Abstract
Leukemia cells escape BCR-ABL-targeted therapy by developing mutations such as T315I in the p210BCR-ABL fusion protein in Philadelphia chromosome-positive chronic myeloid leukemia (CML). While most effort has been focused on development of new tyrosine kinase inhibitors (TKIs), enrichment of these small molecule inhibitors in the tumor tissue can also have a profound impact on treatment outcomes. Here, we report that a 2-hour exposure of the T315I mutant CML cells to 10 μM of the multi-kinase inhibitor TG101209 suppressed BCR-ABL-independent signaling and caused cell cycle arrest at G2/M. Further increase in drug concentration to 17.5 μM blocked phosphorylation of the mutant BCR-ABL kinase and its downstream JAK2 and STAT5. The effective dosage to overcome therapy resistance identified in an in vitro setting serves as a guidance to develop the proper drug formulation for in vivo efficacy. A targeted formulation was developed to achieve sustained bone marrow TG101209 concentration at or above 17.5 μM for effective killing of CML cells in vivo. Potent inhibition of leukemia cell growth and extended survival were observed in two murine models of CML treated with 40 mg/kg intravenously administered targeted TG101209, but not with the untargeted drug at the same dosage. Our finding provides a unique approach to develop treatments for therapy-resistant CML.
Keywords: multi-kinase inhibitor, in vitro-in vivo correlation, bone marrow targeting, therapy-resistance, chronic myeloid leukemia
Introduction
Philadelphia chromosome-positive chronic myeloid leukemia (CML) is caused by constitutive activation of the oncogenic p210BCR-ABL tyrosine kinase as result of a reciprocal translocation between chromosomes 9 and 22 (1). Therefore, CML treatment in clinic has been focused on blocking kinase activity of the fusion protein with tyrosine kinase inhibitors (TKIs) such as imatinib, dasatinib, nilotinib, and recently, ponatinib (2–5). However, patients develop resistance to these targeted therapy drugs (6, 7). Among the potential mechanisms for therapy resistance include development of new mutations in the fusion gene such as the T315I mutation (7), unfavorable pharmacokinetics and biodistribution of TKIs (8, 9) and a protective bone marrow microenvironment (10, 11). Strategies to address these critical issues will be effective in treating CML.
To identify a dose range for effective cell killing, CML cells have traditionally been treated with TKIs for 24~72 hours in cell culture (12–14). However, it is impossible to maintain the therapeutic magnitude and duration of the drugs in vivo due to their rapid drug metabolism and clearance, as the plasma half-lives for dasatinib and nilotinib are 2 hours and 1 hour in mice, respectively (15, 16). Although the IC50 value for nilotinib on inhibition of Ba/F3 cells overexpressing the BCR-ABL fusion protein in cell culture is less than 10 nM (14), a daily dosage of 75~100 mg/kg is needed to treat animals in order to achieve a desirable therapeutic efficacy in murine CML models (17, 18). Since the peak plasma drug concentration could already reach 14 μM at a 25 mg/kg treatment dosage (16), which is over 1,000 folds of the IC50 value in cell culture, the peak plasma concentration at these therapeutic dosages will be even higher. Thus, the in vitro cell growth inhibition study provided little guidance on the design of in vivo efficacy studies. Therefore, more reliable approaches are needed to predict therapeutic outcome based on the cell killing data.
In the current study, we applied TG101209 to treat CML cells that are resistant to BCR-ABL targeted therapy in order to 1) establish an in vitro - in vivo correlation on treatment dosage, and 2) develop an effective treatment for therapy-resistant CML. The TG compounds (TG101209 and TG101348) were originally developed as inhibitors of the JAK2/STAT5 signaling (19, 20). STAT5 is one of the critical mediators for CML initiation, maintenance and TKI resistance (21). Upon BCR-ABL inhibition, CML progenitor cells depend on high levels of cytokine-mediated JAK2/STAT5 activation for continued viability inside the bone marrow (22). So targeting the JAK2/STAT5 signaling with inhibitors such as TG101209 is an ideal approach to prevent CML cell escape from BCR-ABL-targeted therapy. Interestingly, a recent study indicated that TG101209 could also inhibit the p210BCR-ABL tyrosine kinase activity (21). Thus, TG101209 might serve as a multi-kinase inhibitor to block p210BCR-ABL tyrosine kinase-dependent and independent pathways. Since CML cells carrying a T315I mutation in the BCR-ABL gene (p210T315I) are resistant to imatinib and dasatinib (6), and clinical cases of resistance to ponatinib have also been identified (7), we applied cells with overexpressed p210T315I to test drug efficacy in this study. We performed transient treatments of murine myeloid 32D cells overexpressing p210T315I with TG101209 in cell culture, and identified the concentration range where CML cells were sensitive to TG101209 treatment. We then developed a bone-targeted formulation to achieve bone marrow TG101209 concentration at or above the effective concentration range in a sustained manner in vivo so as to effectively kill leukemia cells. Subsequently, we applied two murine leukemia models to demonstrate therapeutic efficacy.
Materials and Methods
Cell culture
32D cells overexpressing wide-type BCR-ABL (32Dp210WT) or T315I mutant BCR-ABL (32Dp210T315I) were generated by infecting cells with retroviruses carrying a wild-type or mutant p210BCR-ABL gene, respectively. The cell lines were kindly provided to us by Dr. Ralph B. Arlinghaus in 2011 from the M.D. Anderson Cancer Center, Houston, Texas (23). All cell lines were routinely tested for cellular morphology and microbial presence by microscopic observation. No authentication of the described cell lines was performed by the authors. 32Dp210WT and 32Dp210T315I cells were cultured in the RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and penicillin/streptomycin.
Cell proliferation assay
32Dp210WT and 32Dp210T315I cells were seeded in 6-well plates, and incubated with increasing concentrations of TG101209 (Selleck Chemicals, Houston, TX). Drug was washed out by centrifugation after 2 hours of treatment, and cells were resuspended in the growth medium and seeded in a 96-well plate at a seeding density of 4,000 cells/well. Cell proliferation was measured 48 hours later using a cell counting kit-8 (CCK8) viability assay (Dojindo Molecular Technologies Inc., Kumamoto, Japan). Half maximal effective concentration (EC50) was calculated as the mean of three independent experiments. All values were normalized to the negative control.
Western blot analysis
32Dp210WT and 32Dp210T315I cells were seeded in 6-well plates, and treated with increasing concentrations of TG101209 or vehicle for 2 hours. Cells were then washed with phosphate buffer saline (PBS) and lysed in the radioimmunoprecipitation assay (RIPA) buffer (Pierce, Thermo Fisher). Equal amount of proteins was applied for Western blot analysis. Primary antibodies specific for the following proteins and phosphoproteins were used for detection: JAK2, phospho-JAK2 (Y1007/1008), STAT5, phospho-STAT5 (Y694), histone H3, phospho-histone H3 (ser10), cleaved caspase-3, and cleaved poly(ADP-ribose) polymerase (PARP) from Cell Signaling Technologies; and c-ABL, phospho-c-ABL (Y412), and Aurora B from Abcam.
Cell cycle analysis by flow cytometry
32Dp210T315I cells were plated in triplicates in 6-well plates supplied with 2 mL growth medium. Cells were treated with 5, 10, or 25 μM of TG101209 for 2 hours before switching back to growth medium. Cell growth was maintained for 24 to 48 hours thereafter. Cells were washed with PBS, and fixed in 70% ethanol overnight at −20°C. For flow cytometric analysis, cells were washed twice with PBS, resuspended in the PI/RNase staining buffer (BD Pharmingen™) and incubated for 30 min at room temperature before they were applied for analysis with a BD LSRII flow cytometer (BD Sciences).
Cytostaining for cell division analysis
32Dp210T315I cells were treated with TG101209 at 10 μM for 2 hours followed by incubation in growth medium for 48 hours. They were fixed with 4% paraformaldehyde in phosphate buffer for 20 min, transferred to a cover slip by centrifugation, and permeabilized with 0.2% Triton X-100 in PBS for 5 min. Cytoskeleton was stained with Alexa Fluor® 488 Phalloidin (Invitrogen) for 20 min, and nuclei were stained with DAPI for 5 min. Images of the stained cells were captured with a Nikon A1 spectral confocal microscope.
Aurora B kinase activity assay
Cell lysates from post-treatment samples were incubated with a primary anti-Aurora B antibody (Abcam) overnight at 4°C. Twenty microliters of protein A/G agarose magnetic beads (Thermal Scientific) were added into 500 μL cell lysate and incubated for 2 hours at 4°C. The pellet was washed three times with a pH 7.5 kinase assay buffer (20 mM Tris, 10 mM MgCl2, 5 mM glycerophosphate, 0.1 mg/mL BSA, 2 mM dithiothreitol), and resuspended in 30 μL kinase buffer supplemented with 200 μM ATP and 2 μg histone H3.1 protein (Biolabs Inc.). The mixture was incubated for 30 min at 30°C to allow protein phosphorylation, and terminated with a SDS sample buffer before applying for Western blot analysis for histone H3.
Preparation of bone-targeted TG101209 micelles
Bone-targeted TG101209 micelles were prepared by encapsulating TG101209 in alendronate-polyethyleneglycol-polylactic acid (PEG-PLA) using a co-solvent evaporation method (24). Briefly, 8 mg TG101209 was mixed with 50 mg methoxy PEG2000-PLA2000 and alendronate-conjugated PEG-PLA (molar ratio: 3:2), and dissolved in 0.5 mL acetone. This solution was added drop-wise into 10 mL distilled water under stirring. The mixture was then vigorously stirred at 20°C for 2 hours, and residual acetone was removed under vacuum. The micelle solution was filtered through a 0.45 mm syringe filter and subsequently lyophilized.
Pharmacokinetics and biodistribution properties of TG101209
Pharmacokinetics of TG101209 in solution or micelles was evaluated in C3H mice. For free TG101209, the compound was dissolved in acetate solution (pH 4.0) containing 20% Tween 80 and administrated by oral gavage (100 mg/kg). Blood samples were collected at the 0.5, 1, 2, 3, 4, and 6 hour timepoints. For TG101209 micelles, the CML mice were treated with untargeted (40 mg/kg) or targeted (20 or 40 mg/kg) TG101209 by intravenous administration, and blood samples were collected at 0.25, 0.5, 1, 2, 3, 4, and 6 hour time points. TG101209 was extracted from plasma by mixing methanol with plasma (9:1, v/v) followed by centrifugation at 21,000×g for 10 min.
Biodistribution analysis was carried out after mice were treated i.v. or p.o. with TG101209. Mice dosed with the drug intravenously were euthanized 0.5, 2, or 6 hours later, and those with oral gavage were sacrificed 2, 4, or 6 hours later. Major organs including lung, liver, heart, kidney, spleen, femur and tibia were collected for drug content analysis. The samples were homogenized and centrifuged at 21,000×g for 10 min. Methanol was added to the supernatant (9:1, v/v) to remove proteins by centrifugation at 21,000×g for 10 min. Femur and tibia were decalcified in 0.5 M ethylenediaminetetraacetic acid (EDTA) at pH 7.2 for 48 hours before homogenization. TG101209 concentration in plasma and tissue samples was measured with high-performance liquid chromatography (HPLC).
Detection of protein phosphorylation in tissue samples
One million GFP-positive 32Dp210T315I cells were inoculated into each C3H mouse by tail vein injection. Three days after inoculation, mice were treated intravenously every other day with 40 mg/kg untargeted or targeted TG101209 for 6 days. In the control group, imatinib was administrated by daily oral gavage (150 mg/kg) for 6 days. Spleen and bone marrow samples were collected 24 hours after the last dosing for Western blot analysis, flow cytometry, and immunohistochemical staining. To measure phosphorylated proteins in cells isolated from bone marrow, cells were fixed and permeabilized with the BD Perm/Wash™ buffer by following the manufacture’s protocol. To analyze histone H3 phosphorylation, cells were stained with a PE-conjugated anti-p-Histone H3 (ser10) antibody. To detect pSTAT5, cells were incubated with an anti-pSTAT5 antibody (Cell Signaling) followed by incubating with a secondary PE-conjugated goat anti-rabbit immunoglobulin.
Efficacy evaluation in murine models of leukemia
Two murine leukemia models were applied in this study. The C3H syngeneic leukemia model was developed by intravenous injection of 1×106 32Dp210T315I cells. The bone marrow transplantation models were developed by inoculating p210WT or p210T315I retrovirus-infected bone marrow cells into sub-lethally irradiated NOD/SCID mice. Briefly, C3H donor mice were treated daily with 5-fluorouracil (200 mg/kg) for 4 days. Mice were euthanized by CO2 and bone marrow cells were harvested from the tibia and femur by flushing with PBS. Bone marrow cells were resuspended at 5 million/mL in DMEM containing 10% FBS, 5% WEHI-medium, 10 ng/mL recombinant murine IL-3, 20 ng/mL recombinant murine IL-6, 100 ng/mL recombinant murine stem cell factor, 100U/mL Pen/Strep, and 2 mM L-glutamine. The cells were infected with 2 rounds of Mig1-BCR-ABL-GFP or Mig1-BCR-ABL-T315I-GFP retroviruses in the presence of 8 μg/mL of polybrene, and infection efficiency was determined by flow cytometry 24 hours after the second round of retrovirus infection. The infected bone marrow cells were then injected into the sub-lethally irradiated (600 cGy) recipient NOD/SCID mice at 1×106 cells/mouse.
To evaluate therapeutic efficacy in the C3H leukemia model, mice were treated beginning the second day after cell inoculation with (1) imatinib (150 mg/kg, p.o. daily), (2) vehicle (empty targeted micelles, i.v., every other day), (3) untargeted TG101209 micelles (40 mg/kg, i.v., every other day), (4) targeted TG101209 micelles (40 mg/kg, i.v., every other day), or (5) free TG101209 in DMSO (40 mg/kg, i.p., every other day). To examine therapeutic efficacy in the bone marrow transplantation models, leukemia development was confirmed based on peripheral blood cell counts and GFP+ cells in peripheral blood one week after transplantation. Mice were then treated with (1) imatinib (150 mg/kg, p.o., daily), (2) untargeted TG101209 micelles (40 mg/kg, i.v., every other day), (3) targeted TG101209 micelles (20 mg/kg, i.v., every other day), or (4) targeted TG101209 micelles (40 mg/kg, i.v., every other day). Percentage of circulating GFP+ leukemia cells in peripheral blood was examined by flow cytometry on days 15 and 25. Mice were euthanized on signs of morbidity, and Kaplan-Meier plots were generated based on animal survival.
In vivo toxicity analysis of targeted TG101209
C3H mice were treated with targeted TG101209 micelles (20 mg/kg or 40 mg/kg, i.v., every other day) for two weeks. The untreated mice were as control. The animals were observed for signs of toxicity throughout the experiment. Water and food consumption were recorded weekly. Body weight of the mice was measured every there days. On day 7 and day 14, blood samples were collected for hematology analysis. At the end of the 2-week treatment, the major organs were collected and histological analyses were performed.
Statistical analysis
Statistical analyses were performed with the GraphPad Prism 5 software (GraphPad Software, Inc., CA, USA). For all in vivo analysis, sample sizes were chosen to ensure adequate power to detect a pre-specified effect size. P-values of less than 0.05 and 0.01 were considered statistically significant and very significant, respectively. Data are presented as means ± SD.
RESULTS
TG101209 suppresses growth and promotes apoptosis of 32Dp210T315I cells in vitro
Resistance to the second-generation p210BCR-ABL TKI dasatinib was confirmed in 32Dp210T315I cells by Western blot analysis. While low nanomolar concentrations of dasatinib was potent enough to inhibit phosphorylation of BCR-ABL, JAK2, and STAT5 in 32Dp210WT cells after 2 hours treatment, this drug was not effective in blocking phosphorylation of these proteins in the mutant line even at micromolar concentrations (Supplementary Fig. 1). In contrast, TG101209 treatment suppressed phosphorylation of these proteins in both 32Dp210WT and 32Dp210T315I cells (Fig. 1A). Interestingly, phosphorylation of all three proteins was inhibited within the same drug concentration range in the same cell line, although the IC50 value was 7-fold as high in 32Dp210T315I (IC50 17.5 μM) as in 32Dp210WT (IC50 2.5 μM) cells (Fig. 1B). This observation indicates that activation of BCR-ABL, JAK2, and STAT5 might be mediated through a common signal transduction pathway. It has been previously reported that STAT5 phosphorylation is mediated by the upstream p210BCR-ABL in CML cells, an event independent of JAK2 activity (21). Our result indicates that JAK2 phosphorylation in 32Dp210WT and 32Dp210T315I cells is most likely also controlled by the BCR-ABL kinase activity. It is important to note that low micromolar plasma drug concentrations are commonly detected in animal studies treated with the more potent analogue TG101348 and in the clinic (25, 26).
Figure 1.
Treatment with TG101209 for 2 hours inhibits phosphorylation of protein kinases in 32Dp210WT and 32Dp210T315I cells and suppresses their growth. (A) Dose-dependent inhibition of phosphorylation of BCR-ABL, JAK2 and STAT5 in 32Dp210WT and 32Dp210T315I cells after 2-hour treatment with TG101209. (B) Dose-dependent curves on inhibition of protein phosphorylation based on Western blot analysis. Relative ratio on phospho/total protein = phospho/total protein in the treated cells / phospho/total protein in the control cells. (C) Time-dependent recovery of protein phosphorylation in cells after a 2-hour treatment with TG101209 at the IC50 concentration (2.5 μM for 32Dp210WT and 17.5 μM for 32Dp210T315I). (D) Dose-dependent inhibition of cell growth after 2 hours of treatment with TG101209 followed by incubation in growth medium for 48 hours. Cell viability is normalized to the control cells.
We treated 32Dp210WT and 32Dp210T315I cells with TG101209 at their respective IC50 values for 2 hours before switching cells to a drug-free growth medium, and tracked JAK2 and STAT5 phosphorylation status in the next 24 hours. Phosphorylation of both proteins was inhibited in the first 3 hours after drug treatment, but gradually recovered thereafter (Fig. 1C). We also measured cell viability 48 hours later. Interestingly, the half maximal effective concentration (EC50) value matched with the IC50 value for inhibition of phosphorylation in 32Dp210WT cells (EC50 3.15 μM), but was 37% less than the IC50 value in 32Dp210T315I cells (EC50 10.9 μM) (Fig. 1D). The discrepancy between IC50 and EC50 in 32Dp210T315I cells indicates that TG101209 might have inhibited BCR-ABL-independent signaling and triggered cell death before reaching an effective concentration for inhibition of BCR-ABL, JAK2, and STAT5 activities.
TG101209 inhibits Aurora B kinase activity and induces cell cycle arrest in 32Dp210T315I cells
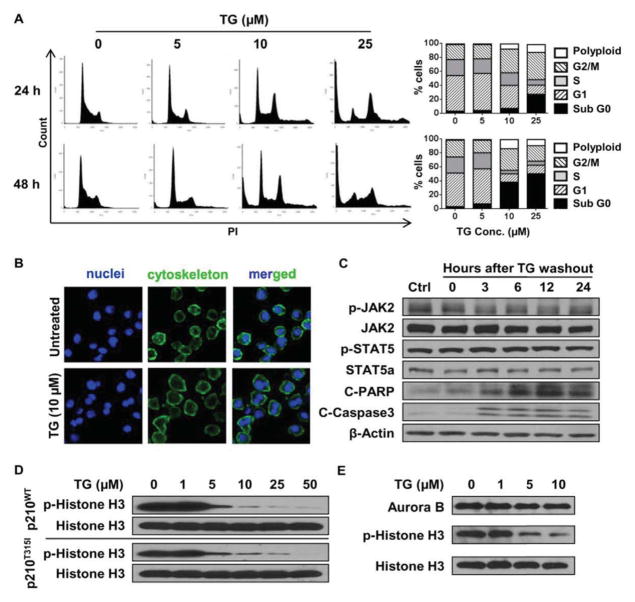
We treated 32Dp210T315I cells with increasing concentrations of TG101209 in order to identify factors critical for 32Dp210T315I cell growth and viability. Surprisingly, a 2-hour incubation with 10 μM TG101209 was already very effective in cell cycle blockage as indicated by increased percentage of cells in G2/M and accumulation of polyploids (Fig. 2A). Cytostaining of cells treated with 10 μM TG101209 for 2 hours revealed enlarged overall cell size and enrichment of multinucleated cells (Fig. 2B). Drug treatment also triggered cell apoptosis as indicated by increased levels of the G0 population (Fig. 2A) and cleaved PARP and caspase 3 (Fig. 2C). As expected, phosphorylation of p210T315I, JAK2 or STAT5 was not blocked at 10 μM TG101209 (Fig. 1A).
Figure 2.
TG101209 triggers cell cycle arrest and cell apoptosis by blocking BCR-ABL-independent signaling in 32Dp210T315I cells. (A) Flow cytometry analysis on cell cycle of 32Dp210T315I cells treated with TG101209 for 2 hours followed by incubation in growth medium for 24 or 48 hours. (B) Nuclear and cytoskeleton staining of 32Dp210T315I cells after a 2-hour treatment with 10 μM TG101209 followed by incubation in growth medium for 48 hours. Nuclei are stained with DAPI (in blue) and cytoskeleton is stained with Alexa Fluor488 Phalloidin (in green). (C) Western blot analysis on phosphorylation status of JAK2 and STAT5, and cleaved PARP (C-PARP) and cleaved caspase 3 (C-Caspase 3) in 32Dp210T315I cells treated with 10 μM TG101209 for 2 hours followed by incubation in growth medium for the indicated time. (D) Western blot analysis on dose-dependent inhibition of histone H3 (ser10) phosphorylation in 32Dp210WT and 32Dp210T315I cells after TG101209 treatment for 2 hours. (E) Immunoprecipitation followed by Western blot analysis for Aurora B kinase activity in 32Dp210T315I cells treated with 10 μM TG101209 for 2 hours.
It has been previously reported that TG101209 can inhibit the activities of multiple protein kinases including an Aurora kinase (19). Aurora kinase inhibitors achieved clinical response in CML patients bearing T315I mutated BCR-ABL (27). Interestingly, blockage of Aurora kinases is an effective approach for cell cycle arrest (28). Selective Aurora B inhibition can block histone H3 phosphorylation at serine 10, and lead to G2/M arrest, polyploidy and apoptosis (28, 29), a result similar to treatment with 10 μM TG101209. We applied a selective aurora B inhibitor, barasertib, to treat CML cells to confirm the cell killing effect. When 32Dp210WT and 32Dp210T315I cells were treated with barasertib for 2 hours, p-histone H3 (ser10) was inhibited at 1 μM in both cell lines (Supplementary Fig. 2A). 32Dp210T315I cells were blocked in G2/M phase, and the ratio of polyploidy and apoptotic cells increased after 2-hour barasertib treatment followed by 24 and 48 hours incubation (Supplementary Fig. 2B and C). We next examined whether Aurora B kinase was involved in TG101209-mediated cell killing. 32Dp210WT and 32Dp210T315I cells were treated with increasing concentrations of TG101209 for 2 hours, and Aurora B kinase activity was analyzed based on phosphorylation of the kinase substrate histone H3 at the Ser10 residue (30). Potent inhibition of histone H3 phosphorylation was achieved at 5 μM concentration in both cell lines (Fig. 2D), indicating that Aurora B kinase activity was effectively inhibited by TG101209. Since phosphorylation of p210T315I, JAK2 or STAT5 was not affected by TG101209 below 10 μM (Fig. 1A), this result also demonstrated that Aurora B kinase activity was independent of the BCR-ABL signaling in 32Dp210T315I cells. To confirm inhibition of kinase activity directly, we treated 32Dp210T315I cells with increasing concentrations of TG101209 for 2 hours, immuno-precipitated Aurora B kinase from cell lysates, and then measured the protein level and kinase activity of Aurora B. While the expression level of Aurora B kinase was unchanged after drug treatment, its ability to phosphorylate histone H3 was dramatically reduced (Fig. 2E). Thus, Aurora B kinase mediates cell cycle blockage in 32Dp210T315I cells treated with TG101209.
A bone-targeted TG101209 significantly enhances drug accumulation inside the bone marrow
In order to reach a high tissue drug concentration so as to effectively block activities of key kinases in CML cells inside the bone marrow, we developed an intravenously injectable bone-targeted formulation of TG101209 for in vivo studies (Fig. 3A). Briefly, the hydrophobic TG101209 was encapsulated in PEG-PLA micelles, and the surface of the micelles was conjugated with alendronate molecules as the bone-targeting moieties. Binding between bisphosphonate drugs including alendronate and the mineral hydroxyapatite in the bone has been well studied (31), and application of bisphosphonates as bone-targeting moieties has been previously documented (32). The untargeted micelles had an average size of 29.9 nanometers and a surface charge of −4.37 mV (Supplementary Table 1). Alendronate-conjugation did not dramatically alter the size of micelles; however, the surface charge of micelles decreased to −38.2 mV owing to the negatively charged phosphate groups in the targeting moiety.
Figure 3.
Targeted TG101209 has favorable pharmacokinetics and bone marrow distribution profiles in leukemia-bearing C3H mice. (A) Schematic illustration of untargeted (mPEG-PLA micelles) and bone-targeted (alendronate-PEG-PLA micelles) TG101209 formulations. (B) Time-dependent changes in plasma drug concentration in leukemia-bearing C3H mice. (C) Accumulation of TG101209 in bone marrow of leukemia-bearing C3H mice after dosed with different formulations of TG101209.
Mice bearing 32Dp210T315I CML were dosed i.v. with TG101209 in untargeted or targeted formulation (40 mg/kg untargeted TG101209 and 20~40 mg/kg targeted TG101209), and pharmacokinetics and tissue biodistribution were compared between the treatment groups. These parameters were also compared to mice treated p.o. with 100 mg/kg of the free drug, as oral administration of TG101209 at this dosage has been explored in preclinical studies (11, 19, 33, 34). Overall, mice treated intravenously with targeted or untargeted micelles showed similar pattern on changes in plasma drug concentration (Fig. 3B), and had similar pharmacokinetic parameters (Supplementary Table 2). Mice in the 20 mg/kg targeted TG101209 treatment group had a comparable area under the curve (AUC) with those in the 100 mg/kg oral gavage treatment group, in line with a previous report that the orally administrated TG101209 has a bioavailability of 23% in mice (19).
As expected, majority of the drug accumulated in the liver, lung, and spleen in all treatment groups regardless of the route of administration, and the highest drug concentrations in the liver and spleen were detected in the oral gavage group (Supplementary Fig. 3). However, bone marrow biodistribution analysis revealed striking difference among the different treatment groups (Fig. 3C). As with the oral gavage group (100 mg/kg), intravenous administration with untargeted micelles at the 40 mg/kg dosage only briefly raised bone marrow TG101209 concentration above 10 μM, but not the 17.5 μM threshold for inhibition of p210T315I/JAK2/STAT5. It is thus unlikely that phosphorylation status of these proteins is altered in the bone marrow CML cells in the untargeted formulation or oral administration treatment groups. In contrast, bone marrow drug concentration could already reach 17.5 μM in mice treated i.v. with the low dosage (20 mg/kg) targeted micelles, and a high bone marrow drug concentration was maintained for over 2 hours in mice dosed with the high dosage (40 mg/kg) targeted micelles. Based on the high bone marrow drug concentration level, it is anticipated that treatment with 40 mg/kg targeted micelles can potently and sustainably block signaling from both Aurora B kinase and p210T315I/JAK2/STAT5 so as to effectively kill CML cells inside the bone marrow.
Potent inhibition of key signal transduction pathways by targeted TG101209 in murine model of p210T315I CML
A murine model of CML was generated by inoculating 32Dp210T315I cells i.v. into C3H mice. Mice were treated daily with imatinib (150 mg/kg, p.o.) or every other day with untargeted or bone-targeted TG101209 micelles (40 mg/kg, i.v.) for 6 days, and the spleen and bone marrow samples were collected 24 hours after the last treatment for expression and histological analyses. Mice in the imatinib treatment group served as controls, and twice a day treatment with imatinib at the indicated dosage is a common practice for preclinical studies on CML (35, 36). Western blot analysis revealed no alteration on phosphorylation status of p210T315I/JAK2/STAT5 in the spleen from imatinib treatment (Fig. 4A). However, phosphorylation of these proteins was effectively blocked in the spleen by untargeted or bone-targeted TG101209 (Fig. 4A and 4C), correlating with the high tissue drug concentration in this organ (Supplementary Fig. 3). In comparison, phosphorylation of p210T315I/JAK2/STAT5 and histone H3 proteins was effectively blocked in bone marrow by bone-targeted, but not untargeted TG101209 (Fig. 4B). In addition, flow cytometry was performed to examine inhibition of STAT5 and histone H3 phosphorylation in leukemia cells isolated from the bone marrow in different treatment groups. While treatment with imatinib or untargeted TG101209 had limited effect on the phosphorylation status of STAT5 and histone H3, complete inhibition of phosphorylation in these two proteins was observed in leukemia cells from the 40 mg/kg targeted TG101209 treatment group (Fig. 4D), correlating with drug concentration analysis in bone marrow (Fig. 3C).
Figure 4.
Targeted TG101209 potently inhibits protein kinases in vivo. (A) Western blot analysis for protein phosphorylation in spleen samples harvested 24 hours after drug treatment. (B) Western blot analysis for protein phosphorylation in bone marrow samples. (C) Immunohistochemistry for p-Histone H3 and p-STAT5 in spleen samples harvested 24 hours after drug treatment. All scale bars represent a size of 100 μm. (D) Flow cytometry analysis on p-STAT5 and p-Histone H3 (ser10) levels in leukemia cells isolated from bone marrow of post-treatment C3H mice.
Inhibition of STAT5 phosphorylation might provide additional benefit for CML treatment by suppressing stimuli from the bone marrow microenvironment. In a recent study, we have shown that, in response to TKI treatment, bone marrow mesenchymal stromal cells secretes cytokines which activates signal transduction pathways leading to therapy resistance by the leukemia cells (37). We incubated 32Dp210T315I cells in growth medium with or without supplemented interleukin 3 (IL-3), and treated them with ponatinib or TG101209. Ponatinib treatment inhibited phosphorylation of p210BCR-ABL and the downstream JAK2 and STAT5 in the absence of IL-3, but was not effective in blocking IL-3-mediated JAK2/STAT5 phosphorylation; in contrast, TG101209 treatment abolished both p210BCR-ABL –mediated and IL-3-mediated STAT5 phosphorylation (Supplementary Fig. 4A). As a result, TG101209 treatment effectively killed leukemia cells regardless of presence of IL-3, while cells showed resistance to ponatinib treatment in the presence of IL-3 (Supplementary Fig. 4B–C).
Targeted TG101209 offers significant survival benefit in murine models of p210T315I CML
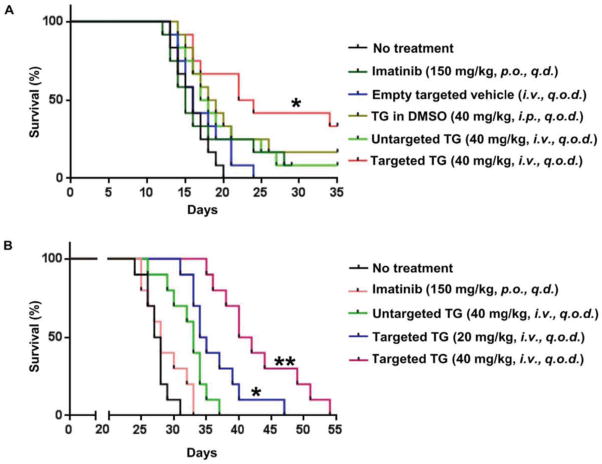
Survival benefit from targeted delivery of TG101209 was evaluated in two murine models of p210T315I CML. In the C3H CML model, the syngeneic 32Dp210T315I CML cells grew aggressively inside the body, and all untreated mice succumbed to leukemia growth 12 ~ 20 days after cell inoculation, with a median survival of 16 days (Fig. 5A). No survival benefit was observed from daily treatment with 150 mg/kg imatinib or every other day treatment with 40 mg/kg free TG101209 or untargeted TG101209 micelles. In contrast, significant therapeutic benefit was achieved in mice treated with 40 mg/kg targeted TG101209 (Fig. 5A).
Figure 5.
Treatment with bone-targeted TG101209 extends survival in two murine models of CML. (A) Kaplan-Meier plot of C3H mice with 32Dp210T315I syngeneic leukemia (n=12). Treatment was initiated on the second day after leukemia cell inoculation. Treatment schedules are as labeled. (*, p<0.05) (B) Kaplan-Meier plot of NOD-scid mice with bone marrow transplanted p210T315I leukemia (n=10). Treatment was initiated 7 days after bone marrow transplantation. Treatment schedules are as labeled (*: p<0.05; **: p<0.01).
The bone marrow transplantation CML model was more sensitive to TG101209 treatment. This CML animal model was generated by inoculating p210T315I retrovirus-infected C3H bone marrow cells into sub-lethally irradiated NOD/SCID mice. Peripheral blood samples were applied to monitor leukemia progression during treatment, and significantly reduced number of CML cells was observed from the targeted TG101209 micelle treatment groups (Supplementary Fig. 5). All mice in the non-treatment group died from leukemia growth 25 ~ 32 days after bone marrow transplantation (Fig. 5B). Imatinib treatment did not offer any survival benefit on the CML-bearing NOD/SCID mice, with a median survival time of 27.5 days (Fig. 5B). Treatment with untargeted or the low-dosage targeted micelles provided a moderate but statistically significant survival benefit over the non-treatment group. Their median survival times were 33 and 34.5 days, respectively. Extension of survival was most likely from cell cycle blockage as a result of inhibition of Aurora B kinase activity, since bone marrow drug levels in these two groups would not be high enough to exert sustained inhibition of p210T315I/JAK2/STAT5 (Fig. 3C). The most significant survival benefit was obtained in mice treated with the high dose targeted TG101209 micelles, with a median survival time of 42 days (Fig. 5B), demonstrating the power of blocking both p210T315I/JAK2/STAT5 activities and cell cycle in suppressing CML. We also applied the same treatments on p210WT CML in the bone marrow transplant model. As expected, imatinib (150 mg/kg, daily) treatment significantly extended animal survival, and the best therapeutic outcome was from treatment with the targeted TG101209 (40 mg/kg, every other day) (Supplementary Fig. 6).
Toxicity evaluation on TG101209
Since daily treatments with high oral doses (100 and 200 mg/kg) of TG101209 could cause severe toxicity such as anemia (11, 33), we carried out in vitro and in vivo studies to evaluate potential toxicity from the bone-targeted formulations. In the in vitro study, cell viability and colony formation ability were monitored in bone marrow cells from C3H mice upon transient treatment with increasing concentrations of TG101209. No significant impact from therapeutic dosages of TG101209 was observed (Supplementary Fig. 7). In the in vivo study, C3H mice were dosed every other day with bone-targeted TG101209, and no changes on blood chemistry or histology were detected (Fig. 6). Thus, bone marrow enrichment of the drug resulted in dramatically decreased overall treatment dosage and significantly reduced drug exposure to normal tissues, which caused no detectable toxicity in our study.
Figure 6.
Targeted TG101209 has minimum sub-acute toxicity. (A) Blood chemistry and peripheral blood cell count in C3H mice treated with 20 and 40 mg/kg of targeted TG101209 (i.v., every other day) for 2 weeks. (B) Histological analysis of tissue blocks from major organs in post-treatment C3H mice. Representative sections of liver and spleen by H&E staining are presented.
Human disease relevance of the targeted TG101209
In order to determine whether enhanced bone marrow drug accumulation could be translated into improved therapeutic efficacy in clinic, we isolated leukemia cells from peripheral blood in CML patients at the blast crisis stage and treated them with increasing concentrations of TG101209. All patients had gone through multiple rounds of treatment, and had become resistant to the first or second generation CML TKIs in clinic. 2-hour TG101209 treatment was effective in blocking phosphorylation of p210BCR-ABL, JAK2, STAT5, and histone H3 at 2.5~25 μM in all samples (Supplementary Fig. 8), indicating that the patients would benefit from the bone marrow-targeted drug formulation in clinic.
Discussion
Multiple TKIs have been developed for the treatment of Philadelphia chromosome positive leukemia so far; however, most patients eventually develop therapy resistance and hence disease relapse (38). The underlying mechanism for therapy resistance includes frequent mutations in the BCR-ABL gene (7, 39), activation of BCR-ABL-independent signal transductions pathways (40–42), protection of leukemia cells by the bone marrow microenvironment (11, 37, 43, 44). In addition, poor pharmacokinetic profile and biodistribution of the therapeutic agent contribute to development of therapy resistance (8). It is thus important to take into consideration of the multiple factors in developing an effective therapeutic strategy for CML. In this study, we have demonstrated that the effective dosage to overcome CML therapy resistance can be identified in an in vitro setting, and the information can serve as a guidance to develop the proper drug formulation for in vivo efficacy studies. Our results have revealed there exist two groups of proteins that are vital for CML cell survival, one is regulated by p210BCR-ABL tyrosine kinase activity (e. g., STAT5) and the other is independent of the p210BCR-ABL signaling (e. g., Aurora B kinase). Since CML cells bearing a wild-type BCR-ABL gene are sensitive to low-dose TG101209 treatments, there is no need to overload the patients with the drug during treatment. Consequently, drug effect on the second group of proteins is minimal. In patients with a mutant BCR-ABL gene, however, the p210T315I tyrosine kinase is more resistant to treatment, and activities of the second group proteins are modulated by the small molecule inhibitor at concentrations below the threshold for inhibition of p210T315I/JAK2/STAT5. Thus, TG101209 treatment results in two stages of responses in the mutant cells: cell cycle arrest and apoptosis due to blockage of p210T315I-independent proteins at a relatively low drug concentration, and enhanced cell death caused by inhibition of p210T315I/JAK2/STAT5 once the threshold concentration is reached. Our results have shown that potent inhibition of both groups of proteins with a high bone marrow concentration of TG101209 is essential for effective treatment of therapy-resistant CML.
We have previously demonstrated JAK2 activation by the p210BCR-ABL tyrosine kinase activity in CML cells (45), and have proposed JAK2 as a potential target for drug intervention in CML (46). We have also shown that one of the JAK2 TKIs, ON044580, inhibits both JAK2 and p210BCR-ABL kinase activities in in vitro assays using their respective peptide substrates (23). Multiple JAK2 TKIs have since then been applied in combination with BCR-ABL TKI for CML treatment (22, 47). Interestingly, the cell killing effect from TG101209 and TG101348 seems to be predominantly from direct inhibition of the p210BCR-ABL kinase rather than JAK2 (21). In the current study, we have observed synchronized blockage of phosphorylation of BCR-ABL, JAK2, and STAT5 kinases in both wild-type and T315I mutant cells by TG101209. The increased drug concentration needed for inhibition of p210T315I/JAK2/STAT5 in the mutant cells supports the notion of direct inhibition of the p210T315I tyrosine kinase by the TKIs. It is unknown whether other JAK2 TKIs in various stages of drug development have a similar spectrum of target inhibition and consequently mechanism of action.
Small molecule JAK TKIs have commonly been dosed through oral administration for the treatment of myelofibrosis and hematopoietic neoplasia in preclinical (19, 33, 48) and clinical studies (49, 50). Our analysis indicates that only a very small portion of the drug can reach the bone marrow, and majority of the drug is accumulated in the liver, lung, and spleen in mice treated with 100 mg/kg TG101209 by oral gavage. Thus, the body needs to be overloaded with a high dosage of the drug in order to achieve a therapeutic level inside the target organ (i.e., the bone marrow). However, sustained inhibition of the JAK/STAT pathway in the major organs may cause severe side effects (11). In comparison, the gap between in vitro activity and in vivo efficacy of TG101209 was bridged by identifying the effective concentration in vitro and achieving this concentration in bone marrow in vivo. The bone-targeted formulation is very efficient in delivering drug to the bone marrow. With this new formulation, targeted therapy is achieved through targeted delivery of the TKI molecules. Bone enrichment of the drug with significantly decreased drug exposure to normal tissues resulted in remarkable therapeutic efficacy and minimal toxicity in our study.
In summary, we have demonstrated that incubation of the therapy-resistant CML cells with TG101209 in the 10~25 μM range blocks BCR-ABL-dependent and -independent signaling and triggers cell death in vitro. The information has guided us to develop a bone marrow-targeted approach to enrich drug in the tissue and effectively treat CML. This in vitro-in vivo correlation strategy for development of effective cancer treatment might be applicable to diseases beyond CML in clinic.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by grants from the Leukemia and Lymphoma Society, Golfers Against Cancer, and NCI 1R01CA193880-01A1 (H. Shen).
The authors would like to thank Drs. Yu Mi and Guohui Wang for their assistance.
Footnotes
Disclosure of Conflicts: The authors declare no competing financial interests.
References
- 1.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–30. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 3.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 4.Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–88. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.le Coutre P, Ottmann OG, Giles F, Kim DW, Cortes J, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood. 2008;111:1834–9. doi: 10.1182/blood-2007-04-083196. [DOI] [PubMed] [Google Scholar]
- 6.Cortes J, Hochhaus A, Hughes T, Kantarjian H. Front-line and salvage therapies with tyrosine kinase inhibitors and other treatments in chronic myeloid leukemia. J Clin Oncol. 2011;29:524–31. doi: 10.1200/JCO.2010.31.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014;26:428–42. doi: 10.1016/j.ccr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi N, Miura M, Scott SA, Niioka T, Sawada K. Pharmacokinetics of dasatinib for Philadelphia-positive acute lymphocytic leukemia with acquired T315I mutation. J Hematol Oncol. 2012;5:23. doi: 10.1186/1756-8722-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glekas AP, Pillarsetty NK, Punzalan B, Khan N, Smith-Jones P, Larson SM. In vivo imaging of Bcr-Abl overexpressing tumors with a radiolabeled imatinib analog as an imaging surrogate for imatinib. journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2011;52:1301–7. doi: 10.2967/jnumed.110.085050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiwase DK, White DL, Powell JA, Saunders VA, Zrim SA, Frede AK, et al. Blocking cytokine signaling along with intense Bcr-Abl kinase inhibition induces apoptosis in primary CML progenitors. Leukemia. 2010;24:771–8. doi: 10.1038/leu.2009.299. [DOI] [PubMed] [Google Scholar]
- 11.Traer E, MacKenzie R, Snead J, Agarwal A, Eiring AM, O’Hare T, et al. Blockade of JAK2-mediated extrinsic survival signals restores sensitivity of CML cells to ABL inhibitors. Leukemia. 2012;26:1140–3. doi: 10.1038/leu.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–12. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–5. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 14.Ray A, Cowan-Jacob SW, Manley PW, Mestan J, Griffin JD. Identification of BCR-ABL point mutations conferring resistance to the Abl kinase inhibitor AMN107 (nilotinib) by a random mutagenesis study. Blood. 2007;109:5011–5. doi: 10.1182/blood-2006-01-015347. [DOI] [PubMed] [Google Scholar]
- 15.Kamath AV, Wang J, Lee FY, Marathe PH. Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): a potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer chemotherapy and pharmacology. 2008;61:365–76. doi: 10.1007/s00280-007-0478-8. [DOI] [PubMed] [Google Scholar]
- 16.Xia B, Heimbach T, He H, Lin TH. Nilotinib preclinical pharmacokinetics and practical application toward clinical projections of oral absorption and systemic availability. Biopharmaceutics & drug disposition. 2012;33:536–49. doi: 10.1002/bdd.1821. [DOI] [PubMed] [Google Scholar]
- 17.Weisberg E, Azab AK, Manley PW, Kung AL, Christie AL, Bronson R, et al. Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia. 2012;26:985–90. doi: 10.1038/leu.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisberg E, Wright RD, McMillin DW, Mitsiades C, Ray A, Barrett R, et al. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Molecular cancer therapeutics. 2008;7:1121–9. doi: 10.1158/1535-7163.MCT-07-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardanani A, Hood J, Lasho T, Levine RL, Martin MB, Noronha G, et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia. 2007;21:1658–68. doi: 10.1038/sj.leu.2404750. [DOI] [PubMed] [Google Scholar]
- 20.Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29:789–96. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hantschel O, Warsch W, Eckelhart E, Kaupe I, Grebien F, Wagner KU, et al. BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia. Nat Chem Biol. 2012;8:285–93. doi: 10.1038/nchembio.775. [DOI] [PubMed] [Google Scholar]
- 22.Gallipoli P, Cook A, Rhodes S, Hopcroft L, Wheadon H, Whetton AD, et al. JAK2/STAT5 inhibition by nilotinib with ruxolitinib contributes to the elimination of chronic phase CML CD34+ cells in vitro and in vivo. Blood. 2014 doi: 10.1182/blood-2013-12-545640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samanta AK, Chakraborty SN, Wang Y, Schlette E, Reddy EP, Arlinghaus RB. Destabilization of Bcr-Abl/Jak2 Network by a Jak2/Abl Kinase Inhibitor ON044580 Overcomes Drug Resistance in Blast Crisis Chronic Myelogenous Leukemia (CML) Genes Cancer. 2010;1:346–59. doi: 10.1177/1947601910372232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mu CF, Balakrishnan P, Cui FD, Yin YM, Lee YB, Choi HG, et al. The effects of mixed MPEG-PLA/Pluronic (R) copolymer micelles on the bioavailability and multidrug resistance of docetaxel. Biomaterials. 2010;31:2371–9. doi: 10.1016/j.biomaterials.2009.11.102. [DOI] [PubMed] [Google Scholar]
- 25.Wernig G, Kharas MG, Okabe R, Moore SA, Leeman DS, Cullen DE, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13:311–20. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Xu CR, Shamiyeh E, Liu F, Yin JY, von Moltke LL, et al. A randomized, placebo-controlled study of the pharmacokinetics, pharmacodynamics, and tolerability of the oral JAK2 inhibitor fedratinib (SAR302503) in healthy volunteers. Journal of clinical pharmacology. 2014;54:415–21. doi: 10.1002/jcph.218. [DOI] [PubMed] [Google Scholar]
- 27.Giles FJ, Swords RT, Nagler A, Hochhaus A, Ottmann OG, Rizzieri DA, et al. MK-0457, an Aurora kinase and BCR-ABL inhibitor, is active in patients with BCR-ABL T315I leukemia. Leukemia. 2013;27:113–7. doi: 10.1038/leu.2012.186. [DOI] [PubMed] [Google Scholar]
- 28.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–7. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 29.Xie H, Lee MH, Zhu F, Reddy K, Peng C, Li Y, et al. Identification of an Aurora kinase inhibitor specific for the Aurora B isoform. Cancer research. 2013;73:716–24. doi: 10.1158/0008-5472.CAN-12-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirota T, Lipp JJ, Toh BH, Peters JM. Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature. 2005;438:1176–80. doi: 10.1038/nature04254. [DOI] [PubMed] [Google Scholar]
- 31.Giger EV, Castagner B, Leroux JC. Biomedical applications of bisphosphonates. J Control Release. 2013;167:175–88. doi: 10.1016/j.jconrel.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 32.Swami A, Reagan MR, Basto P, Mishima Y, Kamaly N, Glavey S, et al. Engineered nanomedicine for myeloma and bone microenvironment targeting. P Natl Acad Sci USA. 2014;111:10287–92. doi: 10.1073/pnas.1401337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo MC, Peterson LF, Yan M, Cong X, Hickman JH, Dekelver RC, et al. JAK inhibitors suppress t(8;21) fusion protein-induced leukemia. Leukemia. 2013;27:2272–9. doi: 10.1038/leu.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waibel M, Solomon VS, Knight DA, Ralli RA, Kim SK, Banks KM, et al. Combined Targeting of JAK2 and Bcl-2/Bcl-xL to Cure Mutant JAK2-Driven Malignancies and Overcome Acquired Resistance to JAK2 Inhibitors. Cell Rep. 2013;5:1047–59. doi: 10.1016/j.celrep.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo H, Quan H, Xie C, Xu Y, Fu L, Lou L. HH-GV-678, a novel selective inhibitor of Bcr-Abl, outperforms imatinib and effectively overrides imatinib resistance. Leukemia. 2010;24:1807–9. doi: 10.1038/leu.2010.169. [DOI] [PubMed] [Google Scholar]
- 36.Floris G, Debiec-Rychter M, Sciot R, Stefan C, Fieuws S, Machiels K, et al. High efficacy of panobinostat towards human gastrointestinal stromal tumors in a xenograft mouse model. Clin Cancer Res. 2009;15:4066–76. doi: 10.1158/1078-0432.CCR-08-2588. [DOI] [PubMed] [Google Scholar]
- 37.Mallampati S, Leng X, Ma H, Zeng J, Li J, Wang H, et al. Tyrosine kinase inhibitors induce mesenchymal stem cell-mediated resistance in BCR-ABL+ acute lymphoblastic leukemia. Blood. 2015;125:2968–73. doi: 10.1182/blood-2014-05-576421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hare T, Zabriskie MS, Eiring AM, Deininger MW. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nature reviews Cancer. 2012;12:513–26. doi: 10.1038/nrc3317. [DOI] [PubMed] [Google Scholar]
- 39.Deininger MW, Hodgson JG, Shah NP, Cortes JE, Kim DW, Nicolini FE, et al. Compound mutations in BCR-ABL1 are not major drivers of primary or secondary resistance to ponatinib in CP-CML patients. Blood. 2015 doi: 10.1182/blood-2015-08-660977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- 41.Gregory MA, Phang TL, Neviani P, Alvarez-Calderon F, Eide CA, O’Hare T, et al. Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl. Cancer Cell. 2010;18:74–87. doi: 10.1016/j.ccr.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma L, Shan Y, Bai R, Xue L, Eide CA, Ou J, et al. A therapeutically targetable mechanism of BCR-ABL-independent imatinib resistance in chronic myeloid leukemia. Science translational medicine. 2014;6:252ra121. doi: 10.1126/scitranslmed.3009073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt T, Kharabi Masouleh B, Loges S, Cauwenberghs S, Fraisl P, Maes C, et al. Loss or inhibition of stromal-derived PIGF prolongs survival of mice with imatinib-resistant Bcr-Abl1(+) leukemia. Cancer Cell. 2011;19:740–53. doi: 10.1016/j.ccr.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Zhang B, Li M, McDonald T, Holyoake TL, Moon RT, Campana D, et al. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-beta-catenin signaling. Blood. 2013;121:1824–38. doi: 10.1182/blood-2012-02-412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson-Rawls J, Liu J, Laneuville P, Arlinghaus RB. P210 Bcr-Abl interacts with the interleukin-3 beta c subunit and constitutively activates Jak2. Leukemia. 1997;11(Suppl 3):428–31. [PubMed] [Google Scholar]
- 46.Samanta AK, Lin H, Sun T, Kantarjian H, Arlinghaus RB. Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res. 2006;66:6468–72. doi: 10.1158/0008-5472.CAN-06-0025. [DOI] [PubMed] [Google Scholar]
- 47.Okabe S, Tauchi T, Katagiri S, Tanaka Y, Ohyashiki K. Combination of the ABL kinase inhibitor imatinib with the Janus kinase 2 inhibitor TG101348 for targeting residual BCR-ABL-positive cells. J Hematol Oncol. 2014;7:37. doi: 10.1186/1756-8722-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhagwat N, Koppikar P, Keller M, Marubayashi S, Shank K, Rampal R, et al. Improved targeting of JAK2 leads to increased therapeutic efficacy in myeloproliferative neoplasms. Blood. 2014;123:2075–83. doi: 10.1182/blood-2014-01-547760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verstovsek S, Passamonti F, Rambaldi A, Barosi G, Rosen PJ, Rumi E, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer. 2014;120:513–20. doi: 10.1002/cncr.28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cervantes F, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Sirulnik A, Stalbovskaya V, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122:4047–53. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.