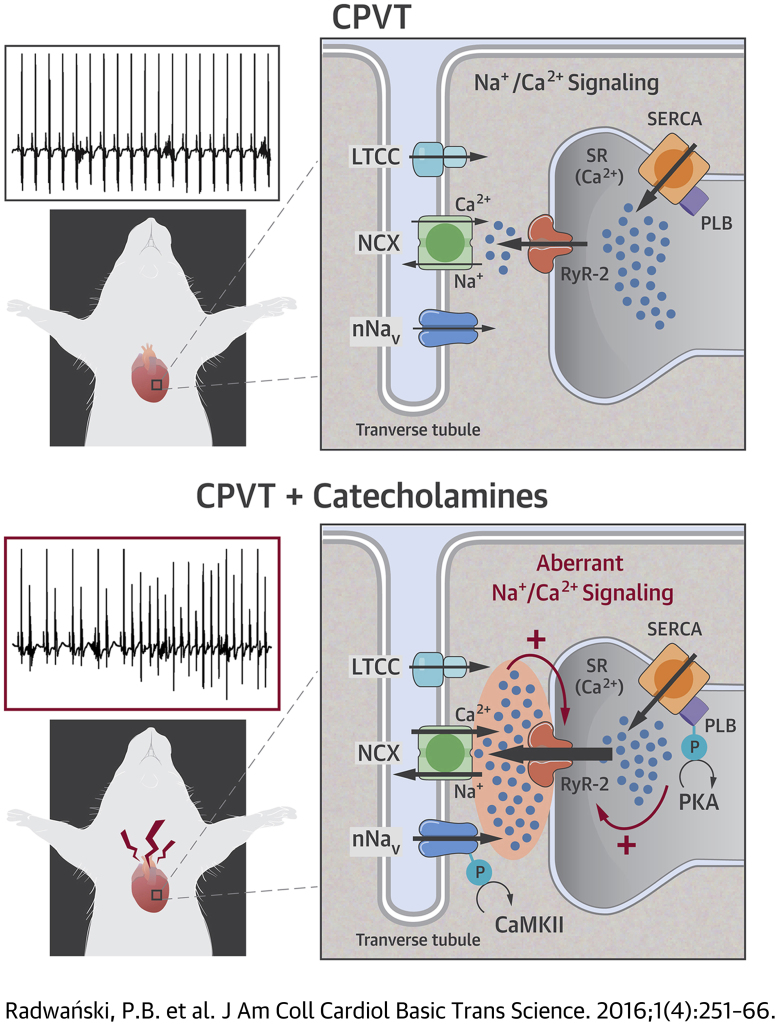
Summary
Although triggered arrhythmias including catecholaminergic polymorphic ventricular tachycardia (CPVT) are often caused by increased levels of circulating catecholamines, the mechanistic link between β-adrenergic receptor (AR) stimulation and the subcellular/molecular arrhythmogenic trigger(s) is unclear. Here, we systematically investigated the subcellular and molecular consequences of β-AR stimulation in the promotion of catecholamine-induced cardiac arrhythmias. Using mouse models of cardiac calsequestrin-associated CPVT, we demonstrate that a subpopulation of Na+ channels, mainly the neuronal Na+ channels (nNav), colocalize with ryanodine receptor 2 (RyR2) and Na+/Ca2+ exchanger (NCX) and are a part of the β-AR-mediated arrhythmogenic process. Specifically, augmented Na+ entry via nNav in the settings of genetic defects within the RyR2 complex and enhanced sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA)-mediated SR Ca2+ refill is both an essential and a necessary factor for arrhythmogenesis. Furthermore, we show that augmentation of Na+ entry involves β-AR–mediated activation of CAMKII, subsequently leading to nNav augmentation. Importantly, selective pharmacological inhibition as well as silencing of Nav1.6 inhibit myocyte arrhythmic potential and prevent arrhythmias in vivo. Taken together, these data suggest that the arrhythmogenic alteration in Na+/Ca2+ handling evidenced ruing β-AR stimulation results, at least in part, from enhanced Na+ influx through nNav. Therefore, selective inhibition of these channels and of Nav1.6 in particular can serve as a potential antiarrhythmic therapy.
Key Words: β-adrenergic receptor, diastolic Ca2+ release, neuronal Na+ channels, ventricular arrhythmias
Abbreviations and Acronyms: β-PMTX, β-pompilidotoxin; β-AR, β-adrenergic receptor; CaMKII, Ca2+/calmodulin-dependent protein kinase II; CASQ2, calsequestrin; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCR, diastolic Ca2+ release; INa, Na+ current; ISO, isoproterenol; nNav, neuronal Na+ channels; NCX, Na+/Ca2+ exchange; PLB, phospholamban; Ril, riluzole; RyR2, ryanodine receptor 2; SR, sarcoplasmic reticulum; SERCA2a, sarcoplasmic reticulum Ca2+-ATPase 2a; TTX, tetrodotoxin; VT, ventricular tachycardia; WT, wild type
Visual Abstract
Highlights
-
•
CPVT is often caused by increased levels of circulating catecholamines; however, the mechanistic link between β-AR stimulation and the subcellular/molecular arrhythmogenic trigger(s) is unclear.
-
•
In both CPVT and wild type mice, a subpopulation of Na+ channels (nNav) colocalize with RyR2 and NCX.
-
•
Augmented Na+ entry via nNav and enhanced SR Ca2+-ATPase (SERCA)-mediated SR Ca2+ refill are both essential and necessary for CPVT.
-
•
Augmentation of Na+ entry involves β-AR–mediated activation of Ca2+/CAMKII.
-
•
Selective pharmacological blockade as well as silencing of Nav1.6 inhibit myocyte arrhythmic potential and prevent arrhythmias in vivo.
Cardiac arrhythmias are a leading cause of death in the United States (1). Arrhythmias caused by abnormal impulse generation are often associated with aberrant diastolic Ca2+ release (DCR) through dysregulated ryanodine receptor 2 (RyR2) Ca2+ release channels. This is especially evident when genetic defects in the RyR2 complex—either the RyR2 itself or 1 of the regulatory proteins associated with the channel (i.e., calmodulin, calsequestrin [CASQ2], triadin and/or calstabin)—facilitate aberrant DCR 2, 3, 4, 5. In particular, recent findings demonstrate that either dysfunction or loss of cardiac calsequestrin (CASQ2), an intra-sarcoplasmic reticulum (SR) Ca2+-binding protein and a regulator of RyR2, impairs the ability of RyR2s to deactivate and become refractory following systolic Ca2+ release 6, 7, 8, 9, 10, 11, 12. This compromised refractoriness of Ca2+ release, in turn, permits the RyR2 channels to reopen during diastole, causing DCR to activate depolarizing membrane currents, resulting in pro-arrhythmic delayed afterdepolarizations 10, 13, 14, 15. Independent of the underlying etiology, compromised RyR2 function is a hallmark of catecholaminergic polymorphic ventricular tachycardia (CPVT).
Episodes of cardiac arrhythmias in CPVT patients are precipitated by emotional stress or exercise, which are associated with increased levels of circulating catecholamines 2, 11, 16. In accordance with the clinical presentation of a vast majority of these arrhythmias, β-blocker therapy is the mainstay of treatment for cardiac rhythm disorders (17). Recent years have witnessed research endeavors that have focused on alterations in Ca2+ handling and their roles in precipitating triggered arrhythmias; however, the precise mechanistic link between β-adrenergic receptor (β-AR) stimulation and arrhythmogenesis in Ca2+-mediated arrhythmias remains elusive. Several targets for phosphorylation, including Cav1.2, phospholamban (PLB), and RyR2, may be involved in the arrhythmogenic effects of β-AR stimulation (15). For instance, protein kinase A phosphorylation of PLB will accelerate SR Ca2+-ATPase 2a (SERCA2a)–mediated Ca2+ refilling of the SR, thereby providing adequate substrate for aberrant DCR (18). However, it is unclear whether phosphorylation of RyR2 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) plays a role in the pathogenesis of cardiac arrhythmias (19). Surprisingly, considering the Ca2+-dependent nature of CPVT, these patients often respond to treatment with Na+-channel blockers such as flecainide 20, 21, 22. It has been proposed that flecainide may exert its antiarrhythmic effect through a direct effect on RyR2 (23); however, this would not explain the effect of other Na+-channel blockers on aberrant Ca2+ handling 24, 25. Recently, we suggested that a subset of Na+ channels, mainly the neuronal Na+ channels (nNav), are present in the transverse (T)-tubule, near Ca2+ handling machinery (26). These channels were initially described in neurons (hence their eponym) and are characterized by a high sensitivity to tetrodotoxin (TTX) 24, 27, 28, 29. However, little is known about the pro-arrhythmic interaction between nNav and aberrant Ca2+ handling during β-AR stimulation as well as the effects flecainide may have on this cross talk. Furthermore, because there are multiple nNav isoforms expressed in cardiac myocytes 30, 31, 32 their specific roles need to be characterized.
In this present study, we have systematically investigated the subcellular and molecular consequences of β-AR stimulation in the promotion of catecholamine-induced cardiac arrhythmias. Because, in certain variants of human CPVT, CASQ2 may be virtually absent or may exist at very low levels due to missense or other mutations, knocking out or mutating CASQ2 in a mouse realistically mimics the phenotype of human disease 2, 33. Therefore, to investigate the role of Na+/Ca2+ signaling, we used well-established murine models of CVPT in which arrhythmogenic oscillation of intracellular Ca2+ and membrane potential are caused by depletion or dysfunction in CASQ2 (CASQ2 null and R33Q, respectively) 6, 14, 26. We report that, in the setting of dysregulated RyR2 channels, catecholamines promote aberrant DCR by facilitating SR Ca2+ refilling while enhancing nNav-mediated persistent Na+ current (INa), respectively, forming the functional basis for catecholamine-induced polymorphic ventricular tachycardia (CPVT).
Methods
All animal procedures were approved by The Ohio State University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 2011).
Genetically-engineered mouse models
All genetically-engineered mice used in our study were homozygous for their respective mutations and/or deletions. Cardiac calsequestrin (CASQ2) null mice (on mixed background) (34) were crossbred with: 1) mice conditionally overexpressing SERCA2a in a doxycycline-dependent manner (on FVB/N background) (35); or 2) RyR2 S2814A mice (on C57BL/6 background; generous gift from Dr. Xander Wehrens) (36). The genotypes of the crossbred mice were confirmed by polymerase chain reactions (PCR) (for CASQ2, reverse tetracycline transactivator driven by the cardiac specific α-myosin heavy chain promoter [35], tetracycline response element-SERCA2a (35), and RyR2 S2814A mutation) using tail deoxyribonucleic acid. To induce the overexpression of SERCA2a, animals received doxycycline diet (Harlan TD 09295 1000 ppm Doxycycline Diet 2018, Harlan, Indianapolis, Indiana) for 14 to 21 days. We also used cardiac CASQ2-R33Q as well as wild type (WT) mice (both on C57BL/6 background) to examine the role of Nav1.6 and Na+/Ca2+ exchange (NCX) in aberrant Na+/Ca2+ signaling (26).
Myocyte isolation, confocal Ca2+ imaging, and Na+ current recordings
Ventricular myocytes were obtained by enzymatic isolation from 3- to 9-month-old mice of both sexes. Mice were anesthetized with isoflurane, and after a deep level of anesthesia was reached, the heart was rapidly removed and perfused via a Langendorff as previously described 14, 26. Peak INa was recorded using an internal solution that contained (in mmol/l): 10 NaCl, 20 tetraethylammonium chloride, 123 CsCl, 1 MgCl2, 0.1 Tris guanosine-5'-triphosphate, 5 Mg adenosine triphosphate, 10 HEPES, and 10 BAPTA (pH 7.2, CsOH). For persistent INa recordings, we substituted BAPTA with 1 mmol/l EGTA and maintained free Ca2+ 100 nmol/l with CaCl2. The extracellular bathing solution for peak INa contained (in mmol/l): 10 NaCl, 130 tetraethylammonium chloride, 4 CsCl, 0.4 CaCl2, 2 MgCl2, 0.05 CdCl2, 10 HEPES, and 10 glucose. The extracellular bathing solution for persistent INa recordings contained (in mmol/l): 140 NaCl, 4 CsCl, 1 CaCl2, 2 MgCl2, 0.05 CdCl2, 10 HEPES, 10 glucose, 0.03 niflumic acid, 0.004 strophanthidin, and 0.2 NiCl2. The pH was maintained at 7.4 with CsOH for both types of solutions. Whole-cell capacitance and series resistance compensation (≥60%) was applied along with leak subtraction. Signals were filtered with 10 kHz Bessel filter, and INa was then normalized to membrane capacitance. Late INa was estimated by integrating INa between 50 and 450 ms.
Electrical field stimulation experiments were performed using the following external solution (in mmol/l): 140 NaCl, 5.4 KCl, 1.0 CaCl2, 0.5 MgCl2, 10 HEPES, and 5.6 glucose (pH 7.4, NaOH). To assess the SR Ca2+ load, 20 mmol/l caffeine was applied at the end of the experiments. Intracellular Ca2+ cycling was monitored by a Nikon A1 laser scanning confocal microscope (Nikon Instruments Inc., Melville, New York). For intact myocytes, we used the cytosolic Ca2+-sensitive indicators Fluo-3 AM. For more reliable measurements of SR Ca2+ release from inside the SR in control and isoproterenol-treated CPVT cardiomyocytes, we performed experiments in Figure 1 using a low-affinity Ca2+ indicator Fluo-4FF-AM. The fluorescent probes were excited with the 488-nm line of an argon laser, and emission was collected at 500 to 600 nm. Fluo-3/Fluo-4FF fluorescence was recorded in the line scan mode of the confocal microscope. For Ca2+ wave recordings, myocytes were paced at 0.3 Hz using extracellular platinum electrodes to obtain DCR frequency. Any DCR event (i.e., wave, wavelet) that increased cell-wide fluorescence intensity above 10% of the signal generated by the preceding stimulated Ca2+ transient was included in the analysis. The fluorescence emitted was expressed as F/F0, where F is the fluorescence at time (t), and F0 represents the background signal. All experiments were performed at room temperature (26°C).
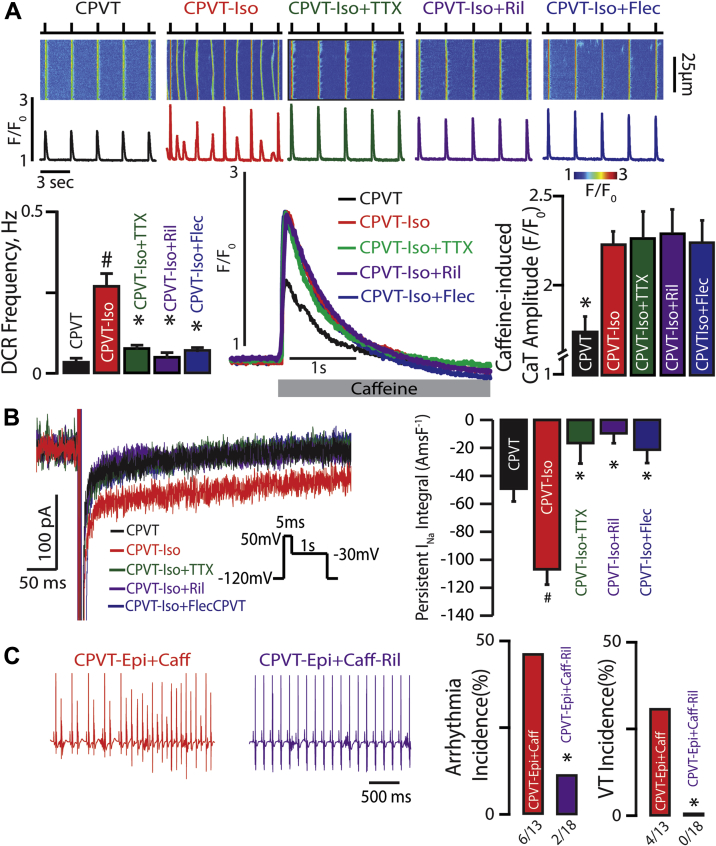
Figure 1.
β-AR Simulation Increases Propensity for CPVT by Augmenting TTX-Sensitive nNav-Mediated Late INa
(A) Effect of β-adrenergic receptor (β-AR) stimulation on neuronal Na+ channel (nNav) blockade and Ca2+ handling. (Top) Representative examples of the line-scan images and corresponding Ca2+ transients (CaT) recorded in catecholaminergic polymorphic ventricular tachycardia (CPVT) ventricular cardiomyocytes loaded with Ca2+ indicator, Fluo-4FF AM, and paced at 0.3 Hz. Cells were treated with isoproterenol (Iso) (100 nmol/l) and tetrodotoxin (TTX) (100 nmol/l), riluzole (Ril) (10 μmol/l), or flecainide (Flec) (2.5 μmol/l). β-AR stimulation with Iso promotes diastolic Ca2+ release (DCR) events in the form of Ca2+ waves relative to untreated CPVT cardiomyocytes (n = 166 and n = 34 cells, respectively; #p < 0.001 Wilcoxon rank sum test). TTX, Ril, and Flec significantly decreased DCR frequency in CPVT cardiomyocytes exposed to Iso (n = 109, 48, and 66 cells, respectively; p < 0.001 Kruskal-Wallis test; *p = 0.003, ∗p < 0.001, and ∗p = 0.032 Wilcoxon rank sum test for TTX, Ril, and Flec vs. ISO, respectively). (Bottom) Representative caffeine (Caff)-induced (20 mmol/l) CaT. ISO significantly increased caffeine-induced CaT relative to untreated CPVT cardiomyocytes (n = 13 and n = 11 cells, respectively; *p = 0.005 Wilcoxon rank sum test). This elevation in caffeine-induced CaT persisted despite concomitant treatment with TTX, Ril, and Flec (n = 11, 13, and 10 cells, respectively; p = 0.99 Kruskal-Wallis test). (B) Effect of β-AR stimulation and subsequent nNav blockade of persistent Na+ current (INa). Representative traces of persistent INa elicited using the protocol are shown in the inset. Iso enhanced persistent INa in CPVT cardiomyocytes (n = 18 and n = 21 cells, respectively; #p = 0.004 Wilcoxon rank sum test). This response to Iso was completely abolished upon addition of TTX, Ril, or Flec (n = 9, 7, and 9 cells, respectively; p < 0.001 Kruskal-Wallis test; *p < 0.001 Wilcoxon rank sum test for each treatment group vs. ISO). Summary data presented as persistent INa integral amp-ms/F (AmsF−1). (C) Effect of β-AR stimulation on nNav-mediated ventricular arrhythmias in vivo. Representative electrocardiography (ECG) recordings of CPVT mice after catecholamine challenge with intraperitoneal injection of epinephrine (1.5 mg/kg) and caffeine (120 mg/kg; red ECGs). A subset of mice was pre-treated with Ril (15 mg/kg; purple ECGs). Arrhythmia and ventricular tachycardia (VT) incidence (%) in CPVT mice exposed to catecholamine challenge during Na+-channel blockade with riluzole (n = 13 vs. n = 18 CPVT-Epi+Caff vs. CPVT-Epi+Caff-Ril treated mice. *p = 0.043 and *p = 0.023 Fisher exact test for arrhythmia and VT incidence, respectively).
Confocal microscopy of immunolabeled myocytes
Isolated ventricular myocytes were prepared for immunofluorescence as well as proximity ligation assay (PLA) as described previously (26). PLA is a histochemical/cytochemical and confocal microscopy technique for determining when specific proteins are colocalized within <40 nm (37). Briefly, cells were plated on laminin-coated glass coverslips, fixed with 4% paraformaldehyde (5 min), permeabilized with 0.1% Triton X-100, and washed with PBS. Endogenous immunoglobulin was blocked using a mouse-on-mouse blocking reagent (M.O.M. kit, Vector Laboratories, Burlingame, California) for 1 h at room temperature and subsequently incubated with primary antibodies (Nav1.1, 1.3, 1.6, 1.5: 1:32, 1:32, 1:50, and 1:50, respectively, for nNavs, Alomone, Jerusalem, Israel; Nav1.5 was a generous gift from Dr. Peter Mohler; and RyR2 1:100 and NCX 1:50 were from Pierce Antibodies, Rockford, Illinois) overnight at 4°C. After washing for immunofluorescence, goat secondary antibodies (antimouse and antirabbit) conjugated to Alexa Fluor (Life Technologies, Grand Island, New York) were added for 1 h, whereas the PLA reactions were carried out using appropriate Duolink secondary antibodies (Sigma, St. Louis, Missouri) according to the manufacturer’s instructions. The sensitivity of PLA was assessed by staining for Nav1.5 (1:50, a generous gift from Dr. Peter Mohler) and Connexin 43 (1:100, Millipore, Billerica, Massachusetts) (Supplemental Figure 1), which were previously demonstrated to colocalize at the intercalated disc (37).
Silencing ribonucleic acid
Targeting silencing ribonucleic acid (siRNA) was purchased from Santa Cruz (Santa Cruz Biotechnology, Inc., Dallas, Texas). We used a previously validated approach of intraperitoneal injection (1.5 mg/kg) mixed with an equal volume of siPORT amine (Ambion, Thermo Fisher Scientific, Waltham, Massachusetts,) in the live animal (38). We administered the siRNA every 24 h for 2 days. Silencing efficacy was evaluated 72 h after initiation of therapy by quantitative real-time (qRT) PCR as well as protein analysis.
Quantitative real-time PCR
Hearts were collected 72 h after initiations of siRNA therapy (n = 3 per each group). Total ribonucleic acid (RNA) was prepared from cells using an RNA Purification Kit (Norgen Biotek, Thorold, Ontario, Canada) in accordance with the manufacturer’s instructions. Total RNA was subjected to qRT PCR. RNA levels were analyzed using the TaqMan Gene Expression Assays in accordance with the manufacturer's instructions (scn1a: Mm00450580_m1, scn3a: Mm00658167_m1, scn5a: Mm01342518_m1, and scn8a: Mm00488110_m1, Life Technologies). RNA concentrations were determined with a NanoDrop 20000 (Thermo Fisher Scientific, Waltham, Massachusetts). Samples were normalized to OAZ1 for mRNAs (Life Technologies). Gene expression levels were quantified using the ABI Prism 7900HT Sequence detection system (Life Technologies). Comparative real-time PCR was performed in triplicate. Relative expression was calculated using the comparative Ct method.
Immunoblots
Heart tissue lysates, following quantitation by the bicinchoninic acid (BCA) assay (Pierce), were loaded into 4% to 15% pre-cast TGX gels (Bio-Rad) and transferred to nitrocellulose membranes. Membranes were blocked for >1 h at room temperature in 3% bovine serum albumin and incubated in primary antibody overnight at 4°C. Primary antibodies included: Nav1.6 (1:500, Alomone) and glyceraldehyde 3-phosphate dehydrogenase (1:5,000, Fitzgerald, Acton, Massachusetts). Secondary antibodies used were donkey antimouse-horseradish peroxidase (HRP) and donkey antirabbit-HRP (Jackson Laboratory, Farmington, Connecticut). Densitometric analysis was performed using Image Lab software (Bio-Rad Laboratories, Hercules, California), and all data was normalized to glyceraldehyde 3-phosphate dehydrogenase.
Electrocardiographic recordings
Continuous electrocardiographic (ECG) recordings (PL3504 PowerLab 4/35, ADInstruments, Sydney, Australia) were obtained from mice anesthetized with isoflurane (1.0% to 1.5%) as previously described (26). Briefly, after baseline recording (5 min), a subset of animals received either riluzole (15 mg/kg) or β-pompilidotoxin (β-PMTX) (30 mg/kg). After 5 min, those animals that were pre-treated with β-PMTX received vehicle, riluzole, or flecainide (20 mg/kg). After an additional 5 to 10 min, animals were exposed to an intraperitoneal epinephrine (1.5 mg/kg) and caffeine (120 mg/kg) challenge, and ECG recording continued for 10 min. We also obtained continuous ECG recordings from CPVT-SERCA mice pre- and post-doxycycline induction. After baseline recording (5 min), each CPVT-SERCA mouse received only β-PMTX (30 mg/kg) intraperitoneally, and ECG recording continued for 10 min. ECG recordings were analyzed using the LabChart 7.3 program (ADInstruments). Arrhythmia was defined as bigeminy or frequent ectopic ventricular activity, whereas ventricular tachycardia (VT) was defined as 3 or more premature ectopies.
Reagents
Unless otherwise stated, all chemicals were purchased from Sigma, Torcis (Bristol, United Kingdom), Focus Biomolecules (Plymouth Meeting, Pennsylvania), Cusabio (Wuhan, China), Medchemexpress LLC (Monmouth Junction, New Jersey), Millipore, or Alomone. Fluorescent dyes were purchased from Molecular Probes (Eugene, Oregon).
Data analysis
INa analysis was performed using pCLAMP9 software (Molecular Devices, Sunnyvale, California). Line scanning images of Ca2+ were normalized for baseline fluorescence (14). The Ca2+ imaging data were processed using ImageJ (NIH, Bethesda, Maryland) and Origin 7.0 software OriginLab Corporation, Northampton, Massachusetts. Confocal micrographs of PLA signal were low pass filtered (Gaussian) and thresholded to generate a black and white mask of the whole myocyte. This was used to calculate myocyte area. The unfiltered image was then thresholded using Otsu's method, followed by nearest-neighbor cluster detection to segment the PLA punctae. The punctae within the whole cell mask areas were counted to determine the density of PLA punctae within the cell (per μm2). Statistical analysis of the data was performed using a Wilcoxon signed rank test and Wilcoxon rank sum test for paired and nonpaired continuous data, respectively, or a Kruskal-Wallis test. The Šidák correction was applied to adjust for multiple comparisons. Fisher exact or McNemar tests were used to test differences in VT incidence. On the basis of our previous observations of mice with high incidence of VT (≥70%) (26), 4 CASQ2-R33Q or other high-VT incidence mice/group were required to have an 80% chance of detecting, as significant at the 5% level, a decrease in the VT incidence from 70% in the control group to 0% in the treatment group. However, due to a lower VT incidence in the CASQ null mice (39), a total sample size of 30 mice in that group was needed. All statistical analyses were performed using Origin 7.0 or R (R Foundation for Statistical Computing, Vienna, Austria). All values are reported as mean ± SEM unless otherwise noted. A p value <0.05 was considered statistically significant.
Results
β-AR stimulation is necessary for aberrant DCR
In this study, we used mouse models of cardiac calsequestrin-associated CPVT. Consistent with the dependence of arrhythmia in CPVT patients on β-AR stimulation, CPVT murine myocytes presented only a few incidents of aberrant DCR in the absence of isoproterenol (ISO) (Sigma) (Figure 1A, black trace and bars). Addition of ISO (100 nmol/l) markedly increased the frequency of arrhythmogenic DCRs (Figure 1A, red traces and bars). This effect of ISO was accompanied by a significant increase in the SR Ca2+ content (Figure 1A, red trace and bar). The ISO-dependent increase in the frequency of arrhythmogenic DCRs could, therefore, be attributed to: 1) increase in the SR Ca2+ content (via phosphorylation of PLB and/or Cav1.2); 2) altered RyR2 function (via phosphorylation of RyR2 at S2814); or 3) augmented nNav-dependent local Na+/Ca2+ signaling (26).
β-AR simulation increases propensity for CPVT by augmenting TTX-sensitive nNav-mediated persistent INa
In addition to the predominant TTX-resistant cardiac Na+ channels (Nav1.5) localized predominantly at the intercalated disc and lateral membrane 26, 37, cardiac myocytes express several types of TTX-sensitive nNav localized in the cardiac T-tubule 26, 30, 31. The nNav blockade with 100 nmol TTX (Tocris Bioscience, Avonmouth, United Kingdom) significantly decreased the frequency of ISO-promoted DCRs (Figure 1A, green traces and bars). Recently, Na+ channel inhibitors flecainide and riluzole emerged as effective therapies in CPVT models 23, 26. Interestingly, riluzole (10 μmol/l, Sigma) and flecainide (2.5 μmol/l, Sigma) both also reduced DCR frequency (Figure 1A, purple and blue traces and bars). Notably, consistent with previous reports 25, 26, none of the aforementioned interventions (i.e., TTX, riluzole, or flecainide) was associated with alterations in the SR Ca2+ content (Figure 1A). Taken together, these findings suggest that, in the setting of dysregulated RyR2 function, increased Na+ influx through nNav during the post-systolic phase may contribute to the arrhythmogenesis evidenced in this model upon β-AR stimulation. To examine the possibility of increased Na+ flux through nNav during β-AR stimulation, we assessed persistent INa in CPVT and WT cardiomyocytes. Exposure to ISO (100 nmol/l) elicited persistent INa both in CPVT and WT myocytes (Figure 1B, Supplemental Figure 2, respectively, red traces and bars). Notably, this current was sensitive to 100 nmol/l TTX (Figure 1B, Supplemental Figure 2, green traces and bars), riluzole, as well as flecainide (Figure 1B, purple and blue traces and bars, respectively), despite the 2 former agents exhibiting only a fraction of flecainide’s total peak INa blocking potential (Supplemental Figure 3).
Next, we examined the effect of nNav-mediated persistent INa on CPVT in vivo. A catecholamine challenge composed of caffeine (Sigma) and epinephrine (Sigma) induced frequent ventricular arrhythmias, which degenerated into polymorphic VT (Figure 1C, red ECG and bars). Consistent with the notion of β-AR–mediated TTX-sensitive persistent INa contributing to pro-arrhythmic DCR, the vast majority of CPVT animals tested remained in sinus rhythm when pre-treated with riluzole (Figure 1C, purple ECG and bars). Therefore, augmentation of Na+ influx through nNav by catecholamines appears to be necessary for the pro-arrhythmic aberrant Na+/Ca2+ signaling in CPVT.
TTX-sensitive nNaV-mediated persistent INa augmentation and increased SR Ca2+ load are necessary and sufficient for arrhythmias in CPVT
To determine whether augmentation of nNav-mediated Na+ influx alone (independent of β-AR stimulation) is sufficient for inducing arrhythmogenic DCR, we induced persistent INa via nNav augmentation with β-PMTX (40) in CPVT cardiomyocytes (Figure 2A). Persistent INa induced by 40 μmol/l β-PMTX (Alomone) was completely reversed by TTX (100 nmol/l), riluzole (10 μmol/l) as well as flecainide (2.5 μmol/l) (Figure 2A). Stimulation of nNav channels by β-PMTX (30 mg/kg intraperitoneally) in the absence of catecholamine challenge, however, failed to induce VT in vivo (Figure 2D).
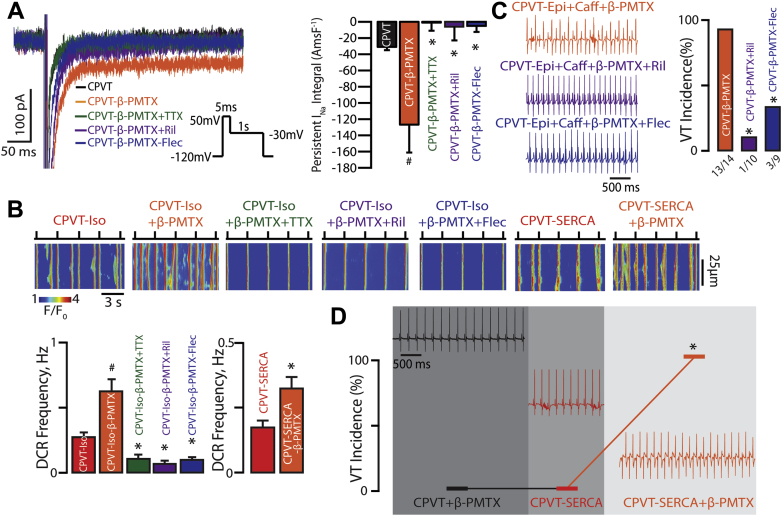
Figure 2.
TTX-Sensitive nNav-Mediated Persistent INa Augmentation in Conjunction With Increased SR Ca2+ Load Contribute to CPVT
(A) Slowed inactivation of nNav with β-PMTX results in TTX-sensitive persistent INa in CPVT mice. Representative traces of persistent INa recorded in CPVT cardiomyocytes. Direct augmentation of nNav-mediated persistent INa with β-PMTX (40 μmol/l) in CPVT myocytes increased persistent INa relative to the control group (n = 12 and n = 31 cells, respectively; #p = 0.004 Wilcoxon rank sum test). β-PMTX–induced persistent INa was reduced by 100 nmol/l TTX, 10 μmol/l Ril, and 2.5 μmol/l Flec (n = 8, 6, and 5 cells; p < 0.001 Kruskal-Wallis test; *p = 0.002, ∗p = 0.039, ∗p = 0.003 Wilcoxon rank sum test vs. β-PMTX alone, respectively). (B) Pharmacological augmentation of nNav-mediated persistent INa promotes DCR in CPVT. Representative line-scan images obtained from CPVT cardiomyocytes and those conditionally overexpressing SERCA2a (CPVT-SERCA) that were loaded with Ca2+ indicator, Fluo-3 AM, and paced at 0.3 Hz. Concomitant application of Iso (100 nmol/l) and β-PMTX (40 μmol/l) further promoted DCR frequency in CPVT cardiomyocytes relative to CPVT myocytes exposed to Iso alone (n = 79 and 166 cells, respectively, #p = 0.001 Wilcoxon rank sum test). Addition of TTX (n = 38), Ril (n = 61), or Flec (n = 70) significantly reduced Iso/β-PMTX–promoted DCRs (p < 0.001 Kruskal-Wallis test; *p < 0.001 Wilcoxon rank sum test for each experimental group vs. ISO + β-PMTX). In the absence of catecholamines, CPVT-SERCA cardiomyocytes exposed to β-PMTX evidenced greater DCR frequency relative to the untreated ones (n = 80 and 83 cells, respectively; *p = 0.01 Wilcoxon rank sum test). (C) Representative ECG recordings of CPVT mice after catecholamine challenge with intraperitoneal injection of epinephrine (1.5 mg/kg) and caffeine (120 mg/kg) and pre-treatment with β-PMTX (30 mg/kg), β-PMTX+Ril (15 mg/kg), or β-PMTX+Flec (20 mg/kg). VT incidence in CPVT mice exposed to catecholamine challenge during various interventions (n = 14, 10, and 9 mice for CPVT-β-PMTX, CPVT-β-PMTX+Ril, and CPVT-β-PMTX+Flec, respectively; *p < 0.001 and ∗p = 0.005 Fisher exact test for CPVT-β-PMTX vs. CPVT-β-PMTX+Ril and CPVT-β-PMTX vs. CPVT-β-PMTX+Flec, respectively). (D) Representative ECG recordings and summary VT incidence of CPVT+SERCA mice before and after doxycycline-induced SERCA2a overexpression and in the presence or absence of β-PMTX (30 mg/kg; n = 6; *p = 0.031 McNemar test for CPVT-SERCA vs. CPVT-SERCA+β-PMTX). All experiments in CPVT-SERCA mice were conducted in the absence of epinephrine and caffeine. Before induction of SERCA2a overexpression, all 6 mice were exposed to β-PMTX. After 2 to 3 weeks of doxycycline-supplemented diet, all 6 mice (horizontal line connecting the hash marks that represent VT incidence) were assessed for arrhythmias, after which they were again exposed to β-PMTX. Abbreviations as in Figure 1.
Of note, β-PMTX further promoted DCR in the presence of ISO on the cellular level (Figure 2B). This resulted in over 90% VT incidence in the CPVT mice undergoing concomitant β-PMTX treatment and catecholamine challenge (Figure 2C, orange ECG and bar). Confirming the involvement of nNav in this pro-arrhythmic process, Na+-channel blockade—both selective and nonselective—significantly reduced DCR and VT incidence in β-PMTX exposed, catecholamine challenged myocytes and animals, respectively, which was independent of changes in SR Ca2+ load (Figures 2B and 2C, Supplemental Figure 4A, green, purple and blue bars, and ECGs). Thus, stimulation of nNav alone, although necessary, is not sufficient to reproduce the proarrhythmic action of catecholamines in CPVT.
To test whether increased SR Ca2+ content is another necessary condition for arrhythmogenesis in CPVT, we performed experiments in CPVT mice that conditionally overexpress SERCA2a (CPVT-SERCA) (35). Even without β-AR stimulation, CPVT-SERCA myocytes evidenced comparable SR Ca2+ load to ISO-exposed CPVT myocytes (Supplemental Figure 4A) and significantly more arrhythmic DCR events relative to ISO-naive CPVT myocytes (Figures 1A and 2B). However, this was insufficient to promote VT in vivo (Figure 2D, red ECG and bar). Importantly, augmentation of Na+ flux through nNav with β-PMTX in ISO-naive CPVT-SERCA myocytes was sufficient to significantly increase aberrant DCR on the cellular level, relative to untreated CPVT-SERCA myocytes (Figure 2B) This, in turn, precipitated VT in all the CPVT-SERCA mice exposed to β-PMTX (Figure 2D, orange ECG and bar). Of note, in 2 instances when SERCA2a overexpression was reversed in CPVT-SERCA mice by stopping the doxycycline-rich diet for 14 days, exposure to β-PMTX failed to induce VT. Taken together, these results suggest that nNav-mediated persistent INa combined with genetically impaired RyR2 function and enhanced SR Ca2+ refill are necessary and sufficient for the arrhythmogenic phenotype responsible for CPVT.
Proarrythmic effect of β-AR stimulation on TTX-sensitive persistent INa augmentation involves CaMKII phosphorylation of nNav and is independent of RyR2 phosphorylation
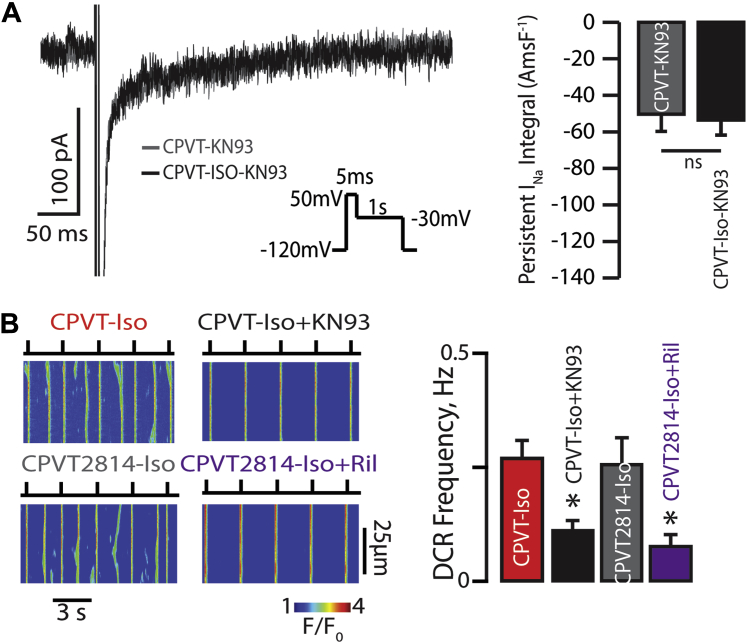
The aforementioned finding that β-AR stimulation promotes Na+ influx through nNav suggests that catecholamines may modulate nNav function through phosphorylation. Recently, Na+ channels have been shown to be subject to phosphorylation by CaMKII 41, 42. To investigate the role of CaMKII-mediated modulation of Na+/Ca2+ signaling in CPVT, we pharmacologically or genetically perturbed CaMKII signaling in CPVT cardiomyocytes. First, we observed that pharmacological blockade of CaMKII with KN93 (1 μmol/l, Sigma) prevented ISO-induced persistent INa (Figure 3A). Second, KN93 significantly reduced ISO-promoted DCR in CPVT myocytes (Figure 3B, black and red bars, respectively). These results suggested that CaMKII promotes aberrant Na+/Ca2+ signaling by augmenting Na+ influx through nNav. To examine the potential direct effects of CaMKII phosphorylation on RyR2 function in CPVT, we used CPVT-S2814A mice in which RyR2 is rendered nonphosphorylatable by CaMKII at S2814 (36). Cardiomyocytes isolated from CPVT-S2814A mice evidenced similar frequency of ISO-promoted DCR relative to those isolated from CPVT mice (Figure 3B, red and gray bars, respectively). Furthermore, the frequency of these aberrant DCRs was significantly reduced by Na+ blockade with riluzole (Figure 3B, purple bar). Notably, none of the aforementioned interventions affected SR Ca2+ load (Supplemental Figure 4B). Thus CaMKII-mediated Na+ influx through nNav can modulate DCR independently of RyR2 phosphorylation.
Figure 3.
Proarrhythmic Effect of β-AR Stimulation on TTX-Sensitive Persistent INa Augmentation Involves CAMKII Phosphorylation of nNav and Is Independent of RyR2 Phosphorylation
(A) The effect of β-AR stimulation on persistent INa is mediated through CaMKII. Representative traces of persistent INa recorded in CPVT cardiomyocytes exposed to CaMKII inhibitor KN93 (1 μmol/l) before and after treatment with 100 nmol/l Iso (n = 9 cells for both groups; p = 0.14 Wilcoxon signed rank test). (B) CaMKII modulates DCR independent of RyR2 phosphorylation at S2814. Representative line-scan images recorded in CPVT ventricular cardiomyocytes as well as those expressing RyR2 that cannot be phosphorylated by CaMKII at site 2814 (S2814A). Myocytes were loaded with Ca2+ indicator, Fluo-3 AM, and paced at 0.3 Hz. CaMKII inhibition with 1 μmol/l KN93 reduced DCR frequency in Iso-treated CPVT cardiomyocytes (n = 166 and n = 105, respectively; *p = 0.001 Wilcoxon rank sum test). CPVT2814 cardiomyocytes did not evidence altered DCR frequency relative to Iso-treated CPVT myocytes; however, exposure of Iso-treated CPVT2814 cardiomyocytes to Ril 10 μmol/l significantly reduced DCR frequency relative to Iso alone (n = 57 and n = 61 cells, respectively; *p = 0.003 Wilcoxon rank sum test). Abbreviations as in Figure 1.
Arrhythmogenesis in CPVT depends on Nav1.6-mediated persistent INa
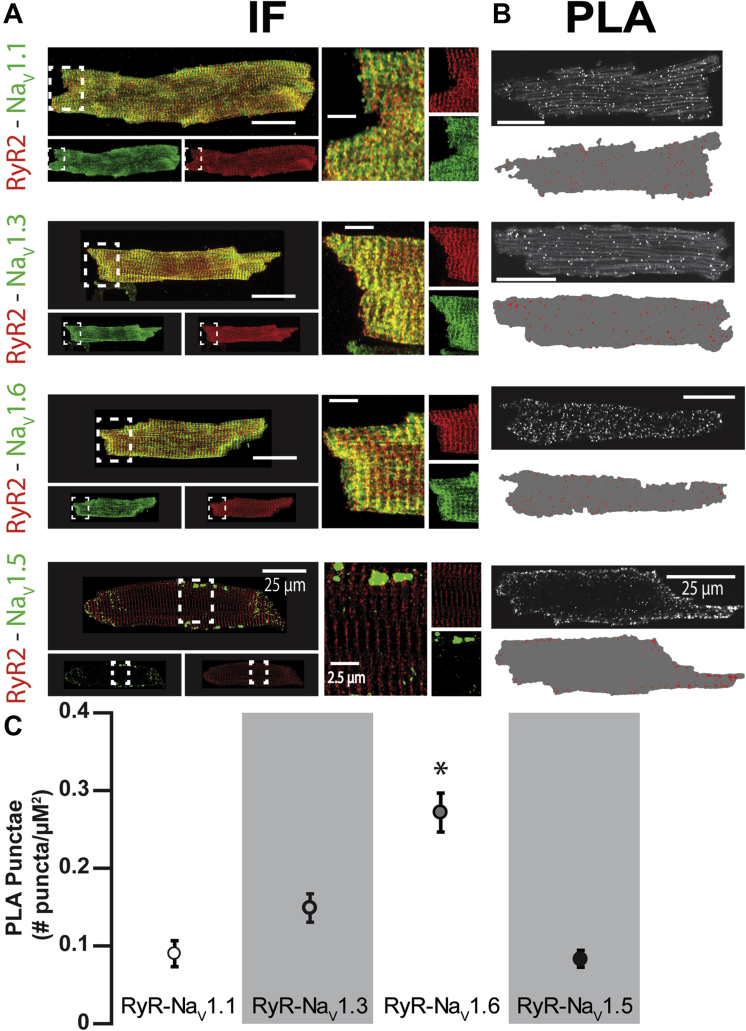
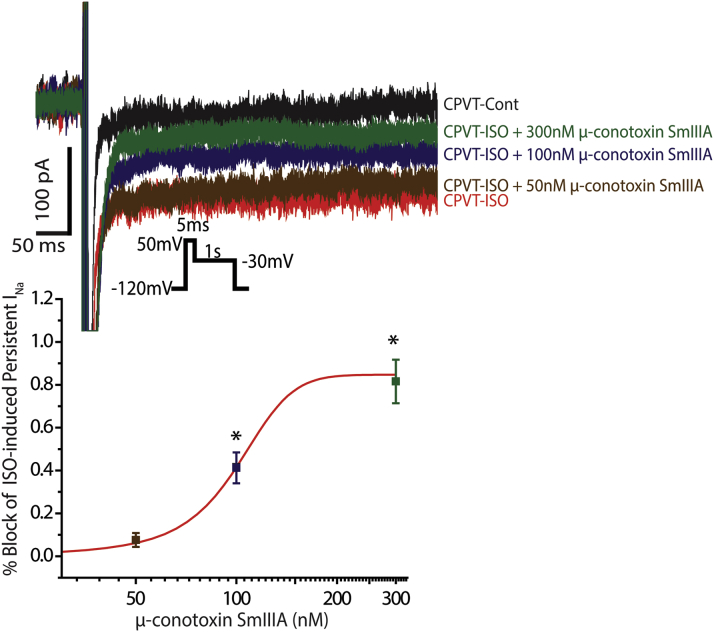
As we have previously demonstrated (26), cardiac myocytes contain several types of Na+ channels, including TTX-sensitive nNav (Nav1.1, Nav1.3, and Nav1.6) as well as the TTX-resistant Nav1.5. The former are located in the vicinity of RyR2 in the junctional microdomain, and the latter, in the lateral membrane and the intercalated discs (Figure 4A). To more precisely examine the localization of these channels with respect to RyR2 in CPVT, we performed a PLA (37). We found that all Na+ channel isoforms were closely colocalized (within <40 nm [37]) with RyR2 (Figure 4B); however, Nav1.5 appeared to be primarily colocalizing with RyR2 in the cell periphery, whereas the nNavs exhibited a more diffuse pattern of colocalization. Specifically, Nav1.6 evidenced the highest degree of colocalization with RyR2 relative to the other nNav isoforms (Figure 4C). The pattern and degree of colocalization of Nav1.6 with RyR2 were similar between myocytes isolated from WT and CPVT hearts, whereas this was not the case for Nav1.1 and 1.3 (Supplemental Figure 5). These data, in the context of recent work suggesting a role for Nav1.6 in progression of demyelinating disease (43), led us to hypothesize a mechanistic role for Nav1.6 in CPVT. Further, the nNaV inhibitor riluzole may exert a therapeutic effect in amyotrophic lateral sclerosis, a demyelinating disorder, through the blockade of Nav1.6 (44). We therefore examined the functional role of Nav1.6 in CPVT. To test this, first we conducted a dose response experiment with μ-conotoxin SmIIIA, which can discriminate between TTX-sensitive Na+ channel isoforms (45). Specifically, at very low nmol/l concentrations, μ-conotoxin SmIIIA inhibits Nav1.1 and Nav1.3 (45). Despite the putative inhibition of Nav1.1 and Nav1.3, 50 nmol/l μ-conotoxin SmIIIA (Cusabio, Wuhan, China) did not significantly alter ISO-induced persistent INa in CPVT myocytes (Figure 5). However, a concentration of μ-conotoxin SmIIIA near the IC50 for Nav1.6 (100 nmol/l) (45) partially reduced ISO-induced persistent INa, whereas 300 nmol/l μ-conotoxin SmIIIA virtually abolished this ISO-induced phenomenon (Figure 5). These data suggest that Nav1.6 can potentially contribute to the ISO-induced persistent INa and arrhythmias in CPVT. To examine this possibility further in both cardiac myocytes and in vivo we used a selective Nav1.6 inhibitor, 4,9-anhydro-TTX (Focus Biomolecules) 46, 47. Notably, ISO-induced persistent INa in CPVT cardiomyocytes was sensitive to 300 nmol/l 4,9-anhydro-TTX (Figure 6A), suggesting that this ISO-promoted persistent INa is for the most part carried by Nav1.6.
Figure 4.
Neuronal Na+ Channels and RyR2 Colocalize to the Same Discrete Subcellular Regions
(A) Representative confocal micrographs of isolated CPVT ventricular myocytes labeled for RyR2 (red) with various Nav isoforms (Nav1.x, green) often resulted in an overlap between the immunofluorescent (IF) signals (yellow) when overlaid. (Right) Close-up views of regions highlighted by dashed white boxes. (B) Representative confocal micrographs of ventricular myocytes isolated from CPVT mice showing fluorescent proximity ligation assay (PLA) signal for RyR2 with different nNav isoforms (NaV1.x). Below each image are the results of digital segmentation, with the cell mask in gray and PLA signal in red. (C) Plot of average number of PLA punctae/μm2 (p < 0.001 Kruskal-Wallis test; *p = 0.002, ∗p = 0.019, ∗p < 0.001 Wilcoxon rank sum test for Nav1.6 vs. Nav1.1, 1.3, and 1.5, respectively; n = 1,231, 1,223, 1,291, and 2,848 punctae from 7, 6, 12, and 7 cells for Nav1.1, 1.3, 1.5, and 1.6, respectively). Abbreviations as in Figure 1.
Figure 5.
Dose Response of ISO-Induced Persistent INa in CPVT Cardiomyocytes to nNav Blockade With μ-Conotoxin SmIIIA
(Top) Representative traces of persistent INa recorded in CPVT cardiomyocytes exposed to ISO (100 nmol/l) and subsequent increasing concentrations of μ-conotoxin SmIIIA (50, 100, and 300 nmol/l). (Bottom) Summary of ISO-induced persistent INa dose response to μ-conotoxin SmIIIA. ISO-induced persistent INa was not significantly affected by 50 nmol/l μ-conotoxin SmIIIA (n = 10 for both CPVT-ISO and CPVT-ISO+50 nmol/l μ-conotoxin SmIIIA; p = 1 Wilcoxon rank sum test), partially inhibited by 100 nmol/l (n = 9; *p = 0.048 Wilcoxon rank sum test vs. CPVT-ISO) and almost completely blocked by 300 nmol/l (n = 6; *p = 0.003 Wilcoxon rank sum test vs. CPVT-ISO). Abbreviations as in Figure 1.
Figure 6.
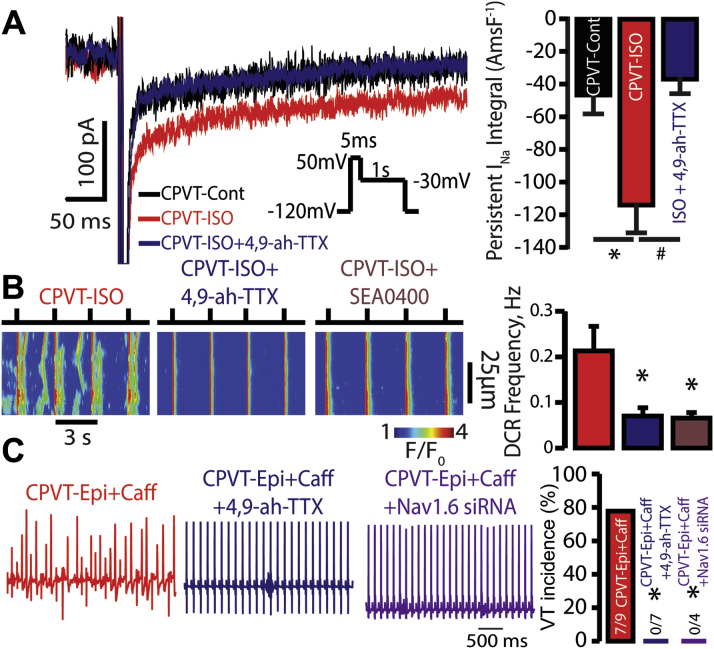
Arrhythmogenesis in CPVT Depends on Nav1.6-Mediated Persistent INa
(A) The effect of Nav1.6 blockade on ISO-induced persistent INa. Representative traces of persistent INa pre- and post-exposure to 100 nmol/l ISO in CPVT cardiomyocytes (p = 0.007 Kruskal-Wallis test, n = 6 for both; *p = 0.035 Wilcoxon rank sum test vs. CPVT control). The persistent INa response to ISO was abolished by 300 nmol/l 4,9-anhydro-TTX (4,9-ah-TTX) (n = 5; #p = 0.016 Wilcoxon rank sum test vs. CPVT-ISO). (B) Nav1.6 and NCX blockade reduce DCR. Representative line-scan images recorded in CPVT ventricular cardiomyocytes that were loaded with Ca2+ indicator, Fluo-3 AM, and were paced at 0.3 Hz. Nav1.6 inhibition with 300 nmol/l 4,9-ah-TTX as well as NCX inhibition with SEA0400 (1 μmol/l) reduced DCR frequency in ISO-treated CPVT cardiomyocytes (n = 99, 123, and 74 cells for ISO-4,9-ah-TTX, ISO-SEA0400, and ISO-treated cells, respectively; p = 0.026 Kruskal-Wallis test; *p = 0.031 and *p = 0.032 Wilcoxon rank sum test for ISO-4,9-ah-TTX and ISO-SEA0400 vs. ISO, respectively). (C) Representative ECG recordings and summary VT incidence of CPVT mice exposed to catecholamine challenge (epinephrine and caffeine) as well as those with pharmacological or genetic inactivation of Nav1.6. Pre-treatment with 4,9-ah-TTX (750 μg/kg intraperitoneally) or administration of siRNA selectively targeting Nav1.6 prevented VT during catecholamine challenge (n = 9, 7, and 4 mice for CPVT-Epi+Caff, CPVT-Epi+Caff +4,9ah-TTX, and CPVT- Epi+Caff+Nav1.6 siRNA, respectively; *p = 0.003 and *p = 0.021 Fisher exact test for CPVT- Epi+Caff +4,9ah-TTX and CPVT- Epi+Caff+Nav1.6 siRNA vs. CPVT-Epi+Caff, respectively). Abbreviations as in Figure 1.
Furthermore, the addition of 300 nmol/l 4,9-anhydro-TTX to CPVT myocytes reduced the frequency of ISO-promoted DCRs (Figure 6B). Similarly, pre-treatment of CPVT mice with 4,9-anhydro-TTX (750 μg/kg) markedly reduced VT vulnerability during catecholamine challenge (Figure 6C, blue ECG and bar). Notably, this intervention had no significant effect on SR Ca2+ load (Supplemental Figure 6). We further addressed the role of Nav1.6 in CPVT by an siRNA approach to selectively target Nav1.6 (Supplemental Figure 7). CPVT mice injected with siRNA against Nav1.6 showed a marked decrease in arrhythmia episodes during catecholamine challenge (Figure 6C, purple ECG and bar). Taken together, these results suggest that Nav1.6 may be in part involved in CPVT-related arrhythmogenesis, which likely involves NCX.
Last, to assess the potential role of NCX in the Na+/Ca2+ signaling, we examined the structural correlation between NCX and nNavs as well as the functional effect of NCX inhibition on aberrant DCR. We found with the aid of PLA that NCX colocalizes with the TTX-sensitive nNav isoforms (Supplemental Figure 8). Furthermore, NCX inhibition with SEA0400 (48) (1 μmol/l, MedChem Express, Monmouth Junction, New Jersey) had a similar effect on DCR relative to that observed with 4,9-anhydro-TTX (Figure 6B). Therefore, these data suggest that NCX may be a component of the pro-arrhythmic interaction between nNavs and RyR2 that, in part, may be responsible for CPVT.
Discussion
Cardiac arrhythmias are often precipitated by catecholamine release during physical or emotional stress. The role of β-AR stimulation is particularly evident in inherited forms of cardiac arrhythmia such as CPVT, where genetic defects in the RyR2 complex (i.e., RyR2, CaM, CASQ2, TRD, and/or calstabin) alter RyR2 channel function and facilitate arrhythmogenic, aberrant DCR 2, 3, 4, 5. Specifically, in the normal heart after each systolic Ca2+ release, RyR2s become refractory via a process that involves a decrease in the SR luminal Ca2+ (10). An intra-SR Ca2+ buffering protein, CASQ2, has been implicated in this process, acting as a Ca2+ buffer and a luminal Ca2+ sensor that regulates RyR2 gating 7, 8, 9, 11, 12, 15. Therefore, CPVT-associated mutations in CASQ2 impair the ability of the RyR2 channel to deactivate during the diastolic phase, thereby making RyR2s prone to premature activation that result in DCR 10, 13, 14, 15. This defective RyR2 gating and the resulting DCR, which are evidenced in CPVT, are enhanced by β-AR stimulation. Despite the critical role of β-AR stimulation as an arrhythmia trigger, the precise mechanisms that link β-AR signaling to arrhythmogenesis remain elusive. Here, we demonstrate that augmented Na+ entry via nNav in the settings of the genetically compromised RyR2s and enhanced SR Ca2+ refill are essential and necessary for the arrhythmogenesis during β-AR stimulation in CPVT. Furthermore, we show that augmentation of Na+ entry involves β-AR–mediated activation of CAMKII, subsequently leading to nNav augmentation. Importantly, selective inhibition of Nav1.6 effectively prevents arrhythmia in vivo, thus potentially presenting a clinically useful antiarrhythmic approach.
Recently we and others have suggested that nNav may facilitate excitation-contraction coupling and contribute to aberrant local Na+/Ca2+ signaling, that, in part, may contribute to cardiac arrhythmias 24, 26, 49, 50, 51, 52. On the basis of these studies, we set out to determine whether β-AR stimulation augments nNav-mediated Na+ entry and thereby facilitates Ca2+ influx via the NCX that, in turn, may stimulate arrhythmogenic DCR through RyR2s. Here, we show that Na+ influx via nNav is not merely a compounding factor, but rather that augmentation of this Na+ influx plays a key role in mediating the proarrhythmic effect of β-AR stimulation in CPVT. Specifically, our findings highlight a distinct nanodomain where nNav are in close proximity (<40 nm) to RyR2s (Figure 4) and NCX (Supplemental Figure 8), and where β-AR–augmented Na+ entry enhances aberrant Na+/Ca2+ signaling, including DCR, thus resulting in CPVT. Of note, the amplitude of the nNav-mediated persistent INa was similar between WT and CPVT myocytes both at baseline and in the presence of ISO (Figures 1 and 6, Supplemental Figure 2). Thus, putative “physiological” β-AR augmentation of nNav activity can become arrhythmogenic in a setting of genetically compromised RyR2 in CPVT.
Stimulation of β-AR has been previously shown to affect intracellular Na+ influx both early and late after a depolarizing stimulus 53, 54. In the case of peak INa, Yarbrough et al. (53) suggested that this phenomenon is coordinated by caveolin-3. Recently, caveolin-3 has been demonstrated to coordinate local nanodomain β2-AR–mediated regulation of L-type Ca2+ channels in the T-tubules 55, 56. However, future studies will need to address the role of caveolin-3 compartmentation on regulation of β-AR–mediated signaling of subpopulations of Na+ channels in various cellular compartments.
Our structural and functional studies make a very compelling case for the involvement of nNav in the arrhythmogenic process. However, this does not preclude the cardiac isoform of the Na+ channels (Nav1.5) from contributing to arrhythmogenesis. In fact, early reports have described late INa as a component of the cardiac INa that can be inhibited by ranolazine (54). This late INa was presumably carried by Nav1.5 and is a reflection of cell-wide sarcolemmal Nav1.5 activity. Here, we show that Nav1.5 is present in the core-compartment of cardiomyocytes, presumably in the T-tubules, albeit its presence in that compartment is very limited (Figure 4). However, nNav, which include Nav1.6, are the predominant isoforms present within these distinct nanodomains (Figure 4) and are responsible for the persistent INa phenotype during β-AR stimulation (Figures 1 and 6, Supplemental Figure 2). In this vein, nonselective (Nav1.5 and nNav) inhibition with flecainide (57), despite having similar effect on persistent INa, more profoundly affected peak INa relative to 100 nmol/l TTX (Supplemental Figure 3), a concentration that completely blocks nNav although mostly sparing Nav1.5 24, 27, 28, 29. These data would further suggest that because 10 μmol/l riluzole inhibits both peak and persistent INa to a similar extent as 100 nmol/l TTX, it may perhaps elicit its DCR-stabilizing effect through blockade of nNav. However, future studies will need to determine the specific Na+ channel isoforms blocked by this agent.
At least 3 isoforms of nNav have been identified in the heart as follows: Nav1.1, Nav1.3, and Nav1.6 (Figure 4) 26, 30, 31, 32, 58. To examine whether a particular nNav isoform is essential for both aberrant Na+/Ca2+ signaling and in vivo arrhythmia in CPVT, we used structural and functional assays. PLA as well as pharmacological and silencing approaches (Figure 4B, Supplemental Figure 5, and Figures 5 and 6, respectively) pointed to the involvement of Nav1.6 in the arrhythmogenic process. Moreover, WT and CPVT myocytes exhibited a similar degree of Nav1.6 and RyR2 colocalization (Figure 4, Supplemental Figure 5), in contrast to changes in RyR2 colocalization observed with other nNav isoforms; thus, the bulk of ISO-promoted late INa in WT and CPVT is likely carried by Nav1.6. Taken together, these findings are consistent with the prevalence of this Na+ channel isoform in cardiomyocytes 26, 30, 31, 32, 58, its substantial persistent current 59, 60, and its localization in the T-tubules in the vicinity of the RyR2 (Figure 4). Furthermore, the persistent INa that was generated by Nav1.6 during application of ISO was modulated by CAMKII-dependent nNav augmentation (Figure 3). Although the exact CaMKII phosphorylation site(s) in Nav1.6 or the other nNavs are not known, there are consensus CaMKII phosphorylation sites in these channels that correspond to DI-II linker conforming to Arg/Lys-X-X-Ser/Thr (61). In particular, S571 in Nav1.5 appears to be conserved in TTX-sensitive nNavs, suggesting that this might be the putative CaMKII phosphorylation site; however, future studies will need to determine the particular phosphorylation site(s) responsible for catecholamine-mediated augmentation of persistent INa.
What other factors, apart from nNav stimulation, are critical to arrhythmogenesis in CPVT? To address this question, we omitted exposure to catecholamines and selectively slowed nNav inactivation with β-PMTX (40) to mimic β-AR–induced nNav augmentation in CPVT mice with inducible SERCA2a overexpression. These studies suggested that enhanced SR Ca2+ refilling or phosphorylation of effector sites such as RyR2 may be necessary for arrhythmogenesis in cardiac CASQ2-associated CPVT. Further experiments where we inhibited nNav activity in CPVT mice exposed to β-AR stimulation that were deficient in RyR2 CaMKII phosphorylation (S2814A) revealed that CaMKII phosphorylation of RyR2 does not play a pivotal role in CASQ2-associated CPVT. Taken together, these data suggest a novel conceptual framework for β-AR–promoted arrhythmogenesis (Figure 7). Mainly, the cross talk among nNav, NCX, and RyR2 may play a critical role in triggering aberrant DCR during β-AR stimulation in CPVT. Furthermore, it is likely that a similar mechanism may contribute to arrhythmogenesis in other genetic and acquired forms of catecholamine-dependent arrhythmias. Likewise, there is evidence to suggest that CPVT is associated with DCR in the atria as well 34, 62. Further, aberrant Ca2+ release events are also observed in atria of patients with various forms of atrial fibrillation (63). Thus, it is very likely that the mechanism described herein may apply to the atrium as well. However, future studies will need to address the involvement of such aberrant Na+/Ca2+ signaling in atrial as well as ventricular variants of genetic and acquired forms of catecholamine-dependent arrhythmias.
Figure 7.
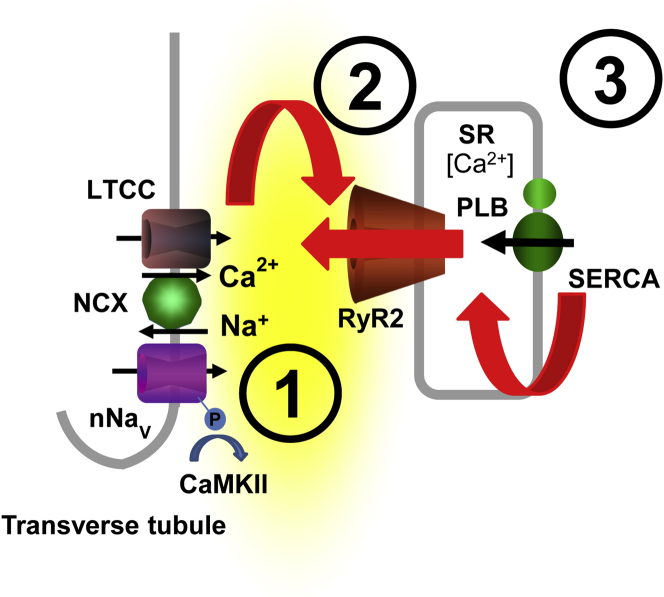
Arrhythmogenic Targets of β-AR Stimulation Responsible for CPVT
During the initiation of excitation-contraction (EC) coupling, Ca2+ influx through the L-type Ca2+ channels (LTCC) results in activation of RyR2 and subsequent Ca2+ release from the SR (68). On the basis of the present study, in the presence of β-adrenergic receptor (β-AR) agonist isoproterenol: ① CaMKII-dependent augmentation of Na+ influx during the post-systolic phase (i.e., persistent Na+ current) may facilitate diastolic Ca2+ release (DCR) by enhancing Na+-Ca2+ exchange (NCX)–dependent Ca2+ accumulation in the dyadic cleft (i.e., space between the sarcolemma and the RyR2); ② this nanodomain Ca2+ accumulation in turn promotes DCR via Ca2+-induced Ca2+ release from the RyR2 that are sensitized due to genetic defects in the RyR2 complex (i.e., RyR2, CaM, CASQ2, TRD, and/or calstabin) 2, 3, 4, 5; which along with ③ increased SR Ca2+ load play a critical role in triggering aberrant DCR during β-AR stimulation in CPVT. Abbreviations as in Figure 1.
Although Na+-channel blockade, with flecainide in particular, has been shown to be effective in management of CPVT 21, 23, the mechanism through which it alleviates arrhythmia remains to be clarified. Initially, the antiarrhythmic effect of flecainide was attributed to the direct inhibition of the RyR2 (23). Subsequent studies have suggested that it reduces the availability of cardiac-type Na+ channels (NaV1.5), thus preventing the development of triggered activity (64). Here, we propose an additional and novel antiarrhythmic mechanism for flecainide in CPVT as follows: antagonizing catecholamine-dependent augmentation of Na+ influx via nNavs in general, and Nav1.6 in particular. Considering that altered RyR2 function contributes to acquired arrhythmias of various etiologies, including ischemic and nonischemic cardiomyopathy (65), inhibition of nNav can potentially be applied to treat these diverse conditions. Interestingly, although non–isoform-selective Na+ channel inhibition initially appeared to be beneficial in the management of Ca2+-mediated arrhythmias due to myocardial infarction (20), it has proven to be pro-arrhythmic and enhance the risk of arrhythmic death in patients with structural heart disease, evidently due to reduced electrical excitability of the myocardium 66, 67. In this context, nNav appears to be a particularly suitable antiarrhythmic target, where the antiarrhythmic effect of selective nNav blockade can be uncoupled from the proarrhythmic effect of reduced cellular excitability associated with Nav1.5 inhibition. Taken together, our study brings well established findings on the global plane regarding the efficacy of Na+ channel as well as β-AR blockers under 1 mechanistic umbrella. Specifically, the novel catecholamine-mediated arrhythmogenic mechanism described herein relies on the maintenance of enhanced SR Ca2+ load in the setting of genetically compromised RyR2 along with augmentation of nNav activity. The combination of these factors promotes aberrant Na+/Ca2+ signaling, resulting in DCR and arrhythmias in vivo. Selective inhibition of nNavs in general, and Nav1.6 in particular, may represent effective treatment for a wide range of arrhythmias associated with altered RyR2 function and sympathetic stimulation.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In a mouse heart, catecholamines promote pro-arrhythmic aberrant diastolic Ca2+ release (DCR) by enhancing neuronal Na+ channel (nNav)-mediated persistent Na+ current (INa) along with maintaining adequate SR Ca2+ load. Thus, these form the functional basis for CPVT.
TRANSLATIONAL OUTLOOK: Na+ channel blockade has been shown to be effective in management of CPVT. Considering that altered RyR2 function contributes to both genetic and acquired arrhythmias of various etiologies, including ischemic and nonischemic cardiomyopathy, inhibition of nNav can potentially be applied to treat these diverse conditions. Interestingly, although non-isoform-selective Na+ channel inhibition initially appeared beneficial in the management of Ca2+-mediated arrhythmias due to myocardial infarction, it has proven to be pro-arrhythmic and enhance the risk of arrhythmic death in patients with structural heart disease evidently due to reduced availability of Nav1.5 and the consequent loss of myocardial excitability. In this context, nNav appears to be particularly suitable antiarrhythmic target, where the antiarrhythmic effect of selective nNav blockade can be uncoupled from the proarrhythmic effect of reduced cellular excitability associated with Nav1.5 inhibition.
Acknowledgments
The authors would like to thank Ms. Megan Koleske for critical reading of the manuscript. Furthermore, they would like to thank Dr. Igor Kubasov and Dr. Dan Bobkov (Sechenov Institute of Evolutionary Physiology and Biochemistry, Russian Academy of Sciences) for their valuable suggestions on the manuscript and help with quantitative image analysis.
Footnotes
This work was supported by National Institutes of Health grants R01-HL074045 and R01-HL063043 (to Dr. Györke); K99-HL127299 (to Dr. Radwański); R01-HL084583, R01-HL083422, and R01- HL075649 (to Dr. Mohler); and R01-HL114893 (to Dr. Hund). Additionally funding was provided by the Russian Science Foundation (N15-15-20008), which was used to support the image analysis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Contributor Information
Przemysław B. Radwański, Email: Przemyslaw.Radwanski@osumc.edu.
Sándor Györke, Email: Sandor.Gyorke@osumc.edu.
Appendix
References
- 1.Kong M.H., Fonarow G.C., Peterson E.D. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794–801. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knollmann B.C., Chopra N., Hlaing T. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priori S.G., Napolitano C., Tiso N. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 4.Iyer V., Hajjar R.J., Armoundas A.A. Mechanisms of abnormal calcium homeostasis in mutations responsible for catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2007;100:e22–e31. doi: 10.1161/01.RES.0000258468.31815.42. [DOI] [PubMed] [Google Scholar]
- 5.Marx S.O., Marks A.R. Dysfunctional ryanodine receptors in the heart: new insights into complex cardiovascular diseases. J Mol Cell Cardiol. 2013;58:225–231. doi: 10.1016/j.yjmcc.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens S.C.W., Terentyev D., Kalyanasundaram A., Periasamy M., Györke S. Intra-sarcoplasmic reticulum Ca2+ oscillations are driven by dynamic regulation of ryanodine receptor function by luminal Ca2+ in cardiomyocytes. J Physiol. 2009;587:4863–4872. doi: 10.1113/jphysiol.2009.175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin J., Valle G., Nani A. Ryanodine receptor luminal Ca2+ regulation: swapping calsequestrin and channel isoforms. Biophys J. 2009;97:1961–1970. doi: 10.1016/j.bpj.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beard N.A., Casarotto M.G., Wei L., Varsányi M., Laver D.R., Dulhunty A.F. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys J. 2005;88:3444–3454. doi: 10.1529/biophysj.104.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin J., Valle G., Nani A. Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J Gen Physiol. 2008;131:325–334. doi: 10.1085/jgp.200709907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radwański P.B., Belevych A.E., Brunello L., Carnes C.A., Györke S. Store-dependent deactivation: cooling the chain-reaction of myocardial calcium signaling. J Mol Cell Cardiol. 2013;58:77–83. doi: 10.1016/j.yjmcc.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terentyev D., Nori A., Santoro M. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 12.Knollmann B.C. New roles of calsequestrin and triadin in cardiac muscle. J Physiol. 2009;587:3081–3087. doi: 10.1113/jphysiol.2009.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornyeyev D., Petrosky A.D., Zepeda B., Ferreiro M., Knollmann B., Escobar A.L. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J Mol Cell Cardiol. 2012;52:21–31. doi: 10.1016/j.yjmcc.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunello L., Slabaugh J.L., Radwanski P.B. Decreased RyR2 refractoriness determines myocardial synchronization of aberrant Ca2+ release in a genetic model of arrhythmia. Proc Natl Acad Sci U S A. 2013;110:10312–10317. doi: 10.1073/pnas.1300052110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Györke S. Molecular basis of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2009;6:123–129. doi: 10.1016/j.hrthm.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Di Barletta M.R., Viatchenko-Karpinski S., Nori A. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2006;114:1012–1019. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- 17.Priori S.G., Aliot E., Blomstrom-Lundqvist C., for the European Society of Cardiology Update of the guidelines on sudden cardiac death of the European Society of Cardiology. Eur Heart J. 2003;24:13–15. doi: 10.1016/s0195-0668x(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 18.Domeier T.L., Maxwell J.T., Blatter L.A. β-adrenergic stimulation increases the intra-sarcoplasmic reticulum Ca2+ threshold for Ca2+ wave generation. J Physiol. 2012;590:6093–6108. doi: 10.1113/jphysiol.2012.236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobrev D., Wehrens X.H.T. Role of RyR2 phosphorylation in heart failure and arrhythmias: controversies around ryanodine receptor phosphorylation in cardiac disease. Circ Res. 2014;114:1311–1319. doi: 10.1161/CIRCRESAHA.114.300568. discussion 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The CAPS Investigators The Cardiac Arrhythmia Pilot Study. Am J Cardiol. 1986;57:91–95. doi: 10.1016/0002-9149(86)90958-6. [DOI] [PubMed] [Google Scholar]
- 21.Van der Werf C., Kannankeril P.J., Sacher F. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011;57:2244–2254. doi: 10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knollmann B.C. Power and pitfalls of using transgenic mice to optimize therapy for CPVT—a need for prospective placebo-controlled clinical trials in genetic arrhythmia disorders. Heart Rhythm. 2010;7:1683–1685. doi: 10.1016/j.hrthm.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe H., Chopra N., Laver D. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radwański P.B., Greer-Short A., Poelzing S. Inhibition of Na+ channels ameliorates arrhythmias in a drug-induced model of Andersen-Tawil syndrome. Heart Rhythm. 2013;10:255–263. doi: 10.1016/j.hrthm.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Sikkel M.B., Collins T.P., Rowlands C. Flecainide reduces Ca(2+) spark and wave frequency via inhibition of the sarcolemmal sodium current. Cardiovasc Res. 2013;98:286–296. doi: 10.1093/cvr/cvt012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radwański P.B., Brunello L., Veeraraghavan R. Neuronal Na+ channel blockade suppresses arrhythmogenic diastolic Ca2+ release. Cardiovasc Res. 2015;106:143–152. doi: 10.1093/cvr/cvu262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie J.M., Rogart R.B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- 28.Renaud J.F., Kazazoglou T., Lombet A. The Na+ channel in mammalian cardiac cells. Two kinds of tetrodotoxin receptors in rat heart membranes. J Biol Chem. 1983;258:8799–8805. [PubMed] [Google Scholar]
- 29.Satin J., Kyle J.W., Chen M. A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science. 1992;256:1202–1205. doi: 10.1126/science.256.5060.1202. [DOI] [PubMed] [Google Scholar]
- 30.Maier S.K.G., Westenbroek R.E., McCormick K.A., Curtis R., Scheuer T., Catterall W.A. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–1427. doi: 10.1161/01.CIR.0000121421.61896.24. [DOI] [PubMed] [Google Scholar]
- 31.Westenbroek R.E., Bischoff S., Fu Y., Maier S.K.G., Catterall W.A., Scheuer T. Localization of sodium channel subtypes in mouse ventricular myocytes using quantitative immunocytochemistry. J Mol Cell Cardiol. 2013;64:69–78. doi: 10.1016/j.yjmcc.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin X., Liu N., Lu J. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart Rhythm. 2011;8:1923–1930. doi: 10.1016/j.hrthm.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzi N., Liu N., Napolitano C. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- 34.Lou Q., Belevych A.E., Radwański P.B. Alternating membrane potential/calcium interplay underlies repetitive focal activity in a genetic model of calcium-dependent atrial arrhythmias. J Physiol. 2015;593:1443–1458. doi: 10.1113/jphysiol.2014.280784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suarez J., Gloss B., Belke D.D. Doxycycline inducible expression of SERCA2a improves calcium handling and reverts cardiac dysfunction in pressure overload-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2004;287:H2164–H2172. doi: 10.1152/ajpheart.00428.2004. [DOI] [PubMed] [Google Scholar]
- 36.Van Oort R.J., McCauley M.D., Dixit S.S. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhett J.M., Ongstad E.L., Jourdan J., Gourdie R.G. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol. 2012;245:411–422. doi: 10.1007/s00232-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mezzaroma E., Toldo S., Farkas D. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu B., Ho H.-T., Velez-Cortes F. Genetic ablation of ryanodine receptor 2 phosphorylation at Ser-2808 aggravates Ca(2+)-dependent cardiomyopathy by exacerbating diastolic Ca2+ release. J Physiol. 2014;592:1957–1973. doi: 10.1113/jphysiol.2013.264689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiavon E., Stevens M., Zaharenko A.J., Konno K., Tytgat J., Wanke E. Voltage-gated sodium channel isoform-specific effects of pompilidotoxins. FEBS J. 2010;277:918–930. doi: 10.1111/j.1742-4658.2009.07533.x. [DOI] [PubMed] [Google Scholar]
- 41.Hund T.J., Koval O.M., Li J. A β(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashpole N.M., Herren A.W., Ginsburg K.S. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J Biol Chem. 2012;287:19856–19869. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craner M.J., Newcombe J., Black J.A., Hartle C., Cuzner M.L., Waxman S.G. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc Natl Acad Sci U S A. 2004;101:8168–8173. doi: 10.1073/pnas.0402765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sierra Bello O., Gonzalez J., Capani F., Barreto G.E. In silico docking reveals possible Riluzole binding sites on Nav1.6 sodium channel: implications for amyotrophic lateral sclerosis therapy. J Theor Biol. 2012;315:53–63. doi: 10.1016/j.jtbi.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Wilson M.J., Yoshikami D., Azam L. μ-conotoxins that differentially block sodium channels NaV1.1 through 1.8 identify those responsible for action potentials in sciatic nerve. Proc Natl Acad Sci U S A. 2011;108:10302–10307. doi: 10.1073/pnas.1107027108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosker C., Lohberger B., Hofer D., Steinecker B., Quasthoff S., Schreibmayer W. The TTX metabolite 4,9-anhydro-TTX is a highly specific blocker of the Na(v1.6) voltage-dependent sodium channel. Am J Physiol Cell Physiol. 2007;293:C783–C789. doi: 10.1152/ajpcell.00070.2007. [DOI] [PubMed] [Google Scholar]
- 47.Hargus N.J., Nigam A., Bertram E.H., 3rd, Patel M.K. Evidence for a role of Nav1.6 in facilitating increases in neuronal hyperexcitability during epileptogenesis. J Neurophysiol. 2013;110:1144–1157. doi: 10.1152/jn.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bourgonje V.J.A., Vos M.A., Ozdemir S. Combined Na(+)/Ca(2+) exchanger and L-type calcium channel block as a potential strategy to suppress arrhythmias and maintain ventricular function. Circ Arrhythm Electrophysiol. 2013;6:371–379. doi: 10.1161/CIRCEP.113.000322. [DOI] [PubMed] [Google Scholar]
- 49.Lin X., O’Malley H., Chen C. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. J Physiol. 2015;593:1389–1407. doi: 10.1113/jphysiol.2014.277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mishra S., Reznikov V., Maltsev V.A., Undrovinas N.A., Sabbah H.N., Undrovinas A. Contribution of sodium channel neuronal isoform Nav1.1 to late sodium current in ventricular myocytes from failing hearts. J Physiol. 2015;593:1409–1427. doi: 10.1113/jphysiol.2014.278259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biet M., Morin N., Lessard-Beaudoin M. Prolongation of action potential duration and QT interval during epilepsy linked to increased contribution of neuronal sodium channels to cardiac late Na+ current: a potential mechanism for sudden death in epilepsy. Circ Arrhythm Electrophysiol. 2015;8:912–920. doi: 10.1161/CIRCEP.114.002693. [DOI] [PubMed] [Google Scholar]
- 52.Torres N.S., Larbig R., Rock A., Goldhaber J.I., Bridge J.H.B. Na+ currents are required for efficient excitation-contraction coupling in rabbit ventricular myocytes: a possible contribution of neuronal Na+ channels. J Physiol. 2010;588:4249–4260. doi: 10.1113/jphysiol.2010.194688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yarbrough T.L., Lu T., Lee H.-C., Shibata E.F. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ Res. 2002;90:443–449. doi: 10.1161/hh0402.105177. [DOI] [PubMed] [Google Scholar]
- 54.Dybkova N., Wagner S., Backs J. Tubulin polymerization disrupts cardiac β-adrenergic regulation of late INa. Cardiovasc Res. 2014;103:168–177. doi: 10.1093/cvr/cvu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nikolaev V.O., Moshkov A., Lyon A.R. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 56.Wright P.T., Nikolaev V.O., O’Hara T. Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J Mol Cell Cardiol. 2014;67:38–48. doi: 10.1016/j.yjmcc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramos E., O’Leary M.E. State-dependent trapping of flecainide in the cardiac sodium channel. J Physiol. 2004;560:37–49. doi: 10.1113/jphysiol.2004.065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maier S.K.G., Westenbroek R.E., Schenkman K.A., Feigl E.O., Scheuer T., Catterall W.A. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci U S A. 2002;99:4073–4078. doi: 10.1073/pnas.261705699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rush A.M., Dib-Hajj S.D., Waxman S.G. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J Physiol. 2005;564:803–815. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y., Yu F.H., Sharp E.M., Beacham D., Scheuer T., Catterall W.A. Functional properties and differential neuromodulation of Na(v)1.6 channels. Mol Cell Neurosci. 2008;38:607–615. doi: 10.1016/j.mcn.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marionneau C., Lichti C.F., Lindenbaum P. Mass spectrometry-based identification of native cardiac Nav1.5 channel α subunit phosphorylation sites. J Proteome Res. 2012;11:5994–6007. doi: 10.1021/pr300702c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faggioni M., Savio-Galimberti E., Venkataraman R. Suppression of spontaneous ca elevations prevents atrial fibrillation in calsequestrin 2-null hearts. Circ Arrhythm Electrophysiol. 2014;7:313–320. doi: 10.1161/CIRCEP.113.000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wakili R., Voigt N., Kääb S., Dobrev D., Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121:2955–2968. doi: 10.1172/JCI46315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu N., Denegri M., Ruan Y. Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity. Circ Res. 2011;109:291–295. doi: 10.1161/CIRCRESAHA.111.247338. [DOI] [PubMed] [Google Scholar]
- 65.Belevych A.E., Radwański P.B., Carnes C.A., Györke S. “Ryanopathy”: causes and manifestations of RyR2 dysfunction in heart failure. Cardiovasc Res. 2013;98:240–247. doi: 10.1093/cvr/cvt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Starmer C.F., Lastra A.A., Nesterenko V.V., Grant A.O. Proarrhythmic response to sodium channel blockade. Theoretical model and numerical experiments. Circulation. 1991;84:1364–1377. doi: 10.1161/01.cir.84.3.1364. [DOI] [PubMed] [Google Scholar]
- 67.Echt D.S., Liebson P.R., Mitchell L.B. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 68.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.