Abstract
Objective
Controversy persists regarding the perioperative management of clopidogrel among patients undergoing carotid endarterectomy (CEA). This study examined the effect of preoperative dual antiplatelet therapy (aspirin and clopidogrel) on in-hospital CEA outcomes.
Methods
Patients undergoing CEA in the Vascular Quality Initiative were analyzed (2003–2014). Patients on clopidogrel and aspirin (dual therapy) were compared with patients taking aspirin alone preoperatively. Study outcomes included reoperation for bleeding and thrombotic complications defined as transient ischemic attack (TIA), stroke, or myocardial infarction. Secondary outcomes were in-hospital death and composite stroke/death. Univariate and multivariable analyses assessed differences in demographics and operative factors. Propensity score-matched cohorts were derived to control for subgroup heterogeneity.
Results
Of 28,683 CEAs, 21,624 patients (75%) were on aspirin and 7059 (25%) were on dual therapy. Patients on dual therapy were more likely to have multiple comorbidities, including coronary artery disease (P < .001), congestive heart failure (P < .001), and diabetes (P < .001). Patients on dual therapy were also more likely to have a drain placed (P < .001) and receive protamine during CEA (P < .001). Multivariable analysis showed that dual therapy was independently associated with increased reoperation for bleeding (odds ratio [OR], 1.71; 95% confidence interval [CI], 1.20–2.42; P = .003) but was protective against TIA or stroke (OR, 0.61; 95% CI, 0.43–0.87; P = .007), stroke (OR, 0.63; 95% CI, 0.41–0.97; P = .03), and stroke/death (OR, 0.66; 95% CI, 0.44–0.98; P = .04). Propensity score matching yielded two groups of 4548 patients and showed that patients on dual therapy were more likely to require reoperation for bleeding (1.3% vs 0.7%; P = .004) but less likely to suffer TIA or stroke (0.9% vs 1.6%; P = .002), stroke (0.6% vs 1.0%; P = .04), or stroke/death (0.7% vs 1.2%; P = .03). Within the propensity score-matched groups, patients on dual therapy had increased rates of reoperation for bleeding regardless of carotid symptom status. However, asymptomatic patients on dual therapy demonstrated reduced rates of TIA or stroke (0.6% vs 1.5%; P < .001), stroke (0.4% vs 0.9%; P = .01), and composite stroke/death (0.5% vs 1.0%; P = .02). Among propensity score-matched patients with symptomatic carotid disease, these differences were not statistically significant.
Conclusions
Preoperative dual antiplatelet therapy was associated with a 40% risk reduction for neurologic events but also incurred a significant increased risk of reoperation for bleeding after CEA. Given its observed overall neurologic protective effect, continued dual antiplatelet therapy throughout the perioperative period is justified. Initiating dual therapy in all patients undergoing CEA may lead to decreased neurologic complication rates.
Persistent controversy surrounds the use of dual antiplatelet therapy with aspirin and clopidogrel (Plavix; Sanofi, Bridgewater, NJ), an important and increasingly used treatment combination in the medical management of carotid artery stenosis and coronary artery disease. Accordingly, surgeons are frequently confronted with the question of whether to continue clopidogrel therapy at the time of carotid endarterectomy (CEA). Some choose to discontinue clopidogrel due to a perceived increased risk of hemorrhagic complications.1 Alternatively, others continue clopidogrel preoperatively, citing concern for potentially increased risk of perioperative thrombotic complications, including stroke or myocardial infarction (MI), if clopidogrel is discontinued. This debate reflects conflicting reports in the scientific literature.2–7
Such variation was highlighted by a recent survey of European vascular surgeons which revealed that 55% would stop clopidogrel before CEA in asymptomatic patients and 43% would stop clopidogrel even among symptomatic patients.1 To date, no large scale, national studies have analyzed the magnitude and implications of the competing risks associated with management of anti-platelet medications at the time of CEA. Because most surgeons are comfortable performing CEA in a patient taking aspirin alone due to its beneficial antithrombotic effects and minimal bleeding risk,1,8 we sought to examine the effect of clopidogrel as a component of dual antiplatelet therapy. Therefore, the purpose of this study was to determine the effect of dual antiplatelet therapy on major bleeding and thrombotic complications in patients undergoing CEA in contemporary practice.
METHODS
Database and cohort assembly
A retrospective analysis of all patients undergoing CEA from 2003 to 2014 in the Vascular Quality Initiative (VQI) database was performed. The VQI maintains a prospective registry wherein trained clinical data abstractors, nurses, and physicians enter data regarding patient characteristics, operative details, and outcomes encompassing >150 variables. The VQI expanded rapidly during the study period and is currently used by ~350 centers and 2600 physicians. The database is audited annually, using hospital claims data, to ensure that all procedures are entered by participating centers.9,10 Because the VQI contains deidentified patient data, patient consent and Institutional Review Board approval are not applicable.
To study patients undergoing elective, initial CEA only, patients with a history of prior ipsilateral CEA or carotid artery stent were excluded. Patients undergoing urgent/emergency surgery were also excluded, as were patients undergoing concurrent procedures such as coronary artery bypass grafting.
Exposure and outcome measures
Patients were first categorized by antiplatelet medication use. The VQI records perioperative medications based on whether the patient received the medication ≤48 hours before surgery; for example, if a patient was on dual antiplatelet therapy but clopidogrel was stopped 5 days before, that patient would be categorized as being on aspirin alone at the time of CEA. To isolate the risks and benefits of clopidogrel, we compared patients on aspirin and clopidogrel to those on aspirin alone. These two groups represented 83% of the overall sample of CEAs and comprised the study cohort. Patients on no antiplatelet medications or on clopidogrel monotherapy were excluded. A very small number of patients (~0.5% of the sample) were also excluded who were on antiplatelet medications other than aspirin or clopidogrel (prasugrel, ticlopidine, ticagrelor, or other).
The primary outcomes studied were major bleeding complications and thrombotic complications. Major bleeding complications were defined as reoperation for bleeding. The occurrence of neck hematoma (not requiring reoperation) could not be analyzed because this outcome is not collected in the VQI. Thrombotic complications were defined as the occurrence of stroke, transient ischemic attack (TIA), or MI in the postoperative period. Secondary outcomes were in-hospital death and composite stroke/death.
Statistical analysis
Patient demographics and operative factors were compared between groups using a t-test (two-group) for continuous variables and the χ2 test for categorical or dichotomous variables. Categorical outcomes were compared using the χ2 test. Separate multivariable logistic regression models were used to determine factors associated with the following outcomes: reoperation for bleeding, TIA or stroke, ipsilateral TIA or stroke, any stroke, MI, in-hospital death, and composite stroke/death.
For propensity score matching between the dual antiplatelet therapy group and the aspirin alone group, a propensity score was derived for each patient based on logistic regression of patient demographic and operative factors that were associated with dual antiplatelet therapy use. Only variables that were collected in the preoperative or immediate perioperative period were used for propensity score derivation. Additional propensity score-matched analyses were performed within symptomatic and asymptomatic subgroups. One-to-one nearest neighbor matching with a caliper of 0.01 was used to create two matched cohorts from the derived propensity score.11 The balance of covariates between groups was verified by comparing demographic and operative factors using the χ2 test. Standardized differences between covariates were also determined to assess for balance.11,12 All P values are two-tailed and were considered statistically significant if <.05. Data were analyzed using Stata 11.2 software (StataCorp LP, College Station, Tex).
RESULTS
Demographic and operative factors
Of 28,683 CEAs, 21,624 patients (75%) were on aspirin and 7059 (25%) were on dual therapy. There were multiple demographic differences between patients on dual antiplatelet therapy and patients on aspirin alone (Table I). Patients on dual therapy were more likely to have symptomatic carotid artery disease (31% vs 22%; P < .001), coronary artery disease (39% vs 26%; P < .001), or to have undergone prior coronary artery bypass grafting or another coronary intervention (49% vs 30%; P < .001). They also had higher rates of multiple comorbid conditions, including hypertension, congestive heart failure, chronic obstructive pulmonary disease, diabetes, and renal dysfunction. Patients on dual therapy were also more likely to be on preoperative β-blockers (69% vs 63%; P < .001) or statins (85% vs 80%; P < .001) and less likely to be on preoperative anticoagulants (3.1% vs 9.0%; P < .001). Further, cardiac stress testing demonstrated an abnormal result in 11% of patients on dual therapy compared with 8% of patients on aspirin alone (P < .001).
Table I.
Unmatched and propensity score-matched comparison of demographics and operative factors in patients undergoing carotid endarterectomy (CEA) in the Vascular Quality Initiative (VQI), stratified by dual antiplatelet therapy use
| Characteristica |
Unmatched
|
Propensity score matched
|
||||
|---|---|---|---|---|---|---|
| Aspirin (n = 21,624) | Aspirin + clopidogrel (n = 7059) | P value | Aspirin (n = 4548) | Aspirin + clopidogrel (n = 4548) | P value | |
| Demographics | ||||||
| Gender | ||||||
| Male | 12,870 (59.5) | 4460 (63.2) | <.001 | 2860 (62.9) | 2858 (62.8) | .965 |
| Female | 8754 (40.4) | 2598 (36.8) | ||||
| White race | 20,392 (94.3) | 6515 (92.3) | <.001 | 4200 (92.4) | 4187 (92.1) | .611 |
| Age, years | 70.4 ± 9.1 | 69.6 ± 9.4 | <.001 | |||
| Age category | ||||||
| <40 years | 15 (0.1) | 8 (0.1) | .257 | 3 (0.1) | 5 (0.1) | .479 |
| 40–49 years | 290 (1.3) | 137 (1.9) | <.001 | 60 (1.3) | 81 (1.8) | .075 |
| 50–59 years | 2355 (10.9) | 904 (12.8) | <.001 | 509 (11.2) | 529 (11.6) | .510 |
| 60–69 years | 7027 (32.5) | 2364 (33.5) | .123 | 1544 (34.0) | 1505 (33.1) | .386 |
| 70–79 years | 8255 (38.2) | 2541 (36.0) | .001 | 1720 (37.8) | 1698 (37.3) | .634 |
| 80–89 years | 3518 (16.3) | 1051 (14.9) | .006 | 685 (15.1) | 691 (15.1) | .861 |
| ≥89 years | 164 (0.8) | 54 (0.8) | .956 | 27 (0.6) | 39 (0.9) | .138 |
| Ipsilateral neurologic symptoms | 4792 (22.2) | 2193 (31.2) | <.001 | 1338 (29.4) | 1317 (29.0) | .628 |
| History of neck radiation | 291 (1.4) | 93 (1.3) | .863 | 64 (1.4) | 67 (1.5) | .790 |
| Hypertension | 19,054 (88.2) | 6414 (90.9) | <.001 | 4177 (91.8) | 4132 (90.9) | .093 |
| Coronary artery disease | 5645 (26.1) | 2772 (39.3) | <.001 | 1673 (36.8) | 1640 (36.1) | .472 |
| Prior CABG or coronary intervention | 6482 (30.3) | 3441 (49.4) | <.001 | 2224 (48.9) | 2212 (48.6) | .801 |
| Congestive heart failure | 1820 (8.4) | 773 (11.0) | <.001 | 480 (10.6) | 505 (11.1) | .399 |
| Smoking (prior or current) | 16,509 (76.5) | 5395 (76.5) | .881 | 3492 (76.8) | 3479 (76.6) | .820 |
| COPD | 4382 (20.3) | 1591 (22.6) | <.001 | 1008 (22.2) | 1018 (22.4) | .801 |
| Diabetes | 7068 (32.7) | 2694 (38.2) | <.001 | 1752 (38.5) | 1742 (38.3) | .829 |
| Creatinine ≥1.8% | 1045 (5.0) | 409 (5.9) | .001 | 234 (5.3) | 243 (5.5) | .688 |
| Dialysis | 172 (0.8) | 84 (1.2) | .002 | 55 (1.2) | 59 (1.3) | .706 |
| Pre-op medication regimen | ||||||
| β-blockers | 13,700 (63.4) | 4865 (69.0) | <.001 | 3044 (66.9) | 3033 (66.7) | .807 |
| Statin use | 17,182 (79.5) | 6025 (85.4) | <.001 | 3893 (85.6) | 3896 (85.7) | .929 |
| Anticoagulation | 1139 (9.0) | 146 (3.1) | <.001 | 147 (3.2) | 142 (3.1) | .765 |
| Stress test | ||||||
| Not done | 13,828 (64.2) | 4379 (62.3) | <.001 | 2904 (63.9) | 2870 (63.1) | .657 |
| Normal result | 6002 (27.8) | 1879 (26.7) | 1207 (26.5) | 1218 (26.8) | ||
| Abnormal result | 1726 (8.0) | 776 (11.0) | 437 (9.6) | 460 (10.1) | ||
| Operative factors | ||||||
| Drain | 6812 (39.9) | 2867 (46.1) | <.001 | 2026 (44.6) | 2085 (45.8) | .214 |
| Heparin | 21,366 (99.0) | 34,047 (98.9) | .741 | 4492 (98.8) | 4491 (98.8) | .925 |
| Protamine | 12,742 (59.1) | 4800 (68.2) | <.001 | 3104 (68.3) | 3108 (68.3) | .928 |
| Dextran | 2893 (13.4) | 859 (12.2) | .009 | 597 (13.1) | 619 (13.6) | .498 |
| Type | ||||||
| Conventional | 18,817 (87.5) | 6100 (87.0) | .308 | 3905 (85.9) | 3871 (85.1) | .311 |
| Eversion | 2698 (12.5) | 912 (13.0) | 643 (14.1) | 677 (14.9) | ||
| Shunt | 11,017 (51.1) | 4029 (57.3) | <.001 | 2560 (56.3) | 2519 (55.4) | .387 |
| Patch | 19.198 (88.9) | 6320 (89.9) | .031 | 4127 (90.7) | 4098 (90.1) | .301 |
| Post-op hypertensionb | 3250 (15.1) | 1145 (16.3) | .015 | 728 (16.0) | 775 (17.0) | .185 |
| Post-op hypotensionb | 2247 (10.4) | 785 (11.2) | .082 | 487 (10.7) | 491 (10.8) | .892 |
CABG, Coronary artery bypass graft; COPD, chronic obstructive pulmonary disease.
Categorical data are shown as number (%) and continuous data as mean ± standard deviation.
Requiring intravenous medication.
Analysis of operative factors revealed that surgical drains were placed in 46% of patients on dual antiplatelet therapy compared with 40% of patients on aspirin alone (P < .001). Dual-therapy patients were also more likely to receive protamine (68% vs 59%; P < .001). Shunts were used more frequently in dual therapy patients (57% vs 51%; P < .001). In the postoperative period, patients on dual therapy were more like to require intravenous medications to control hypertension (16% vs 15%; P = .015).
Crude and multivariable analysis
Crude analysis of major bleeding complications and thrombotic complications revealed that patients on dual antiplatelet therapy were significantly more likely to require reoperation for bleeding, with a reoperation rate of 1.2% compared with 0.7% in the aspirin-alone group (P < .001; Table II and Fig 1). No significant crude differences in thrombotic complications were seen between the two groups except for postoperative MI, which was more common in patients on dual therapy (1.2% vs 0.8%; P = .001).
Table II.
Major bleeding and thrombotic complications in unmatched and propensity score-matched patients undergoing carotid endarterectomy (CEA) in the Vascular Quality Initiative (VQI), stratified by dual antiplatelet therapy use
|
Unmatched
|
Propensity score matched
|
|||||
|---|---|---|---|---|---|---|
| Crude outcomes | Aspirin alone (n = 21,624), No. (%) | Clopidogrel + aspirin (n = 7059), No. (%) | P valuea | Aspirin alone (n = 4548), No. (%) | Clopidogrel + aspirin (n = 4548), No. (%) | P value |
| Major bleeding complications | ||||||
| RTOR for bleeding | 158 (0.7) | 84 (1.2) | <.001 | 32 (0.7) | 59 (1.3) | .004 |
| Thrombotic complications | ||||||
| TIA or stroke | 280 (1.3) | 72 (1.0) | .069 | 72 (1.6) | 39 (0.9) | .002 |
| Ipsilateral TIA or stroke | 209 (1.0) | 59 (0.8) | .322 | 56 (1.2) | 34 (0.8) | .02 |
| Any stroke | 180 (0.8) | 47 (0.7) | .171 | 44 (1.0) | 27 (0.6) | .04 |
| Post-op MI | 164 (0.8) | 84 (1.2) | .001 | 35 (0.8) | 43 (1.0) | .4 |
| Death | 44 (0.2) | 18 (0.3) | .417 | 14 (0.3) | 9 (0.2) | .3 |
| Stroke/death | 206 (1.0) | 57 (0.8) | .266 | 53 (1.2) | 33 (0.7) | .03 |
MI, Myocardial infarction; RTOR, return to operating room; TIA, transient ischemic attack.
P values for categorical variables were determined using the χ2 test.
Fig 1.
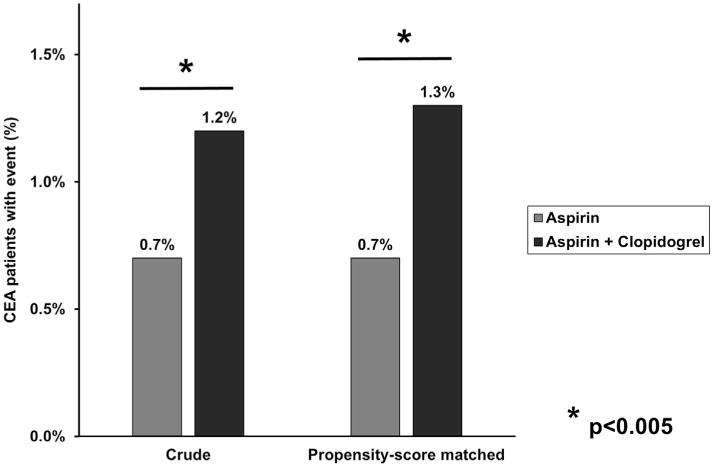
Rates of reoperation for bleeding in patients undergoing carotid endarterectomy (CEA) in the Vascular Quality Initiative (VQI), stratified by dual antiplatelet therapy use. Crude and propensity score-matched analyses are shown.
Multivariable analysis demonstrated that dual antiplatelet therapy was significantly associated with reoperation for bleeding with an odds ratio (OR) of 1.71 (95% confidence interval [CI], 1.20–2.42; P = .003; Table III). The only more powerful predictor was preoperative anticoagulant use (OR, 2.02; 95% CI, 1.23–3.31; P = .006). Notably, the only protective factor was protamine, with an OR of 0.45 (95% CI, 0.32–0.63; P < .001). Also of note, surgical drain placement did not protect against reoperation for bleeding, with an OR of 1.06 (95% CI, 0.76–1.48; P = .72).
Table III.
Independent predictors of reoperation for bleeding derived from multivariable logistic regression model
| Variable | OR (95% CI) | P value |
|---|---|---|
| Pre-op anticoagulant use | 2.02 (1.23–3.31) | .006 |
| Dual antiplatelet therapy | 1.71 (1.20–2.42) | .003 |
| Prior CABG or coronary intervention | 1.57 (1.06–2.33) | .02 |
| COPD | 1.47 (1.01–2.13) | .04 |
| Protamine | 0.45 (0.32–0.63) | <.001 |
CABG, Coronary artery bypass graft; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Separate additional multivariable models were constructed for each of the thrombotic outcomes, and the OR of dual antiplatelet therapy use was determined for each (Table IV). Dual therapy was found to be protective against TIA or stroke (OR, 0.61; 95% CI, 0.43–0.87; P = .007), any stroke (OR, 0.63; 95% CI, 0.41–0.97; P = .04), and composite stroke/death (OR, 0.66; 95% CI, 0.44–0.98; P = .04). Despite the observed difference in crude rates of postoperative MI between groups, there was no difference in MI rates on multivariable analysis (OR, 1.08; 95% CI, 0.75–1.57; P = .71).
Table IV.
Aggregate results of separate multivariable logistic regression models for each of the outcomes
| Outcome | Dual antiplatelet therapy OR (95% CI)a | P value |
|---|---|---|
| TIA or stroke | 0.61 (0.43–0.87) | .007 |
| Any stroke | 0.63 (0.41–0.97) | .04 |
| Post-op MI | 1.08 (0.74–1.57) | .71 |
| Death | 0.69 (0.32–1.47) | .34 |
| Stroke/death | 0.66 (0.44–0.98) | .04 |
CI, Confidence interval; MI, myocardial infarction; OR, odds ratio; TIA, transient ischemic attack.
The ORs represent the effect of dual antiplatelet therapy in each model.
Effect of reoperation for bleeding
To determine the clinical effect of reoperation for bleeding, outcomes were compared among patients who required reoperation and those who did not, irrespective of antiplatelet medication use. Overall, only 242 patients required reoperation, representing 0.8% of the study cohort. However, patients requiring reoperation for bleeding had significantly worse outcomes in every measured outcome. In particular, reoperation incurred higher rates of stroke (3.7% vs 0.8%; P < .001), MI (6.2% vs 0.8%; P < .001), and death (2.5% vs 0.2%; P < .001; Table V).
Table V.
Clinical implications of reoperation for bleedinga
| Outcome | RTOR for bleeding, No. (%) | No RTOR, No. (%) | P valueb |
|---|---|---|---|
| Patients, No. | 242 (0.8) | 28,441 (99.2) | <.001 |
| TIA or stroke | 17 (7.0) | 335 (1.2) | |
| Ipsilateral TIA or stroke | 13 (5.4) | 255 (0.9) | |
| Any Stroke | 9 (3.7) | 218 (0.8) | |
| Post-op MI | 15 (6.2) | 233 (0.8) | |
| Death | 6 (2.5) | 56 (0.2) | |
| Stroke/death | 12 (5.0) | 251 (0.9) |
MI, Myocardial infarction; RTOR, return to the operating room; TIA, transient ischemic attack.
Rates of major complications in patients who required reoperation for bleeding compared with patients who did not.
P values for categorical variables were determined using the χ2.
Propensity score-matched analysis
To optimally correct for significant sample heterogeneity, propensity score matching was performed, yielding two matched groups of 4548 patients. Despite the multiple differences in patient comorbidities and operative factors in the unmatched groups, propensity score matching successfully eliminated all differences between groups (Table I and Supplementary Table I, online only). Outcomes were then analyzed in the propensity score-matched cohorts, revealing that higher rates of reoperation for bleeding persisted among patients taking dual antiplatelet therapy (1.3% vs 0.7%; P = .004; Table II and Fig 1). However, compared with patients taking aspirin alone, patients on dual therapy experienced decreased rates of TIA or stroke (0.9% vs 1.6%; P = .002), ipsilateral TIA or stroke (0.8% vs 1.2%; P = .02), any stroke (0.6% vs 1.0%; P = .04), and composite stroke/death (0.7% vs 1.2%; P = .03; Fig 2).
Fig 2.
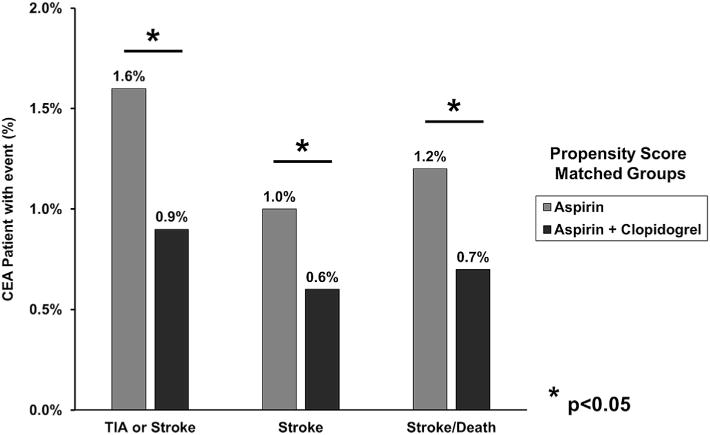
Rates of neurologic outcomes in propensity score-matched patients undergoing carotid endarterectomy (CEA) in the Vascular Quality Initiative (VQI), stratified by dual antiplatelet therapy use. Stroke, Any stroke; TIA, transient ischemic attack.
To determine the clinical effect of reoperation for bleeding in propensity score-matched cohorts, we analyzed thrombotic outcomes in patients who required reoperation for bleeding. On the basis of preoperative antiplatelet medication use, patients requiring reoperation for bleeding did not exhibit differences in TIA or stroke (3.4% dual therapy vs 9.4% aspirin alone; P = .2), any stroke (3.4% dual therapy vs 3.1% aspirin alone; P =.9), MI (8.5% dual therapy vs 6.3% aspirin alone; P = .7), or composite stroke/death (6.8% dual therapy vs 3.1% aspirin alone; P = .5).
Given that protamine exerted a strong protective effect in preventing reoperation for bleeding in multivariable analysis, we determined reoperation rates in the subset of propensity score-matched patients who received protamine (aspirin alone, n = 3104; dual therapy, n = 3108). Although protamine administration decreased rates of reoperation for bleeding in all patients, regardless of dual therapy use, a trend toward higher reoperation rates persisted in patients on dual antiplatelet therapy (0.9% vs 0.5%; P = .06).
To better determine the severity of neurologic events in each group, patients who experienced any TIA or stroke (0.9% in the dual therapy group and 1.6% in the aspirin alone group) were then further stratified by event type. Patients on dual therapy experienced significantly lower rates of TIA (0.3% vs 0.6% overall; P = .01) and major stroke (0.3% vs 0.6% overall; P = .03). In addition, there was also a trend toward decreased minor stroke rates in patients taking dual therapy, although this failed to reach statistical significance (0.2% vs 0.4%; P = .1).
Propensity score-matched analysis, stratified by symptom status
Outcomes in propensity score-matched groups were stratified based on preoperative neurologic symptom status (Figs 3 and 4). Asymptomatic patients had a persistent elevated risk of reoperation for bleeding associated with dual therapy (1.2% dual therapy vs 0.7% aspirin alone; P = .05). This effect was more pronounced in the symptomatic group (1.7% dual therapy vs 0.8% aspirin alone; P = .03). However, the protective effect of dual therapy against thrombotic complications was most evident in patients with asymptomatic carotid disease. Asymptomatic patients on dual therapy demonstrated reduced rates of TIA or stroke (0.6% dual therapy vs 1.5% aspirin alone; P < .001), any stroke (0.4% dual therapy vs 0.9% aspirin alone; P = .01), and composite stroke/death (0.5% dual therapy vs 1.0% aspirin alone; P = .02). By comparison, this effect was diminished among patients with symptomatic carotid disease. Symptomatic patients taking dual therapy revealed reduced rates of TIA or stroke (1.4% dual therapy vs 1.7% aspirin alone; P = .6), any stroke (1.1% dual therapy vs 1.2% aspirin alone; P = .9), and composite stroke/death (1.2% dual therapy vs 1.5% aspirin alone; P = .5), but none of these differences were statistically significant.
Fig 3.
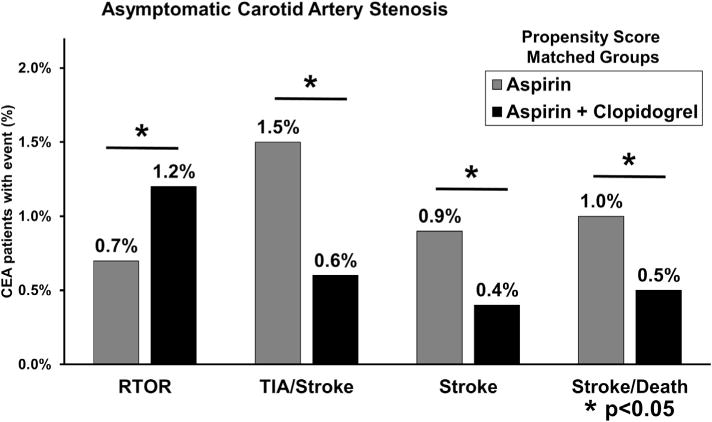
Rates of bleeding and thrombotic complications in propensity score matched patients with asymptomatic carotid artery stenosis undergoing carotid endarterectomy (CEA) in the Vascular Quality Initiative (VQI). RTOR, Return to the operating room for bleeding; stroke, any stroke; TIA, transient ischemic attack.
Fig 4.
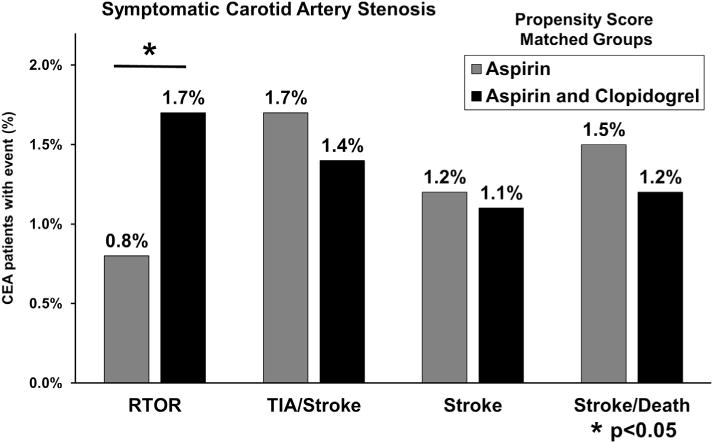
Rates of bleeding and thrombotic complications in propensity score-matched patients with asymptomatic carotid artery stenosis undergoing carotid endarterectomy (CEA) in the Vascular Quality Initiative (VQI). RTOR, Return to the operating room for bleeding; stroke, any stroke; TIA, transient ischemic attack.
Propensity score-matched analysis, additional symptom status models
To further explore whether patients with asymptomatic carotid disease differentially benefited from dual antiplatelet therapy compared with symptomatic patients, propensity score-matched groups were constructed separately for asymptomatic patients and symptomatic patients.
For asymptomatic patients, propensity score matching yielded two groups of 3220 patients that were well matched with regards to preoperative and intraoperative factors, except for increased patch use in patients on aspirin alone (90% dual therapy vs 91% aspirin alone; P = .04; Supplementary Table II, online only). Asymptomatic patients on dual therapy exhibited lower rates of TIA or stroke (0.6% dual therapy vs 1.3% aspirin alone; P = .003) and any stroke (0.3% dual therapy vs 0.8% aspirin alone; P = .03). However, there was no significant difference in stroke/death rates (0.5% dual therapy vs 0.8% aspirin alone; P = .1; Supplementary Table III, online only).
Propensity score matching of symptomatic patients yielded two groups of 1306 patients that were well-matched without any significant imbalance (Supplementary Table IV, online only). There were no differences in thrombotic complications in propensity score-matched symptomatic patients (Supplementary Table V, online only).
Reoperation for bleeding was related to dual antiplatelet therapy use in the symptomatic propensity score-matched analysis (1.5% dual therapy vs 0.6% aspirin alone; P = .02); however, there was no significant difference in the asymptomatic subgroup (1.2% dual therapy vs 1.0% aspirin alone; P = .5).
DISCUSSION
In this analysis of nearly 30,000 CEAs, patients taking dual antiplatelet therapy demonstrated a significantly reduced risk of major thrombotic complications, including stroke and TIA, leading to an overall protective effect against composite stroke/death, despite a concomitant small but significant increased risk of reoperation for bleeding. Although sequelae for reoperation for bleeding were not benign, with increased observed rates of MI, stroke, and death, the overall magnitude of dual therapy protection against stroke and stroke/death outweighed the bleeding risks.
These findings are supported by a randomized clinical trial in which 100 patients (all on aspirin) underwent CEA for symptomatic carotid artery stenosis and were randomized to receive clopidogrel vs placebo on the night before surgery. The group receiving clopidogrel had a 10-fold reduced risk of having >20 transcranial-detected cerebral emboli in the postoperative period but also had longer incision closure times (an indirect measure of hemostasis).13 The protective neurologic effects of clopidogrel have also been suggested for urgent CEA14 and in other series of patients with symptomatic carotid stenosis.15,16 Furthermore, emerging evidence by Batchelder et al17 suggests that initiating clopidogrel in patients who have recently suffered a TIA associated with 50% to 99% carotid artery stenosis can significantly reduce the risk of recurrent neurologic events before definitive CEA, thus recommending its use in this presumptively vulnerable patient population.
The perceived increased bleeding risk associated with dual antiplatelet therapy has been studied multiple times with conflicting results. A previous report from the Vascular Study Group of New England demonstrated no increased bleeding risk associated with dual antiplatelet therapy use, but this analysis was not specific to CEA.7 Other small series have alternately supported or refuted the association of dual antiplatelet therapy with bleeding complications for CEA.2–6 On the basis of our current analysis, there is an increased risk of bleeding complications associated with dual antiplatelet therapy use, and surgeons should be aware of this when performing CEA in these patients.
Interestingly, our current study also confirmed that the use of protamine to reverse heparin during CEA has a powerful protective effect against reoperation for bleeding, a finding consistent with previously published studies by Patel et al18 and Stone et al.19 In our current study, the administration of protamine to reverse heparin in patients taking dual therapy was associated with observed reoperation for bleeding rates of <1%. These findings further highlight that the optimal perioperative management of patients undergoing CEA calls for continued dual antiplatelet therapy in combination with intraoperative protamine administration.
The multivariable models convincingly show that dual antiplatelet therapy is independently associated with increased bleeding complications and decreased neurologic complications at the time of CEA. This risk modification is independent of symptom status. Interestingly, our propensity score-matched subgroups paradoxically demonstrated that asymptomatic patients derived the greatest clinical benefit from dual therapy compared with those with antecedent carotid symptoms. Although the symptomatic cohort demonstrated trends toward decreased neurologic complications, this finding did not achieve statistical significance. Symptomatic patients are at higher risk of neurologic complications in the postoperative period, which may be less easily modified by dual antiplatelet therapy. In addition to the clearly demonstrated protective perioperative neurologic effects independent of symptom status, we recommend dual therapy in symptomatic patients to reduce the risk of preoperative stroke after initial symptoms, as others have shown.17 We also recommend meticulous attention to hemostasis when performing CEA in patients on dual therapy due to the increased risk of reoperation for bleeding.
Overall, our results support the continuation of dual antiplatelet therapy at the time of CEA in patients with important indications for its use. Our results cannot determine whether dual therapy should be initiated preoperatively in all patients undergoing CEA. If clopidogrel is initiated at the time of CEA, there is currently no evidence establishing the duration of therapy. Alcocer et al20 reported that patients on dual therapy after CEA for asymptomatic disease demonstrated higher rates of all-cause mortality over a median follow-up of >4 years, suggesting that the risks of dual antiplatelet therapy may extend beyond the perioperative beneficial effects observed in this report.
This study has several intrinsic limitations. First, this is a multi-institutional registry, and as a result, clinical decisions regarding the need for reoperation for bleeding are not standardized. However, we believe there is little room for significant clinical variation in the management of such severe bleeding events.
Second, patients stopped taking clopidogrel 2 to 7 days before surgery may still have had residual dual antiplatelet effects, although they would have been categorized in the aspirin monotherapy group. This could potentially lead to an underestimation of the effects reported here.
In addition, only elective CEAs were included to best isolate the patients in whom decisions regarding perioperative antiplatelet medication management are most relevant. As a result, these data cannot be used to determine the effects of dual antiplatelet therapy in urgent or emergency CEA.
Finally, propensity score matching may not account for unmeasured differences in patient groups, such as the indication for antiplatelet therapy. However, >9000 patients were matched across multiple preoperative patient characteristics and intraoperative variables, minimizing the possibility of marked differences between cohorts. In this registry-based analysis, we are unable to account for clopidogrel resistance, although we believe this effect would be both rare and distributed evenly among the patients included in the analysis.
CONCLUSIONS
Compared with aspirin alone, dual antiplatelet therapy with clopidogrel was associated with increased rates of reoperation for bleeding. Despite this finding, dual therapy was independently associated with a substantially decreased risk of in-hospital TIA, stroke, and combined stroke/death, an effect that was most evident in asymptomatic patients. Furthermore, this study confirmed the protective effect of protamine use against significant bleeding complications at the time of CEA. Despite an increased bleeding risk, dual antiplatelet therapy should be continued given its significant protective neurologic effect at the time of CEA. On the basis of these findings, potential consideration for initiating dual therapy in all CEA patients is likely beneficial.
Supplementary Material
Footnotes
Presented as a plenary presentation at the 2015 Vascular Annual Meeting of the Society for Vascular Surgery, Chicago, Ill, June 17–20, 2015.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvascsurg.org.
AUTHOR CONTRIBUTIONS
Conception and design: DJ, PG, MC, BN, ER, RP, JC, DS
Analysis and interpretation: DJ, PG, JC, DS
Data collection: PG, JC, DS
Writing the article: DJ, DS
Critical revision of the article: DJ, PG, MC, BN, ER, RP, JC, DS
Final approval of the article: DJ, PG, MC, BN, ER, RP, JC, DS
Statistical analysis: DJ, PG, DS
Obtained funding: Not applicable
Overall responsibility: DS
References
- 1.Hamish M, Gohel MS, Shepherd A, Howes NJ, Davies AH. Variations in the pharmacological management of patients treated with carotid endarterectomy: a survey of European vascular surgeons. Eur J Vasc Endovasc Surg. 2009;38:402–7. doi: 10.1016/j.ejvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Baracchini C, Gruppo M, Mazzalai F, Lorenzetti R, Meneghetti G, Ballotta E. Predictors of neck bleeding after eversion carotid endarterectomy. J Vasc Surg. 2011;54:699–705. doi: 10.1016/j.jvs.2011.03.262. [DOI] [PubMed] [Google Scholar]
- 3.Saadeh C, Sfeir J. Discontinuation of preoperative clopidogrel is unnecessary in peripheral arterial surgery. J Vasc Surg. 2013;58:1586–92. doi: 10.1016/j.jvs.2013.05.092. [DOI] [PubMed] [Google Scholar]
- 4.Morales Gisbert SM, Sala Almonacil VA, Zaragoza Garcia JM, Genoves Gasco B, Gomez Palones FJ, Ortiz Monzon E. Predictors of cervical bleeding after carotid endarterectomy. Ann Vasc Surg. 2014;28:366–74. doi: 10.1016/j.avsg.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Payne DA, Twigg MW, Hayes PD, Naylor AR. Antiplatelet agents and risk factors for bleeding postcarotid endarterectomy. Ann Vasc Surg. 2010;24:900–7. doi: 10.1016/j.avsg.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum A, Rizvi AZ, Alden PB, Tretinyak AS, Graber JN, Goldman JA, et al. Outcomes related to antiplatelet or anticoagulation use in patients undergoing carotid endarterectomy. Ann Vasc Surg. 2011;25:25–31. doi: 10.1016/j.avsg.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Stone DH, Goodney PP, Schanzer A, Nolan BW, Adams JE, Powell RJ, et al. Clopidogrel is not associated with major bleeding complications during peripheral arterial surgery. J Vasc Surg. 2011;54:779–84. doi: 10.1016/j.jvs.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liapis CD, Bell PR, Mikhailidis D, Sivenius J, Nicolaides A, Fernandes e Fernandes J, et al. ESVS guidelines Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg. 2009;37(4 Suppl):1–19. doi: 10.1016/j.ejvs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 9.The Vascular Quality Initiative. Available at: http://www.vascularqualityinitiative.org/. Accessed July 25, 2015.
- 10.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW, et al. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion: 1101–2. [DOI] [PubMed] [Google Scholar]
- 11.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Payne DA, Jones CI, Hayes PD, Thompson MM, London NJ, Bell PR, et al. Beneficial effects of clopidogrel combined with aspirin in reducing cerebral emboli in patients undergoing carotid endarterectomy. Circulation. 2004;109:1476–81. doi: 10.1161/01.CIR.0000121739.05643.E6. [DOI] [PubMed] [Google Scholar]
- 14.Tsivgoulis G, Kerasnoudis A, Krogias C, Vadikolias K, Meves SH, Heliopoulos I, et al. Clopidogrel load for emboli reduction in patients with symptomatic carotid stenosis undergoing urgent carotid endarterectomy. Stroke. 2012;43:1957–60. doi: 10.1161/STROKEAHA.112.657916. [DOI] [PubMed] [Google Scholar]
- 15.Naylor AR, Sayers RD, McCarthy MJ, Bown MJ, Nasim A, Dennis MJ, et al. Closing the loop: a 21-year audit of strategies for preventing stroke and death following carotid endarterectomy. Eur J Vasc Endovasc Surg. 2013;46:161–70. doi: 10.1016/j.ejvs.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Sharpe RY, Dennis MJ, Nasim A, McCarthy MJ, Sayers RD, London NJ, et al. Dual antiplatelet therapy prior to carotid endarterectomy reduces post-operative embolisation and thromboembolic events: post-operative transcranial Doppler monitoring is now unnecessary. Eur J Vasc Endovasc Surg. 2010;40:162–7. doi: 10.1016/j.ejvs.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Batchelder A, Hunter J, Cairns V, Sandford R, Munshi A, Naylor AR. Dual antiplatelet therapy prior to expedited carotid surgery reduces recurrent events prior to surgery without significantly increasing peri-operative bleeding complications. Eur J Vasc Endovasc Surg. 2015;50:412–9. doi: 10.1016/j.ejvs.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Patel RB, Beaulieu P, Homa K, Goodney PP, Stanley AC, Cronenwett JL, et al. Shared quality data are associated with increased protamine use and reduced bleeding complications after carotid endarterectomy in the Vascular Study Group of New England. J Vasc Surg. 2013;58:1518–24.e1. doi: 10.1016/j.jvs.2013.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone DH, Nolan BW, Schanzer A, Goodney PP, Cambria RA, Likosky DS, et al. Protamine reduces bleeding complications associated with carotid endarterectomy without increasing the risk of stroke. J Vasc Surg. 2010;51:559–64. 564.e1. doi: 10.1016/j.jvs.2009.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcocer F, Novak Z, Combs BR, Lowman B, Passman MA, Mujib M, et al. Dual antiplatelet therapy (clopidogrel and aspirin) is associated with increased all-cause mortality after carotid revascularization for asymptomatic carotid disease. J Vasc Surg. 2014;59:950–5. doi: 10.1016/j.jvs.2013.10.087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


