Abstract
Genome-wide association studies (GWAS) have identified many genetic susceptibility loci for colorectal cancer (CRC). However, variants in these loci explain only a small proportion of familial aggregation, and there are likely additional variants that are associated with CRC susceptibility. Genome-wide studies of gene-environment interactions may identify variants that are not detected in GWAS of marginal gene effects. To study this, we conducted a genome-wide analysis for interaction between genetic variants and alcohol consumption and cigarette smoking using data from the Colon Cancer Family Registry (CCFR) and the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO). Interactions were tested using logistic regression. We identified interaction between CRC risk and alcohol consumption and variants in the 9q22.32/HIATL1 (Pinteraction = 1.76×10−8; permuted p-value 3.51x10-8) region. Compared to non-/occasional drinking light to moderate alcohol consumption was associated with a lower risk of colorectal cancer among individuals with rs9409565 CT genotype (OR, 0.82 [95% CI, 0.74–0.91]; P = 2.1×10−4) and TT genotypes (OR,0.62 [95% CI, 0.51–0.75]; P = 1.3×10−6) but not associated among those with the CC genotype (p = 0.059). No genome-wide statistically significant interactions were observed for smoking. If replicated our suggestive finding of a genome-wide significant interaction between genetic variants and alcohol consumption might contribute to understanding colorectal cancer etiology and identifying subpopulations with differential susceptibility to the effect of alcohol on CRC risk.
Author Summary
Alcohol consumption and smoking are associated with CRC risk. We performed a genome-wide analysis for interaction between genetic variants and alcohol consumption and cigarette smoking to identify potential new genetic regions associated with CRC. About 8,000 CRC cases and 8,800 controls were included in alcohol-related analysis and over 11,000 cases and 11,000 controls were involved in smoking-related analysis. We identified interaction between variants at 9q22.32/HIATL1 and alcohol consumption in relation to CRC risk (Pinteraction = 1.76×10−8). If replicated our suggested finding of the interaction between genetic variants and alcohol consumption might contribute to understanding colorectal cancer etiology and identifying subpopulations with differential susceptible to the effect of alcohol on CRC risk.
Introduction
Colorectal cancer (CRC) is the third-most common cancer in men and the second most common cancer in women worldwide [1]. Both environmental and genetic factors are involved in the development of CRC [2–7]. Since 2007, genome-wide association studies (GWAS) have identified about 50 loci associated with CRC risk[8–11]. However, only a small portion of the familial aggregation of CRC is explained by these identified genetic loci, and additional variants associated with CRC susceptibility are more likely to be identified through analyses of interactions between genes and environmental risk factors [12, 13]. Single nucleotide polymorphisms (SNP) that impact only a subgroup of the population or have opposite effects in different subgroups are likely to produce weak main effects that cannot be easily detected by marginal association testing of the SNPs. However, these variants may be identified by testing for interactions between SNP and environmental risk factors (genome-wide interaction analysis) [14, 15]. These findings may provide etiologic insight into CRC and identify potentially susceptible subpopulations [14, 15].
There is compelling evidence from epidemiologic studies that alcohol consumption and cigarette smoking are associated with risk of CRC [16–25]. Both alcohol consumption and cigarette smoking influence disease risk through pathways involving multiple gene products and regulatory elements, providing potential for biological interactions [26–28]. Accordingly, alcohol consumption and smoking are important lifestyle factors to study interactions with genetic variants. In this study, we performed a genome-wide interaction analysis using the large datasets from the Colon Cancer Family Registry (CCFR) and the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO) [3] to identify SNPs that modify the effects of alcohol and smoking on CRC risk.
Results
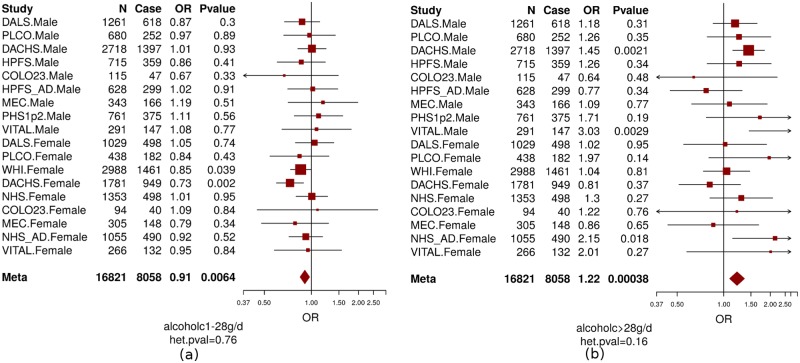
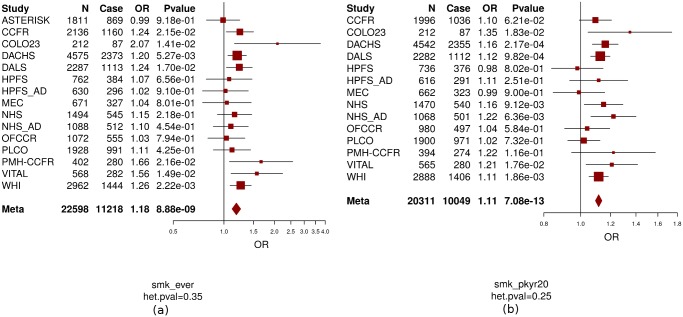
In this study, we included 14 studies from the Colon Cancer Family Registry (CCFR) and the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO) as described previously [3, 29, 30] and in the S1 Text and S1 and S2 Tables. Basic characteristics of the participants, stratified by study center, are described in S1 and S2 Tables, respectively. We were able to harmonize measures of alcohol consumption across 8,058 cases and 8,765 controls and measures of smoking across up to 11,219 cases and 11,382 controls. As seen for other common diseases, such as cardiovascular diseases, alcohol consumption shows a different effect with CRC risk depending on the level of alcohol consumed. Heavy alcohol intake (>2 standard drinks per day) has been shown to be associated with increased risk of CRC [16, 17, 31] while light-to-moderate drinking (<2 standard drinks per day) may have little effect [18, 19] or reduce risk of CRC [16, 20–22] compared to non-drinkers. Consistent with these previous publications [16–22, 31] we observed an inverse association with CRC risk for light-to-moderate drinkers (OR = 0.91, P = 0.006, Fig 1A) but a positive association for heavy drinkers (OR = 1.22, P = 0.0004, Fig 1B) compared with non-/occasional drinkers. Modeling alcohol using this categorical approach fitted the association between alcohol intake and CRC risk better than the continuous variable based on the Akaike Information Criterion (AIC) which was 12.42 smaller for the model including the two categorical variables compared with the model including the continuous variable (AIC = 23123.72 for continuous alcohol and AIC = 23111.3 for categorical alcohol)[32]. Given the opposite effect of light/moderate alcohol drinking vs. heavy drinking, it is critical that analyses further investigating the impact of alcohol on CRC, such as interaction analysis do this separately for light/moderate and heavy drinking. Ever-smokers and pack-years of cigarette smoking were positively associated with CRC risk (OR = 1.18 for ever vs. never smokers, P = 8.9×10−9; OR = 1.11 per 10 pack-years increase, P = 7.1×10−13, Fig 2A and 2B). None of the smoking and alcohol variables showed evidence of heterogeneous associations across studies (Pheterogeneity>0.16).
Fig 1. The association between CRC and alcohol consumption (non-/occasional drinkers [reference group]; light-to-moderate drinkers [a]; and heavy drinkers[b]).
Men and women were analyzed separately in each study and age and study site (if applicable) were adjusted in model. Non-/occasional drinkers: drinking < 1 gram of alcohol per day; light-to-moderate drinkers: drinking 1–28 grams of alcohol per day ([a] alcoholc1-28g/d); and heavy drinkers: drinking >28 grams of alcohol per day ([b] alcoholc>28g/d). OR: odds ratio; N = total number of subjects; case = number of cases. Colon23: Hawaii Colorectal Cancer Studies 2 and 3; DACHS: Darmkrebs: Chancen der Verhütung durch Screening; DALS: Diet, Activity and Lifestyle Study; HPFS: Health Professionals Follow-up Study; HPFS_AD: Health Professionals Follow-up Study for colorectal adenoma; MEC: Multiethnic Cohort Study; NHS: Nurses’ Health Study; NHS_AD: Nurses’ Health Study for colorectal adenoma; PHS: Physicians’ Health Study; PLCO: Prostate, Lung, Colorectal and Ovarian Cancer; Screening Trial; VITAL: VITamins And Lifestyle; WHI: Women’s Health Initiative. het.pval: p value of heterogeneity.
Fig 2. The association between CRC and smoking (ever vs. never smokers [a]; pack-years of smoking [b]).
Never smokers were assigned the value 0 for pack-years of smoking. OR: odds ratio; OR for pack-years of smoking is based on per 20 pack-years increase. Age, sex (if applicable), and study site (if applicable) were adjusted in model. ASTERISK: The French Association STudy Evaluating RISK for sporadic colorectal cancer; CCFR: Colon Cancer Family Registry; Colon23: Hawaii Colorectal Cancer Studies 2 and 3.; DACHS: Darmkrebs: Chancen der Verhütung durch Screening; DALS: Diet, Activity and Lifestyle Study; HPFS:Health Professionals Follow-up Study; HPFS_AD: Health Professionals Follow-up Study for colorectal adenoma; MEC: Multiethnic Cohort Study; NHS: Nurses’ Health Study; NHS_AD: Nurses’ Health Study for colorectal adenoma; OFCCR: Ontario Familial Colorectal Cancer Registry; PMH-CCFR: Postmenopausal Hormone study- Colon Cancer Family Registry; PLCO: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; VITAL: VITamins And Lifestyle; WHI: Women’s Health Initiative. CCFR is a collaborating study with GECCO. smk_ever: ever smokers; smk_pkyr20: pack-years of smoking; het.pval: p value of heterogeneity.
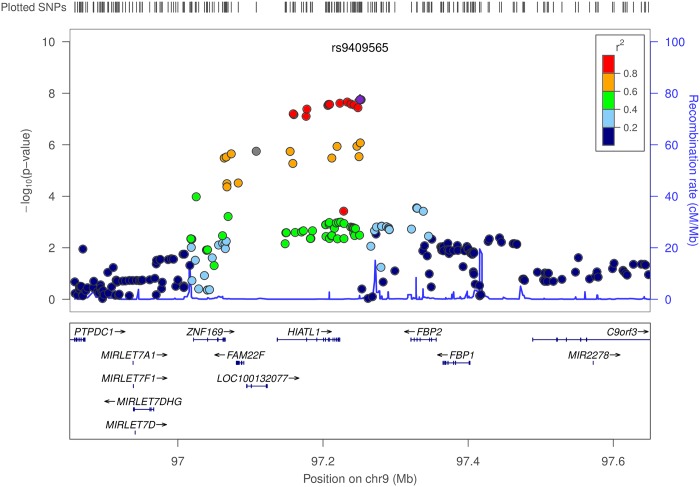
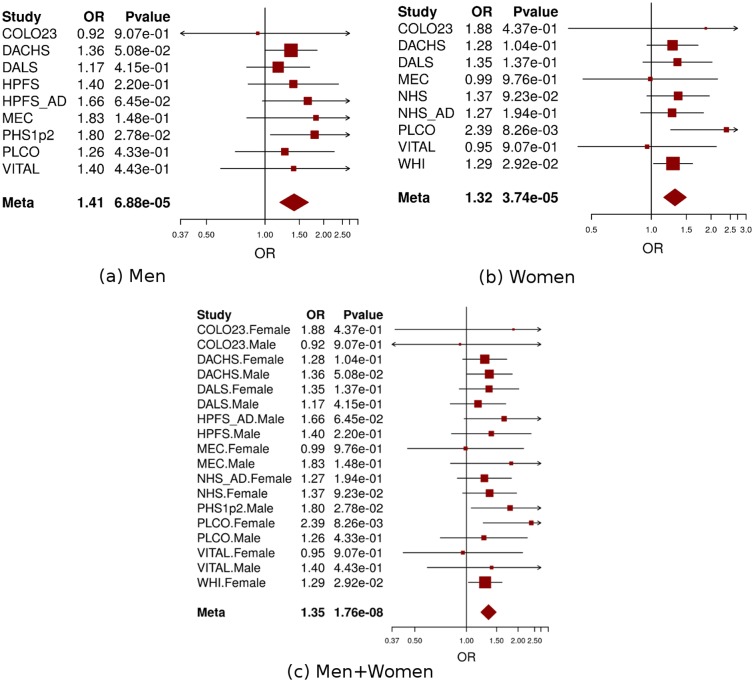
Using conventional logistic regression including multiplicative interaction terms, we identified genome-wide significant interactions (at P<5×10−8) between 11 SNPs at the 9q22.32/HIATL1 (Hippocampus Abundant Transcript-Like 1) locus and light-to-moderate drinking with no evidence of heterogeneity across studies (Pheterogeneity>0.5 for any of the 11 SNPs) (S3 Table, Fig 3). All 11 SNPs were common variants with minor allele frequency (MAF) between 0.31–0.34 and genotyped or imputed with high accuracy (imputation r2>0.98, S3 Table). The most significant SNP was rs9409565 with Pinteraction = 1.76×10−8; permuted p-value 3.51x10-8 (Table 1, Fig 4C). The genetic variant was located in an intergenic region (28kb downstream of HIATL1 and 70kb downstream of FBP2, Fig 3). All the other 10 genome-wide significant SNPs were in strong linkage disequilibrium (LD) with rs9409565 (LD r2>0.8, S3 Table, Fig 3) and some of them were located within the gene HIATL1. The observed interaction for rs9409565 was similar in men and women and by cancer site (colon vs rectum) (Fig 4A and 4B, S4 Table). We did not observe any genome-wide significant interaction between any SNP and heavy drinking. No inflation was observed in the genome-wide SNP × alcohol interaction analysis (the inflation factor λ = 0.99 and 1.00 for light-to-moderate drinkers and heavy drinkers, respectively). To evaluate the potential confounding[33] by other lifestyle and environmental risk factors of the interactions between rs9409565 and light-to-moderate alcohol consumption in relation to CRC risk, we adjusted for smoking status (ever vs. never) and BMI (two variables have the highest correlation r = 0.15 and 0.13 with alcohol consumption in our data), as well as exercise, fruit and vegetable consumption in the conventional case-control logistic regression model. Our results did not change (multivariate adjusted interaction p-value = 4.34x10-8).
Fig 3. Regional association plot for the interaction analyses between moderate alcohol drinking and SNPs at 9q22.32/HIATL1.
The–log10 of p values (left y-axis) are plotted against the SNP genomic position based on NCBI build 37 (x-axis); the estimated recombination rate from 1000 Genomes Project European populations are on the right y-axis and plotted in blue. The most significant SNP was denoted with purple diamond. SNPs are colored to reflect correlation with the most significant SNP. Gene annotations are from the UCSC genome browser. Gene FAM22F is also known as NUTM2F.
Table 1. Stratification analysesa by genotypes of rs9409565 for the association between alcohol consumption and CRC.
| Genotype | Non/occasional drinkers | Light-to-moderate drinkers | P interationb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Case (n) | Control (n) | OR | Case (n) | Control (n) | OR (95% CI) | P value | |||
| rs9409565 | CC | 1,365 | 1,593 | 1.0 | 1,638 | 1,717 | 1.11 (1.00–1.23) | 5.9E-02 | |
| CT | 1,495 | 1,574 | 1.0 | 1,646 | 2,002 | 0.82 (0.74–0.91) | 2.1E-04 | ||
| TT | 425 | 387 | 1.0 | 434 | 590 | 0.62 (0.51–0.75) | 1.3E-06 | ||
| 1.76E-08 | |||||||||
a: non/occasional drinkers as the reference group. Non-/occasional drinkers: drinking < 1 gram of alcohol per day; light-to-moderate drinkers: drinking 1–28 grams of alcohol per day. Men and women were analyzed separately in each study and age, study site (if applicable), and population structure were adjusted in model.
b: P value of interaction term between SNP and alcohol consumption, permuted p-value = 3.51x10-8 (p value of heterogeneity = 0.96).
Fig 4. Forest plot for meta-analysis of interaction analysis for rs9409565 and light-to-moderate drinking among men (a), women (b) and combined (c).
Odds ratios (ORs) and 95% confidence intervals (95% CI) are presented for the multiplicative interaction between each additional copy of the count (or tested) allele (C) and light-to-moderate vs. non/occasional drinkers. The box sizes are proportional in size to the inverse of the variance for each study, and the lines visually depict the confidence interval. Results from the fixed-effects meta-analysis are shown as diamonds. The width of the diamond represents the confidence interval. P value of heterogeneity for (a), (b), and (c) is 0.93, 0.78, and 0.96, respectively.
When stratified by genotype rs9409565, light-to-moderate alcohol consumption (compared to non/occasional alcohol consumption) significantly decreased CRC risk in individuals with CT genotype (prevalence, 45% vs 49%; OR, 0.82 [95% CI, 0.74–0.91]; P = 2.1×10−4) and TT genotype (prevalence, 42% vs 52%; OR,0.62 [95% CI, 0.51–0.75]; P = 1.3×10−6) but not in those with CC genotype (P = 0.059) (Table 1, S5 Table). The association between alcohol intake and CRC was also not heterogeneous within each genotype strata (p-heterogeneity > 0.73; S1 Fig).
We also estimated absolute risks of CRC based on Surveillance, Epidemiology, and End Results (SEER) age-adjusted incidence rates (Table 2). Compared with non/occasional drinking, light-to-moderate drinking was associated with 14.0 fewer CRC cases per 100,000 individuals carrying the rs9409565-CT genotype per year; 35.5 fewer CRC cases per 100,000 individuals carrying the rs9409565-TT genotype per year.
Table 2. Absolute riska of CRC for alcohol consumption among individuals with different genotypes of rs9409565.
| Alcohol consumption | rs9409565 genotype | ||
|---|---|---|---|
| TT | CT | CC | |
| Non/occasional drinking | 93.3 (79.9–106.5) b | 79.7 (74.0–85.3) | 69.7 (64.6–74.8) |
| Light-to-moderate drinking | 57.8 (50.1–65.43) | 65.7 (61.6–69.9) | 77.1 (72.2–82.0) |
a: Absolute risk calculation was based on Surveillance, Epidemiology, and End Results (SEER) age-adjusted CRC incidence rates between 1982–2011 among the White population of 74.5 per 100,000 men and women per year.
b: the number of CRC cases per 100,000 individuals (95% CI).
Using the Cocktail method as a two-step method that may improve power we did not observe any genome-wide significant SNP×alcohol interactions. Further, we did not observe any genome-wide significant interactions for SNP×smoking (smoking history and pack-years of smoking) using logistic regression or the Cocktail method.
Gene expression analyses
The SNP rs9409565 showing a significant interaction with alcohol is located in an intergenic region between HIATL1 and FBP2. As there is a recombination hotspot lying between rs9409565 and FPB2 (Fig 3), we focused the gene expression analysis on HIATL1, which is expressed in normal colon and rectal tissue. [34, 35] Furthermore, based on our gene expression data for 35 colorectal cancer cases (S2 Text), the expression levels of the HIATL1 gene was significantly higher in tumor tissues compared with adjacent normal tissues (paired student t test, P<7.2×10−5, S2 Fig). This finding is consistent with a previous study [36] which is included in the UCSC Cancer Genomics Browser[37–39] and show that human colon tumors (n = 100) significantly over-expressed HIATL1 compared to normal colon tissues (n = 5) [36] (Fisher exact test: P = 0.03). Similarly, we were able to reproduce this observation in 50 independent paired colorectal adenocarcinoma and adjacent normal samples from The Cancer Genome Atlas (TCGA) (paired student t test, P = 0.02, S2 Fig). Furthermore, we observed that HIATL1 showed significant differential expression across various levels of lifetime alcohol consumption in the colon tumor tissues (n = 28, ANOVA test P = 0.03, S3 Fig) and also had differential gene expression across levels of alcohol consumption at reference time (the year before enrollment) in the normal colon tissues (n = 33) at P = 0.06 from ANOVA test (S4 Fig). In addition, for rs9409565 and rs9409567 (LD r2 = 1.0 in CEU population), the two most significant SNPs at 9q22.32/HIATL1, are cis-acting quantitative trait loci (eQTL) for HIATL1 expression in lymphoblastoid cell lines (P<7.0×10−6) and monocytes (P<5.8×10−12) [40, 41], which is consistent with previously published eQTL results from GTEx, Genevar[42], Westra et al., and Lappalainen et al. showing that this these SNPs tag an eQTL locus in lymphoblastoid cells and related anatomical sources (including spleen, whole blood, esophagus muscularis, and sun-exposed skin) with p values ranging from 7x10-138 to 4x10-6 (S8 Table). In contrast, evaluation of eQTL in both normal (GTEx) and cancer colorectal tissue from TCGA for the rs9409565 locus (r2> = 0.2 in Phase 3 1000 genomes EUR data) did not show any significant eQTL. The inability to detect an eQTL is likely because the enhancer tagged by the locus is active in some but not all cancer cell lines and the current reference cancer transcriptome data may not be large enough or molecularly representative of our study population S5 Fig). Furthermore, we investigated whether any of the tagging SNPs are located in variant enhancer loci (VEL)reported by Akhtar-Zaidi et al.[43] using ChIP-seq (H3k27ac) enhancer signals. We observed that four of the variants (rs28406858, rs7042481, rs7858082, and rs9409510) in LD with rs9409565 (LD r2≥0.6) were positioned within three gained cancer-specific VEL (S6 Fig).
Discussion
We identified a suggestive interaction between variants at 9q22.32/HIATL1 and light-to-moderate alcohol consumption in relation to CRC risk. This is the first genome-wide significant GxE interaction reported for alcohol intake and risk of CRC and warrants replication in independent studies. Evidence for overlap between the discovered 9q22.32/HIATL1 region with VEL as well as gene expression results support the relevance of the 9q22.32/HIATL1 region for CRC risk.
Gene expression analyses indicated that a) SNPs identified in our study impact HIATL1 expression, b) HIATL1 is involved in signaling pathways related to CRC and expression differs between normal and tumor CR tissue, and c) HIATL1 expression in colon tissue differs by alcohol consumption. The most significant variant rs9409565 is correlated with 142 variants (LD r2≥0.5 in Phase 3 1000 Genomes European populations), which spanned across intronic regions and approximately 50kb downstream and 75kb upstream of HIATL1. Nine of these variants (including rs9409550, rs4744345, rs9409546, rs9409778, and rs639276, all with interaction P<5×10−8) fall within a transcriptionally active region in normal colon, rectal and duodenal mucosa [44] as defined by epigenetic signals.[45] Furthermore, these variants fall in a region of enriched enhancer signal; although we note that currently available ChIP-seq data are not able to identify a putative transcription factor binding site at any of the tagged SNPs (S6 Fig). In support of our findings that HIATL1 expression is higher in tumor than adjacent normal colorectal tissue, ChIP-seq (H3k27ac) enhancer signals suggest that this locus implicates a gained enhancer present in CR tumors that is absent in normal crypt cells (S6 Fig). In summary, multiple data points suggest that the genetic variants we identified to interact with alcohol on CRC risk are located in regulatory regions impacting the expression of HIATL1 and that HIATL1 expression varies by alcohol consumption.
HIATL1 is a member of the solute carrier (SLC) group of membrane transport, which enables the directed movement of substances (such as peptides, amino acids, proteins, metals, and neurotransmitters) into or out of cells and plays an important role in a variety of cellular functions [46, 47]. Although the detailed function of HIATL1 remains elusive, this gene was found to be expressed in a large range of animal species and it is highly evolutionarily conserved [48], suggesting an potentially important functional role. Transporter proteins are commonly upregulated in many cancers [49, 50] and take part in nutrient signaling to the mTOR pathway [51] which is an important signaling pathway in apoptosis and cancer [52–54]. Alcohol may modify the effects of HIATL1 on CRC risk through its influence on the gene expression of HIATL1. Nonetheless, the precise mechanism(s) of the interaction between alcohol and HIATL1 on CRC risk remains unclear and further studies are needed.
Our Cocktail method for detecting G×E interactions did not identify the statistical interaction detected by the conventional logistic regression analysis because rs9409565 did not show strong statistical evidence for association with CRC risk in the marginal association analyses (P = 0.54, OR = 1.014) or with alcohol consumption (P = 0.22). Accordingly, this SNP was ranked low in step 1 of the Cocktail method, resulting in very stringent alpha-threshold for the interaction term in step 2. Although the conventional logistic regression analysis tends to be less powerful overall for genome-wide interaction analysis compared with the Cocktail method [14, 55], it has greater power to detect an association if the marginal association of the SNP on disease or the correlation of the SNP with environmental factor are weak as it was the case for the observed interaction. In addition, no association between rs9409565 and alcohol consumption excluded the possibility that the observed interaction was due to the dependence between them [56]. We also explored the effect of rs9409565 and alcohol using other potentially more powerful single step approaches and observed a similar interaction effect in the Empirical Bayesian analysis[57] and a weaker interaction effect in the case-only analysis[58], which may be explained by the non-significant differential effect of alcohol on CRC in individual carrying the CC genotype (S6 Table).
To investigate if genome-wide interaction may help identifying variants that would be missed we looked up the marginal association of rs9409565 in the largest GWAS[59] which is about twice as large as our study and showed an OR for rs9409565 of 0.975 (95%CI 0.946–1.007, p-value 0.127). Accordingly, the variant by itself showed only weak evidence for association with CRC. This may not be surprising given that it is estimated that the sample sizes required to identify GxE interaction vs. main effects is at least 4x larger[60]. Our study has several strengths, including the large sample size, environmental exposure assessment in well-characterized populations, and standardized harmonization of environmental data across studies. Further, there is no evidence of heterogeneity across studies for our findings, indicating our results are not dominated by one or a few studies and, indeed, represent evidence across all studies. There are also some limitations. Because amassing sufficient study power for genome-wide interaction analysis is a challenge, we combined all studies in the analysis to gain the greatest power[61] instead of dividing studies into discovery and replication sets. Although we do not have a replication set, the consistency of our findings across all studies and the independent evidence from different types of gene expression data and bioinformatics analyses support a novel interaction for CRC risk between alcohol intake and variants in the 9q22.32/HIATL1 region. Our analyses focused on current alcohol consumption, rather than lifetime alcohol use, which may cause misclassification of a certain portion of alcohol users. Both differential and non-differential misclassifications of alcohol consumption levels tend to lead to underestimation of interaction parameters (e.g. leading to non-significant interaction term between SNP and alcohol intake) [62], accordingly, we may have missed some true interactions. However, it is unlikely that this led to false positives for the interactions observed. Because, there is no strong evidence that the type of alcohol (usually defined as wine, beer and hard liquor) has a differential impact on CRC[63] we have not investigated interaction between genetic variants and type of alcohol. As we preformed genome-wide interaction testing for two environmental risk factors (smoking and alcohol consumption), additional adjustment for multiple comparisons may be needed. However, we note that the observed interaction at 9q22.32/HIATL1 would remain borderline significant (alpha threshold = 5×10−8/2 = 2.5×10−8). The small numbers of heavy drinkers, particular in women, impeded the reliable estimation of interaction parameters and limited our power to identify significant interaction between SNP and heavy drinking. We focused gene expression analysis on HIATL1 because rs9409565 is located in an intergenic region between HIATL1 and FBP2 and further there is a recombination hotspot lying between rs9409565 and FPB2. If we expand gene expression analyses for all genes 500kb upstream or downstream 500kb of rs9409565 in the 35 pairs of colorectal tumor-normal tissue samples (S2 Text) we observed no significant result after false discovery rate (FDR) correction. The most significant results were for MIRLET7F which has a p value of 0.001 for testing differential gene expression across various levels of lifetime alcohol consumption in normal tissues and PTPDC1 which has a p value of 0.002 for testing differential gene expression across various levels of alcohol consumption at reference time. Further studies are needed to confirm our findings.
Alcohol has a particularly detrimental effect on several cancers, possibly including CRC, in Asian subpopulations with genetic determined alcohol sensitivity[64–66]. However, as we have focused our analysis on European descent populations and did not observe significant differences of the alcohol-CRC association between studies (phet = 0.16–0.76) we do not expect major underlying differences of the effect of alcohol in our study populations.
We did not perform stratification analyses by anatomical sites for our genome-wide GxE interaction analysis because the association of CRC with alcohol consumption (S7 Table) and smoking [23] did not vary according to anatomical site within the large bowel. Although we did observe potential interactions for alcohol consumption, we did not observe statistical evidence for genome-wide SNP x smoking interactions. This may be because smoking has a weaker association with CRC compared with alcohol intake [24, 26, 67], so we may have been underpowered even with more than 10,000 cases and 10,000 controls. We also may not have properly captured the most relevant smoking variables, such as duration of smoking or time since quitting smoking. The association between smoking and CRC risk are strongest for tumors that display certain molecular features such as microsatellite instability (MSI)-high and CpG island methylator phenotype (CIMP)-positive [68, 69]. Because of the lack of MSI or CIMP data in several studies, we cannot perform stratification analysis by tumor characteristics for smoking-related analyses.
We note that it would be too early to make any recommendation on alcohol intake from our findings even after independent replication given that such recommendation need to be considered in context of the effect of alcohol on all diseases. Furthermore, it will be important to investigate the interactions between alcohol and genetic variants in larger studies to comprehensively evaluate the full impact of genetic variation on the effect of alcohol on colorectal cancer risk.
In summary, we identified a tentative novel interaction for CRC risk between alcohol intake and variants at 9q22.32/HIATL1. Further replication and functional studies are required to confirm our findings and understand the biologic implications of the interaction. This, in turn, could provide further insight into CRC etiology and may identify potentially susceptible subpopulations.
Materials and Methods
Ethics statement
The overall project was reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Review Board (approval number: 6501 and 3995). Each study was approved by the local IRB [University of Hawaii Human Studies Program (Colo23 and MEC); University of Utah Institutional Review Board (DALS); Partners Human Research Committee (NHS and PHS); Harvard School of Public Health Institutional Review Board (HPFS); Fred Hutchinson Cancer Research Center Institutional Review Board (VITAL, overall study); Ethics Committee of the Medical Faculty of the University of Heidelberg (DKFZ); NCI Special Studies Institutional Review Board (PLCO)]. For each participating study, participants or the next of kin in the case of deceased participants, provided either written informed consent to participate (Colo23, DACHS, DALS, MEC, PHS, PLCO, VITAL, WHI) or they provided implied written consent by the return of the mailed questionnaires (NHS, HPFS). Additional consent to review medical records was obtained through signed written consent.
Study population
We included 14 study centers from the CCFR and GECCO as described in the S1 Text and S1 and S2 Tables. All colorectal cancer cases were defined as colorectal adenocarcinoma and confirmed by medical records, pathologic reports, or death certificates. We included advanced colorectal adenoma, a well-defined colorectal cancer precursor [70, 71], from two studies (S1 Text). Advanced adenoma was defined as an adenoma 1 cm or larger in diameter and/or with tubulovillous, villous, or high-grade dysplasia/carcinoma-in-situ histology. Colorectal adenoma cases were confirmed by medical records, histopathology, or pathologic reports. Controls for adenoma cases had a clean sigmoidoscopic or colonoscopic examination. All participants provided informed consent and studies were approved by their respective Institutional Review Boards.
Genotyping, quality assurance/quality control and imputation
Average sample and SNP call rates, and concordance rates for blinded duplicates have been previously published [3]. In brief, genotyped SNPs were excluded based on call rate (< 98%), lack of Hardy-Weinberg Equilibrium in controls (HWE, p < 1 x 10−4), and low minor allele frequency (MAF<0.05). We imputed the autosomal SNPs of all studies to the Northern Europeans from Utah (CEU population) in HapMap II. SNPs were restricted based on per-study minor allele count > 5 and imputation accuracy (R2 > 0.3). After imputation and quality-control (QC) exclusion, approximately 2.7M SNPs were used in analysis.
All analyses were restricted to individuals of European ancestry, defined as samples clustering with the Utah residents with Northern and Western European ancestry from the CEPH collection population in principal component analysis [72], including the HapMap II populations as reference.
Alcohol consumption and smoking information
All information on basic demographics and environmental risk factors were collected through interviews or through self-administered questionnaires. Data for all studies were centrally harmonized at the data coordinating center. We used the risk-factor information at the reference time, which varied across studies (S1 Text). A multi-step data-harmonization procedure which is described in detail in Hutter et al. [29] was applied to reconcile differences in individual study questionnaires. We converted consumption of alcoholic beverages into grams of alcohol per day (g/day) by summing the alcohol content of each beverage consumed per day. To test if the categorical or continuous variable fitted the association between alcohol intake and CRC risk better we used Akaike Information Criterion (AIC) to compare both models. With our sample size a model with an AIC that is 6 points smaller than the other model is considered a better fitting model[32]. According to this analysis and consistent with previously described risk profiles [16, 17, 19–22, 73], we grouped study participants as non-/occasional drinkers (drinking < 1 g/day); light-to-moderate drinkers (drinking 1–28 g/day); and heavy drinkers (drinking >28 g/day, one standard drinking is approximately equal to 14 grams of alcohol). We coded these categories using indicator variables for the genome-wide interaction analysis. Smoking history was defined as never- and ever-smoking; pack-years of smoking was calculated by multiplying the average number of packs of cigarettes smoked per day by smoking duration (years). Smoking history (ever vs. never smoking) and pack-years (treated as a continuous variable) of smoking were used in genome-wide interaction analysis, separately.
Statistical analysis
Statistical analyses of all data were conducted centrally at the GECCO coordinating center on individual-level data to ensure a consistent analytical approach. Unless otherwise indicated, we adjusted for age at the reference time, sex (when appropriate), center (when appropriate), and the first three principal components from EIGENSTRAT to account for potential population substructure. The alcohol and smoking variables were coded as described above. Each directly genotyped SNP was coded as 0, 1, or 2 copies of the variant allele. For imputed SNPs, we used the expected number of copies of the variant allele (the “dosage”), which has been shown to give unbiased test statistics [74]. Genotypes were treated as continuous variables (i.e. log-additive effects). Each study was analyzed separately using logistic regression models and study-specific results were combined using fixed-effects meta-analysis methods to obtain summary odds ratios (ORs) and 95% confidence intervals (CIs) across studies. We calculated the heterogeneity p-values using Woolf’s test [75]. Quantile-quantile (Q-Q) plots were assessed to determine whether the distribution of the p-values was consistent with the null distribution (except for the extreme tail). Subjects with missing data for SNPs or environmental factors were excluded from the relevant analyses. Considering the potential male-female difference in alcohol metabolism[76, 77] and the different levels of alcohol consumption between sexes, we conducted the genome-wide interaction analysis for alcohol separately for men and women and used fixed effects meta-analysis to combine their results. All analyses were conducted using the R software (Version 3.0.1).
Two statistical methods that leverage SNPs and environmental factors interaction (G×E interaction) were used to detect potential disease associated loci. First, we used conventional case-control logistic regression analysis including G×E interaction term(s). As the alcohol consumption variable has three categories there are two interaction terms in the statistical models. Based on an increasing number of publications [78–83] providing a detailed discussion on the appropriate genome-wide significance threshold, which all arrive at similar values in the range of 5 x 10-7to 5 x 10−8 for European populations, we decided to use an alpha level of 5 x 10−8 as the genome-wide significance threshold, assuming about 1 million independent tests across the genome (0.05/1,000,000 = 5 x 10−8). For significant results we used permutation approach to determine the empirical p-value. We defined the number of permutation needed as 1/p-value (i.e., for a p-value of 5 x 10−8 1/5E-08 = 20,000,000). We permutated the case-control status 1/p-value times and calculated the p values for the interaction from each meta-analyses to calculate the permuted p-value.
Second, we used our recently developed Cocktail method.[55] In brief, this method consists of two-steps: a screening step to prioritize SNPs and a testing step for GxE interaction. For the screening step, we ranked and prioritized variants through a genome-wide screen of each of the 2.7M SNPs (referred to as “G”) by the maximum of the two test statistics from marginal association testing of Gs on disease risk [84], and correlation testing between G and exposure (E) in cases and controls combined.[85] Based on the ranks of these SNPs from screening, we used a weighted hypothesis framework to partition SNPs into ordered groups and assigned each group an alpha-level cut-off, with higher ranked groups from the screening stage having less stringent alpha-level cut-offs for interaction [86, 87]. The second step of the Cocktail method is the testing step. We used either case-control (CC) or case-only (CO) logistic regression to calculate a p-value for the interaction. If the G was assigned based on its low marginal association P value in the screening tests, we used CO test; if it was ranked because of a low correlation screening p-value, we used CC tests. We compared the test step p-value to the alpha-level cutoff for each SNP in a given group.
We calculated absolute risks for each genotype of the SNP showing significant G×E interaction. Briefly, based upon the Surveillance, Epidemiology, and End Results (SEER) age-adjusted colorectal cancer incidence rate (denoted by “I”) between 1982–2011 among the White population of 42.9 per 100,000 men and women per year, we estimated the reference incidence rate of colorectal cancer (denoted by “I_{reference}”) using the following formula: I_{reference} = I/(P(AA, non-E) + OR{Aa, non-E}×P(Aa, non-E) + OR{aa, non-E}×P(aa, non-E) + OR{AA, E}×P(AA, E) + OR{Aa, E}×P(Aa, E)) + OR{aa, E}×P(aa, E)), where P(genotype, E (or non-E)) is the prevalence of light-to-moderate drinking (or non/occasional drinking) in each corresponding genotype category among controls (non-cases). Based on this reference incidence rate of colorectal cancer (i.e., I_{reference}), we further calculated absolute colorectal cancer incidence rates within each subgroup defined by genotype of the SNP according to a light-to-moderate drinking or non/occasional drinking by multiplying the I_{reference} with each corresponding OR. Bootstrap methods were used to calculate the 95% CI of absolute risk estimates [88].
Expression analyses
We used different types of gene expression data to examine putative expression of genes identified in our genome-wide interaction analysis, and to determine biological plausibility that the variants identified might impact CRC risk. First, we searched the Genotype-Tissue Expression project (GTEx) portal (http://www.broadinstitute.org/gtex/searchGenes)[34] and the Human Protein Atlas (http://www.proteinatlas.org)[35] to establish whether the implicated genes and corresponding proteins are expressed in human colon/rectal tissues. Second, we used several eQTL databases including the Browser at University of Chicago (http://eqtl.uchicago.edu/Home.html),the Genevar (GENe Expression VARiation) at the Wellcome Trust Sanger Institute (http://www.sanger.ac.uk/resources/software/genevar) [42], HaploReg (http://www.broadinstitute.org/mammals/haploreg/haploreg.php) (PMID:22064851), and the GTEx Portal Version 4(http://gtexportal.org/home/) (PMID: 26484569) to investigate whether any of the implicated SNPs may impact the expression of the nearby genes. A cis-eQTL analysis was also performed in TCGA COAD data in 356 Caucasian samples that have demographic and clinical data for 15,008 genes (S1 Text). Third, we analyzed expression data for the implicated genes from 35 pairs of colorectal tumor-normal tissue samples included in the ColoCare Cohort (S2 Text) as well as expression data from the Cancer Genome Atlas (TCGA; http://cancergenome.nih.gov) in 50 pairs of colorectal adenocarcinoma-normal tissue samples. We searched the UCSC Cancer Genomics Browser (https://genome-cancer.ucsc.edu) [37–39] to examine whether the implicated genes showed evidence of differential expression in colorectal tumor tissue and normal tissue. Last, we used the publically available data in the Gene Expression Omnibus site (http://www.ncbi.nlm.nih.gov/geo/) [89, 90] and the gene expression data from normal colon (n = 33) and tumor (n = 28) tissue in the ColoCare Cohort (S2 Text) to investigate whether the expression of implicated genes are correlated with alcohol/smoking history.
Bioinformatics analysis
We explored potential functional annotations for the SNPs that showed evidence for interactions with either smoking or alcohol in our genome-wide interaction analyses. As detailed in S1 Text, we queried multiple bioinformatics databases using the UCSC genome browser (http://genome.ucsc.edu), HaploReg (http://www.broadinstitute.org/mammals/haploreg/haploreg.php), and literature review of published enhancer signatures of colon cancer.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
The 4 probes for HIATL1 all showed that HIATL1 expression was significantly higher in tumor tissue than in normal tissue (Paired t test, P = 4.4×10−9 to 7.2×10−5); the results from two probes that uniquely match HIATL1 transcript were shown in a (P = 7.2×10−5) & b (P = 5.1×10−7); These results were replicated in the colorectal tumor-normal-matched samples from TCGA (c,d) (P = 0.025). In figures a, b, and c each line represent a colorectal cancer case connecting the values of gene expression in adjacent normal tissue to tumor tissue from that same case. In figure d the log2 transformed mean expression with 95% confidence interval is shown with a line connecting values of gene expression in tumor and adjacent normal tissue.
(DOCX)
The lifetime alcohol consumption was categorized into four groups ([0, 4.7), [4.7,12.5), [12.5, 25.3), & > = 25.3 grams of alcohol/day). Y axis: normalized and log2 transformed values of gene expression; X axis: the lifetime alcohol consumption (grams of alcohol/day). Each dot in the figure represented a single sample.
(DOCX)
The alcohol consumption at reference time was categorized into four groups ([0, 4.7), [4.7, 12.5), [12.5, 25.3), & > = 25.3 grams of alcohol/day). Y axis: normalized and log2 transformed values of gene expression; X axis: the lifetime alcohol consumption (grams of alcohol/day). Each dot in the figure represented a single sample. (a) and (b) represented the results from two probes uniquely matching HIATL1 transcript.
(DOCX)
Individual genotypes are plotted on a strip chart, where observed and permuted P values are labeled. r: Spearman's rho; P: observed P value; Pemp: p value of 10,000 permutations.
(DOCX)
rs9409565 (shown as green bar) is correlated with 142 variants (r2≥0.5 in 1000 Genomes Phase 3 European populations). The tagged variants span across intronic regions and approximately 50kb downstream and 75kb upstream of HIATL1. Eigtheen of these variants fall within a transcriptionally active region in colorectal tissue, and four of these variants (rs7042481,rs7858085, rs9409510, rs28406858) are positioned within three variant enhancer loci (VEL, shown as orange bars).
(DOCX)
Rs28406858 is shown as the orange bar and highlighted in blue. The variant is positioned in both a variant enhancer locus in the first intron of HIATL1 and a protein binding site for ELF1. Bioinformatic annotation suggests this variant is a strong candidate for functional follow-up.
(DOCX)
Acknowledgments
We thank Dr. Wei Sun for providing eQTL results on TCGA data. We thank Dr. Peter Scacheri for his guidance on the variant enhancer loci analysis.
ASTERISK: We are very grateful to Dr. Bruno Buecher without whom this project would not have existed. We also thank all those who agreed to participate in this study, including the patients and the healthy control persons, as well as all the physicians, technicians and students.
DACHS: We thank all participants and cooperating clinicians, and Ute Handte-Daub, Renate Hettler-Jensen, Utz Benscheid, Muhabbet Celik and Ursula Eilber for excellent technical assistance.
GECCO: The authors would like to thank all those at the GECCO Coordinating Center for helping bring together the data and people that made this project possible. The authors also acknowledge COMPASS (Comprehensive Center for the Advancement of Scientific Strategies) at the Fred Hutchinson Cancer Research Center for their work harmonizing the GECCO epidemiological data set. The authors acknowledge Dave Duggan and team members at TGEN (Translational Genomics Research Institute), the Broad Institute, and the Génome Québec Innovation Center for genotyping DNA samples of cases and controls, and for scientific input for GECCO.
HPFS, NHS and PHS: We would like to acknowledge Patrice Soule and Hardeep Ranu of the Dana Farber Harvard Cancer Center High-Throughput Polymorphism Core who assisted in the genotyping for NHS, HPFS, and PHS under the supervision of Dr. Immaculata Devivo and Dr. David Hunter, Qin (Carolyn) Guo and Lixue Zhu who assisted in programming for NHS and HPFS, and Haiyan Zhang who assisted in programming for the PHS. We would like to thank the participants and staff of the Nurses' Health Study and the Health Professionals Follow-Up Study, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
PLCO: The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute, the Screening Center investigators and staff or the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Mr. Tom Riley and staff, Information Management Services, Inc., Ms. Barbara O’Brien and staff, Westat, Inc., and Drs. Bill Kopp, Wen Shao, and staff, SAIC-Frederick. Most importantly, we acknowledge the study participants for their contributions to making this study possible. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI.
PMH: The authors would like to thank the study participants and staff of the Hormones and Colon Cancer study.
WHI: The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
GECCO is supported by National Cancer Institute, National Institutes of Health, U.S. Department of Health, and Human Services (U01 CA137088; R01 CA059045; R01 CA120582). ASTERISK is supported by a Hospital Clinical Research Program (PHRC), the Regional Council of Pays de la Loire, the Groupement des Entreprises Françaises dans la Lutte contre le Cancer (GEFLUC), the Association Anne de Bretagne Génétique and the Ligue Régionale Contre le Cancer (LRCC). COLO2&3 is supported by National Institutes of Health (R01 CA60987). CCFR is supported by National Institutes of Health (UM1 CA167551) and through cooperative agreements with members of the Colon Cancer Family Registry and P.I.s. This genome wide scan was supported by the National Cancer Institute, National Institutes of Health by U01 CA122839 and R01 CA143237 to Graham Casey. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the CFRs, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CFR. The following Colon CFR centers contributed data to this manuscript and were supported by National Institutes of Health: Australasian Colorectal Cancer Family Registry (U01/U24 CA097735), Ontario Registry for Studies of Familial Colorectal Cancer (U01/U24 CA074783), and Seattle Colorectal Cancer Family Registry (U01/U24 CA074794). DACHS is supported by German Research Council (Deutsche Forschungsgemeinschaft, BR 1704/6-1, BR 1704/6-3, BR 1704/6-4 and CH 117/1-1), and the German Federal Ministry of Education and Research (01KH0404 and 01ER0814). DALS is supported by National Institutes of Health (R01 CA48998 to MLS). HPFS is supported by the National Institutes of Health (P01 CA 055075, UM1 CA167552, R01 137178, R01 CA 151993, R35 CA197735, K07 CA190673, and P50 CA 127003), NHS by the National Institutes of Health (R01 CA137178, P01 CA 087969, UM1 CA186107, R01 CA151993, R35 CA197735, K07190673, and P50 CA 127003,) and PHS by the National Institutes of Health (R01 CA042182). MEC is supported by National Institutes of Health (R37 CA54281, P01 CA033619, and R01 CA63464). OFCCR is supported by National Institutes of Health through funding allocated to the Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783, see CCFR section above). Additional funding toward genetic analyses of OFCCR includes the Ontario Research Fund, the Canadian Institutes of Health Research, and the Ontario Institute for Cancer Research through generous support from the Ontario Ministry of Research and Innovation. PLCO is supported by Intramural Research Program of the Division of Cancer Epidemiology and Genetics and supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS. Additionally, a subset of control samples were genotyped as part of the Cancer Genetic Markers of Susceptibility (CGEMS) Prostate Cancer GWAS (Yeager, M et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet 2007 May;39(5):645-9), Colon CGEMS pancreatic cancer scan (PanScan) (Amundadottir, L et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009 Sep;41(9):986-90, and Petersen, GM et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010 Mar;42(3):224-8), and the Lung Cancer and Smoking study (Landi MT, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009 Nov;85(5):679-91). The prostate and PanScan study datasets were accessed with appropriate approval through the dbGaP online resource (http://cgems.cancer.gov/data/) accession numbers phs000207.v1.p1 and phs000206.v3.p2, respectively, and the lung datasets were accessed from the dbGaP website (http://www.ncbi.nlm.nih.gov/gap) through accession number phs000093.v2.p2. Funding for the Lung Cancer and Smoking study was provided by National Institutes of Health (NIH), Genes, Environment and Health Initiative (GEI) Z01 CP 010200, NIH U01 HG004446, and NIH GEI U01 HG 004438. For the lung study, the GENEVA Coordinating Center provided assistance with genotype cleaning and general study coordination, and the Johns Hopkins University Center for Inherited Disease Research conducted genotyping. PMH is supported by National Institutes of Health (R01 CA076366 to P.A. Newcomb). VITAL is supported byNational Institutes of Health (K05 CA154337). WHI is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J, S.H., Bray F, Forman D, Mathers C and Parkin DM, GLOBOCAN 2008 v1.2. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet], 2008(Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr).
- 2.Peters U, et al. , Meta-analysis of new genome-wide association studies of colorectal cancer risk. Hum Genet, 2012. 131(2): p. 217–34. 10.1007/s00439-011-1055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters U, et al. , Identification of Genetic Susceptibility Loci for Colorectal Tumors in a Genome-Wide Meta-analysis. Gastroenterology, 2013. 144(4): p. 799–807 e24. 10.1053/j.gastro.2012.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenstein P, et al. , Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med, 2000. 343(2): p. 78–85. 10.1056/NEJM200007133430201 [DOI] [PubMed] [Google Scholar]
- 5.Tenesa A and Dunlop MG, New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat Rev Genet, 2009. 10(6): p. 353–8. 10.1038/nrg2574 [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, et al. , Colorectal cancer. Lancet, 2010. 375(9719): p. 1030–47. 10.1016/S0140-6736(10)60353-4 [DOI] [PubMed] [Google Scholar]
- 7.Brenner H, Kloor M, and Pox CP, Colorectal cancer. Lancet, 2014. 383(9927): p. 1490–502. 10.1016/S0140-6736(13)61649-9 [DOI] [PubMed] [Google Scholar]
- 8.Peters U, Bien S, and Zubair N, Genetic architecture of colorectal cancer. Gut, 2015. 10.1136/gutjnl-2013-306705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Tassan NA, et al. , Erratum: A new GWAS and meta-analysis with 1000Genomes imputation identifies novel risk variants for colorectal cancer. Sci Rep, 2015. 5: p. 12372 10.1038/srep12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemire M, et al. , A genome-wide association study for colorectal cancer identifies a risk locus in 14q23.1. Hum Genet, 2015. 134(11–12): p. 1249–1262. 10.1007/s00439-015-1598-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng C, et al. , Identification of Susceptibility Loci and Genes for Colorectal Cancer Risk. Gastroenterology, 2016. 10.1053/j.gastro.2016.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas D, Gene—environment-wide association studies: emerging approaches. Nat Rev Genet, 2010. 11(4): p. 259–72. 10.1038/nrg2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Ijzendoorn MH, et al. , Gene-by-environment experiments: a new approach to finding the missing heritability. Nat Rev Genet, 2011. 12(12): p. 881; author reply 881. 10.1038/nrg2764-c1 [DOI] [PubMed] [Google Scholar]
- 14.Gauderman WJ, et al. , Finding novel genes by testing G x E interactions in a genome-wide association study. Genet Epidemiol, 2013. 37(6): p. 603–13. 10.1002/gepi.21748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutter CM, et al. , Gene-environment interactions in cancer epidemiology: a National Cancer Institute Think Tank report. Genet Epidemiol, 2013. 37(7): p. 643–57. 10.1002/gepi.21756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho E, et al. , Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med, 2004. 140(8): p. 603–13. 10.7326/0003-4819-140-8-200404200-00007 [DOI] [PubMed] [Google Scholar]
- 17.Fedirko V, et al. , Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol, 2011. 22(9): p. 1958–72. 10.1093/annonc/mdq653 [DOI] [PubMed] [Google Scholar]
- 18.Wei EK, et al. , Comparison of risk factors for colon and rectal cancer. Int J Cancer, 2004. 108(3): p. 433–42. 10.1002/ijc.11540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longnecker MP, et al. , A meta-analysis of alcoholic beverage consumption in relation to risk of colorectal cancer. Cancer Causes Control, 1990. 1(1): p. 59–68. 10.1007/BF00053184 [DOI] [PubMed] [Google Scholar]
- 20.Fekjaer HO, Alcohol-a universal preventive agent? A critical analysis. Addiction, 2013. 108(12): p. 2051–7. 10.1111/add.12104 [DOI] [PubMed] [Google Scholar]
- 21.Bergmann MM, et al. , The association of pattern of lifetime alcohol use and cause of death in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. International Journal of Epidemiology, 2013. 42(6): p. 1772–1790. 10.1093/ije/dyt154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kontou N, et al. , Alcohol consumption and colorectal cancer in a Mediterranean population: a case-control study. Dis Colon Rectum, 2012. 55(6): p. 703–10. 10.1097/DCR.0b013e31824e612a [DOI] [PubMed] [Google Scholar]
- 23.Gong J, et al. , A pooled analysis of smoking and colorectal cancer: timing of exposure and interactions with environmental factors. Cancer Epidemiol Biomarkers Prev, 2012. 21(11): p. 1974–85. 10.1158/1055-9965.EPI-12-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botteri E, et al. , Smoking and colorectal cancer: a meta-analysis. JAMA, 2008. 300(23): p. 2765–78. 10.1001/jama.2008.839 [DOI] [PubMed] [Google Scholar]
- 25.Liang PS, Chen TY, and Giovannucci E, Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer, 2009. 124(10): p. 2406–15. 10.1002/ijc.24191 [DOI] [PubMed] [Google Scholar]
- 26.Varela-Rey M, et al. , Alcohol, DNA methylation, and cancer. Alcohol Res, 2013. 35(1): p. 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oyesanmi O, et al. , Alcohol consumption and cancer risk: understanding possible causal mechanisms for breast and colorectal cancers. Evid Rep Technol Assess (Full Rep), 2010(197): p. 1–151. [PMC free article] [PubMed] [Google Scholar]
- 28.Cleary SP, et al. , Cigarette smoking, genetic variants in carcinogen-metabolizing enzymes, and colorectal cancer risk. Am J Epidemiol, 2010. 172(9): p. 1000–14. 10.1093/aje/kwq245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutter CM, et al. , Characterization of gene-environment interactions for colorectal cancer susceptibility loci. Cancer Res, 2012. 72(8): p. 2036–44. 10.1158/0008-5472.CAN-11-4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newcomb PA, et al. , Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev, 2007. 16(11): p. 2331–43. 10.1158/1055-9965.EPI-07-0648 [DOI] [PubMed] [Google Scholar]
- 31.Research, W.C.R.F.A.I.f.C., Continuous Update Project Report Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. 2011, Washington, DC: AICR. [Google Scholar]
- 32.Hilbe JM, Negative Binomial Regression. 2nd ed 2011: Cambridge University Press; 10.1017/CBO9780511811852 [DOI] [Google Scholar]
- 33.Vanderweele TJ, Ko YA, and Mukherjee B, Environmental confounding in gene-environment interaction studies. Am J Epidemiol, 2013. 178(1): p. 144–52. 10.1093/aje/kws439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Consortium GT, The Genotype-Tissue Expression (GTEx) project. Nat Genet, 2013. 45(6): p. 580–5. 10.1038/ng.2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhlen M, et al. , Towards a knowledge-based Human Protein Atlas. Nat Biotechnol, 2010. 28(12): p. 1248–50. 10.1038/nbt1210-1248 [DOI] [PubMed] [Google Scholar]
- 36.Kaiser S, et al. , Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biology, 2007. 8(7). 10.1186/gb-2007-8-7-r131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman M, et al. , The UCSC Cancer Genomics Browser: update 2013. Nucleic Acids Res, 2013. 41(Database issue): p. D949–54. 10.1093/nar/gks1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanborn JZ, et al. , The UCSC Cancer Genomics Browser: update 2011. Nucleic Acids Res, 2011. 39(Database issue): p. D951–9. 10.1093/nar/gkq1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, et al. , The UCSC Cancer Genomics Browser. Nat Methods, 2009. 6(4): p. 239–40. 10.1038/nmeth0409-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeller T, et al. , Genetics and beyond—the transcriptome of human monocytes and disease susceptibility. PLoS One, 2010. 5(5): p. e10693 10.1371/journal.pone.0010693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veyrieras JB, et al. , High-Resolution Mapping of Expression-QTLs Yields Insight into Human Gene Regulation. Plos Genetics, 2008. 4(10). 10.1371/journal.pgen.1000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang TP, et al. , Genevar: a database and Java application for the analysis and visualization of SNP-gene associations in eQTL studies. Bioinformatics, 2010. 26(19): p. 2474–6. 10.1093/bioinformatics/btq452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akhtar-Zaidi B, et al. , Epigenomic enhancer profiling defines a signature of colon cancer. Science, 2012. 336(6082): p. 736–739. 10.1126/science.1217277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chadwick LH, The NIH Roadmap Epigenomics Program data resource. Epigenomics, 2012. 4(3): p. 317–324. 10.2217/epi.12.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffman MM, et al. , Unsupervised pattern discovery in human chromatin structure through genomic segmentation. Nat Methods, 2012. 9(5): p. 473–U88. 10.1038/nmeth.1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlessinger A, et al. , Comparison of human solute carriers. Protein Science, 2010. 19(3): p. 412–428. 10.1002/pro.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoglund PJ, et al. , The Solute Carrier Families Have a Remarkably Long Evolutionary History with the Majority of the Human Families Present before Divergence of Bilaterian Species. Molecular Biology and Evolution, 2011. 28(4): p. 1531–1541. 10.1093/molbev/msq350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sreedharan S, et al. , Long evolutionary conservation and considerable tissue specificity of several atypical solute carrier transporters. Gene, 2011. 478(1–2): p. 11–18. 10.1016/j.gene.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 49.Nakanishi T and Tamai I, Solute Carrier Transporters as Targets for Drug Delivery and Pharmacological Intervention for Chemotherapy. Journal of Pharmaceutical Sciences, 2011. 100(9): p. 3731–3750. 10.1002/jps.22576 [DOI] [PubMed] [Google Scholar]
- 50.Okudaira H, et al. , Putative Transport Mechanism and Intracellular Fate of Trans-1-Amino-3-F-18-Fluorocyclobutanecarboxylic Acid in Human Prostate Cancer. Journal of Nuclear Medicine, 2011. 52(5): p. 822–829. 10.2967/jnumed.110.086074 [DOI] [PubMed] [Google Scholar]
- 51.Fan XT, et al. , Impact of system L amino acid transporter 1 (LAT1) on proliferation of human ovarian cancer cells: A possible target for combination therapy with anti-proliferative aminopeptidase inhibitors. Biochemical Pharmacology, 2010. 80(6): p. 811–818. 10.1016/j.bcp.2010.05.021 [DOI] [PubMed] [Google Scholar]
- 52.Laplante M and Sabatini DM, mTOR signaling at a glance. Journal of Cell Science, 2009. 122(20): p. 3589–3594. 10.1242/jcs.051011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoeffer CA and Klann E, mTOR signaling: At the crossroads of plasticity, memory and disease. Trends in Neurosciences, 2010. 33(2): p. 67–75. 10.1016/j.tins.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoncu R, Efeyan A, and Sabatini DM, mTOR: from growth signal integration to cancer, diabetes and ageing. Nature Reviews Molecular Cell Biology, 2011. 12(1): p. 21–35. 10.1038/nrm3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu L, et al. , Powerful cocktail methods for detecting genome-wide gene-environment interaction. Genet Epidemiol, 2012. 36(3): p. 183–94. 10.1002/gepi.21610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dudbridge F and Fletcher O, Gene-environment dependence creates spurious gene-environment interaction. Am J Hum Genet, 2014. 95(3): p. 301–7. 10.1016/j.ajhg.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mukherjee B and Chatterjee N, Exploiting gene-environment independence for analysis of case-control studies: an empirical Bayes-type shrinkage estimator to trade-off between bias and efficiency. biometrics, 2008. 64(3): p. 685–694. 10.1111/j.1541-0420.2007.00953.x [DOI] [PubMed] [Google Scholar]
- 58.Piegorsch WW, Weinberg CR, and Taylor JA, Non-hierarchical logistic models and case-only designs for assessing susceptibility in population-based case-control studies. Stat Med, 1994. 13(2): p. 153–162. 10.1002/sim.4780130206 [DOI] [PubMed] [Google Scholar]
- 59.Schumacher FR, et al. , Genome-wide association study of colorectal cancer identifies six new susceptibility loci. Nat Commun, 2015. 6: p. 7138 10.1038/ncomms8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith PG and Day NE, The design of case-control studies: the influence of confounding and interaction effects. Int. J Epidemiol, 1984. 13(3): p. 356–365. 10.1093/ije/13.3.356 [DOI] [PubMed] [Google Scholar]
- 61.Skol AD, et al. , Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet, 2006. 38(2): p. 209–213. 10.1038/ng1706 [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Closas M, Thompson WD, and Robins JM, Differential misclassification and the assessment of gene-environment interactions in case-control studies. Am J Epidemiol, 1998. 147(5): p. 426–433. 10.1093/oxfordjournals.aje.a009467 [DOI] [PubMed] [Google Scholar]
- 63.Society AC, Cancer Facts and Figures 2014. 2014: Altanta, GA. [Google Scholar]
- 64.Eriksson CJ, Genetic-epidemiological evidence for the role of acetaldehyde in cancers related to alcohol drinking. Adv Exp Med Biol, 2015. 815: p. 41–58. 10.1007/978-3-319-09614-8_3 [DOI] [PubMed] [Google Scholar]
- 65.Guo XF, et al. , Meta-analysis of the ADH1B and ALDH2 polymorphisms and the risk of colorectal cancer in East Asians. Intern Med, 2013. 52(24): p. 2693–9. 10.2169/internalmedicine.52.1202 [DOI] [PubMed] [Google Scholar]
- 66.Chen B, et al. , A critical analysis of the relationship between aldehyde dehydrogenases-2 Glu487Lys polymorphism and colorectal cancer susceptibility. Pathol Oncol Res, 2015. 21(3): p. 727–33. 10.1007/s12253-014-9881-8 [DOI] [PubMed] [Google Scholar]
- 67.Houlston RS and Cogent, COGENT (COlorectal cancer GENeTics) revisited. Mutagenesis, 2012. 27(2): p. 143–151. 10.1093/mutage/ger059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogino S, et al. , Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol, 2013. 26(4): p. 465–84. 10.1038/modpathol.2012.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogino S, et al. , Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut, 2011. 60(3): p. 397–411. 10.1136/gut.2010.217182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brenner H, et al. , Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut, 2007. 56(11): p. 1585–1589. 10.1136/gut.2007.122739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kinzler KW and Vogelstein B, Lessons from hereditary colorectal cancer. Cell, 1996. 87(2): p. 159–70. 10.1016/S0092-8674(00)81333-1 [DOI] [PubMed] [Google Scholar]
- 72.Price AL, et al. , Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet, 2006. 38(8): p. 904–9. 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 73.Alavanja MC, Brownson RC, and Benichou J, Estimating the effect of dietary fat on the risk of lung cancer in nonsmoking women. Lung Cancer, 1996. 14 Suppl 1: p. S63–S74. 10.1016/S0169-5002(96)90211-1 [DOI] [PubMed] [Google Scholar]
- 74.Jiao S, et al. , The Use of Imputed Values in the Meta-Analysis of Genome-Wide Association Studies. Genet Epidemiol, 2011. 35(7): p. 597–605. 10.1002/gepi.20608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woolf B, On estimating the relation between blood group and disease. Ann Hum Genet, 1955. 19(4): p. 251–3. 10.1111/j.1469-1809.1955.tb01348.x [DOI] [PubMed] [Google Scholar]
- 76.Lieber CS, in Gender differences in alcohol metabolism and susceptibility In Wilsnack RW, Wilsnack SC (eds). Gender and alcohol. New Brunswick, NJ: Rutgers Center of Alcohol Studies. [Google Scholar]
- 77.Frezza M, et al. , High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med, 1990. 322(2): p. 95–9. 10.1056/NEJM199001113220205 [DOI] [PubMed] [Google Scholar]
- 78.International HapMap, C., A haplotype map of the human genome. Nature, 2005. 437(7063): p. 1299–320. 10.1038/nature04226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wellcome Trust Case Control, C., Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature, 2007. 447(7145): p. 661–78. 10.1038/nature05911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Risch N and Merikangas K, The future of genetic studies of complex human diseases. Science, 1996. 273(5281): p. 1516–1517. 10.1126/science.273.5281.1516 [DOI] [PubMed] [Google Scholar]
- 81.Hoggart CJ, et al. , Genome-wide significance for dense SNP and resequencing data. Genet Epidemiol, 2008. 32(2): p. 179–85. 10.1002/gepi.20292 [DOI] [PubMed] [Google Scholar]
- 82.Pe'er I, et al. , Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol, 2008. 32(4): p. 381–5. 10.1002/gepi.20303 [DOI] [PubMed] [Google Scholar]
- 83.Dudbridge F and Gusnanto A, Estimation of significance thresholds for genomewide association scans. Genet Epidemiol, 2008. 32(3): p. 227–234. 10.1002/gepi.20297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kooperberg C and LeBlanc M, Increasing the power of identifying gene x gene interactions in genome-wide association studies. Genet Epidemiol, 2008. 32(3): p. 255–263. 10.1002/gepi.20300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murcray CE, Lewinger JP, and Gauderman WJ, Gene-environment interaction in genome-wide association studies. Am J Epidemiol, 2009. 169(2): p. 219–26. 10.1093/aje/kwn353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roeder K and Wasserman L, Genome-Wide Significance Levels and Weighted Hypothesis Testing. Stat Sci, 2009. 24(4): p. 398–413. 10.1214/09-STS289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ionita-Laza I, et al. , Genomewide weighted hypothesis testing in family-based association studies, with an application to a 100K scan. Am J Hum Genet, 2007. 81(3): p. 607–14. 10.1086/519748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Efron B, 1977 Rietz Lecture—Bootstrap Methods—Another Look at the Jackknife. Annals of Statistics, 1979. 7(1): p. 1–26. [Google Scholar]
- 89.Edgar R, Domrachev M, and Lash AE, Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res, 2002. 30(1): p. 207–10. 10.1093/nar/30.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barrett T, et al. , NCBI GEO: archive for functional genomics data sets—10 years on. Nucleic Acids Res, 2011. 39(Database issue): p. D1005–10. 10.1093/nar/gkq1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
The 4 probes for HIATL1 all showed that HIATL1 expression was significantly higher in tumor tissue than in normal tissue (Paired t test, P = 4.4×10−9 to 7.2×10−5); the results from two probes that uniquely match HIATL1 transcript were shown in a (P = 7.2×10−5) & b (P = 5.1×10−7); These results were replicated in the colorectal tumor-normal-matched samples from TCGA (c,d) (P = 0.025). In figures a, b, and c each line represent a colorectal cancer case connecting the values of gene expression in adjacent normal tissue to tumor tissue from that same case. In figure d the log2 transformed mean expression with 95% confidence interval is shown with a line connecting values of gene expression in tumor and adjacent normal tissue.
(DOCX)
The lifetime alcohol consumption was categorized into four groups ([0, 4.7), [4.7,12.5), [12.5, 25.3), & > = 25.3 grams of alcohol/day). Y axis: normalized and log2 transformed values of gene expression; X axis: the lifetime alcohol consumption (grams of alcohol/day). Each dot in the figure represented a single sample.
(DOCX)
The alcohol consumption at reference time was categorized into four groups ([0, 4.7), [4.7, 12.5), [12.5, 25.3), & > = 25.3 grams of alcohol/day). Y axis: normalized and log2 transformed values of gene expression; X axis: the lifetime alcohol consumption (grams of alcohol/day). Each dot in the figure represented a single sample. (a) and (b) represented the results from two probes uniquely matching HIATL1 transcript.
(DOCX)
Individual genotypes are plotted on a strip chart, where observed and permuted P values are labeled. r: Spearman's rho; P: observed P value; Pemp: p value of 10,000 permutations.
(DOCX)
rs9409565 (shown as green bar) is correlated with 142 variants (r2≥0.5 in 1000 Genomes Phase 3 European populations). The tagged variants span across intronic regions and approximately 50kb downstream and 75kb upstream of HIATL1. Eigtheen of these variants fall within a transcriptionally active region in colorectal tissue, and four of these variants (rs7042481,rs7858085, rs9409510, rs28406858) are positioned within three variant enhancer loci (VEL, shown as orange bars).
(DOCX)
Rs28406858 is shown as the orange bar and highlighted in blue. The variant is positioned in both a variant enhancer locus in the first intron of HIATL1 and a protein binding site for ELF1. Bioinformatic annotation suggests this variant is a strong candidate for functional follow-up.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.