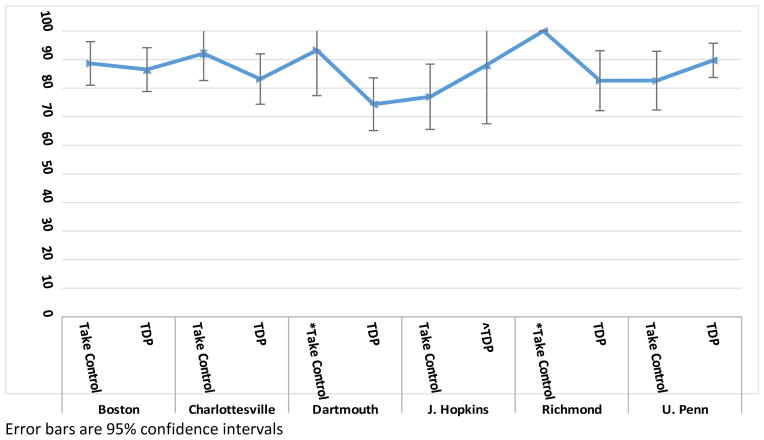
When averaged across clinical sites, the Take Control and TDP groups had statistically similar visit participation rates (mean [95% CI]): Take Control = 86.5% (82.2–90.8) vs. TDP = 83.4% (79.7–87.1); p=.369). The group differences varied by clinical site, with the Take Control group having slightly higher visit participation rates than the TDP group in four of the sites and lower rates in two of the sites (Table 5, Figure 4). The groups also showed slight within-site variability by site (Table 5). For example, the SDs were similar in the Take Control and TDP groups within one site (Boston), lower in the Take Control than in the TDP group in two sites (Dartmouth and Richmond), and higher in the Take Control than in the TDP group in three other sites (Johns Hopkins, Charlottesville, and U Penn). Finally, the groups had statistically similar between-site variability (SD: Take Control = 8.2% vs. TDP = 5.5%; p=.368).
Table 5.
Visit Participation Rate - Take Control vs Therapist-Delivered Platform
| Boston | Take Control | 38 | 88.6 | 23.8 | 3.9 | 81.0 | – | 96.3 |
| TDP | 38 | 86.5 | 23.9 | 3.9 | 78.8 | – | 94.1 | |
| Charlottesville | Take Control | 27 | 92.1 | 24.9 | 4.8 | 82.7 | – | 101.6 |
| TDP | 17 | 83.2 | 18.4 | 4.5 | 74.4 | – | 92.0 | |
| Dartmouth | *Take Control | 17 | 93.3 | 18.3 | 4.4 | 84.5 | – | 102.0 |
| TDP | 39 | 74.4 | 29.2 | 4.7 | 65.1 | – | 83.6 | |
| J. Hopkins | Take Control | 34 | 77.0 | 33.7 | 5.8 | 65.6 | – | 88.4 |
| ^TDP | 13 | 88.1 | 18.8 | 5.2 | 77.8 | – | 98.4 | |
| Richmond | *Take Control | 9 | 100.0 | 0.0 | 0.0 | 100.0 | – | 100.0 |
| TDP | 23 | 82.6 | 25.5 | 5.3 | 72.1 | – | 93.1 | |
| U. Penn | Take Control | 34 | 82.6 | 30.3 | 5.2 | 72.4 | – | 92.9 |
| TDP | 34 | 89.7 | 17.6 | 3.0 | 83.8 | – | 95.7 |
Figure 4.
Visit Participation Rate - Take Control vs Therapist-Delivered Platform
5.0 Discussion
Behavioral platforms are necessary components of any placebo-controlled trial assessing the efficacy of a medication for AUD. Unfortunately, therapists may introduce nonspecific treatment effects by inadvertently treating the placebo group differently from the active medication group. Such bias can make it difficult to assess the true therapeutic effect of the medication. Although the effect sizes generally are small to moderate [25], any variability in the therapy between treatment groups may mask detection of a medication’s actual effect.
A computer-delivered behavioral platform, Take Control, was designed to decrease the potential bias related to therapist interactions, increase the potential for detecting a medication effect, and decrease the cost of trial implementation. Data on key performance measures from four NCIG-funded RCTs were analyzed to compare Take Control with two TDPs. Take Control was comparable to TDP on placebo response rate, medication adherence, and participant retention. Take Control was also comparable to TDP on measures of between-site variability for placebo response rate, medication adherence, and participant retention. Given its performance on these key measures, Take Control appears to be a promising tool for testing AUD medications in RCTs. Take Control may also help to reduce trial start-up costs. Development costs for Take Control averaged $10,000 for each trial compared with the high cost of training, certification and monitoring of therapist-driven therapies (estimated at $200,000 per trial) [26]. NIAAA intends to make Take Control (in its present form) available to researchers at no cost.
5.1 Limitations
There are several limitations of this study due to the fact that this was a retrospective, descriptive and exploratory analyses of data collected over four separate clinical trials. First, the benefit of Take Control on medication adherence relative to TDP may be underestimated in the present study. Medication in the TDP trials was packaged and distributed to participants in blister packs with once per day dosing, whereas pill bottles were used in the Take Control trials with twice per day dosing. Data suggests that blister packs may have a positive effect on adherence compared with pill bottles [27] and less frequent dosing is associated with better adherence than more frequent dosing [28]. Second, the entry criteria in the NCIG studies were slightly different from one study to another. The most notable difference between the Take Control and TDP studies was the minimum drinking entry criterion. The two studies with TDPs required very heavy drinking and the two studies with Take Control required only heavy drinking. It is possible that subjects with very heavy drinking have a greater disease severity and may be more prone to non-adherence and dropout than subjects with lesser disease severity. However, despite the differences in inclusion criteria, the actual levels of alcohol consumption at baseline only differed slightly between the Take Control and TDP trials (Table 1). Third, although within-site variability was reported for retention, adherence and placebo response, the small sample sizes comparing Take Control and TDP within a site resulted in a relatively high degree of variability and should be interpreted with caution.
6.0 Summary
There is a clear need for further development and testing of a computerized behavioral platform such as Take Control for use in pharmacotherapy trials for the treatment of AUD. Additional research is needed to evaluate the performance of Take Control in other alcohol pharmacotherapy trials. Furthermore, a randomized trial could directly compare Take Control to another behavioral therapy such as Medical Management or BBCET to evaluate the impact of a therapist driven vs. computerized therapy on clinical outcomes.
Acknowledgments
The authors thank Barbara Vann of CSR Incorporated for her excellent editorial comments.
7.0 Funding
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [Contracts HHSN275200900005C and HHSN275201400001I].
Appendix A. Content of Take Control by treatment session
NCIG 004 – 7 Module Format
| Session 1 – Thinking About Change? |
| Module one focuses on encouraging drinkers to consider the pros and cons of change and encouraging medication adherence. |
| *Session 2 – How Much is Too Much? |
| Module two focuses on providing education about what constitutes a standard drink, defining “at-risk” drinking limits, providing normative feedback regarding rates of drinking within the US, educating drinkers about the health risks of heavy drinking, reviewing the symptoms of alcohol dependence, setting a drinking goal, reviewing behavioral strategies for cutting back on drinking, and encouraging medication adherence. |
| *Session 3 – Tips and Strategies |
| Module three focuses on reviewing behavioral strategies for cutting back on drinking (e.g., counting and measuring drinks, spacing drinks, finding alternatives to drinking) and encouraging medication adherence. |
| Session 4 – Meeting Your Goals |
| Module four focuses on educating drinkers about strategies for reducing drinking (e.g., handling triggers, coping with urges, refusing offers to drink), encouraging drinkers to seek out sober support, and encouraging medication adherence. |
| Session 5 – Obstacles |
| Module five focuses on encouraging drinkers to anticipate risks and to plan coping strategies ahead of time by changing risky thinking that rationalizes continued drinking, and encouraging medication adherence. |
| Session 6 – Module Review |
| Module six reviews modules one through four and is intended to reinforce strategies learned during the course of treatment. |
| Session 7 – Treatment Options |
| Module seven provides drinkers with an overview of treatment options including mutual support groups, addiction specialists, and medications commonly used to treat problem drinking. |
Modules 2 and 3 were combined for NCIG-003 to yield a 6-module version of Take Control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amdur RJ, Biddle CJ. The placebo controlled clinical trial. In: Amdur RJ, Bankert EA, editors. Institutional Review Board: Management and Function. Jones and Bartlett; Sudbury, MA: 2006. pp. 441–449. [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States. JAMA. 2000;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Rehm J, Gmel G, Sempos CT, Trevisan M. Alcohol-related morbidity and mortality. Alcohol Res Health. 2003;27:39–51. [PMC free article] [PubMed] [Google Scholar]
- 4.Miller WR, Wilbourne PL. Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97:265–277. doi: 10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- 5.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. [accessed 4.11.2016];ICH harmonised tripartite guideline, choice of control group and related issues in clinical trials E10. 2002 http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E10/Step4/E10_Guideline.pdf.
- 6.Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug Alcohol Depend. 2004;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waller G. Evidence-based treatment and therapist drift. Behaviour Research and Therapy. 2009;47:119–127. doi: 10.1016/j.brat.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Miller MR, Moyers TB, Arciniega L, Ernst D, Forcehimes A. Training, supervision and quality monitoring of the COMBINE study behavioral interventions. Journal of Studies on Alcohol. 2005;(Supplement no 15):188–195. doi: 10.15288/jsas.2005.s15.188. [DOI] [PubMed] [Google Scholar]
- 9.Carroll KM. Manual-guided psychosocial treatment: a new virtual requirement for pharmacotherapy trials? Arch Gen Psychiat. 1997;54:923–928. doi: 10.1001/archpsyc.1997.01830220041007. [DOI] [PubMed] [Google Scholar]
- 10.Baer JS, Ball SA, Campbell BK, Miele GM, Schoener EP, Tracy K. Training and fidelity monitoring of behavioral interventions in multi-site addictions research: A review. Drug and alcohol dependence. 2007;87(2–3):107–118. doi: 10.1016/j.drugalcdep.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller WR, Moyers TB. The forest and the trees: relational and specific factors in addiction treatment. Addiction. 2015;110:410–413. doi: 10.1111/add.12693. [DOI] [PubMed] [Google Scholar]
- 12.Johnson BA, DiClemente CC, Ait-Daoud N, Stoks SM. Brief Behavioral Compliance Enhancement Treatment (BBCET) manual. In: Johnston BA, Ruiz P, Galanter M, editors. Handbook of clinical alcoholism treatment. Lippincott Williams & Wilkins; Baltimore, MD: 2003. pp. 282–301. [Google Scholar]
- 13.Volpicelli JR, Pettinati HM, McLellan AT, O’Brien CP. Combining medication and psychosocial treatments for addictions: the BRENDA approach. Guilford Press; New York: 2001. [Google Scholar]
- 14.Pettinati HM, Weiss RD, Dundon W, et al. A structured approach to medical management: A psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. Journal of Studies on Alcohol. 2015;(Supplement 15):170–178. doi: 10.15288/jsas.2005.s15.170. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. Journal of the American Medical Association. 2007;298(14):1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- 16.Volpicelli JR, Volpicelli LA, O’Brien CP. Medical management of alcohol dependence: Clinical use and limitations of naltrexone treatment. Alcohol and Alcoholism. 1995;30(6):789–798. [PubMed] [Google Scholar]
- 17.Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent. 2011;71:552–563. doi: 10.1111/j.1752-7325.2011.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute on Alcohol Abuse and Alcoholism (NIAAA) Rethinking drinking. National Institutes of Health; Bethesda, MD: 2009. NIH publication no. 09–3770. [Google Scholar]
- 19.Litten RZ, Ryan ML, Fertig JB, et al. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7(4):277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan ML, Falk DE, Fertig JB, et al. A phase 2, double-blind, placebo-controlled trial shows efficacy of ABT-436, a V1b antagonist, for treatment of alcohol dependence. doi: 10.1038/npp.2016.214. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litten RZ, Fertig JB, Falk DE, et al. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate in very heavy drinking alcohol-dependent patients. Alcoholism, Clinical and Experimental Research. 2012;36(3):406–416. doi: 10.1111/j.1530-0277.2011.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fertig JB, Ryan ML, Falk DE, Litten RZ, Mattson ME, Ransom J, Rickman WJ, Scott C, Ciraulo D, Green AI, Tiouririne NA, Johnson B, Pettinati H, Strain EC, Devine E, Brunette MF, Kampman KA, Tompkins D, Stout R the NCIG 002 Study Group. A double-blind, placebo-controlled trial assessing the efficacy of levetiracetam extended-release in very heavy drinking alcohol-dependent patients. Alcoholism: Clinical and Experimental Research. 2012;36:1421–1430. doi: 10.1111/j.1530-0277.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- 24.Miller W. Form 90: A structured assessment interview for drinking and related behaviors (Test Manual) National Institute on Alcohol Abuse and Alcoholism; Bethesda, Maryland: 1996. NIH publication no. 96-4004. [Google Scholar]
- 25.Magill M, Ray LA. Cognitive-behavioral treatment with adult alcohol and illicit drug users: A meta-analysis of randomized controlled trials. J Stud Alcohol Drugs. 2009;70(4):516–527. doi: 10.15288/jsad.2009.70.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institute on Alcohol and Alcoholism. [accessed 4.11.2016];New technology boosts clinical study design for alcohol medications team. 2016 http://www.niaaa.nih.gov/news-events/news-noteworthy/new-technology-boosts-clinical-study-design-alcohol-medications-team.
- 27.Zedler BK, Joyce A, Murrell L, Kakad P, Harpe S. A pharmacoepidemiologic anaylsis of the impact of calendar packaging on adherence to self-administered medication for long-term use. Clin Thera. 2011;33(5):581–597. doi: 10.1016/j.clinthera.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. American Journal of Managed Care. 2009;15(6):e22–e33. [PubMed] [Google Scholar]