Abstract
Objective
This study investigates the impact of isometric contraction of anterior cervical muscles on cervical lordosis.
Methods
29 volunteers were randomly assigned to an anterior head translation (n=15) or anterior head flexion (n=14) group. Resting neutral lateral cervical x-rays were compared to x-rays of sustained isometric contraction of the anterior cervical muscles producing anterior head translation or anterior head flexion.
Results
Paired sample t-tests indicate no significant difference between pre and post anterior head translation or anterior head flexion. Analysis of variance suggests that gender and peak force were not associated with change in cervical lordosis. Chamberlain’s to atlas plane line angle difference was significantly associated with cervical lordosis difference during anterior head translation (p=0.01).
Conclusion
This study shows no evidence that hypertonicity, as seen in muscle spasms, of the muscles responsible for anterior head translation and anterior head flexion have a significant impact on cervical lordosis.
Keywords: anterior head flexion, anterior cervical muscles, anterior head translation, forward head posture, hypolordosis, muscle spasm, cervical biomechanics, neutral lateral cervical radiograph, hypertonicity
INTRODUCTION
Proper cervical curves in the sagittal plane have been shown to be an important clinical outcome of health [1–3]. Recent surgical and non-surgical studies have emphasized that patients with less cervical lordosis have statistically significant increases in neck, upper thoracic, and shoulder pain, as well as overall inferior health outcomes [4–8]. As cervical lordosis decreases from normal, so to does this increase the risks of continuous abnormal stress and strain loads on the vertebrae and intervertebral discs (IVD) resulting in bone remodeling, arthritis, and IVD and ligament damage; tensile stresses in the nervous system resulting in various neuromusculoskeletal conditions; and stress and strain variations of vascular tissue resulting in ischemia, reduced perfusion of the spinal cord and surrounding tissues, and nerve conduction failure [9].
Claims have been made in radiology texts, papers, and reports that cervical kyphosis and hypolordosis can be caused by muscle spasms or hypertonicity in the anterior cervical muscles that produce anterior head translation and anterior head flexion, particularly when there has been a sustained trauma [6–8, 10–18]. Gehweiler, et al. [10] and Clark, et al. [11] claim that cervical kyphosis is the result of muscle spasm. Kettner and Guebert [12] and Deltoff and Kogon [13] make the same claim without providing data. Pederson [14] and Yochum and Rowe [15] reference Jugl, et al. [16] in support of this claim. Rechtman, et al. stated that “flattening of a cervical lordosis should be evaluated, carefully, especially in medicolegal problems, before being attributed to muscular spasm, as has been mentioned so commonly in radiological reports. The muscular response associated with loss of cervical lordosis remains for further clarification” [17]. Helliwell, et al. found no relationship between muscle spasm and loss of cervical lordosis. They suggested that muscle spasm would cause an increased lordosis due to the larger volume and larger moment arms of the posterior extensor muscles of the cervical spine [18]. However, to date there have been no published data that show the impact of anterior cervical muscle spasms on the cervical curve.
Muscle spasm is the “involuntary sustained contraction of muscle that prohibits joint motion in the direction opposite the afflicted muscle’s action” [19]. The muscles producing anterior head translation include the sternocleidomastoid (SCM) and cervical scalene (anterior, medius, and posterior) muscles [19]. Activation of these muscles produces lower cervical flexion and upper cervical extension producing the anterior translation of the head [19]. The muscles producing anterior head flexion include the SCM and cervical scalene muscles. Activation of these muscles produces upper and lower cervical flexion producing the anterior flexion of the head [19].
This pilot study employs a pre-post design and is the first using isometric contraction of the anterior cervical muscles producing anterior head translation and anterior head flexion to determine the effects on cervical lordosis. The objective of this study is to provide objective measurements quantifying any changes in cervical lordosis associated with these isometric contractions.
MATERIALS & METHODS
The anterior cervical muscles were activated by contracting the muscles involved in anterior head translation (+TzH) or anterior head flexion (+RxH). These actions were tested independently of each other. The subjects that met the inclusion criteria were able to be weight-bearing for the radiographic imaging and able to forcibly contract the muscles that produce anterior head translation or anterior head flexion in a sustained isometric contraction for 15 seconds.
Anterior Head Translation
Fifteen subjects volunteered to perform anterior head translation. A resting neutral lateral cervical (NLC) radiograph (Figure 1A) was followed by a NLC radiograph while the subjects performed isometric anterior head translation without causing displacement of their head from its original position (Figure 1B). The subjects were instructed to stand in a natural, resting posture for the first radiograph and to maintain their posture until completion of the second radiograph. For the second radiograph, the subjects raised both arms and placed the Hoggan Health microFET handheld dynamometer (Salt Lake City, UT, USA) (Figure 2A & 2B) against their forehead with both hands and were instructed to forcibly perform anterior head translation with maximum effort for approximately 15 seconds while providing enough counter-pressure with the dynamometer to maintain their posture. The microFET handheld dynamometer is a force evaluation and testing (FET) device. It provides objective, valid, and reliable peak force and duration of force measurements. Prior to radiographs, CLA Insight™ Surface Electromyography (sEMG) sensors (Bethany Beach, DE, USA) were placed over the SCM and cervical scalene muscles to record the muscle activity. Surface EMG assesses muscle activity by measuring amplitude and is used as an objective, quantifiable measure of the changes in muscle activity [20]. Amplitude refers to the signal level measured in microvolts. Increased microvolts represent increased muscle activity [20]. The sEMG was used to ensure activation of the SCM and scalenes muscles. The second film was taken and the anterior head translation force was recorded from the dynamometer.
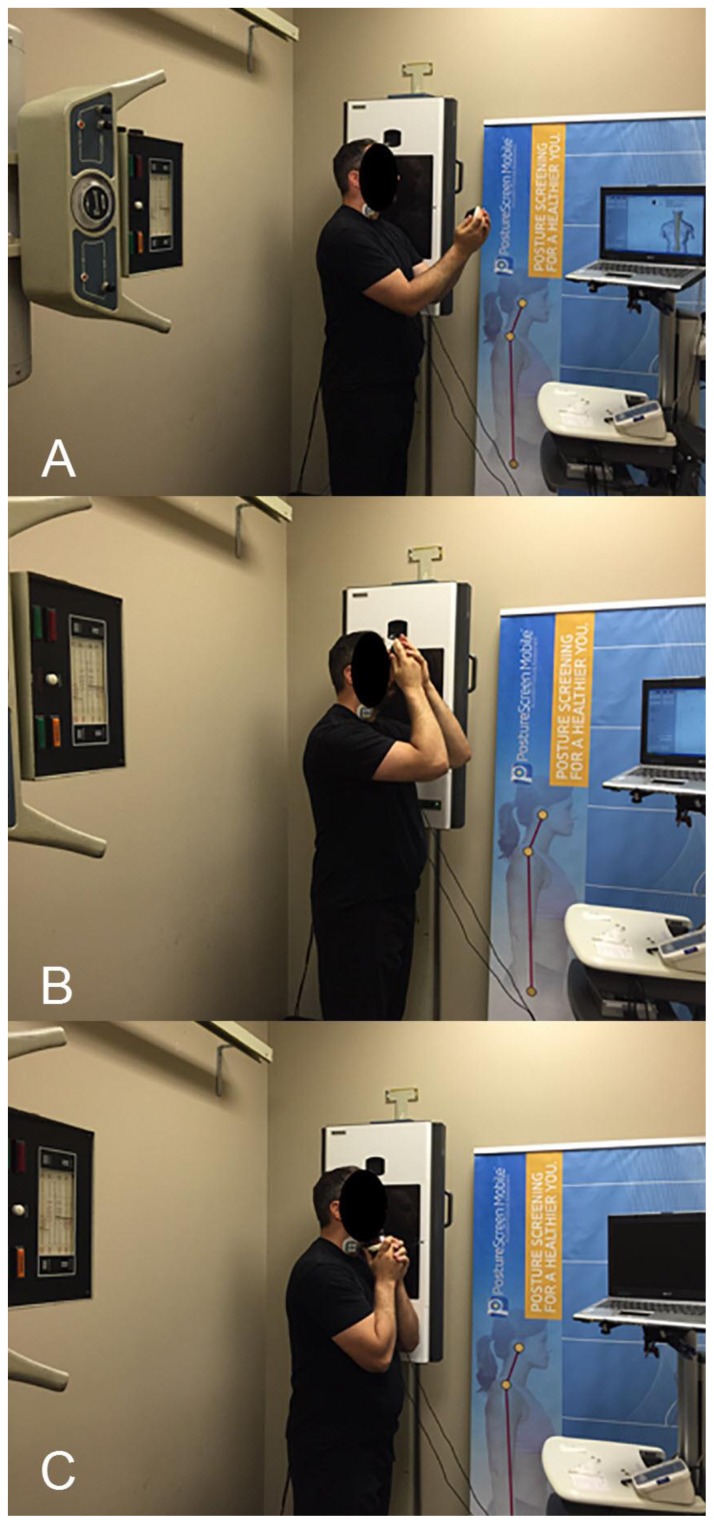
Figure 1.
A) is an image depicting a subject positioned for the resting neutral lateral cervical (NLC) x-ray. The subject is standing in a comfortable, weight-bearing position perpendicular to the x-ray Bucky. The subject is holding the Hoggan Health microFET handheld dynamometer (Salt Lake City, UT) anterior to his head to account for the posture during isometric anterior head translation or anterior head flexion. CLA Insight™ Surface Electromyography (sEMG) sensors are attached to the anterior aspect of the neck to determine activity of the sternocleidomastoid (SCM) and cervical scalene muscles, which are responsible for anterior head translation and anterior head flexion.
B) is an image depicting a subject positioned for the NLC x-ray while performing isometric anterior head translation. The subject is standing in a comfortable, weight-bearing position perpendicular to the x-ray Bucky. The subject is holding the Hoggan Health microFET handheld dynamometer (Salt Lake City, UT) against the anterior aspect of his forehead to produce isometric anterior head translation. CLA Insight™ sEMG sensors are attached to the anterior aspect of the neck to validate activity of the SCM and cervical scalene muscles, which are responsible for anterior head translation and anterior head flexion.
C) is an image depicting a subject positioned for the NLC x-ray while performing isometric anterior head flexion. The subject is standing in a comfortable, weight-bearing position perpendicular to the x-ray Bucky. The subject is holding the Hoggan Health microFET handheld dynamometer (Salt Lake City, UT) against the inferior aspect of his chin to produce isometric anterior head flexion. CLA Insight™ sEMG sensors are attached to the anterior aspect of the neck to validate activity of the SCM and cervical scalene muscles, which are responsible for anterior head translation and anterior head flexion.
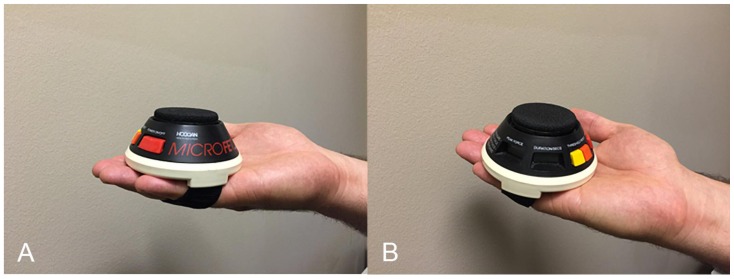
Figure 2.
A is the Hoggan Health microFET handheld dynamometer (Salt Lake City, UT). The microFET handheld dynamometer is a Force Evaluation and Testing (FET) device. It provides objective, valid, and reliable peak force and duration of force measurements.
B is the Hoggan Health microFET handheld dynamometer (Salt Lake City, UT). The microFET handheld dynamometer is a FET device. It provides objective, valid, and reliable peak force and duration of force measurements.
Head Flexion
Fourteen subjects volunteered to perform anterior head flexion. A resting NLC radiograph (Figure 1A) was followed by a NLC radiograph while the subjects performed isometric anterior head flexion without causing displacement of their head from its original position (Figure 1C). The subjects were instructed to stand in a natural, resting posture for the first radiograph and to maintain their posture until completion of the second radiograph. For the second radiograph, the subjects raised both arms and placed the Hoggan Health microFET handheld dynamometer (Salt Lake City, UT, USA) (Figure 2A & 2B) under their chin with both hands and were instructed to forcibly perform anterior head flexion with maximum effort for approximately 15 seconds while providing enough counter-pressure with the dynamometer to maintain their posture. The sEMG sensors were placed over the SCM and cervical scalene muscles to record the muscle activity. The second film was taken and the anterior head flexion force was recorded from the dynamometer.
Radiograph Analysis
Radiographic analysis is an evidence-based, valid way to assess spinal alignment and postural abnormalities. Spinal radiographs were taken with the subjects in a normal, neutral, weight-bearing position. Spinal radiographs were used to analyze cervical spinal structure to quantify cervical alignment.
The subjects’ NLC radiographs were analyzed using the Harrison Posterior Tangent Method. The Harrison Posterior Tangent method is a valid and reliable x-ray line drawing method [21–24] in accordance with the Harrison Spinal Model, which is a valid geometric spinal model [25–28]. From NLC x-rays, cervical angles are measured by drawing a line at the posterior of each vertebral body from C2 to C7. Measurements of a spinal region provide the absolute rotation angle (ARA). Cervical ARA is measured using the posterior tangent of C2 and C7. Global anterior or posterior translations of the spine can be measured by drawing a vertical line from an inferior landmark and measuring the distance to a superior landmark perpendicular to the vertical line. Anterior or posterior translation of the cervical spinal region is measured using the posterior, inferior body of C7 as the inferior landmark and the posterior, superior body of C2 as the superior landmark.
Spinal radiographic analysis involved the right-hand Cartesian coordinate system to “describe translations and rotations of the head around x, y, and z-axes, in the coronal, sagittal, and transverse planes” [29]. A shorthand method is used for documenting spinal alignment. In these spinal listings, the positive or negative sign indicates the direction of translation in or rotation around the x, y, and z-axes in the frontal, sagittal, and horizontal plane, respectively. The first letter denotes translation (T) or rotation (R). The second letter denotes the axis per the Cartesian coordinate system in or around which the T or R takes place. The third letter denotes the body region (head is H, thoracic cage is T, and pelvis is P) with respect to the body region below.
Radiographs were digitized and then analyzed with PostureRay® X-Ray Electronic Medical Records (EMR) Software (Trinity, FL, USA) using the Harrison Posterior Tangent Method according to the Harrison Spinal Model to provide spinal mensuration and comparison analyses. The pre and post radiographs for the isometric anterior head translation group (Figure 3) and the anterior head flexion group (Figure 4) were assessed for cervical ARA from C2 to C7 (ARA C2–C7), anterior or posterior head translation from C2 to C7 (TzH C2–C7), and Chamberlain’s to atlas plane line (APL) angle. Chamberlain’s to APL angle was measured to compare pre and post films to determine whether any significant head extension took place between pre and post films. Harrison et al. found that cervical extension of 14 degrees or less has no significant effect on ARA C2–C7 [30]. The clinicians marking the films were blinded from whether the film being marked was either the pre or post film. Each measurement was then entered into a spreadsheet by another clinician and sent off for statistical analysis.
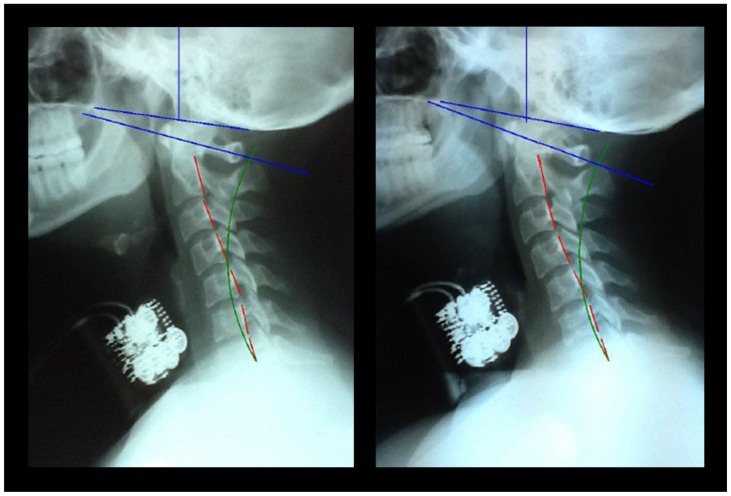
Figure 3.
Resting NLC (left) and NLC with isometric anterior head translation (right) x-ray views of subject 14A (a 21-year-old female weighing 131 pounds). Radiographic settings include: 30 mAs, 200 mA, 68 kVp with a 72 inch focal-film distance (FFD), central ray (CR) at C4, and 7 inch by 10 inch cropping. Chamberlain’s line is the blue line originating at the posterior aspect of the hard palate and ending at the opisthion of the skull. The atlas plane line (APL) is the blue line drawn through the anterior aspect of the anterior aspect of the atlas (C1) and the vertical center of the posterior arch of C1. The red lines represent the actual posterior tangent of the cervical vertebral bodies from C2–C7 of the subject. The green line represents the ideal spinal curvature as defined by the Harrison Spinal Model. The artifacts located anterior to C3–C6 bodies are the sEMG sensors.
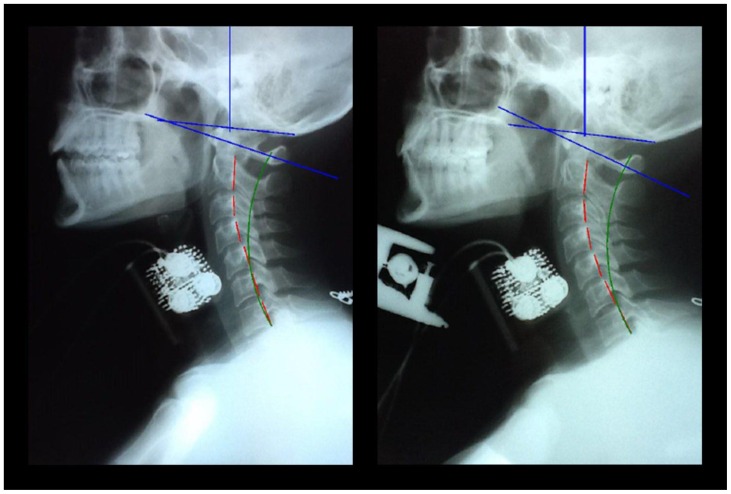
Figure 4.
Resting NLC (left) and NLC with isometric anterior head flexion (right) x-ray views of subject 7B (a 24-year-old female weighing 101 pounds). Radiographic settings include: 30 mAs, 200 mA, 68 kVp with a 72 inch FFD, CR at C4, and 7 inch by 10 inch cropping. Chamberlain’s line is the blue line originating at the posterior aspect of the hard palate and ending at the opisthion of the skull. The APL is the blue line drawn through the anterior aspect of the anterior aspect of C1 and the vertical center of the posterior arch of C1. The red lines represent the actual posterior tangent of the cervical vertebral bodies from C2–C7 of the subject. The green line represents the ideal spinal curvature as defined by the Harrison Spinal Model. The artifacts located anterior to C3–C5 bodies are the sEMG sensors. The artifact located inferior and anterior to the chin is the dynamometer.
Statistical Methods
Resting NLC radiographs were analyzed and compared with NLC radiographs with isometric anterior head translation for ARA C2–C7 and TzH C2–C7 (Table 1A) and Chamberlain’s to APL angle (Table 1B). Resting NLC radiographs were analyzed and compared with NLC radiographs with isometric anterior head flexion for ARA C2–C7 and TzH C2–C7 (Table 2A) and Chamberlain’s to APL angle (Table 2B). The difference in the ARA C2–C7 between the pre and post radiographs was calculated and assessed for statistical significance. Summary statistics for patient characteristics were calculated using means or proportions, as appropriate (Table 3). In order to address whether there was a significant difference between pre and post ARA C2–C7, unadjusted paired t-tests were performed for the anterior head translation and anterior head flexion groups (Table 4). The tests were conducted against a null hypothesis that the difference in pre and post ARA C2–C7 was 0 degrees. Simple linear regression models were developed for each group to determine whether peak force, TzH C2–C7, gender, or Chamberlain’s to APL angle had an affect on any difference in ARA C2–C7 (Table 5). If any variable was found to be significant, a follow-up analysis for ARA C2–C7 adjusted for the significant variable(s) was performed. The correlation between the difference in ARA C2–C7 and the difference in TzH C2–C7 was determined (Table 6). Additionally, the effects of different values of Chamberlain’s to APL angle difference on the mean ARA C2–C7 for the anterior head translation group was assessed (Table 7). All tests of hypotheses were two-tailed and conducted at a significance level of 0.05. In addition to p-values, 95% confidence intervals (CI) were determined.
Table 1a.
Global analysis of x-ray 1 (pre) and x-ray 2 (post) ARA C2–C7 and translation from C2–C7 (TzH C2–C7) for subject 14A (a 21-year-old female weighing 131 pounds). X-ray 1 and 2 values are compared to normal values as per the Harrison Spinal Model.
| Normal Values | X-ray 1 Values | Versus Normal | X-ray 2 Values | Versus Normal | |
|---|---|---|---|---|---|
| ARA C2–C7 | −42.0° | −2.4° | 94.3% | −8.5° | 79.8% |
| Translation C2–C7 | 0.0 mm | 26.4 mm | 26.4 mm | 29.9 mm | 29.9 mm |
ARA = Absolute Rotational Angle of Measurement
Table 1b.
Cervical morphology of x-ray 1 (pre) and x-ray 2 (post) in terms of Chamberlain’s line to APL values (°) for subject 14A (a 21-year-old female weighing 131 pounds). X-ray 1 and 2 values are compared to each other to determine whether any significant head extension (>14°) occurred between pre and post films that would produce a change in cervical sagittal spinal alignment.
| X-ray 1 Values | X-ray 2 Values | |
|---|---|---|
| C0–C1 Chamberlain’s to APL | 6.8° | 9.8° |
APL = Atlas Plane Line
Table 2a.
Global analysis of x-ray 1 (pre) and x-ray 2 (post) ARA C2–C7 and TzH C2–C7 for subject 7B (a 24-year-old female weighing 101 pounds). X-ray 1 and 2 values are compared to normal values as per the Harrison Spinal Model.
| Normal Values | X-ray 1 Values | Versus Normal | X-ray 2 Values | Versus Normal | |
|---|---|---|---|---|---|
| ARA C2–C7 | −42.0° | −24.5° | 41.7% | −31.8° | 24.3% |
| Translation C2–C7 | 0.0 mm | 17.8 mm | 17.8 mm | 20.5 mm | 20.5 mm |
ARA = Absolute Rotational Angle of Measurement
Table 3.
Statistics for patient characteristics calculated using means or proportions, as appropriate.
| Characteristic | Anterior Head Translation Group (n = 15) | Anterior Head Flexion Group (n = 14) |
|---|---|---|
|
| ||
| Sex | ||
| Male | 9 (60.0%) | 6 (40.0%) |
| Female | 5 (35.7%) | 9 (64.3%) |
|
| ||
| Race, n (%) | ||
| Caucasian | 13 (86.7%) | 11 (78.6%) |
| African American | 1 (6.7%) | 1 (7.1%) |
| Pacific Islander | 1 (6.7%) | 0 (0%) |
| Puerto Rican | 0 (0%) | 2 (14.3%) |
|
| ||
| Mean Age (years) | 28.27 (±5.01) | 27.5 (±2.62) |
|
| ||
| Mean ARA C2–C7 (°) | ||
| Pre | −18.06 (±13.85) | −12.66 (±12.32) |
| Post | −15.93 (±18.60) | −17.04 (±13.20) |
| Difference (Post – Pre) | 2.13 (±9.41) | −4.37 (±8.23) |
|
| ||
| Mean Peak force (lbs.) | 11.80 (±6.91) | 15.00 (±10.01) |
|
| ||
| Mean Chamberlain’s to APL Angle (°) | ||
| Pre | 3.48 (±6.77) | 4.41 (±4.21) |
| Post | 1.37 (±6.73) | 4.30 (±6.39) |
| Difference (Post-Pre) | −2.11 (±4.84) | −0.11 (±2.84) |
|
| ||
| Mean TzH C2–C7 (mm) | ||
| Pre | 16.90 (±11.41) | 13.88 (±9.39) |
| Post | 20.74 (±13.04) | 19.95 (±9.31) |
| Difference (Post- Pre) | 3.84 (±3.31) | 5.07 (±6.62) |
Table 4.
Table summarizing whether there was a significant difference between pre and post ARA C2–C7. Unadjusted paired t-tests were performed for the anterior head translation and anterior head flexion groups. The tests were conducted against a null hypothesis that the difference in pre and post ARA C2–C7 was 0 degrees. Tests of hypotheses were two-tailed and conducted at a significance level of 0.05. In addition to p-values, 95% CI were determined.
| Group | Mean ARA C2–C7 Difference (°) | 95% Confidence Interval (CI) | Test Statisticsa | p-value |
|---|---|---|---|---|
| Anterior Head Translation | 2.13 | (−3.08, 7.34) | 0.88 | 0.39 |
| Anterior Head Flexion | −4.37 | (−9.12, 0.38) | −1.99 | 0.07 |
Tested against null hypothesis that there is no difference between pre and post ARA C2–C7
Table 5.
Table summarizing simple linear regression models developed for each group to determine whether peak force, TzH C2–C7, gender, or Chamberlain’s to APL angle had an effect on any difference in ARA C2–C7. The tests were conducted against a null hypothesis that there is no linear association between any variable and ARA C2–C7 difference. Tests of hypotheses were conducted at a significance level of 0.05.
| Group | Variable | Test Statistica | p-value |
|---|---|---|---|
|
| |||
| Anterior Head Translation | Gender | 0.05 | 0.82 |
| Peak Force (lbs.) | 0.22 | 0.65 | |
| TzH C2–C7 Difference (mm) | 2.03 | 0.18 | |
| Chamberlain’s to APL Angle Difference (°) | 10.40 | 0.01 | |
|
| |||
| Anterior Head Flexion | Gender | 0.82 | 0.38 |
| Peak Force (lbs.) | 2.18 | 0.17 | |
| TzH C2–C7 Difference (mm) | 0.81 | 0.39 | |
| Chamberlain’s to APL Angle Difference (°) | 1.72 | 0.21 | |
Tested against null hypothesis that there is no linear association between the variable and ARA C2–C7 difference
Table 6.
Correlation between the difference in ARA C2–C7 and the difference in TzH C2–C7 and Chamberlain’s to APL angle difference.
| Group | Variable | Pearson Correlation Coefficient | p-value |
|---|---|---|---|
|
| |||
| Anterior Head Translation | TzH C2–C7 Difference | 0.37 | 0.18 |
| Chamberlain’s to APL Angle Difference | −0.67 | 0.01 | |
|
| |||
| Anterior Head Flexion | TzH C2–C7 Difference | −0.25 | 0.39 |
| Chamberlain’s to APL Angle Difference | 0.35 | 0.21 | |
Table 7.
Effects of different values of Chamberlain’s to APL angle difference on the mean ARA C2–C7 for the anterior head translation group.
| Chamberlain’s to APL Angle Difference (°) | Mean ARA C2–C7 Difference (°) | 95% CI |
|---|---|---|
| 2 | −3.19 | (−8.59, 2.22) |
| 1 | −1.89 | (−6.76, 2.98) |
| 0 | −0.60 | (−5.05, 3.86) |
| −1 | 0.70 | (−3.47, 4.87) |
| −2 | 1.88 | (−2.07, 6.05) |
RESULTS
Among the 15 participants in the anterior head translation group, 9 (60%) were male with a mean age of 28.27 years. 13 (86.7%) were Caucasian, and the mean difference in ARA C2–C7 was 2.13 degrees. The mean isometric anterior head translation peak force was 11.80 lbs, the mean difference in TzH C2–C7 was 3.84 millimeters (mm), and the mean difference in Chamberlain’s to APL angle was −2.11 degrees. Among the 14 participants in the anterior head flexion group, 5 (35.7%) were male with a mean age of 27.50 years. 11 (78.6%) were Caucasian, and the mean difference in ARA C2–C7 was −4.37 degrees. The mean isometric anterior head flexion peak force was 15.00 lbs, the mean difference in TzH C2–C7 was 5.07 mm, and the mean difference in Chamberlain’s to APL angle was −0.11 degrees (Table 3).
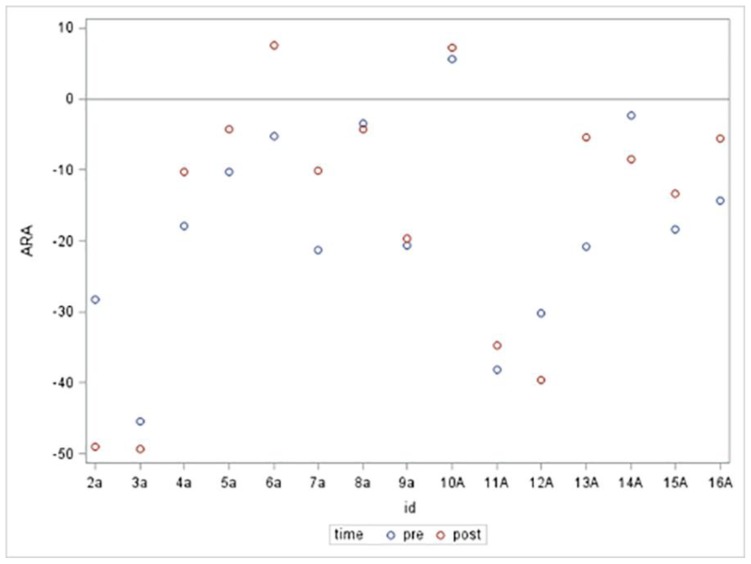
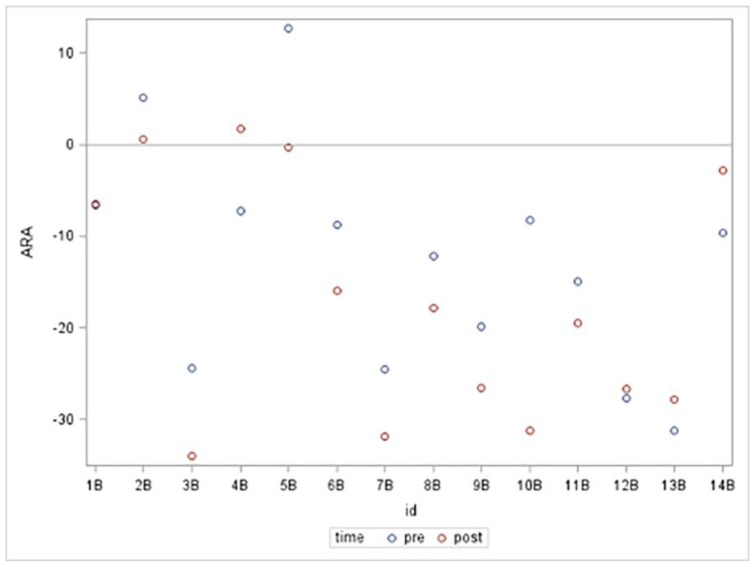
In the anterior head translation group, 4 of the 15 subjects had an increase in lordosis and 1 of the 15 subjects went from a lordosis to kyphosis (Figure 5). In the anterior head flexion group, 9 of the 14 subjects had an increase in lordosis and 1 of the 14 subjects went from lordosis to kyphosis (Figure 6).
Figure 5.
Absolute Rotational Angle from C2 to C7 (ARA C2–C7) Pre and Post Anterior Head Translation
Presented is a scatter plot graph of the ARA C2–C7 for the 15 subjects in the anterior head translation group. Subjects’ assigned identification (ID) numbers are represented on the x-axis and ARA C2–C7 (°) is represented on the y-axis. The blue plots represent the resting NLC ARA C2–C7 values prior to isometric anterior head translation. The red plots represent the NLC ARA C2–C7 values during a sustained maximum isometric anterior head translation.
Figure 6.
ARA C2–C7 Pre and Post Anterior Head Flexion
Presented is a scatter plot graph of the ARA C2–C7 for the 14 subjects in the anterior head flexion group. Subjects’ assigned ID numbers are represented on the x-axis and ARA C2–C7 (°) is represented on the y-axis. The blue plots represent the resting NLC ARA C2–C7 values prior to isometric anterior head flexion. The red plots represent the NLC ARA C2–C7 values during a sustained maximum isometric anterior head flexion.
The paired sample t-tests indicated that there was not a significant difference between pre and post mean ARA C2–C7 for the anterior head translation and anterior head flexion groups (Table 4). Analysis of Variance (ANOVA) suggested that gender and peak force were not associated with ARA C2–C7 difference in either of the two groups. However, the Chamberlain’s to APL angle difference was significantly associated with ARA C2–C7 difference in the anterior head translation group (Chamberlain’s to APL angle difference=10.40°, p=0.01) (Table 5).
A further analysis of the effect of TzH difference suggested that when the difference was positive (post TzH value was greater than the pre TzH value) there was a significant mean difference in ARA C2–C7. When the TzH C2–C7 difference was 20.0 mm, the estimated mean ARA C2–C7 difference was 6.24 degrees (95%CI: 1.43, 11.05), and when the TzH C2–C7 difference was 10.0 mm, the estimated mean ARA C2–C7 difference was 5.72 degrees (95% CI: 0.85, 10.60) (Table 6). Finally, Chamberlain’s to APL angle difference showed a significant correlation with ARA C2–C7 (r=−0.67, p=0.01). When the Chamberlain’s to APL angle difference became more negative (increased posterior head extension) the ARA C2–C7 difference became more positive (increased cervical lordosis) (Table 7).
DISCUSSION
This study is the first using objective measurements and statistical data to determine whether hypertonicity of anterior cervical muscles can have a structural impact on the cervical spine. These data do not show evidence that hypertonicity of anterior cervical muscles elicited from anterior head translation and anterior head flexion have a significant impact on cervical spinal alignment.
Proposed Mechanisms of Cervical Hypolordosis or Kyphosis
There are various proposed mechanisms of cervical hypolordosis or kyphosis that are supported by significant data. Thakar et al. found that patients with hypolordosis showed significant atrophy and dysfunction of deep neck flexors and extensors and concluded that spinal ligamentous laxity supersedes the influence of spinal musculature [31]. Cervical spinal ligament injury may contribute to cervical hypolordosis. Hyperflexion-hyperextension injuries, such as whiplash associated disorders (WAD), of the cervical spine result in such injuries. Tominaga et al. found that “for all whiplash-exposed ligaments, the average failure elongation exceeded the average physiological elongation and the whiplash-exposed ligaments had significantly lower failure force” [32]. Weaker, lax ligaments decrease cervical spine stability resulting in structural abnormalities. Cervical hypolordosis, kyphosis, or anterior head translation can create a cascade of weakened cervical ligaments and discs yielding further increased hypolordosis, kyphosis, or anterior head translation. Harrison et al. concluded that “spinal arthritis and disc disease (SADD) is commonly found at areas of altered stress and strain [and] translations of the cervical spine posture is an unhealthy position as it will accelerate the development or progression of SADD due to altered and increased spinal loads experienced by the spine tissues, up to 4.25 times greater compared to the normal, healthy lordosis of the cervical spine” [33]. In addition to the cervical spine, other spinal regions can affect cervical alignment. In a review of literature, Ames et al. concluded that “cervical sagittal alignment is related to thoracolumbar spinal alignment and T1 slope, regional disability, and general health” [34].
Strengths and Limitations:
The dynamometry provided valid data of force output from anterior head translation and anterior head flexion and the sEMG provided valid data of activation of the SCM and cervical scalene muscles responsible for anterior head translation and anterior head flexion.
Muscle spasm is the “involuntary sustained contraction of muscle that prohibits joint motion in the direction opposite the afflicted muscle’s action” [19] While this study examined the effects of voluntary muscle contraction, the physiological effect of muscular contraction or hypertonicity does not change with respect to a voluntary or involuntary mechanism of action.
In clinical scenarios, the head can move. People with muscle spasms of anterior cervical muscles that produce head flexion and anterior head translation will move accordingly. However, antalgic spinal posture is different than normal spinal posture. The purpose of this study was to determine whether muscle hypertonicity could structurally change spinal alignment. As such, muscle hypertonicity was isolated. Additionally, sagittal posture is not a valid assessment tool for spinal alignment. Clinically, patients may present with the same sagittal cervical posture, but may differ in hyperlordotic, hypolordotic, straight, buckling, and kyphotic sagittal cervical spinal alignment. As such, maintained sagittal posture between pre and post radiographs does not predict sagittal spinal alignment.
The method of having the subjects raise their arms to hold the dynamometer against their heads for the isometric contraction could pose an argument for a source of error contributing to unintentional movement in the subjects’ spinal postures between radiographs. A significant anterior or posterior head translation would affect cervical lordosis. However, any significant change in head flexion and translation was measured and accounted for. Statistical analyses determined that there was no significant difference in pre and post ARA C2–C7 in the anterior head translation and anterior head flexion groups.
Additionally, the sample size is modest. Future studies can improve upon this pilot by including larger groups of people and creating an apparatus to fix the body in space so that any isometric contraction is ensured to have a net zero effect on sagittal posture.
CONCLUSION
The results of this study are in direct conflict with over fifty years of radiographic reports, physiological texts, and articles stating that the loss or reversing of the cervical lordosis are caused by cervical muscle spasms. No other quantitative study has been performed to date to confirm or deny these claims. This study provides evidence that may be beneficial to healthcare practitioners who wish to better understand the etiology and management of cervical spine.
TEACHING POINT
Hypertonicity of anterior cervical muscles may not decrease cervical lordosis as shown by the findings of this pilot study and the lack of supporting evidence in the literature.
Table 2b.
Cervical morphology of x-ray 1 (pre) and x-ray 2 (post) in terms of Chamberlain’s line to APL values (°) for subject 7B (a 24-year-old female weighing 101 pounds). X-ray 1 and 2 values are compared to each other to determine whether any significant head extension (>14°) occurred between pre and post films that would produce a change in cervical sagittal spinal alignment.
| X-ray 1 Values | X-ray 2 Values | |
|---|---|---|
| C0–C1 Chamberlain’s to APL | 12.5° | 18.4° |
APL = Atlas Plane Line
ABBREVIATIONS
- °
degree
- ANOVA
analysis of variance
- APL
atlas plane line
- ARA C2–C7
absolute rotational angle from C2 to C7
- C1
atlas
- CI
confidence interval
- CR
central ray
- EMR
Electronic Medical Records
- FET
force evaluation and testing
- FFD
film-focal distance
- ID
identification
- kVp
peak kilovolt
- mA
milliamp
- mAs
milliamp second
- mm
millimeter
- n
number of subjects
- NLC
neutral lateral cervical
- p
p-value
- r
Pearson coefficient
- SADD
spinal arthritis and disc disease
- SCM
sternocleidomastoid
- sEMG
surface electromyography
- TzH C2–C7
anterior or posterior head translation from C2 to C7
- WAD
whiplash associated disorder
Spinal Alignment Shorthand
- +/− _ _ _
direction of movement per the Cartesian coordinate system
- T_ _
Translation along an axis per the Cartesian coordinate system
- R _ _
Rotation around an axis per the Cartesian coordinate system
- _x_
x-axis (in the body’s frontal plane) per the Cartesian coordinate system
- _y_
y-axis (in the body’s sagittal plane) per the Cartesian coordinate system
- _z_
z-axis (in the body’s transverse plane) per the Cartesian coordinate system
- _ _ P
Pelvis (in relation to the feet)
- _ _ T
Thoracic cage (in relation to the pelvis)
- _ _ H
Head (in relation to the thoracic cage)
- +TzH
Anterior Head Translation
- +RxH
Anterior Head Flexion
REFERENCES
- 1.Jones AMM, Stringer EA, Wong DA. Plated cervical fusions yield better outcomes. Trans Orthop Res Soc. 1998;22:524. [Google Scholar]
- 2.Kawakami M, Tamaki T, Yoshida M, et al. Axial symptoms and cervical alignments after cervical anterior spinal fusion for patients with cervical myelopathy. J Spinal Disord. 1999;2:50–6. [PubMed] [Google Scholar]
- 3.Matsunaga S, Sakou T, Sunahara N, et al. Biomechanical analysis of buckling alignment of the cervical spines. Predictive value for subaxial subluxation after occipitocervical fusion. Spine. 1997;22:765–71. doi: 10.1097/00007632-199704010-00011. [DOI] [PubMed] [Google Scholar]
- 4.Lowery G. Three-dimensional screw divergence and sagittal balance: a personal philosophy relative to cervical biomechanics. Spine: State of the Art Reviews. 1996;10:343–56. [Google Scholar]
- 5.Katsuura A, Hukuda S, Imanaka T, et al. Anterior cervical plate used in degenerative disease can maintain cervical Lordosis. J Spinal Disorders. 1996;9:470–6. [PubMed] [Google Scholar]
- 6.Kawakami M, Tamaki T, Yoshida M, et al. Axial symptoms and cervical alignments after cervical anterior spinal fusion for patients with cervical myelopathy. J Spinal Disorders. 1999;12:50–6. [PubMed] [Google Scholar]
- 7.Jones AMM, Stringer EA, Wong DA. Plated cervical fusions yield better outcomes. Orthopaedic Transactions. 1999;22(2):524. [Google Scholar]
- 8.Kai Y, Oyama M, Kurose S, et al. Traumatic thoracic outlet syndrome. Orthop Traumatol. 1998;47:1169–71. [Google Scholar]
- 9.Harrison DD, Troyanovich SJ, Harrison DE, Janik TJ, Murphy DJ. A normal sagittal spinal configuration: a desirable clinical outcome. J Manipulative Physiol Ther. 1996;19(6):398–405. [PubMed] [Google Scholar]
- 10.Gehweiler JA, Clark WN, Schaaf RE, et al. Cervical spine trauma: the common combines conditions. Radiology. 1979;130:77–86. doi: 10.1148/130.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Clark WN, Gehweiler JA, Laib R. Twelve significant signs of cervical spine trauma. Skeletal Radiol. 1979;3:201–5. [Google Scholar]
- 12.Kettner NW, Guebert GM. The radiology of cervical spine injury. J Manipulative and Physiol Ther. 1991;14:518–26. [PubMed] [Google Scholar]
- 13.Deltoff MN, Kogon PL. The portable skeletal x-ray library. St. Louis: Mosby; 1998. p. 247. [Google Scholar]
- 14.Pederson PL. Review of cervical trauma in relation to cervical hypolordosis. Eur J Chiropr. 1990;38:141–7. [Google Scholar]
- 15.Yochum TR, Rowe LJ. Essentials of skeletal radiology. I. Baltimore: Williams & Wilkins; 1987. p. 169. [Google Scholar]
- 16.Juhl JH, Miller SM, Roberts GW. Roentgenographic variations in the normal cervical spine. Radiology. 1962;78:591–7. [Google Scholar]
- 17.Rechtman AM, Boreadis Borden AG, Gershon-Cohen J. The lordotic curve of the cervical spine. Clin Orthop. 1961;20:208–16. [PubMed] [Google Scholar]
- 18.Helliwell PS, Evans PF, Wright V. The straight cervical spine: does it indicate muscle spasm? J Bone Joint Surg. 1994;76:103–10. [PubMed] [Google Scholar]
- 19.Liebenson C. Rehabilitation of the spine: a practitioner’s manual. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 20.Kent C. Surface electromyography in the assessment of changes in paraspinal muscle activity associated with vertebral subluxation: a review. JVSR. 1997;1(3):1–8. [Google Scholar]
- 21.Jackson BL, Harrison DD, Robertson GA, Barker WF. Chiropractic Biophysics Lateral Cervical Film Analysis. J Manipulative Physiol Ther. 1993;16(6):384–91. [PubMed] [Google Scholar]
- 22.Harrison DE, Harrison DD, Cailliet R, Troyanovich SJ, Janik TJ, Holland B. Cobb Method or Harrison Posterior Tangent Method: Which is Better for Lateral Cervical Analysis? Spine. 2000;25(16):2072–8. doi: 10.1097/00007632-200008150-00011. [DOI] [PubMed] [Google Scholar]
- 23.Harrison DE, Holland B, Harrison DD, Janik TJ. Further Reliability Analysis of the Harrison Radiographic Line Drawing Methods: Crossed ICCs for Lateral Posterior Tangents and AP Modified Risser-Ferguson. J Manipulative Physiol Ther. 2002;25:93–8. doi: 10.1067/mmt.2002.121411. [DOI] [PubMed] [Google Scholar]
- 24.Harrison DE, Harrison DD, Colloca CJ, Betz JW, Janik TJ, Holland B. Repeatability Over Time of Posture, X-ray Positioning, and X-ray Line Drawing: An Analysis of Six Control Groups. J Manipulative Physiol Ther. 2003;26(2):87–98. doi: 10.1067/mmt.2003.15. [DOI] [PubMed] [Google Scholar]
- 25.Harrison DD, Janik TJ, Troyanovich SJ, Holland B. Comparisons of Lordotic Cervical Spine Curvatures to a Theoretical Ideal Model of the Static Sagittal Cervical Spine. Spine. 1996;21(6):667–75. doi: 10.1097/00007632-199603150-00002. [DOI] [PubMed] [Google Scholar]
- 26.Harrison DD, Janik TJ, Troyanovich SJ, Harrison DE, Colloca CJ. Evaluations of the Assumptions Used to Derive an Ideal Normal Cervical Spine Model. J Manipulative Physiol Ther. 1997;20(4):246–56. [PubMed] [Google Scholar]
- 27.Harrison DD, Harrison DE, Janik TJ, Cailliet R, Haas JW, Ferrantelli J, Holland B. Modeling of the Sagittal Cervical Spine as a Method to Discriminate Hypo-Lordosis: Results of Elliptical and Circular Modeling in 72 Asymptomatic Subjects, 52 Acute Neck Pain Subjects, and 70 Chronic Neck Pain Subjects. Spine. 2004;29:2485–92. doi: 10.1097/01.brs.0000144449.90741.7c. [DOI] [PubMed] [Google Scholar]
- 28.McAviney J, Schulz D, Richard Bock R, Harrison DE, Holland B. Determining a clinical normal value for cervical lordosis. J Manipulative Physiol Ther. 2005;28:187–93. doi: 10.1016/j.jmpt.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Janik TJ, Harrison DE, Harrison DD, Payne MR, Coleman RR, Holland B. Reliability of lateral bending and axial rotation with validity of a New Method to determine Axial Rotations on AP Radiographs. J Manipulative Physiol Ther. 2001;24(7):445–8. [PubMed] [Google Scholar]
- 30.Harrison DE, Harrison D, Janik T, Holland B, Siskin L. Slight Head Extension: does it change the sagittal cervical curve? Eur Spine J. 2001;10:149–53. doi: 10.1007/s005860000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thakar S, Mohan D, Furtado SV, Sai Kiran NA, Dadlani R, Aryan S, Rao AS, Hegde AS. Paraspinal muscle morphometry in cervical spondylotic myelopathy and its implications in clinicoradiological outcomes following central corpectomy: clinical article. J Neurosurg Spine. 2014;21(2):223–30. doi: 10.3171/2014.4.SPINE13627. [DOI] [PubMed] [Google Scholar]
- 32.Tominaga Y, Ndu AB, Coe MP, Valenson AJ, Ivancic PC, Ito S, Rubin W, Panjabi MM. Neck ligament strength is decreased following whiplash trauma. BMC Musculoskelet Disord. 2006;7:103. doi: 10.1186/1471-2474-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison DE, Jones EW, Janik TJ, Harrison DD. Evaluation of axial and flexural stresses in the vertebral body cortex and trabecular bone in lordosis and two sagittal cervical translation configurations with an elliptical shell model. J Manipulative Physiol Ther. 2002;25(6):391–401. doi: 10.1067/mmt.2002.126128. [DOI] [PubMed] [Google Scholar]
- 34.Ames CP, Blondel B, Scheer JK, Schwab FJ, Le Huec JC, Massicotte EM, Patel AA, Traynelis VC, Kim HJ, Shaffrey CI, Smith JS, Lafage V. Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine. 2013;38(22):S149–60. doi: 10.1097/BRS.0b013e3182a7f449. [DOI] [PubMed] [Google Scholar]