Abstract
Biomarkers have the potential to expedite drug development, increase patient safety, and optimize clinical response. Yet few have achieved regulatory qualification. A survey was conducted to clarify industry's perspective on biomarker qualification and identify the most promising biomarkers for drug development. The results across toxicities/clinical areas highlight challenges in regulatory qualification, although early prioritization and alignment on an evidentiary standard framework are key factors in facilitating biomarker development and qualification.
THE NEED FOR A TRANSPARENT AND MULTIPLE‐STAKEHOLDER APPROACH TO BIOMARKER QUALIFICATION
Drug development remains costly and time‐intensive,1, 2, 3 and it is clear that improvements in the drug development process have not kept pace with biomedical and technical advances.4, 5, 6 Recent initiatives to expedite the drug development process have included an increased focus on drug development tools such as novel clinical and nonclinical biomarkers.7, 8, 9, 10, 11 Biomarkers are measurable physiological properties that can be evaluated as indicators of “normal biologic processes, pathologic processes, or biological responses to a therapeutic intervention.”4 The use of biomarkers can enhance drug development in a number of ways.12, 13, 14, 15, 16, 17 For example, by signaling previously unobserved drug toxicity in nonclinical trials, new translational biomarkers may increase safety of phase III trials. The potential to further enhance safety in clinical trials may be realized through the effective monitoring of novel acute toxicity biomarkers that can register reversible toxicity, thus enabling participant withdrawal before the onset of permanent tissue damage.18, 19 Beyond advantages related to safety, the results of nonclinical trials may clarify biochemical processes underlying disease pathology, thereby aiding in the development of targeted medications and safer, more efficient drug development.20 Furthermore, nonclinical biomarkers that accurately reflect human physiological responses may salvage compounds that are safe and effective in humans, but would otherwise have been abandoned due to negative findings with older nonclinical safety assays.21, 22 By revealing physiological differences among patients, biomarkers with increased sensitivity and specificity also have the potential to enable the production of safe medications targeted to particular patient subpopulations.23, 24
In order for a biomarker to be considered during the development process for a therapeutic, it must either have been approved through direct interactions with US Food and Drug Administration (FDA) staff or through the FDA's Biomarker Qualification program.4 Biomarker qualification is defined by the FDA as “a conclusion that within the stated context of use, a biomarker can be relied upon to have a specific interpretation and application in drug development and regulatory review.”4 Despite the promise of biomarkers in facilitating the identification and approval of safe and effective drugs, and even with multiyear coordinated efforts from government, industrial, academic, and nonprofit sectors through consortia such as the Biomarker Consortium and the Critical Path Institute (C‐Path), only five sets of biomarkers (three nonclinical, two clinical) have been qualified25, 26, 27 since the inception of the Biomarkers Qualification Program in 2007.28 Possible explanations for this slow evolution include the prohibitive costs incurred by pharmaceutical companies to independently develop both biomarkers and the tools to effectively measure them,17 the long timeline29 and complexities inherent to gaining regulatory approval of biomarkers,16 and the challenges in translating nonclinical biomarkers to clinical biomarkers. Such difficulties can include the lack of universally accepted tools and measurement techniques (e.g., the lack of an effective clinical analog for histological analysis). The resulting uncertain evidentiary standards further complicate the regulatory approval process. Meetings between key stakeholders such as the Pharmaceutical Research and Manufacturers of America (PhRMA); FDA workshop of 2007,30 the Howard Hughes Medical Institute; FDA Biomarkers Workshop of 2013; the PDUFA V public meeting in September of 2014,31 and the more recent FDA, C‐Path, and the University of Maryland Center of Excellence in Regulatory Science and Innovation (M‐CERSI) meeting in August of 2015 are positive steps towards addressing these limitations to biomarker development and qualification.
In January 2014, the Center for Drug Evaluation and Research (CDER) finalized guidance documents to outline and clarify the evaluation process the CDER uses in its decision‐making on biomarker qualification.4 At the same time, the CDER and the National Institutes of Health encouraged industry partners to gather data on the needs and challenges in biomarker development and use, and the related impact biomarkers are perceived to have on drug development. A recent publication spearheaded by the FDA summarizes the updated biomarker qualification processes and highlights the importance of coordinating efforts from multiple stakeholders, stating “we need to have the help of industry, government entities, and academia to help us determine what levels of evidence befit different types of biomarkers, based on their context of use.”26
In response, PhRMA and the Biotechnology Industry Organization (BIO) commissioned a survey of industry experts to better understand industry's current perspective on biomarker qualification. The survey sought to identify the following: challenges to biomarker qualification, high‐interest contexts of use for biomarkers, evidentiary standards for high‐interest contexts of use, and potential biomarkers of organ toxicity and clinical efficacy that appear most promising and urgent for drug development. A “context of use” (COU) is a complete and precise statement that describes the appropriate use of the biomarker, and how the qualified biomarker is applied in drug development and regulatory review.
Here we provide a general overview of industry experience with biomarker qualification through a brief summary of key survey results. Survey findings highlight both the importance of biomarkers to expedite drug development and the challenges associated with achieving qualification. Finally, we present recommendations to increase the efficiency of biomarker development and qualification.
SURVEY RESULTS: EXPERT INSIGHTS ON BIOMARKER DEVELOPMENT
In April 2014, we collected survey responses from 24 PhRMA and BIO member‐companies comprised of 18 publicly traded (nine mega cap, seven large cap, and two mid cap) and six privately financed companies.
The survey was designed by PhRMA and BIO staff in collaboration with third‐party consultants, and consisted of ∼300 discrete query elements. For both nonclinical and clinical safety biomarkers, as well as clinical efficacy biomarkers, respondents were asked to rate the barriers to (i.e., scientific, technical, and regulatory “complexity”), as well as the potential benefits of (i.e., projected increase of efficiency in drug development), regulatory qualification. In addition, respondents identified the highest interest COUs for these biomarkers, as well as the related evidentiary standards (ES) that should support regulatory qualification for those specific COUs. Lastly, respondents were permitted free text responses to explain and expand upon their rating choices and selections. Responses for the topics above were collected and considered for safety and clinical efficacy biomarkers separately (Figure 1).
Figure 1.
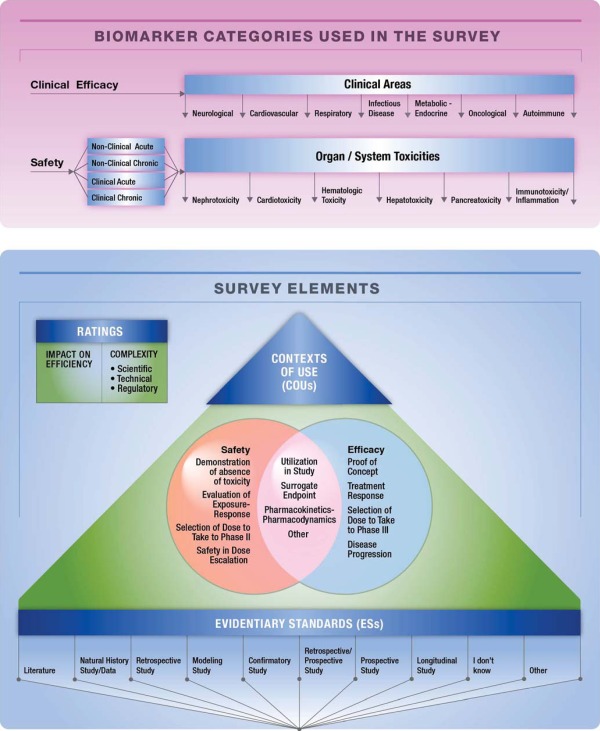
Survey design. Survey participants were asked to rate the complexity of biomarker qualification and the impact qualified biomarkers may have on the efficiency of drug development. They were also asked to select the most important context of use (COU), and the most correspondent relevant supporting evidentiary standards (ESs) for qualification of a biomarker within a particular efficacy or safety area. Participants were each asked to identify and consider acute and chronic, and clinical and nonclinical safety and efficacy biomarkers relevant to their disciplines. Biomarkers of safety spanned the six given toxicity areas. Biomarkers of clinical efficacy spanned over the seven given therapeutic areas.
The ideal biomarker in any category would have a high score for positive impact on the efficiency of drug development with a relatively low score in complexity. The industry survey found that most safety and efficacy biomarkers scored high on both dimensions, indicating that most biomarkers considered to have high value may be limited by the scientific, regulatory, or technical complexity required for regulatory qualification. Exceptions included the following: nonclinical acute and chronic hematologic toxicity biomarkers had low scores for both efficiency and complexity; clinical acute and chronic pancreatoxicity biomarkers were thought to be highly complex to qualify, while having low potential impact on the efficiency of drug development. The free response sections of the survey revealed that researchers believed effective biomarkers for hematologic toxicity already existed, and that pancreatoxicity occurred too rarely to warrant investigation of new biomarkers.
Clinical efficacy biomarkers for cardiovascular disorders were considered highly complex to qualify and were thought to have medium potential to improve efficiency of drug development. Although no biomarkers in this survey scored high in impact and low in complexity, the qualification of biomarkers in certain therapeutic areas consistently showed high scores with respect to potential positive impact on drug development for the treatment of particular disorders, including: heart failure, coronary heart disease, Alzheimer's disease, multiple sclerosis, solid tumors, and systemic lupus erythematosus.
Participants reported that regulatory complexity posed a greater challenge to biomarker qualification than scientific or technical complexity for a number of biomarkers. Of the safety biomarkers sharing this characteristic, clinical acute biomarkers for hepatotoxicity and nephrotoxicity were perceived to have a high potential to increase efficiency of drug development (Figure 2 a). According to the free responses, efficiency of drug development would increase by allowing differentiation between adverse and nonadverse organ responses, and also through earlier identification of drug‐related tissue damage, possibly while it was still reversible. In contrast, biomarkers for pancreatoxicity and cardiotoxicity were reported to have a low or intermediate potential, respectively, to increase the efficiency of drug development (Figure 2 a)—pancreatoxicity, for reasons already discussed, and cardiotoxicity because sensitive and specific biomarkers for this organ toxicity are already well established. Regulatory complexity appeared to be the most significant challenge to the qualification of clinical efficacy biomarkers for metabolic/endocrine and cardiovascular disorders as well (Figure 2 e).
Figure 2.
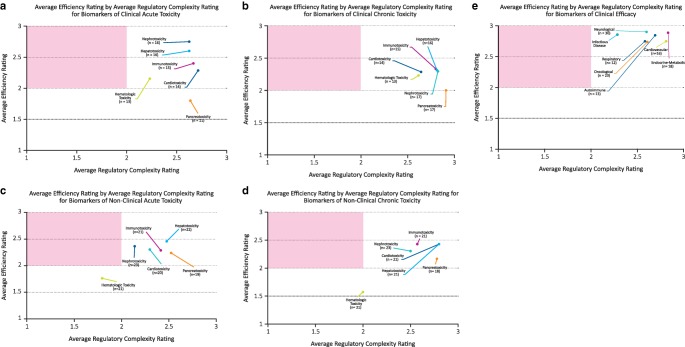
Efficiency and complexity. Average efficiency by average regulatory complexity rating for safety and efficacy biomarkers. The x‐axis reflects the mean respondent rating for barriers to regulatory qualification, or “regulatory complexity” for biomarkers in a particular safety or efficacy category. The y‐axis reflects mean respondent rating for the projected increase in efficiency of drug development, were the biomarker(s) under consideration to gain regulatory qualification. The red quadrant of each graph represents the location of “ideal” biomarkers, or biomarkers that have the potential to have a high impact on the efficiency of drug development, but whose qualification is associated with a low level of regulatory complexity.
When asked to identify the most useful COU for nonclinical safety biomarkers, “to demonstrate absence of toxicity without histopathology” was the most frequently selected, and was deemed most important for acute and chronic cardiotoxicity, acute hematologic toxicity, acute hepatotoxicity, and acute and chronic nephrotoxicity. For clinical safety biomarkers, the most consistently selected COU was “demonstration of absence of toxicity,” being cited as most important for all organ toxicities except acute nephrotoxicity. However, the COU most frequently cited for clinical efficacy varied by clinical area. For example, “treatment response” was most commonly noted for infectious diseases and autoimmune, oncological, and respiratory disorders. Free responses from survey participants indicated that since the disorders in these clinical areas are often characterized by heterogeneous symptoms and show variable responses to drugs, biomarkers that enable early and effective evaluation of treatment will result in cheaper and more streamlined clinical trials. In contrast, “surrogate endpoint” was most often selected as the most appropriate COU for cardiovascular disorders. Most cardiovascular disorder survey respondents who selected “surrogate endpoint” studied heart failure, and unsurprisingly noted that qualified biomarkers would result in speedier and safer clinical trials. “Utilization in study” was most frequently noted for neurological disorders and tied with “proof of concept” as one of the two COU selections most frequently cited for metabolic disorders (Figure 3). For both clinical areas, participants selecting “utilization in study” as the most significant COU noted that qualified biomarkers granting the ability to enroll specific patient subpopulations from a studied disorders would result in faster, cheaper, and more informative clinical trials, thus increasing the speed to market of effective treatment options. Respondents who selected “proof of concept” most frequently for metabolic disorders indicated in their free responses that such biomarkers would increase understanding of currently ambiguous disease mechanisms.
Figure 3.
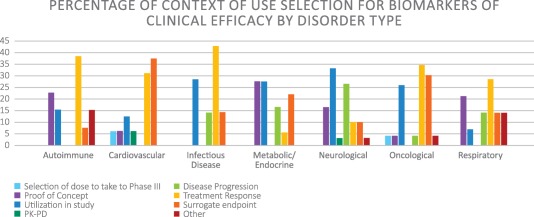
Distribution of context of use selection for efficacy biomarkers by clinical area.
After the COU was selected, respondents were asked to select the combination of ESs that they felt were sufficient to support the COU. For acute nonclinical toxicity biomarkers with a COU of “demonstrated absence of toxicity without histopathology,” the majority of the ESs selected were “literature,” “confirmatory studies,” and “prospective/retrospective studies” (Figure 4). However, for chronic nonclinical toxicity biomarkers with the same COU, there was an increased selection of “longitudinal studies,” in addition to the evidentiary standards that were selected for the acute nonclinical safety biomarkers (Figure 4). Responses to questions posed about clinical biomarkers for efficacy (rather than safety) had a different pattern due to the specific needs of the disease areas, as well as small responses rates that made generalization difficult. For example, “utilization in study” was selected as the most important COU for both neurological as well as metabolic‐endocrine disorders. However, “retrospective/prospective studies,” “literature,” and “natural history” were the three most frequently cited ESs to support this COU for neurological disorders, while “confirmatory” and “prospective” studies were the most frequently cited ESs to support the COU for metabolic‐endocrine disorders. There were also some interesting signals related to the selection of highest‐impact COUs for particular disorders within therapeutic areas. For example, within the autoimmune therapeutic area, the majority of respondents reported the need for efficacy biomarkers for treatments of systemic lupus erythematosus (SLE) indicated an interest in having a biomarker qualified for “proof of concept.” This is consistent with the need to fully define the underlying causes and progression of SLE.32 In addition, there was strong support for a qualified biomarker in Alzheimer's disease that could be used to enroll specific populations in clinical trials; in lung cancer, the desire for qualified biomarkers that could predict treatment response was most frequently identified. Due to the limited number of participant responses for each disease within a particular clinical efficacy area, it is difficult to interpret the significance of the above differences.
Figure 4.
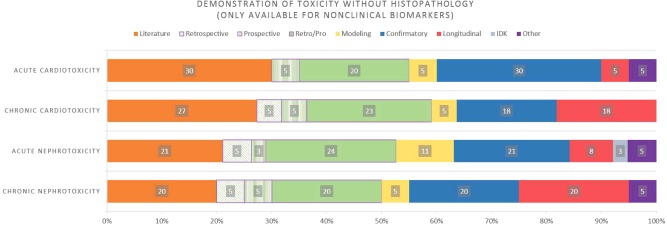
Distribution of evidentiary standard selection for the context of use, “demonstration of toxicity without histopathology” for nonclinical safety biomarkers. Evidentiary standard options for selection were: Literature, Retrospective Studies, Prospective Studies, Retrospective/Prospective Studies, Modeling Studies, Confirmatory Studies, Longitudinal Studies, I Don't Know (IDK), and Other.
BROAD IMPLICATIONS OF SURVEY RESULTS
Industry experts remain largely engaged and continue to believe in the promise of qualified biomarkers to expedite and improve the efficiency of drug development. Even so, as seen through the survey results and free responses from survey participants, regulatory and scientific complexity remain a significant challenge to regulatory qualification of biomarkers. The survey results do show agreement among responders about the most urgent needs for qualified biomarkers in specific organs/systems and disease areas. Furthermore, there is good baseline agreement on general evidentiary standards for specific COUs.
SURVEY RESULTS IN THE CONTEXT OF THE FDA BIOMARKER QUALIFICATION REGULATORY PROCESS
As mentioned above, in July of 2015, the FDA and CDER published an article on the current state of biomarker qualification and its associated regulatory processes.26 The authors emphasized the need for increasing regulatory scrutiny as the significance of a biomarker increased from exploratory to decisional. However, they also acknowledged a lack of definition of appropriate evidentiary standards for different kinds of biomarkers or for particular COUs.
Our findings suggest that such definitions are indeed possible, but may need to be considered in light of 1) the kind of biomarker they are meant to support: safety or efficacy, and 2) the therapeutic area in which the biomarker will be applied. Such definitions have the potential to significantly clarify the regulatory process for biomarker qualification and, thus, speed the development of safe and effective drugs.
FUTURE DIRECTIONS
The question remains, then, how can we improve the efficiency and predictability of regulatory qualification of biomarkers? As has been pointed out most recently by the CDER,26 the answer likely lies in the expansion of dialog between the FDA and scientific experts from academia, patient organizations, government, the nonprofit sector, and industry.
By adopting the following two goals, we hope the ensuing dialog optimizes the predictability and consistency of regulatory qualification. Our survey has clearly shown that not all biomarkers have equal potential to enhance the efficiency of drug development. Therefore, it is pivotal to initiate dialog among scientific experts to first enable identification of specific biomarkers that hold the promise to expedite the delivery of drugs to patients in need. To achieve true improvement, the second goal should be to establish an “evidentiary standards framework” wherein consistently agreed‐upon levels of evidence are applied to regulate qualification of biomarkers of a given type, or within particular clinical areas.
To improve transparency, ensure public documentation, and facilitate targeted discussion, PhRMA, BIO, and the FDA remain in ongoing discussions regarding these survey data, their implications, and how to best publicly disseminate additional findings.
ACKNOWLEDGEMENTS
Financial support was provided by PhRMA and BIO to FaegreBD Consulting to aid in survey development and administration and to Prescott Medical Communications Group to assist in data analysis and manuscript development.
References
- 1. Morgan, S. , Grootendorst, P. , Lexchin, J. , Cunningham, C. & Greyson, D. The cost of drug development: a systematic review. Health Policy 100, 4–17 (2011). [DOI] [PubMed] [Google Scholar]
- 2. Sertkaya, A. , Birkenbach, A. , Berlind, A. & Eyraud, J . Examination of Clinical Trial Costs and Barriers for Drug Development Final <http://aspe.hhs.gov/report/examination-clinical-trial-costs-and-barriers-drug-development> (2014). Accessed 29 July 2015. [Google Scholar]
- 3. DiMasi, J.A. , Hansen, R.W. & Grabowski, H.G. The price of innovation: new estimates of drug development costs. J. Health Econ. 22, 151–185 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Center for Drug Evaluation and Research C. Guidance for Industry and FDA Staff Qualification Process for Drug Development Tools <http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm230597.pdf> (2014). Accessed 13 January 2015.
- 5. Lesko, L.J. , Rowland, M. , Peck, C.C. & Blaschke, T.F. Optimizing the science of drug development: opportunities for better candidate selection and accelerated evaluation in humans. Pharmaceut. Res. 17, 1335–1344 (2000). [DOI] [PubMed] [Google Scholar]
- 6. Mirnezami, R. , Nicholson, J. & Darzi, A. Preparing for precision medicine. N. Engl. J. Med. 366, 489–491 (2012). [DOI] [PubMed] [Google Scholar]
- 7. Campion, S. et al The current status of biomarkers for predicting toxicity. Expert Opin. Drug Metab. Toxicol. 9, 1391–1408 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kelloff, G.J. et al Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer—a plan to move forward. Clin. Cancer Res. 12, 3661–3697 (2006). [DOI] [PubMed] [Google Scholar]
- 9. Park, J.W. et al Rationale for biomarkers and surrogate end points in mechanism‐driven oncology drug development. Clin. Cancer Res. 10, 3885–3896 (2004). [DOI] [PubMed] [Google Scholar]
- 10. Javitt, D.C. , Spencer, K.M. Thaker, G.K. , Winterer, G. & Hajós, M. Neurophysiological biomarkers for drug development in schizophrenia. Nat. Rev. Drug Discov. 7, 68–83 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frank, R. & Hargreaves, R. Clinical biomarkers in drug discovery and development. Nat. Rev. Drug Discov. 2, 566–580 (2003). [DOI] [PubMed] [Google Scholar]
- 12. Wagner, J.A. Overview of biomarkers and surrogate endpoints in drug development. Dis. Markers 18, 41–46 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Growdon, J.H. Incorporating biomarkers into clinical drug trials in Alzheimer's disease. J. Alzheimers Dis. 3, 287–292 (2001). [DOI] [PubMed] [Google Scholar]
- 14. Altar, C.A. The Biomarkers Consortium: on the critical path of drug discovery. Clin. Pharmacol. Ther. 83, 361–364 (2008). [DOI] [PubMed] [Google Scholar]
- 15. Lathia, C.D. Biomarkers and surrogate endpoints: how and when might they impact drug development? Dis. Markers 18, 83–90 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sistare, F.D. & DeGeorge, J.J. Promise of new translational safety biomarkers in drug development and challenges to regulatory qualification. Biomarkers Med. 5, 497–514 (2011). [DOI] [PubMed] [Google Scholar]
- 17. Marrer, E. & Dieterle, F. Promises of biomarkers in drug development—a reality check. Chem. Biol. Drug Des. 69, 381–394 (2007). [DOI] [PubMed] [Google Scholar]
- 18. Muller, P.Y. & Dieterle, F. Tissue‐specific, non‐invasive toxicity biomarkers: translation from preclinical safety assessment to clinical safety monitoring. Expert Opin. Drug Metab. Toxicol. 5, 1023–1038 (2009). [DOI] [PubMed] [Google Scholar]
- 19. Moore, E. , Bellomo, R. & Nichol, A. Biomarkers of acute kidney injury in anesthesia, intensive care and major surgery: from the bench to clinical research to clinical practice. Minerva Anestesiol. 76, 425–440 (2010). [PubMed] [Google Scholar]
- 20. White, T.J. , Clark, A.G. & Broder, S. Genome‐based biomarkers for adverse drug effects, patient enrichment and prediction of drug response, and their incorporation into clinical trial design. J. Pers. Med. 3, 177–185 (2006). [DOI] [PubMed] [Google Scholar]
- 21. Stevens, J.L. & Baker, T.K. The future of drug safety testing: expanding the view and narrowing the focus. Drug Discov. Today 14, 162–167 (2009). [DOI] [PubMed] [Google Scholar]
- 22. Ashrafian, H. , McKenna, W.J. & Watkins, H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ. Res. 109, 86–96 (2011). [DOI] [PubMed] [Google Scholar]
- 23. Paul, S.M. et al How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat. Rev. Drug Discov. 9, 203–214 (2010). [DOI] [PubMed] [Google Scholar]
- 24. Trusheim, M.R. , Berndt, E.R. & Douglas, F.L. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat. Rev. Drug Discov. 6, 287–293 (2007). [DOI] [PubMed] [Google Scholar]
- 25. U.S. Food and Drug Administration F. Biomarker Qualification Program . <http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm284076.htm>. (2014). Accessed 31 December 2014.
- 26. Amur, S. , LaVange, L. , Zineh, I. , Buckman‐Garner, S. & Woodcock, J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin. Pharmacol. Ther. 98, 34–46 (2015). [DOI] [PubMed] [Google Scholar]
- 27. Qualification of Biomarker—Plasma Fibrinogen in Studies Examining Exacerbations and/or All‐Cause Mortality for Patients With Chronic Obstructive Pulmonary Disease; Draft Guidance for Industry <https://www.federalregister.gov/articles/2015/07/07/2015-16563/qualification-of-biomarker-plasma-fibrinogen-in-studies-examining-exacerbations-andor-all-cause> (2015). Accessed 29 July 2015.
- 28. Goodsaid, F. & Frueh, F. Biomarker qualification pilot process at the US Food and Drug Administration. AAPS J. 9, E105–E108 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goodsaid, F. & Mattes, W.B. The Path From Biomarker Discovery to Regulatory Qualification. 1st ed. (Elsevier, Oxford, UK, 2013). [Google Scholar]
- 30. Altar, C.A. et al A prototypical process for creating evidentiary standards for biomarkers and diagnostics. Clin. Pharmacol. Ther. 83, 368–371 (2008). [DOI] [PubMed] [Google Scholar]
- 31. Advancing the Use of Biomarkers and Pharmacogenomics; Notice of Public Meeting; Request for Comments (Docket No. FDA‐2014‐N‐0926, Document Number: 2014‐17090) <https://federalregister.gov/a/2014‐17090> (2014).
- 32. Bartels, C.M. & Krause, R. Systemic Lupus Erythematosus (SLE) (Medscape Reference, New York, 2011). [Google Scholar]


