Abstract
Primary bone lymphoma (PBL) is an uncommon extra nodal disease that represents about 1–3% of lymphoma cases. Imaging findings are variable and non-specific. Computed tomography may demonstrate lytic lesions with sequestra and periosteal reaction. On magnetic resonance imaging, lesions are T1WI hypointense and T2WI hyperintense, related to peritumoral edema or bone marrow replacement. Rarely lesions may have associated fibrosis and show a more hypointense signal pattern on T2WI. After administration of contrast, PBL tends to enhance avidly. We present a case of a 24 years old African American female patient with history of back pain. Initial imaging examinations showed lesions involving the T12 and T11 vertebral bodies with initial negative biopsy results. One month later, the patient returned with worsening back pain, and the follow up studies depicted collapse of the T12 vertebral body. A diagnosis of anaplastic large cell lymphoma in T12 was made. A brief review of the literature, imaging and pathological findings, and treatment options are also discussed.
Keywords: Vertebral lymphoma, bone lymphoma, anaplastic large cell lymphoma, computed tomography, magnetic resonance, pathological fracture
CASE REPORT
A 24 years old African American female presented with a 2-month history of thoracolumbar back pain. She was seen in the Emergency Department (ED) twice and at each visit was discharged home with a diagnosis of lumbar sprain. She returned again to the Emergency Department for continuous pain and a Computed Tomography (CT) was performed and depicted lytic lesions involving the left T12 vertebral body (Figure 1 and 2), with a geographic pattern without peripheral sclerosis and narrow transition zone. No osteoid or chondroid matrix and no fluid – fluid levels were noticed. There was also concern for an early lytic lesion of the inferior left T11 vertebral body, sparing the intervertebral disc, raising concern for tuberculous spondylitis or metastatic disease. The patient had recent negative PPD, no previous history of malignancy, and denied constitutional symptoms. She denied bowel or bladder incontinence, numbness of extremities, or radicular symptoms. After an initial negative laboratory work up and negative chest radiograph, she was discharged home from the ED with neurosurgery follow-up. No neurosurgical intervention was performed since she was stable at that time and further work up was recommended. During the following week, the patient went through an extensive workup of the suspicious thoracic spine lesion including CT of the abdomen and pelvis, Magnetic Resonance (MR) of the thoracic and lumbar spine with gadolinium, and CT guided bone biopsy of the T12 lesion. The initial MR showed diffuse T1 marrow signal near isointense to paraspinal muscle likely reflecting hematopoetic marrow in this young female patient. Within the left T12 vertebral body, there were multiple lesions that were slightly hyperintense to the adjacent marrow signal (Figure 3). T2 weighted images showed diffuse abnormal hyperintense signal within the left T12 vertebral body and mild T2 hyperintense signal of the T11 left inferior vertebral with a normal T11–T12 intervertebral disc (Figure 4). Mild thickening of the prevertebral soft tissues at these levels was also noted. STIR image also depicted increased signal intensity of the T12 body and T11 inferior endplate (Figure 5). T1WI with contrast showed avid enhancement of the T11 and T12 vertebral bodies and prevertebral soft tissues (Figure 6). CT guided T12 bone biopsy was performed using a left transpedicular approach and pathology results were negative for malignancy (Figure 7). However, the results showed fibrosis and chronic inflammatory changes with differential considerations of a reactive process adjacent to true lesional tissue versus a benign reparative process (such as chronic osteomyelitis). Based on these findings, repeat biopsy was suggested. However, the patient opted for close follow up imaging.
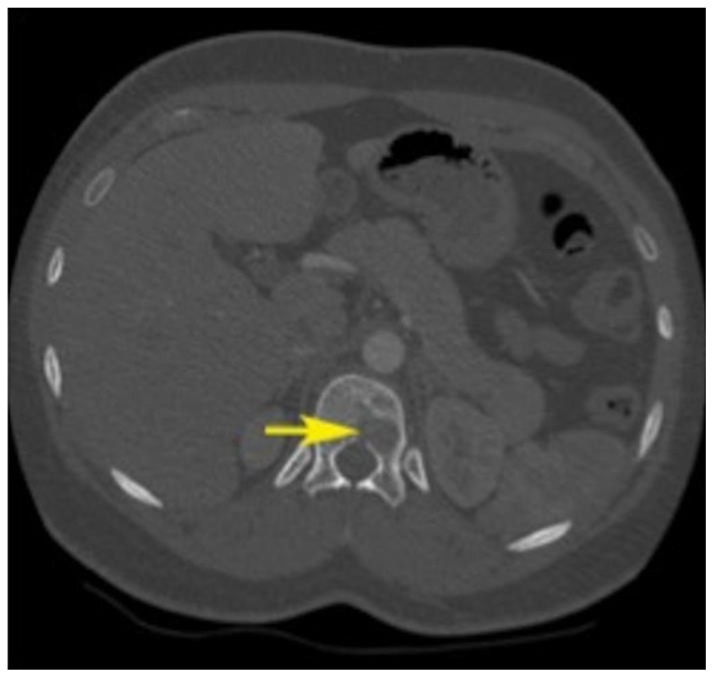
Figure 1.
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine.
Findings: Axial CT image (bone window) at the T12 level depicts multiple lytic lesions within the left aspect of the vertebral body (arrow). These lesions show a narrow transition zone and geographic pattern (well-defined margins without peripheral sclerosis). No osteoid or chondroid matrix and no fluid-fluid levels are noticed.
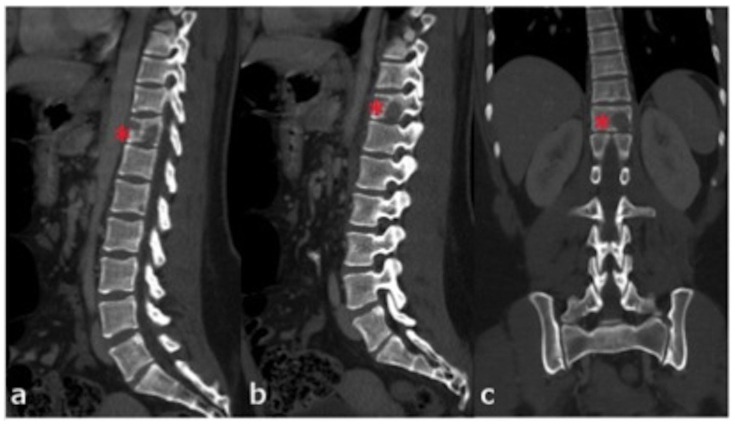
Figure 2.
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine.
Findings: a–c: Multiplanar reconstructions in the sagittal (a and b) and coronal (c) planes again show multiple lytic, well-defined lesions within the T12 vertebral body (red asterisks) as described in figure 1.
Technique: mA 248, KVp 120, 0.63 slice thickness, with 100 ml, Omnipaque 350.
Figure 3.
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine.
Findings: a) Sagittal T1WI without contrast shows heterogeneous signal of the T12 vertebral body with an area of oblique oriented linear hypointense signal, which may represent a pathologic fracture (arrow). There is also increased mild thickening of the prevertebral soft tissues. b and c) Axial T1WI without contrast shows multifocal hyperintense well-defined lesions within the left side of the vertebral body (red asterisks).
Technique: 3T GE magnet, TR: 483.336, TE: 16.224, FOV: 32 cm, 4.0 mm thick.
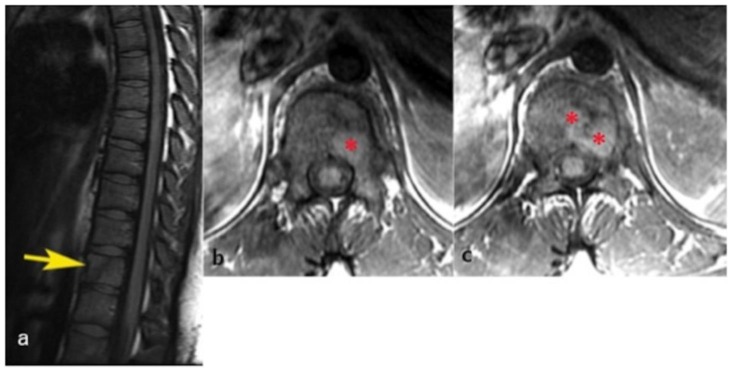
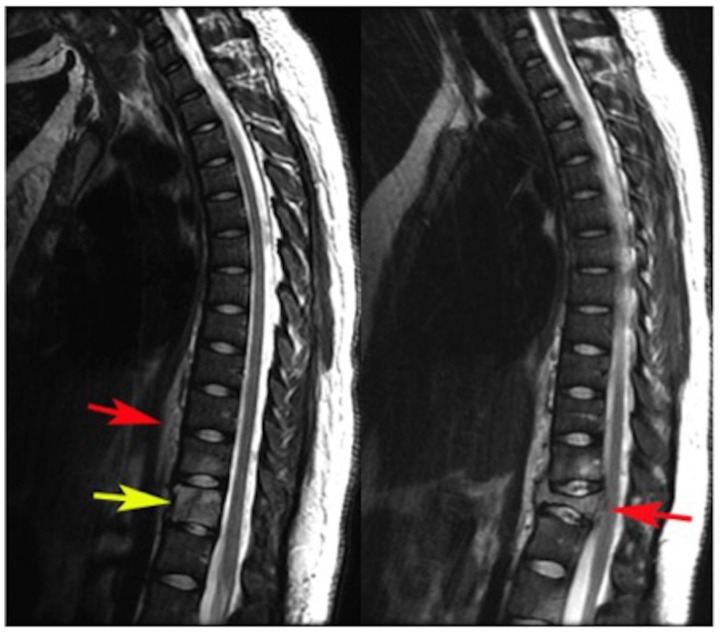
Figure 4.
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine.
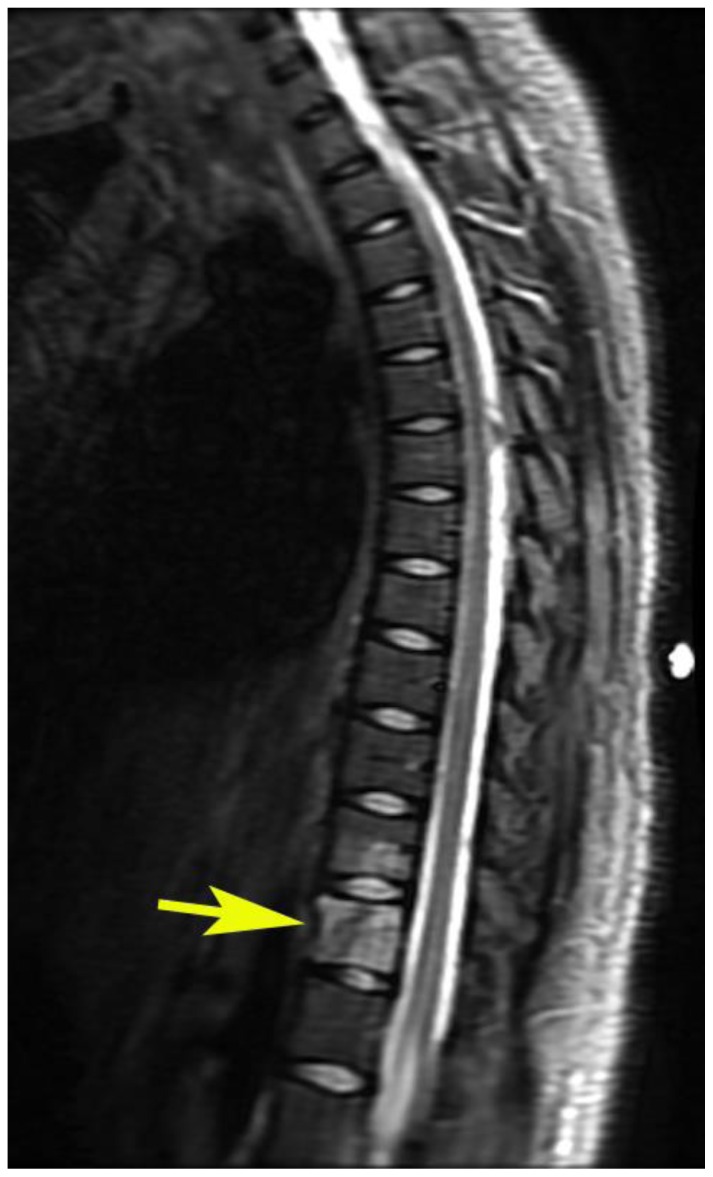
Findings: Sagittal T2WI demonstrates an oblique oriented linear hypointense signal which may represent a pathologic fracture, increased signal intensity of the body of T12 (yellow arrow) and also increased signal in the inferior aspect of T11. The visualized intervertebral discs are normal in signal with minimal disc height loss at the T12–L1 level. Increased signal intensity and thickness of the prevertebral soft tissues from T9 to T12 is also depicted (red arrow).
Technique: 3T GE Magnet, TR: 4183.34 TE: 123, FOV: 32 cm
Figure 5.
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine.
Findings: Sagittal STIR also depicts increased signal intensity of the body of T12 (arrow) and T11. The alterations previously mentioned within the prevertebral soft tissues from T9 to T12 are also depicted.
Technique: 3T GE Magnet, TR: 4850 TE: 41.292, FOV: 32 cm
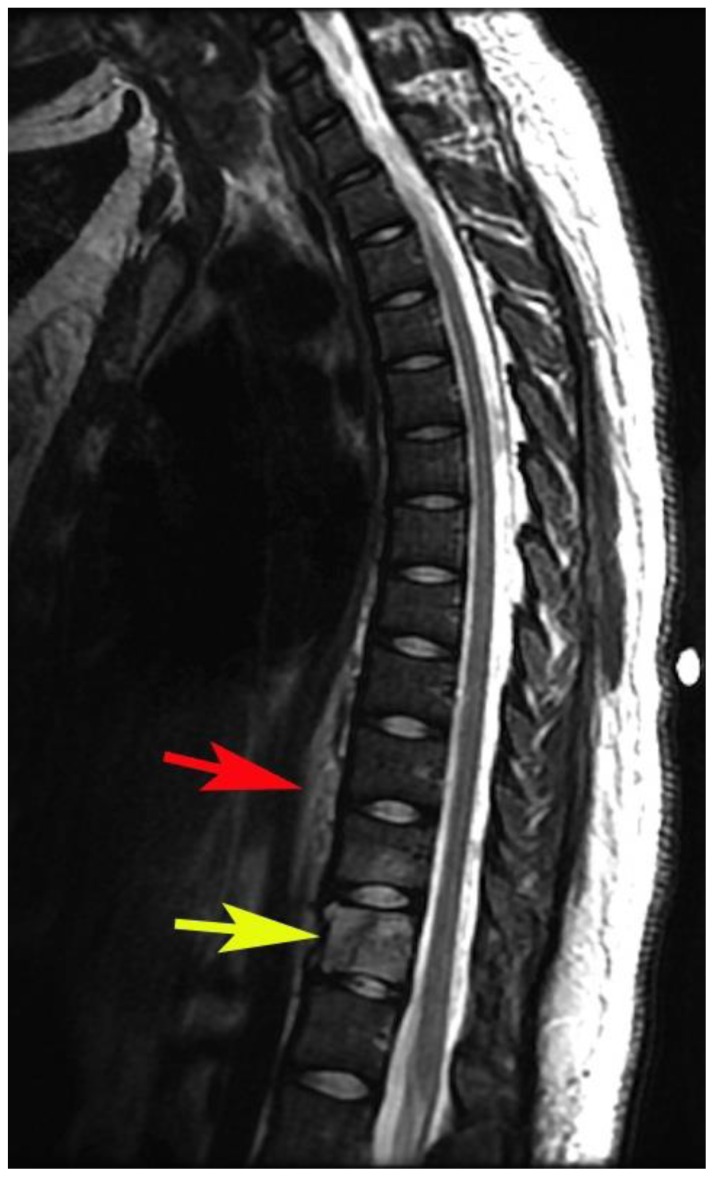
Figure 6.
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine.
Findings: a) and b) Sagittal T1WI with contrast and without fat saturation show intense enhancement of T11, T12 and prevertebral component. There is no enhancement of the intervertebral discs. c) Axial T1WI fat saturated with gadolinium (15 ml, MultiHance) shows enhancement of the lesion within the body of T12 (yellow arrow), ventral epidural space and prevertebral soft tissues (red arrowheads).
Technique: 3T GE Magnet, TR: 616.668, TE: 9.36, FOV: 18 cm)
Figure 7.
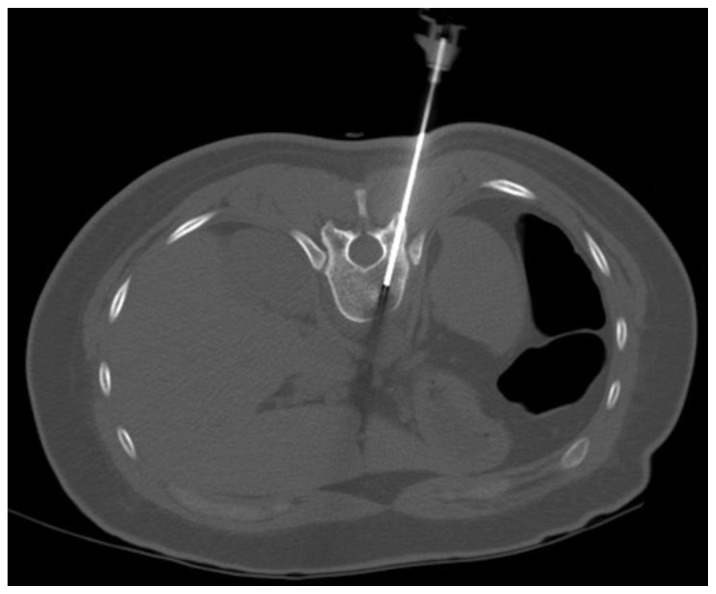
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine.
Findings: Axial CT image without contrast depicts the CT guided first bone biopsy with the tip of the needle inside the lytic lesion from a left transpedicular approach. Even then, the results were negative.
Technique: 181 mA, 120 KVp, 5.0 mm slice thickness.
After several weeks, the patient represented for progressive severity of thoracolumbar pain. X-rays revealed a compression fracture of T12 (figure 8). Repeat MRI showed progression of the T12 lesion with a pathologic compression fracture and focal thoracic kyphosis. There was also slight progression of T2 hyperintense signal within the T11 vertebral body (Figure 9). After contrast administration, T1WI showed avid enhancement of T11, T12 and perivertebral soft tissues, as well as new areas of enhancement in the L1 and L2 vertebral bodies (Figure 10). Fluoro-2-deoxy-D-glucose (FDG)-Positron Emission Tomography-CT (PET-CT) revealed disease in T12 vertebral body (Figure 11 a). It also depicted increased FDG uptake in the T11 and L2 vertebral body (Figure 11 b–d). No PET avid lymphadenopathy or suspicious extraskeletal FDG uptake was depicted. A second CT guided biopsy of the T12 vertebral body was performed and the diagnosis of Anaplastic Large Cell Lymphoma, anaplastic lymphoma kinase positive (ALK+), was made (Figure 12). CT guided biopsy of the T11 vertebral body was also obtained and showed normocellular bone marrow with trilineage hematopoiesis, relatively increased erythropoiesis, and mild megaloblastoid changes. The etiology of megaloblastoid changes in the erythroid precursors was unclear, but could be due to medication effect, nutritional deficiency, or the presence of lymphoma in another anatomic location. The patient received radiation therapy at the T11–T12 level followed by spine stabilization surgery. The outcome was satisfactory without any complications. The patient then continued with her second cycle of the chemotherapy treatment.
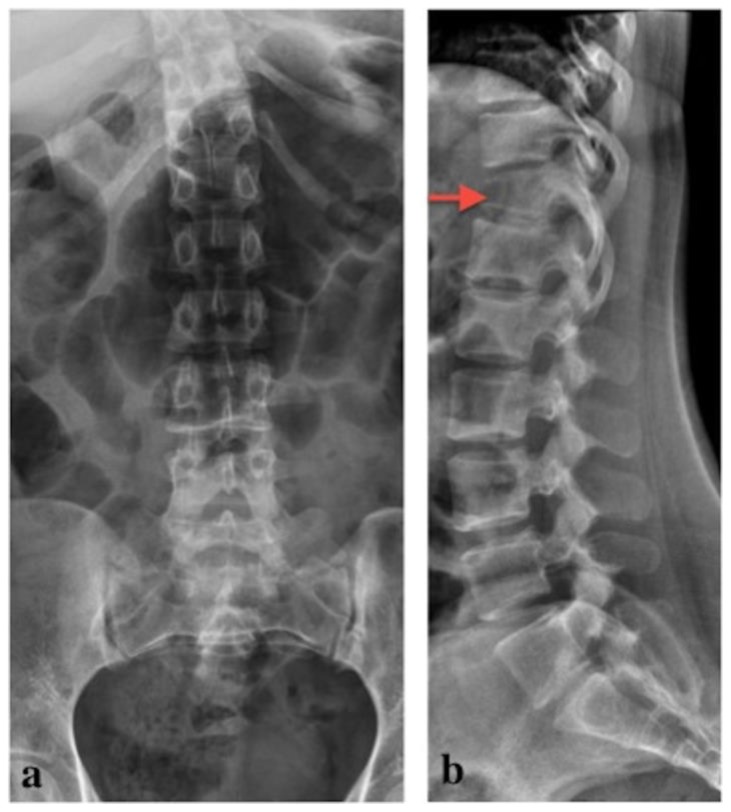
Figure 8.
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine. Follow up study one month later.
Findings: X-ray images: a) anteroposterior and b) lateral projection. Decrease in height and anterior wedging of the T12 vertebral body compatible with compression fracture, which is evident only in the lateral projection (red arrow in b).
Technique: kVp: 70; mAs: 50.
Figure 9.
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine. Follow up study one month later.
Findings: Sagittal T2WI. a) Initial study (for comparison) and b) follow up study one month later shows interval collapse of T12, with focal kyphosis, vertebral malaligment and indentation of the anterior margin of the thecal sac (arrow). Hyperintensity of T11. Increased signal intensity and thickness of the prevertebral soft tissues from T8 to L1.
Technique: 3T GE Magnet, TR: 3066.67, TE: 122.96, FOV: 32 cm.
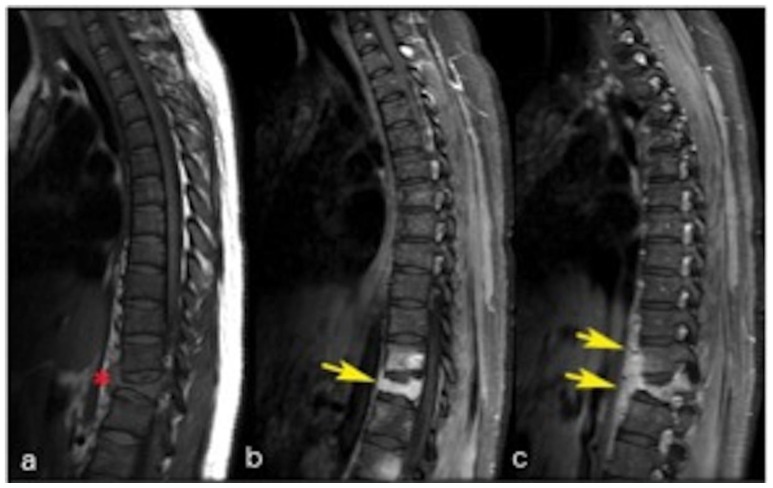
Figure 10.
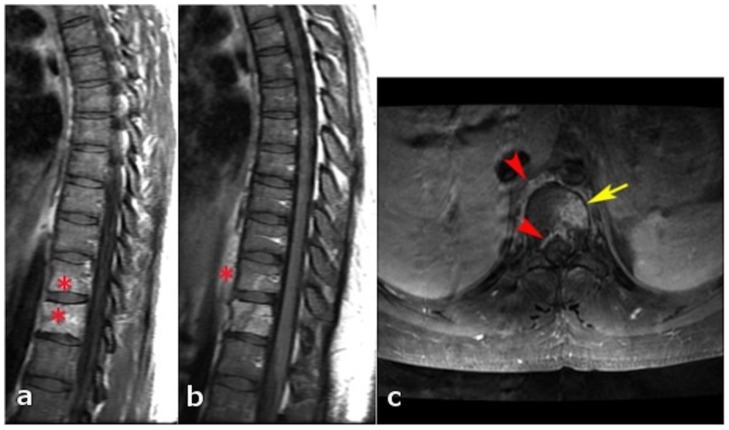
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine.
Findings: a) Sagittal T1WI without contrast shows hypointense prevertebral component (red asterisk); b and c) Sagittal T1WI with contrast (15 ml, MultiHance) and fat saturation (in different levels than figure a), shows intense enhancement of the T12 lesion and the prevertebral component (yellow arrows). Enhancement of T11 is also noticed. New patchy enhancement is also seen in the L1 and L2 vertebral bodies. There is relative preservation of the intervertebral discs without pathologic enhancement.
Technique: 3T GE Magnet, TR: 916.668, TE: 16.02, FOV: 32 cm.
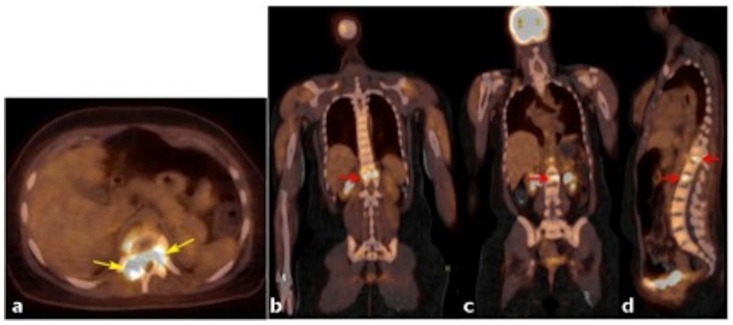
Figure 11.
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine.
Findings: a) PET/CT axial fused image and b–d) PET/CT fused images in the coronal plane (a and b) and sagital plane (c) show large lytic lesion in T12 with intense FDG uptake (yellow arrows in figure 11, red arrow in figure 12a and the superior red arrow in c). Max SUV was of 12.1. An additional area of focal increased uptake within L2 is depicted in figure 12b (red arrow) and 12c (inferior red arrow). A transitional vertebral body was identified.
Technique: radiopharmaceutical: 11.96 mCi F-18 FDG IV in right antecubital fossa; low dose CT without contrast administration, mA: 108, KVp: 120, FOV: 60.0 cm, 5.00 slice thickness.
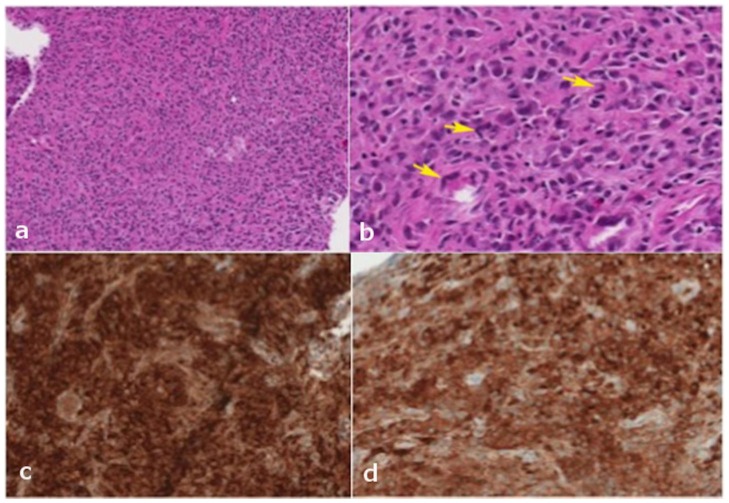
Figure 12.
24 years old female patient: Diagnosis of Anaplastic Large Cell Lymphoma ALK + of the thoracic spine.
Findings: a) Histopathological findings: Medium power: The majority of the specimen consists of fragments of tissue without distinct architecture containing sheets of small lymphocytes, plasma cells, histiocytes, and abundant large atypical cells. These large atypical cells have varying amounts of pink cytoplasm, single or multiple nuclei with irregular nuclear contours, and frequent eosinophilic nucleoli. b) High power: The large atypical cells display pleomorphic morphology. Occasional cells have a horseshoe-shaped nucleus (yellow arrows), and some multinucleated cells have a “wreath-like” configuration of their nuclei. Rare mitotic figures are identified. c) High power: Panel of immunohistochemical stains show that the atypical cells are strongly positive for CD30 (some cells show Golgi staining), and are variably positive for CD4 and CD43. d) High power: The majority of these cells also depict strong nuclear and cytoplasmic staining for ALK-1.
DISCUSSION
Among patients under 30 years of age, the majority of spinal tumors are benign, however, malignant tumors may also be found in young adults and children. Conventional radiography continues to have a role in the initial evaluation of patients with nonspecific symptoms related to the spine. However, CT and MR are required to further characterize a bone lesion and to determine the presence and extent of surrounding soft tissue involvement. Whenever evaluating a bone neoplasm, it is important to remember those features which, may help to narrow the differential diagnosis such as the age of the patient, lesion matrix, zone of transition, the specific bones affected, and some secondary findings such as fluid – fluid levels.
Etiology & Demographics
PBL is an uncommon extra nodal disease that represents about 1–3% of the cases of lymphoma with a male predominance (8:1). The peak prevalence is between the 5th and 7th decades of life [1,2]. Oberling first described it as reticulum cell sarcoma in 1928 [3]. Based on their series in 1939, Parker and Jackson [4,5] established PBL as a distinct clinical entity. The majority of the cases are diffuse large – B cell type (PBDLBCL). This type is further subdivided into two subtypes according to the different stages of B – cell maturation, which are germinal center B-cell like, and activated B-cell like (or non-germinal center). B – cell lymphoma more frequently arises from alterations during the germinal center phase [6]. Primary bone lymphoma of non-B – Cell origin includes primary T – Cell, Natural Killer Cell and Hodgkin Disease. According to the World Health Organization (WHO), a primary bone lymphoma is confined to bone without lymph node or visceral disease. It more commonly affects elderly and middle age patients, but cases of both Hodgkin and non-Hodgkin bone lymphomas have been described in younger adults and children [7,8,9]. The category of anaplastic large cell lymphomas (ALCL) are included within the list of Mature T-cell and NK-cell neoplasms. In the 2001 WHO classification, ALK + and ALK − neoplasms within the same group were considered as the same entity. However, the 2008 WHO classification concluded that ALK + ALCL should be distinguished as a different entity. ALK + ALCL is found mainly in pediatric and young adult patients and has a better prognosis than ALK – ALCL [10]. These are tumors of T – cell origin and are extremely rare, except in Japan, where they represent 10% of PBL [11].
Clinical & Imaging Findings
Clinically, patients with bone lymphoma may present with insidious and intermittent pain, fever, weight loss, swelling and a palpable mass. The radiographic appearance is variable and non-specific. Some cases may have near normal radiographs, while other cases may depict lytic (most common pattern) or blastic-sclerotic lesions, cortical disruption, pathological fractures and soft tissue masses. A combination of patterns may also be seen. It is important to take into account that patients with negative radiographic findings and persistent symptoms should undergo additional imaging modalities. CT may demonstrate bony sequestra and periosteal reaction (onion – peel appearance or discontinuous and sunburst pattern which suggest a more aggressive behavior and poorer prognosis) [11,12]. On MR, lesions are T1WI hypointense and T2WI hyperintense, unless there are changes associated with fibrosis. Short tau inversion recovery (STIR) and T2WI often show hyperintense signal related to peritumoral edema and/or bone marrow replacement. After intravenous administration of gadolinium, the lesions tend to enhance avidly [11,12]. Radionuclide bone scans depict intense uptake in the majority of the cases [13]. Absent uptake of 67Ga after completion of treatment denotes a good response and outcome even in cases where CT and MR findings persist for up to one year after treatment [14]. Fluoro-2-deoxy-D-glucose (FDG) Positron Emission Tomography (PET)/CT has more sensitivity (97%) and specificity (100%) than CT alone for staging (87% and 85% respectively), restaging (Sensitivity: 96% and Specificity: 99% for FDG PET/CT; Sensitivity: 61% and Specificity: 89% for CT) and is very useful for therapy response assessment. The FDG uptake depends on many factors including the histologic subtype, grade, local perfusion, and proliferation index, among others. Extranodal lymphomas including bone lymphoma demonstrate intense FDG uptake [15, 16, 17, 18].
Despite having multiple imaging modalities, biopsy and immunohistochemical studies remain essential for definitive diagnosis. For this purpose, CT guided percutaneuos biopsy has proven to be a safe and reliable way to obtain sufficient samples [19, 20, 21].
Microscopically, primary diffuse large B-cell lymphoma of bone depicts a diffuse growth pattern with thickened or normal trabeculae. The lymphoma cells are atypical or polymorphous large cells, with prominent nucleoli and often multilobulated nuclei. Fine chromatin, infiltration by mature T cells; fine reticular fibers and fibrosis have also been identified between the cells. The presence of fibrotic components may give the lymphoma a sarcomatous appearance. Other histological subtypes of lymphomas such as Anaplastic Large Cell, peripheral T – cell lymphoma not otherwise specified, Hodgkin lymphoma, marginal zone, small lymphocytic, lymphoplasmacytic and primary follicular lymphomas are uncommon. Regarding the anaplastic type, it tends to affect the axial skeleton. The large cells are characterized by multiple prominent nucleoli, and a pleomorphic nucleus, which often has a “horseshoe” shape (hallmark cells). Some cells may have multiple nuclei in a circular pattern “wreath cells”. These lymphomas are positive for CD30 and most of them depict reactivity to an anaplastic lymphoma kinase (ALK – 1) antibody. Expression of cytotoxic proteins, epithelial membrane antigen and staining for CD45 are variable [22]. The increased availability of immunohistochemical stains has allowed for better classification of some lymphomas according to phenotypes. For example, stains for BCL-6 and CD10 are considered germinal center markers. In a study conducted by Heyning et al [23], inmunohistochemical analysis for cluster of differentiation 10 (CD10), B cell lymphoma – 6 (BCL-6), multiple myeloma – 1 (MUM-1), BCL-2, CD30, CD44 and p53 was acquired in 36 patients with primary bone lymphoma in order to determine the possible germinal center origin and to evaluate the prognostic value of the markers. They identified 19 cases with a germinal center phenotype, 8 cases with a non-germinal center phenotype and 9 cases with an indeterminate phenotype. Unfortunately, they found neither significant difference in the survival rates independent of the tumor phenotype or any statistically significant effect on prognosis by individual markers. Other inmunohistochemical markers, such as the 100% Soluble in ammonium sulfate at neutral pH (S100), friend leukaemia integration 1 (FLI1), CD1a, and CD99, may also help to differentiate a primary bone lymphoma from other tumors such as Ewing sarcoma [22].
Treatment & Prognosis
The control of primary bone lymphoma depends on many factors including the patient’s age, stage at the time of the diagnosis and the selected therapy. Surgical treatment is reserved for patients with unstable spinal fractures and those at risks of a myelopathy, with the purpose to restore function and alleviate pain, but it should not delay chemotherapy [24]. Chemotherapy may be used alone with a regimen that includes doxorubicin like cyclophosphamide, doxorubicin, vincristine and prednisone for 6 to 8 cycles. Another alternative is just 3 cycles of chemotherapy plus localized radiotherapy. Miller et al [25] found that the combined treatment is better than chemotherapy alone for patients with intermediate or high-grade non-Hodgkin lymphoma. Their study demonstrated that the use of a combined treatment is advantageous because it allows the number of chemotherapy cycles to be reduced and therefore reducing the risk of associated complications such as cardiotoxicity, myelosuppression and neutropenia. This therapy may also increase the progression-free survival and overall survival. For patients with stage I and II PBL, combined therapy has also demonstrated better results than radiotherapy alone according to Barbieri et al [26]. Bone lymphomas have a better response to treatment and a better prognosis in comparison with other bone tumors.
Differential Diagnoses
The main differential considerations include secondary osseous lymphoma (widespread lymphoma with secondary involvement of the bone), other subtypes of lymphoma (diffuse large B-cell lymphoma, NK/T-cell lymphoma, Burkitt’s, follicular and lymphoplasmacytic) [4,5], osteosarcoma, metastases, Ewing sarcoma [11] and granulomatous infection such as tuberculosis [7].
a) Osteosarcomas
Osteosarcomas are sarcomas with osteoid immature matrix and woven osteoid. The peak prevalence occurs during the 4th decade. Within the spine, the majority of the cases arise in the posterior elements. Radiographic and CT findings depict an aggressive lesion, with a permeative or moth eaten pattern and a wide zone of transition and soft tissue mass. On T1WI and T2WI, the mineralized components are hypointense and the soft tissue components enhance after the contrast administration. Lytic lesions and fluid-fluid levels may be seen in telangiectatic osteosarcoma [27]. Bone scan and FDG PET/CT show increased uptake.
b) Ewing’s sarcoma
Ewing’s sarcoma is a round cell sarcoma of the bone. The spine is affected in only 5% of the cases. Radiographic findings are permeative or moth eaten pattern of bone destruction, wide zone of transition, and vertebra plana. Disc height and endplates are usually preserved when two adjacent vertebral bodies are affected, which may help to differentiate from a bacterial spondylodiscitis. CT shows soft tissue lesions with central necrosis and heterogeneous enhancement. Sclerotic lesions are rare. Lesions have low to intermediate signal intensity on T1WI and intermediate to high signal intensity on T2WI [1]. Bone scans and FDG PET/CT show increased radiotracer uptake.
c) Metastases
Metastases may be lytic or sclerotic and the imaging characteristics vary accordingly. Lytic lesions are hypointense on T1WI and hyperintense on T2WI compared to non-affected marrow. Blastic lesions are hypointense on T1WI and T2WI. Lytic lesions usually enhance after contrast administration. There is increased uptake of Tc99m (in areas of bone production) and increased uptake of FDG PET/CT.
d) Granulomatous infections
Granulomatous infections such as tuberculous spondylitis depict endplate irregularity, bone destruction, reactive sclerosis, epidural soft tissue masses, paravertebral abscesses and vertebral collapse. The intervertebral discs may or may not be affected. Diffuse enhancement of the epidural components and rim enhancement of the paravertebral collections are depicted in CT. Lesions are hypointense on T1WI and hyperintense on T2WI and STIR. Dural, epidural, disc and marrow enhancement may be seen, as well asrim enhancing abscesses and diffusely enhancing phlegmon within the soft tissue components. There is increased radionuclide uptake on bone scan and gallium scans, and increased FDG uptake on PET/CT [28,29]. A granulomatous infection may mimic an aggressive neoplastic process. Histopathological and microbiological evaluation is required for definitive diagnosis [7].
In this case, the first CT guided biopsy result showed no malignant process despite imaging showing the needle traversing the lytic lesion. This result likely represented sampling of a perilesional reactive process seen as bone demineralization on CT. The second MR examination showed interval collapse of the T12 vertebral body, epidural extension of disease, and progressive intense enhancement, as well as new areas of enhancement in the L1 and L2 vertebral bodies, findings that were highly suggestive of an aggressive neoplastic process. Eosinophilic granuloma was less likely because of the patient’s age. There was no history of trauma and since this patient had recent previous MR and CT examinations, a compression fracture with epidural hematoma was not included within the differential diagnosis. From an imaging standpoint, this case may be differentiated from a granulomatous or pyogenic osteomyelitis by the absence of paravertebral abscesses with ring enhancement, calcifications within a chronic abscess, and preservation of the intervertebral discs. A Gallium scan will depict increased radionuclide uptake in an infectious process of the spine and paravertebral soft tissues.
TEACHING POINT
Anaplastic large cell lymphomas are a rare subtype PBL of T – cell origin, with imaging findings that are non-specific and suggestive of an aggressive process. Although the majority of spinal tumors are benign among patients under 30 years of age and a granulomatous infection may mimic this entity, the histopathological and/or microbiological analysis can differentiate these processes and give the definitive diagnosis.
Table 1.
Summary table for Anaplastic Large Cell Bone Lymphoma.
| Etiology | Neoplasm of T cell origin. |
| Incidence | 1 – 3% of all lymphomas. |
| Gender Ratio | Male preponderance (8:1). |
| Age Predilection | Wide range (4 – 63 years). Reported mean peak prevalence between the 5th and 7th decade (references 1, 2, 9). |
| Risk Factors | Advance age, male gender, immunosuppression, and radiation exposure. |
| Treatment | Chemotherapy + localized radiation, surgical for selected cases (to restore function and alleviate pain without delay the chemotherapy). |
| Prognosis | Better prognosis than other bone tumors. |
| Findings on Imaging |
|
Table 2.
Differential diagnosis table for Bone Lymphoma.
| XR | CT | MR | Pattern of contrast | Scintigraphy | PET | |
|---|---|---|---|---|---|---|
| Lymphoma |
|
|
|
|
|
|
| Osteosarcoma |
|
|
|
|
|
|
| Ewing’s sarcoma |
|
|
|
|
|
|
| Metastasis |
|
|
|
|
|
|
| Osteomyelitis (Pott’s disease) |
|
|
|
|
|
|
ABBREVIATIONS
- ALCL
Anaplastic large cell lymphoma
- ALK +
Anaplastic lymphoma kinase positive
- BCL
B cell lymphoma
- CD
Cluster of differentiation
- CT
Computed Tomography
- FDG PET
Fluoro-2-deoxy-D-glucose Positron Emission Tomography
- FLI
Friend leukaemia integration
- MR
Magnetic Resonance
- MUM
Multiple myeloma
- NHL
Non-Hodgkin Lymphoma
- PBDLBCL
Primary bone diffuse large B cell lymphoma
- PBL
Primary bone lymphoma
- S100
100% Soluble in ammonium sulfate at neutral pH (derivation of name)
- STIR
Short tau inversion recovery
- WHO
World Health Organization
- WI
Weighted Image
REFERENCES
- 1.Rodallec MH, Feydy A, Larousserie F, et al. Diagnostic imaging of solitary tumors of the spine:What to Do and Say. Radiographics. 2008;28:1019–1041. doi: 10.1148/rg.284075156. [DOI] [PubMed] [Google Scholar]
- 2.Motamedi K, Ilaslan H, Seeger L. Imaging of the lumbar spine neoplasms. Semin Ultrasound CT MRI. 2004;25:474–489. doi: 10.1053/j.sult.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Oberling C. Les reticulosacomes et les reticuloendotheliosarcomes de la osseuse (sarcomes d’Ewing) Bull Assoc Fr Etude Cancer. 1928;17:259–296. [Google Scholar]
- 4.Parker F, Jackson H., Jr Primary reticulum cell sarcoma of bone. Surg Gynecol Obstet. 1939;68:45. [Google Scholar]
- 5.Mikhaeel NG. Primary bone lymphoma. Clin Oncol. 2012;24:366–370. doi: 10.1016/j.clon.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Guerard EJ, Bishop MR. Overview of Non-Hodgkin’s Lymphoma. Dis Mon. 2012;58:208–218. doi: 10.1016/j.disamonth.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Khor LK, Wang S, Lu S-J. Anaplastic large cell lymphoma of the vertebra masquerading as tuberculous spondylitis:potential pitfalls of conventional imaging. Intern Emerg Med. 2012;7:573–577. doi: 10.1007/s11739-012-0868-8. [DOI] [PubMed] [Google Scholar]
- 8.Uehara M, Tahashi J, Hirabayashi H, et al. Hodgkin’s disease of the thoracic vertebrae. Spine J. 2013;13:e59–e63. doi: 10.1016/j.spinee.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Zhao XF, Young KH, Frank D, et al. Pediatric primary bone lymphoma-diffuse large B-cell lymphoma morphologic and immunohistochemical characteristics of 10 cases. Am J Clin Pathol. 2007;127:47–54. doi: 10.1309/LRQVTE5NM8B9ANLY. [DOI] [PubMed] [Google Scholar]
- 10.Campo E, Swerdlow S, Harris N, Pileri S, Stein H, Jaffe E. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011 May 12;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. Prepublished online 2011 Feb 7. PMID: 21300984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan A, Shirkhoda A, Tehranzadeh F, Armin A, Irwin R, Kimberly Les. Primary bone lymphoma:radiographic-MR imaging correlation. Radiographics. 2003;23:1371–1387. doi: 10.1148/rg.236025056. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill J, Finlay K, Jurriaans E, Friedman L. Radiological manifestations of skeletal lymphoma. Curr Probl Diagn Radiol. 2009;38:228–236. doi: 10.1067/j.cpradiol.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Kwee TC, de Klerk John MH, Nievelstein RAJ. Imaging in bone marrow involvement in lymphoma:state of the art and future directions. ScientificWorldJournal. 2011;11:391–402. doi: 10.1100/tsw.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Israel O, Mekel M, Bar-Shalom R, et al. Bone lymphoma:67Ga scintigraphy and CT for prediction of outcome after treatment. J Nucl Med. 2002;43:1295–1303. [PubMed] [Google Scholar]
- 15.Paes FM, Kalkanis DG, Sideras PA, Serafini AN. FDG PET/CT of extranodal involvement in Non-Hodgkin Lymphoma and Hodgkin Disease. Radiographics. 2010;30:269–291. doi: 10.1148/rg.301095088. [DOI] [PubMed] [Google Scholar]
- 16.La Fougère C, Hundt W, Bröckel N, et al. Value of PET/CT versus PET and CT performed as separate investigations in patients with Hodgkin’s disease and non-Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging. 2006;33:1417–1425. doi: 10.1007/s00259-006-0171-x. [DOI] [PubMed] [Google Scholar]
- 17.Terasawa T, Nihashi T, Hotta T, Nagai H. 18F-FDG PET for posttherapy assessment of Hodgkin’s Disease and aggressive Non-Hodgkin Lymphoma:a systematic review. J Nucl Med. 2008;49:13–21. doi: 10.2967/jnumed.107.039867. [DOI] [PubMed] [Google Scholar]
- 18.Kwee TC, Kwee RM, Nievelstein RAJ. Imaging in staging of malignant lymphoma:a systematic review. Blood. 2008;111:504–516. doi: 10.1182/blood-2007-07-101899. [DOI] [PubMed] [Google Scholar]
- 19.Maciel MJ, Tyng CJ, Vieira Pinto Barbosa PN, et al. Computed tomography-guided percutaneous biopsy of bone lesions: rate of diagnostic success and complications. Radiol Bras. 2014;47( 5):269–274. doi: 10.1590/0100-3984.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hau MA, Kim JI, Kattapuram S, et al. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skeletal Radiol. 2002;31:349–353. doi: 10.1007/s00256-002-0474-3. [DOI] [PubMed] [Google Scholar]
- 21.Espinosa LA, Jamadar DA, Jacobson JA, et al. CT-guided biopsy of bone:a radiologist’s perspective. AJR. 2008;190:W283–W289. doi: 10.2214/AJR.07.3138. [DOI] [PubMed] [Google Scholar]
- 22.Bhagavathi S, Fu K. Primary bone lymphoma. Arch Pathol Lab Med. 2009;133(11):1868–1871. doi: 10.5858/133.11.1868. [DOI] [PubMed] [Google Scholar]
- 23.Heyning FH, Hogendoom PCM, Kramer MHH, Holland CTQ, Dreef E, Jansen PM. Primary lymphoma of bone:extranodal lymphoma with favourable survival independent of germinal centre, post-germinal centre or indeterminate phenotype. J Clin Pathol. 2009;62:820–824. doi: 10.1136/jcp.2008.063156. [DOI] [PubMed] [Google Scholar]
- 24.Scoccianti G, Rigacci L, Puccini B, et al. Primary lymphoma of bone: outcome and role of surgery. Int Orthop (SICOT) 2013;37:2437–2442. doi: 10.1007/s00264-013-2055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller TP, Dahlberg S, Cassidy JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade Non-Hodgkin’s Lymphoma. N Engl J Med. 1998 Jul 2;339(1):21–26. doi: 10.1056/NEJM199807023390104. [DOI] [PubMed] [Google Scholar]
- 26.Barbieri E, Cammelli S, Mauro F, et al. Primary Non-Hodgkin’s Lymphoma of the bone:treatment and analysis of prognostic factors for stage I and Stage II. Int J Radiat Oncol Biol Phys. 2004;59(3):760–764. doi: 10.1016/j.ijrobp.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Ilaslan H, Sundaram M, Unni KK, Shives T. Primary vertebral osteosarcoma: imaging findings. Radiology. 2004;230:697–702. doi: 10.1148/radiol.2303030226. [DOI] [PubMed] [Google Scholar]
- 28.Ross JS, Brant-Zawadzki M, Moore K, Crim J, Chen M, Katzman GL. Diagnostic imaging:spine. 1st Edition. Salt Lake City; Amirsys Inc.: 2004. Part III Infection and inflammatory disorders (Section 1:10–13); Part IV Neoplasm, Cysts, and Other Masses (Section 1:6–13,42–45,50–57). [Google Scholar]
- 29.Osborn AG, Ross JS, Salzman KL, et al. Brain and spine. 1st Edition. Salt Lake City: Amirsys Inc.; 2009. Expert differential diagnosis. Part II Bony lesion, aggresive (Section 3:24–27) [Google Scholar]