Abstract
Extramammary Paget disease (EMPD) is an uncommon malignancy. It manifests either in the primary form in the skin as an intraepithelial neoplasm, or in secondary form as pagetoid (intraepithelial) spread of an underlying internal carcinoma to the skin. Although local invasion and recurrence of primary extramammary Paget disease are relatively frequent, widespread metastases are rare. As such, there are very few reports and little characterization of the radiologic features of widespread spinal metastases. To our knowledge, there are no prior reports of a metastatic extramammary Paget disease presenting as a painful pathologic vertebral body compression fracture. We report the radiological features of a case of primary extramammary Paget disease with subsequent spinal metastases presenting as a painful compression fracture.
Keywords: Extramammary Paget disease, spinal metastases, spine MRI
CASE REPORT
A 57-year-old male presented to an outside institution with a perineal rash (Fig. 1). He was initially prescribed topical corticosteroids for presumptive dermatitis without benefit. Two years later, he was referred to dermatology at our institution. Punch biopsy of the lesion demonstrated large intraepidermal pagetoid cells consistent with EMPD.
Figure 1.
58-year-old male with spinal metastases of extramammary Paget disease.
Findings: Hypopigmented region (arrow) of perineal skin with smaller erythematous areas.
A staging PET/CT revealed no hypermetabolism of the primary lesion and no evidence of metastatic disease (Fig. 2). Absence of an underlying gastrointestinal or genitourinary cancer was consistent with this patient’s disease as the primary, rather than secondary, form of EMPD. Thirteen months after initial pathologic diagnosis, the patient presented to our emergency department with severe, acute exacerbation of back pain, which had been gradual in onset during the preceding 2 months.
Figure 2.
58-year-old male with spinal metastases of extramammary Paget disease.
Findings: PET/CT whole body Maximum Intensity Projection frontal image. Demonstrates no hypermetabolism to suggest local or distant metastatic disease.
Technique: F-18 FDG, 15.0 millicuries. Time of acquisition,60 minutes.
Imaging Findings
Radiographs of the spine demonstrated multilevel lytic lesions with a pathologic compression fracture of the T8 vertebral body (Fig. 3). A right shoulder radiograph demonstrated an additional axial skeletal lytic lesion in the scapula (Fig. 4). CT of the abdomen and pelvis showed multiple presumed splenic and hepatic metastases (Fig 5, 6), as well as numerous predominantly lytic metastases of the axial skeleton (Fig. 7). MRI of the spine showed diffuse, patchy T1 hypointensity and T2 mild hyperintensity, with avid enhancement of the vertebrae. The T8 vertebral body fracture findings included mild epidural extension, compatible with metastatic infiltration and pathologic fracture (Fig. 8).
Figure 3.
58-year-old man with spinal metastases of extramammary Paget disease.
Findings: Lateral radiograph of the thoracic spine shows multilevel lytic lesions with pathologic compression fracture and mild vertebral body height loss at T8 (arrow).
Figure 4.
58-year-old man with spinal metastases of extramammary Paget disease.
Findings: AP radiograph of the right shoulder reveals a large lytic lesion (arrow) of the superior right scapula representing additional axial skeletal metastasis of extramammary Paget disease.
Figure 5.
58-year-old man with spinal metastases of extramammary Paget disease.
Findings: Axial enhanced CT of the pelvis in portal venous phase shows a low attenuation lesion in the liver with peripheral enhancement consistent with a presumed metastasis.
Technique: 120 kVp, 271 mAs, 3.0 mm slice thickness, 140 mL Iohexol 300 administered intravenously at injection rate of 4 mL/second. Portal Venous Phase.
Figure 6.
58-year-old man with spinal metastases of extramammary Paget disease.
Findings: Axial enhanced CT of the pelvis in portal venous phase shows a low attenuation lesion in the spleen, consistent with a presumed metastasis.
Technique: 120 kVp, 271 mAs, 3.0 mm slice thickness, 140 mL Iohexol 300 administered intravenously at injection rate of 4 mL/second. Portal Venous Phase.
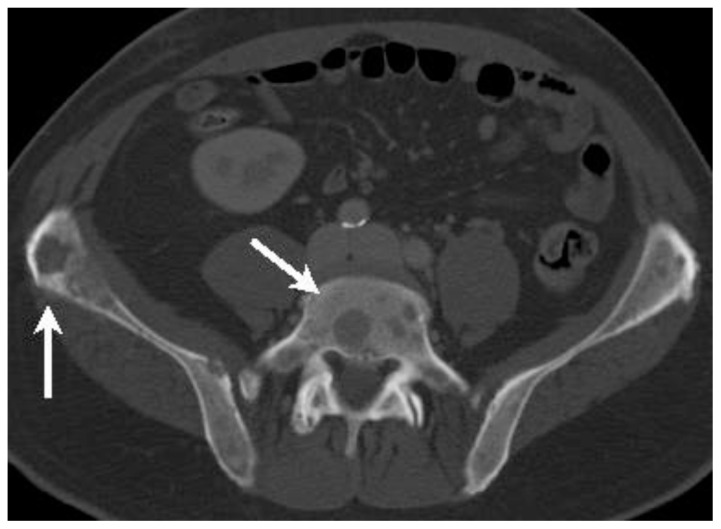
Figure 7.
58-year-old man with spinal metastases of extramammary Paget disease.
Findings: Axial enhanced CT of the pelvis in portal venous phase shows numerous predominantly lytic lesions (arrows) throughout the axial skeleton, here depicted in the L5 vertebra and iliac bones.
Technique: 120 kVp, 271 mAs, 3.0 mm slice thickness, 140 mL Iohexol 300 administered intravenously at injection rate of 4 mL/second.
Figure 8.
58-year-old man with spinal metastases of extramammary Paget disease.
Findings: precontrast T1-weighted (a), inversion recovery (b), and postcontrast T1-weighted (c) sagittal MR images of the spine showed diffuse, patchy T1 hypointensity and T2 hyperintensity, with avid enhancement of the vertebrae. Mild epidural extension and height loss at T8 (arrow) are compatible with metastatic infiltration and pathologic fracture.
Technique: 1.5 T Siemens Magnetom Espree, T1-weighted images (TR=500, TE=9.2); inversion recovery images (TR=3400, TE=43, inversion time=170); postcontrast T1-weighted images after intravenous administration of 14.4 mL Multihance (TR=409, TE=9.2).
CT-guided bone marrow biopsy was performed in a region of the right ilium with lytic lesions (Fig. 7). Histopathological analysis revealed extensive involvement of the bone marrow tissue by adenocarcinoma including clusters of large atypical cells morphologically consistent with EMPD (Fig. 9). Cytokeratin 7 stain was strongly and diffusely positive within the neoplastic cells, further supporting the diagnosis of metastatic EMPD (Fig. 10).
Figure 9.
58-year-old male with spinal metastases of extramammary Paget disease.
Findings: Clusters of atypical glandular epithelial cells (arrow) within the bone marrow.
Technique: H&E stain, original magnification ×20.
Figure 10.
58-year-old male with spinal metastases of extramammary Paget disease.
Findings: Strongly positive CK7 stain within clusters of atypical glandular epithelial cells (arrow) within the bone marrow, significant for involvement of the bone marrow by adenocarcinoma morphologically consistent with extramammary Paget disease.
Technique: Keratin 7 immunostain, original magnification ×20.
Management
The patient underwent Mohs surgery of the perineum, a surgical technique in which layers of cancer-containing skin are progressively removed and examined under a microscope until the margins are clear, after biopsy of his original presenting skin lesion revealed EMPD. At surgery, the patient was found to have multifocal, extensive regional disease. The dermatologist determined that negative margins were unlikely to be achieved by further surgical resection, and radiation therapy was performed as an alternative, given high likelihood of local recurrence. Upon discovery of his osseous spinal metastases, palliative radiation to the spine and systemic chemotherapy with paclitaxel were administered. He also underwent vertebroplasty of the pathologic T8 vertebral body fracture.
Follow-up
Progression of metastases was rapid in our patient, with a documented absence of FDG-avid metastases only 13 months prior to discovery of widespread metastases. Only 5 months after discovery of spinal metastases, the patient developed liver failure and sepsis, opted for palliative care, and died.
DISCUSSION
Etiology & Demographics
EMPD often presents as persistent perineal dermatitis affecting older men [1–2]. The genital region is affected because primary EMPD arises from pluripotent stem cells in the genital skin. EMPD can represent a primary epidermal adenocarcinoma in some patients, though it can also herald the presence of an underlying adenocarcinoma of the gastrointestinal or genitourinary tract. Immunohistochemical assays may be useful in discriminating primary EMPD from EMPD secondary to anorectal adenocarcinoma, according to one recently published study. [3] The differentiation between primary and secondary EMPD may also be made by exclusion or confirmation of an underlying gastrointestinal or genitourinary adenocarcinoma, usually with imaging studies and/or colonoscopy. Histopathological features of Paget disease of the breast and EMPD are similar. However, Paget disease of the breast is characterized by adenocarcinoma arising in lactiferous ducts and extending into epidermis rather than arising directly within epidermis [4].
It is important for radiologists who perform image-guided biopsies to understand that EMPD is a form of cutaneous adenocarcinoma that may uncommonly metastasize to internal organs to facilitate radiologic-pathologic correlation of biopsy results. The primary form of disease is initially limited to epidermis, but may undergo dermal invasion, increasing risk of metastasis to regional, usually inguinal, lymph nodes [2, 4–5]. Clinical lymphadenopathy, if present, has been shown to strongly correlate with lymph node metastasis. [6]. Distant metastases, however, are rare [2].
Clinical & Imaging Findings
There are only a few prior reports of cases of EMPD with skeletal metastasis [7–18]. Most of these focus on chemotherapy regimens or histopathological analysis with minimal attention to appearance on imaging studies. Of the 3 case reports we found with images of skeletal metastases, all reported use of PET/CT for initial staging [8, 10–11]. These studies found that metastatic disease is hypermetabolic without further characterization of degree of FDG avidity on PET/CT. No separate CT images were displayed to characterize lesions as lytic, blastic, or mixed. These reports do not describe use of other imaging modalities, such as radiographs or MRI. As is typical of many other types of metastatic disease, the current case demonstrates its osseous metastases to show geographic T1 hypointensity, T2 hyperintensity, and avid enhancement on MRI. Metastatic lesions in this case were predominantly lytic on CT. As with other lytic metastasis, these could manifest on plain film when larger than approximately 1cm with greater than 50% bone destruction, and with an absent pedicle.
Pathologic vertebral body compression fracture may be a feature metastatic EMPD. While imaging features have a limited ability to differentiate between benign and malignant compression fractures, an associated epidural or paraspinal mass, extension of signal into the pedicles, a convex posterior vertebral body contour, and abnormal signal throughout the vertebral body can suggest a pathologic fracture. Alternatively, a gas or fluid-filled cleft is commonly present with benign osteoporotic fractures [19]. The radiologist may need to have a high index of suspicion for an underlying malignancy in patients with a vertebral compression fracture and underlying EMPD even if extra-osseous extension is absent such as in our case.
We demonstrate multimodality imaging of skeletal metastases of EMPD, including radiographs, CT, and MRI. Comparison of our case with other reported cases reveals a similar pattern of disease, with widely disseminated skeletal metastases throughout the axial skeleton and a paucity of appendicular skeletal involvement.
Treatment & Prognosis
EMPD is often treated with topical corticosteroids initially due to the nonspecific dermatologic appearance [2]. Intraepidermal confinement of disease has a good prognosis with surgical excision, but dermal invasion confers a worse prognosis, with greater association with metastasis [1]. EMPD may also be locally aggressive and can recur even with seemingly negative margins, although positive margins increase the likelihood of recurrence [1]. Disease recurrence at the primary site after resection and larger or multifocal primary disease also increase the risk of metastases.
Radiation therapy can be an effective adjunctive treatment for some cases of more extensive local disease not amenable to complete surgical resection [2]. Because distant metastasis of EMPD is rare, optimal chemotherapy agents and regimen have not been established. Case reports in the oncology literature describe multiple individual experiences with various chemotherapy regimens, but prognosis of distal metastasis is poor [8–9].
Slow progression of local disease with more rapid spread of metastases was a typical time course in prior reports [1–13]. However, there are no prior reports of pathologic vertebral body compression fracture. Our case also demonstrates that back pain in patients with a history of previously localized EMPD can be a sign of metastatic disease. Back pain in such patients should prompt imaging for diagnosis and potential biopsy.
Differential Diagnosis
The differential diagnosis for spinal metastases of EMPD primarily includes metastases of other primary cancers and myeloma. Given the paucity of relevant reports, we know of no uniquely characteristic feature of spinal metastases of EMPD to allow reliable discrimination from other spinal metastases. Identification of the primary cancer is of paramount importance. However, our case suggests some distinguishing features may be useful. For example, multiple myeloma may present with expansile or punched out lesions or a diffuse variegated MRI pattern. Blastic metastases may present with sclerotic lesions. Other metastases may be hypometabolic, hypoenhancing or, like melanoma, demonstrate T1 hyperintensity. These specific features were absent in our case and, if present, may prompt consideration of an underlying malignancy other than EMPD. While diffuse homogenous bone marrow replacement with decreased T1 signal was not present in the current case or other case reports, diffuse lytic metastasis could potentially manifest this pattern. However, many alternative causes for diffusely decreased T1 signal exist such as multiple myeloma, diffuse blastic metastasis, myelofibrosis, or anemia. Biopsy of spinal metastasis can confirm the association with the primary cancer, as in our case.
TEACHING POINT
Extramammary Paget disease, an uncommon intraepithelial cancer in older patients, has rarely been reported to metastasize to the spine. New osseous pain or lesions in patients with extramammary Paget disease should prompt consideration of metastatic disease, which has manifested as lytic osseous lesions on CT, hypermetabolic lesions on PET, and avidly enhancing, T1 hypointense and mildly T2 hyperintense lesions on MRI.
Table 1.
Summary table for spine metastases from extramammary Paget disease.
| Etiology | Primary intraepithelial neoplasm or pagetoid (intraepithelial) spread of underlying internal carcinoma; subsequent metastasis |
| Incidence | Rare; only a few other case reports |
| Gender ratio | Slightly greater incidence in men in published cases |
| Age predilection | Typically >60 years of age |
| Risk factors | Finding of dermal invasion at initial resection of local extramammary Paget disease increases risk of metastasis |
| Treatment | Palliative radiation and chemotherapy |
| Prognosis | Poor |
| Findings on imaging | Radiography and CT: Lytic osseous lesions +/− pathologic fractures PET: hypermetabolic spine lesions MRI: Patchy or geographic vertebral lesions that are T1 hypointense and T2 hyperintense to normal marrow; lesions avidly enhance; +/− pathologic fractures |
Table 2.
Differential diagnosis table for multiple vertebral lesions.
| Diagnosis | Radiograph | CT | PET | MRI |
|---|---|---|---|---|
| Spinal metastasis of extramammary Paget disease | Geographic lytic lesions, +/− compression fracture | Geographic lytic lesions with destruction of marrow, cortex, +/− compression fractures | Hypermetabolic | T1 hypointense, T2 hyperintense to normal marrow, geographic lesions with avid enhancement, +/− compression fracture |
| Multiple myeloma - spine | Diffuse osteopenia; multifocal, often purely lytic “punched out” lesions, may be expansile, +/− compression fractures | Multifocal lytic lesions with destruction of marrow and cortex, may be expansile, +/− compression fractures | Hypermetabolic in active disease | T1 hypointense, T2 hyperintense to normal marrow, enhancement; can have diffuse marrow involvement or variegated pattern |
| Other spinal metastases | Variable; lytic lesions; multiple sclerotic lesions if blastic metastases | Various findings specific to entity, such as sclerotic lesions of (blastic) metastatic prostate cancer | Variable; lytic metastases may be nonhypermetabolic; blastic metastases generally hypermetabolic, but treated blastic metastases can be nonhypermetabolic | Variable characteristics on T1, T2; some findings specific to entity, such as precontrast T1 hyperintensity of metastatic melanoma |
ABBREVIATIONS
- CT
computed tomography
- EMPD
extramammary Paget disease
- FDG
fluorodeoxyglucose
- MRI
magnetic resonance imaging
- PET/CT
positron emission tomography/computed tomography;
REFERENCES
- 1.Hatta N, Yamada M, Hirano T, Fujimoto A, Morita R. Extramammary Paget’s disease: Treatment, prognostic factors and outcome in 76 patients. Br J Dermatol. 2008;158:313–8. doi: 10.1111/j.1365-2133.2007.08314.x. [DOI] [PubMed] [Google Scholar]
- 2.Zollo JD, Zeitouni NC. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br J Dermatol. 2000;142(1):59–65. doi: 10.1046/j.1365-2133.2000.03242.x. [DOI] [PubMed] [Google Scholar]
- 3.Perrotto J, Abbott JJ, Ceilley RI, Ahmed I. The role of immunohistochemistry in discriminating primary from secondary extramammary Paget disease. Am J Dermatopathol. 2010;32(2):137–143. doi: 10.1097/DAD.0b013e3181b71481. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease. J Clin Pathol. 2000;53:742–749. doi: 10.1136/jcp.53.10.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusatake K, Harada Y, Mizumoto K, Keneko S, Nihara H, Chinuki Y, Morita E. Usefulness of sentinel lymph node biopsy for detection of metastasis in the early stage of extramammary Paget disease. Eur J Dermatol. 2015;25(2):156–61. doi: 10.1684/ejd.2015.2534. [DOI] [PubMed] [Google Scholar]
- 6.Fujisawa Y, Yoshino K, Kiyohara Y, Kadono T, Murata Y, Uhara H, et al. The role of sentinel lymph node biopsy in the management of invasive extramammary Paget’s disease: mu.lti-center retrospective study of 151 patients. J Dermatol Sci. 2015;79(1):38–42. doi: 10.1016/j.jdermsci.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Moretto P, Nair VJ, El Hallani S, et al. Management of penoscrotal extramammary Paget disease: case series and review of the literature. Curr Oncol. 2013;20(4):e311–320. doi: 10.3747/co.20.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vornicova O, Hershkovitz D, Yablonski-Peretz T, Ben-Itzhak O, Keidar Z, Bar-Sela G. Treatment of Metastatic Extramammary Paget’s Disease Associated With Adnexal Adenocarcinoma, With Anti-HER2 Drugs Based on Genomic Alteration ERBB2 S310F. The Oncologist. 2014;19:1006–7. doi: 10.1634/theoncologist.2014-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beleznay KM, Levesque MA, Gill S. Response to 5-fluorouracil in metastatic extramammary Paget disease of the scrotum presenting as pancytopenia and back pain. Curr Oncol. 2009;16(5):81–83. doi: 10.3747/co.v16i5.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy IS, Swain M, Gowrishankar S, Murthy DN. Primary extramammary Paget’s disease with extensive skeletal metastases. Indian J Dermatol Venereol Leprol. 2012;78:89–92. doi: 10.4103/0378-6323.90954. [DOI] [PubMed] [Google Scholar]
- 11.Cho SB, Yun M, Lee M, Chung KY. Variable patterns of positron emission tomography in the assessment of patients with extramammary Paget’s disease. J Am Acad Dermatol. 2005;52( 2):353–5. doi: 10.1016/j.jaad.2004.10.864. [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Gong K, Zhang X, Yang Y, Na Y. Extramammary Paget’s disease of scrotum—report of 25 cases and literature review. Urologic Oncology: Seminars and Original Investigations. 2010;28:28–33. doi: 10.1016/j.urolonc.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Cappuccini F, Tewari K, Rogers LW, DiSaia PJ. Extramammary Paget’s Disease of the Vulva: Metastases to the Bone Marrow in the Absence of an Underlying Adenocarcinoma--Case Report and Literature Review. Gynecol Onc. 1997;66:146–50. doi: 10.1006/gyno.1997.4735. [DOI] [PubMed] [Google Scholar]
- 14.Umebayashi Y. Distal phalangeal metastasis of extramammary Paget’s disease. J Dermatol. 2004 Jan;31(1):63–5. doi: 10.1111/j.1346-8138.2004.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 15.Piura B, Rabinovich A, Dhani R. Extramammary Paget’s disease of the vulva: report of five cases and review of the literature. Eur J Gynaecol Oncol. 1999;20(2):98–101. [PubMed] [Google Scholar]
- 16.Kariya K, Tsuji T, Schwartz RA. Trial of low-dose 5-fluorouracil/cisplatin therapy for advanced extramammary Paget’s disease. Dermatol Surg. 2004;30(2 pt 2):341–4. doi: 10.1111/j.1524-4725.2004.30093.x. [DOI] [PubMed] [Google Scholar]
- 17.Takahagi S, Noda H, Kamegashira A, et al. Metastatic extramammary Paget’s disease treated with paclitaxel and trastuzumab combination chemotherapy. J Dermatol. 2009;36(8):457–61. doi: 10.1111/j.1346-8138.2009.00676.x. [DOI] [PubMed] [Google Scholar]
- 18.Renaud-Vilmer C, Cavelier-Balloy B, Boazec L, Moquelet P, Bagot M. [Metastatic extramammary Paget’s disease of the scrotum]. Ann Dermatol Venereol. 2012;139(5):387–90. doi: 10.1016/j.annder.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Lane JL, Maus TP, Wald JT, Thielen KR, Bobra S, Luetmer PH. Intravertebral clefts opacified during vertebroplasty: pathogensis, technical implications, and prognostic significance. Am J Neuroradiol AJNR. 2002;23:1642–1646. [PMC free article] [PubMed] [Google Scholar]