Abstract
For several decades, we have known that epigenetic regulation is disrupted in cancer. Recently, an increasing body of data suggests epigenetics might be an intersection of current cancer research trends: next generation sequencing, immunology, metabolomics, and cell aging. The new emphasis on epigenetics is also related to the increasing production of drugs capable of interfering with epigenetic mechanisms and able to trigger clinical responses in even advanced phase patients. In this review, we will use myeloid malignancies as proof of concept examples of how epigenetic mechanisms can trigger or promote oncogenesis. We will also show how epigenetic mechanisms are related to genetic aberrations, and how they affect other systems, like immune response. Finally, we will show how we can try to influence the fate of cancer cells with epigenetic therapy.
Introduction
Over the past two decades, the connection between cancer and epigenetic regulation has been a promising venue for research. From the first evidence of the epigenetic silencing of tumor suppressor genes’ promotors, we now have a more complex and multidimensional picture, integrating several layers of (dys)regulated DNA methylation, histone modification, and micro RNA modulation. Maybe most important, epigenetic regulation has emerged as an intersection of several key hallmarks of cancer such as immunology, metabolism, or aging [1,2].
Many of these discoveries were initially described in context of hematological malignancies, and, acknowledging significant exceptions, their counterparts in solid tumors have not been so easy to demonstrate. Similarly, the benefit of epigenetic targeting has been identified in myelodysplastic syndromes and acute myeloid leukemias with the use of DNA hypomethylating agents and, to a lesser extent, histone deacetylase inhibitors. As our tools to study epigenetics progressed, our arsenal of epigenetic-targeted drugs started to expand. Hematology is at the cutting edge of research on the development of drugs targeting epigenetic regulators, including DOT1L, BET proteins, LSD1, and IDH1/2 inhibitors.
In this review, we will present the current trends in epigenetic research encompassing the biology of epigenetics, interactions with other cancer mechanisms, and drug development. Research on myeloid malignancies will be used to illustrate these different topics.
Mechanism of Epigenetics
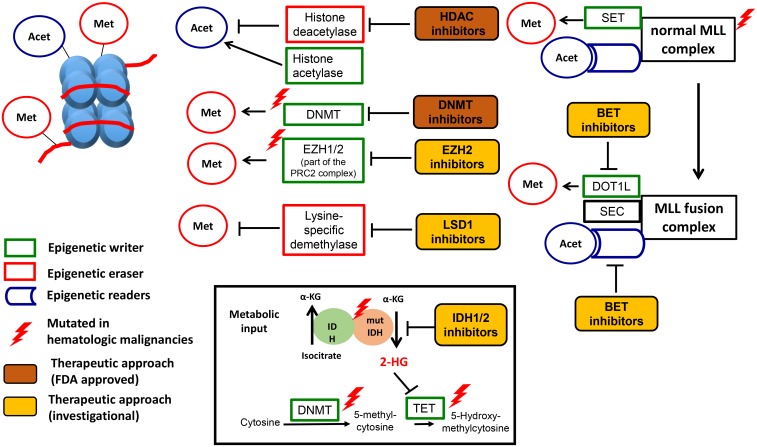
Epigenetics is defined as heritable changes in gene expression that are not due to any alteration in the DNA sequence [3,4]. Epigenetic modifications are placed by epigenetic writers and removed by erasers in a dynamic but highly regulated manner [5]. Many different DNA and histone modifications have been identified to determine the epigenetic landscape (Fig 1) [6–8].
Fig 1. Epigenetics in hematological malignancies.
Epigenetic regulation, dysregulation and therapeutic targets. DNA (red) forms a complex with histone proteins (light blue) to form nucleosomes. Each nucleosome consists of DNA wrapped around a unit of eight histone proteins. Epigenetic regulation: Epigenetic marks are placed both on the DNA and histones by epigenetic writers (in green). DNMT3A, TET2, EZH1/2, and the histone acetylase are examples of epigenetic writers. EZH2 is part of the Polycomb Repressive Complex 2 (PRC2), which also contains ASXL1, EED, SUZ12, and RBAP48. Epigenetic marks are removed by epigenetic erasers (in red), e.g., Lysine specific demythylases (LSD) and histone deacetylases (HDAC). IDH1/2 provide metabolic input by providing a-KG, which is an important substrate for the catalytic domain of other epigenetic regulators like TET2. Finally, epigenetic marks are recognized by epigenetic regulators though special reader domains (in blue), which lead to the recruitment of epigenetic regulators to DNA and histones. Examples of reader domains are the plant homeodomain (PHD) finger proteins and the bromodomain and extraterminal (BET) family of proteins. The BET family has four members, including bromodomain-containing proteins 2, 3, and 4 (BRD2, BRD3, and BRD4) and BRDT. The wild-type mixed-lineage leukemia (MLL) gene is post-translationally cleaved into N-terminal and C-terminal fragments that re-associate to form the MLL complex. The C-terminal fragment contains a SET domain, which methylates H3K4, and the N-terminal fragment contains PHD fingers and a bromodomain, which serve as reader domains. Epigenetic dysregulation: The complex epigenetic regulatory program is disturbed in hematologic malignancies by mutations in epigenetic regulators (indicated by red thunderbolt) or by the recruitment of large multi-protein complexes like the MLL fusion complex (purple circle). Translocations involving the MLL gene account for the vast majority of infantile and approximately 10% of adult leukemias. Following translocation, a fragment of the N-terminal portion of MLL is fused in frame with a translocation partner, leading to the formation of novel MLL-fusion protein complexes, including the super elongation complex (SEC) and the DOT1-Like Histone H3K79 Methyltransferase (DOT1L) complex. The DOT1L complex leads to misdirected H3K79 methylation, which has been shown to sustain the expression of key pro-leukemic genes such as the HOXA genes and MEIS1. The SEC complex phosphorylates RNA polymerase II (POL II) facilitating its recruitment to the promoters of crucial oncogenes such as MYC, BCL2, and CDK6. Metabolic dysregulation is caused by IDH1/2 mutations, which leads to the production of an abnormal metabolite in the cell, 2-hydroxyglutarate (2HG), which can inhibit the hydroxylation of 5-mC by TET2. Therapeutic targeting: Food and Drug Administration (FDA)-approved are DNMT3A inhibitors for AML and MDS and HDAC inhibitors for T cell lymphoma and multiple myeloma, respectively (in orange). Several investigational drugs (in yellow) are in different stages of preclinical and clinical development. Adopted from Semin Hematol. 2015 Jul;52(3):172–83 [8]
The DNA (5-cytosine)-methytransferases (DNMTs) add methyl groups to cytosine in CpG dinucleotides in DNA and the TET family of proteins catalyze 5-methylcytosine to 5-hydroxymethylcytosine [9–11].
Histone acetylation is associated with elevated transcription, while deacetylated histones are associated with gene repression. Acetylation removes the positive charge on the histones, which leads to a decrease in the interaction of the N termini of histones with the negatively charged phosphate groups of DNA. Subsequently condensed chromatin (heterochromatin) is transformed into a more relaxed structure (euchromatin), which leads to increased levels of gene transcription [6]. Histone acetylases and histone deacetylases (HDACs) add and remove acetyl groups from histones and are critical regulators of gene expression [12].
Methyltransferase Enhancer of Zeste Homologue 2 (EZH2) is an integral part of the polycomb repressive complex 2 (PRC2), which maintains transcriptional silencing through posttranslational histone modifications [11]. Transcriptional silencing is initiated by recruitment of PRC2, which, through EZH2, induces mono-,di-, and trimethylation of lysine 27 of histone H3 (H3K27). PRC1 recognizes H3K27me3 and mediates ubiquitylation (Ub) of lysine 119 of histone H2A (H2AK119), which is thought to lead to the recruitment of DNMTs to target loci and silencing of gene expression (Fig 1) [13].
Lastly, metabolic input is mediated by the isocitrate dehydrogenase enzyme (IDH), which catalyzes the conversion of isocitrate to alpha-ketogluatarate (α-KG) [14]. Dioxygenase enzymes, which include the TET family of enzymes and the Jumonji –C domain-containing (JMJC) family of histone lysine demethylases, are α-KG dependent enzymes (Fig 1) [15].
The application of new molecular techniques, namely, next generation sequencing (NGS) coupled with DNA methylation profiling as well as chromatin immunoprecipitation (ChIP-Seq) [16–18] and epigenome editing technology based on CRISPR-Cas9 approaches [19,20], allowed researchers to characterize the impact of epigenetic modification not only on promotors but on the entire genome [21]. Typical patterns of histone modifications exhibited at promoters and regulatory domains (insulators, enhancers, repressors) have been identified [22]. Many chromatin regulators also survey the epigenetic landscape using specialized domains to dock at specific domains within the genome, leading to recruitment of functional complexes regulating DNA transcription [5,23]. Many “writers” and “erasers” possess this chromatin “reader” ability in addition to their catalytic activity and respond to information conveyed by upstream signaling cascades. These regulators use complex three-dimensional binding pockets (e.g., bromodomain, PHD finger), which allow readers with similar binding domains to dock at different modified residues or at the same amino acid displaying a different modification state (Fig 1) [24]. The multifaceted mechanism that chromatin readers use to decipher the epigenetic landscape is exemplified by the fact that many readers have more than one reader domain, and binding to chromatin is influenced by neighboring histone modifications [5,25]. Single-cell epigenetic profiling will further promote our understanding of epigenetic regulation by addressing the issue of epigenetic heterogeneity of cancer [26].
Role in Cancer
Epigenetic dysregulation manifests in cancer with global DNA hypomethylation, causing genomic instability as well as silencing of specific tumor suppressor genes and of microRNA (miRNA) genes by hypermethylation [4,27–31].
Large-scale studies of DNA methylation have detected extensive hypomethylated genomic regions in gene-poor areas in cancer cells and demonstrated that the degree of hypomethylation of genomic DNA increases as the tumor progresses from a benign proliferation of cells to an invasive cancer [32,33].
Global hypomethylation promotes tumorigenesis by the generation of chromosomal instability (promoting chromosomal deletions and rearrangements), reactivation of transposable elements (further disrupting the genome), and loss of imprinting [34–39]. Furthermore, gene inactivation through hypermethylation of the CpG islands in the promoter region has been identified for many tumor suppressor genes, including the retinoblastoma tumor-suppressor gene (Rb), the von Hippel-Lindau tumor-suppressor gene (VHL), p16INK4a, the breast-cancer susceptibility gene 1 (BRCA1), and the MutL homolog 1 gene (hMLH1) [4,28,40–43]. Profiles of hypermethylation of the CpG islands in tumor-suppressor genes are specific to the cancer type so that each tumor can be assigned a specific, defining DNA “hypermethylome.” [44–46]. In acute myelogenous leukemia (AML), large-scale, genome-wide DNA methylation profiling reveals the existence of distinct DNA methylation patterns and identifies novel, biologically, and clinically relevant defined AML subgroups [47]. For example, the function of the basic leucine zipper transcription factor CCAAT/enhancer binding protein-α (C/EBPα), one of the crucial transcription factors for myeloid cell development, is frequently abrogated in AML by mutations but also through epigenetic modification through hypermethylation of the CEBPA promoter [48–52].
Furthermore, hypermethylation of CCCTC-binding factor (CTCF) sites has been shown to disrupt the function of insulators, which separate different genomic loops from each other [53–55]. In IDH mutated gliomas, this mechanism leads to the close interaction of FIP1L1 gene and Platelet-Derived Growth Factor Receptor, Alpha Polypeptide (PDGFRA) gene, which are normally confined to separate loop domains [53]. This allows the constitutive enhancer FIP1L1 to interact aberrantly with PDGFRA, a prominent glioma oncogene. This has not been so far demonstrated in hematological malignancies.
Importantly, epigenetic integrity itself can be disrupted in two different ways. Epigenetic regulators can be directly mutated, or they can be epigenetically modified, leading to a positive feedback and a drift from a tightly regulated epigenetic set point. This leads to a growth advantage of cancer cells [56]. In most solid tumors, epigenetic mutations are rather rare; they are mainly found in hematologic malignancies, rare childhood cancers, and highly aggressive solid tumors like glioblastoma multiforme [57]. Much of what we know about the epigenetic dysregulation in cancer has been elucidated by studying hematologic malignancies, because most direct epigenetic mutations (both in epigenetic writers/erasers and writers) are found in hematologic cancers.
Mutations Involving Epigenetic Writers/Erasers
Many mutations in epigenetic regulators have been described [10,11,58–60] (see Table 1 for detailed review of mutational frequency, mechanism, and prognostic relevance of these mutations). Mutations in regulators of DNA methylation/hydoxymethylation are found in the DNA (5-cytosine)-methytransferase 3A (DNMT3A) [11,61–64] and the TET family of proteins (Fig 1) [63,65–68].
Table 1. Mutations in epigenetic regulators in myeloid malignancies.
| Gene | Mutational frequency in myeloid malignancies | Mechanism | Impact on outcome |
|---|---|---|---|
| Mutations in DNA modifying enzymes | |||
| DNMT3A [61] |
|
DNMT3A possesses DNA methyltransferase activity, which leads to the addition of a methyl group at the 5-position of cytosine of DNA 5-methylcytosine [5mC]. DNMT3A mutations result either in premature truncation of the protein product (nonsense or frameshift mutations), or occur at a single amino acid, R882 (60% of mutations). In most cases, one DNMT3A allele remains wild-type, as haploinsufficiency seems sufficient to contribute to myeloid transformation. | Adverse risk in patients with CN-AML and FLT3-ITD mutations [62]. Improved outcome with high dose daunorubicin [63]. Single study showed adverse prognosis of DNMT3A mutations in MDS [64]. There is no known prognostic importance, if any, in patients with MPN [11,67]. |
| TET2 [65] |
|
TET2 possesses DNA dioxygenase activity, which leads to the conversion of the methyl group at the 5-position of cytosine of DNA 5- methylcytosine [5mC] to 5-hydroxy-methylcytosine [5hmC]. TET2 enzymes are dependent on Fe(II) and α-ketoglutarate (α-KG). | Adverse risk in patients with CN-AML independent from FLT3-ITD mutational status [63,66]; no clear prognostic importance in MDS and MPN [67,87]. |
| Mutations in histone modifying enzymes | |||
| EZH2 [59,68,69] |
|
EZH2 is the catalytic subunit of the PcG Repressor Complex 2 (PRC2), a highly conserved Histone H3K27 methyltransferase. EZH2 mutations have a complex role, as they result both in gain and loss of function. EZH2 may serve a dual purpose as an oncogene and tumor-suppressor gene. [69]. Biological effects of mutations unclear as EZH2 conditional knockout leads to minimal myeloid haematopoietic defects [11]. | Adverse risk in all studies to date (AML, MDS, and MPD) [11,68]. |
| ASXL1 [11,70] |
|
Unclear whether ASXL1 mutations confer a loss or gain of function. Their role in mammalian haematopoietic-specific context is not known [74,75]. | Adverse risk in patients with CN-AML, intermediate risk AML [88], and MDS [87]. Significantly associated with RUNX1 and NPM1 mutations. |
| Mutations in enzymes regulating metabolic input | |||
| IDH1/2 [14,76,77,89] |
|
IDH converts isocitrate to α-KG, which is essential for TET2 function and mutated IDH has neomorphic enzymatic activity, which converts α-KG to 2-HG (“oncometabolite”). IDH1/2 mutations share a mutual exclusivity with TET2 mutations. IDH1/2 mutations are significantly associated with NPM1 mutations. | Conflicting studies about the prognostic relevance of IDH mutations [79]. |
Mutations affecting histone modification are found in the Methyltransferase Enhancer of Zeste Homologue 2 (EZH2) [59,68,69] and the additional sex combs such as 1 transcriptional regulator (ASXL1) [11,70]. Apart from playing a role in myeloid malignancies [68,69], EZH2 mutations (at codon 641) have been found to be common in follicular and diffuse large B-cell lymphomas of germinal center origin and are a promising target in these lymphomas [71–73]. The role of ASXL1 mutations in myeloid malignancies is less well understood. ASXL1 is not thought to possess enzymatic activity [74] but may be important for the recruitment of EZH2 and the stability of the PRC2 complex as demonstrated in co-immunoprecipitation experiments (Fig 1) [75].
Mutations in IDH have been discovered first in glioblastoma and then in AML (Fig 1) [14,76–78]. IDH2 mutations at the active enzyme site at position R172 and R140 confer a gain-of-function and result in a neomorphic enzymatic activity of the mutated IDH enzyme: mutant IDH1/2 catalyzes the conversion of alpha ketoglutarate to beta-hydroxyglutarate (2-HG) [79]. Supra-normal levels of intracellular 2-HG lead to competitive inhibition of α -KG dependent epigenetic regulators like TET2 and subsequently to hypermethylation of DNA as well as histones and a blockade of cellular differentiation [80,81]. Importantly, IDH and TET2 seem to be almost entirely mutually exclusive, supporting the common mechanism of action of both mutations [82].
The discovery of IDH mutations has led to the concept that “oncometabolites” like 2-HG play a major role in tumorigenesis, further underscored by the interaction of epigenetics and metabolomics in cancer.
Apart from being mutated, epigenetic writers and erasers can be aberrantly recruited by fusion proteins, which are formed by chromosomal translocation. The fusion proteins PML-RARa and AML1-ETO found in patients with t(15;17) and t(8;21) AML are the two most prominent examples. Both fusion proteins recruit multiprotein complexes including both HDACs and DNMTs to alter transcription, repress differentiation genes, and drive leukemogenesis [83–85]. Recently, the ecotropic viral integration site 1 (EVI1), a DNA binding zinc-finger transcription factor, has been shown to direct a unique recurrent DNA methylation signature in AML by specifically recruiting DNMTs and HDACs to target promoters [86].
Indirect Effects of Epigenetic Enzyme Mutations- Impact on Epigenetic Reader Domains
Rearrangement of the Histone-lysine N-methyltransferase 2A/mixed-lineage leukemia gene (KTM2A/MLL1) is found in approximately 5% of ALL cases and around 5% to 10% of AML cases in adults. This rearrangement results in aggressive leukemia with poor prognosis and is often refractory to conventional therapies [90,91]. Central to each of the translocations seems to be abnormal transcriptional elongation involving abnormal recruitment of histone reader proteins [5,92]. Many of the common translocation partners of MLL (including AF9, ENL, AF4, and ELL) are critical members of the super elongation complex, which contains the positive transcription elongation factor b (SEC-P-TEFb complex) (Fig 1) [93–95]. The SEC-P-TEFb complex phosphorylates RNA polymerase II, facilitating transcriptional elongation, leading to transcription of crucial oncogenes like myc and bcl-2.
The SEC-P-TEFb uses “reader domains” in the form of bromodomain and extraterminal proteins (BET proteins) in order to bind to acetylated histones on chromatin. BET reader proteins can be targeted by small molecule inhibitors (BETi) (Fig 1) [96–98]. In addition to the ability of MLL1 fusion proteins to recruit transcriptional machinery such as the SEC, MLL1 rearrangement promotes gene expression by elevating local H3K79me2 levels [99,100]. The only known enzyme in mammals that catalyzes methylation of H3K79 is DOT1L (disruptor of telomeric silencing 1-like), and the MLL1 fusion proteins may directly recruit DOT1L to MLL1 fusion target loci, leading to activation of homeobox A (HOXA) cluster genes, which induce leukemic transformation of hematopoietic progenitors. Their high expression is a hallmark in MLL1 rearranged leukemias [101–103]. Currently, there are several DOT1L inhibitors in development (Fig 1) [100,104,105].
Interaction of Epigenetics with Other Hallmarks of Cancer
Recurrent genetic alterations in AML can be functionally categorized matching the hallmarks of cancer described by Hanahan and Weinberg [1,106]. Disruption in epigenetic regulation has been found to collaborate with these hallmarks in cancer development in many ways [56,107–114].
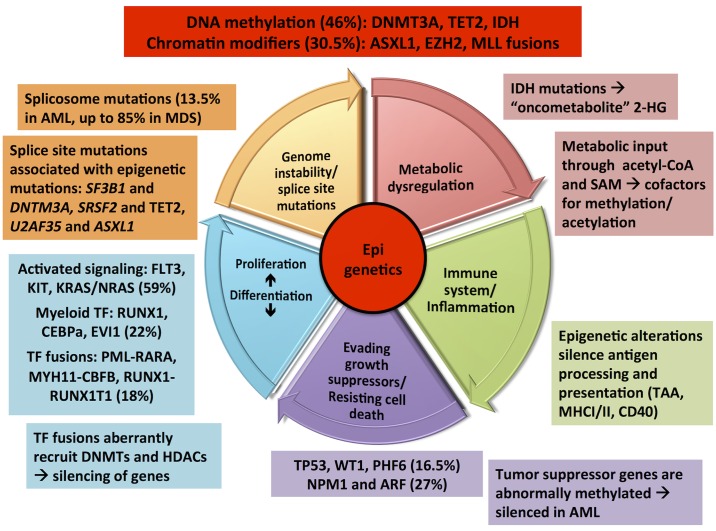
Fig 2 shows an overlay of the proposed cancer hallmarks and the recurrent genetic mutations of AML with epigenetic dysregulation at the center of these complex interactions. Certain mutations seem to collaborate while others are mutually exclusive. For example, there is a strong association between mutations in the epigenetic regulator DNMT3A, FLT3 (activating signaling), and NPM1 (tumor suppressor). DNMT3A mutations may occur early in leukemogenesis and cause genetic instability, which is prone to FLT3, NPM1 mutations. On the other hand, mutual exclusivity exists among transcription factor fusion genes, NPM1, RUNX1, TP53, and CEBPA.
Fig 2. Epigenetic dysregulation as a hallmark of cancer.
Overlay graphic synthesizing concepts from “Hallmarks of cancer: the next generation” by Hannahan and Weinberg [1] and “A panoramic view of acute myeloid leukemia [106]. Mutational frequency of different functional classes of mutations in parenthesis. Abbreviations: FLT-3: Fms-like tyrosine kinase 3; KRAS: Kirsten rat sarcoma viral oncogene homolog; NRAS: Neuroblastoma rat sarcoma viral oncogene homolog; TF: Transcription factor; RUNX1: Runt-related transcription factor 1; CEBPa: CCAAT/enhancer-binding protein alpha; PML-RARA: fusion of the promyelocytic leukemia (PML) gene on chromosome 15 to the retinoic acid receptor (RAR) gene on chromosome 17; CBFB-MYH11: chromosomal rearrangements involving the core-binding factor, beta subunit (CBFB) gene on chromosome 16p13.1 and the Myosin, heavy chain 11, smooth muscle (MYH11) gene on chromosome 16q22; RUNX1-RUNX1T1: chromosomal rearrangements involving the RUNX1 gene on chromosome 21 and the Runt-related transcription factor 1 translocated to 1 (RUNX1T1) gene on chromosome 8. TP53: Tumor protein 53; WT1: Wilms tumor protein 1; PHF6: PHD Finger Protein 6; NPM1: Nucleophosmin 1; ARF: ADP-ribosylation factor 1; TAA: Tumor associated antigen; MHC I/II: Major histocompatibility complex I/II; SAM: S-Adenosyl Methionine.
New research focuses on the intersection of cancer epigenetics and the newly characterized cancer hallmarks of cancer immunology [115], metabolism [116,117], and alternative m-RNA processing/splicing. Immunosuppressive microenvironment and epigenetic alterations are known to silence/downregulate all steps of antigen processing and presentation machinery (APM) in cancer cells, including tumor-associated antigens, human leukocyte antigens, and accessory/co-stimulatory molecules [118]. Epigenetic drugs have shown to up-regulate all the elements in the antigen presenting machinery, e.g., the expression of tumor associated antigens (TAA), MHC I and MHC II molecules as well as co-stimulatory surface markers like CD40, CD80 and ICAM1 [115,119–122]. There is significant evidence based on preclinical in vitro and in vivo models supporting combination therapy using epigenetic modulators and immunotherapy.
Prime examples of the interaction of the epigenome with metabolism are mutations in IDH/IDH2, which lead to accumulation of the oncometabolite 2-HG as well as acetyl CoA and S-adenosylmethionine (SAM), connecting nutritional status to gene expression through their role as donors/coenzymes for histone acetylation and DNA/histone methylation, respectively [123–125].
Mutations in splicing factors are observed in up to 85% of myeloid neoplasms with myelodysplastic features [126]. Chromatin structure and epigenetic histone modifications may act as key regulators of alternative splicing [127]. Histone marks are non-randomly distributed in the genome and are enriched specifically in exons relative to their flanking intronic regions [128]. There might be direct physical crosstalk between chromatin and the splicing machinery via an adaptor complex [129]. Importantly, each splice gene mutation seems to be associated with one concomitant mutation in a gene involved in epigenetic regulation of transcription. SF3B1, SRSF2 /ZRSR2, and U2AF35 mutations are enriched in patients with DNMT3A, TET2, and ASXL1, respectively [130]. On the other hand, mutations in the splicing factor genes U2AF1 and SRSF2 cause dysfunctional processing of pre-mRNA and reduced EZH2 expression [69].
The cohesin complex is important in mediating proper sister chromatid cohesion and separation from S phase to M phase in mitosis as well as in regulating transcription through genome-wide chromatin organization. Mutations of proteins of the complex are frequently found in myeloid neoplasms [131]; they collectively occur in approximately 15% of AML cases and other myeloid malignancies [132]. Leukemia-associated cohesion mutations have been found to impair differentiation and enforce stem cell programs in human stem and progenitor cells by demonstrating increased chromatin accessibility of stem cell regulators like the Runt-related transcription factor 1 (RUNX1) and GATA2 [133,134].
Epigenetic Therapy
Current Therapies
To date, epigenetic therapies have been limited to targeting epigenetic writers in the form of DNA methyltransferase inhibitors (DNMTi) and epigenetic erasers in the form of histone deacetylase inhibitors (HDACi) (Fig 1). The Food and Drug Administation (FDA) approved two DNMTi (azacitidine and decitabine) for the treatment of MDS [135] and several HDACi (vorinostat, romidepsin, belinostat, and panobinostat) for the treatment of cutaneous T-cell lymphoma and multiple myeloma [136–140], respectively. Due to their pleitropic effects, it has been difficult to confirm the mechanism of action of DNMTi and HDACi.
Emerging Future Therapies
Isoform Specific HDAC Inhibitors
Current research focuses on developing specific therapy by using isoform-specific HDACi [141]. For example, the class I HDAC inhibitor entinostat was recently awarded by the FDA a breakthrough therapy status for patients with metastatic, estrogen receptor-positive breast cancer based on data from the phase II ENCORE 301 study (NCT00676663) [142].
Novel Epigenetic-Targeted Pharmacologic Agents
Several new agents targeting epigenetic writers and erasers are in development, including EZH2 inhibitors [73,143], protein methyltransferase inhibitors (PMT inhibitors) [144], and histone lysine demethylases (KDM inhibitors) [145]. Several phase I/II clinical trials will be dedicated to studying the effect of these new agents in patients (see Table 2).
Table 2. Selection of ongoing clinical trials evaluating epigenetic targeted therapies in hematologic malignancies.
| Clinical Trial | Intervention | Malignancy studied |
|---|---|---|
| EZH2 inhibitors | ||
| NCT02395601 | Phase 1 Study: EZH2 inhibitor CPI-1205 | Progressive B-cell lymphomas |
| NCT01897571 | Phase 1/2 Study: EZH2 inhibitor E7438 | B-cell lymphomas and advanced solid tumors |
| KDM inhibitors | ||
| NCT02261779 | Phase 1/2 Study: ATRA + tranylcypromine (TCP) an irreversible monoamine-oxidase (MAO) and Lysin-specific demethylase (LSD) inhibitor | Relapsed/refractory AML |
| IDH2 inhibitors | ||
| NCT01915498 | Phase 1/2 Study: reversible inhibitor of mutant IDH2 AG-221 | Advanced hematologic malignancies with IDH2 mutation |
| NCT02273739 | Phase 1/2 Study: reversible inhibitor of mutant IDH2 AG-221 | Advanced solid tumors (glioma) and angioimmunoblastic T-cell lymphoma |
| BET1/DOT1L inhibitors | ||
| NCT01943851 | Phase 1/2 Study: BET inhibitor GSK525762 | Relapsed/refractory hematologic malignancies (leukemias, myeloproliferative neoplasms, lymphomas, and myelomas) |
| NCT02158858 | Phase 1 Study: BET inhibitor CPI-0610 | AML, myelodysplastic syndromse, myeloproliferative neoplasms, myelofibrosis |
| NCT02308761 | Phase 1 Study: BET inhibitor TEN-010 | AML, myelodysplastic syndromse |
| NCT01684150 | Phase 1 Study: second generation DOT1L inhibitor EPZ-5676 | AML/ALL/MLL with MLL1 rearrangements (including 11q23 or partial tandem duplications) in adult patients |
| NCT02141828 | Phase 1 Study: second generation DOT1L inhibitor EPZ-5676 | AML/ALL with MLL1 rearrangements (including 11q23 or partial tandem duplications) in pediatric patients |
| Combination treatment with cancer vaccines | ||
| NCT01483274 | Phase 1 study: Decitabine + donor lymphocyte infusion + Vaccine (autologous dendritic cells) | AML with relapse after allogeneic stem cell transplantation |
| Combination treatment with immune checkpoint inhibitors | ||
| NCT02281084 | Phase 2 Study: Durvalumab (PD-L1 inhibitor) + CC-486 (oral azacitidine) | Myelodysplastic syndromes |
| NCT02530463 | Phase 2 Study: Nivolumab (PD-1 inhibitor) and/or Ipilimumab (CTLA-4 inhibitor) + azacitidine | Myelodysplastic syndromes |
This list is not complete but presents a selection of clinical trials by the authors of this manuscript meant to illustrate the different strategies.
IDH inhibitors seem to be particularly promising [77,89,144]. Early results of AG-221, an inhibitor of mutant IDH2, showed that from 48 patients with advanced AML/MDS with an IDH2 mutation, 20 patients had evidence of an objective response (eight complete remissions) (see Table 2) [146,147]. Other compounds like pan IDH and IDH1 inhibitors (AG-120) are in development.
Exciting new data also comes from drugs developed to target leukemias harboring MLL1 translocations, BET inhibitors, and DOT1L inhibitors (see Table 2) [5,100,104]. The MLL fusion protein can aberrantly recruit multiprotein complexes including SEC and DOT1L, activating important oncogenic genes like HOXA cluster genes, c-myc, bcl-2, and others, but can be interrupted by targeting the reader proteins BET within SEC or DOT1L directly. Interestingly, BET inhibitors and DOT1L inhibitors are also effective in vitro in a variety of other leukemias [148] and hematologic malignancies such as multiple myeloma or Burkitt lymphoma [149,150], for which HOXA or c-myc activation are key drivers of the disease.
Combination Strategies
In combining epigenetic agents with cytotoxic chemotherapy, the reactivation of tumor-suppressor genes and restoration of DNA-repair pathways by epigenetic drugs results in more chemo-sensitive cells. These cells can then be targeted by another type of therapy [151]. Initial studies combining epigenetic agents with chemotherapy showed disappointing results [152], though further studies suggest that the timing of epigenetic therapy matters and that it might be able to reverse resistance to chemotherapy [153–156].
Initial approaches focused on combining epigenetic agents with cytokine-based immunotherapy and vaccination with tumor cells or peptide vaccines [118,157–159]. With the dawn of the checkpoint inhibitors CTLA-4 and PD-1/ PDL-1 to stimulate the immune system in solid malignancies, the combination of immune checkpoint inhibitors with epigenetic therapy has been promising in preclinical models [160–162]. Several ongoing phase-I/II clinical trials are dedicated to investigating the effect of combining epigenetic agents with immunotherapy (see Table 2) [115,161].
Discussion/Conclusion
We have come a long way in understanding the epigenetic network from the initial model of epigenetic regulation: DNA gets methylated, recruits histone deacetylases, and the two systems button down chromatin and silence expression. With the availability of NSG, CHIP-Seq, and Crisp-cas9 technologies, we now understand that epigenetics involves a complex and dynamic interplay of writers, erasers, and readers, which act not only on promoters but on many regulatory elements, including enhancers and repressors, forming a three-dimensional network of regulation.
The impact of epigenetic alterations in cancer is also complex. Many mutations in epigenetic writers, erasers, and readers have been identified as promoting cancer development. Furthermore, epigenetics has been recognized as lying at the heart of multiple hallmarks of cancer, interacting with cell cycle promotion, cancer metabolism, neo angiogenesis and the immune system. Although not able to induce leukemia [163,164], a dysregulated epigenome allows other mutations to occur, giving cancer cells a growth advantage over normal cells. Unsurprisingly, epigenetic mutations are starting to get used as biomarkers in hematologic malignancies and have been found to be associated with poor prognosis.
The parallel development of epigenetic therapy mirrors the evolution of biology. The “historical” DNMTi and HDACi are registered in hematological cancers, but their mode of action is not fully understood, while more recently, NGS-driven drug discovery has led to the development of real targeted agents focused on epigenetic writers and IDH. Several important questions have to be answered: What is the optimal duration of therapy with epigenetic agents? Several of these agents might require longer exposure to have a therapeutic effect compared to traditional cytotoxic chemotherapy and small molecule tyrosine kinase inhibitors [58,73,104,143]. Second, can we develop biomarkers to predict response to epigenetic therapy? CpG island methylation signatures have only been mildly successful in predicting therapy response [56,165,166]. Furthermore, as is the case for the majority of tumors sensitive to BET and DOT1L inhibition, epigenetic therapeutic targets are not necessarily mutated in sensitive tumor types. And although a central theme of BET inhibition seems to be c-myc downregulation, there are multiple cancer cell lines that overexpress c-myc but do not respond to BETi [5,150], as c-myc downregulation does not predict a response [96,98]. Therefore, simple mutational screening or gene expression profiling may not provide a predictor of response and might require large drug screening studies to test sensitivities [167,168]. Last, but not least, will there be a role for chemoprevention similar to using statins in heart disease? As epigenetics is recognized as a very early driver for cancer progression, this holds promise for both improved early diagnosis and therapy of cancer [56]. Will it be possible in the future to identify patients early in the course of their disease and treat even before development of overt cancer based on their epigenetic profile? There is some evidence that this might be possible in colorectal and cervical cancer [169,170]. As single mutations in epigenetic regulators cannot induce cancer on their own, single epigenetic agents will only be part of the cure. AML and cancer in general is a multi-step process, and targeting a single defect (as has been seen with Flt-3 inhibitors) is not sufficient to control cancer. In that context, one of the most promising approaches is using epigenetic therapy in combination with other therapies targeting different hallmarks of cancer, including traditional chemo- and radiation therapy as well as immunotherapy, which is currently changing the paradigm of therapy in solid malignancies [171]. Although most data are generated in vitro and in mouse models, there is evidence that the use of epigenetic drugs improves the antitumor activity of immune checkpoint inhibitors. As the overall effectiveness of immunotherapy is still far from optimal—only a minority of treated patients achieve long-term clinical benefit [172]—and there are poorly immunogenic tumors like AML, epigenetic therapy could serve as an essential part of future combination immune therapy [118]. There might be two sides to the coin in terms of pleiotropic effects of epigenetic agents: initially viewed as a weakness, it might prove to be an advantage in the light of combination therapy.
Understanding the impact that epigenetics has on cancer biology, diagnosis, and therapy is complex and fascinating and holds great promise for the future.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 3.Holliday R. The inheritance of epigenetic defects. Science. 1987;238(4824):163–70. 10.1126/science.3310230 [DOI] [PubMed] [Google Scholar]
- 4.Esteller M. Epigenetics in cancer. The New England journal of medicine. 2008;358(11):1148–59. 10.1056/NEJMra072067 [DOI] [PubMed] [Google Scholar]
- 5.Dawson MA, Kouzarides T, Huntly BJ. Targeting epigenetic readers in cancer. The New England journal of medicine. 2012;367(7):647–57. 10.1056/NEJMra1112635 [DOI] [PubMed] [Google Scholar]
- 6.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 7.Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146(6):1016–28.; PubMed Central PMCID: PMC3176443. 10.1016/j.cell.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim TK, Gore SD, Zeidan AM. Epigenetic Therapy in Acute Myeloid Leukemia: Current and Future Directions. Seminars in hematology. 2015;52(3):172–83. 10.1053/j.seminhematol.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics. 2003;33 Suppl:245–54. 10.1038/ng1089 [DOI] [PubMed] [Google Scholar]
- 10.Woods BA, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Immunol Rev. 2015;263(1):22–35. 10.1111/imr.12246 [DOI] [PubMed] [Google Scholar]
- 11.Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nature reviews Cancer. 2012;12(9):599–612. 10.1038/nrc3343 [DOI] [PubMed] [Google Scholar]
- 12.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. The Journal of clinical investigation. 2014;124(1):30–9.; PubMed Central PMCID: PMCPMC3871231. 10.1172/JCI69738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkel P, Dupret B, Le Bourhis X, Angrand PO. Diverse involvement of EZH2 in cancer epigenetics. Am J Transl Res. 2015;7(2):175–93.; PubMed Central PMCID: PMCPMC4399085. [PMC free article] [PubMed] [Google Scholar]
- 14.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer cell. 2010;17(3):225–34.; PubMed Central PMCID: PMC2849316. 10.1016/j.ccr.2010.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer cell. 2010;18(6):553–67.; PubMed Central PMCID: PMC4105845. 10.1016/j.ccr.2010.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellegrini M, Ferrari R. Epigenetic analysis: ChIP-chip and ChIP-seq. Methods in molecular biology. 2012;802:377–87. 10.1007/978-1-61779-400-1_25 [DOI] [PubMed] [Google Scholar]
- 17.Capell BC, Berger SL. Genome-wide epigenetics. The Journal of investigative dermatology. 2013;133(6):e9 10.1038/jid.2013.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Jeltsch A. The application of next generation sequencing in DNA methylation analysis. Genes (Basel). 2010;1(1):85–101.; PubMed Central PMCID: PMCPMC3960863. 10.3390/genes1010085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zentner GE, Henikoff S. Epigenome editing made easy. Nature biotechnology. 2015;33(6):606–7. 10.1038/nbt.3248 [DOI] [PubMed] [Google Scholar]
- 20.Rusk N. CRISPRs and epigenome editing. Nature methods. 2014;11(1):28 10.1038/nmeth.2775 [DOI] [PubMed] [Google Scholar]
- 21.Rando OJ, Chang HY. Genome-wide views of chromatin structure. Annual review of biochemistry. 2009;78:245–71.; PubMed Central PMCID: PMC2811691. 10.1146/annurev.biochem.78.071107.134639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–37. 10.1016/j.cell.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 23.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell research. 2011;21(3):381–95.; PubMed Central PMCID: PMC3193420. 10.1038/cr.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nature structural & molecular biology. 2007;14(11):1025–40. 10.1038/nsmb1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nature reviews Molecular cell biology. 2007;8(12):983–94. 10.1038/nrm2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bheda P, Schneider R. Epigenetics reloaded: the single-cell revolution. Trends in cell biology. 2014;24(11):712–23. 10.1016/j.tcb.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 27.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301(5895):89–92. 10.1038/301089a0 [DOI] [PubMed] [Google Scholar]
- 28.Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, Burger PC, et al. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nature medicine. 1995;1(7):686–92. 10.1038/nm0795-686 [DOI] [PubMed] [Google Scholar]
- 29.Sakai T, Toguchida J, Ohtani N, Yandell DW, Rapaport JM, Dryja TP. Allele-specific hypermethylation of the retinoblastoma tumor-suppressor gene. American journal of human genetics. 1991;48(5):880–8.; PubMed Central PMCID: PMC1683063. [PMC free article] [PubMed] [Google Scholar]
- 30.Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer cell. 2006;9(6):435–43. 10.1016/j.ccr.2006.04.020 [DOI] [PubMed] [Google Scholar]
- 31.Lujambio A, Ropero S, Ballestar E, Fraga MF, Cerrato C, Setien F, et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer research. 2007;67(4):1424–9. 10.1158/0008-5472.CAN-06-4218 [DOI] [PubMed] [Google Scholar]
- 32.Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nature genetics. 2005;37(8):853–62. 10.1038/ng1598 [DOI] [PubMed] [Google Scholar]
- 33.Fraga MF, Herranz M, Espada J, Ballestar E, Paz MF, Ropero S, et al. A mouse skin multistage carcinogenesis model reflects the aberrant DNA methylation patterns of human tumors. Cancer research. 2004;64(16):5527–34. 10.1158/0008-5472.CAN-03-4061 [DOI] [PubMed] [Google Scholar]
- 34.Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science. 2003;300(5618):455 10.1126/science.1083557 [DOI] [PubMed] [Google Scholar]
- 35.Karpf AR, Matsui S. Genetic disruption of cytosine DNA methyltransferase enzymes induces chromosomal instability in human cancer cells. Cancer research. 2005;65(19):8635–9. 10.1158/0008-5472.CAN-05-1961 [DOI] [PubMed] [Google Scholar]
- 36.Bestor TH. Transposons reanimated in mice. Cell. 2005;122(3):322–5. 10.1016/j.cell.2005.07.024 [DOI] [PubMed] [Google Scholar]
- 37.Feinberg AP. Imprinting of a genomic domain of 11p15 and loss of imprinting in cancer: an introduction. Cancer research. 1999;59(7 Suppl):1743s–6s. [PubMed] [Google Scholar]
- 38.Kaneda A, Feinberg AP. Loss of imprinting of IGF2: a common epigenetic modifier of intestinal tumor risk. Cancer research. 2005;65(24):11236–40. 10.1158/0008-5472.CAN-05-2959 [DOI] [PubMed] [Google Scholar]
- 39.Holm TM, Jackson-Grusby L, Brambrink T, Yamada Y, Rideout WM 3rd, Jaenisch R. Global loss of imprinting leads to widespread tumorigenesis in adult mice. Cancer cell. 2005;8(4):275–85. 10.1016/j.ccr.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 40.Greger V, Passarge E, Hopping W, Messmer E, Horsthemke B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet. 1989;83(2):155–8. 10.1007/BF00286709 [DOI] [PubMed] [Google Scholar]
- 41.Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(21):9700–4.; PubMed Central PMCID: PMCPMC44884. 10.1073/pnas.91.21.9700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92(7):564–9. 10.1093/jnci/92.7.564 [DOI] [PubMed] [Google Scholar]
- 43.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. The New England journal of medicine. 2003;349(21):2042–54. 10.1056/NEJMra023075 [DOI] [PubMed] [Google Scholar]
- 44.Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, et al. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nature genetics. 2000;24(2):132–8. 10.1038/72785 [DOI] [PubMed] [Google Scholar]
- 45.Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer research. 2001;61(8):3225–9. [PubMed] [Google Scholar]
- 46.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nature reviews Genetics. 2007;8(4):286–98. 10.1038/nrg2005 [DOI] [PubMed] [Google Scholar]
- 47.Figueroa ME, Lugthart S, Li Y, Erpelinck-Verschueren C, Deng X, Christos PJ, et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer cell. 2010;17(1):13–27.; PubMed Central PMCID: PMCPMC3008568. 10.1016/j.ccr.2009.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frohling S, Schlenk RF, Stolze I, Bihlmayr J, Benner A, Kreitmeier S, et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22(4):624–33. 10.1200/JCO.2004.06.060 [DOI] [PubMed] [Google Scholar]
- 49.Pabst T, Mueller BU, Zhang P, Radomska HS, Narravula S, Schnittger S, et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nature genetics. 2001;27(3):263–70. 10.1038/85820 [DOI] [PubMed] [Google Scholar]
- 50.Wouters BJ, Jorda MA, Keeshan K, Louwers I, Erpelinck-Verschueren CA, Tielemans D, et al. Distinct gene expression profiles of acute myeloid/T-lymphoid leukemia with silenced CEBPA and mutations in NOTCH1. Blood. 2007;110(10):3706–14.; PubMed Central PMCID: PMCPMC2077318. 10.1182/blood-2007-02-073486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hollink IH, van den Heuvel-Eibrink MM, Arentsen-Peters ST, Zimmermann M, Peeters JK, Valk PJ, et al. Characterization of CEBPA mutations and promoter hypermethylation in pediatric acute myeloid leukemia. Haematologica. 2011;96(3):384–92.; PubMed Central PMCID: PMCPMC3046269. 10.3324/haematol.2010.031336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figueroa ME, Wouters BJ, Skrabanek L, Glass J, Li Y, Erpelinck-Verschueren CA, et al. Genome-wide epigenetic analysis delineates a biologically distinct immature acute leukemia with myeloid/T-lymphoid features. Blood. 2009;113(12):2795–804.; PubMed Central PMCID: PMCPMC2945920. 10.1182/blood-2008-08-172387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529(7584):110–4.; PubMed Central PMCID: PMCPMC4831574. 10.1038/nature16490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang JY, Song SH, Yun J, Jeon MS, Kim HP, Han SW, et al. Disruption of CTCF/cohesin-mediated high-order chromatin structures by DNA methylation downregulates PTGS2 expression. Oncogene. 2015;34(45):5677–84. 10.1038/onc.2015.17 [DOI] [PubMed] [Google Scholar]
- 55.Hnisz D, Weintraub AS, Day DS, Valton AL, Bak RO, Li CH, et al. Activation of proto-oncogenes by disruption of chromosome neighborhoods. Science. 2016;351(6280):1454–8. 10.1126/science.aad9024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nature reviews Cancer. 2013;13(7):497–510.; PubMed Central PMCID: PMC4636434. 10.1038/nrc3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ichimura K, Pearson DM, Kocialkowski S, Backlund LM, Chan R, Jones DT, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341–7.; PubMed Central PMCID: PMCPMC2743214. 10.1215/15228517-2009-025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdel-Wahab O, Levine RL. Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood. 2013;121(18):3563–72.; PubMed Central PMCID: PMC3643757. 10.1182/blood-2013-01-451781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conway O'Brien E, Prideaux S, Chevassut T. The epigenetic landscape of acute myeloid leukemia. Adv Hematol. 2014;2014:103175; PubMed Central PMCID: PMCPMC3980839. 10.1155/2014/103175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen H, Laird PW. Interplay between the cancer genome and epigenome. Cell. 2013;153(1):38–55.; PubMed Central PMCID: PMCPMC3648790. 10.1016/j.cell.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, et al. DNMT3A mutations in acute myeloid leukemia. The New England journal of medicine. 2010;363(25):2424–33.; PubMed Central PMCID: PMCPMC3201818. 10.1056/NEJMoa1005143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thiede C. Mutant DNMT3A: teaming up to transform. Blood. 2012;119(24):5615–7. 10.1182/blood-2012-04-423905 [DOI] [PubMed] [Google Scholar]
- 63.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. The New England journal of medicine. 2012;366(12):1079–89.; PubMed Central PMCID: PMCPMC3545649. 10.1056/NEJMoa1112304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walter MJ, Ding L, Shen D, Shao J, Grillot M, McLellan M, et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia. 2011;25(7):1153–8.; PubMed Central PMCID: PMCPMC3202965. 10.1038/leu.2011.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abdel-Wahab O, Mullally A, Hedvat C, Garcia-Manero G, Patel J, Wadleigh M, et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114(1):144–7.; PubMed Central PMCID: PMCPMC2710942. 10.1182/blood-2009-03-210039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chou WC, Chou SC, Liu CY, Chen CY, Hou HA, Kuo YY, et al. TET2 mutation is an unfavorable prognostic factor in acute myeloid leukemia patients with intermediate-risk cytogenetics. Blood. 2011;118(14):3803–10. 10.1182/blood-2011-02-339747 [DOI] [PubMed] [Google Scholar]
- 67.Tefferi A, Pardanani A, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia. 2009;23(5):905–11.; PubMed Central PMCID: PMCPMC4654629. 10.1038/leu.2009.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu F, Li X. The role of histone methyltransferase EZH2 in myelodysplastic syndromes. Expert review of hematology. 2012;5(2):177–85. 10.1586/ehm.12.5 [DOI] [PubMed] [Google Scholar]
- 69.Lund K, Adams PD, Copland M. EZH2 in normal and malignant hematopoiesis. Leukemia. 2014;28(1):44–9. 10.1038/leu.2013.288 [DOI] [PubMed] [Google Scholar]
- 70.Abdel-Wahab O, Dey A. The ASXL-BAP1 axis: new factors in myelopoiesis, cancer and epigenetics. Leukemia. 2013;27(1):10–5. 10.1038/leu.2012.288 [DOI] [PubMed] [Google Scholar]
- 71.Ryan RJ, Nitta M, Borger D, Zukerberg LR, Ferry JA, Harris NL, et al. EZH2 codon 641 mutations are common in BCL2-rearranged germinal center B cell lymphomas. PLoS ONE. 2011;6(12):e28585; PubMed Central PMCID: PMCPMC3237460. 10.1371/journal.pone.0028585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nature genetics. 2010;42(2):181–5.; PubMed Central PMCID: PMCPMC2850970. 10.1038/ng.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492(7427):108–12. 10.1038/nature11606 [DOI] [PubMed] [Google Scholar]
- 74.Aravind L, Iyer LM. The HARE-HTH and associated domains: novel modules in the coordination of epigenetic DNA and protein modifications. Cell cycle. 2012;11(1):119–31.; PubMed Central PMCID: PMCPMC3272235. 10.4161/cc.11.1.18475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdel-Wahab O, Adli M, LaFave LM, Gao J, Hricik T, Shih AH, et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer cell. 2012;22(2):180–93.; PubMed Central PMCID: PMCPMC3422511. 10.1016/j.ccr.2012.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. The Journal of experimental medicine. 2010;207(2):339–44.; PubMed Central PMCID: PMCPMC2822606. 10.1084/jem.20092506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stein EM. IDH2 inhibition in AML: Finally progress? Best practice & research Clinical haematology. 2015;28(2–3):112–5. 10.1016/j.beha.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 78.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. The New England journal of medicine. 2009;360(8):765–73.; PubMed Central PMCID: PMC2820383. 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Green CL, Evans CM, Zhao L, Hills RK, Burnett AK, Linch DC, et al. The prognostic significance of IDH2 mutations in AML depends on the location of the mutation. Blood. 2011;118(2):409–12. 10.1182/blood-2010-12-322479 [DOI] [PubMed] [Google Scholar]
- 80.Wang F, Travins J, DeLaBarre B, Penard-Lacronique V, Schalm S, Hansen E, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340(6132):622–6. 10.1126/science.1234769 [DOI] [PubMed] [Google Scholar]
- 81.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer cell. 2011;19(1):17–30.; PubMed Central PMCID: PMC3229304. 10.1016/j.ccr.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaidzik VI, Paschka P, Spath D, Habdank M, Kohne CH, Germing U, et al. TET2 mutations in acute myeloid leukemia (AML): results from a comprehensive genetic and clinical analysis of the AML study group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(12):1350–7. 10.1200/JCO.2011.39.2886 [DOI] [PubMed] [Google Scholar]
- 83.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nature reviews Drug discovery. 2014;13(9):673–91. 10.1038/nrd4360 [DOI] [PubMed] [Google Scholar]
- 84.Hug BA, Lazar MA. ETO interacting proteins. Oncogene. 2004;23(24):4270–4. 10.1038/sj.onc.1207674 [DOI] [PubMed] [Google Scholar]
- 85.Rice KL, de The H. The acute promyelocytic leukaemia success story: curing leukaemia through targeted therapies. Journal of internal medicine. 2014;276(1):61–70. 10.1111/joim.12208 [DOI] [PubMed] [Google Scholar]
- 86.Lugthart S, Figueroa ME, Bindels E, Skrabanek L, Valk PJ, Li Y, et al. Aberrant DNA hypermethylation signature in acute myeloid leukemia directed by EVI1. Blood. 2011;117(1):234–41.; PubMed Central PMCID: PMCPMC3037746. 10.1182/blood-2010-04-281337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364(26):2496–506. Epub 2011/07/01.; PubMed Central PMCID: PMC3159042. 10.1056/NEJMoa1013343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Metzeler KH, Becker H, Maharry K, Radmacher MD, Kohlschmidt J, Mrozek K, et al. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytogenetically normal AML within the ELN Favorable genetic category. Blood. 2011;118(26):6920–9.; PubMed Central PMCID: PMCPMC3245212. 10.1182/blood-2011-08-368225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levis M. Targeting IDH: the next big thing in AML. Blood. 2013;122(16):2770–1. 10.1182/blood-2013-09-522441 [DOI] [PubMed] [Google Scholar]
- 90.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nature reviews Cancer. 2007;7(11):823–33. 10.1038/nrc2253 [DOI] [PubMed] [Google Scholar]
- 91.Meyer C, Schneider B, Jakob S, Strehl S, Attarbaschi A, Schnittger S, et al. The MLL recombinome of acute leukemias. Leukemia. 2006;20(5):777–84. 10.1038/sj.leu.2404150 [DOI] [PubMed] [Google Scholar]
- 92.Mohan M, Lin C, Guest E, Shilatifard A. Licensed to elongate: a molecular mechanism for MLL-based leukaemogenesis. Nature reviews Cancer. 2010;10(10):721–8. 10.1038/nrc2915 [DOI] [PubMed] [Google Scholar]
- 93.Mueller D, Garcia-Cuellar MP, Bach C, Buhl S, Maethner E, Slany RK. Misguided transcriptional elongation causes mixed lineage leukemia. PLoS Biol. 2009;7(11):e1000249; PubMed Central PMCID: PMC2774266. 10.1371/journal.pbio.1000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, et al. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Molecular cell. 2010;37(3):429–37.; PubMed Central PMCID: PMC2872029. 10.1016/j.molcel.2010.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer cell. 2010;17(2):198–212.; PubMed Central PMCID: PMC2824033. 10.1016/j.ccr.2009.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478(7370):529–33.; PubMed Central PMCID: PMC3679520. 10.1038/nature10509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–73.; PubMed Central PMCID: PMC3010259. 10.1038/nature09504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–8.; PubMed Central PMCID: PMC3328300. 10.1038/nature10334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krivtsov AV, Feng Z, Lemieux ME, Faber J, Vempati S, Sinha AU, et al. H3K79 methylation profiles define murine and human MLL-AF4 leukemias. Cancer cell. 2008;14(5):355–68.; PubMed Central PMCID: PMC2591932. 10.1016/j.ccr.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen CW, Armstrong SA. Targeting DOT1L and HOX gene expression in MLL-rearranged leukemia and beyond. Experimental hematology. 2015;43(8):673–84.; PubMed Central PMCID: PMC4540610. 10.1016/j.exphem.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer cell. 2011;20(1):66–78.; PubMed Central PMCID: PMC3329803. 10.1016/j.ccr.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Milne TA, Martin ME, Brock HW, Slany RK, Hess JL. Leukemogenic MLL fusion proteins bind across a broad region of the Hox a9 locus, promoting transcription and multiple histone modifications. Cancer research. 2005;65(24):11367–74. 10.1158/0008-5472.CAN-05-1041 [DOI] [PubMed] [Google Scholar]
- 103.Ferrando AA, Armstrong SA, Neuberg DS, Sallan SE, Silverman LB, Korsmeyer SJ, et al. Gene expression signatures in MLL-rearranged T-lineage and B-precursor acute leukemias: dominance of HOX dysregulation. Blood. 2003;102(1):262–8. 10.1182/blood-2002-10-3221 [DOI] [PubMed] [Google Scholar]
- 104.Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer cell. 2011;20(1):53–65.; PubMed Central PMCID: PMC4046888. 10.1016/j.ccr.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Daigle SR, Olhava EJ, Therkelsen CA, Basavapathruni A, Jin L, Boriack-Sjodin PA, et al. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood. 2013;122(6):1017–25.; PubMed Central PMCID: PMC3739029. 10.1182/blood-2013-04-497644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen SJ, Shen Y, Chen Z. A panoramic view of acute myeloid leukemia. Nature genetics. 2013;45(6):586–7. 10.1038/ng.2651 [DOI] [PubMed] [Google Scholar]
- 107.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nature genetics. 2009;41(12):1350–3.; PubMed Central PMCID: PMC2958040. 10.1038/ng.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Timp W, Levchenko A, Feinberg AP. A new link between epigenetic progenitor lesions in cancer and the dynamics of signal transduction. Cell cycle. 2009;8(3):383–90. 10.4161/cc.8.3.7542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fuks F, Milner J, Kouzarides T. BRCA2 associates with acetyltransferase activity when bound to P/CAF. Oncogene. 1998;17(19):2531–4. 10.1038/sj.onc.1202475 [DOI] [PubMed] [Google Scholar]
- 110.Esteve PO, Chin HG, Pradhan S. Human maintenance DNA (cytosine-5)-methyltransferase and p53 modulate expression of p53-repressed promoters. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(4):1000–5.; PubMed Central PMCID: PMC544618. 10.1073/pnas.0407729102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Campbell PM, Szyf M. Human DNA methyltransferase gene DNMT1 is regulated by the APC pathway. Carcinogenesis. 2003;24(1):17–24. 10.1093/carcin/24.1.17 [DOI] [PubMed] [Google Scholar]
- 112.Coomber BL, Yu JL, Fathers KE, Plumb C, Rak JW. Angiogenesis and the role of epigenetics in metastasis. Clinical & experimental metastasis. 2003;20(3):215–27. [DOI] [PubMed] [Google Scholar]
- 113.Lujambio A, Esteller M. How epigenetics can explain human metastasis: a new role for microRNAs. Cell cycle. 2009;8(3):377–82. 10.4161/cc.8.3.7526 [DOI] [PubMed] [Google Scholar]
- 114.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–92.; PubMed Central PMCID: PMC3894624. 10.1016/j.cell.2007.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maio M, Covre A, Fratta E, Di Giacomo AM, Taverna P, Natali PG, et al. Molecular Pathways: At the Crossroads of Cancer Epigenetics and Immunotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21(18):4040–7. 10.1158/1078-0432.CCR-14-2914 [DOI] [PubMed] [Google Scholar]
- 116.Carrer A, Wellen KE. Metabolism and epigenetics: a link cancer cells exploit. Current opinion in biotechnology. 2015;34:23–9. 10.1016/j.copbio.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaelin WG Jr., McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153(1):56–69.; PubMed Central PMCID: PMC3775362. 10.1016/j.cell.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sigalotti L, Fratta E, Coral S, Maio M. Epigenetic drugs as immunomodulators for combination therapies in solid tumors. Pharmacology & therapeutics. 2014;142(3):339–50. 10.1016/j.pharmthera.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 119.Coral S, Parisi G, Nicolay HJ, Colizzi F, Danielli R, Fratta E, et al. Immunomodulatory activity of SGI-110, a 5-aza-2'-deoxycytidine-containing demethylating dinucleotide. Cancer immunology, immunotherapy: CII. 2013;62(3):605–14. 10.1007/s00262-012-1365-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Srivastava P, Paluch BE, Matsuzaki J, James SR, Collamat-Lai G, Karbach J, et al. Immunomodulatory action of SGI-110, a hypomethylating agent, in acute myeloid leukemia cells and xenografts. Leukemia research. 2014;38(11):1332–41. 10.1016/j.leukres.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fratta E, Sigalotti L, Colizzi F, Covre A, Nicolay HJ, Danielli R, et al. Epigenetically regulated clonal heritability of CTA expression profiles in human melanoma. Journal of cellular physiology. 2010;223(2):352–8. 10.1002/jcp.22040 [DOI] [PubMed] [Google Scholar]
- 122.Fonsatti E, Nicolay HJ, Sigalotti L, Calabro L, Pezzani L, Colizzi F, et al. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2'-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(11):3333–8. 10.1158/1078-0432.CCR-06-3091 [DOI] [PubMed] [Google Scholar]
- 123.Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer discovery. 2013;3(7):730–41. 10.1158/2159-8290.CD-13-0083 [DOI] [PubMed] [Google Scholar]
- 124.Cai L, Sutter BM, Li B, Tu BP. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Molecular cell. 2011;42(4):426–37.; PubMed Central PMCID: PMC3109073. 10.1016/j.molcel.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaochar S, Tu BP. Gatekeepers of chromatin: Small metabolites elicit big changes in gene expression. Trends in biochemical sciences. 2012;37(11):477–83.; PubMed Central PMCID: PMC3482309. 10.1016/j.tibs.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478(7367):64–9. 10.1038/nature10496 [DOI] [PubMed] [Google Scholar]
- 127.Luco RF, Allo M, Schor IE, Kornblihtt AR, Misteli T. Epigenetics in alternative pre-mRNA splicing. Cell. 2011;144(1):16–26.; PubMed Central PMCID: PMCPMC3038581. 10.1016/j.cell.2010.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kolasinska-Zwierz P, Down T, Latorre I, Liu T, Liu XS, Ahringer J. Differential chromatin marking of introns and expressed exons by H3K36me3. Nature genetics. 2009;41(3):376–81.; PubMed Central PMCID: PMCPMC2648722. 10.1038/ng.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luco RF, Pan Q, Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T. Regulation of alternative splicing by histone modifications. Science. 2010;327(5968):996–1000.; PubMed Central PMCID: PMCPMC2913848. 10.1126/science.1184208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Damm F, Kosmider O, Gelsi-Boyer V, Renneville A, Carbuccia N, Hidalgo-Curtis C, et al. Mutations affecting mRNA splicing define distinct clinical phenotypes and correlate with patient outcome in myelodysplastic syndromes. Blood. 2012;119(14):3211–8. 10.1182/blood-2011-12-400994 [DOI] [PubMed] [Google Scholar]
- 131.Leeke B, Marsman J, O'Sullivan JM, Horsfield JA. Cohesin mutations in myeloid malignancies: underlying mechanisms. Exp Hematol Oncol. 2014;3:13; PubMed Central PMCID: PMCPMC4046106. 10.1186/2162-3619-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kon A, Shih LY, Minamino M, Sanada M, Shiraishi Y, Nagata Y, et al. Recurrent mutations in multiple components of the cohesin complex in myeloid neoplasms. Nature genetics. 2013;45(10):1232–7. 10.1038/ng.2731 [DOI] [PubMed] [Google Scholar]
- 133.Mazumdar C, Shen Y, Xavy S, Zhao F, Reinisch A, Li R, et al. Leukemia-Associated Cohesin Mutants Dominantly Enforce Stem Cell Programs and Impair Human Hematopoietic Progenitor Differentiation. Cell stem cell. 2015;17(6):675–88.; PubMed Central PMCID: PMCPMC4671831. 10.1016/j.stem.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mullenders J, Aranda-Orgilles B, Lhoumaud P, Keller M, Pae J, Wang K, et al. Cohesin loss alters adult hematopoietic stem cell homeostasis, leading to myeloproliferative neoplasms. The Journal of experimental medicine. 2015;212(11):1833–50.; PubMed Central PMCID: PMCPMC4612095. 10.1084/jem.20151323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Garcia-Manero G, Fenaux P. Hypomethylating agents and other novel strategies in myelodysplastic syndromes. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(5):516–23.; PubMed Central PMCID: PMC3056493. 10.1200/JCO.2010.31.0854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109(1):31–9. 10.1182/blood-2006-06-025999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(29):4485–91. 10.1200/JCO.2010.28.9066 [DOI] [PubMed] [Google Scholar]
- 138.Foss FM, Zinzani PL, Vose JM, Gascoyne RD, Rosen ST, Tobinai K. Peripheral T-cell lymphoma. Blood. 2011;117(25):6756–67. 10.1182/blood-2010-05-231548 [DOI] [PubMed] [Google Scholar]
- 139.Laubach JP, Moreau P, San-Miguel JF, Richardson PG. Panobinostat for the Treatment of Multiple Myeloma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21(21):4767–73. 10.1158/1078-0432.CCR-15-0530 [DOI] [PubMed] [Google Scholar]
- 140.O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(23):2492–9. 10.1200/JCO.2014.59.2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Balasubramanian S, Verner E, Buggy JJ. Isoform-specific histone deacetylase inhibitors: the next step? Cancer letters. 2009;280(2):211–21. 10.1016/j.canlet.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 142.Yardley D. A. I-K R, Klein P.. Results of ENCORE 301, a randomized, phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive (ER+) breast cancer progressing on a nonsteroidal aromatase inhibitor (AI). J Clin Oncol. 2011;29(suppl 27; abstr 268). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Knutson SK, Wigle TJ, Warholic NM, Sneeringer CJ, Allain CJ, Klaus CR, et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nature chemical biology. 2012;8(11):890–6. 10.1038/nchembio.1084 [DOI] [PubMed] [Google Scholar]
- 144.Kaniskan HU, Konze KD, Jin J. Selective inhibitors of protein methyltransferases. Journal of medicinal chemistry. 2015;58(4):1596–629.; PubMed Central PMCID: PMC4345896. 10.1021/jm501234a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rotili D, Mai A. Targeting Histone Demethylases: A New Avenue for the Fight against Cancer. Genes & cancer. 2011;2(6):663–79.; PubMed Central PMCID: PMC3174264. 10.1177/1947601911417976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stein Eytan M, Altman Jessica K, Collins Robert, DeAngelo Daniel J. 115 AG-221, an Oral, Selective, First-in-Class, Potent Inhibitor of the IDH2 Mutant Metabolic Enzyme, Induces Durable Remissions in a Phase I Study in Patients with IDH2 Mutation Positive Advanced Hematologic Malignancies 2014. [Google Scholar]
- 147.DiNardo C. S EM, Altman J.K., Collins R., DeAngelo D.J., Fathi A.T., et al. AG-221, an oral, selective, first-in-class, potent inhibitor of the IDH2 mutant enzyme, induced durable responses in a phase 1 study of IDH2 mutation-positive advanced hematologic malignancies. Haematol Eur Hematol Assoc Annu Meet, 100 (s1) (2015), p. 569 [abstr)]. [Google Scholar]
- 148.Deshpande AJ, Deshpande A, Sinha AU, Chen L, Chang J, Cihan A, et al. AF10 regulates progressive H3K79 methylation and HOX gene expression in diverse AML subtypes. Cancer cell. 2014;26(6):896–908.; PubMed Central PMCID: PMC4291116. 10.1016/j.ccell.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–17.; PubMed Central PMCID: PMC3187920. 10.1016/j.cell.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(40):16669–74.; PubMed Central PMCID: PMC3189078. 10.1073/pnas.1108190108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nature reviews Drug discovery. 2006;5(1):37–50. 10.1038/nrd1930 [DOI] [PubMed] [Google Scholar]
- 152.Schwartsmann G, Schunemann H, Gorini CN, Filho AF, Garbino C, Sabini G, et al. A phase I trial of cisplatin plus decitabine, a new DNA-hypomethylating agent, in patients with advanced solid tumors and a follow-up early phase II evaluation in patients with inoperable non-small cell lung cancer. Investigational new drugs. 2000;18(1):83–91. [DOI] [PubMed] [Google Scholar]
- 153.Fillmore CM, Xu C, Desai PT, Berry JM, Rowbotham SP, Lin YJ, et al. EZH2 inhibition sensitizes BRG1 and EGFR mutant lung tumours to TopoII inhibitors. Nature. 2015;520(7546):239–42.; PubMed Central PMCID: PMC4393352. 10.1038/nature14122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Balch C, Nephew KP. Epigenetic targeting therapies to overcome chemotherapy resistance. Advances in experimental medicine and biology. 2013;754:285–311. 10.1007/978-1-4419-9967-2_14 [DOI] [PubMed] [Google Scholar]
- 155.Candelaria M, Gallardo-Rincon D, Arce C, Cetina L, Aguilar-Ponce JL, Arrieta O, et al. A phase II study of epigenetic therapy with hydralazine and magnesium valproate to overcome chemotherapy resistance in refractory solid tumors. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2007;18(9):1529–38. 10.1093/annonc/mdm204 [DOI] [PubMed] [Google Scholar]
- 156.Appleton K, Mackay HJ, Judson I, Plumb JA, McCormick C, Strathdee G, et al. Phase I and pharmacodynamic trial of the DNA methyltransferase inhibitor decitabine and carboplatin in solid tumors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(29):4603–9. 10.1200/JCO.2007.10.8688 [DOI] [PubMed] [Google Scholar]
- 157.Kozar K, Kaminski R, Switaj T, Oldak T, Machaj E, Wysocki PJ, et al. Interleukin 12-based immunotherapy improves the antitumor effectiveness of a low-dose 5-Aza-2'-deoxycitidine treatment in L1210 leukemia and B16F10 melanoma models in mice. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9(8):3124–33. [PubMed] [Google Scholar]
- 158.Simova J, Pollakova V, Indrova M, Mikyskova R, Bieblova J, Stepanek I, et al. Immunotherapy augments the effect of 5-azacytidine on HPV16-associated tumours with different MHC class I-expression status. British journal of cancer. 2011;105(10):1533–41.; PubMed Central PMCID: PMC3242529. 10.1038/bjc.2011.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shen L, Ciesielski M, Ramakrishnan S, Miles KM, Ellis L, Sotomayor P, et al. Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS ONE. 2012;7(1):e30815; PubMed Central PMCID: PMC3267747. 10.1371/journal.pone.0030815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Covre A, Coral S, Nicolay H, Parisi G, Fazio C, Colizzi F, et al. Antitumor activity of epigenetic immunomodulation combined with CTLA-4 blockade in syngeneic mouse models. Oncoimmunology. 2015;4(8):e1019978; PubMed Central PMCID: PMC4570131. 10.1080/2162402X.2015.1019978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Covre A, Coral S, Di Giacomo AM, Taverna P, Azab M, Maio M. Epigenetics meets immune checkpoints. Seminars in oncology. 2015;42(3):506–13. 10.1053/j.seminoncol.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 162.Christiansen AJ, West A, Banks KM, Haynes NM, Teng MW, Smyth MJ, et al. Eradication of solid tumors using histone deacetylase inhibitors combined with immune-stimulating antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(10):4141–6.; PubMed Central PMCID: PMC3054015. 10.1073/pnas.1011037108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nature genetics. 2012;44(1):23–31.; PubMed Central PMCID: PMC3637952. 10.1038/ng.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brustle A, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488(7413):656–9.; PubMed Central PMCID: PMC4005896. 10.1038/nature11323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Silber JR, Bobola MS, Blank A, Chamberlain MC. O(6)-methylguanine-DNA methyltransferase in glioma therapy: promise and problems. Biochimica et biophysica acta. 2012;1826(1):71–82.; PubMed Central PMCID: PMC3340514. 10.1016/j.bbcan.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rodriguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nature medicine. 2011;17(3):330–9. 10.1038/nm.2305 [DOI] [PubMed] [Google Scholar]
- 167.Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483(7391):570–5.; PubMed Central PMCID: PMC3349233. 10.1038/nature11005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–7.; PubMed Central PMCID: PMC3320027. 10.1038/nature11003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299(5613):1753–5. 10.1126/science.1080902 [DOI] [PubMed] [Google Scholar]
- 170.Teschendorff AE, Widschwendter M. Differential variability improves the identification of cancer risk markers in DNA methylation studies profiling precursor cancer lesions. Bioinformatics. 2012;28(11):1487–94. 10.1093/bioinformatics/bts170 [DOI] [PubMed] [Google Scholar]
- 171.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–14. 10.1016/j.cell.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, et al. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2013;24(8):2174–80.; PubMed Central PMCID: PMC4081656. 10.1093/annonc/mdt161 [DOI] [PMC free article] [PubMed] [Google Scholar]