Abstract
Most cases of pancreatoblastoma, a rare malignant epithelial tumor of the pancreas, are seen in the pediatric population. The rarity of pancreatoblastoma, the similar radiologic findings to those seen in other pancreatic lesions, and its histopathologic heterogeneity, make its preoperative diagnosis in adults a real challenge. We report ultrasound, computed tomography and magnetic resonance imaging correlative findings of a histologically proven pancreatoblastoma in a 37-year-old woman. Pancreatoblastoma should be considered in the differential diagnosis of a pancreatic mass presenting uncommon imaging features.
Keywords: Pancreatoblastoma, pancreaticoblastoma, infantile-type carcinoma of the pancreas, infantile pancreatic carcinoma, pancreas, US, CT, MRI
CASE REPORT
A 37-year-old female who had a dystocic labour with severe blood loss requiring transfusion, presented herself to emergency department 2 weeks later complaining of fatigue and prostration. Physical examination revealed a tender epigastric lump. Laboratory results were unremarkable except for severe anemia, with a hemoglobin level of 5,4 g/dL (normal range − 12,1 to 15,6 g/dL).
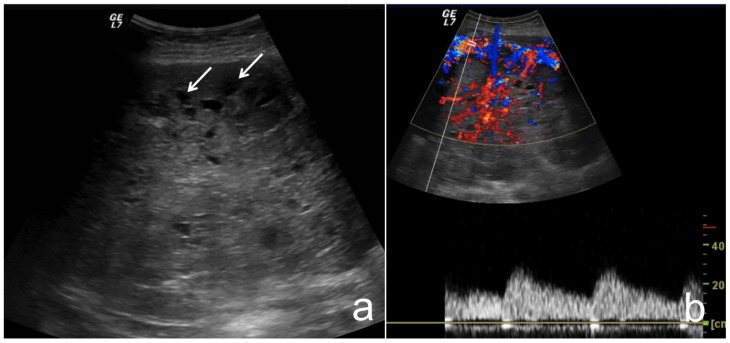
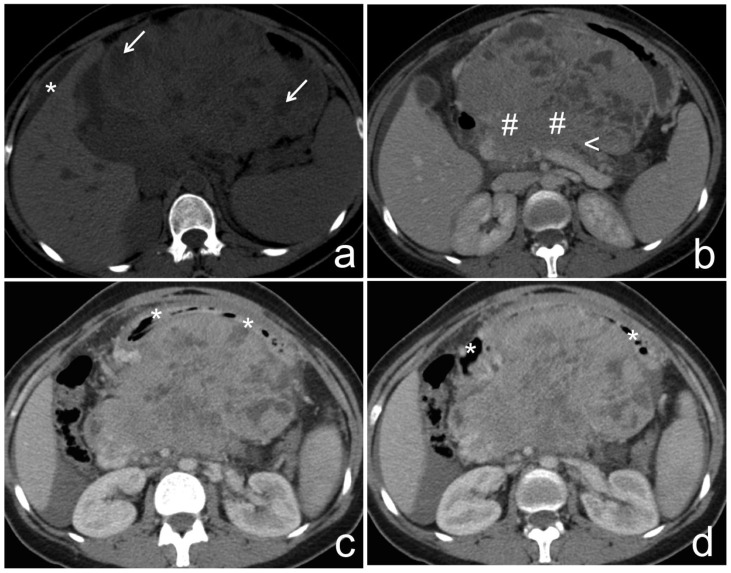
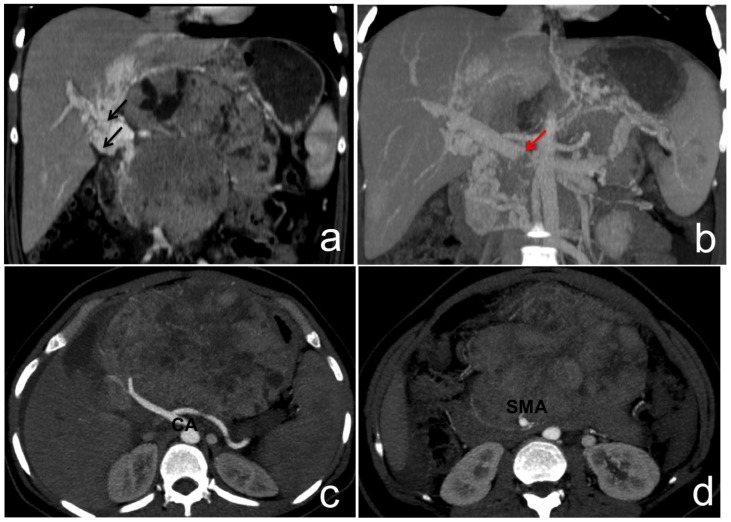
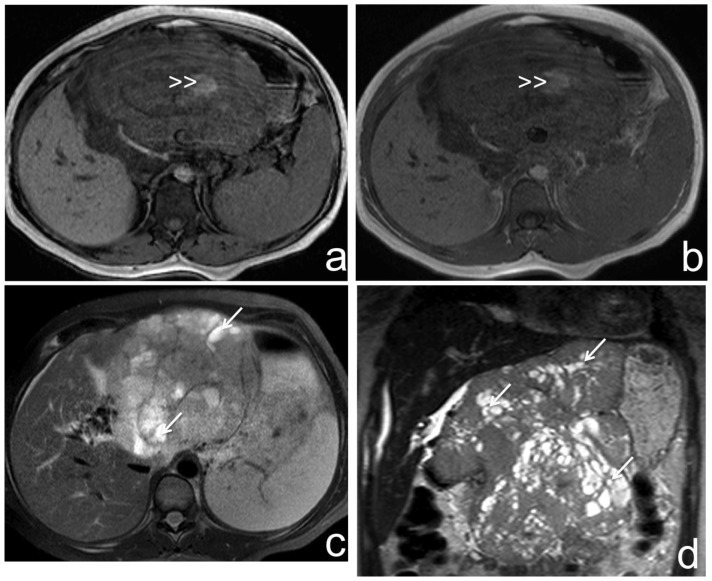
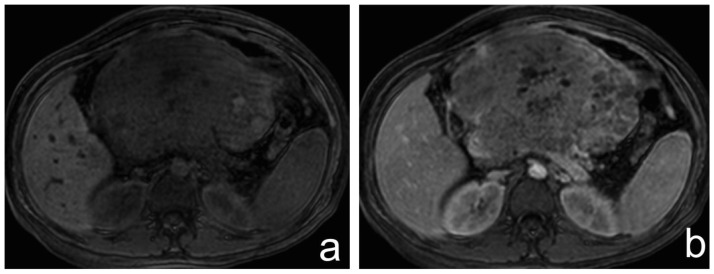
Abdominal ultrasound (US) revealed a voluminous, heterogeneous, epigastric mass, predominantly hypoechoic, with cystic cavities (fig. 1a), highly vascularized at Doppler-US (fig. 1b). Abdomino-pelvic computed tomography (CT) showed a large and heterogeneous mass due to the presence of cystic and solid areas, with partially well-defined and lobulated margins (fig. 2a), and mild contrast enhancement (fig. 2b). The mass was intra-abdominal and centered at the epigastrium, measuring 11,4×16,7×17,0 cm (antero-posterior, transverse and longitudinal axis, respectively). It was completely independent from the uterus and ovaries, which had normal postpartum appearance. The mass deviated neighboring structures, making it difficult to identify its origin; the duodenal arch was anteriorly deviated, suggesting a retroperitoneal origin (fig. 2c, 2d). The pancreatic contour blended with the mass, without cleavage plane between them, and causing distal pancreatic ductal dilatation (fig. 2b). The portal vein showed cavernous transformation, and the celiac axis and the superior mesenteric artery were encased by the tumor (fig. 3). Magnetic resonance imaging (MR) was performed to improve the characterization of the lesion. There were focal areas of high signal intensity on T1-weighted images consistent with intratumoral hemorrhage (fig. 4a, 4b), and on T2-weigthed images the mass was heterogeneously hyperintense compared to the liver (fig. 4c, 4d). After contrast, solid areas showed moderate enhancement (fig. 5). Neither adenopathies nor distant metastases were found.
Figure 1.
37-year-old female with pancreatoblastoma.
a) Transverse transabdominal B-mode US image through the epigastrium shows a large heterogeneous mass, predominantly hypoechoic with small cystic cavities (white arrow). b) Transverse transabdominal Color Doppler US image shows that the mass has intense arterial and venous flow.
Technique: US (GE Logic 7 Pro), curved transducer, 3.5 MHz
Figure 2.
37-year-old female with pancreatoblastoma.
a) Non-contrast-enhanced CT, axial reconstruction, shows a large intra-abdominal mass, centered at the epigastric region, presenting partially well-defined and lobulated contours, measuring 11,4×16,7×17,0 cm (antero-posterior, transverse and longitudinal axis, respectively). The mass is predominantly solid, with internal low-density cavities presumably cystic (white arrow). Note peri-hepatic ascites (asterisk).
b–d) Portal phase enhanced CT, axial reconstruction, shows that the solid component of the mass enhances, and is in continuity with the pancreatic body (cardinal). Note distal pancreatic ductal dilatation (arrowhead), suggesting its involvement. The mass pushed away the neighboring structures. The duodenal arch (asterisk) was anteriorly deviated, suggesting a retroperitoneal origin.
Technique: MCTD (Philips, Brilliance, 6), 120 kV, 155 mAs, 5 (a) and 3 mm slice thickness (b–d), 100 ml of iobitridol 350 mg/ml contrast media.
Figure 3.
37-year-old female with pancreatoblastoma.
a) and b) Portal phase enhanced CT image, coronal reconstruction and MIP reformation respectively, demonstrate cavernous transformation (black arrow) of the portal vein. Note the portal vein luminal defect (red arrow), suggesting its thrombosis.
c) and d) Arterial phase enhanced CT, axial MIP reformations at celiac axis and at proximal superior mesenteric artery planes, respectively. Celiac axis and at its branches (CA) and superior mesenteric artery (SMA), are encased by tumor, but still patent.
Technique: MCTD (Philips, Brilliance, 6), 158 mAs, 120 kV, 3 mm slice thickness (image a) and 10mm reformations (c–d), 100 ml of iobitridol 350 mg/ml
Figure 4.
37-year-old female with pancreatoblastoma.
a) and b) Axial T1-weighted out and in phase MR images respectively, show a large, heterogeneous mass with hyperintense areas suggesting hemorrhage (double arrowhead).
c) and d) Axial and coronal T2-weighted MR images show a large heterogeneous mass with predominantly solid intermediate signal intensity and hyperintense cystic areas (white arrow), presenting lobulated and partially well-defined borders.
Technique: 1,5 Tesla MRI (Philips Medical Systems Achieva). Image a: TR 115,7, TE 2,3, non-contrast, 7,5 mm slice thickness. Image b: TR 115,7, TE 4,6, non-contrast, 7,5 mm slice thickness. Image c: TR 1887, TE 70, non-contrast, 7,5 mm slice thickness. d: TR 746,6, TE 91, 4,5 mm slice thickness.
Figure 5.
37-year-old female with pancreatoblastoma.
a and b) Axial T1-weighted fat saturated MR image before and after gadolinium administration, respectively. The solid components show moderate enhancement.
Technique: 1,5 Tesla MRI (Philips Medical Systems Achieva). Image a: TR 3,6, TE 1,8, 5 mm slice thickness, non-contrast. Image b: TR 3,6, TE 1,8, 5 mm slice thickness following 5 ml of gadobutrol 1 mmol/ml, late arterial phase.
A preoperative diagnosis of a solid-pseudopapillary neoplasm of the pancreas was suggested based on the patient’s age and gender, and on imaging findings.
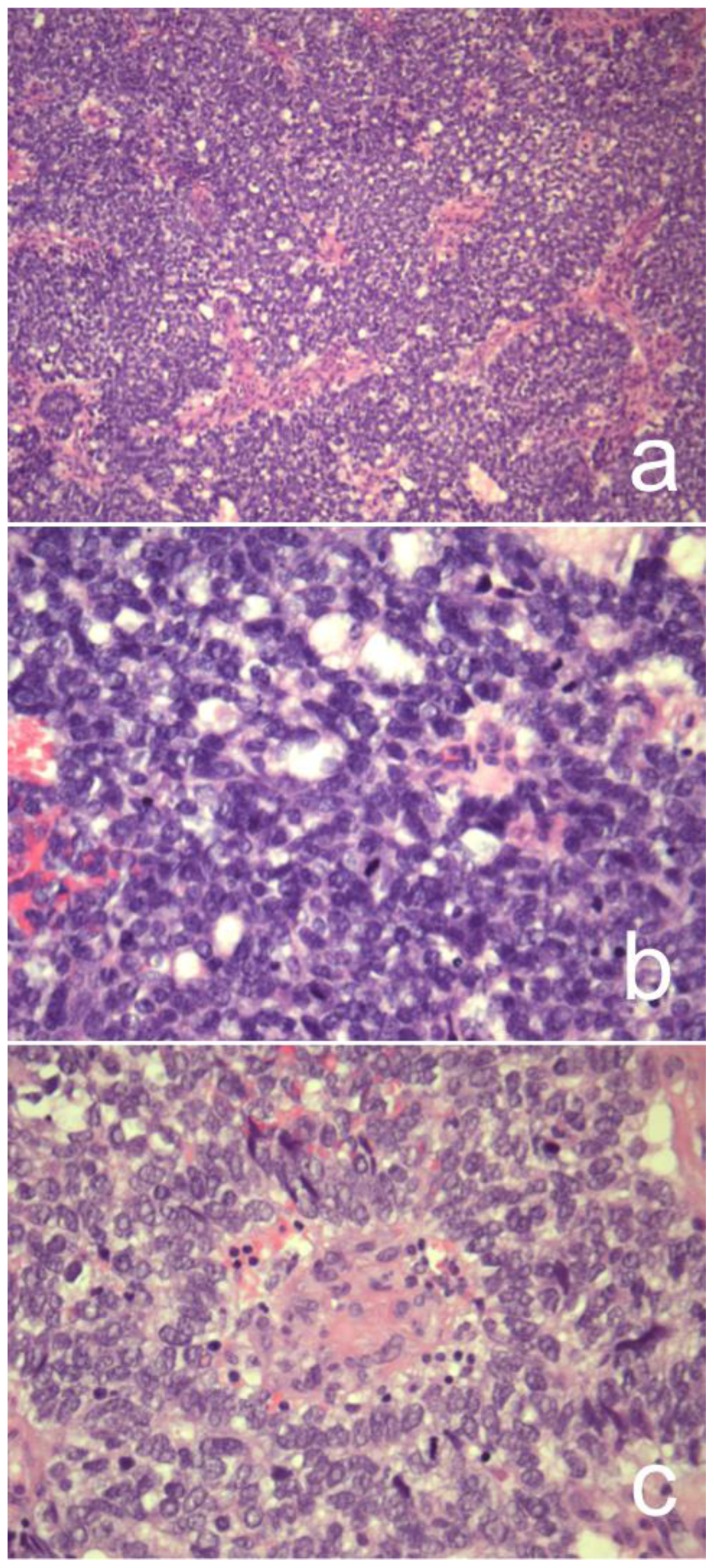
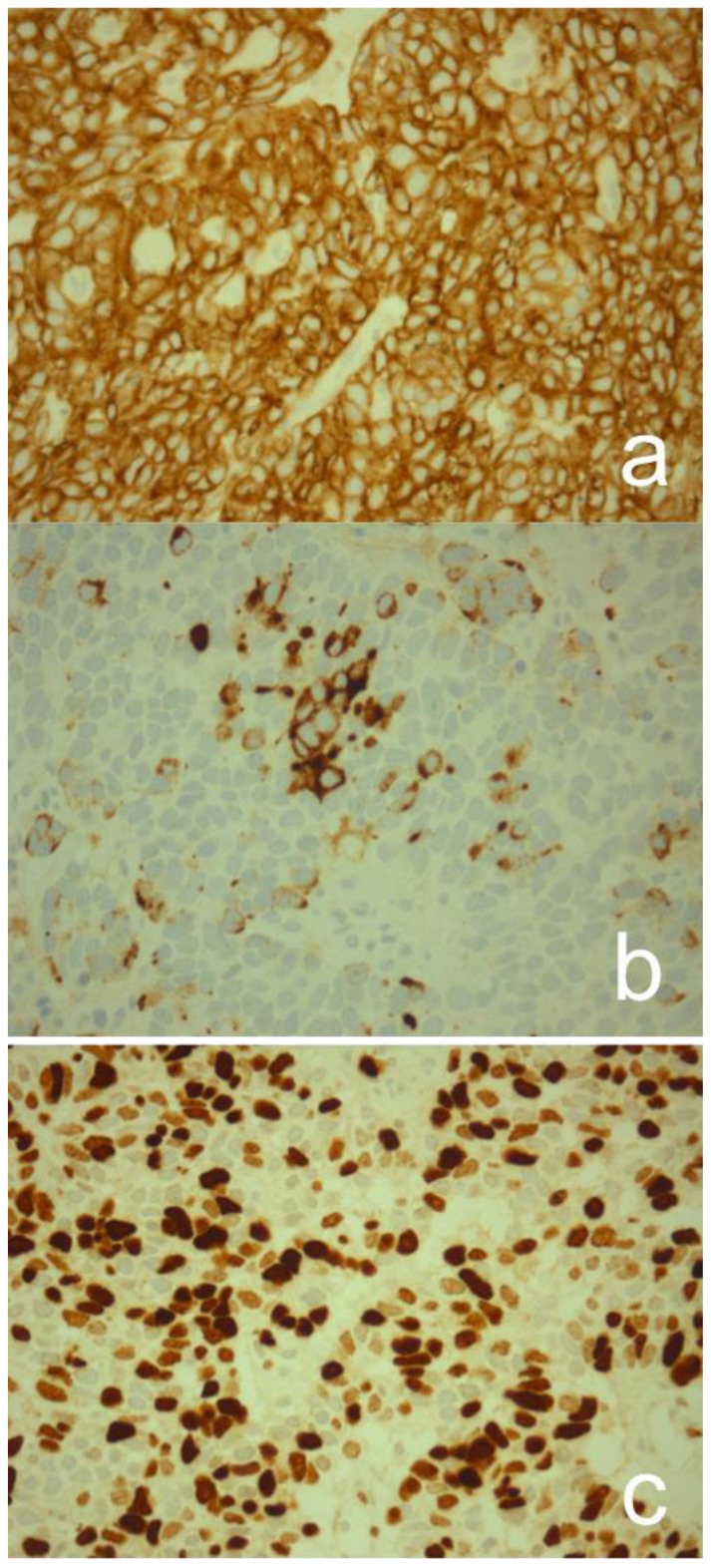
The initial percutaneous tru-core biopsy of the lesion was compatible with a solid-pseudopapillary neoplasm of the pancreas. Then, the patient was sent to a more experienced institution in hepatobiliary pathology for intraoperative evaluation of resectability. Surgical exploration of the pancreatic mass revealed a large tumor with gastric and transverse colon invasion, and also portal vein invasion with secondary portal hypertension, without arterial invasion. A wedge surgical biopsy and a portal-caval shunt were performed. At histopathological analysis of this biopsy, diagnostic features of pancreatoblastoma were recognized. Microscopy showed abundant small tumor cells that exhibited an organoid growth pattern with focal acinar features, separated by cellular stroma. In a few foci, the tumor cells had an epithelioid appearance with clusters of squamoid cells and occasional keratinization (fig. 10). Immunohistochemical staining showed a positive reaction for epithelial markers, namely cytokeratin (CK)AE1/AE3, calmodulin (CaM)53, CK903, CK7 and focally the neuroendocrine marker chromogranin A (fig. 11). Tumor cells stained negative for CK20, vimentin, cluster of differentiation (CD)10, rhodamine phalloidin (RP) and nuclear β-catenin. Ki-67 labeling index was greater than 60% (fig. 11).
The morphologic and immunophenotypic features, together with evidence of mixed acinar differentiation and squamoid corpuscles, supported the diagnosis of pancreatoblastoma.
After surgery, the patient was referred to the oncology department for palliative chemotherapy. She died one year after the initial presentation.
DISCUSSION
Etiology & Demographics
Pancreatoblastoma, formerly known as infantile pancreatic carcinoma, owes its name to similarities of the tumor to the incompletely differentiated acini of fetal pancreatic tissue at 7 weeks of gestation [1, 2].
The precise etiology and pathogenesis are unknown. Although most cases are sporadic, associations with genetic syndromes (Beckwith-Wiedemann syndrome and familial adenomatous polyposis) and with genetic mutations (alterations in the Wnt signaling pathway and allelic loss on chromosome 11p) have been described [3, 4].
Pancreatoblastoma is a rare neoplasm with an annual incidence of around 0.004 new cases per 100,000 population [5]. It occurs mostly in pediatric patients between the ages of 1 and 8 years, where it accounts for almost 25% of pancreatic neoplasms [1]. Adult pancreatoblastoma presents an almost equal incidence between males and female and presents no significant predilection for head, body or tail. It is usually indolent, but exhibits a malignant behavior with local invasion, recurrence, and occasionally, with distant metastasis [1]. Adult pancreatoblastoma is extremely rare, with a total of 35 cases reported in the literature, and generally presenting a more aggressive behavior [6, 7].
To our knowledge, there is no documented association between pancreatoblastoma and pregnancy in literature, and this finding in the puerperium is likely to be coincidental.
Clinical & Imaging Findings
Clinical presentation varies and is non-specific. They often remain occult until they are large. Adult patients mainly present with abdominal pain, weight loss or an abdominal mass [8]. Anorexia, change in bowel habits, and jaundice are other common symptoms [9].
Alpha-fetoprotein is high in two-thirds of children, but is not consistently elevated in adults [10]. Other tumor markers such as Carcinoembryonic Antigen (CEA) and Cancer Antigen (CA)19-9 are not helpful in adult pancreatoblastoma. Liver function test abnormalities [11] and anemia are sometimes reported [12].
Imaging features of pancreatoblastoma in adults have been infrequently reported, but no studies suggest significant differences between adult and pediatric patients [6, 10]. They are usually described as large, well-defined masses, with partially circumscribed and lobulated margins, without a cleavage plane with the pancreas. Their internal architecture is heterogeneous with solid and cystic components [11, 12]. At US, the most common finding is a mixed echogenicity solid mass [9, 13]. Its heterogeneity manifests as a solid mass with low attenuation multiloculated elements at CT, and as a solid mass presenting areas with low to intermediate signal intensity on T1-weighted images and high signal on T2-weighted images [9, 14]. Enhancement is a common feature on contrast-enhanced CT and MRI. MRI better delineates intratumoral hemorrhage and necrotic areas. On the other hand, CT better demonstrates calcifications, as small, punctate, clustered, or rim like hyperdense foci [11, 12].
Pancreatoblastoma is a slow growing tumor, very large in size at the time of diagnosis [11], making depiction of organ origin very challenging for radiologists. A multi-step approach should ensue. The first step is to decide whether the tumor is intra or retroperitoneal located. Anterior displacement of retroperitoneal organs, such as the kidneys, adrenal glands, ureters, ascending and descending colon, pancreas, and some portions of the duodenum, strongly suggests a retroperitoneal origin. Retroperitoneal masses may also displace retroperitoneal major vessels. The second step is to decide if the lesion originates or not from a retroperitoneal major organ. If not, it suggests a primary retroperitoneal tumor arising mainly from the mesodermal system or from the nervous system. Some radiologic signs are helpful in determining tumor origin: 1) the “beak sign” – when a mass deforms the edge of an adjacent organ, suggesting that the mass arises from that organ; 2) the “phantom organ sign” - when a large mass arises from a small organ, the organ sometimes becomes undetectable; 3) the “positive embedded organ sign” - when a mass is in close contact with an organ by a sclerotic contact surface, it is likely that the tumor originates from that the involved organ [15, 16].
In the present case, there was anterior and right displacement of the first and second duodenal segments, proposing a retroperitoneal origin. The pancreas was embedded in the lesion and there was pancreatic ductal dilatation, favoring a pancreatic origin instead of a primary retroperitoneal tumor.
Pathological Features
Pre-operative pathologic diagnosis is difficult because tumor cellular heterogeneity with multiple cellular lines of differentiation overlap with other tumors, hence explaining the discordance between the results from the tru-cut and the open biopsies.
The distinctive pathologic features of pancreatoblastoma are the combined mixture of primitive acinar, endocrine, islet cell, and ductal elements, reminiscent of the fetal pancreas [8]. The most characteristic histologic finding, and an important clue to the correct diagnosis, is the presence of squamoid corpuscles [9, 17]. These appear as variably sized foci of squamoid cells, which occasionally keratinize.
The immunohistochemical staining reflects the distinctive cellular differentiation of the tumor. Markers of ductal differentiation stain positive in approximately half of the cases. The neuroendocrine component stains positive for neuroendocrine markers. Pancreatoblastoma demonstrates prominent staining for markers of acinar differentiation such as trypsin and chymotrypsin [6].
Treatment & Prognosis
The prognosis of pancreatoblastoma in adults is uniformly poor, with frequent local infiltration of surrounding tissues and metastatic disease [9, 11]. Pancreatoblastoma invades adjacent structures such as the spleen, colon, duodenum, portal vein, superior mesenteric vessels, peripancreatic soft tissue, common bile duct and nerves. Biliary and pancreatic ductal dilatation can occasionally be present [11, 12]. Metastases are also frequent, with the liver being the most common involved organ. Other sites include lymph nodes, lungs, bone and peritoneum [18].
The only curative treatment is complete resection. When this is not possible or when there are distant metastases, radiotherapy or chemotherapy may be offered. In general, despite aggressive treatment, pancreatoblastoma in adults is associated with poorer outcome than in children, with a median survival time of 5 months in patients with unresected tumours, to 20 months in patients treated with chemoradiotherapy and surgery [6].
Differential Diagnosis
Facing a puerperal female with anemia and abdominal lump at emergency, the first thought is a gynecological cause. Ultrasound excluded this hypothesis and found a large abdominal heterogeneous vascularized mass. CT better delineated its retroperitoneal origin, most likely from pancreas, with which showed no cleavage plane.
The main differential considerations towards a large exophytic tumor in pancreas with solid and cystic-necrotic areas are solid pseudopapillary neoplasm, serous microcystic cystadenoma, mucinous pancreatic neoplasms, non-functioning endocrine neoplasm, acinar cell carcinoma, adenocarcinoma and pseudocyst.
Solid pseudopapillary neoplasm
Solid pseudopapillary neoplasms are low-grade malignancies typically diagnosed in young women. They are approximately equally distributed within the tail and the head of the pancreas [19]. Usually they are very large at presentation, containing solid and cystic areas, with a thick, well-defined capsule and sometimes calcifications. They exhibit a heterogeneous signal on both T1-weighted and T2-weighted MR imaging [7, 20]. Its enhancement pattern can contribute to the diagnosis: peripheral rim enhancement in their thick fibrous capsule and progressive heterogeneous fill-in on dynamic enhanced images [4, 21].
Serous cystadenoma
Serous cystadenomas, usually found in older women and typically located in the pancreatic head, are characteristically composed of multiple tiny cystic spaces separated by thin septa, eventually calcified [9]. Presumably because of the small diameter of the associated microcysts (<2mm) it can mimic a solid mass, especially on CT. On T1-weighted MR images, it may appear as a heterogeneous hypointense mass with hyperintense foci related to prior hemorrhage, and on T2-weighted images shows hyperintense signal intensity with low-signal-intensity central areas due to scar formation and hypointense septa that may radiate toward an enhancing central scar [22]. This radial central scar is not a feature of pancreatoblastoma.
Mucinous cystic neoplasm
Mucinous cystic neoplasms, which include mucinous cystadenoma/cystoadenocarcinoma and intraductal papillary mucinous neoplasm (IPMN), are predominantly cystic. Mucinous cystadenoma mostly presents in the tail or body of the pancreas, while IPMN is located in the pancreatic head, and patients are usually over 60 years old. Although the mucin-filled cystic spaces are typically hyperintense on T2-weighted images and hypointense on T1-weighted images, mucin occasionally results in high signal intensity on both T1- and T2-weighted images [23]. Calcifications and fluid-fluid level from hemorrhage can also exist; capsule enhancement is typically delayed [20].
Non-functioning endocrine neoplasm
Non-functioning endocrine neoplasms appear in older patients and have an equal gender distribution. They may appear cystic, contain calcifications, and show areas of internal hemorrhage [24]. Non-functioning endocrine neoplasm are low in signal intensity on fat-suppressed T1-weighted images, exhibit high signal intensity on T2-weighted images, and show marked ring or diffuse heterogeneous enhancement on immediate gadolinium-enhanced gradient-echo images [25]. Imaging features that help distinguish pancreatoblastoma from endocrine tumor of the pancreas are the different signal intensity on T1-weighted images and the different contrast enhancement pattern, with non-functioning endocrine neoplasm being hypervascularized [24].
Acinar cell carcinoma
Acinar cell carcinomas affect older patients and predominantly male. They can be very large, well circumscribed and necrotic; metastases are often present [11, 26].
Ductal adenocarcinoma
Although ductal adenocarcinoma is the most common primary pancreatic malignancy, it is rarely a differential of pancreatoblastoma in daily practice. Pancreatic adenocarcinoma is seen in older patients, it is often small in size at the time of diagnosis and very rarely exhibit necrosis, hemorrhage or calcifications [11].
Pancreatic pseudocyst
Pancreatic pseudocyst, the most common single cystic lesion of the pancreas, may undergo internal hemorrhage, mimicking pancreatoblastoma [22]. However, there are no solid components, a history of pancreatitis is almost always present, and inflammatory changes in the peripancreatic fat and irregular pancreatic dilatation should be looked for. Pseudocysts are typically located in the tail or body of the pancreas [27].
TEACHING POINT
The preoperative diagnosis of pancreatoblastoma in adults is very challenging because of its rarity, the similar radiologic findings to those seen in other pancreatic lesions, and its histopathologic heterogeneity. Nevertheless, it should be considered in tumors presenting solid and cystic components in order to reach an early detection and proper management.
Figure 6.
37-year-old female with pancreatoblastoma.
Microscopic pathology of haematoxylin-eosin staining, original magnification ×100 (a) and ×400 (b–c). Microscopy showed abundant small tumor cells that exhibited an organoid growth pattern with focal acinar features. There are a few squamoid corpuscles.
Figure 7.
37-year-old female with pancreatoblastoma.
Microscopic pathology of immunohistochemical staining for cytokeratin AE1/AE3 (a), chromogranin A (b) and Ki-67 (c) - original magnification ×100.
Immunohistochemical staining showed a positive reaction for cytokeratin AE1/AE3, and focally positive reaction for chromogranin A. Ki-67 (MIB-1) labeling index was > 60%.
Table 1.
Differential diagnosis table for pancreatoblastoma
| Local morphology | X-Ray | US | CT | MRI | Contrast enhancement | |
|---|---|---|---|---|---|---|
| Pancreatoblastoma | No predilection for head, body or tail. Large with partially circumscribed and lobulated margins. |
Sometimes small, punctate, clustered, or rim like Ca2+ | Mixed echogenicity solid mass | Solid mass with low attenuation multiloculated elements | Solid mass presenting areas with low to intermediate signal intensity on T1-wi and high signal on T2-wi | Enhancement in arterial and portal venous phases |
| Solid Pseudo-papillary tumor | Tail (43%) | Sometimes curvilinear Ca2+ | Very large, encapsulated, heterogeneous (hypoechoic solid and anechoic cystic areas) | Well-circumscribed, encapsulated, round or lobulated lesion Variable internal architecture depending on the degree of hemorrhage |
Hemorrhage: high SI onT1-wi, low or inhomogeneous SI on T2-wi Solid component: low SI on T1-wi, high SI on T2-wi |
Early, peripheral enhancement Progressive heterogeneous fill-in |
| Non-functioning endocrine neoplasm | Small or large in size | May contain Ca2+ | Homogeneously hypoechoic lesion | Isoattenuating to the parenchyma, but may appear cystic Distant metastasis |
Low SI or isointensity on T1-wi High to isointense on T2-wi |
Enhancement in arterial phase |
| Serous cystadenoma | Head Innumerable small cyst |
Central calcified scar | Multiple millimetric hypoechoic or anechoic cysts | Honeycomb pattern of multiple millimetric cysts | Hypointense on T1 and hyperintense on T2-wi clustered cysts | Parietal enhancement |
| Ductal adenocarcinoma | Head Ill-defined lesion with contour deformity of the gland |
Ca2+ very rare | Hypoechoic lesion Dilated pancreatic duct Atrophic gland |
Isodense to the parenchyma Dilated pancreatic duct and atrophic gland Obliteration of peripancreatic fat Contiguous organ invasion, vascular invasion and distant metastases |
Low SI on T1-wi Variable SI on T2-wi Contiguous organ invasion and distant metastases |
Poor or non-enhancing lesion |
| Mucinous cystic neoplasm | Tail Uni/multilocular cyst |
Sunburst Ca2+ | Hypoechoic macrocystic (cystadenoma) and with pancreatic duct dilatation (IPMN) | Hypodense uni- or multilocular, peripheral calcification (cystadenoma) pancreatic duct dilatation (IPMN) metastasis (mucinous adenoCa) | High SI on T2 and low on T1-wi (but variable with mucin concentration) Fluid-fluid level from hemorrhage can exist. |
Enhancement of the internal septa and cyst wall typically delayed |
| Pancreatic pseudocyst | Tail and body | May be calcified peripherally | Anechoic or hypoechoic, pancreatic calcifications might be seen | Hypodense, pancreatic calcifications and inflammatory changes in the peripancreatic fat might be seen | T1: hypointense, T2: hyperintense, Debris or hemorrhage can change the intensity | Might have mild enhancement of the thin fibrous capsule, no inner enhancement |
| Acinar cell carcinoma | Can be very large, well circumscribed and necrotic | Ca2+ usually not evident | Hypoechoic lesion | Mild hypodense appearance with a focal cystic/necrotic component | Predominantly T1 hypointense and T2 iso to hyperintense, with central cystic areas when large | Hypovascular lesion |
Table 2.
Summary table for pancreatoblastoma
| Etiology | Unknown; most cases sporadic |
| Incidence | Around 0.004 new cases per 100,000 population |
| Gender ratio | Almost equal distribution in adults |
| Age predilection |
|
| Risk factors |
|
| Treatment |
|
| Prognosis |
|
| Findings on imaging |
|
ACKNOWLEDGEMENTS
We thank our colleague Rosa Cardoso for sharing this case, Joana Pinto and Graça Pereira for manuscript revision.
ABBREVIATIONS
- CA
cancer antigen
- CaM
calmodulin
- CD
Cluster of differentiation
- CEA
carcinoembryonic antigen
- CK
cytokeratin
- CT
computed tomography
- IPMN
intraductal papillary mucinous neoplasm
- MRI
magnetic resonance imaging
- RP
rhodamine phalloidin
- SPIR
spectral presaturation by Inversion Recovery
- TE
echo time
- THRIVE
T1-weighted high resolution isotropic volume examination
- TR
repetition time
- US
ultrasound
REFERENCES
- 1.Salman B, Brat G, Yoon YS, Hruban RH, Singhi AD, Fishman EK, Herman JM, et al. The diagnosis and surgical treatment of pancreatoblastoma in adults: A case series and review of the literature. J Gastrointest Surg. 2013;17:2153–2161. doi: 10.1007/s11605-013-2294-2. [DOI] [PubMed] [Google Scholar]
- 2.Horie A, Yano Y, Kotoo Y, Miwa A. Morphogenesis of pancreatoblastoma, infantile carcinoma of the pancreas: report of two cases. Cancer. 1977;39:247–54. doi: 10.1002/1097-0142(197701)39:1<247::aid-cncr2820390138>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Kohda E, Iseki M, Ikawa H, Endoh M, Yokoyama J, Mukai M, Hata J, et al. Pancreatoblastoma: Three original cases and review of the literature. Acta Radiol. 2000;41:334–7. doi: 10.1080/028418500127345604. [DOI] [PubMed] [Google Scholar]
- 4.Palmucci S, Uccello A, Leone G, et al. Rare pancreatic neoplasm: MDCT and MRI features of a typical Solid Pseudopapillary Tumor. Journal of Radiology Case Reports. 2012;6(1):17–24. doi: 10.3941/jrcr.v6i1.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argon A, Çelik A, Oniz H, Ozok G, Barbet FY. Pancreatoblastoma, a Rare Childhood Tumor: A Case Report. Turk Patoloji. 2014;11:1–5. doi: 10.5146/tjpath.2014.01268. [DOI] [PubMed] [Google Scholar]
- 6.Omiyale AO. Clinicopathological review of pancreatoblastoma in adults. Gland Surgery. 2015;4(4):322–328. doi: 10.3978/j.issn.2227-684X.2015.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low G, Panu A, Millo N, Leen E. Multimodality Imaging of Neoplastic and Non-neoplastic Solid Lesions of the Pancreas. Radiographics. 2011;31(4):993–1015. doi: 10.1148/rg.314105731. [DOI] [PubMed] [Google Scholar]
- 8.Rosebrook JL, Glickman JN, Mortele KJ. Pancreatoblastoma in an adult woman: Sonography, CT and dynamic gadolinium-enhanced MRI features. AJR. 2005;184:S78–S81. doi: 10.2214/ajr.184.3_supplement.01840s78. [DOI] [PubMed] [Google Scholar]
- 9.Charlton-Ouw KM, Kaiser CL, Tong GX, Allendorf JD, Chabot JA. Revisiting metastatic adult pancreatoblastoma: A case and review of the literature. J Pancreas. 2008;9(6):733–738. [PubMed] [Google Scholar]
- 10.Rajpal S, Warren RS, Alexander M, Yeh BM, Grenert JP, Hintzen S. Pancreatoblastoma in an adult: case report and review of the literature. J Gastrointest Surg. 2006;10:829–36. doi: 10.1016/j.gassur.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Savastano S, d’Amore ESG, Zuccatotto D, Banzato O, Begheto M, Famengo B. Pancreatoblastoma in an adult patient: A case report. J Pancreas. 2009;10(2):192–195. [PubMed] [Google Scholar]
- 12.Ozcan HN, Oguz B, Sen HS, Akyuz C, Haliloglu M. Imaging features of primary malignant pancreatic tumors in children. AJR. 2014;203(3):662–667. doi: 10.2214/AJR.13.12300. [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Kim IO, Kim WS, et al. CT and US findings of pancreatoblastoma. J Comput Assist Tomogr. 1996;20:370–4. doi: 10.1097/00004728-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Montemarano H, Lonergan GJ, Bulas DI, Selby DM. Pancreatoblastoma: imaging findings in 10 patients and review of the literature. Radiology. 2000;214:476–82. doi: 10.1148/radiology.214.2.r00fe36476. [DOI] [PubMed] [Google Scholar]
- 15.Brennan C, Kajal D, Khalili K, Ghai S. Solid malignant retroperitoneal masses – a pictorial review. Insights Imag. 2014;5:53–65. doi: 10.1007/s13244-013-0294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino M, Hayakawa K, Minami M, Yamamoto A, Ueda H, Tokasu K. Primary retroperitoneal neoplasms: CT and MR Imaging findings with anatomic and pathologic diagnostic clues. RadioGraphics. 2003;23:45–57. doi: 10.1148/rg.231025037. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Tang N, Liu Y, Wang E. Pancreatoblastoma in an adult. Indian J Pathol Microbiol. 2015;58(1):93–5. doi: 10.4103/0377-4929.151199. [DOI] [PubMed] [Google Scholar]
- 18.Balasundaram C, Luthra M, Chavalitdhamrong D, et al. Pancreatoblastoma: a rare tumor still evolving in clinical presentation and histology. JOP. 2012;13:301–3. [PubMed] [Google Scholar]
- 19.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200(6):965–72. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Cantisani V, Mortele KJ, Levy A, Glickman JN, Ricci P, Passariello R, Ros PR, Silverman SG. MR Imaging features of solid pseudopapillary tumor of the pancreas in adult and pediatric patients. AJR. 2003;181:395–401. doi: 10.2214/ajr.181.2.1810395. [DOI] [PubMed] [Google Scholar]
- 21.Wang D-B, Wang Q-B, Chai W-M, et al. Imaging features of solid pseudopapillary tumor of the pancreas on multi-detector row computed tomography. World J Gastroenterol. 2009;15(7):829–35. doi: 10.3748/wjg.15.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kehagias D, Smyrniotis V, Gouliamos A, Vlahos L. Cystic pancreatic neoplasms: Computed tomography and Magnetic resonance imaging findings. Int J Pancreatol. 2000;28:223–230. doi: 10.1385/IJGC:28:3:223. [DOI] [PubMed] [Google Scholar]
- 23.Minami M, Itai Y, Ohtomo K. Cystic endoplasms of the pancreas: comparison of MR imaging with CT. Radiology. 1989;171:53–56. doi: 10.1148/radiology.171.1.2928546. [DOI] [PubMed] [Google Scholar]
- 24.Takeshita K, Furui S, Makita K, Yamauchi T, Irie T, Tsuchiya K, Kusano S, et al. Cystic islet cell tumors: radiologic findings in three cases. Abdom Imaging. 1994;19:225–228. doi: 10.1007/BF00203512. [DOI] [PubMed] [Google Scholar]
- 25.Buetow PC, Parrino TV, Buck JL, Pantograg-Brown L, Ros PR, Dachman AH, Cruess DF. Islet cell tumors of the pancreas: pathologic–imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR. 1995;165:1175–1179. doi: 10.2214/ajr.165.5.7572498. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Kim YK, Jang KT, Lim JH. Intraductal growing acinar cell carcinoma of the pancreas. Abdom Imaging. 2013;38(5):1115–9. doi: 10.1007/s00261-013-9993-8. [DOI] [PubMed] [Google Scholar]
- 27.Federle MP, Jeffrey RB, Desser TS, et al. Abdomen. 1st ed. Amirsys Inc; 2004. Pancreatic Pseudocyst, in Diagnostic Imaging; pp. 24–27. [Google Scholar]