Abstract
Background
In recent years, the primate malaria Plasmodium knowlesi has emerged in human populations throughout South East Asia, with the largest hotspot being in Sabah, Malaysian Borneo. Control efforts are hindered by limited knowledge of where and when people get exposed to mosquito vectors. It is assumed that exposure occurs primarily when people are working in forest areas, but the role of other potential exposure routes (including domestic or peri-domestic transmission) has not been thoroughly investigated.
Methodology/Principal Findings
We integrated entomological surveillance within a comprehensive case-control study occurring within a large hotspot of transmission in Sabah, Malaysia. Mosquitoes were collected at 28 pairs households composed of one where an occupant had a confirmed P. knowlesi infection within the preceding 3 weeks (“case”) and an associated “control” where no infection was reported. Human landing catches were conducted to measure the number and diversity of mosquitoes host seeking inside houses and in the surrounding peri-domestic (outdoors but around the household) areas. The predominant malaria vector species was Anopheles balabacensis, most of which were caught outdoors in the early evening (6pm - 9pm). It was significantly more abundant in the peri-domestic area than inside houses (5.5-fold), and also higher at case than control households (0.28±0.194 vs 0.17±0.127, p<0.001). Ten out of 641 An. balabacensis tested were positive for simian malaria parasites, but none for P. knowlesi.
Conclusions/Significance
This study shows there is a possibility that humans can be exposed to P. knowlesi infection around their homes. The vector is highly exophagic and few were caught indoors indicating interventions using bednets inside households may have relatively little impact.
Author Summary
The primate knowlesi malaria has now emerged in human populations throughout South East Asia. Our limited knowledge of where and when people get exposed to the vector (Anopheles balabacensis) has resulted in poor control measures, although it is assumed that exposure occurs primarily when people are working in forest areas. We investigated the role of peri-domestic (outdoors but around the household) and domestic transmission. Mosquitoes were collected at 28 pairs households composed of one where an occupant had a confirmed knowlesi malaria infection and an associated “control” where no infection was reported. Most of the vectors were caught outdoors from 6pm - 9pm. The vectors were also significantly more abundant in the peri-domestic area than inside houses (5.5-fold), and also higher at case than control households. Ten Anopheles (out of 641) were found positive for primate malaria parasites. This study shows that humans can be exposed to knowlesi infection around their homes. Given the vectors are mainly outdoor biters, interventions using insecticide treated bednets inside households may have relatively little impact. A paradigm shift in control methods is required to reduce infection of this primate malaria.
Introduction
The success story of reducing malaria worldwide [1] has been marred by a few notable exceptions where bulk of disease is caused by zoonotic “neglected” malaria species with atypical transmission that makes them less easy to control. Zoonotic malaria, such as Plasmodium knowlesi from the long tailed macaque (Macaca fascicularis) in SE Asia [2], and P. brasilanum from NewWorld monkeys in South America [3], are a growing public health problem.
In South East Asia, the long tailed macaque harbours at least five simian malarias, namely, P. coatneyi, P. inui, P. fieldi, P. cynomolgi and P. knowlesi [4,5]. Plasmodium knowlesi is presently the main zoonotic malaria with the greatest public health importance, especially in Sabah, Malaysian Borneo which has recorded the highest growing number of P. knowlesi cases in humans, and most of these cases are clustered within one district, Kudat in the North eastern region [6,7]. Plasmodium knowlesi is morphologically similar to P. malariae and had been misdiagnosed as such for a long time [2]. A first case of naturally acquired human infection with P. cynomolgi has also been reported from peninsular Malaysia [8]. Thus it is a possibility that other primate parasites may also be soon contributing to human cases as previously predicted [9].
In Sabah, it has been confirmed that An. balabacensis is the primary vector [10] and the long tail macaques, which are the natural reservoir hosts for simian malaria parasites are present. Furthermore, Kudat district has many secondary forest areas surrounded by hilly areas, oil palm estates and rubber plantations which in general serve as habitats not only for long-tail macaques but also for Anopheles species. Close interaction between monkeys, mosquitoes and human increases the chances of being infected with P. knowlesi.
The vectors of P. knowlesi malaria in Malaysia comprise of five Anopheles species of the Leucospyrus group namely: An. hackeri, An. latens, An. cracens, An. introlatus and An. balabacensis [10–15]. In Vietnam Anopheles dirus of the Dirus group was recorded as the vector [16,17]. These vectors are found mainly in the forests and are outdoor biters, and likely to have low susceptibility to frontline control strategies which typically involve use of insecticides in homes.
In Malaysia, the National Malaria Eradication Program was launched in 1967, followed by state-wide malaria control programs during the 1970s and 1980s. Consequently, great reductions in malaria prevalence were recorded, from 240,000 in 1961 to around 50,000/year during the 1980s [11,18]. The success of the eradication programs was also reflected in Sabah, East Malaysia where malaria notifications decreased sharply, from peak notifications of 33,153 and 15,877 during 1994–1995 for P. falciparum and P. vivax respectively to 605 and 628 respectively in 2011. Similarly notifications of P. malariae/P. knowlesi also fell from a peak of 614 in 1994 to <100/year in the late 1990s/early 2000s [6].
Although Malaysia has shown considerable success in the control of human malaria and is on target towards elimination of malaria by 2020 [19], notifications of suspected P. knowlesi cases have increased from 59 notifications in 2004 to 996 in 2013, an overall increase of over 16-fold [6]. According to the Malaysian Ministry of Health, P. knowlesi is the predominant species occurring in the country comprising 62% of the cases in 2013 [20]. It was suggested that the increase in number of P. knowlesi notification in Kudat maybe be due to high awareness of knowlesi infection among physicians and availability of better diagnostic tools to identify this malaria parasite [20]. In other words what is reported now represents the true infection rate as compared in the late 1990s and early 2000s when P. knowlesi cases were misdiagnosed as P. malariae. However, recent findings by Fornace et al. had demonstrated a clear link between land use change and P. knowlesi incidence, which strongly supports the idea that this is not just a problem of poor diagnosis/changing awareness, but a real epidemiological change [21].
The frontline vector control methods practiced in Malaysia under the malaria elimination programme are same as those used in several other endemic settings within the region i.e. the application of insecticides in houses either through use of Long Lasting Insecticide Treated Nets (LLINs) or Indoor Residual Spraying (IRS). However there is little evidence to support that this is effective against P. knowlesi vectors.
Thus there is a need for more detailed entomological investigation to assess the relative importance of exposure to mosquito vectors at or away from home, and to design control measures accordingly. Our working hypothesis is that given An balabacensis is the primary vector, we would expect that infection risk is higher when they are present. Towards this end, a case-control entomological study was conducted to determine if P. knowlesi infection risk is linked to exposure to vectors in domestic and peri-domestic settings. Specific comparisons were included to test for differences in vector abundance, species composition, biting time and infection rate at case and control households. Further aims were to assess and characterize the biting behavior of P. knowlesi vectors near homes (time and place of biting e.g outdoors vs indoors), and to evaluate the potential for spread of other primate malarias in domestic settings. These findings will be useful for the control programme in designing vector control measures.
Methods
Selection of P. knowlesi cases for the study
The study was conducted in Kudat district which is located in the northeasternn tip of Sabah (6°53'14.35" N 116°49'25.10" E) and covers an area of approximately 1,300km2 with a population of 84,000 people of predominantly Rungus ethnicity (2010 National Census). The climate is tropical and the area is mainly coastal, with a maximum elevation of 250 metres above sea level. Forest cover is highly fragmented and substantial deforestation has occurred through conversion of forest to agricultural land [21, 22]. The majority of the population lives in small villages (mean population 160±15, S1 Table) and the main livelihood activities are small scale farming and plantation work.
Plasmodium knowlesi is the main cause of human malaria in Kudat and, due to this relatively high incidence, this area is the focus for a number of interdisciplinary studies on the biomedical, environmental and social risk factors for P. knowlesi (http://malaria.lshtm.ac.uk/MONKEYBAR). This includes a case control study, in which clinical malaria cases were recruited from the district hospital and visited at their homes within two weeks of initial diagnosis [22]. As there is mandatory reporting and referral of all malaria cases to the district hospital, the majority of symptomatic cases are captured by hospital systems.
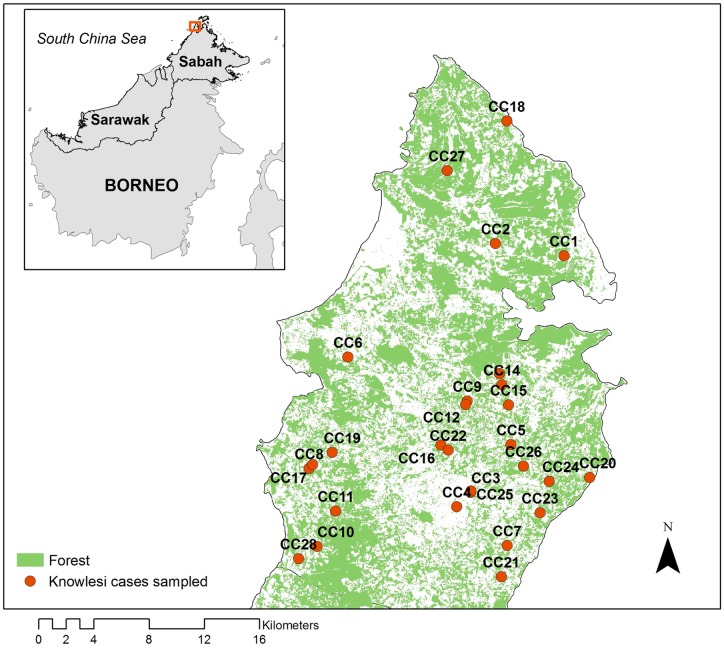
Approximately 180 P. knowlesi cases were identified through this active case surveillance between 2013–14; of which we randomly selected a subgroup of 28 for further entomological follow up (representing cases reporting February, July, 2014 from 23 different villages: Fig 1, S1 Table). The cases came from the age group 19–74 years old with a mean of 44. There was a preponderance of males amongst the cases, and many (78.6%) worked in agricultural sector, taking at least about 10–30 minutes to walk to their work place (Table 1)
Fig 1. Distribution of P. knowlesi cases in Kudat District used in this study.
Table 1. Demographic information of the cases and controls.
The controls represent one individual selected randomly from each “control” household and is not age-based.
| Case | Control | ||||
|---|---|---|---|---|---|
| Demographic features | N | % | N | % | |
| sex | Males | 26 | 93 | 22 | 79 |
| Females | 2 | 7 | 6 | 21 | |
| Mean age (range) | 44 (19–74) | 40 (2–72) | |||
| Agricultural sector | Rubber estate | 13 | 79 | 6 | 57 |
| Oil palm plantation | 5 | 2 | |||
| Coconut plantation | 2 | 2 | |||
| Vegetable garden | 1 | 6 | |||
| Fruit orchard | 1 | — | |||
| Non-agricultural sector | House wife | 1 | 14 | 4 | 32 |
| Others | 3 | 5 | |||
| Others | Unspecified | 1 | 7 | — | 11 |
| Not working | 1 | 3 | |||
| Time to get to work place | 0 (house wives, unemployed) | 1 | 4 | 8 | 29 |
| 10 min (agricultural) | 15 | 54 | 5 | 18 | |
| 30 min (agricultural) | 8 | 29 | 12 | 43 | |
| 2 hr (agricultural, construction worker) | 1 | 4 | 2 | 7 | |
| Unspecified | 3 | 11 | 1 | 4 | |
Additionally, a matched “control” household was recruited in the vicinity of the case household for study which shared similar environmental characteristics in terms of surrounding vegetation and terrain, but where no occupants had reported with any malaria infection within the study period as indicated by records from the hospital and interviewing the residents. From the group of potential “control” households identified in the vicinity, one was randomly selected using a random table. The final choice was also dependent on the owner’s agreement. Within two weeks of the case detection, the selection of control house was accomplished and entomological work initiated.
Data on the types of crops or vegetation surrounding households was collected, as well as the distance between each pair of case and control household (S2 Table). The mean distance between the case and the control houses was 255±48 m (18–1000 m). As the villages were generally small in area, occasionally a control house could be located in a neighbouring village. Of the 28 case-control household pairs, five pairs occurred within the same village.
Collection of Anopheles using human landing catch
For all pairs of households, mosquito sampling was conducted by four workers each at case and control simultaneously on the same nights. At these pairs, sampling was conducted for one to three nights depending on the owner’s permission and logistic constraints.
Indoor collections were conducted at one station in the living room of houses (H), whereas outdoor collections were conducted at three selected stations (S1, S2 & S3) within the garden area surrounding the house. The distance of the stations from the house was 24±1.7 m for the case and 19±0.8 m for the control household. These outdoor stations were selected based on information provided by the family members about where they were most likely to spend time outdoors in the evening. Mosquitoes were baited using human landing catch (HLC) method, but only Anopheles spp were collected for further analysis, whilst other species were killed and discarded at the site. Here a volunteer collected mosquitoes by exposing his lower legs (from knee downwards) and collecting all mosquitoes that land upon them in a plastic specimen tube (2 cm diameter X 6 cm) which had a small piece of moist tissue at the bottom. Each station was manned by one person who would collect Anopheles for 12 hours straight (1800 to 0600 hr), and there was rotation of workers. The HLC workers at each case and control house were regularly monitored by a supervisor. The Anopheles were kept individually in a tube with a label which had information on the place, date, hour, location (indoors vs outdoors) and station of collection. The mosquito samples collected were recorded by hour in order to estimate the biting profile over course of night. The next morning the samples were taken to the laboratory to be processed.
Identification of Anopheles species
Anopheles specimens were identified the next morning in the laboratory based on morphology characters using published identification keys. The key of Sallum et al. [23] was used for Leucosphyrus group, whereas keys developed by Rattanarithikul et al. [24] were used for other groups. The identified specimens were kept individually in a sterile 1.5mL microfuge tube and stored in -20°C until used for molecular analysis.
Total DNA extraction and PCR of malaria parasites
Each Anopheles specimen was cut into two parts: head-thorax and abdomen, and placed separately inside an autoclaved mortar and the tissue homogenized using pestle. The total DNA was extracted from each part following the method of Phillips and Simon [25] and stored in -30°C until PCR analysis. Detection of malaria parasites in the Anopheles specimens was performed using the nested PCR Plasmodium genus-specific method described by Singh et al. [26]. When a sample was found positive for malaria parasites, a second nested PCR was performed to determine the Plasmodium spp. using species specific primers in singleplex PCR [4, 8, 26,27]. Primers of nine species of Plasmodium namely P. coatneyi, P. inui, P. fieldi, P. cynomolgi, P. knowlesi, P. falciparum, P. vivax, P. malarie and P. ovale were used in this study. All these species have been recorded in Malaysia although P. ovale is an imported species, while P. knowlesi is the prevalent simian malaria infecting man.
Both PCR 1 and PCR 2 were performed with 25μl final volume. The reaction components were prepared by mixing 5.0μl of 5X PCR buffer (Promega), 0.5μl of dNTPs (10mM) mixture (Promega), 3.0μl of 25mM MgCl2 (Promega), 1.0μl each of 10μM forward and reverse primers, 0.3μl of Taq DNA polymerase (5U/μl), 2.0μl of DNA template and sterile dH2O up to 25μl final volume. After the first PCR was completed, 2.0μl of the first PCR product was used as a template in the second PCR. The PCR conditions used were: an initial denaturation at 95°C for 5 min, followed by 35 cycles of 94°C for 1 min, annealing for 1 min and 72°C for 1 min, and a final extension at 72°C for 5 min. The annealing temperature was set based on the optimum temperature of the primers (S3 Table).
Statistical analysis
Statistical analysis was conducted using R programming language for statistical analysis (version 3.2.2). Generalised linear mixed models (GLMM) were constructed to test for variation in the abundance of Anopheles between case and control houses, and indoor and peri-domestic settings. In the analysis, household type (case or control) and location (indoor or outdoor) were considered as fixed effects, while month, night and sampling station (site) as random effects. To identify the best model, both negative binomial and Poisson distributions, interaction between type and location, as well as zero inflation were fitted. Tukey's Post Hoc test was used to compare mean between fix effects (household type and location) as well interaction between these two effects.
We also analysed the proportion of mosquitoes that were caught feeding outdoors (Po), and the proportion of human exposure to An. balabacensis (Pe).
The proportion of mosquitoes that were caught outdoors (Po) was calculated as
| (1) |
where O and I are respectively the number of mosquitoes caught biting outdoors and indoors during 6 pm– 6 am.
From interviewing the residents and observation, more than 50% of the villagers would be indoors by 8 pm, and out the next morning by 5 am as they go to the plantations to work. The proportion of human exposure to An. balabacensis (Pe) is thus calculated as the proportion of bites that happen outdoors during the time when people are likely to be outdoors, out of the sum of bites expected throughout the night as humans move between indoor and outdoor areas of their home:
| (2) |
where O18-20, 05 h represents the mosquitoes caught biting from 6–8 pm, and 5-6am, and I20-5h represents the number caught indoors between 8 pm– 5 am. This would give a comparison between the proportion of bites people exposed to when outdoors between case and control households.
GLMM with a binomial distribution and a logit link function was used to obtain the binary estimates of Po and Pe. In these models, household type (case or control) was fitted as a fixed effect, while sampling station of the case as a random effect.
Ethical clearance
This project was approved by the NMRR Ministry of Health Malaysia (NMRR-12-786-13048). All volunteers who carried out mosquito collections signed informed consent forms and were provided with antimalarial prophylaxis during participation. House owners also gave permission to use their houses for collection of mosquitoes.
Results
Employment in agricultural sector among case and control households
Among those who worked in the agricultural sector (Table 1), more than double the number cases were employed on rubber or oil palm plantations than controls, while more controls worked in the vegetable farms than cases.
Composition of Anopheles spp.
A total of 793 Anopheles belonging to 12 species were caught during the period of study, with An. balabacensis being the dominant species (81% of total), followed by An. maculatus, An. barbumbrosus, and An. donaldi (Table 2). Overall, more An. balabacensis were caught at case (total 392 or 1.81 bites per man per night) than control houses (total 249 or 1.15 bites per man per night). Ten and 9 different Anopheles species were collected at case and control houses respectively, compared to only 6 and 2 indoor collections from case and control. Higher numbers were recorded at Kg. Tinukadan Laut (CC24), Kpg. Paradason B (CC26), Kpg. Nangka (CC5) (S1 Fig).
Table 2. Anopheles species caught outdoors and indoors at the case and control houses.
| Anopheles spp. | Indoors | outdoors | total | % | ||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||
| An. balabacensis Baisas | 18 | 16 | 374 | 233 | 641 | 80.83 |
| An. maculatus Theobald | 1 | 1 | 12 | 35 | 49 | 6.18 |
| An. barbumbrosus Strickland & Chowdhury | 2 | 0 | 24 | 11 | 37 | 4.67 |
| An. donaldi Reid | 1 | 0 | 7 | 26 | 34 | 4.29 |
| An. subpictus Grassi | 2 | 0 | 2 | 8 | 12 | 1.51 |
| An. tessellatus Theobald | 0 | 0 | 2 | 6 | 8 | 1.01 |
| An. peditaeniatus Leicester | 0 | 0 | 2 | 1 | 3 | 0.38 |
| An. whartoni Reid | 0 | 0 | 0 | 3 | 3 | 0.38 |
| An. umbrosus Theobald | 0 | 0 | 2 | 0 | 2 | 0.25 |
| An. indefinitus Ludlow | 1 | 0 | 1 | 0 | 2 | 0.25 |
| An. kochi Dönitz | 0 | 0 | 1 | 0 | 1 | 0.13 |
| An. baezai Gater | 0 | 0 | 0 | 1 | 1 | 0.13 |
| Total species | 6 | 2 | 10 | 9 | - | - |
| Total individuals | 25 | 17 | 427 | 324 | 793 | 100.00 |
| Percentage | 5.5 | 5.0 | 95.5 | 95.0 | ||
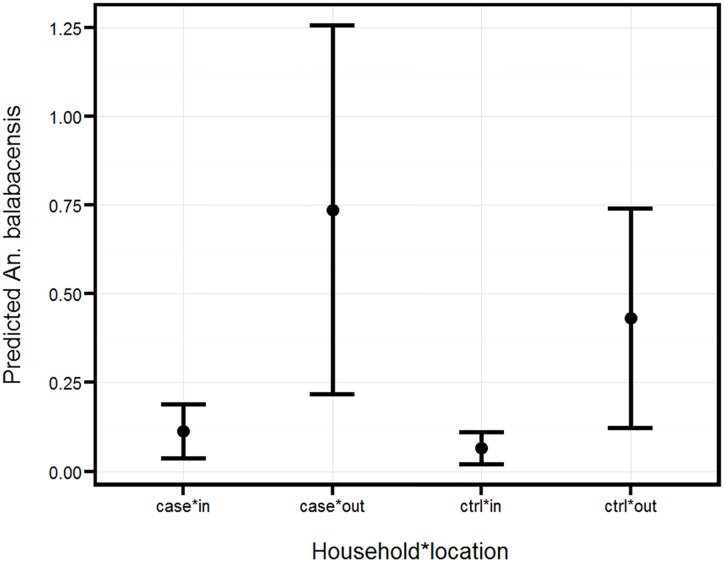
GLMM analysis indicated that the negative binomial distribution gave a better fit than Poisson distribution. The log-likelihood values were for negative binomial and Poisson distribution -513 vs -529 respectively, while the Akaike information criterion (AIC) values were 1043 vs 1068. Adopting the model with a negative binomial distribution, the abundance of An. balabacensis was found to vary significantly between case and control (case 0.28±0.194 vs control 0.17±0.127, z = 4.62, p<0.001), and between the surrounding peri-domestic area and inside the house (0.56± 0.394 versus 0.09±0.063, z = 9.09, p<0.001) (Fig 2). The interaction between house type and location was not significant (z = 0.8, P>0.05)
Fig 2. Mean number ± SE of An. balabacensis as predicted by GLMM.
The value represents number caught per house per night from the case and control houses outdoors and indoors.
Biting time of An. balabacensis
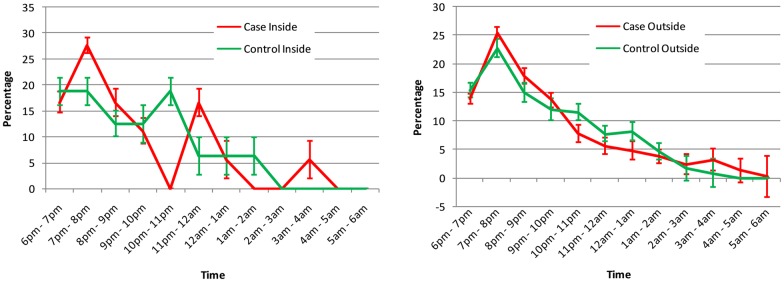
More than 50% of An. balabacensis mosquitoes were caught biting in the early evening (6pm - 9pm) with the peak hour between 7pm - 8pm (Fig 3). After 8pm, the number rapidly decreased with approximately 84% of the total nightly catch being accumulated by midnight. Nevertheless, one or two individuals of An. balabacensis could still be caught until dawn.
Fig 3. Biting time of An. balabacensis mosquitoes caught indoors and outdoors at the case and control houses.
Data represent percentage of An. balabacensis (out of total collected) for each hour for the whole study period. Error bar represent 95% standard error of the mean.
In general, the proportion of bites taken by An. balabacensis outdoors (Po) was very high (>95%), and did not vary between case and control households (Table 3). Similarly the proportion of human exposure to bites (Pe) did not vary between case and control households.
Table 3. GLMM analysis of proportion of An. balabacensis biting occurring outdoors (Po) and proportion of human outdoor exposure to mosquito bites (6–8 pm and 5–6 am) (Pe).
In the analyses household (case or control) was considered as the fixed effect.
| Parameter | Coefficients of GLMM | Proportion of biting for household type | Tukey’s test case vs control | ||
|---|---|---|---|---|---|
| Intercept | Household | Case | Control | ||
| Po | 3.343 | -0.258 | 0.9659 | 0.9563 | p>0.05 |
| Pe | 4.074 | -0.469 | 0.9833 | 0.9735 | p>0.05 |
Infection rate of An. balabacensis
A total of 793 Anopheles individuals were tested by molecular method and only ten An. balabacensis (out of 641 or 1.56%) were found to be positive for malaria parasites. Seven of them were caught at case houses (6 outdoors and one indoors), and 3 at control houses (all outdoors, Table 4). All these mosquitoes were found to be positive for simian malaria parasites (P. coatneyi, P. inui, P.cynomologi & P. fieldi); but none were with either the dominant zoonotic parasite reported in the area (P. knowlesi) or any human-specific Plasmodium. Ninety percent of infected An. balabacensis was caught biting outdoors between 6pm - 10pm (9 out of 10), and with one infected individual caught between 1am - 2am.
Table 4. Plasmodium spp. found in the infected An. balabacensis by PCR analysis.
| Plasmodium spp. | No. infected | House | Site | Time | Reference No. |
|---|---|---|---|---|---|
| P. coatneyi | 1 | Control | Outdoor | 7pm | CC16 |
| P. inui | 2 | Case | Outdoor | 7pm | CC23 |
| Case | Outdoor | 1am | CC24 | ||
| P. cynomolgi | 3 | Case | Outdoor | 8pm | CC8 |
| Case | Outdoor | 9pm | CC26 | ||
| Control | Outdoor | 7pm | CC24 | ||
| P. inui + P. fieldi | 2 | Case | Outdoor | 6pm | CC15 |
| Case | Indoor | 7pm | CC15 | ||
| P. inui + P. cynomolgi | 1 | Case | Outdoor | 9pm | CC23 |
| P. inui + P. fieldi + P. cynomolgi | 1 | Control | Outdoor | 8pm | CC15 |
The proportion of infected An. balabacensis caught from case houses (7/392 or 1.79%) was slightly higher than at control houses (3/249 or 1.20%). However, the sample sizes were too low for any robust statistical analysis of differences.
Discussion
We conducted a randomized case-control field study to test the hypothesis that there is an association between P. knowlesi infection risk and higher exposure to mosquito vectors in peri-domestic (outdoors surrounding houses) and within domestic (inside house) settings. Although 12 Anopheles species were caught by HLC, only 4 were detected in reasonably high abundance: An. balabacensis, An. maculatus, An. barbumbrosus and An. donaldi. Of these, only An. balabacensis and An. donaldi have been previously implicated as malaria vectors of human malaria in Sabah [18,28]. In contrast, An. maculatus is the main malaria vector of human malaria in peninsular Malaysia [29]. Anopheles balabacensis appears to be a widespread species found in almost all sites, although significantly high numbers were caught in Kpg Tinukadan Laut (CC24) especially in those areas near to forest fringes. About 95% of An. balabacensis was caught outdoors, similar to what was previously recorded (a ratio of outdoor:indoor catch of 24:1) in Kuala Penyu, another district in Sabah [28]. A recent study conducted in Banggi Island situated north of Sabah and in Kg Paradason in the interior of Kudat district [10] also revealed that An. balabacensis was the predominant species collected, followed by An. donaldi in both sites. However, the next most abundant species was An. vagus, in Banggi, but An. barbirostris group in Kg Paradason. Anopheles maculatus was not caught in Banggi.
GLMM analysis indicated a significant difference between the number of vectors caught at case and control houses, and between outdoor and indoor catches. The primary vector of P. knowlesi in the area, An. balabacensis, was present at higher abundance at households where cases were reported, which would suggest a higher risk at the case houses. Furthermore, as 90% of the infected mosquitoes were caught outdoors, it is likely that peri-domestic infection is an important risk factor. Although the indoor number of infective mosquitoes caught was small, getting infected indoors cannot be discounted.
In Sarawak, it was postulated that humans were likely to acquire infection of P. knowlesi from being bitten by infected An. latens while hunting in the forest or as they return from the farm around dusk since in their study no infective mosquitoes were obtained from the village [13]. However, in Sabah clustering of cases among family members have been reported and they postulated that people could be infected around their homes [30]. A recent study also in Sabah showed the presence of asymptomatic cases of P. knowlesi occurring among the community in Kudat [31]. Thus, the result of this study seems to support the hypothesis that it is also possible for people to be infected in and around their homes. Although An. balabacensis is highly exophagic with only one infected individual found indoors we should not dismiss the fact that the possibility of indoor infection does exist. Thus we need to possibly expand our paradigm about transmission of P. knowlesi to include the possibility of peri-domestic infection, and conduct further studies to evaluate simultaneously the infection risk in and around households, as well as in forest areas, so the relative contribution of all these routes could be formally quantified.
Many areas in Kudat district have undergone deforestation and clearance of vegetation for crop plantations, but it appears that An. balabacensis has remained the dominant species, with the exception of Kinabatangan area of Sabah where An. balabacensis was replaced by An. donaldi as main malaria vector as a result of deforestation and malaria control activity [18]. This suggests that the abundance of An. balabacensis in Kudat district was not greatly affected by the environmental changes. The impact of forest disturbance such as logging has been shown to increase the abundance of this disease vector and may partly explain the rapid rise in P. knowlesi cases in Sabah [32].
The feeding time of An. balabacensis appears to have changed since late 1960s when most of the area in Kudat district was still covered with forest. A study conducted then [29] showed that An. balabacensis was actively biting human at late night (10pm onwards), compared to early night with peak hour between 7pm to 8pm recorded now. In fact, this species starts biting human outdoors as soon as it starts to get dark. This change in feeding time could be due to An. balabacensis adapting to more people staying closer to forest fringe as more forested areas are cleared for crop plantation and housing. This could also be due to the introduction of insecticide treated bednets. Further study will be needed to confirm this.
Although we did not obtain an An balabacensis individual infected with P. knowlesi, as only ten individuals were found Plasmodium positive albeit for other simian malarias, this could be a sampling error. As such, we are unable to make a conclusive prediction about infection risk at case households. Given the generally low rates of P. knowlesi infection in the vector (eg 13/1482 or 0.88%, data also collected in Kudat) [10], thousands of samples are needed to obtain strong evidence to show that P. knowlesi was not present, and/or to compare infection rates between case and control households. Furthermore, as the densities of vectors in these settings are generally low, it would not have been feasible to achieve this sample size within the one year time span that the case control study was running. What data collected here do show however is that the primary vector is present at higher abundance in peridomestic settings where cases are reporting, on which basis the possibility of peridomestic transmission cannot be dismissed. This also indicates that people are routinely exposed to a variety of different primate malarias around their home; but that to date, only a couple (knowlesi and cynomologi) seem capable to causing any clinical infection. More research needs to be carried out to determine why these two primate malarias succeeded where the others fail so we can be proactive in the fight against future new simian malarias infecting man.
The difference between bites per man per night between case and control houses is 0.66 (1.81–1.15) which works out to be 241more bites per person in a year for the case house. Since the infective proportion of vector is 0.88 [10], this is equivalent to a higher entomological inoculation rate (EIR) of 2.12. In addition, more than double the number cases worked in rubber or oil palm plantations than controls. Perhaps these two factors may help explain why there was P. knowlesi infection in the case houses. However more research is needed to validate this.
As most P. knowlesi cases have been recorded from villages close to where macaques abound, and given that the primary vectors species, An balabacensis bite outdoors, a new paradigm in managing this malaria is needed. More attention should be focused on the ecology and biology of An. balabacensis in order to develop more effective control methods if the control or elimination of P. knowlesi malaria in Kudat district is to be successful. The current malaria control programme using ITNs might not have the desired impact as this species is mainly an exophagic species, and infection is more likely to occur outdoors in peri-domestic settings, in plantations and forest.
Supporting Information
(PDF)
(PDF)
(PDF)
(TIF)
(PDF)
Acknowledgments
We acknowledge the Universiti Malaysia Sabah for all the research facilities provided, Dr. Matthew J. Grigg for the case information, Mr. Fazreen and Mr. Nemran for helping in the field work.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by: Medical Research Council, Natural Environment Research Council, Economic and Social Research Council, and Biotechnology and Biosciences Research Council for the funding received for this project through the Environmental & Social Ecology of Human Infectious Diseases Initiative (ESEI), MRC Grant Number: G1100796. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. (2015) The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, et al. (2004) A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363: 1017–1024. 10.1016/S0140-6736(04)15836-4 [DOI] [PubMed] [Google Scholar]
- 3.Lalremruata A, Magris M, Vivas-Martinez S, Koehler M, Esen M, Kempaiah P, et al. (2015) Natural infection of Plasmodium brasilianum in humans: Man and monkey share quartan malaria parasites in the Venezuelan Amazon. EBioMedicine 2: 1186–1192. 10.1016/j.ebiom.2015.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KS, Divis PC, Zakaria SK, Matusop A, Julin RA, Conway DJ, et al. (2011) Plasmodium knowlesi: reservoir hosts and tracking the emergence in humans and macaques. PLoS Pathog 7: e1002015 10.1371/journal.ppat.1002015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KS, Vythilingam I (2013) Plasmodium knowlesi: Emergent human malaria in Southeast Asia In: Lim YAL, Vythilingam I, editors. Parasites and their vectors A special focus on Southeast Asia Heidelberg: Springer; 10.1007/978-3-7091-1553-4_2 [DOI] [Google Scholar]
- 6.William T, Rahman HA, Jelip J, Ibrahim MY, Menon J, Grigg MJ, et al. (2013) Increasing incidence of Plasmodium knowlesi malaria following control of P. falciparum and P. vivax Malaria in Sabah, Malaysia. PLoS Negl Trop Dis 7: e2026 10.1371/journal.pntd.0002026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber BE, William T, Jikal M, Jilip J, Dhararaj P, Menon J, et al. (2011) Plasmodium knowlesi malaria in children. Emerg Infect Dis 17: 814–820. 10.3201/eid1705.101489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM (2014) First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J 13: 68 10.1186/1475-2875-13-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coatney GR (1968) Simian malarias in man: facts, implications, and predictions. Am J Trop Med Hyg 17: 147–155. [DOI] [PubMed] [Google Scholar]
- 10.Wong ML, Chua TH, Leong CS, Khaw LT, Fornace K, Wan-Sulaiman WY, et al. (2015) Seasonal and Spatial Dynamics of the Primary Vector of Plasmodium knowlesi within a Major Transmission Focus in Sabah, Malaysia. PLoS Negl Trop Dis 9: e0004135 10.1371/journal.pntd.0004135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vythilingam I, Lim YA, Venugopalan B, Ngui R, Leong CS, Wong ML, et al. (2014) Plasmodium knowlesi malaria an emerging public health problem in Hulu Selangor, Selangor, Malaysia (2009–2013): epidemiologic and entomologic analysis. Parasit Vectors 7: 436 10.1186/1756-3305-7-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wharton RH, Eyles DE (1961) Anopheles hackeri, a vector of Plasmodium knowlesi in Malaya. Science 134: 279–280. 10.1126/science.134.3474.279 [DOI] [PubMed] [Google Scholar]
- 13.Vythilingam I, Tan CH, Asmad M, Chan ST, Lee KS, Singh B (2006) Natural transmission of Plasmodium knowlesi to humans by Anopheles latens in Sarawak, Malaysia. Trans R Soc Trop Med Hyg 100: 1087–1088. 10.1016/j.trstmh.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 14.Tan CH, Vythilingam I, Matusop A, Chan ST, Singh B (2008) Bionomics of Anopheles latens in Kapit, Sarawak, Malaysian Borneo in relation to the transmission of zoonotic simian malaria parasite Plasmodium knowlesi. Malar J 7: 52 10.1186/1475-2875-7-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiram AI, Vythilingam I, NoorAzian YM, Yusof YM, Azahari AH, Fong MY (2012) Entomologic investigation of Plasmodium knowlesi vectors in Kuala Lipis, Pahang, Malaysia. Malar J 11: 213 10.1186/1475-2875-11-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakazawa S, Marchand RP, Quang NT, Culleton R, Manh ND, Maeno Y (2009) Anopheles dirus co-infection with human and monkey malaria parasites in Vietnam. Int J Parasitol 39: 1533–1537. 10.1016/j.ijpara.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 17.Marchand RP, Culleton R, Maeno Y, Quang NT, Nakazawa S (2011) Co-infections of Plasmodium knowlesi, P. falciparum, and P. vivax among Humans and Anopheles dirus Mosquitoes, Southern Vietnam. Emerg Infect Dis 17: 1232–1239. 10.3201/eid1707.101551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vythilingam I, Chan ST, Shanmugratnam C, Tanrang H, Chooi KH (2005) The impact of development and malaria control activities on its vectors in the Kinabatangan area of Sabah, East Malaysia. Acta Trop 96: 24–30. 10.1016/j.actatropica.2005.06.022 [DOI] [PubMed] [Google Scholar]
- 19.Rundi C (2011) Malaria Elimination in Malaysia. Third annual APMEN technical and business meeting, 9–12. May 2011; Kota Kinabalu, Malaysia. 2011.
- 20.William T, Jelip J, Menon J, Anderios F, Mohammad R, Awang Mohammad TA, et al. (2014) Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malar J 13: 390 10.1186/1475-2875-13-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fornace KM, Abidin TR, Alexander N, Brock P, Grigg MJ, Murphy A, et al. (2016) Association between Landscape Factors and Spatial Patterns of Plasmodium knowlesi Infections in Sabah, Malaysia. Emerg Infect Dis 22: 201–208. 10.3201/eid2202.150656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigg MJ, William T, Drakeley CJ, Jelip J, von Seidlein L, Barber BE, et al. (2014) Factors that are associated with the risk of acquiring Plasmodium knowlesi malaria in Sabah, Malaysia: a case-control study protocol. BMJ Open 4: e006004 10.1136/bmjopen-2014-006004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallum MAM, Peyton EL, Harrison BA, Wilkerson RC (2005) Revision of the Leucosphyrus group of Anopheles (Cellia) (Diptera, Culicidae) Rev Bras entomol 49: 1–152. 10.1590/S0085-56262005000500001 [DOI] [Google Scholar]
- 24.Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Coleman RE (2006) Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian J Trop Med Public Health 37 Suppl 2: 1–128. [PubMed] [Google Scholar]
- 25.Phillips A, Simon C (1995) Simple, efficient, and nondestructive DNA extraction protocols for arthropods Annals of the Entomological Society of America 88 281–283. 10.1093/aesa/88.3.281 [DOI] [Google Scholar]
- 26.Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA (1999) A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg 60: 687–692. [DOI] [PubMed] [Google Scholar]
- 27.Imwong M, Tanomsing N, Pukrittayakamee S, Day NPJ, White NJ and Snounou G (2009). Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J Clinical Microbiol 47: 4173–4175. 10.1128/JCM.00811-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hii JL (1985) Evidence for the existence of genetic variability in the tendency of Anopheles balabacensis to rest in houses and to bite man. Southeast Asian J Trop Med Public Health 16: 173–182. [PubMed] [Google Scholar]
- 29.Reid JA (1968) Anopheline mosquitoes of Malaya and Borneo Staples Printers Limited at their Kettering, Northants: Government of Malaysia; 520 p. [Google Scholar]
- 30.Barber BE, William T, Dhararaj P, Anderios F, Grigg MJ, Yeo TW, et al. (2012) Epidemiology of Plasmodium knowlesi malaria in north-east Sabah, Malaysia: family clusters and wide age distribution. Malar J 11: 401 10.1186/1475-2875-11-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fornace KM, Nuin NA, Betson M, Grigg MJ, William T, Anstey NM, et al. (2016) Asymptomatic and Submicroscopic Carriage of Plasmodium knowlesi Malaria in Household and Community Members of Clinical Cases in Sabah, Malaysia. J Infect Dis 213: 784–787. 10.1093/infdis/jiv475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brant HL, Ewers RM, Vythilingam I, Drakeley C, Benedick S, Mumford JD (2016) Vertical stratification of adult mosquitoes (Diptera: Culicidae) within a tropical rainforest in Sabah, Malaysia. Malar J 15:370 10.1186/s12936-016-1416-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper.