Abstract
Ectomycorrhizal fungi (EMF) represent one of the major guilds of symbiotic fungi associated with roots of forest trees, where they function to improve plant nutrition and fitness in exchange for plant carbon. Many groups of EMF exhibit preference or specificity for different plant host genera; a good example is the genus Suillus, which grows in association with the conifer family Pinaceae. We investigated genetics of EMF host-specificity by cross-inoculating basidiospores of five species of Suillus onto ten species of Pinus, and screened them for their ability to form ectomycorrhizae. Several Suillus spp. including S. granulatus, S. spraguei, and S. americanus readily formed ectomycorrhizae (compatible reaction) with white pine hosts (subgenus Strobus), but were incompatible with other pine hosts (subgenus Pinus). Metatranscriptomic analysis of inoculated roots reveals that plant and fungus each express unique gene sets during incompatible vs. compatible pairings. The Suillus-Pinus metatranscriptomes utilize highly conserved gene regulatory pathways, including fungal G-protein signaling, secretory pathways, leucine-rich repeat and pathogen resistance proteins that are similar to those associated with host-pathogen interactions in other plant-fungal systems. Metatranscriptomic study of the combined Suillus-Pinus transcriptome has provided new insight into mechanisms of adaptation and coevolution of forest trees with their microbial community, and revealed that genetic regulation of ectomycorrhizal symbiosis utilizes universal gene regulatory pathways used by other types of fungal-plant interactions including pathogenic fungal-host interactions.
Author Summary
Ectomycorrhizal fungi (EMF) comprise the dominant group of symbiotic fungi associated with plant roots in temperate and boreal forests. We examined host-specificity and gene-expression of five EMF Suillus species that exhibited strong patterns of mycorrhizal compatibility/incompatibility with either white pines (Pinus subg. Strobus) or hard pines (subg. Pinus). Using RNA-Seq, we identified conserved transcriptomic responses associated with compatible versus incompatible Pinus-Suillus species pairings. Comparative metatranscriptomic analysis of compatible vs. incompatible pairings allowed us to identify unique sets of fungal and plant genes associated with symbiont recognition and specificity. Comparativ transcriptomic study of the Suillus-Pinus system provides insight into the core functions involved in ectomycorrhizal symbiosis, and the mechanisms by which host-symbiont pairs recognize one another.
Introduction
Growing evidence has shown that many symbiotic plant-microbial associations including pathogenic as well as mutualistic symbioses are governed by similar genetic interaction mechanisms [1,2]. For example, in many groups of pathogenic fungi and oomycetes, coevolution with their plant hosts has resulted in typical 'arms-race' patterns of interactions, in which pathogens evolve batteries of effectors that suppress plant defense responses, while plants evolve modified receptors that sense microbial molecules and reactivate plant defense responses [3]. The molecular functions of several fungal and oomycete effectors involved in host-pathogen recognition have recently been elucidated. For instance, cysteine-rich avirulence genes (Avr) have been identified in several fungi including Cladosporium fulvum and Melampsora lini [4, 5], while Avr1b was isolated from the oomycete Phytophthora sojae [6]. Studying the functions of these effectors is a challenging task, because of the highly divergent nature of effectors in diverse taxa of pathogenic microbes and the lack of similarity of the sequences of these effectors to other proteins in public databases. Plant defense proteins that perceive microbial effectors include nucleotide-binding leucine-rich repeat (NB-LRR) proteins [1, 7, 8] and cell membrane receptors (e.g. phosphatidylinositol 3-P) [9]. These receptors can be activated by direct binding of effectors or modified by effector-associated proteins, leading to a plant-defense response.
Mutualistic plant-fungal interactions, including arbuscular mycorrhizae and ectomycorrhizae, also share similar conserved genetic interaction mechanisms with other symbiotic plant-fungal systems [10–12]. Over 30 plant families are known to form ectomycorrhizal associations with over 80 lineages (250 genera) of fungi [13]. A highly diverse community of EMF form the dominant guild of soil microbes in most of the world's forests [14,15], where they provide their plant hosts with essential resources (N, P, H2O) as well as protection from pathogens, in exchange for photosynthetically fixed carbon [16].
Details about molecular interactions between EMF and their plant hosts are emerging. Recent studies have identified differentially expressed genes associated with EMF symbiosis for several EMF-plant interactions including Pisolithus microcarpus with Eucalyptus [17], Paxillus involutus with Betula [18], and Laccaria bicolor with different Populus spp. [2]. One of these genes, a small secreted protein (MiSSP7) produced by the ectomycorrhizal basidiomycete Laccaria bicolor, functions as a critical effector for compatible mycorrhizal interaction with Populus. MiSSP7 was shown to be imported into plant nuclei where it suppresses plant host defenses, enabling mycorrhiza formation. Other recent studies also demonstrated that jasmonic acid (JA) and related plant defense-activated compounds are produced by Populus in response to signals from their symbiont [19,20]. These results suggest a general involvement of JA-mediated and other conserved plant signaling pathways for plant-fungal communication during EMF symbiosis. Similar to the mechanisms of EMF interaction in Laccaria [2], plant pathogenic fungi (e.g. M. larici) can also deliver SSPs to multiple cellular compartments in Populus [21]. These studies demonstrate that EMF are able to modulate plant defense system during symbiosis [2,10,21], and suggest that that most plant-microbial associations (including pathogenic and mutualistic interactions) may be governed by similar mechanisms. Unlike biotrophic/necrotrophic parasitisms, mutualistic fungal-plant interactions such as EMF must also establish stable long-term relationships with their living host cells, with benefits to both the fungus and its host. Thus, there is considerable potential for an array of distinct elements to regulate the host-specific communications of symbiosis compared to plant-pathogen interactions.
Many groups of EMF are known to exhibit preference or specificity for different plant host genera [22,23]. A good example of strong host-specificity is the bolete genus Suillus, which grows in association with the conifer family Pinaceae [24,25]. Most species of Suillus form ectomycorrhizae with specific Pinaceae host species (e.g., white pine, douglas fir, larch), suggesting a long history of plant-fungal coevolution in this genus [26–28]. Other examples of EMF with host-specific interactions include Laccaria bicolor, which shows differential host-compatibility with different species of Populus [29], and Paxillus involutus, which favors Betula as a host over Populus [30]. In order to study the molecular basis for host-specificity between different Pinus and Suillus species, we used pairwise plant-fungal bioassays to identify patterns of compatible and incompatible EMF interactions. Compatible EMF interactions are characterized by morphogenesis of plant and fungal tissues leading to development of modified plant short roots with bifurcated root tips that are sheathed by a hyphal mantle over the root epidermal surface, with hyphal ingrowth into the root cortex to form the Hartig-net [31]. In contrast, incompatible EMF interactions fail to induce root morphogenesis, resulting in little or no mycelial growth, and are morphologically indistinguishable from uninoculated (non-symbiotic) roots.
The pace of genetic studies of EMF-plant symbiosis has greatly accelerated by expanding numbers of genome sequencing for many EMF [10]. Though study of most EMF is still hindered by a lack of ‘finished’ genomes, we recently developed a procedure that employs RNA-Seq and de-novo assembly and annotation to characterize the metatranscriptome of EMF associated with Pinus taeda from field-collected mycorrhizal root clusters [32]. Here we apply metatranscriptomic profiling to study compatible versus incompatible mycorrhizal interactions from both plant and fungal perspectives. Our studies demonstrate that Suillus and Pinus each exhibit well-differentiated transcriptomic profiles during compatible and incompatible interactions. Comparison of expression patterns in compatible and incompatible pairings helped us to identify gene sets associated with plant-fungal recognition and establishment of EMF symbiosis.
Results
Host-specific relationships between Suillus and Pinus species
To investigate occurrence of Suillus in natural Pinaceae forests, we first examined patterns of host specificity for Suillus operational taxonomic units (OTUs) detected by a recent survey of North American pine forest soils using next generation amplicon sequence analysis of the ribosomal RNA internal transcribed spacer (ITS) region [14]. Eleven Suillus OTUs detected by that survey (out of a total of >10,000 fungal OTUs detected across North America) exhibit distinct host range patterns corresponding with different Pinaceae hosts (S1 Fig): S. glandulosa with Picea glauca; S.hirtellus and S. cothurnatus with Pinus taeda; S. granulatus, S. spraguei (= S. pictus) and S. americanus with Pinus strobus; and an unidentified Suillus sp. with Pinus monticola. Several Suillus species were observed to be broadly associated with multiple Pinus species, including Suillus brevipes, which is associated with several Pinus spp. across North America (S1 Fig) but was restricted to hosts in the subgenus Pinus (P. ponderosa, P. contorta, P. banksiana, and P. taeda).
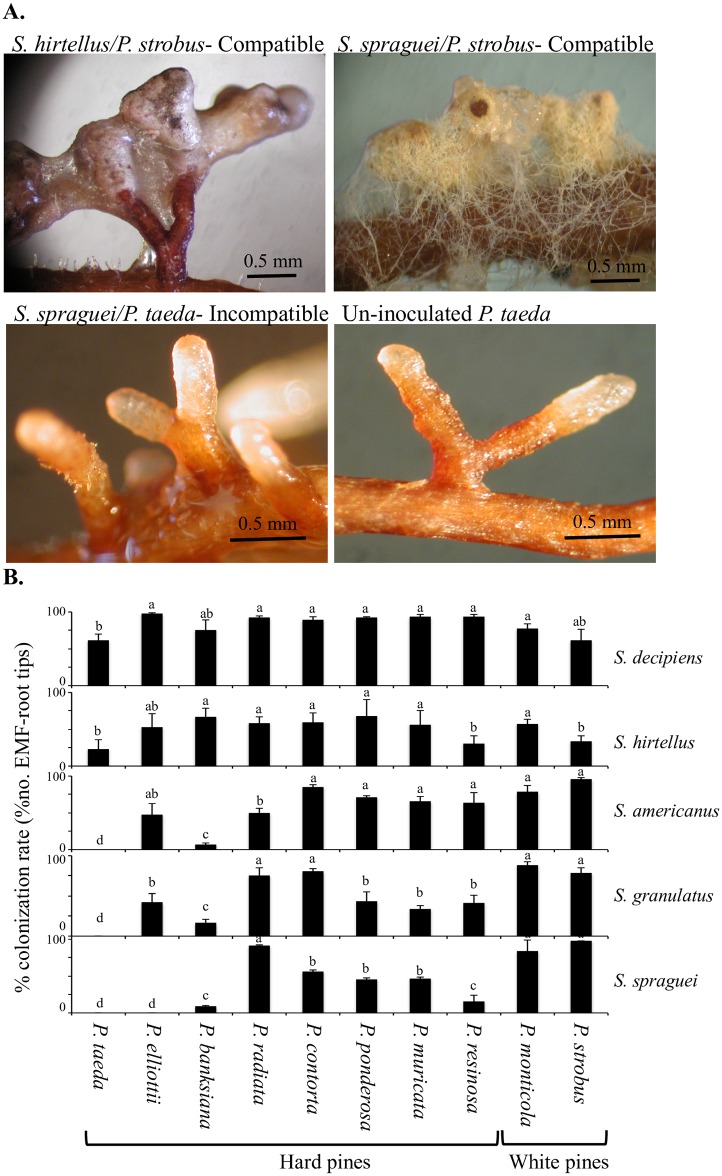
To study host specificity, a plant bioassay was developed using axenically grown pine seedlings inoculated with Suillus basidiospores to establish Suillus-Pinus mycorrhizae in vitro [31]. Seedlings of ten Pinus species were inoculated in all pairwise combinations with basidiospores of five Suillus species and scored for ectomycorrhiza formation after 8 weeks growth. In Pinus, successful formation of ectomycorrhizae (compatible interaction) results in a series of characteristic morphogenetic changes to young root tips that become swollen and bifurcated, and ensheathed by a mycelial mantle which penetrates into the root cortex to form a Hartig net [33] (Fig 1A and S2 Fig). In contrast, incompatible pairings are characterized by little or no colonization of roots by fungal mycelium (both fungal mantle and Hartig-net absent).
Fig 1. Ectomycorrhizal compatibility and incompatibility between Suillus—Pinus species pairings.
(A) During a compatible EMF interaction, Suillus-inoculated roots develop characteristic ectomycorrhizas with short swollen (bifurcated) root tips with well-developed hyphal sheath and Hartig-net (observed in cross-sections of root tips, S1 Fig). Incompatible EMF interactions fail to establish mycorrhizae, with little or no fungal colonization, and are morphologically indistinguishable from un-inoculated (non-mycorrhizal) control roots (also shown). (B) Ectomycorrhizal compatibility between different species of Suillus and Pinus measured as a proportion of EMF root tips versus total bare root tips (n≥3). Tukey test was used to test significance across Pinus species within a Suillus species (P<0.05). Means marked by the same letters were not significantly different. The complete list of fungal cultures and spore prints used is listed in S3 Table.
Basidiospore inoculations of two generalist species, S. hirtellus and S. decipiens, resulted in well-developed (compatible) ectomycorrhizae with most Pinus species (Fig 1B), when S. hirtellus had relatively lower rates of colonization on all hosts. Three white pine specialists (S. granulatus, S. americanus and S. spraguei) readily formed ectomycorrhizae with white pines (P. strobus and P. monticola), but had lower colonization rates on hard pines (e.g. P. banksiana), and failed to form visible ectomycorrhizae on P. taeda (incompatible pairing) (Fig 1B).
Transcriptomic activity of Suillus and Pinus mycorrhizal roots
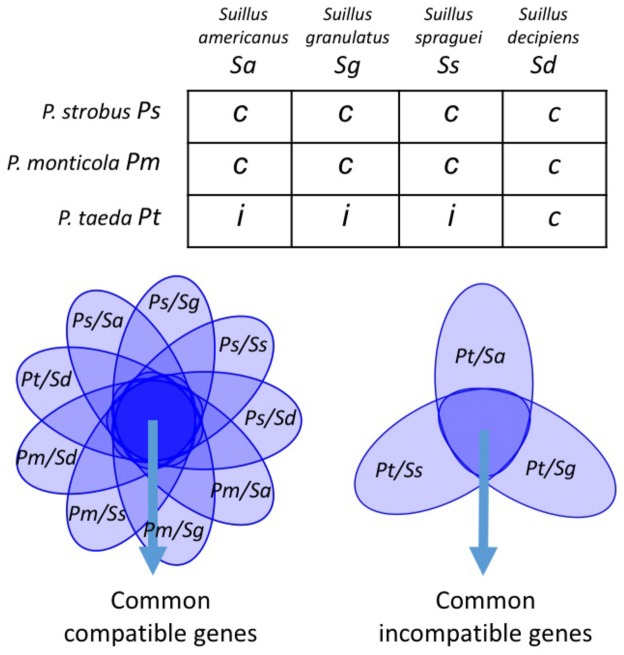
Variation in mycorrhizal compatibility between different Suillus and Pinus species suggests that genetic differences underlie host recognition and specificity during ectomycorrhizal symbiosis. To test this hypothesis, we compared transcriptomic activities across a panel of compatible and incompatible root tip samples formed by inoculation of three Pinus species (P. monticola, P. strobus, P. taeda) with four species of Suillus (S. americanus, S. granulatus, S. spraguei, and S. decipiens). Detailed descriptions of the individual Suillus-Pinus sample pairs, including strains used are listed in S1 Dataset. Transcriptomes from uninoculated pine roots were included as controls (to confirm that Suillus genes were not expressed by uninoculated roots) along with pure cultures of each fungal species (as references for transcriptome assembly). Comparative transcriptome profiling was used to identify candidate genes involved in Pinus-Suillus recognition (Table 1). The computational strategies included a) de novo transcriptome assembly to identify reads representing genes for different rRNA, Suillus, Pinus, and b) comparative transcriptomic analysis to identify common (core) and unique (host-specific) genes involved in symbiosis (see Materials and Methods, and SI text A1-A4; S3–S5 Figs). Unique genes were defined as upregulated genes detected in the RNA contig assembly of one Suillus species, but absent in other species examined. However, whether these genes are truly unique to different Suillus species still need to be determined through whole genome sequencing.
Table 1. Compatible and incompatible Suillus-Pinus species pairings used for comparative transcriptomic analysis.
For each Suillus-Pinus species pair, spore prints from two to three different fruit bodies (biological replicates) were used to inoculate Pinus seedlings. Collection data with source information for Suillus cutures, spore prints (and voucher specimens) are listed in S1 Dataset & S3 Table.
| S. americanus | S. granulatus | S. spraguei | S. decipiens | |
|---|---|---|---|---|
| P. monticola | Compatible interaction. ID of spore prints used: SA0005; SA0010; SA0011 | Compatible interaction. ID of spore prints used: SG0004; SG0009; SG0014 | Compatible interaction. ID of spore prints used: SS0006; SS0012; SS0013 | Compatible interaction. ID of spore prints used: SD0002; SD0003; SD0008 |
| P. strobus | Compatible interaction. (Sa/Ps rep1 to rep3) ID of spore prints used: SA0005; SA0010; SA0011 | Compatible interaction. ID of spore prints used: SG0004; SG0009 | Compatible interaction. ID of spore prints used: SS0006; SS0012; SS0013 | Compatible interaction. ID of spore prints used: SD0002; SD0003; SD0008 |
| P. taeda | Incompatible interaction. ID of spore prints used: SA0005; SA0010 | Incompatible interaction. ID of spore prints used: SG0004; SG0009 | Incompatible interaction. ID of spore prints used: SS0006; SS0012 | Compatible interaction. ID of spore prints used: SD0002; SD0003; SD0008 |
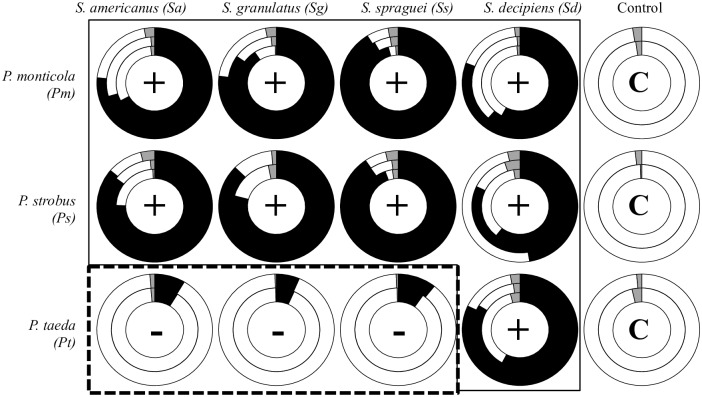
Up to 28 million (M) high quality reads were recovered from inoculated root tips using RNA-Seq (approx. 1 mg root tissue per sample, equal to about ten root tips) (S1 Dataset). Compatible Pinus-Suillus pairs resulted in roughly equal numbers of plant and fungal reads, while incompatible pairs resulted in much lower number of fungal reads compared to the corresponding plant reads (Fig 2). These differences of Suillus/Pinus reads recovered from compatible and incompatible interactions are also consistent with to the higher proportion of fungal biomass present in compatible versus incompatible mycorrhizal pairings. The Suillus transcriptome generated from de novo assembly of pooled data was used to identify 15M (51% of total reads) and 2M (6.1% of total reads) reads from compatible and incompatible reactions, respectively (Fig 2 and S1 Dataset). Approximately 3M (11% of total reads) and 21M (66% of total reads) Pinus transcriptome reads were also recovered from compatible and incompatible pairings, which could be matched to 44% and 69% of publicly available Pinus EST databases (~0.3M ESTs), respectively. In total, 11,029 and 5,947 Suillus contigs were obtained through de novo assembly from compatible and incompatible root samples respectively (S1 Dataset).
Fig 2. Proportion of metatranscriptomic RNASeq reads assigned to Suillus (black), Pinus (white), or other fungi (grey) during compatible (+) vs. incompatible (-) EMF interactions.
Nested circles within graph represent individual pine seedlings (biological replicates) inoculated with basidiospores of different Suillus species. Controls (C) are uninoculated Pinus roots. Details with read numbers and gene annotations are shown in S1 Dataset.
Expression of Suillus genes specific for different species of Pinus
We hypothesized that pairings between different Suillus (and Pinus) species would share common gene expression patterns during compatible vs. incompatible pairings. Similarly, unique gene sets expressed by individual Suillus/Pinus pairings could also be identified (Fig 2). Here we defined “common genes” as the core sets of genes that were upregulated (> 2-fold) in response to compatible hosts; in contrast, “unique genes” were identified as those were only expressed in individual Suillus spp. in response to specific Pinus host species. To test these hypotheses, we used comparative transcriptomic analysis to identify Suillus and Pinus expressed genes during compatible and incompatible ECM interactions of four Suillus species grown with three different hosts, P. monticola, P. strobus, and P. taeda, (Fig 3). To compare gene expression patterns between interacting fungal and host genomes, sequencing reads aligned to either Suillus or Pinus contigs were normalized using DESeq package (ver. 1.14.0) [34]. (Details were provided in Support Information SI A2, S5 Fig). Gene expression biplots revealed strong differences between compatible and incompatible EMF pairings (S6A and S6B Fig). All of the compatible EMF pairings showed similar expression patterns of Suillus genes, even on different hosts (e.g. P. strobus and P. monticola) (S7 and S8 Figs), which suggests that different Suillus species all employ common regulatory pathways across different compatible host species. Significant differences were observed in gene expression between compatible and incompatible reactions (t-test, p-value < 0.01) (Fig 4). On average, 8,765 Suillus contigs were upregulated when they grew with compatible hosts, whereas fewer contigs (1,918 contigs in average) were upregulated from incompatible pairings (S1 Dataset).
Fig 3. Experimental design showing compatible (c) and incompatible (i) mycorrhizal pairings used for RNASeq analysis to identify common compatible and common incompatible gene sets.
After de novo assembly and annotation, common and unique compatible/incompatible gene sets are identified for each species pair of mycobiont (Suillus) and phycobiont (Pinus).
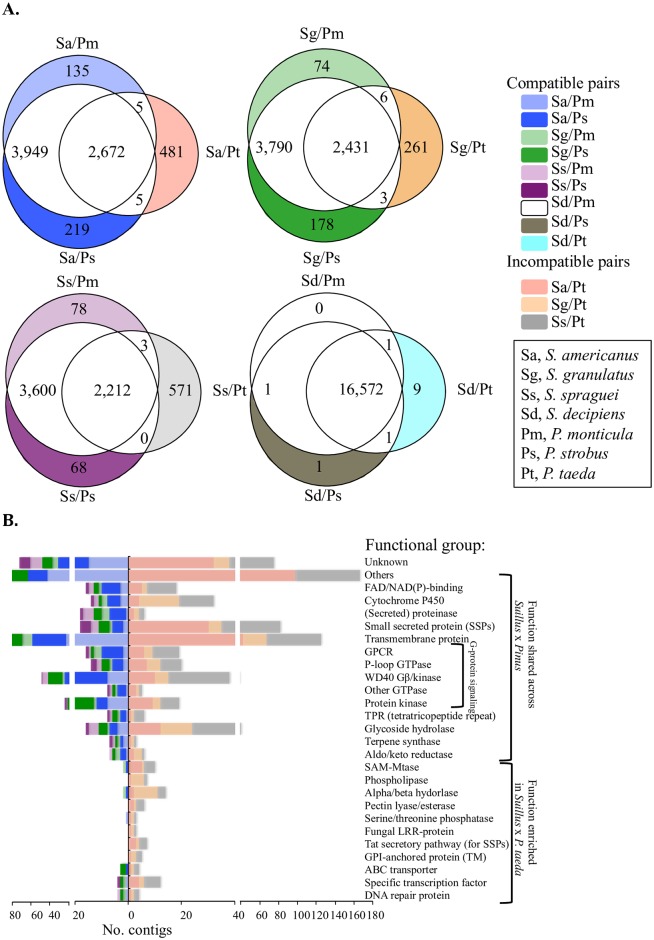
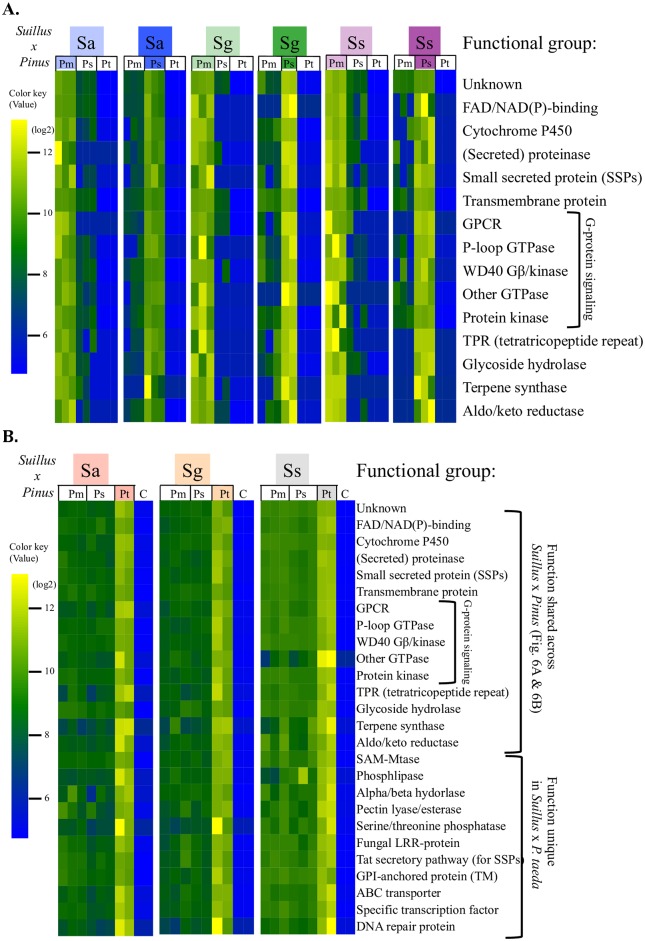
Fig 4. Unique and common Suillus genes that are upregulated in response to different pine hosts.
(A) Venn diagrams illustrating number of shared and unique genes for 4 Suillus species interacting with 3 Pinus hosts. Color backgrounds indicate normalized counts of upregulated host-specific transcripts (“unique genes”) identified from the individual pair combinations. Color coding is same for panels A and B. False discovery rate of 5% was used to identify unique genes with at least twofold change in expression (n = 3 for the compatible pairs and n = 2 for incompatible pairs). (B) Color-bar graph showing normalized functional categories of upregulated genes expressed during compatible and incompatible interactions (from Fig 5A). Abbreviations used for Suillus and Pinus: S. americanus (Sa), S. granulatus (Sg), and S. spraguei (Ss).P. monticola (Pm); P. strobus (Ps); P. taeda (Pt).
Gene expression patterns were analyzed among all individual Suillus-Pinus species pairs to identify common genes involved in both compatible and incompatible interactions (SI text A2; S5 Fig). A majority of Suillus transcripts (~3,800 contigs) were similarly regulated in response to different compatible Pinus species. We compared the sequence identities of these genes across all four Suillus species and identified 231 “common genes” that were upregulated during the compatible mycorrhizal interactions (Fig 3; SI text A3; S1 Dataset). In contrast to common genes expressed during compatible interaction, a smaller number of genes (261–571 genes) were found to be upregulated during incompatible interactions in different Suillus species (Fig 4A). BLASTX search against all four Suillus species only identified seven common genes expressed during incompatible mycorrhizal interactions in all species (S1 Dataset). Functional annotations of these seven common genes identified two GHs (glucoside hydrolase), one F-box, one fatty acid desaturase, one signal transduction receptor, and two genes with unknown functions.
Core functions of Suillus genes involved in host recognition
In contrast to sharing of 231 expressed genes in compatible mycorrhizal interactions, most genes associated with incompatibility were unique to individual Suillus-Pinus species pairs. These included a large number of SSPs, G-proteins, and other genes with little similarity/homology to each other or with other known genes, suggesting that these unique genes for host specificity are highly diverse at the genomic level (Fig 4A, for detailed analysis strategies see SI text A4 and S5 Fig). Unique genes varied among different plant-fungus combinations (from 68 to 571 genes for an individual pair Fig 4B), and were found to represent 14 functional groups (Fig 4B) with similar functions but very low sequence similarity to one another (S1 Dataset). Over two thirds of unique genes expressed by Suillus spp. were related to G-protein signaling, such as G-protein coupled receptor (GPCR), GTPase P-loop, Gβ WD40, and G-protein regulated kinases (Figs 4B and 5), which suggests a strong involvement of G-protein pathways in host-specific recognition. Other differentially expressed Suillus genes were those related to FAD/AND(P) binding, cytochrome P450-related, secretory, catalysis (proteinase/hydrolysis/reductase/terpene synthesis) and nucleus-associated genes.
Fig 5. Comparative gene expression of Suillus genes expressed with different Pinus hosts during compatible (Fig 6A) and incompatible (Fig 6B) mycorrhizal interactions.
Suillus genes identified from Fig 4 were grouped according to function and relative expression rate (SI text A4), and plotted as a heatmap (color coding same as in Fig 4). Suillus/Pinus species pairs with over 500 unique genes are shown in Fig 4A. Only 10 unique genes were identified from S. decipiens/Pinus pairs. These include 2 SSPs, 1 P-loop, 1 WD40 and 6 unknown; their annotations are provided (with other 3 Suillus spp. in S1 Dataset. Significance was determined by normalization of reads across pairings (using DESeq) with false discovery rate (FDR) of 5% using Benjamini-Hochberg test to identify highly expressed transcripts with at least 2-fold change. The color key shows log2 fold changes of the normalized read number. Gene expression in uninoculated P. taeda roots (“C”) is also shown. Complete read count data for all genes and treatments are shown in S1 Dataset.
Of the 261–571 contigs that were strongly upregulated in response to Suillus-Pinus incompatibility (Fig 5A), functional profiling revealed 22 to 28 contigs for shared functions related to tat signaling pathway for exporting small secreted proteins, GPI anchored proteins, fungal LRR-domain proteins, phosphatase, and pectin lyase (Figs 4B and 5B). Expression of these genes was not detected in most compatible pairings.
Putative Suillus effectors for host recognition
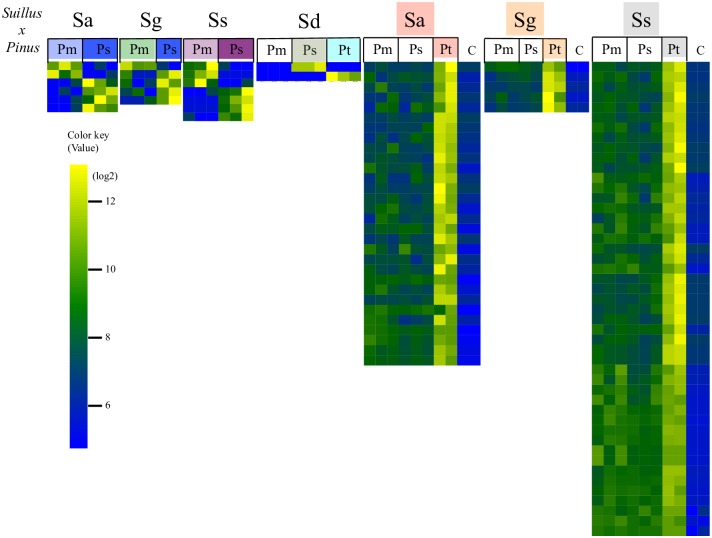
Fungal small-secreted proteins (SSPs) are predicted to be key mycorrhizal effectors for the recognition of EMF by their plant host system. Using domain analysis (SI text A4), SSPs were defined by several criteria including (a) size smaller than 300 amino acid, (b) signal peptide predicted at the N-terminal and extracellular localization activity; (c) absence of transmembrane domains; (d) absence of endoplasmic reticulum retention motifs [12]. 47 Suillus SSP's matching these criteria were upregulated in response to different Pinus hosts (Fig 6). More SSPs were upregulated during incompatible than compatible interactions. At the sequence level, most SSPs are highly diverse and do not share sequence similarity with other SSPs from currently available databases. Most Suillus SSPs were also observed to be highly diverse in their tertiary structure (S8A Fig).
Fig 6. Expression of unique Suillus small-secreted proteins (SSPs) during compatible EMF interactions with Pinus.
Heatmap shows normalized gene expression of SSPs for individual Suillus spp. (Sa, Sg, Ss, or Sd) paired with different Pinus spp. Each gene was significantly overexpressed in one of the pair combinations as determined by comparisons with FDR<0.05 using Benjamini-Hochberg test. Gene expression in uninoculated P. taeda roots (“C”) is also shown. See S1 Dataset for complete gene annotations and read counts.
Unique genes of Pinus associated with Suillus recognition
Comparative transcriptional profiling of Pinus genes across the Suillus-root pairs also identified a large number of pine transcripts with similar expression in response to compatible vs. incompatible EMF pairings (~18,000 contigs; S10 Fig). Overall, a smaller number of Pinus genes (from 253–5452 contigs) were differentially expressed in response to pairings with different species of Suillus. The largest number of upregulated genes was observed for Pinus-S. spraguei interactions compared to other compatible pairs, suggesting the possibility of a greater Pinus response to S. spraguei.
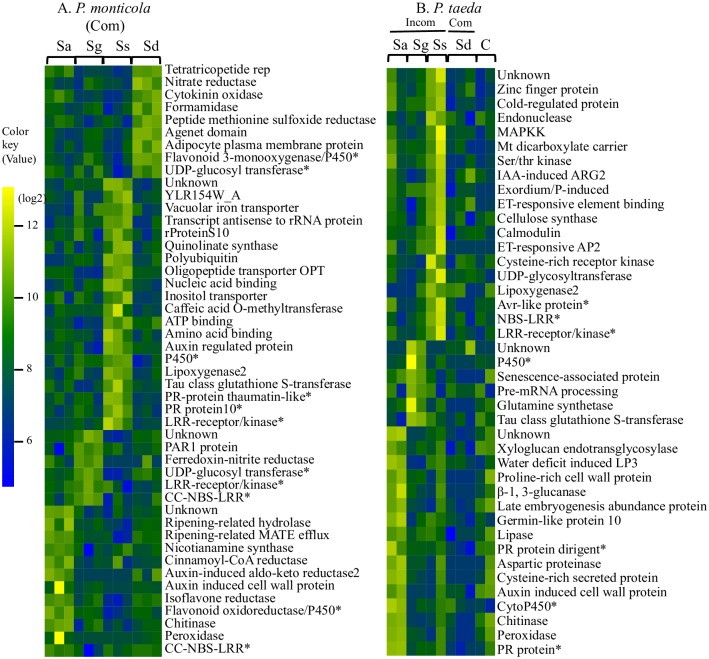
Highly expressed Pinus genes with at least two-fold change (FDR<0.05) were further characterized as “pine unique genes” involved in fungal recognition (expressed by individual Pinus spp. in response to specific species of Suillus) (Fig 7). On average, 20 Pinus contigs were identified as unique genes for every Suillus-pair sample. BLASTX annotation identified sets of unique Pinus genes with common function involved in Suillus recognition, including genes for leucine rich (LRR)- proteins, UDP-glucosyl transferase, and cytochrome P450. Inoculation with S. spraguei also upregulated distinct Pinus genes encoding lipoxygenase 2, suggesting a potential effect on JA pathways for the Pinus-S. spraguei interaction.
Fig 7. Relative expression of Pinus monticola (A) and Pinus taeda (B) functional gene groups in response to individual Suillus species (Sa, Sg, Ss and Sd).
Gene expression in uninoculated P. taeda roots (“C” in Fig 7B) is also shown. Significance was determined using normalized read counts with FDR<0.05 using Benjamini-Hochberg test. Unique functional genes shared across all pairings are marked by an asterisk. Unique Pinus genes were further characterized by functional annotation (SI text A4).
Two different sets of P. taeda genes were found to be expressed during incompatible response including fungal species-specific (Fig 7) and species-nonspecific genes (S11 Fig). Comparative transcriptomic analysis also captured changes in expression patterns of 460 P. taeda genes associated with incompatibility, but these do not appear to be Suillus species-specific (S11A Fig). A number of Pinus genes known to be associated with defense responses were only weakly or not expressed in compatible pairings and uninoculated roots, including genes involved in plant resistance and water stress response including genes for salicylic acid acquired resistance (NDR1), ethylene-responsive transcription factor and RNA helicase, leucine rich proteins (e.g. Cf2.1, receptor kinase), thaumatin-like proteins, dehydrin and water deficit induced-LP3 (S11B Fig).
Discussion
Comparative metatranscriptomic profiling of compatible vs. incompatible Pinus-Suillus interactions reveals several novel aspects of ectomycorrhizal symbiosis: (a) Suillus are transcriptionally active under both compatible and incompatible reactions; (b) Suillus spp. vary in their host specificity with different species of Pinus; (c) Suillus spp. share common sets of genes expressed during compatible and incompatible responses with different Pinus spp.; (d) Individual Pinus-Suillus species pairings induce expression of unique gene sets including genes for small secreted proteins (SSPs)/G-protein signaling pathway (Suillus genes) and LRR/PR proteins (Pinus genes).
We hypothesize that the shared functions among “common genes” contribute to a common role in core mechanisms of host-recognition. In contrast, “unique genes” may be involved in recognition between individual Suillus-Pinus species pairs. During incompatible interactions, these unique genes are largely associated with host recognition, specificity, and incompatibility.
Common genes of Suillus
We identified 231 common genes expressed during compatible mycorrhizal interactions (9 out of 12 Suillus-Pinus species pairings, Fig 3). In contrast, comparative analysis revealed only 7 common genes expressed during incompatible interactions between all three white-pine specialists when paired with loblolly pine (P. taeda). These findings suggest that different Suillus spp. share a common set of genes involved in compatible but not in incompatible responses. These estimates are likely to be higher, however, since our strategy employing de novo assembly and annotation could not detect less abundantly expressed genes without much deeper sequencing or access to a high quality reference genome. Further mapping of compatible/incompatible gene sets to fully-sequenced reference genomes of Suillus and Pinus is likely to reveal additional shared common genes involved in compatible/incompatible interactions.
Host-specific EMF interactions between Pinus and Suillus utilize conserved gene regulatory pathways
We were able to identify several sets of “unique genes” expressed by individual Suillus-Pinus species pairs. Most of these belong to gene regulatory networks associated with host-recognition, and include SSPs and G-proteins of Suillus, and LRR proteins of Pinus. During pathogenic fungal-plant interactions, fungal SSPs are recognized with high specificity by plant LRR-protein receptors [35]. Most Suillus SSPs that we detected are species-specific and lack sequence similarity to other known proteins. In plants, LRR-containing R proteins are able to recognize unique SSPs produced by their fungal pathogens [36,37]. Our study provides evidence for the involvement of SSP-LRR interactions during ectomycorrhizal symbiosis between Suillus and Pinus. Up-regulation of unique fungal SSP and plant LRR genes suggests that EMF and their plant hosts utilize a similar recognition system (SSP-LRR recognition) for species-specific interaction as other pathogenic fungal-plant interaction [6,7,38]. In addition to expression of SSP-LRR genes, upregulation of Pinus genes for jasmonic acid/ethylene (JA/ET) pathways during incompatible interactions (Fig 7 and S10B Fig) also suggests the involvement of these pathways in EMF symbiosis. Interestingly, expression of genes for salicylic acid (SA) mediated pathway that is associated with plant-defense in other hosts such as Populus [39] were not observed during EMF compatibility between Suillus and Pinus.
Fungal G-protein pathways are predicted to have a number of important roles including mating compatibility and pathogenicity [40,41]. Recent evidence for expansion of gene families for WD40-domain proteins and GTPase α in L. bicolor [42,43] also suggests the involvement of G-protein pathway in EMF symbiosis. Detailed characterization of several representative unique genes of Suillus and Pinus are illustrated below.
Unique genes of Suillus
Comparative transcriptomics allowed us to identify several novel Suillus genes involved in EMF symbiosis, including both common as well as unique genes which may share similar functions (e.g. small secreted proteins, G-protein signalling). Coexpression of multiple common and unique genes during EMF-plant symbiosis suggests that many of the unique genes collectively contribute to host specificity. Involvement of multiple unique genes from an individual functional group might explain how species of Suillus exhibit different degrees of host specificity. Gain or loss of function of individual unique genes for Suillus might also result in host-range expansion or restriction. Further studies are necessary to confirm the function of these genes during EMF symbiosis.
In addition to their role in host-specificity, structural analyses suggest a diversity of functional roles for SSPs in Suillus. None of the SSPs we detected had importin α-dependent nuclear localization signals which are necessary for import into the plant nucleus. Many Suillus SSPs do contain a N-glyco motif indicating their role in extracellular activity and cell-cell interactions (examples shown in S9A Fig). Several SSPs identified from S. granulatus were found to contain a short N-terminal motif, RXLR, which has been reported involved in host-cell translocation and phosphatidylinositol 3-phosphate binding [44]. Based upon tertiary structure prediction, most of the Suillus SSPs show no significant sequence similarity to proteins of known structure using protein prediction tools (Phyre2 and I-TASSER) (examples shown in S9A Fig). Many Suillus SSPs do contain one or two L-shaped alpha-helix folds (examples shown in S9A Fig) similar to the structure observed in some avirulence (Avr) genes [45]. L-shaped alpha-helical structures are asymmetric, and are found in diverse proteins that are involved in protein-protein interaction [46]. These characteristics suggest internalization of SSPs into plant cells. Overall, most SSPs identified from Suillus appear species-specific, diverse in their sequence structure, and lacking sequence similarity to known proteins, making it difficult to characterize their function. Further study is needed to understand the molecular basis of SSP function in these mycorrhizal plant systems.
There is a clear requirement for G-protein signaling in host-recognition by Suillus. Over 100 contigs, more than 40% of Suillus “unique genes” in each pair, encode proteins associated with G-protein transduction, including G-protein coupled receptor (GPCR)-like proteins, heterotrimeric GTPases, and kinases. GPCRs, the cell surface proteins, enable Suillus to respond to a variety of extracellular cues and transmit the signals through Gα/Gβγ, and activate downstream effectors. Besides GPCR, a large proportion of gene counts (over 15% of “unique genes”, representing approximately 30 contigs for each pair) were detected with alpha helix transmembrane domains that are likely act as host-specific receptors (Fig 4B and S9B Fig). Although we could not predict which kind of signals these membrane proteins detect, the redundancy of their protein structures in different Suillus spp. (SI text A5, S9B Fig) suggests their potential to perceive a structurally diverse set of compounds released from specific hosts at the symbiotic interface.
Possible downstream responses triggered by G-protein pathways are still unclear. Several “unique genes” for nucleus activities (e.g. DNA helicase and specific transcriptional factors) were identified across different pair samples, hinting at the possibility that G-protein pathways are involved in transcriptional control of EMF symbiosis. In addition to genes for signal transduction, we also identified “unique genes” that regulate enzyme activities of FAD/NAD(P) binding, cytochrome P450, proteinase, glycoside hydrolase and terpene synthase, indicating involvement of certain metabolic pathways in host adaptation. It is unclear whether those enzymes are also regulated by G-protein mediated signaling.
Unique genes of Pinus
At least 20 fungal-specific gene groups were identified from different Pinus spp. (Fig 7). Most of these genes encode proteins known to be directly or indirectly involved in plant defense responses (S1 Dataset). Based on nucleotide sequences, distinct genes for plant LRR-proteins were strongly upregulated in response to inoculation with different species of Suillus, suggesting their function in mediating EMF recognition. These LRR-containing proteins include receptor-like kinase or CC-NBS type, often referred to as “R” (resistance) proteins that mediate recognition of fungal effectors. Our study identified several species-specific LRR-proteins in Pinus that are involved in compatible and incompatible reactions. In addition, a number of enzymes for metabolites (e.g. flavonoids) were uniquely identified in Suillus-Pinus specific pairs. It is unclear whether these enzymes are involved in downstream of LRR-fungal interactions or if parallel interactions could be involved in species-specific recognition.
In summary, metatranscriptomic analyses show that Suillus and Pinus exploit a conserved communication system between symbiotic fungi and their hosts (the "symbiosis tool kit" described by Kohler, et al. [10]). We can envision the following scenario leading to mycorrhiza development between compatible Suillus-Pinus species pairs: 1) During early stages of mycorrhizal initiation, Suillus spp. interact with Pinus roots via small secreted proteins and host-specific G-protein signaling; 2) At the same time, the Pinus host expresses unique sets of plant receptors (e.g. LRR-proteins) that allow the plant to recognize and interact with its EMF; 3) During compatible interactions, plant-fungal recognition is followed by nutrient exchange between the plant and its EMF. Continued adaptation and coevolution between plant/fungal unique genes is predicted to result in different host-specificity outcomes. Altered recognition by the same core system may also result in incompatibility between symbionts.
Suillus genes associated with incompatibility
In addition to common functions of Suillus that regulate the host-specific recognition across the pairs, comparative transcriptomic analysis also identified genes that displayed a distinctive transcriptional response to incompatible hosts. Those not only include transcripts that are absent in compatible pairings (e.g. twin-arginine translocation (tat) pathway, GPI anchored protein, fungal LRR-domain proteins, phosphatase, alpha/beta hydrolase, and pectin lyase) but also genes with three to over 6,000x significantly higher gene count in response to incompatibility (e.g. small-secreted proteins and membrane protein/receptor) (S1 Dataset). Protein domain analysis provides additional evidence for the role of these genes in plant recognition (S9 Fig), indicating active communication by Suillus with both compatible and incompatible hosts. Though mycorrhizae are not established during incompatible interactions (Fig 1A and S1 Fig), gene products associated with incompatible response suggest that Suillus communicates with its pine host. Further analyses of the initial stages of symbiosis are needed to identify more “unique genes” involved in host-recognition, including transiently expressed genes during early stages of ectomycorrhiza formation.
Suillus host specificity and compatibility with white pines
Our results confirm and extend earlier reports of host-range and specificity for Suillus spp. that fruit under white pine forests [24,25,47]. Two EMF species we tested (S. decipiens and S. hirtellus) showed broad host compatibility and were able to form ectomycorrhizae with all 10 different Pinus spp. (Fig 1B). S. decipiens and S. hirtellus are reported to fruit under loblolly pine (P. taeda) and other 2-needle pines [24]. An ecological association between S. hirtellus with P. taeda is also supported by environmental metagenomic sequences from other pine forest soils which were able to detect S. hirtellus within the soil metagenomic community [14,15]. In contrast, S. granulatus, S. spraguei and S. americanus formed abundant ectomycorrhizae only with white pine hosts (P. strobus and P. monticola) and were less compatible or else failed to form ectomycorrhizae with hard pines (Fig 1B). All three specialist Suillus species were also detected under white pine sites using next generation amplicon sequencing [15], though at least one species (S. spraguei) was also detected under jack pine forests (P. banksiana) (S2 Fig).
EMF host compatibility deduced from laboratory experiments may not always reflect compatibility under field conditions [23,48]. Many Suillus species that fruit under a single pine host in nature may still form mycorrhizae with unrelated hosts. For example, Palm and Stewart [48] were able to experimentally synthesize ectomycorrhizae between several Suillus spp. grown with two different pine hosts (P. banksiana and P. strobus), though not every combination was successful. Both of these pines belong to different sections of the genus Pinus (Trifoliae and Qunquefoliae) that diverged 85 MYA ([49], suggesting that other environmental and biological factors also contribute to 'ecological specificity' between Suillus spp. and their hosts [23]. Our findings suggest that specialization of Suillus spp. on white pines at least is under genetic control, and involves mutual signaling between EMF and plant host.
Detection of fungal transcripts during compatible and incompatible Suillus-Pinus pairings suggests that mycorrhizal compatibility is not solely due to failure of basidiospores to germinate, but is the product of bidirectional communication between mycorrhizal fungi and their plant host. Our results also shed some light on the process of host-switching and adaptation by Suillus with different Pinaceae hosts. Phylogenetic studies of Suillus reveal that host specificity with white pines has arisen independently several times [27]. For example, S. decipiens and S. spraguei are each sister clades within the Suillus genus, yet they exhibit different levels of host specificity with different sections of the genus Pinus. Similar patterns of white-pine specialization among unrelated EMF suggest that host specificity is likely the result of very recent genome evolution targeting similar genes for mycorrhizal compatibility. Although our study did not examine intraspecific variation that is known to occur in many Suillus species [50, 51], our use of genetically diverse basidiospores from separate fruit-bodies as inoculum (instead of a pure-culture of a single fungal strain) reflects the way most pines are colonized in nature, and suggests that host-specificity in this instance is largely due to species-level characteristics (instead of within-population variation).
Host specificity of pathogenic fungi vs. EMF
The ability of individual fungal strains to recognize specific plant hosts depends upon specific genes that distinguish compatible fungi from closely related fungal taxa. Results from previous studies on host-specificity in plant-pathogens [52,53] and mutualists [29] suggest that all fungi utilize common molecular mechanisms to recognize compatible/incompatible host plants [12]. The primary gene group involved in this mechanism are the SSPs, key signaling effectors that target the plant apoplast or cytosol, which are recognized by LRR receptors of host plants (host/non-host determination) [1,7]. Successful activation by fungal SSPs results in modulation of plant defenses (compatibility determination), and reprogramming of the host cell metabolism to provide plant nutrients to the fungal symbiont [54]. Similar to biotrophic and nectrotrophic plant pathogenic fungi, ectomycorrhizal fungi appear to employ SSPs to initiate interactions with their plant hosts. Secretion of SSP by biotrophic pathogens can alter SA-triggered immune system [52]; in contrast to EMF, they may primarily target the JA/ET-mediated immunity of their host plants [2;39, this study]. Moreover, plants can trigger a hypersensitive response (localized plant cell death) in response to the SSPs secreted by the biotrophic fungi. We have also observed partial necrosis of root tissues during incompatible Suillus-Pinus pairings (Fig 2), which may be similar to the hypersensitive response, though additional functional studies are needed to test this hypothesis.
Unlike interactions between pathogens and plants that ultimately lead to host cell degradation or death, mutualistic fungi such as EMF must interact with and help to maintain health with cells of their host plant. Thus, the “symbiotic tool kit” for fungal mutualisms must be very different from the “pathogenic tool kit”. The common genes identified from these studies suggesting that over 3,500 key genes that are required for symbiosis between Suillus with Pinus (Fig 5A).
Analysis of EMF mycorrhizae using RNA-seq
In this study we demonstrated how comparative metatranscriptomics enables in-depth exploration of key genes involved in ectomycorrhiza establishment between specific plant-fungus species pairs. These strategies employing next generation sequencing of metatranscriptomes and de novo gene assembly offers a practical solution for the study of plant-fungal interactions in other plant-fungal systems where reference genomes may be unavailable.
Materials and Methods
Field study and Suillus-Pinus pair bioassay
To study the distribution of Suillus in natural Pinaceae forest soils, next generation sequencing was conducted to identify fungal operational taxonomic units (OTUs) of Suillus from the soils collected in Pinaceae forests across the North America. Technical details and data source to generate S1 Fig can be found in Talbot et al. [15]. For mycorrhizal plant bioassays, seeds of different Pinus species were purchased from Sheffield’s Seed Co., Inc. (Locke, NY) (see S3 Table for detailed description). The seeds were surface sterilized in 10% bleach for 10 min, suspended in sterilized water overnight and stratified at 4°C for different time periods prior to germination. Germinated seedlings were planted in sterilized sand and watered using sterile water. Basidiospores of different Suillus spp. were collected as spore deposits from field-collected fruit bodies by placing pilei overnight on wax paper or aluminum foil. Fruit body collection data are given in SI text A6.
A Suillus-Pinus pairwise bioassay was conducted using basidiospore inoculations with six-week old pine seedlings. Ten Pinus species were crossed with five Suillus species for a total of 50 pairwise combinations (replicated three times). Basidiospores (106 spores) were suspended in sterile water with 0.1% Tween-20, and added to sterilized 400 g of autoclaved sand to fill a four inch pot. Seedlings growing in sterile sand (without inoculum) were used as controls for all experiments (and also to check for airborne growth chamber contamination). Seedlings were grown in a growth chamber at 25°C, 80% humidity and fluorescent light at 200 μmol for 12 hours per day. At 180-d post-inoculation, EMF root tips were visualized under a dissection microscope, and percentage of EMF root tips were counted in comparison with bare (uninoculated) root tips.
Sampling
Root tips were harvested from the bioassay pots at 90-d post-inoculation. From each plant, 10 root tips were collected using forceps, frozen in liquid N2 and stored in -80°C for RNA extraction. Four species of Suillus (S. americanus; S. granulatus; S. spraguei (= S. pictus); S. decipiens) and three species of Pinus (P. monticola; P. strobus; P. taeda) were grown in all 12 pairwise combinations (each replicated three times). Root tips collected from uninoculated Pinus species were also included as controls. The controls included six samples for a total of three species of Pinus that were replications for two different seedlings for each species (Table 1).
RNA preparation, cDNA construction and Illumina sequencing
Total RNA was extracted using CTAB/chloroform extraction and LiCl precipitation method as described [32]. The mRNA samples for RNA-seq analysis were performed using a TruSeq RNA sample preparation kit (Illumina, San Diego, CA). The cDNA libraries were sequenced on the Illumina HiSeq 2000 (Illumina, San Diego, CA) instruments in Duke Center for Genomic and Computational Biology (GCB). Thirteen samples were sequenced using a single lane of Illumina run and generated 38Gb of data. The data generated from four lanes were applied for this study. The raw reads were deposited in the NCBI Short Read Archive (accession no. SRP057033).
Sequence assembly and annotation
We employed a genome-free assembly method to sort reads representing genes for different rRNA, Suillus, Pinus, and other genes (S3 and S4 Figs and SI Text A1). The computational workflow for sequence assembly (S3 Fig) was modified after Liao et al. [32]. First, Suillus sequence references were generated using the sequencing reads generated from Suillus fungal cultures, including S. americanus, S. granulatus, S. spraguei and S. decipiens. Next, de novo assembly was applied using Trinity [34]. The quality of the assembled contigs/unigenes for the four Suillus species are listed in S1 Table. The filtered reads (~28 million) were mapped onto four sets of reference sequences using bowtie with default settings (http://bowtie-bio.sourceforge.net/index.shtml), including references of fungal rRNA, 16S rRNA, contigs generated from Suillus cultures, and EST database of P. taeda. Remaining unmapped reads (approximately 3-million) were assembled de novo into contigs using Trinity followed by sorting into fungal and plant reads BlastX. Detailed descriptions of bioinformatics and databases used for three steps are included in SI text A1. The numbers of reads belonging to Suillus, Pinus, rRNA (and others) is shown in S1 Database. Comparative analysis of gene expression was used to evaluate their biological functions. The t-test (P<0.01) was used to identify the genes of Suillus in response to their compatible vs. in compatible hosts (Fig 3). A false discovery rate (FDR) of 5% was used to identify highly expressed transcripts with at least 2-fold change for the common and unique genes of Suillus and Pinus (Figs 4–7).
Transcriptome (EST) databases for S. americanus (19,123 contigs), S. granulatus (15,724 contigs), S. spraguei (18,898 contigs) and S. decipiens (16,871 contigs) were assembled de novo from fungal cultures using RNASeq. Besides the transcriptome references generated in our study (S1 Table), the other reference databases used in this study include: Fungal rRNA (NCBI, UNITE); Bacterial 16S (Ribosomal Database Project, http://rdp.cme.msu.edu); P. taeda EST database (NCBI). The databases were quality filtered using FASTA within the Galaxy web-based package. Detailed protocols for plant and fungal annotation databases are provided in SI text A2-A4.
Supporting Information
FASTQ Quality Trimmer v1.0.0 was used to trim and quality filter reads (cutoff for quality scores <28). Suillus strain IDs (in parentheses) provided for RNASeq sample ID (e.g. S6_16) and fungal strain ID (e.g. EM31). Additional information for sample IDs is described in S1 Dataset, Table 1 and S3 Table.
(DOCX)
All seeds were purchased from Sheffield's Seed Co., Inc., Locke, New York, with exception of P. muricata, which was provided by the Bruns lab, UC-Berkeley.
(DOCX)
Cultures (tissue isolates) were isolated from fresh fruit bodies on MMN media (same media used for maintaining and storing cultures). For each Suillus-Pinus species pair examined (Fig 1B), spore prints from three sporocarps (fruit bodies) were pooled and used to inoculate Pinus seedlings. Voucher sporocarp collections of each species are deposited with the Duke University fungal herbarium.
(DOCX)
OTU frequency (based on the ratio of the counts) of internal transcribed spacer (ITS) sequences of Suillus versus other fungal taxa amplified from soil samples using 454 sequencing strategies [12]. Frequency of Suillus OTUs shown by gray shading (white indicates no Suillus taxa detected). Boxes highlight co-occurrence of Suillus OTUs with P. taeda and other white pines, respectively.
(TIF)
S, fungal sheath, In, interfacial apoplast; HN, Hartig-net; Co, cortical cells (cortex); Ep, epidermis; En, endodermis; X, Xylem.
(TIF)
Detailed descriptions is given in SI text A1. DB = database; D2 = Large subunit (28S) rRNA Divergent domain 2.
(TIF)
Total number of reads after quality trimming = 28 million.
(TIF)
The detailed descriptions are indicated in SI text A2, A3 and A4. Sa, S. americanus; Sg, S. granulatus; Ss, S. spraguei; Pm, P. monticola; Ps, P. strobus; Pt, P. taeda
(TIF)
(A) Principal components analysis of loadings for different Suillus-Pinus species pairings (Suillus/P. monticola in blue; Suillus/P. strobus in red; Suillus/P. taeda in green) based on normalized expression (log10) of Suillus genes (average 12,000 contigs per sample). (B) Volcano plots showing expression of Suillus genes in response to compatible/incompatible Pinus hosts (plotted as log2 fold change versus the –log10 of the adjusted p-value). The horizontal axis is the log2 fold change between of the mean expression value of Suillus genes in different pairs. For each Suillus species, genes upregulated in response to different pine hosts are shown for white pines (P. monticola and P. strobus, red dots) or hard pine (P. taeda, green dots). Read counts of individual gene contigs are listed in S1 Dataset. Additional details of the analysis workflow are given in S3 Fig
(TIF)
The common genes of Suillus in S1 Dataset were further analyzed for their relative expression rate (SI text A3). A false discovery rate (FDR) of 5% using Benjamini-Hochberg test was used to identify highly expressed transcripts with at least 2-fold change for the genes of Suillus in compatible pairs compared to incompatible pairs, un-inoculated control and the free living mycelium (cultures). The color key shows the relative log2 fold changes of the normalized values.
(TIF)
Dots indicate the expression pattern of an individual Suillus gene from Suillus/P. monticola vs. Suillus/P. strobus pairs. The data (normalized expression rates using DESeq package) for all genes are plotted as log2 fold change versus the –log10 of the adjusted p-value. Data were generated based upon average 12,000 contigs. Differentially expressed Suillus genes (dots and the numbers of the genes) shown in response to P. monticola (green) and P. strobus (purple). Black dots represent genes with no significant difference across the comparisons. Cross-comparative expression of deferential expressed genes was analyzed using Wilcox text [13] package to compare Suillus/P. monticola vs. Suillus/P. strobus (n = 3; P<0.01; > 2-fold changes). The counts of contigs are listed in S1 Dataset.
(TIF)
Panels illustrate 20 examples of Suillus genes and their responses to different Pinus hosts. The topographical models were predicted using Protter v 1.0 (http://wlab.ethz.ch/protter/start/). For the ribbon model, the helix (pink) and sheet structures (yellow) are shown. The protein tertiary structures were predicted using I-TASSER v 3.0 [14;15;16]. C-score is a confidence score for estimating the quality of predicted models by I-TASSER (calculated based on significance of threading template alignments and the convergence parameters of the structure assembly simulations). C-score is in the range from -5 to 2, where a C-score of higher value signifies a model with a higher confidence. (A) Small-secreted protein (SSP); (B) G-protein coupled receptor like (GPCR-like). Gene Ontology = GO0007186, G-protein coupled receptor signaling pathway.
(TIF)
Within each panel, dots represent the loading of one pine gene from data sources across four root pairs, including Pinus/S. americanus, Pinus/S. granulatus, Pinus/S. spraguei, Pinus/S. decipiens (n = 3; Wilcox package [12]; P<0.01). Colored dots (red, blue, black) indicate differentially expressed unique pine genes for the samples paired with one Suillus species (labeled in the left side of the graphs) compared to other species of Suillus (blue = gene overrepresented; red = gene underrepresented). Black dots showed the expression of pine genes with no significant difference across the comparisons. SA, S. americanus; SG, S. granulatus; SS, S. spraguei; SD, S. decipiens; Control, un-inoculated roots.
(TIF)
Control, un-inoculated control (2-fold changes; FDR<0.05). The annotated genes and their normalized values are listed in S5 Dateset. (A) SA, S. americanus; SG, S. granulatus; SS, S. spraguei; SD, S. decipiens. (B) Relative expression of top 15 gene groups responsible for incompatibility and absence under compatible interactions.
(TIF)
In this study, under compatible interactions, 17M reads of Suillus were recovered from a compatible pair, however, only around 1.7M reads were recovered under incompatible pairs. To test if the normalizations for the Suillus reads for compatible and incompatible treatments are compatible, a representative sample of compatible pairs (Sa/Ps1) was used to compare the expression patterns between original reads (All data) and randomly reduced reads (Subsets). Sequence reads of three subsets (1.7M) were randomly resampled from the original reads (17M), followed by normalization using DESeq package. The BiocGenerics package was used to generate the plot showing that expression patterns of most genes were not significant different from original Suillus reads versus the three subsets of reduced reads (blue dots). Only 2 to 3 genes showed significant different in their expression patterns (red dots, P<0.01).
(TIF)
Section 1. Numbers of Illumina RNASeq reads of Suillus and Pinus genes recovered from root tip samples; Section 2. The number of contigs recovered from root samples; Section 3. Lists of interactomes identified by comparative transcriptomics (the number of unique genes showed in Fig 4A); Section 4. Published studies describing function of plant gene groups associated with plant defense response; Section 5. Pinus normalized gene expression; Section 6. Sequence counts for 28S rRNA reads (D2 region) recovered from root samples
(XLSX)
Acknowledgments
We gratefully thank Chih-Ming Hsu and Khalid Hameed for assistance with fieldwork and plant inoculations, to Xinnian Dong. Louisa Lieberman, and four external reviewers for helpful and insightful comments on earlier drafts. We also thank Mark Miller for providing Suillus spore prints. Computational support was provided through the Duke University Shared Cluster Resource. Sequencing was performed at the Duke University Center for Genomic and Computational Biology.
Data Availability
The raw reads are deposited in the NCBI Short Read Archive (accession no. SRP057033).
Funding Statement
This material is based upon work supported by the National Science Foundation under Grant No. DBI-10-46052 to RV. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dodds PN, Rathjen JP. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11:539–548. 10.1038/nrg2812 [DOI] [PubMed] [Google Scholar]
- 2.Plett JM, Kemppainen M, Kale SD, Kohler A, Legué V, Brun A, et al. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Cur Biol. 2011;21(14):1197–1203. 10.1016/j.cub.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 3.Chisholm ST, Coaker G, Day B, Staskawicz B. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124(4):803–814. 10.1016/j.cell.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 4.Rivas S, Thomas CM. Molecular interactions between tomato and the leaf mold pathogen Cladosporium fulvum. Annu Rev Phytopathol. 2005;43:395–436. 10.1146/annurev.phyto.43.040204.140224 [DOI] [PubMed] [Google Scholar]
- 5.Catanzariti AM, Dodds PN, Lawrence GJ, Ayliffe MA, Ellis JG. Haustorially expressed secreted proteins from flax rust are highly enriched for avirulence elicitors. Plant Cell. 2005;18(1):243–256. 10.1105/tpc.105.035980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shan W, Cao M, Leung D, Tyler BM. The Avr1b locus of Phytophthora sojae encodes an elicitor and a regulator required for avirulence on soybean plants carrying resistance gene Rps1b. Mol Plant Microbe Interact. 2004;17(4):394–403. 10.1094/MPMI.2004.17.4.394 [DOI] [PubMed] [Google Scholar]
- 7.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- 8.Bonardi V, Tang S, Stallmann A, Roberts M, Cherkis K, Dangl JL. Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. PNAS. 2011;108(39):16463–16468. 10.1073/pnas.1113726108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kale SD, Tyler BM. Entry of oomycete and fungal effectors into plant and animal host cells. Cell Microbiol. 2011;13(12):1839–1848. 10.1111/j.1462-5822.2011.01659.x [DOI] [PubMed] [Google Scholar]
- 10.Kohler A, Kuo A, Nagy LG, Morin E, Barry KW, Buscot F, et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics. 2015;47(4):410–415. 10.1038/ng.3223 [DOI] [PubMed] [Google Scholar]
- 11.Tisserant E, Charron P, Duensing N, dit Frey NF, Gianinazzi-Pearson V, Gilbert LB, et al. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. PNAS. 2013;110(50):20117–20122. 10.1073/pnas.1313452110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Presti, Lo L., Lanver D., Schweizer G., Tanaka S., Liang L., Tollot M., Zuccaro A., Reissmann S., and Kahmann R.. 2015. Fungal effectors and plant susceptibility. Annual Review of Plant biology 66:513–545. 10.1146/annurev-arplant-043014-114623 [DOI] [PubMed] [Google Scholar]
- 13.Tedersoo L, Smith ME. Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol Rev. 2013;27(3–4):83–99. 10.1016/j.fbr.2013.09.001 [DOI] [Google Scholar]
- 14.O’Brien HE, Parrent JL, Jackson JA, Moncalvo JM, Vilgalys R. Fungal community analysis by large-scale sequencing of environmental samples. Appl Environ Microbiol. 2005;71(9):5544–5550. 10.1128/AEM.71.9.5544-5550.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talbot JM, Bruns T, Taylor JW, Smith DP, Branco S, Glassman SI, et al. Endemism and functional convergence across the North American soil mycobiome. PNAS. 2014;111(17):6341–6346. 10.1073/pnas.1402584111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SE, Read DJ. Mycorrhizal symbiosis. 3rd edn Academic Press, London, UK; 2008. [Google Scholar]
- 17.Duplessis S, Courty PE, Tagu D, Martin F. Transcript patterns associated with ectomycorrhiza development in Eucalyptus globulus and Pisolithus microcarpus. New Phytol. 2005;165(2):599–611. 10.1111/j.1469-8137.2004.01248.x [DOI] [PubMed] [Google Scholar]
- 18.Le Quéré A, Wright DP, Söderström B, Tunlid A, Johansson T. Global patterns of gene regulation associated with the development of ectomycorrhiza between birch (Betula pendula Roth.) and Paxillus involutus (Batsch) Fr. Mol Plant-Microbe Interact. 2005;18(7):659–673. 10.1094/MPMI-18-0659 [DOI] [PubMed] [Google Scholar]
- 19.Stein E, Molitor A, Kogel K-H, Waller F. Systemic resistance in Arabidopsis conferred by the mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1. Plant Cell Physiol. 2008;49(11):1747–1751. 10.1093/pcp/pcn147 [DOI] [PubMed] [Google Scholar]
- 20.López-Ráez JA, Verhage A, Fernández I, García JM, Azcón-Aguilar C, Flors V, et al. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J Exp Bot. 2010;61(10):2589–2601. 10.1093/jxb/erq089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petre B, Saunders DG, Sklenar J, Lorrain C, Win J, Duplessis S, Kamoun S. Candidate effector proteins of the rust pathogen Melampsora larici-populina target diverse plant cell compartments. 2015, 28(6):689–700. 10.1094/MPMI-01-15-0003-R [DOI] [PubMed] [Google Scholar]
- 22.Trappe JM. Fungus associates of ectotrophic mycorrhizae. Bot Rev. 1962;28:538–606. 10.1007/BF02868758 [DOI] [Google Scholar]
- 23.Molina R, Trappe JM. Patterns of ectomycorrhizal specificity and potential among Pacific Northwest conifers and fungi. Forest Science. 1982;28(3):423–458. [Google Scholar]
- 24.Smith AH, Thiers HD. A contribution toward a monograph of North American species of Suillus. Ann Arbor. 1964. 116 p. [Google Scholar]
- 25.Moser M. Rohrlinge and Blatterpilze. 4 Auflage Band 11b der Kleinen Kryptogamenflora, ed Gams H (Stuttgart, Gustav Fischer Verlag; ); 1978. [Google Scholar]
- 26.Dahlberg A, Finlay RD. Suillus Ectomycorrhizal Fungi Key Genera in Profile, eds Cairney JWG and Cairney SM (Springer, Berlin: ), 1999. p. 33–64. [Google Scholar]
- 27.Kretzer A, Li Y, Szaro T, Bruns TD. Internal transcribed spacer sequences from 38 recognized species of Suillus sensu lato: Phylogenetic and taxonomic implications. Mycologia. 1996;88(5):776–785. 10.2307/3760972 [DOI] [Google Scholar]
- 28.Read DJ. The mycorrhizal status of Pinus Ecology and Biogeography of Pinus. ed Richardson DM (Cambridge University Press, Cambridge, U.K.), 1998. p. 324–340. [Google Scholar]
- 29.Plett J. M., Tisserant E., Brun A., Morin E., Grigoriev I. V., Kuo A., Martin F., and Kohler A.. 2015. The mutualist Laccaria bicolor expresses a core gene regulon during the colonization of diverse host plants and a variable regulon to counteract host-specific defenses. Molecular Plant-Microbe Interactions 28:261–273. 10.1094/MPMI-05-14-0129-FI [DOI] [PubMed] [Google Scholar]
- 30.Le Quéré A, Schützendübel A, Rajashekar B, Canbäck B, Hedh J, Erland S, Johansson T, and Tunlid A. Divergence in gene expression related to variation in host specificity of an ectomycorrhizal fungus. Mol Ecol. 2004;13:3809–3819. 10.1111/j.1365-294X.2004.02369.x [DOI] [PubMed] [Google Scholar]
- 31.Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N. Working with mycorrhizas in forestry and agriculture. Canberra: Australian Centre for International Agricultural Research (ACIAR); 1996. [Google Scholar]
- 32.Liao H. L., Chen Y., Bruns T. D., Peay K. G., Taylor J. W., Branco S., Talbot J. M., and Vilgalys R.. 2014. Metatranscriptomic analysis of ectomycorrhizal roots reveals genes associated with Piloderma-Pinus symbiosis: improved methodologies for assessing gene expression in situ. Environmental Microbiology … 16:3730–3742. 10.1111/1462-2920.12619 [DOI] [PubMed] [Google Scholar]
- 33.Agerer R. Ed. Colour atlas of ectomycorrhizae Einhorn-Verlag Eduard Dietenberger GmbH. Schwäbisch Gmünd: 1987–1998; pp45–47. [Google Scholar]
- 34.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat Biotechnol. 2011;29:644–652. 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Jiang N, Liu J, Liu W, Wang G-L. The role of effectors and host immunity in plant-necrotrophic fungal interactions. Virulence. 2014;5(7):722–732. 10.4161/viru.29798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bisgrove SR, Simonich MT, Smith NM, Sattler A, Lnnes RW. A disease resistance gene in Arabidopsis with specificity for two different pathogen avirulence genes. Plant Cell. 1994;6(7):927–933. 10.1105/tpc.6.7.927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao SY, Ellwood S, Calis O, Patrick E, Li T, Coleman M, et al. Broad-spectrum mildew resistance in Arabidopsis thaliana mediated by RPW8. Science. 2001;291(5501):118–120. 10.1126/science.291.5501.118 [DOI] [PubMed] [Google Scholar]
- 38.Duplessis S, Major I, Martin F, Séguin. Poplar and pathogen interactions: Insights form Populus genome-wide analyses of resistance and defense gene families and gene expression profiling. Critical Reviews in Plant Science. 2009, 28:309–334 [Google Scholar]
- 39.Plett JM, Khachane A, Ouassou M, Sundberg B, Kohler A, Martin F. Ethylene and jasmonic acid act as negative modulators during mutualistic symbiosis between Laccaria bicolor and Populus roots. New Phytol. 2014;202:270–286. 10.1111/nph.12655 [DOI] [PubMed] [Google Scholar]
- 40.Regenfelder E., Spellig T., Hartmann A., Lauenstein S., Bölker M., and Kahmann R.. 1997. G proteins in Ustilago maydis: transmission of multiple signals? The EMBO Journal 16:1934–1942. 10.1093/emboj/16.8.1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brefort T., Doehlemann G., Mendoza-Mendoza A., Reissmann S., Djamei A., and Kahmann R.. 2009. Ustilago maydis as a Pathogen. Annual review of Phytopathology 47:423–445. 10.1146/annurev-phyto-080508-081923 [DOI] [PubMed] [Google Scholar]
- 42.Martin F, Aerts A, Ahrén D, Brun A, Danchin EG, Duchaussoy F, et al. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature. 2008;452:88–93. 10.1038/nature06556 [DOI] [PubMed] [Google Scholar]
- 43.Rajashekar B., Kohler A., Johansson T., Martin F., Tunlid A. Ahrén D. Expansion of signal pathways in the ectomycorrhizal fungus Laccaria bicolor—evolution of nucleotide sequences and expression patterns in families of protein kinases and RAS GTPases. New Phytol. 2009;183(2):365–379. 10.1111/j.1469-8137.2009.02860.x [DOI] [PubMed] [Google Scholar]
- 44.Kale SD, Gu B, Capelluto DG, Dou D, Feldman E, Rumore A, et al. External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell. 2010;142(2):284–295. 10.1016/j.cell.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 45.Ve T, Williams SJ, Catanzariti AM, Rafiqi M, Rahman M, Ellis JG, et al. Structures of the flax-rust effector AvrM reveal insights into the molecular basis of plant-cell entry and effector-triggered immunity. PNAS. 2013;110(43):17594–17599. 10.1073/pnas.1307614110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burkhard P, Stetefeld J, Strelkov SV. Coiled coils: A highly versatile protein folding motif. Trends Cell Biol. 2001,(2):82–88. 10.1016/S0962-8924(00)01898-5 [DOI] [PubMed] [Google Scholar]
- 47.Hirose D, Shirouzu T, Tokumasu S. Host range and potential distribution of ectomycorrhizal basidiomycete Suillus pictus in Japan. Fungal Ecol. 2010;3(3): 255–260. 10.1016/j.funeco.2009.11.001 [DOI] [Google Scholar]
- 48.Palm ME, Stewart EL. In vitro synthesis of mycorrhizae between presumed specific and nonspecific Pinus + Suillus combinations Mycologia. 1984;76(4):579–600. 10.2307/3793215 [DOI] [Google Scholar]
- 49.Syring J, Willyard A, Cronn R, Liston A. Evolutionary relationships among Pinus (Pinaceae) subsections inferred from multiple low-copy nuclear loci. Am J Bot. 2005;92(12):2086–2100. 10.3732/ajb.92.12.2086 [DOI] [PubMed] [Google Scholar]
- 50.Branco S, Gladieux P, Ellison CE, Kuo A, LaButti K, Lipzen A, et al. Genetic isolation between two recently diverged populations of a symbiotic fungus. Mol Ecol. 2015;24(11)2747–2758. 10.1111/mec.13132 [DOI] [PubMed] [Google Scholar]
- 51.Ruytinx J, Craciun AR, Verstraelen K, Vangronsveld J, Colpaert JV, Verbruggen N. Transcriptome analysis by cDNA-AFLP of Suillus luteus Cd-tolerant and Cd-sensitive isolates. Mycorrhiza. 2011;21(3):145–54. 10.1007/s00572-010-0318-2 [DOI] [PubMed] [Google Scholar]
- 52.Koeck M, Hardham AR, Dodds PN. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell Microbiol. 2011;13(12):1849–1857. 10.1111/j.1462-5822.2011.01665.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glazebrook J. Contrasting mechanisms of defence against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005; 43:205–227. 10.1146/annurev.phyto.43.040204.135923 [DOI] [PubMed] [Google Scholar]
- 54.Plett JM, Daguerre Y, Wittulsky S, Vayssieres A, Deveau A, et al. Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. PNAS. 2014; 111(22):8299–8304 10.1073/pnas.1322671111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FASTQ Quality Trimmer v1.0.0 was used to trim and quality filter reads (cutoff for quality scores <28). Suillus strain IDs (in parentheses) provided for RNASeq sample ID (e.g. S6_16) and fungal strain ID (e.g. EM31). Additional information for sample IDs is described in S1 Dataset, Table 1 and S3 Table.
(DOCX)
All seeds were purchased from Sheffield's Seed Co., Inc., Locke, New York, with exception of P. muricata, which was provided by the Bruns lab, UC-Berkeley.
(DOCX)
Cultures (tissue isolates) were isolated from fresh fruit bodies on MMN media (same media used for maintaining and storing cultures). For each Suillus-Pinus species pair examined (Fig 1B), spore prints from three sporocarps (fruit bodies) were pooled and used to inoculate Pinus seedlings. Voucher sporocarp collections of each species are deposited with the Duke University fungal herbarium.
(DOCX)
OTU frequency (based on the ratio of the counts) of internal transcribed spacer (ITS) sequences of Suillus versus other fungal taxa amplified from soil samples using 454 sequencing strategies [12]. Frequency of Suillus OTUs shown by gray shading (white indicates no Suillus taxa detected). Boxes highlight co-occurrence of Suillus OTUs with P. taeda and other white pines, respectively.
(TIF)
S, fungal sheath, In, interfacial apoplast; HN, Hartig-net; Co, cortical cells (cortex); Ep, epidermis; En, endodermis; X, Xylem.
(TIF)
Detailed descriptions is given in SI text A1. DB = database; D2 = Large subunit (28S) rRNA Divergent domain 2.
(TIF)
Total number of reads after quality trimming = 28 million.
(TIF)
The detailed descriptions are indicated in SI text A2, A3 and A4. Sa, S. americanus; Sg, S. granulatus; Ss, S. spraguei; Pm, P. monticola; Ps, P. strobus; Pt, P. taeda
(TIF)
(A) Principal components analysis of loadings for different Suillus-Pinus species pairings (Suillus/P. monticola in blue; Suillus/P. strobus in red; Suillus/P. taeda in green) based on normalized expression (log10) of Suillus genes (average 12,000 contigs per sample). (B) Volcano plots showing expression of Suillus genes in response to compatible/incompatible Pinus hosts (plotted as log2 fold change versus the –log10 of the adjusted p-value). The horizontal axis is the log2 fold change between of the mean expression value of Suillus genes in different pairs. For each Suillus species, genes upregulated in response to different pine hosts are shown for white pines (P. monticola and P. strobus, red dots) or hard pine (P. taeda, green dots). Read counts of individual gene contigs are listed in S1 Dataset. Additional details of the analysis workflow are given in S3 Fig
(TIF)
The common genes of Suillus in S1 Dataset were further analyzed for their relative expression rate (SI text A3). A false discovery rate (FDR) of 5% using Benjamini-Hochberg test was used to identify highly expressed transcripts with at least 2-fold change for the genes of Suillus in compatible pairs compared to incompatible pairs, un-inoculated control and the free living mycelium (cultures). The color key shows the relative log2 fold changes of the normalized values.
(TIF)
Dots indicate the expression pattern of an individual Suillus gene from Suillus/P. monticola vs. Suillus/P. strobus pairs. The data (normalized expression rates using DESeq package) for all genes are plotted as log2 fold change versus the –log10 of the adjusted p-value. Data were generated based upon average 12,000 contigs. Differentially expressed Suillus genes (dots and the numbers of the genes) shown in response to P. monticola (green) and P. strobus (purple). Black dots represent genes with no significant difference across the comparisons. Cross-comparative expression of deferential expressed genes was analyzed using Wilcox text [13] package to compare Suillus/P. monticola vs. Suillus/P. strobus (n = 3; P<0.01; > 2-fold changes). The counts of contigs are listed in S1 Dataset.
(TIF)
Panels illustrate 20 examples of Suillus genes and their responses to different Pinus hosts. The topographical models were predicted using Protter v 1.0 (http://wlab.ethz.ch/protter/start/). For the ribbon model, the helix (pink) and sheet structures (yellow) are shown. The protein tertiary structures were predicted using I-TASSER v 3.0 [14;15;16]. C-score is a confidence score for estimating the quality of predicted models by I-TASSER (calculated based on significance of threading template alignments and the convergence parameters of the structure assembly simulations). C-score is in the range from -5 to 2, where a C-score of higher value signifies a model with a higher confidence. (A) Small-secreted protein (SSP); (B) G-protein coupled receptor like (GPCR-like). Gene Ontology = GO0007186, G-protein coupled receptor signaling pathway.
(TIF)
Within each panel, dots represent the loading of one pine gene from data sources across four root pairs, including Pinus/S. americanus, Pinus/S. granulatus, Pinus/S. spraguei, Pinus/S. decipiens (n = 3; Wilcox package [12]; P<0.01). Colored dots (red, blue, black) indicate differentially expressed unique pine genes for the samples paired with one Suillus species (labeled in the left side of the graphs) compared to other species of Suillus (blue = gene overrepresented; red = gene underrepresented). Black dots showed the expression of pine genes with no significant difference across the comparisons. SA, S. americanus; SG, S. granulatus; SS, S. spraguei; SD, S. decipiens; Control, un-inoculated roots.
(TIF)
Control, un-inoculated control (2-fold changes; FDR<0.05). The annotated genes and their normalized values are listed in S5 Dateset. (A) SA, S. americanus; SG, S. granulatus; SS, S. spraguei; SD, S. decipiens. (B) Relative expression of top 15 gene groups responsible for incompatibility and absence under compatible interactions.
(TIF)
In this study, under compatible interactions, 17M reads of Suillus were recovered from a compatible pair, however, only around 1.7M reads were recovered under incompatible pairs. To test if the normalizations for the Suillus reads for compatible and incompatible treatments are compatible, a representative sample of compatible pairs (Sa/Ps1) was used to compare the expression patterns between original reads (All data) and randomly reduced reads (Subsets). Sequence reads of three subsets (1.7M) were randomly resampled from the original reads (17M), followed by normalization using DESeq package. The BiocGenerics package was used to generate the plot showing that expression patterns of most genes were not significant different from original Suillus reads versus the three subsets of reduced reads (blue dots). Only 2 to 3 genes showed significant different in their expression patterns (red dots, P<0.01).
(TIF)
Section 1. Numbers of Illumina RNASeq reads of Suillus and Pinus genes recovered from root tip samples; Section 2. The number of contigs recovered from root samples; Section 3. Lists of interactomes identified by comparative transcriptomics (the number of unique genes showed in Fig 4A); Section 4. Published studies describing function of plant gene groups associated with plant defense response; Section 5. Pinus normalized gene expression; Section 6. Sequence counts for 28S rRNA reads (D2 region) recovered from root samples
(XLSX)
Data Availability Statement
The raw reads are deposited in the NCBI Short Read Archive (accession no. SRP057033).