Abstract
Purpose
By applying the principles of real-time biopsy, biomarker-based, adaptively randomized studies in non–small-cell lung cancer (NSCLC) established by the Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) trial, we conducted BATTLE-2 (BATTLE-2 Program: A Biomarker-Integrated Targeted Therapy Study in Previously Treated Patients With Advanced Non-Small Cell Lung Cancer), an umbrella study to evaluate the effects of targeted therapies focusing on KRAS-mutated cancers.
Patients and Methods
Patients with advanced NSCLC (excluding sensitizing EGFR mutations and ALK gene fusions) refractory to more than one prior therapy were randomly assigned, stratified by KRAS status, to four arms: (1) erlotinib, (2) erlotinib plus MK-2206, (3) MK-2206 plus AZD6244, or (4) sorafenib. Tumor gene expression profiling–targeted next-generation sequencing was performed to evaluate predictive and prognostic biomarkers.
Results
Two hundred patients, 27% with KRAS-mutated (KRAS mut+) tumors, were adaptively randomly assigned to erlotinib (n = 22), erlotinib plus MK-2206 (n = 42), MK-2206 plus AZD6244 (n = 75), or sorafenib (n = 61). In all, 186 patients were evaluable, and the primary end point of an 8-week disease control rate (DCR) was 48% (arm 1, 32%; arm 2, 50%; arm 3, 53%; and arm 4, 46%). For KRAS mut+ patients, DCR was 20%, 25%, 62%, and 44% whereas for KRAS wild-type patients, DCR was 36%, 57%, 49%, and 47% for arms 1, 2, 3, and 4, respectively. Median progression-free survival was 2.0 months, not different by KRAS status, 1.8 months for arm 1, and 2.5 months for arms 2 versus arms 3 and 4 in KRAS mut+ patients (P = .04). Median overall survival was 6.5 months, 9.0 and 5.1 months for arms 1 and 2 versus arms 3 and 4 in KRAS wild-type patients (P = .03). Median overall survival was 7.5 months in mesenchymal versus 5 months in epithelial tumors (P = .02).
Conclusion
Despite improved progression-free survival on therapy that did not contain erlotinib for KRAS mut+ patients and improved prognosis for mesenchymal tumors, better biomarker-driven treatment strategies are still needed.
INTRODUCTION
Non–small-cell lung cancer (NSCLC) is the leading cause of cancer-related death and accounts for more than a million deaths per year worldwide.1 The disease is usually diagnosed at later stages, when curative treatment is not available.2 The benefit from platinum-based doublet chemotherapy is modest.3 Lung cancers are biologically and molecularly diverse4 and have various responses to both traditional chemotherapy and targeted therapy designed to address molecular alterations that drive cancer progression.5 The rapid evolution of genomic profiling has dramatically accelerated our knowledge of the diversity of lung cancer4 and has generated the impetus for using genotyping as a guide for clinical care of patients with lung cancer and for creating novel design paradigms in genomics-driven clinical trials.
In the phase II Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) program of personalized medicine (ClinicalTrials.gov numbers NCT00409968, NCT00411671, NCT00411632, NCT00410059, and NCT00410189) previously reported6,7 by our group, we prospectively biopsied tumors and, on the basis of tumor markers, we used adaptive randomization to assign patients with NSCLC to the treatment with the greatest potential benefit on the basis of cumulative data. The trial established the feasibility of performing core biopsies in pretreated patients with advanced disease and of using real-time biomarker analysis for treatment assignments,8 and it represented a major step toward personalizing therapy for patients with NSCLC.
On this basis, the BATTLE-2 trial (BATTLE-2 Program: A Biomarker-Integrated Targeted Therapy Study in Previously Treated Patients With Advanced Non-Small Cell Lung Cancer) capitalized on activity observed with sorafenib,9-11 on enhanced understanding of lung cancer biology, and on the availability of several promising agents, including MK-2206, an allosteric AKT inhibitor,12 and AZD6244, an MEK inhibitor.13 We could thus test novel hypotheses derived from a KRAS-mutant lung cancer mouse model in which combined MEK and PI3K/mammalian target of rapamycin inhibition resulted in synergistic tumor regression14 and also test preclinical information that justified combining erlotinib and MK-220615,16 as a means of overcoming resistance to EGFR inhibitors conferred by continued PI3K pathway activation and hepatocyte growth factor. The goals of the trial were to evaluate efficacy and identify predictive biomarkers for targeted therapies in the first stage, aiming at optimized patient selection for these therapies in the second stage. BATTLE-2 was designed with a particular emphasis on targeting mutant Kirsten rat sarcoma viral oncogene homolog (KRAS mut+) NSCLC refractory to platinum-based regimens. Here we report the results of the first stage of the BATTLE-2 trial.
PATIENTS AND METHODS
Patient Population
Patients with pretreated NSCLC at the University of Texas MD Anderson Cancer Center and Yale Cancer Center who agreed to a baseline tumor biopsy, who had Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2, and who had multiple prior lines of therapy and stable or treated brain metastases were enrolled (details for eligibility are provided in the Data Supplement). Patients were excluded if their tumor harbored EGFR sensitizing mutations or ALK gene fusions, and they were erlotinib or crizotinib naïve. All participants provided written informed consent. The MD Anderson Cancer Center and Yale Cancer Center Institutional Review Boards approved the study. The trial was monitored by an independent data and safety monitoring board.
Study Design
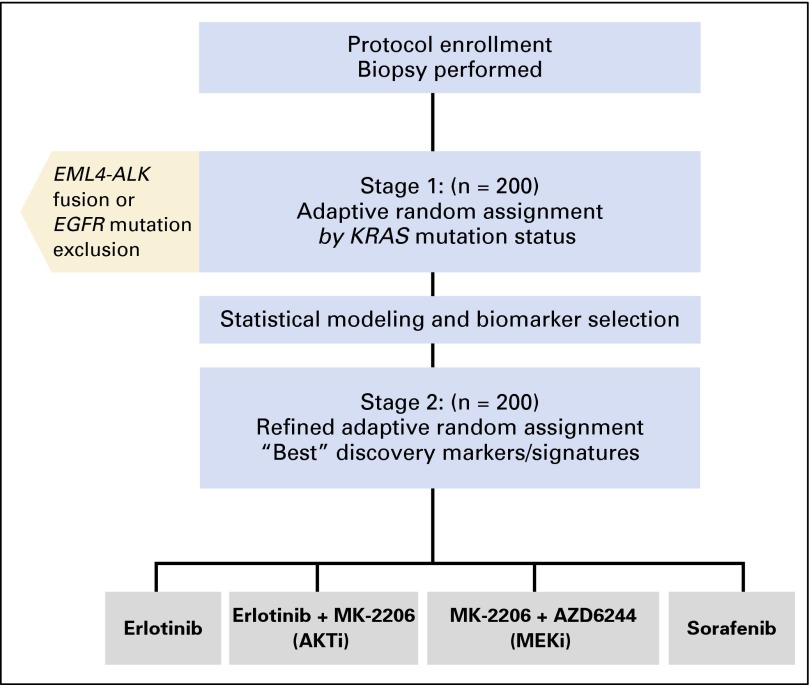
BATTLE-2 was a randomized, phase II, multicenter, open-label study in patients with advanced NSCLC refractory to prior platinum-based chemotherapy (Fig 1). After molecular tumor biomarker assessments, patients were adaptively randomly assigned to four arms: arm 1, erlotinib 150 mg once per day (OSI Pharmaceuticals, Farmingdale, NY; Genentech, San Francisco, CA); arm 2, erlotinib 150 mg once per day and the AKT inhibitor MK-2206 135 mg once per week (Merck, Kenilworth, NJ); arm 3, MEK inhibitor AZD6244 100 mg per day (AstraZeneca, Wilmington, DE) and AKT inhibitor MK-2206 100 mg once per week; and arm 4, sorafenib 400 mg orally twice per day (Bayer, Whippany, NJ). Patients who received prior erlotinib were randomly assigned to one of arms 2, 3, or 4. Tumor evaluation studies were performed after two cycles (one cycle is 28 days) and every two cycles thereafter. KRAS mutation status was a stratification factor. All patients who received at least one cycle of treatment (4 weeks) were considered evaluable for response assessment, and all patients who were randomly assigned were evaluable for safety and survival analyses.
Fig 1.
BATTLE-2 trial schema. AKTi, AKT inhibitor; MEKi, MEK inhibitor.
Biopsy, Molecular Analysis, and Biomarker Profiling
Patients had a mandatory baseline tumor tissue biopsy for biomarker analysis. Written informed consent was obtained from patients before the biopsy, which was performed under computed tomographic or sonographic guidance as previously described,6,8 including management of pneumothorax after the biopsy. Four to five fresh core needle biopsy tumor specimens approximately 1.5 cm long were collected, two of which were formalin-fixed immediately, paraffin embedded, and reviewed for presence, quantity, quality, and histologic type of tumor tissue by the dedicated pathologist. EGFR and KRAS Sanger sequencing (Data Supplement) and ALK fluorescence in situ hybridization testing17 were performed in Clinical Laboratory Improvement Amendments–certified laboratories within 2 weeks. The remaining three core needle biopsies were frozen, stored, and allocated for gene expression analysis by messenger RNA GeneChip Human Gene 1.0 ST Array from Affymetrix (Santa Clara, CA), which tested prospectively predefined signatures, including the epithelial mesenchymal transition (EMT) signature, and DNA-targeted next-generation sequencing (NGS; Foundation Medicine, Cambridge, MA) analysis18 in 140 tumors with sufficient material. Detailed methods are included in the Data Supplement.
Statistical Analysis
The accrual goal of stage 1 of the BATTLE-2 trial was 200 randomly assigned patients, which would allow at least 80% power with a 10% type I error rate to identify effective treatments for arms 2, 3, and 4 compared with arm 1. The overall power is 97.8% with a 20% family-wise type I error, which was chosen to prevent missing any potentially effective treatments; there was a plan to confirm the results in stage 2 and in future studies.19
The primary end point was the 8-week disease control rate (DCR; complete or partial response or stable disease via Response Evaluation Criteria in Solid Tumors [RECIST]),20 a previously validated end point.6,21 A Bayesian logistic regression model was applied to model the 8-week disease control status. Under the null hypothesis, we assumed that the 8-week DCRs were 0.3 for KRAS-wild-type (wt) and 0.1 for KRAS-mutant patients. Under the alternative hypothesis, and presuming one predictive marker per arm, we assumed that the 8-week DCR increased to 80% in the predictive marker–positive patients and remained at 30% in the predictive marker–negative patients. Equal randomization was performed in the first 70 patients. Subsequently, outcome adaptive randomization was used to incorporate the 8-week disease control status, KRAS mutation, and treatment into the calculation of the posterior probability of efficacy for treatments to allow more patients to be assigned to effective therapies and fewer patients to be assigned to less effective therapies. The posterior probability was continuously updated as the data became available. This learn-as-we-go approach leveraged accumulating data to improve outcome and is described in more detail elsewhere.19 Other end points included response rate, progression-free survival (PFS), overall survival (OS), and toxicity. Planned exploratory objectives were each treatment’s efficacy in relation to biomarker profiles. PFS and OS were assessed from the date of drug start to the earliest sign of disease progression (PFS) or death as a result of any cause (PFS and OS). Tumor response was assessed every 8 weeks until disease progression. Toxicity was assessed in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Standard statistical methods included Fisher’s exact test for contingency tables and Kaplan-Meier plots and log-rank test for univariable survival data. We used a logistic regression model in a multivariable analysis to assess the relationship of DCR with clinical factors and a Cox regression to model PFS and OS and interactions between KRAS mutation and erlotinib-containing therapy. SAS version 9.3 (SAS Institute, Cary, NC) and R version 3.2.2 (Foundation for Statistical Computing, Vienna, Austria) were used.
RESULTS
Patient Characteristics
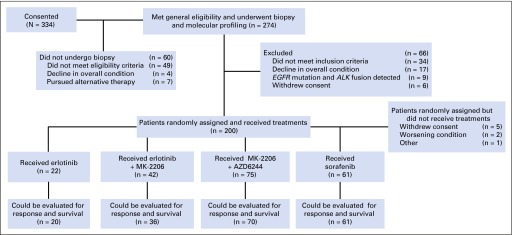
A total of 334 patients provided consent, 60 were never biopsied because they did not fulfill eligibility criteria (n = 49) or had declining overall condition (n = 4) or decided to pursue alternative therapy (n = 7). Of 274 patients biopsied, 66 were not randomly assigned because they no longer fulfilled eligibility criteria (n = 34), they experienced a decline in overall condition (n = 17), they had a tumor that harbored a sensitizing EGFR mutation or an ALK gene fusion (n = 9), or they withdrew consent (n = 6). Randomly assigned and treated patients per treatment arm were 22 (erlotinib), 42 (erlotinib and MK-2206), 75 (MK-2206 plus AZD6244), and 61 (sorafenib; Fig 2). Eight randomly assigned patients never received therapy because they withdrew consent (n = 5), had declining condition (n = 2), or had other reasons (n = 1).
Fig 2.
CONSORT flow diagram of patient population and treatment assignments.
Table 1 lists the distribution of the following patient characteristics: median age, 61 years (range, 26 to 82 years); female sex, 53%; ECOG PS of 0 to 1, 85%; never smoker, 22%; former smoker, 63%; current smoker, 16%; adenocarcinoma, 73.5%; and squamous cell carcinoma, 17.5%. KRAS mutations were present in 54 patients (27%); 75 patients (38%) had prior EGFR tyrosine kinase inhibitor treatment and a median of three prior therapies, with more patients heavily pretreated in arms 2, 3, and 4 (P = .03).
Table 1.
Summary of Patient Characteristics
| Characteristic | Arm 1 (erlotinib) | Arm 2 (erlotinib + MK-2206) | Arm 3 (MK-2206 + AZD6244) | Arm 4 (sorafenib) | Total | P * | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Age, years | |||||||||||
| ≤ 49 | 2 | 9.1 | 2 | 4.8 | 6 | 8.0 | 7 | 11.5 | 17 | 8.5 | .72 |
| 50-59 | 8 | 36.4 | 16 | 38.1 | 27 | 36.0 | 19 | 31.1 | 70 | 35.0 | |
| 60-69 | 10 | 45.5 | 12 | 28.6 | 29 | 38.7 | 24 | 39.3 | 75 | 37.5 | |
| 70+ | 2 | 9.0 | 12 | 28.6 | 13 | 17.3 | 11 | 18.0 | 38 | 19.0 | |
| Median (range) | 61 (34-82) | 62 (43-78) | 61 (26-76) | 62 (35-79) | 61 (26-82) | .94† | |||||
| Sex | |||||||||||
| Female | 10 | 45.5 | 15 | 35.7 | 49 | 65.3 | 32 | 52.5 | 106 | 53.0 | .02 |
| Male | 12 | 54.5 | 27 | 64.3 | 26 | 34.7 | 29 | 47.5 | 94 | 47.0 | |
| Race/ethnicity | |||||||||||
| White | 18 | 81.8 | 40 | 95.2 | 67 | 89.3 | 51 | 83.6 | 176 | 88.0 | .23 |
| Other | 4 | 18.2 | 2 | 4.8 | 8 | 10.7 | 10 | 16.4 | 24 | 12.0 | |
| Smoking status | |||||||||||
| Never | 3 | 13.6 | 8 | 19.0 | 19 | 25.3 | 13 | 21.3 | 43 | 21.5 | .50 |
| Former | 17 | 77.3 | 26 | 61.9 | 48 | 64.0 | 35 | 57.4 | 126 | 63.0 | |
| Current | 2 | 9.1 | 8 | 19.0 | 8 | 10.7 | 13 | 21.3 | 31 | 15.5 | |
| KRAS mut+ | |||||||||||
| No | 16 | 72.7 | 34 | 81.0 | 53 | 70.7 | 43 | 70.5 | 146 | 73.0 | .63 |
| Yes | 6 | 27.3 | 8 | 19.0 | 22 | 29.3 | 18 | 29.5 | 54 | 27.0 | |
| Prior erlotinib therapy | |||||||||||
| No | 22 | 100 | 27 | 64.3 | 42 | 56.0 | 34 | 55.7 | 125 | 62.5 | < .001 |
| Yes | 15 | 35.7 | 33 | 44.0 | 27 | 44.3 | 75 | 37.5 | |||
| ECOG PS | |||||||||||
| 0 | 3 | 13.6 | 4 | 9.5 | 6 | 8.0 | 4 | 6.6 | 17 | 8.5 | .59 |
| 1 | 17 | 77.3 | 31 | 73.8 | 61 | 81.3 | 44 | 72.1 | 153 | 76.5 | |
| 2 | 2 | 9.1 | 7 | 16.7 | 8 | 10.7 | 13 | 21.3 | 30 | 15.0 | |
| No. of prior therapies | |||||||||||
| 1 | 8 | 36.4 | 4 | 9.5 | 11 | 14.7 | 6 | 9.8 | 29 | 14.5 | .14 |
| 2 | 6 | 27.3 | 12 | 28.6 | 13 | 17.3 | 17 | 27.9 | 48 | 24.0 | |
| 3 | 4 | 18.2 | 7 | 16.7 | 21 | 28.0 | 15 | 24.6 | 47 | 23.5 | |
| 4 | 3 | 13.6 | 9 | 21.4 | 16 | 21.3 | 13 | 21.3 | 41 | 20.5 | |
| 5 | 7 | 16.7 | 6 | 8.0 | 3 | 4.9 | 16 | 8.0 | |||
| ≥ 6 | 1 | 4.5 | 3 | 7.1 | 8 | 10.7 | 7 | 11.5 | 19 | 9.5 | |
| Median (range) | 2.0 (1.0-6.0) | 3.0 (1.0-7.0) | 3.0 (1.0-10.0) | 3.0 (1.0-9.0) | 3.0 (1.0-10.0) | .03† | |||||
| Histology | |||||||||||
| Adenocarcinoma | 14 | 63.6 | 27 | 64.3 | 63 | 84.0 | 43 | 70.5 | 147 | 73.5 | .05 |
| Squamous cell | 7 | 31.8 | 11 | 26.2 | 8 | 10.7 | 9 | 14.8 | 35 | 17.5 | |
| Other | 1 | 4.5 | 4 | 9.5 | 4 | 5.3 | 9 | 14.8 | 18 | 9.0 | |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status; KRAS mut+, KRAS mutation.
Fisher’s exact test.
Kruskal-Wallis test.
Efficacy
The overall 8-week DCR in 186 patients eligible for this analysis was 48% (Table 2), median PFS was 2.0 months (95% CI, 1.9 to 2.8 months), median OS was 6.5 months (95% CI, 5.1 to 7.6 months), and 1-year survival was 28%. Median follow-up was 20 months for PFS and 21 months for OS. There were no complete responses and only six partial responses in these heavily pretreated patients, three in arm 3 and three in arm 4. The overall 8-week DCRs were 32% (arm 1), 50% (arm 2), 53% (arm 3), and 46% (arm 4; pairwise Fisher’s exact test compared with arm 1 P = .26, .12, and .30, respectively; Table 2).
Table 2.
Summary of 8-Week Response by Treatment
| Response | Arm 1 (erlotinib) | Arm 2 (erlotinib + MK-2206) | Arm 3 (MK-2206 + AZD6244) | Arm 4 (sorafenib) | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| PR | 0 | 0 | 0 | 0 | 3 | 4 | 3 | 5 | 6 | 3 |
| SD | 6 | 32 | 18 | 50 | 34 | 49 | 25 | 41 | 83 | 45 |
| 8-week DCR (PR + SD) | 6 | 32 | 18 | 50 | 37 | 53 | 28 | 46 | 89 | 48 |
| PD | 13 | 68 | 18 | 50 | 33 | 47 | 33 | 54 | 97 | 52 |
| Not evaluable* | 3 | 6 | 5 | 0 | 14 | |||||
Abbreviations: DCR, disease control rate; PD, progressive disease; PR, partial response; SD, stable disease
Two patients were not evaluable because they did not complete treatment, one because the patient was retrospectively found to be noneligible because the tumor harbored an EGFR-sensitizing mutation that was not detected during screening.
Only PS was associated with improved DCR; the 8-week DCR for PS 0 was 77% versus only 47% for PS 1 and 36% for PS 2 (Fisher’s exact test P = .03; Data Supplement), a significant association even after adjusting for other parameters in a logistic model (Data Supplement).
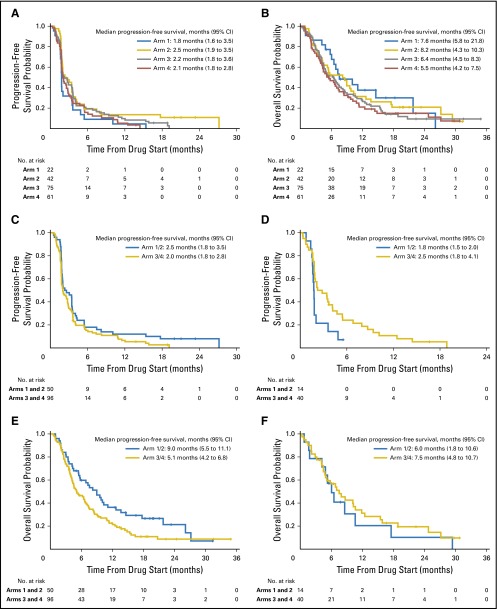
PFS was almost identical among all four arms (1.8, 2.5, 2.2, and 2.1 months for arms 1, 2, 3, and 4, respectively; Fig 3A). OS was not significantly different among the four arms (median, 7.6, 8.2, 6.4, and 5.5 months for arms 1, 2, 3, and 4, respectively; log-rank test P = .46; Fig 3B). In a multivariable Cox model, none of the parameters were significantly associated with PFS, and the only parameter associated with OS was PS (Data Supplement).
Fig 3.
(A) Progression-free survival (PFS) by treatment (P = .17), (B) overall survival (OS) by treatment (P = .46), (C) PFS of patients with KRAS wild-type (wt) tumors by treatment (P = .13), and hazard ratio (HR) for erlotinib-containing treatments versus treatments not containing erlotinib (HR, 0.76; 95% CI, 0.54 to 1.09). (D) PFS of patients with KRAS-mutated (KRAS mut+) tumors by treatment (HR, 1.95; 95% CI, 1.00 to 3.77; P = .04), (E) OS of patients with KRAS wt tumors by treatment (HR, 0.66; 95% CI, 0.45 to 0.97; P = .03), and (F) OS of patients with KRAS mut+ tumors by treatment (HR, 1.26; 95% CI, 0.65 to 2.46; P = .50). All P values were based on two-sided log-rank test.
Biomarkers and Outcomes
Of the 54 KRAS mut+ patients, 52 were evaluable for the prespecified 8-week DCR assessment. There was no significant association between 8-week DCR and KRAS mutation status (Data Supplement).
PFS and OS were not different for patients with KRAS mut+ versus KRAS wt tumors for the whole study (Data Supplement) In KRAS wt patients, there was no difference in PFS between therapy containing erlotinib or not containing erlotinib (hazard ratio [HR] for erlotinib-containing treatments v not containing erlotinib, 0.76; 95% CI, 0.54 to 1.09; P = .13; Fig 3C). Patients with KRAS mut+ tumors experienced a statistically significantly longer PFS if treated with therapy that did not contain erlotinib (HR, 1.95; 95% CI, 1.00 to 3.77; P = .04; Fig 3D). There is a significant qualitative interaction between KRAS mutation and erlotinib-containing therapy (P = .01). Patients with KRAS wt tumors treated with erlotinib-containing therapy had significantly better OS compared with those treated with therapy that did not contain erlotinib (HR, 0.66; 95% CI, 0.45 to 0.97; P = .03; Fig 3E), yet no difference in OS was seen among KRAS mut+ patients between these two treatment groups (HR, 0.26; 95% CI, 0.65 to 2.46; P = .50; Fig 3F), and the influence of the interaction between KRAS mutation and erlotinib-containing therapy on OS was not significant (P = .09). In arm 1, patients with KRAS mut+ tumors had a statistically significantly worse OS than those with KRAS wt tumors (median, 5.5 v 11.1 months; P = .02), but no significant differences were observed for KRAS mut+ compared with KRAS wt tumor-bearing patients in all other arms.
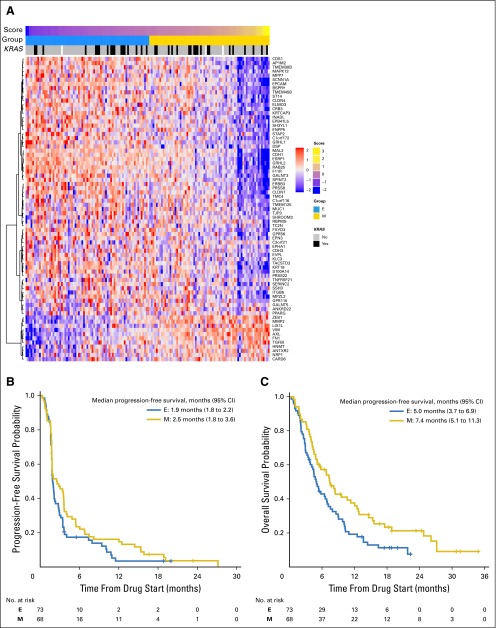
In tumors of 141 randomly assigned patients with adequate material for testing, we examined gene signatures described in the BATTLE study, including a sorafenib sensitivity signature generated from NSCLC cell lines and patient tumor biopsies22 that was not predictive of outcome in this set of patients, as well as an EMT gene signature23 that was associated with resistance in EGFR wt patients who received erlotinib. Patient tumors were scored to classify them as mesenchymal (EMT score > 0; n = 68) or epithelial (EMT score < 0; n = 73). There was no significant association between 8-week DCR and EMT score (Wilcoxon rank sum test P = .72; Data Supplement). EMT gene signature23 analysis (Fig 4A) revealed that PFS was not different in epithelial versus mesenchymal tumors (Fig 4B), whereas analysis by arm revealed improved PFS for patients with mesenchymal tumors treated with the MEK inhibitor (arm 3; P = .04; Data Supplement). A statistically significantly improved OS was seen in patients with mesenchymal tumors (log-rank test P = .02; Fig 4C). The most pronounced effect was found for patients treated with sorafenib and among KRAS mut+ tumors (log-rank test P = .01; Data Supplement). Among rare responders, genomic profiling revealed an exon 19 deletion EGFR mutation on the erlotinib arm not detected at study entry (excluded from DCR analysis; Table 2), a KRAS G12C mutation, an ARAF mutation (R124H; predicted to be associated with sensitivity to MEK inhibition; first responder), an FBXW7 mutation (R479Q), and a short variant of unknown significance in the NOTCH1 gene both predicted to potentially contribute to sensitivity to AKT inhibition24 (second responder) on arm 3 (MK-2206 and AZD6244).
Fig 4.
(A) The epithelial mesenchymal transition gene expression signature classifies BATTLE-2 tumors (all treatment arms) into epithelial (E) and mesenchymal (M). Distribution of KRAS-mutated tumors is shown. (B) Progression-free survival among epithelial and mesenchymal tumors (log-rank test P = .12). (C) Overall survival was superior for patients with mesenchymal tumors (log-rank test P = .02).
Toxicity
Toxicity, especially for the novel arms 2 and 3, was as expected on the basis of prior reports16,25 (Table 3). Average treatment compliance was more than 95% in all arms. There was only one grade 5 event observed in the sorafenib arm: esophageal hemorrhage with a centrally located tumor invading the esophagus and death possibly related to treatment. The most common grade 3 to 4 toxicity in arm 2 was diarrhea (16.7%); in arm 3, maculopapular rash (9.3%), and arm 4 (sorafenib), fatigue (13.1%). Treatment discontinuation rate was 9%, 14%, 13%, and 15% and dose reductions and/or delays were necessary in 18%, 43%, 39%, and 41% in arms 1, 2, 3, and 4, respectively. Nineteen patients (6.9%) experienced biopsy-related pneumothorax, and only two patients (0.7%) required hospitalization for management.
Table 3.
Treatment-Related (including definite, possible, and probable) Adverse Events of All Grades and Grades 3 to 5 Occurring in More Than 10% of Patients in Any Arm
| Adverse Event | Total (n = 200) | Arm 1 (n = 22) | Arm 2 (n = 42) | Arm 3 (n = 75) | Arm 4 (n = 61) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Grades | Grades 3-5 | All Grades | Grades 3-5 | All Grades | Grades 3-5 | All Grades | Grades 3-5 | All Grades | Grades 3-5 | |||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Fatigue | 74 | 37.0 | 16 | 8.0 | 2 | 9.1 | 1 | 4.5 | 15 | 35.7 | 1 | 2.4 | 31 | 41.3 | 6 | 8.0 | 26 | 42.6 | 8 | 13.1 |
| Diarrhea | 73 | 36.5 | 13 | 6.5 | 8 | 36.4 | 2 | 9.1 | 24 | 57.1 | 7 | 16.7 | 26 | 34.7 | 2 | 2.7 | 15 | 24.6 | 2 | 3.3 |
| Rash | ||||||||||||||||||||
| Maculopapular | 70 | 35.0 | 9 | 4.5 | 3 | 13.6 | 0 | 0.0 | 14 | 33.3 | 1 | 2.4 | 29 | 38.7 | 7 | 9.3 | 24 | 39.3 | 1 | 1.6 |
| Acneiform | 61 | 30.5 | 6 | 3.0 | 11 | 50.0 | 1 | 4.5 | 18 | 42.9 | 1 | 2.4 | 29 | 38.7 | 4 | 5.3 | 3 | 4.9 | 0 | 0.0 |
| Nausea | 57 | 28.5 | 5 | 2.5 | 7 | 31.8 | 1 | 4.5 | 13 | 31.0 | 2 | 4.8 | 25 | 33.3 | 1 | 1.3 | 12 | 19.7 | 1 | 1.6 |
| Vomiting | 56 | 28.0 | 5 | 2.5 | 5 | 22.7 | 0 | 0.0 | 10 | 23.8 | 3 | 7.1 | 27 | 36.0 | 2 | 2.7 | 14 | 23.0 | 0 | 0.0 |
| Dry skin | 50 | 25.0 | 4 | 2.0 | 7 | 31.8 | 0 | 0.0 | 12 | 28.6 | 0 | 0.0 | 13 | 17.3 | 3 | 4.0 | 18 | 29.5 | 1 | 1.6 |
| Anorexia | 46 | 23.0 | 1 | 0.5 | 6 | 27.3 | 0 | 0.0 | 16 | 38.1 | 0 | 0.0 | 9 | 12.0 | 0 | 0.0 | 15 | 24.6 | 1 | 1.6 |
| Increased AST | 46 | 23.0 | 5 | 2.5 | 5 | 22.7 | 0 | 0.0 | 5 | 11.9 | 0 | 0.0 | 29 | 38.7 | 5 | 6.7 | 7 | 11.5 | 0 | 0.0 |
| Hyperglycemia | 41 | 20.5 | 3 | 1.5 | 2 | 9.1 | 0 | 0.0 | 11 | 26.2 | 1 | 2.4 | 24 | 32.0 | 2 | 2.7 | 4 | 6.6 | 0 | 0.0 |
| Oral mucositis | 40 | 20.0 | 6 | 3.0 | 3 | 13.6 | 1 | 4.5 | 13 | 31.0 | 3 | 7.1 | 14 | 18.7 | 1 | 1.3 | 10 | 16.4 | 1 | 1.6 |
| Increased alkaline phosphatase | 39 | 19.5 | 2 | 1.0 | 2 | 9.1 | 0 | 0.0 | 10 | 23.8 | 0 | 0.0 | 17 | 22.7 | 1 | 1.3 | 10 | 16.4 | 1 | 1.6 |
| Weight loss | 38 | 19.0 | 1 | 0.5 | 3 | 13.6 | 0 | 0.0 | 11 | 26.2 | 0 | 0.0 | 2 | 2.7 | 0 | 0.0 | 22 | 36.1 | 1 | 1.6 |
| Increased ALT | 32 | 16.0 | 4 | 2.0 | 0 | 0.0 | 0 | 0.0 | 5 | 11.9 | 0 | 0.0 | 22 | 29.3 | 4 | 5.3 | 5 | 8.2 | 0 | 0.0 |
| Pruritus | 22 | 11.0 | 1 | 0.5 | 1 | 4.5 | 0 | 0.0 | 4 | 9.5 | 0 | 0.0 | 8 | 10.7 | 1 | 1.3 | 9 | 14.8 | 0 | 0.0 |
| Other skin and subcutaneous tissue disorders, specify | 19 | 9.5 | 0 | 0.0 | 3 | 13.6 | 0 | 0.0 | 3 | 7.1 | 0 | 0.0 | 8 | 10.7 | 0 | 0.0 | 5 | 8.2 | 0 | 0.0 |
| Dyspnea | 18 | 9.0 | 4 | 2.0 | 1 | 4.5 | 0 | 0.0 | 2 | 4.8 | 1 | 2.4 | 8 | 10.7 | 1 | 1.3 | 7 | 11.5 | 2 | 3.3 |
| Palmar-plantar erythrodysesthesia syndrome | 17 | 8.5 | 5 | 2.5 | 1 | 4.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.3 | 0 | 0.0 | 15 | 24.6 | 5 | 8.2 |
| Dry mouth | 16 | 8.0 | 0 | 0.0 | 1 | 4.5 | 0 | 0.0 | 6 | 14.3 | 0 | 0.0 | 6 | 8.0 | 0 | 0.0 | 3 | 4.9 | 0 | 0.0 |
| Increased blood bilirubin | 13 | 6.5 | 1 | 0.5 | 5 | 22.7 | 0 | 0.0 | 2 | 4.8 | 0 | 0.0 | 1 | 1.3 | 0 | 0.0 | 5 | 8.2 | 1 | 1.6 |
| Hyponatremia | 13 | 6.5 | 3 | 1.5 | 1 | 4.5 | 0 | 0.0 | 3 | 7.1 | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 | 9 | 14.8 | 2 | 3.3 |
| Alopecia | 13 | 6.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 2.4 | 0 | 0.0 | 5 | 6.7 | 0 | 0.0 | 7 | 11.5 | 0 | 0.0 |
| Hypertension | 13 | 6.5 | 3 | 1.5 | 0 | 0.0 | 0 | 0.0 | 1 | 2.4 | 0 | 0.0 | 2 | 2.7 | 0 | 0.0 | 10 | 16.4 | 3 | 4.9 |
| Anemia | 11 | 5.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 4.8 | 0 | 0.0 | 2 | 2.7 | 0 | 0.0 | 7 | 11.5 | 0 | 0.0 |
| Hypoalbuminemia | 10 | 5.0 | 0 | 0.0 | 1 | 4.5 | 0 | 0.0 | 1 | 2.4 | 0 | 0.0 | 1 | 1.3 | 0 | 0.0 | 7 | 11.5 | 0 | 0.0 |
| Dysgeusia | 9 | 4.5 | 0 | 0.0 | 1 | 4.5 | 0 | 0.0 | 5 | 11.9 | 0 | 0.0 | 3 | 4.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Increased creatinine | 9 | 4.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 5 | 11.9 | 0 | 0.0 | 2 | 2.7 | 0 | 0.0 | 2 | 3.3 | 0 | 0.0 |
| Hoarseness | 9 | 4.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.3 | 0 | 0.0 | 8 | 13.1 | 0 | 0.0 |
| Epistaxis | 8 | 4.0 | 0 | 0.0 | 3 | 13.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.3 | 0 | 0.0 | 4 | 6.6 | 0 | 0.0 |
| Hypophosphatemia | 8 | 4.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 8 | 13.1 | 1 | 1.6 |
DISCUSSION
The phase II randomized BATTLE-2 trial confirmed the feasibility of biopsy-mandated, biomarker-based, adaptively randomized clinical study design in patients with pretreated advanced NSCLC. The trial data demonstrated the following key points: there was no significant association between 8-week DCR and KRAS mutation status; patients with KRAS wt tumors treated with erlotinib-containing therapy had better OS compared with those treated with therapy that did not contain erlotinib, whereas patients with KRAS mut+ tumors experienced longer PFS if treated with therapy that did not contain erlotinib and better 8-week DCR with MEK and AKT inhibitor therapy; and mesenchymal gene signature was associated with improved OS.
In all, 334 screened patients were needed to randomly assign 200 patients who reflected the heavily pretreated population with significant comorbid disease and declining PS, also underlying the response rate of 3.2% partially because of a lack of validated predictive markers. The 8-week DCR observed in BATTLE-2 (48%) is similar to that observed in BATTLE-16 (46%) despite the exclusion of erlotinib-naïve patients with EGFR-sensitizing mutations (15% of BATTLE-1 patients).
In BATTLE-2, we prespecified an extremely limited set of markers, and our intent was to use the first half of the study (200 patients) to conduct prospective testing of biomarkers and/or gene signatures. Predictive markers were to be used to guide patient assignments in the second half of the study. Although the design theoretically provided advantages because clear predictive markers did not exist for any of the treatment arms, activity was modest yielding no new predictive markers and not warranting further exploration.
However, several interesting observations were derived from the trial. The EMT signature,23 was not predictive of DCR or PFS in the overall group, but patients with mesenchymal tumors treated with MK-2206 and AZD6244 had improved PFS and those with mesenchymal tumors had improved OS compared with patients with epithelial tumors, an effect mostly driven by treatment with sorafenib. In a recent pan-cancer EMT analysis, there was a trend toward greater sensitivity of mesenchymal cell lines to sorafenib and to drugs that target PDGFR (overexpressed in mesenchymal tumors), consistent with the finding in this study.26 Interestingly, this effect of EMT on OS (all arms, log-rank test P = .02) and among sorafenib-treated patients (log-rank test P = .01) was maintained among patients with KRAS mut+ tumors (Data Supplement). Sorafenib significantly reduces the epigenetic switching of critical EMT-associated genes by potentiating histone acetylation through regulation of expression of histone-modifying enzymes.27 It can inhibit transforming growth factor β1–induced EMT and hepatocyte growth factor–mediated EMT in hepatocytes,28 the latter effect being mediated by inhibition of MAPK signaling, possibly implicated in the improved PFS observed for patients with mesenchymal tumors treated with AZD6244.29
A major focus of BATTLE-2 was exploration of the efficacy of combined AKT and MEK inhibition for KRAS mut+ patients. RAS signaling30 is activated through growth factor receptors or somatic mutations seen in 25% of lung adenocarcinomas, frequently in the context of other co-mutations. RAS has been an elusive target for direct targeting.31 Co-targeting of the Ras/Raf/MEK/ERK and PI3K/AKT parallel pathways on the basis of multiple points of cross-talk and negative feedback interactions32 can blunt compensatory pathway activation leading to antitumor effects. Indeed, in a KRAS-mutant lung cancer mouse model, combined MEK and PI3K/mammalian target of rapamycin inhibition resulted in synergistic effects and tumor regression.14 In this trial, we used two potent selective inhibitors, MK-2206, an AKT inhibitor, and AZD6244, a non-ATP competitive inhibitor of MEK,33 a combination evaluated in a phase I study,34 which was partially run in parallel with our study with encouraging results (23% response rate in KRAS mut+ NSCLC). There were only three partial responses: two had available genomic data and one harbored both a KRAS G12C and an ARAF mutation suggesting multiple inputs in the MAPK signaling pathway and possibly conferring increased sensitivity to MEK inhibition. The observed heterogeneity of response among patients with KRAS-mut+ cancers likely reflects the complex co-mutational landscape of KRAS mut+ tumors that defines biologically distinct subgroups with different therapeutic vulnerabilities.35 Our experience mirrors that of several other trials evaluating combinations of PI3K/AKT and MEK inhibitors25,36-38 that have demonstrated modest activity and poor tolerance to combinations related to on-target inhibition of the MAPK and PI3K pathways in normal tissues.
Complex mutational background tumors encountered in heavily pretreated patients may be better addressed with novel immunotherapy agents39,40 or other combinations of targeted therapy with or without immunotherapy.
The BATTLE-2 study showed the utility of real-time biopsies for broad profiling of tumors that serve as a discovery vehicle for better target selection. We are currently pursuing alternative strategies in targeting KRAS mut+ tumors by incorporating knowledge derived from BATTLE-2.
Supplementary Material
Acknowledgment
We gratefully acknowledge the patients who participated in the study, the physicians that referred and enrolled patients on the study, Ashley White and Emily Duffield for clinical research coordination, Jeffrey Lewis for programming and database management support, and Angela Incassati for editorial assistance. We also thank the Anderson Data Safety and Monitoring Board for monitoring the trial.
Footnotes
Supported by research grants from Merck and Bayer Healthcare Pharmaceuticals (No. 15660) and by Grants No. R01CA155196-01A1 and P30CA01667 (Cancer Center Core Grant) from the National Cancer Institute.
Presented in part at the 49th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, May 31-June 4, 2013, and the 50th Annual Meeting of ASCO, Chicago, IL, May 31-June 3, 2014.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. contributions are found at the end of this article.
Clinical trial information: NCT01248247
See accompanying editorial on page 3595
AUTHOR CONTRIBUTIONS
Conception and design: Vassiliki Papadimitrakopoulou, J. Jack Lee, Anne S. Tsao, Kevin R. Coombes, David J. Mauro, Eric H. Rubin, Waun Ki Hong, Roy S. Herbst
Financial support: David J. Mauro, Eric H. Rubin
Administrative support: John V. Heymach
Provision of study materials or patients: Vassiliki Papadimitrakopoulou, Anne S. Tsao, Frank V. Fossella, Lauren Averett Byers, Scott N. Gettinger, Sarah B. Goldberg, Don L. Gibbons, Alda L. Tam, David J. Mauro, Eric H. Rubin, Roy S. Herbst
Collection and assembly of data: Vassiliki Papadimitrakopoulou, J. Jack Lee, Ignacio I. Wistuba, Anne S. Tsao, Frank V. Fossella, Neda Kalhor, Sanjay Gupta, Lauren Averett Byers, Julie G. Izzo, Scott N. Gettinger, Sarah B. Goldberg, Ximing Tang, Vincent A. Miller, Li Shen, Caimiao Wei, Lixia Diao, Alda L. Tam, Ja Seok Koo, John V. Heymach, Roy S. Herbst
Data analysis and interpretation: Vassiliki Papadimitrakopoulou, J. Jack Lee, Lauren Averett Byers, Julie G. Izzo, Ferdinandos Skoulidis, Don L. Gibbons, Li Shen, Caimiao Wei, Lixia Diao, S. Andrew Peng, Jing Wang, John V. Heymach, Waun Ki Hong, Roy S. Herbst
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The BATTLE-2 Study: A Biomarker-Integrated Targeted Therapy Study in Previously Treated Patients With Advanced Non–Small–Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Vassiliki Papadimitrakopoulou
Consulting or Advisory Role: Clovis Oncology, Genentech, Merck, Biothera, Eli Lilly, Janssen Pharmaceuticals, Gensignia Life Sciences, AstraZeneca, ARIAD Pharmaceuticals, Bristol-Myers Squibb
Research Funding: Merck (Inst), Novartis (Inst), Celgene (Inst), Clovis Oncology (Inst), Bayer Healthcare Pharmaceuticals (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), Janssen Oncology (Inst), Pfizer (Inst)
J. Jack Lee
No relationship to disclose
Ignacio I. Wistuba
Consulting or Advisory Role: Genentech, GlaxoSmithKline, Eli Lilly, Clovis Oncology, Bristol-Myers Squibb, Synta Pharmaceuticals, HTG Molecular Diagnostics, Asuragen, AstraZeneca, ARIAD Pharmaceuticals
Speaker’s Bureau: Pfizer, Boehringer Ingelheim, Medscape
Research Funding: Genentech, Merck, OncoPlex Diagnostics, Myriad Genetics, Bayer Healthcare Pharmaceuticals, Jounce Therapeutics, HTG Molecular Diagnostics, MedImmune, Merck
Anne S. Tsao
Consulting or Advisory Role: Novartis, Boehringer Ingelheim, Astellas Pharma, Genentech, MedImmune, Imedex
Research Funding: MedImmune (Inst), Merck (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Novartis
Frank V. Fossella
No relationship to disclose
Neda Kalhor
No relationship to disclose
Sanjay Gupta
No relationship to disclose
Lauren Averett Byers
Consulting or Advisory Role: AbbVie, Medivation, BioMarin Pharmaceutical, AstraZeneca
Research Funding: Takeda Pharmaceuticals
Julie G. Izzo
No relationship to disclose
Scott N. Gettinger
Consulting or Advisory Role: Janssen Pharmaceuticals, Bristol-Myers Squibb, ARIAD Pharmaceuticals
Research Funding: Bristol-Myers Squibb (Inst), Genentech (Inst), ARIAD Pharmaceuticals (Inst), AstraZeneca/MedImmune (Inst), Boehringer Ingelheim (Inst), Incyte (Inst), Pfizer (Inst)
Sarah B. Goldberg
Consulting or Advisory Role: Clovis Oncology
Research Funding: AstraZeneca, Merck (Inst), Genentech (Inst), Pfizer (Inst)
Ximing Tang
No relationship to disclose
Vincent A. Miller
Employment: Foundation Medicine
Leadership: Foundation Medicine
Stock or Other Ownership: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Royalty for T790M EGFR licensing
Ferdinandos Skoulidis
No relationship to disclose
Don L. Gibbons
Travel, Accommodations, Expenses: AstraZeneca/MedImmune
Li Shen
No relationship to disclose
Caimiao Wei
No relationship to disclose
Lixia Diao
No relationship to disclose
S. Andrew Peng
No relationship to disclose
Jing Wang
No relationship to disclose
Alda L. Tam
Honoraria: Medtronic, Galil Medical
Research Funding: AngioDynamics
Travel, Accommodations, Expenses: Medtronic
Kevin R. Coombes
Patents, Royalties, Other Intellectual Property: Royalties on a patent for BCRP (I)
Ja Seok Koo
No relationship to disclose
David J. Mauro
Employment: Merck Sharp & Dohme
Stock or Other Ownership: Merck Sharp & Dohme
Eric H. Rubin
Employment: Merck
Stock or Other Ownership: Merck
John V. Heymach
Stock or Other Ownership: Cardinal Spine and Pain Management, BioTree Labs
Consulting or Advisory Role: AstraZeneca, AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Medivation, ARIAD Pharmaceuticals, Synta Pharmaceuticals, Oncomed Pharmaceuticals, Novartis, Genentech, Calithera Biosciences
Research Funding: AstraZeneca, GlaxoSmithKline, Bayer Healthcare Pharmaceuticals
Waun Ki Hong
No relationship to disclose
Roy S. Herbst
Honoraria: Eli Lilly, Merck, Pfizer, AstraZeneca/MedImmune, Genentech
Consulting or Advisory Role: Kolltan Pharmaceuticals, DiaTech Oncology, Biothera
Research Funding: Genentech
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Le Chevalier T, Scagliotti G, Natale R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: A meta-analysis of survival outcomes. Lung Cancer. 2005;47:69–80. doi: 10.1016/j.lungcan.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyooka S, Mitsudomi T, Soh J, et al. Molecular oncology of lung cancer. Gen Thorac Cardiovasc Surg. 2011;59:527–537. doi: 10.1007/s11748-010-0743-3. [DOI] [PubMed] [Google Scholar]
- 6.Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: Personalizing therapy for lung cancer. Cancer Discov. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Liu S, Kim ES, et al. Bayesian adaptive design for targeted therapy development in lung cancer: A step toward personalized medicine. Clin Trials. 2008;5:181–193. doi: 10.1177/1740774508091815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tam AL, Kim ES, Lee JJ, et al. Feasibility of image-guided transthoracic core-needle biopsy in the BATTLE lung trial. J Thorac Oncol. 2013;8:436–442. doi: 10.1097/JTO.0b013e318287c91e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. doi: 10.1097/JTO.0000000000000693. Paz-Ares L, Hirsh V, Zhang L, et al: MISSION Trial: A phase III, multi-center, placebo-controlled trial of sorafenib in patients with relapsed or refractory predominantly non-squamous NSCLC after 2 or 3 previous treatment regimens. J Thorac Oncol 10.1097/JTO.0000000000000693 [epub ahead of print on November 6, 2015] [DOI] [PubMed] [Google Scholar]
- 10.Wakelee HA, Lee JW, Hanna NH, et al. A double-blind randomized discontinuation phase-II study of sorafenib (BAY 43-9006) in previously treated non-small-cell lung cancer patients: Eastern Cooperative Oncology Group study E2501. J Thorac Oncol. 2012;7:1574–1582. doi: 10.1097/JTO.0b013e31826149ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dingemans AM, Mellema WW, Groen HJ, et al. A phase II study of sorafenib in patients with platinum-pretreated, advanced (Stage IIIb or IV) non-small cell lung cancer with a KRAS mutation. Clin Cancer Res. 2013;19:743–751. doi: 10.1158/1078-0432.CCR-12-1779. [DOI] [PubMed] [Google Scholar]
- 12.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 13.Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: A randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 14.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 16.Lara PN, Jr, Longmate J, Mack PC, et al. Phase II study of the AKT inhibitor MK-2206 plus erlotinib in patients with advanced non-small cell lung cancer who previously progressed on erlotinib. Clin Cancer Res. 2015;21:4321–4326. doi: 10.1158/1078-0432.CCR-14-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camidge DR, Kono SA, Flacco A, et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res. 2010;16:5581–5590. doi: 10.1158/1078-0432.CCR-10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu X, Chen N, Wei C, et al. Bayesian two-stage biomarker-based adaptive design for targeted therapy development. Stat Biosci. 2014;7:1–30. doi: 10.1007/s12561-014-9124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Lara PN, Jr, Redman MW, Kelly K, et al. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: Results from Southwest Oncology Group randomized trials. J Clin Oncol. 2008;26:463–467. doi: 10.1200/JCO.2007.13.0344. [DOI] [PubMed] [Google Scholar]
- 22.Blumenschein GR, Jr, Saintigny P, Liu S, et al. Comprehensive biomarker analysis and final efficacy results of sorafenib in the BATTLE trial. Clin Cancer Res. 2013;19:6967–6975. doi: 10.1158/1078-0432.CCR-12-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byers LA, Diao L, Wang J, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19:279–290. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren H, Koo J, Guan B, et al. The E3 ubiquitin ligases β-TrCP and FBXW7 cooperatively mediates GSK3-dependent Mcl-1 degradation induced by the Akt inhibitor API-1, resulting in apoptosis. Mol Cancer. 2013;12:146. doi: 10.1186/1476-4598-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolcher AW, Patnaik A, Papadopoulos KP, et al. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemother Pharmacol. 2015;75:183–189. doi: 10.1007/s00280-014-2615-5. [DOI] [PubMed] [Google Scholar]
- 26. doi: 10.1158/1078-0432.CCR-15-0876. Mak MP, Tong P, Diao L, et al: A patient-derived, pan-cancer EMT signature identifies global molecular alterations and immune target enrichment following epithelial-to-mesenchymal transition. Clin Cancer Res 22:609-620, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Chen YL, Ji G, et al. Sorafenib inhibits epithelial-mesenchymal transition through an epigenetic-based mechanism in human lung epithelial cells. PLoS One. 2013;8:e64954. doi: 10.1371/journal.pone.0064954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YL, Lv J, Ye XL, et al. Sorafenib inhibits transforming growth factor β1-mediated epithelial-mesenchymal transition and apoptosis in mouse hepatocytes. Hepatology. 2011;53:1708–1718. doi: 10.1002/hep.24254. [DOI] [PubMed] [Google Scholar]
- 29.Buonato JM, Lazzara MJ. ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Cancer Res. 2014;74:309–319. doi: 10.1158/0008-5472.CAN-12-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young A, Lyons J, Miller AL, et al. Ras signaling and therapies. Adv Cancer Res. 2009;102:1–17. doi: 10.1016/S0065-230X(09)02001-6. [DOI] [PubMed] [Google Scholar]
- 31.Ostrem JM, Peters U, Sos ML, et al. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 33.Banerji U, Camidge DR, Verheul HM, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): A phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–1623. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 34.Tolcher AW, Khan K, Ong M, et al. Antitumor activity in RAS-driven tumors by blocking AKT and MEK. Clin Cancer Res. 2015;21:739–748. doi: 10.1158/1078-0432.CCR-14-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5:860–877. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bedard P, Tabernero J, Kurzrock R, et al: A phase lb, open-label, multicenter, dose-escalation study of the oral pan-PI3K inhibitor BKM120 in combination with the oral MEK1/2 inhibitor GSK1120212 in patients (pts) with selected advanced solid tumors. J Clin Oncol 30, 2012 (suppl; abstr 3003) [Google Scholar]
- 37. Infante JR, Gandhi L, Shapiro G, et al: Phase lb combination trial of a MEK inhibitor, pimasertib (MSC1936369B), and a PI3K/mTOR inhibitor, SAR245409, in patients with locally advanced or metastatic solid tumors. J Clin Oncol 30, 2012 (suppl; abstr TPS3118) [Google Scholar]
- 38. LoRusso P: A first-in-human phase Ib study to evaluate the MEK inhibitor GDC-0973, combined with the pan-PI3K inhibitor GDC-0941, in patients with advanced solid tumors. J Clin Oncol 30, 2012 (suppl; abstr 2566) [Google Scholar]
- 39.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.