Abstract
The use of live attenuated intravesicular Bacillus Calmette-Guerin (BCG) therapy is a generally accepted safe and effective method for the treatment of superficial transitional cell carcinoma (TCC) of the bladder. Although rare, < 5% of patient’s treated with intravesicular BCG therapy may develop potentially serious complications, including localized infections to the genitourinary tract, mycotic aneurysms and osteomyelitis. We present here a case of a 63-year-old male who developed left coronary and multiple peripheral M. Bovis mycotic aneurysms as a late complication of intravesicular BCG therapy for superficial bladder cancer. The patient initially presented with acute onset pain and swelling in the left knee > 2 years following initial therapy, and initial workup revealed a ruptured saccular aneurysm of the left popliteal artery as well as incidental bilateral common femoral artery aneurysms. Following endovascular treatment and additional workup, the patient was discovered to have additional aneurysms in the right popliteal artery and left anterior descending artery (LAD). Surgical pathology and bacterial cultures obtained from the excised femoral aneurysms and surgical groin wounds were positive for Mycobacterium Bovis, and the patient was initiated on a nine-month antimycobacterial course of isoniazid, rifampin and ethambutol. Including the present case, there has been a total of 32 reported cases of mycotic aneurysms as a complication from intravesicular BCG therapy, which we will review here. The majority of reported cases involve the abdominal aorta; however, this represents the first known reported case of a coronary aneurysm.
Keywords: Mycotic aneurysm, BCG, Bacillus Calmette-Guerin, Mycobacterium bovis, intravesicular
CASE REPORT
A 63-year-old male with a 45-pack year smoking history presented to his primary care provider with gross hematuria. Subsequent urological referral and cystoscopy revealed a left lateral bladder mass with suspicious cytology. CT urography was negative for upper tract involvement and the patient underwent transurethral resection of the bladder tumor (TURBT) with final pathology consistent with high grade T1 transitional cell carcinoma (TCC). The patient underwent six cycles of induction BCG therapy from November 2012 through January 2013 and ten cycles of maintenance therapy from March 2013 through August 2014, which he tolerated well during that period.
In March of 2015, the patient presented to the Emergency Department (ED) after the acute onset of left knee pain and swelling. A venous Doppler ultrasound (US) was performed in the ED, demonstrating a complex vascular mass arising from the popliteal artery consistent with a partially thrombosed popliteal aneurysm [Figure 1a, b]. In addition, a partially thrombosed left common femoral aneurysm was identified [Figure 1 c, d]. He received a CTA immediately afterwards, which demonstrated a 5.5 × 6.5 cm ruptured left popliteal aneurysm [Figure 2a] and incidental bilateral common femoral artery (CFA) aneurysms [Figure 2b, c]. Mild scattered calcified atherosclerotic plaque was noted in the inflow vessels, without significant stenosis. The patient was stabilized and subsequently underwent left popliteal artery covered stent placement by vascular surgery and interventional radiology the following day. He had an uneventful post-operative course and was discharged shortly afterwards with future plans for elective repair of the bilateral CFA aneurysms.
Figure 1.
A 63-year-old male with acute left knee pain and swelling due to a ruptured left popliteal artery mycotic aneurysm.
Findings: (a) - Sagittal gray-scale ultrasound (US) of the left popliteal fossa shows a large non-compressible acute hematoma (yellow asterisk). (b) - Transverse color Doppler US of the left medial knee shows a small amount of “ying-yang” flow within a partially thrombosed saccular aneurysm sac (white arrow). (c, d) - Transverse color Doppler US demonstrates an incidental partially thrombosed saccular aneurysm arising from the left common femoral vein (CFV; white asterisk).
Technique: GE Logiq E9 linear 8.4 Mhz transducer.
Figure 2.
A 63 year-old-male with an acute ruptured left popliteal aneurysm.
Findings: (a) - Axial CTA demonstrating a mixed density acute left popliteal hematoma with focal active arterial extravasation (blue arrow). (b, c) - Axial and 3D volume rendered CTA of the lower pelvis demonstrating a left (white arrow) and bilateral (yellow arrow) saccular aneurysms arising from the distal common femoral arteries.
Technique: Siemens SOMATOM Definition Flash, 128 channel, mAs 10,283, kVP 100, slice thickness 3 mm; Contrast material: 125 mL Omnipaque 350 mg/ml injected at a rate of 5 ml/s.
In May of 2015, the patient returned to the ED with acute onset pain and swelling behind the right knee. Doppler US demonstrated a ruptured right popliteal aneurysm in addition to enlarging bilateral CFA aneurysms [Figure 3]. He received endovascular repair of the ruptured popliteal aneurysm with a covered stent the following day, followed by open surgical repair of the CFA aneurysms with bilateral interposition grafts the following week [Figure 4, 5]. Pathology results from the excised femoral aneurysms demonstrated the presence of acid bacilli on AFB stains, consistent with mycobacterial infection. The post-operative course was prolonged and complicated by non-healing bilateral surgical groin wounds and progressive weight loss. On post-op day 12, the patient also developed fevers and chills. These developments led to an extensive but unremarkable workup for potential sources of infection and occult malignancy, including a negative CT of the chest, abdomen and pelvis and multiple negative blood cultures. One month post-op, the patient underwent bilateral groin wound washout with wound VAC placement. Wound cultures obtained at the time of surgery, were positive for Mycobacterium Bovis, consistent with the previous pathology findings. Given the diagnosis of M. Bovis induced mycotic aneurysms, the patient underwent CTA of the head, neck, chest, abdomen and pelvis to screen for additional aneurysms, which were reported as negative for additional aneurysms. Infectious Disease was consulted, who initiated a 9-month course of anti-mycobacterial treatment with Isoniazid, Rifampin and Ethambutol.
Figure 3.
A 63 year-old-male with acute onset right knee pain due to ruptured right popliteal artery mycotic aneurysm.
Findings: (a) - Sagittal grayscale (left) and color Doppler (right) US demonstrating an acute hematoma (asterisk) adjacent to a 5.7 × 2.7 cm irregular-shaped mycotic aneurysm arising from the popliteal artery (arrow). (b) - Sagittal spectral Doppler US demonstrating a “to-and-fro” flow pattern within the aneurysm sac, compatible with pseudoaneurysm (yellow calipers).
Technique: Protocol: GE Logiq E9 Curvilinear 4 MHz transducer.
Figure 4.
A 63 year-old-male undergoing covered stent placement of a right popliteal mycotic aneurysm.
Findings: (a) - Carbon dioxide digital subtraction angiogram (DSA) demonstrating a saccular aneurysm arising from the above-knee popliteal artery (yellow arrow) with active extravasation (curved black arrow). (b) - A Storq guidewire was used to cross the aneurysm in the mid SFA. (c) - Placement of a 8 mm × 10 cm balloon-expandable Viabahn covered stent. (d) - Post DSA angiogram demonstrating successful exclusion of the aneurysm sac following placement of the covered stent.
Technique: Siemens AXIOM Artis, Fluoroscopy time 8.6 minutes, KAP 605 mGym2, kVP 66, Contrast material: Carbon dioxide and Visipaque, hand-injected.
Figure 5.
A 63 year-old-male status post bilateral covered popliteal artery covered stent placement for ruptured mycotic aneurysms.
Findings: (a) - 3D-volume rendered and (b) oblique coronal maximum intensity projection CTA demonstrating patent bilateral covered popliteal stents with successful exclusion of the popliteal aneurysm sacs (arrows).
Technique: GE Lightspeed, 16 channel, mAs 1,066, kVP 120, slice thickness 2.5 mm; Contrast material: 125 mL Omnipaque 350 mg/ml injected at a rate of 5 ml/s.
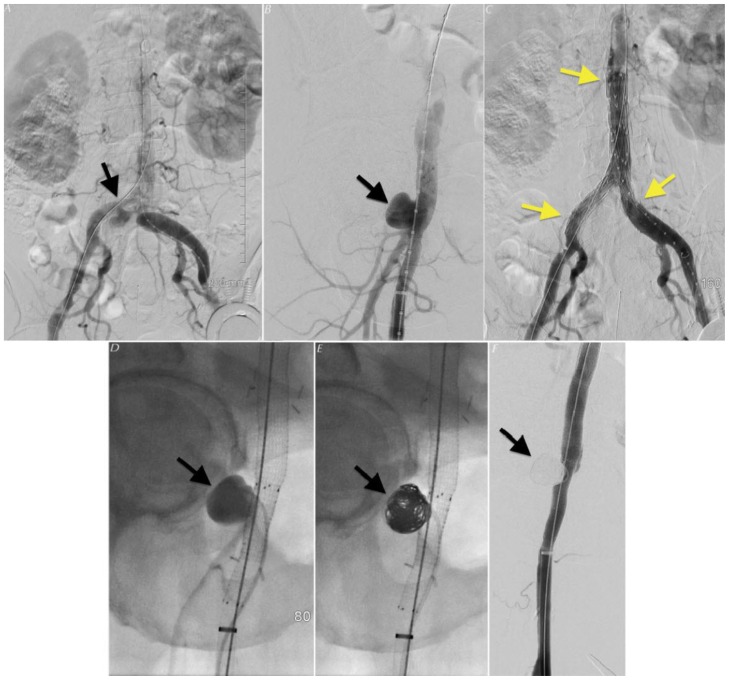
Two months following the initial diagnosis, the patient underwent surveillance CTA of the lower extremities to evaluate patency of the surgical interposition grafts. The results demonstrated patent surgical grafts; however, enlarging mycotic aneurysms were discovered in the proximal right common iliac artery and at the distal attachment site of the right surgical interposition graft [Figure 6]. The following week, the patient underwent endovascular repair with a bifurcated distal aortic stent graft and coil embolization of the right common iliac aneurysm [Figure 7]. There was no evidence of new or enlarging aneurysms on 2-month follow-up CTA [Figure 8].
Figure 6.
A 63-year-old-male status undergoing surveillance CTA status post bilateral interposition femoral vein grafts and bilateral popliteal covered stent placement.
Findings: (a, b) - Axial CTA; (c, d) - 3D-volume rendered CTA; (e) - coronal CTA through the right common femoral artery demonstrating incidental saccular pseudoaneurysms arising from the proximal (white arrow) and distal (yellow arrow) attachment sites of the surgical interposition grafts.
Technique: GE Lightspeed, 16 channel, mAs 56,703, kVP 120, slice thickness 2.5 mm; Contrast material: 125 mL Omnipaque 350 mg/ml injected at a rate of 5 ml/s.
Figure 7.
A 63-year-old-male undergoing placement of a bifurcated aortoiliac stent graft and coil embolization of a distal right CFA mycotic pseudoaneurysm.
Findings: (a, b, d) - Coronal oblique DSA demonstrating saccular mycotic aneurysms arising from the (a, arrow) proximal and (b and d, arrow) distal attachment sites of a common femoral surgical interposition graft. (c) - Coronal DSA following successful placement of an Endologix AFX bifurcated aortoiliac stent graft. (e) - Coronal DSA showing a deployment of coils into the distal aneurysm sac. (f) - Post-coil DSA demonstrating successful exclusion of the distal mycotic aneurysm sac.
Technique: Siemens AXIOM Artis, Fluoroscopy time 37.8 minutes, KAP 6,036 mGym2, kVP 66–73, Contrast material: 30 mL Omnipaque 300 mg/mL.
Figure 8.
63 year-old-male undergoing surveillance CTA following coil-embolization of a right CFA mycotic aneurysm.
Findings: (a) - Curved planar CTA; (b) - Oblique coronal CTA; (c) - 3D-volume rendered CTA demonstrating patent right common femoral surgical interposition grafts (arrows) with successful coil exclusion of a mycotic aneurysm at the distal graft attachment site (asterisk).
Technique: GE Lightspeed, 16 channel, mAs 28,497, kVP 120, slice thickness 2.5 mm; Contrast material: 125 mL Omnipaque 350 mg/ml injected at a rate of 5 ml/s.
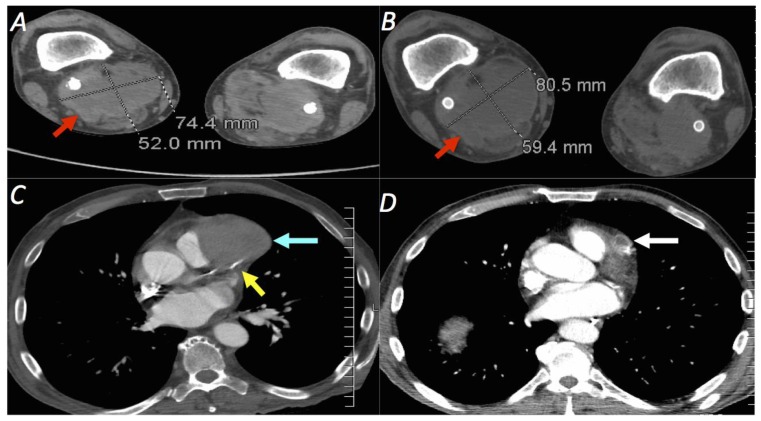
Over the next month, the patient had complaints of increased pain and swelling behind the right knee. CTA of the lower extremities was performed which showed an enlarging hematoma in the right popliteal fossa [Figure 9a, b]. In addition, there appeared to be an enlarging partially thrombosed aneurysm arising from the mid LAD, which, in retrospect, was present on screening CTA performed two months earlier [Figure 9 c, d]. The patient subsequently received a dedicated coronary CTA to further evaluate this new finding. Results demonstrated a large saccular aneurysm with sluggish internal flow arising from the mid LAD with resultant mass effect on the left ventricular outflow tract [Figure 10]. In lieu of the newly discovered LAD aneurysm, cardiothoracic surgery was consulted who deemed that no surgical intervention was warranted at that time due to the poor functional and nutritional status of the patient. Alternatively, a coronary catheter angiogram was performed by interventional cardiology to evaluate the possibility of stent placement; however, stent placement was precluded by occlusion of the mid LAD at the aneurysm site [Figure 11].
Figure 9.
A 63 year-old-male undergoing CTA of the lower extremities for increasing pain and swelling behind the right knee.
Findings: (a, b) - Axial CTA demonstrating an enlarging 8.1 × 5.9 cm right popliteal hematoma (red arrow) surrounding a patent popliteal stent graft. The hematoma previously measured 7.4 × 5.2 cm three weeks earlier. (c, d) - Axial CTA of the lower chest demonstrates a 5.7 × 4.1 cm thrombosed aneurysm (cyan arrow) which appears to arise from the LAD (yellow arrow). In retrospect, this aneurysm was present on screening CTA of the chest performed 49 days earlier and measured 1.6 × 1.6 cm at that time (white arrow).
Technique: GE Lightspeed, 16 channel, mAs 10,400, kVP 120, slice thickness 2.5 mm; Contrast material: 125 mL Omnipaque 350 mg/ml injected at a rate of 5 ml/s.
Figure 10.
A 63 year-old-male undergoing coronary CTA for evaluation of a left coronary artery mycotic aneurysm.
Findings: (a–d) - Axial pre-contrast (a), arterial-phase (b) and 3D-volume rendered (c, d) coronary CTA demonstrating a large saccular aneurysm with sluggish internal flow (blue arrow) arising from the mid left anterior descending (LAD) artery (yellow arrow). Significant mass effect and compression on the left ventricular outflow tract (LVOT) is noted (purple arrow).
Technique: Siemens SOMATOM Definition Flash, 128 channel, mAs 1,683, kVP 120, slice thickness 0.75 mm; Contrast material: 97 mL Omnipaque 350 mg/ml injected at a rate of 5 ml/s.
Figure 11.
A 63 year-old-male undergoing left main coronary catheter angiography.
Findings: (a–d) - Dynamic images obtained during injection of the left main coronary artery (white arrow) demonstrates a focal caliber change in the mid LAD (blue arrow). An amorphous collection of contrast is noted adjacent to the mid LAD, which progressively fills a large saccular aneurysm sac arising from the mid LAD (curved red arrow). There is no opacification of the LAD distal to the aneurysm site, indicating occlusion of the distal LAD. The left circumflex artery appears normal (curved yellow arrow).
Technique: Fluoroscopy time 6.2 minutes; DAP 49 mGy-m2; Contrast material: 70 mL Omnipaque 350 mg/ml.
Given the rapid interval growth of the LAD mycotic aneurysm and the patient’s poor functional status, his long-term prognosis was deemed poor. As such, the patient was placed on the palliative care service and converted to a DNR status. He continues to receive antiomycobacterial therapy in attempt to prevent interval mycotic aneurysm growth and formation. Currently the patient is undergoing subacute rehabilitation with the aims of improving his functional and nutritional status for the future possibility of potential surgical repair.
DISCUSSION
Etiology & Demographics
Bacillus Calmette-Guerin (BCG) is a live attenuated strain of Mycobacterium bovis that was initially developed as a vaccination against Mycobacterium tuberculosis infection. In 1976, BCG was introduced as a primary treatment for superficial transitional cell carcinoma (TCC) of the urinary bladder with a reported cure rate of 70% [1]. Intravesicular BCG therapy is generally considered safe with the most common reported side effects of low-grade fever, dysuria, urinary frequency and malaise with a typical onset within 2–4 hours following therapy and resolving within 48 hours [2, 3, 24, 29]. In < 5% of cases, more serious localized or disseminated infections due to BCG may occur. The largest reported series followed 2,602 patients who received BCG therapy. In this group, granulomatous prostatitis occurred in 0.9% of patients, hepatitis occurred in 0.7% of patients and pneumonitis in 0.7% of patients [3]. The onset of symptoms following initial treatment is variable and some authors have divided complications into early (< 6 months) and late complications (> 6 months) [4, 5]. A retrospective review of 41 patients who developed treatment complications demonstrated that slightly more than half (25/41) of patients were considered early-onset and tended to manifest with non-specific systemic symptoms (fever, chills, malaise and weight loss) due to an inflammatory response. On the contrary, the remaining 16/42 patients who developed late-onset disease tended to develop more localized infections due to BCG reactivation, with the most common site involving the genitourinary tract followed by the vascular system, skeleton and retroperitoneal soft tissues [4, 24].
The incidence of BCG-induced mycotic aneurysms following intravesicular infusion is exceedingly rare. Including the current case, there have only been 32 reported cases, with all but one occurring in middle-aged or elderly men [Table 3]. The majority of reported cases (26/32) involve the thoracic or abdominal aorta [6–33, Table 1]. Approximately one-fourth of reported cases involve the peripheral vascular system of the lower extremities (9/32) and there have been three reported cases involving the carotid arteries [Table 1]. To our knowledge, the current patient marks the first reported case of a Mycobacterium bovis mycotic aneurysm of a coronary artery following intravesicular BCG therapy for bladder cancer.
Table 3.
Summary of BCG-induced Mycobacterium bovis mycotic aneurysms
| Etiology | Localized or disseminated infection by live BCG. Exact mechanism for mycotic aneurysm formation unknown. |
| Incidence | Very rare (<< 1%); 32 total reported cases |
| Gender ratio | M >> F (31 M, 1 F in reported literature) |
| Age predilection | Middle-aged/elderly; Mean age 71 in reported literature |
| Location | Aorta (infrarenal > suprarenal) > iliac, femoral > popliteal > carotid > coronary |
| Risk factors | Urogenital trauma (i.e. difficult bladder catheterizations, cystitis, recent biopsy or surgery), immunocompromised |
| Clinical findings | Fever of unknown origin, unintentional weight loss, abdominal/back pain, abdominal mass, ruptured aneurysm |
| Treatment | Long course (6–12 months) anti-mycobacterial antibiotics; Surgical or endovascular repair of aneurysms |
| Prognosis | Variable (highly dependent on location of aneurysm, clinical status of patient, co-morbidities) |
| Imaging findings | Rapidly enlarging saccular aneurysms in medium-large sized arteries, possible rupture |
Table 1.
Literature review. Reported cases of BCG-induced M. bovis aneurysms
| First author | Age | Gender | Location of aneurysm(s) | Antibiotics | Intervention | Outcome |
|---|---|---|---|---|---|---|
| Woods6 | 62 | F | AAA | + | Surgical graft | Healthy |
| Bornet7 | 74 | M | Bilateral femoral | + | Surgical graft | Healthy |
| Deresiewicz8 | 67 | M | AAA, iliac | − | Surgical graft | Died (disseminated infection) |
| Izes9 | 69 | M | Aortic arch | − | None | Died (palliative care, untreated) |
| Wolf10 | 80 | M | AAA | − | Surgical graft | Alive, developed aortoenteric fistula |
| Hellinger11 | 71 | M | AAA | + | Surgical graft | Healthy |
| Rozenblit12 | 76 | M | AAA | + | Stent graft | Died (myocardial infarction) |
| Damm13 | 71 | M | AAA | + | Surgical graft | Healthy |
| LaBerge14 | 75 | M | AAA | NS | Surgical graft | NS |
| Seelig15 | 58 | M | AAA | + | Surgical graft | Healthy |
| Seelig15 | 71 | M | AAA, popliteal | + | Surgical graft | Healthy |
| Farber16 | 74 | M | Femoral | + | Prosthesis removal | Healthy |
| Geldmacher17 | 68 | M | Carotid | + | Surgical graft | NS |
| Kamphuis18 | 65 | M | AAA | − | Surgical graft | Died (post-operative shock) |
| Wada19 | 75 | M | AAA, femoral | − | Surgical graft | Healthy |
| Witjes20 | 67 | M | Popliteal | + | Surgical graft | Healthy |
| Dahl21 | 69 | M | AAA | + | Surgical graft | Healthy |
| Harding22 | 80 | M | AAA | + | Surgical graft | Healthy |
| Safdar2 | 79 | M | AAA | + | Surgical graft | Healthy |
| Costiniuk23 | 75 | M | AAA, femoral | + | Surgical graft | Healthy |
| Coscas5 | 79 | M | Thoracic aorta, Iliac, PT, carotids | + | None | Died (cancer progression) |
| Maundrell24 | 75 | M | AAA (juxtarenal) | + | Surgical (Ax-bifem bypass) | Died (aneurysm rupture) |
| Psoinos25 | 69 | M | AAA | + | Surgical graft | Healthy |
| Roylance26 | 77 | M | AAA (aortoenteric fistula) | − | Surgical graft | Died (post-operative AKI, respiratory failure) |
| Samadian27 | 94 | M | AAA (osteodiscitis) | + | NS | NS |
| Santbergen28 | 58 | M | AAA (infected EVAR stent graft) | + | Surgical graft removal | Healthy |
| Mizoguchi29 | 81 | M | AAA (infected EVAR stent graft) | + | Surgical graft removal, surgical bypass | NS |
| Kuy30 | 73 | M | Carotid | NS | Surgical graft | NS |
| Davis31 | 64 | M | Thoracoabdominal aorta (multi) | + | Surgical graft | Healthy |
| Seastedt32 | 70 | M | Thoracic aorta | + | Surgical graft | NS |
| Floros33 | 57 | M | AAA | + | Endovascular stent graft | NS (patient left AMA) |
| Present case | 63 | M | Popliteal (bl), CFA, LAD | + | Stent grafts (popliteal,CFA) | ABX, Palliative care |
Total # cases: 32
AAA = Abdominal aortic aneurysm; NS = not specified; EVAR = Endovascular aneurysm repair; PT = posterior tibial; CFA = common femoral artery; LAD = left anterior descending
The exact mechanism for mycotic aneurysm formation is unknown. Potential proposed mechanisms for BCG infection include direct intimal colonization from hematogenous spread, metastatic implantation through the vasa-vasorum or local vascular extension from an adjacent infectious site [5, 22]. Accordingly, the most important known risk factor for BCG infection is disruption of the urogenital mucosa at the time of bladder instillation, including traumatic indwelling catheter insertion, recent biopsy, surgery or active cystitis [2, 22]. Some authors have proposed the use of prophylactic isoniazid at the time of BCG instillation, however this has not been shown to be effective [34]. Most authors now advise delaying BCG therapy for several weeks following cystitis, bladder resection, biopsy or difficult catheterizations [22].
Clinical & Imaging Findings
The diagnosis of M. bovis mycotic aneurysms is difficult and remains elusive owing to the nonspecific clinical findings and relatively low incidence of occurrence. Diagnosis is often delayed, with the mean time of diagnosis occurring 23 months (range 4–69 months) following BCG therapy [5]. Patients usually present with fever of unknown origin, weight loss, a palpable abdominal mass and rarely signs/symptoms of aneurysm rupture. The gold standard for definitive diagnosis is bacterial cultures; however, this requires a minimum incubation period of 6–8 weeks and acid-fast stains require a minimum of 10,000 organisms per gram of tissue to yield positivity [5, 26].
The imaging findings are relatively straightforward but also nonspecific, demonstrating typical findings for mycotic or inflammatory aneurysms such as saccular aneurysms of medium or large-caliber arteries and associated perivascular inflammation [Tables 1, 2]. Catheter angiography remains the gold standard for imaging diagnosis of mycotic aneurysms, but its use is variable based on local expertise and limited by invasiveness. Multidetector CT angiography is the current imaging modality of choice for the evaluation of suspected mycotic aneurysms, with the advantages of rapid image acquisition, high sensitivity, widespread availability, high spatial resolution and multi-planar capabilities [35]. Mycotic aneurysms commonly appear as saccular dilatations of arteries not commonly involved by atherosclerosis (i.e. peripheral arteries), although the infrarenal abdominal aorta is still the most common reported site of mycotic aneurysms. Associated findings of perivascular inflammatory change and relative lack of atheromatous wall calcifications increases the specificity for mycotic aneurysm [Table 2]. MR angiography demonstrates similar findings as CTA but is limited by long acquisition times, availability, motion susceptibility and relatively lower spatial resolution [35]. As in our patient, ultrasound is mainly limited to mycotic aneurysms of the peripheral arteries and is as not reliable at diagnosing visceral or abdominal aortic aneurysms
Table 2.
Differential table for Mycobacterium bovis mycotic aneurysms
| Mycobacterium bovis mycotic aneurysm | Inflammatory aneurysm | Atherosclerotic aneurysm | Contained aneurysm rupture | Aortoenteric fistula | |
|---|---|---|---|---|---|
| Clinical history |
|
|
|
|
|
| X-ray |
|
|
|
|
|
| US |
|
|
|
|
|
| CTA |
|
|
|
|
|
| MRI |
|
|
|
|
|
| Angiography |
|
|
|
|
|
Treatment & Prognosis
The treatment of severe systemic BCG reactions includes a combination of medical and surgical/interventional therapy. The International Bladder Cancer Group recommends discontinuation of BCG therapy, initiation of high-dose fluoroquinolones, corticosteroids and daily antimycobacterial therapy with isoniazid, rifampin and ethambutol for six months duration [24]. It is well established that this particular strain of Mycobacterium is resistant to pyrazinamide [36]. In regards to treatment of the aneurysm itself, the mainstay of therapy in the reported literature has been surgical stent-grafting, or less commonly surgical bypass procedures. Of the 32 reported cases 68% (22/32) underwent a surgical graft procedure [Table 1]. As endovascular aneurysm repairs are becoming increasingly more popular for the treatment of non-mycotic aneurysms due to decreased peri-operative morbidity and mortality, some have suggested that endovascular repair should be the exclusive means for treating mycotic aneurysms [37]. The data on endovascular repair of mycotic M. bovis aneurysms, however, is scarce. To our knowledge, there have only been two reported cases of primary repair by endovascular stent-graft placement [12, 33]. One patient was treated with EVAR for a mycotic AAA and died due to a MI as a peri-operative complication [12]. The other patient was also treated with an EVAR but lost to follow-up (left the hospital against medical advice, 33]. With regards to treatment of peripheral M. bovis mycotic aneurysms, all of the previous reported cases have undergone surgical treatment as the primary means of repair with generally good results [7, 8, 15, 16, 17, 19, 20, 23, 30]. To our knowledge, the current patient marks the first reported case of primary endovascular repair with covered stents for peripheral BCG aneurysms. Furthermore, this also marks the first known reported case of a BCG-induced coronary aneurysm. As such, no established guidelines for treatment exist and the prognosis remains unknown.
Differential Diagnosis
The main differential diagnosis for mycotic aneurysms on imaging is relatively limited and includes atherosclerotic aneurysms, inflammatory aneurysms, contained aneurysm rupture and aortoenteric fistulas [Table 2]. Once mycotic aneurysms are suspected, it is very important to correlate findings with a detailed history and physical. Although rare, any patient with a recent or prior history of BCG therapy for bladder cancer should yield a lower threshold for the consideration M. bovis mycotic aneurysm in these patients.
Atherosclerotic aneurysm
This is the most common etiology for aneurysm and typically occurs in males older than 50 years of age with atherosclerotic risk factors (i.e. hypertension, smoking, diabetes). The most common location is the infrarenal aorta and manifests on imaging as fusiform dilatation with calcified and fibrofatty wall plaque. Furthermore, if an aneurysm is encountered within a coronary artery, it is most likely to be atherosclerotic in etiology.
Inflammatory aneurysm
Inflammatory aneurysms typically occur in patients with similar risk factors to atherosclerotic aneurysms, but are generally found about a decade earlier and present with systemic inflammatory symptoms (i.e. fever, weight loss, elevated ESR). Similar to atherosclerotic aneurysms, inflammatory aneurysms present with fusiform dilatation of the infrarenal aorta; however, imaging will also demonstrate signs of inflammation (i.e. thickening aortic wall, peri-vascular stranding and/or fluid).
Contained aneurysm rupture
Patients with aneurysms of any etiology may occasionally present with a contained rupture. Clinically, patients will often present with acute onset abdominal or back pain with or without hemodynamic instability. The most reliable sign on imaging is the presence of focal wall discontinuity within a calcified aneurysm wall with associated peri-aneurysmal fluid. Conventional catheter angiography might demonstrate active arterial extravasation if the rupture is ongoing.
Aortoenteric fistula
Defined as an abnormal fistulous connection between small bowel and the aorta, this typically occurs in the setting of prior retroperitoneal surgery, most commonly surgical AAA repair. Patients may present acutely with a brisk lower gastrointestinal bleed. Imaging will demonstrate loss of the normal fat plane between small bowel and abdominal aorta, and if present, gas within the aorta is highly suggestive of aortoenteric fistula.
TEACHING POINT
Bacillus Calmette-Guerin (BCG) is a generally safe and accepted method for the treatment of superficial transitional cell carcinoma (TCC) of the bladder. In rare instances, BCG-activation may result in the serious complication of mycotic aneurysm formation, which typically manifests months-to-years following therapy as mycotic AAA, or less commonly mycotic peripheral aneurysms. Therapy consists of cessation of ongoing BCG therapy, antimycobacterial therapy and surgical/endovascular aneurysm repair.
ABBREVIATIONS
- AAA
Abdominal aortic aneurysm
- BCG
Bacillus Calmette-Guerin
- CFA
Common femoral artery
- CTA
Computed tomographic angiography
- DNR
Do not resuscitate
- ED
Emergency department
- EVAR
Endovascular aneurysm repair
- LAD
Left anterior descending
- TCC
Transitional cell carcinoma
- TURBT
Transuretheral resection of bladder tumor
- US
Ultrasound
REFERENCES
- 1.Schellhammer PF, Ladaga LE, Fillion MB. Bacillus Calmette-Guerin for superficial transitional cell carcinoma of the bladder. J Urol. 1986;135:261–4. doi: 10.1016/s0022-5347(17)45603-5. [DOI] [PubMed] [Google Scholar]
- 2.Safdar N, Abad CL, Kaul DR, Jarrard D, Saint S. An unintended consequence. N Engl J Med. 2008;358:1496–501. doi: 10.1056/NEJMcps0706711. [DOI] [PubMed] [Google Scholar]
- 3.Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW, Soloway MS, Steg A, Debruyne FM. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez OY, Musher DM, Brar I, Furgeson S, Boktour MR, Septimus EJ, Hamill RJ, Graviss EA. Spectrum of bacilli Calmette-Guerin (BCG) infection after intravesical BCG immunotherapy. Clin Infect Dis. 2003;36:140–8. doi: 10.1086/344908. [DOI] [PubMed] [Google Scholar]
- 5.Coscas R, Arlet JB, Belhomme D, Fabiani JN, Pouchot J. Multiple mycotic aneurysms due to Mycobacterium bovis after intravesical bacillus Calmette-Guerin therapy. J Vasc Surg. 2009;50:1185–90. doi: 10.1016/j.jvs.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Woods JM, 4th, Schellack J, Stewart MT, Murray DR, Schwartzman SW. Mycotic abdominal aortic aneurysm induced by immunotherapy with bacille Calmette-Guerin vaccine for malignancy. J Vasc Surg. 1988;7:808–10. doi: 10.1067/mva.1988.avs0070808. [DOI] [PubMed] [Google Scholar]
- 7.Bornet P, Pujade B, Lacaine F, Bazelly B, Paquet JC, Roland J, Huguier M. Tuberculous aneurysm of the femoral artery: a complication of bacilli Calmette-Guerin vaccine immunotherapy – a case report. J Vasc Surg. 1989;10:688–92. [PubMed] [Google Scholar]
- 8.Deresiewicz RL, Stone RM, Aster JC. Fatal disseminated mycobacterial infection following intravesical bacillus Calmette-Guerin. J Urol. 1990;144:1331–3. doi: 10.1016/s0022-5347(17)39732-x. [DOI] [PubMed] [Google Scholar]
- 9.Izes JK, Bihrle W, 3rd, Thomas CB. Corticosteroid-associated fatal mycobacterial sepsis occurring 3 years after instillation of intravesical bacillus Calmette-Guerin. J Urol. 1993;150:1498–1500. doi: 10.1016/s0022-5347(17)35824-x. [DOI] [PubMed] [Google Scholar]
- 10.Wolf YG, Wolf DG, Higginbottom PA, Dilley RB. Infection of a ruptured aortic aneurysm and an aortic graft with bacilli Calmette-Guerin after intravesical administration for bladder cancer. J Vasc Surg. 1995;22:80–4. doi: 10.1016/s0741-5214(95)70092-7. [DOI] [PubMed] [Google Scholar]
- 11.Hellinger WC, Oldenburg A, Alvarez S. Vascular and other serious infections with Mycobacterium bovis after bacillus of Calmette-Guerin therapy for bladder cancer. South Med J. 1995;88:1212–6. doi: 10.1097/00007611-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rozenbilt A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rozenbilt G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical Calmette-Guerin therapy. AJR. 1996;167:711–3. doi: 10.2214/ajr.167.3.8751686. [DOI] [PubMed] [Google Scholar]
- 13.Damm O, Briheim G, Hagstrom T, Jonsson B, Skau T. Ruptured mycotic aneurysm of the abdominal aorta: a serious complication of intravesical instillation bacillus Calmette-Guerin therapy. J Urol. 1998;159:984. doi: 10.1016/s0022-5347(01)63796-0. [DOI] [PubMed] [Google Scholar]
- 14.LaBerge JM, Kerlan RK, Jr, Reilly LM, Chuter TA. Diagnosis please. Case 9: mycotic pseudoaneurysm of the abdominal aorta in association with mycobacterial psoas abscess – a complication of BCG therapy. Radiology. 1999;211:81–5. doi: 10.1148/radiology.211.1.r99ap4081. [DOI] [PubMed] [Google Scholar]
- 15.Seelig MH, Oldenburg WA, Klinger PJ, Blute ML, Pairolero PC. Mycotic vascular infections of large arteries with Mycobacterium bovis after intravesical bacillus Calmette-Guerin therapy: a case report. J Vasc Surg. 1999;29:377–81. doi: 10.1016/s0741-5214(99)70391-5. [DOI] [PubMed] [Google Scholar]
- 16.Farber A, Grigoryants V, Palac DM, Chapman T, Cronenwett JL, Powell RJ. Primary aortoduodenal fistula in a patient with a history of intravesical therapy for bladder cancer with bacillus Calmette-Guerin: a review of primary aortoduodenal fistula without abdominal aortic aneurysm. J Vasc Surg. 2001;33:868–73. doi: 10.1067/mva.2001.112327. [DOI] [PubMed] [Google Scholar]
- 17.Geldmacher H, Taube C, Markert U, Kirsten DK. Nearly fatal complications of cervical lymphadenitis following BCG immunotherapy for superficial bladder cancer. Respiration. 2001;68:420–1. doi: 10.1159/000050539. [DOI] [PubMed] [Google Scholar]
- 18.Kamphuis JT, Buiting AG, Misere JF, van Berge Henegouwen DP, van Soolingen D, Rensma PL. BCG immunotherapy: be cautious of granulomas. Disseminated BCG infection and mycotic aneurysm as late complications of intravesical BCG instillations. Neth J Med. 2001;58:71–5. doi: 10.1016/s0300-2977(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 19.Wada S, Watanabe Y, Shiono N, Masuhara H, Hamada S, Ozawa T, Fujii T, Yokomuro H, Kawasaki M, Yoshihara K, Koyama N. Tuberculous abdominal aortic pseudoaneurysm penetrating the left psoas muscle after BCG therapy for bladder cancer. Cardiovasc Surg. 2003;11:231–5. doi: 10.1016/s0967-2109(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 20.Witjes JA, Vriesema JL, Brinkman K, Bootsma G, Barentsz JO. Mycotic aneurysm of the popliteal artery as a complication of intravesical BCG therapy for superficial bladder cancer. Case report and literature review. Urol Int. 2003;71:430–2. doi: 10.1159/000074100. [DOI] [PubMed] [Google Scholar]
- 21.Dahl T, Lange C, Odegard A, Bergh K, Osen SS, Myhre HO. Ruptured abdominal aortic aneurysm secondary to tuberculous spondylitis. Int Angiol. 2005;24:98–101. [PubMed] [Google Scholar]
- 22.Harding GE, Lawlor DK. Ruptured mycotic abdominal aortic aneurysm secondary to Mycobacterium bovis after intravesical treatment with bacillus Calmette-Guerin. J Vasc Surg. 2007;46:131–4. doi: 10.1016/j.jvs.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 23.Costiniuk CT, Sharapov AA, Rose GW, Veinot JP, Desajardins M, Brandys TM, Suh KN. Mycobacterium bovis abdominal aortic and femoral artery aneurysms following intravesical bacillus Calmette-Guerin therapy for bladder cancer. Cardiovasc Pathol. 2010;19:e29–32. doi: 10.1016/j.carpath.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Maundrell J, Fletcher S, Roberts P, Stein A, Lambie M. Mycotic aneurysm of the aorta as a complication of Bacillus Calmette-Guerin instillation. J R Coll Physicians Edinb. 2011;41:114–6. doi: 10.4997/JRCPE.2011.203. [DOI] [PubMed] [Google Scholar]
- 25.Psoinos CM, Simons JP, Baril DT, Robinson WP, Schanzer A. A Mycobacterium bovis mycotic abdominal aortic aneurysm resulting from bladder cancer treatment, resection and reconstruction with a cryopreserved aortic graft. Vasc Endovascular Surg. 2013;47:61–4. doi: 10.1177/1538574412463973. [DOI] [PubMed] [Google Scholar]
- 26.Roylance A, Mosley J, Jameel M, Sylvan A, Walker V. Aorto-enteric fistula development secondary to mycotic abdominal aortic aneurysm following intravesical bacillus Calmette-Guerin (BCG) treatment for transitional cell carcinoma of the bladder. Int J Surg Case Rep. 2013;4:88–90. doi: 10.1016/j.ijscr.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samadian S, Phillips FM, Deeab D. Mycobacterium bovis vertebral osteomyelitis and discitis with adjacent mycotic abdominal aortic aneurysm caused by intravesical BCG therapy: a case report in an elderly gentleman. Age Ageing. 2013;42:129–31. doi: 10.1093/ageing/afs164. [DOI] [PubMed] [Google Scholar]
- 28.Santbergen B, Vriens PH, de Lange WC, Van Kasteren ME. Combined infection of vertebroplasty and aortic graft after intravesical BCG treatment. BMJ Case Rep. 2013 doi: 10.1136/bcr-2012-008161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizoguchi H, Ilda O, Dohi T, Tomoda K, Kimura H, Inoue K, Iwata T, Tei K, Miura T. Abdominal aortic aneurysmal and endovascular device infection with iliopsoas abscess caused by Mycobacterium bovis as a complication of intravesical bacillus Calmette-Guerin therapy. Ann Vasc Surg. 2013;27:e1–5. doi: 10.1016/j.avsg.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Kuy S, Dua A, Desai SS, Baraniewski H, Lee CJ. Ruptured mycobacterial aneurysm of the carotid artery. Perspect Vasc Surg Endovasc Ther. 2013;25:53–6. doi: 10.1177/1531003513512870. [DOI] [PubMed] [Google Scholar]
- 31.Davis FM, Miller DJ, Newton D, Arya S, Escobar GA. Successful treatment of a mycotic multifocal thoracoabdominal aortic aneurysm as a late sequelae of intravesical bacillus Calmette-Guerin therapy: case report and literature review. Ann Vasc Surg. 2015;29:e9–13. doi: 10.1016/j.avsg.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Seastedt KP, Ahmad U, Lau C, Ruggeri-Weigel P, Tsang HC, Hartman BJ, Girardi LN. Mycotic thoracic aortic aneurysm after intravesical bacillus Calmette-Guerin treatment. Ann Thorac Surg. 2015;99:2210–2. doi: 10.1016/j.athoracsur.2014.07.083. [DOI] [PubMed] [Google Scholar]
- 33.Floros N, Meletiadis K, Kusenack U, Zirngibl H, Kamper L, Haage P, Dreger NM. Ruptured mycotic aortic aneurysm after bacilli Calmette-Guerin therapy. Ann Vasc Surg. 2015;15:488–4. doi: 10.1016/j.avsg.2015.03.060. [DOI] [PubMed] [Google Scholar]
- 34.Vegt PD, van der Meijden AP, Sylvester R, Brausi M, Holtl W, de Balincourt C. Does isoniazid reduce side effects of intravesical bacillus Calmette-Guerin therapy in superficial bladder cancer? Interim results of European Organization for Research and Treatment of Cancer Protocol 30911. J Urol. 1997;157:1246–9. [PubMed] [Google Scholar]
- 35.Lee W, Mossop PJ, Little AF, Fitt GJ, Vrazas JI, Hoang JK, Hennessy OF. Infected (mycotic) aneurysms: Spectrum of imaging appearance and management. Radiographics. 2008;28:1853–68. doi: 10.1148/rg.287085054. [DOI] [PubMed] [Google Scholar]
- 36.Van der Meijden AP. Practical approaches to the prevention and treatment of adverse reactions to BCG. Eur Urol. 1995;27(Suppl 1):23–8. doi: 10.1159/000475205. [DOI] [PubMed] [Google Scholar]
- 37.Sorelius K, Mani K, Bjorck M, Nyman R, Wanhainen A. Endovascular repair of mycotic aortic aneurysms. J Vasc Surg. 2009;50:269–74. doi: 10.1016/j.jvs.2009.01.001. [DOI] [PubMed] [Google Scholar]