Abstract
The aim of this study was to assess the expression of β-catenin, transcription factor-4 (TCF-4) and sclerostin in the subchondral bone of patients with primary knee osteoarthritis (OA). Tibial plateau specimens from patients with OA who underwent total knee arthroplasty were classified into the early stage (n=15), intermediate stage (n=13) and late stage (n=17) groups using the Mankin score. Structural parameters, including total articular cartilage (TAC), subchondral bone plate (SCP) thickness and trabecular bone volume (BV/TV), were assessed using Image-Pro Plus 6.0 analysis software. Subsequently, β-catenin and sclerostin expression levels in subchondral bone were determined by immunohistochemistry. In addition, the mRNA and protein levels of β-catenin, TCF-4 and sclerostin were evaluated by RT-qPCR and western blot analysis, respectively. As regards the cartilage and subchondral bone structural parameters, TAC was reduced, while SCP thickness and BV/TV were increased due to OA, with significant differences observed among the different stages (all P<0.05). The results of immunohistochemistry revealed that the β-catenin levels in the intermediate- and late-stage samples were significantly increased, while the levels of sclerostin were markedly decreased compared with the values in the early-stage samples (all P<0.05). Compared with the intermediate-stage samples, the sclerostin levels were decreased, and SCP thickness and the β-catenin levels were increased in the late-stage samples (all P<0.05). The results of RT-qPCR and western blot analysis revealed that the β-catenin and TCF-4 mRNA and protein levels in the intermediate- and late-stage samples were significantly increased, while sclerostin expression was significantly decreased compared with the early-stage samples; a similar trend was observed between the intermediate- and late-stage samples (all P<0.05). Finally, the β-catenin and TCF-4 levels positively correlated with the Mankin scores, while there was a negative correlation with sclerostin expression. Our findings demonstrate that sclerostin expression is closely associated with the degree of joint damage in patients with OA, confirming its involvement in the development of OA.
Keywords: β-catenin, osteoarthritis, sclerostin, subchondral bone, Wnt
Introduction
Osteoarthritis (OA), which affects weight-bearing joints and pathologically alters the cartilage and subchondral bone, is a chronic and degenerative disease that causes chronic joint pain and dysfunction (1). Pathologically, OA is characterized by the progressive degeneration of articular cartilage, the abnormal reconstruction of subchondral bone, and subchondral bone sclerosis. OA results from an imbalance between the synthesis and degradation of the cartilage and subchondral bone, secondary to effects by multiple factors. It was previously considered that cartilage degeneration is the disease-initiating factor. Following the progress made in basic research, subchondral bone is now believed to be closely associated with the onset and progression of OA (2), and has thus become a hotspot in the research of OA pathogenesis.
During the development of OA, subchondral bone is pathologically characterized by an abnormal increase in bone turnover. The Wnt/β-catenin signaling pathway is mainly responsible for cell proliferation, differentiation and apoptosis. In recent years, Wnt/β-catenin signaling has been shown to play an important role in maintaining the normal functions of osteocytes and osteoblasts, and regulates bone metabolism. In osteogenesis, Wnt/β-catenin inhibits the differentiation of mesenchymal stem cells into adipocytes, instead promoting their differentiation into osteoblasts. Indeed, Wnt/β-catenin induces osteoblast proliferation and maturation (3), while inhibiting apoptosis. In bone resorption, Wnt/β-catenin promotes osteoprotegerin (OPG) secretion by osteoblasts (4) and simultaneously inhibits receptor activator of nuclear factor-κB ligand (RANKL) secretion (5), which reduces the RANKL/OPG ratio and suppresses bone resorption. In addition, Wnt/β-catenin inhibits apoptosis and activates downstream target genes that regulate bone metabolism (3–5). However, the regulatory effects of the activated Wnt signaling pathway in osteocytes on other cells are not yet fully understood. The activation of Wnt/β-catenin signaling reduces bone resorption and increases osteogenesis.
Sclerostin the secreted protein product of the SOST gene, is a negative regulator of osteoblasts and may be exclusively osteocyte-derived (6). Sclerostin is an antagonist for Wnt signaling in Xenopus embryos and mammalian cells by binding to the extracellular domain of the Wnt coreceptors, LRP5 and LRP6, and disrupting Wnt-induced Frizzled-LRP complex formation. The loss of SOST function likely leads to the hyperactivation of Wnt signaling that underlies bone overgrowth observed in sclerosteosis patients (7–10). We hypothesized that sclerostin production by osteocytes may regulate the linear extent of formation and the induction or maintenance of a lining cell phenotype on bone surfaces. In doing so, sclerostin may act as a key inhibitory signal governing skeletal microarchitecture. Thus, sclerostin is considered an attractive therapeutic target for patients with bone metabolic diseases (11).
In this study, we aimed to assess the levels of β-catenin, transcription factor-4 (TCF-4) and sclerostin in samples from patients with OA of different disease stages, and to clarify the effect of sclerostin and Wnt/β-catenin signaling on OA.
Materials and methods
Sample collection
Fresh tibial plateau specimens were collected from patients with primary OA who underwent total knee arthroplasty at the Third Department of the General Hospital of Ningxia Medical University, Yinchuan, China between 2013 and 2014. A total of 45 medial or lateral tibial plateau samples was collected from 32 patients with OA, including 1 male and 31 females. The median age of the patients was 64.9 years, ranging from 51 to 74 years. OA was diagnosed according to the American College of Rheumatology Society diagnostic criteria (12), and patients with OA secondary to other diseases, such as trauma and connective tissue diseases, were excluded.
This study was approved by the Ethics Committee of the General Hospital of Ningxia Medical University (no. 2015-005), and informed consent was obtained from each patient prior to enrolment.
Macroscopic observations
Tibial plateau material was collected during surgery and immediately observed on a clean bench, for surface appearance and color, and for the presence of cartilage ulcers, cartilage fractures and osteophytes.
Sample processing
Bone and cartilage at the central load-bearing area of the medial or lateral tibial plateau samples were preserved and trimmed into blocks (2.0×2.0×1.0 cm). The bone and cartilage tissues were divided into 2 parts by coronal sectioning. One part, containing cartilage and subchondral bone, was fixed in 10% neutral formalin for 24 h, decalcified in 10% ethylenediaminetetraacetic acid (EDTA) at room temperature, paraffin-embedded, sliced at 4-µm-thick discontinuous sections, and submitted to Safranin O and Fast Green staining or immunohistochemistry. The cartilage was removed from the other part, and the subchondral bone was wrapped with foil paper and stored at −80°C for the detection of mRNA and protein levels.
Mankin scoring
As previously described (13), OA was classified into 3 stages as follows: early-stage (mild degeneration, scores 0–6; n=15), intermediate-stage (moderate degeneration, scores 7–9; n=13), or late-stage (severe degeneration, scores 10–14; n=17).
Structural evaluation of cartilage and subchondral bone
Three sections from each sample were used for Safranin O and Fast Green staining (Hebei Bohai Biotechnology Development Co., Ltd., Hebei, China), with 3 high power fields (magnification, ×4) randomly selected per section for analysis. The Image-Pro Plus 6.0 image analysis software (Media Cybernetics, Bethesda, MD, USA) was employed to assess the structural parameters of cartilage and subchondral bone with morphometrical methods. The assessed parameters included: i) total articular cartilage (TAC), namely the distance between the cartilage surface and bonding line; ii) subchondral bone plate (SCP) thickness, namely the distance from the bonding line to the interface between the subchondral bone plate and trabecular bone; and iii) trabecular bone volume (BV/TV), which was cacluated as follows: (trabecular bone area in the region of measurement)/(trabecular bone area + marrow cavity area) ×100.
Immunohistochemistry
The sections were incubated at 60°C overnight and deparaffinized. Antigen retrieval was performed with 0.1% trypsin for 20 min, and the sections were incubated with 3% H2O2 for 10 min, blocked with goat serum for 10 min, and treated at 37°C for 75 min with rabbit anti-human β-catenin (1:100; ab32572) or anti-human sclerostin (1:50; ab63097) (both from Abcam, Cambridge, MA, USA) monoclonal antibodies in phosphate-buffered saline containing Tween-20 (PBST). Following 3 washes with PBST (5 min), the sections were incubated with HRP-conjugated goat anti-rabbit IgG (1:2,000; ZB-2301; Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd., Beijing, China) at 37°C for 40 min. Visualization was carried out with 3,3′-diaminobenzidine (DAB), and hematoxylin used for counterstaining. Routine dehydration and mounting were performed. In the negative controls, the primary antibody was replaced with PBST. For histological scoring, 3 high power fields (magnification, ×20) were randomly selected from the subchondral bone plate and trabecular bone sections. This was performed independently by 2 experienced pathologists (Dr Anshan Han and Dr Haiping Tian; Ningxia Medical University). The numbers of β-catenin- or sclerostin-positive cells were counted, and positive-to-total cell ratios were considered to be the relative protein expression levels.
Reverse transcription-quantititave PCR (RT-qPCR)
Six samples from each group were randomly selected to assess the mRNA levels of target genes in the subchondral bone specimens. In brief, the subchondral bone samples were thawed, weighed and homogenized in liquid nitrogen. Subsequently, total RNA was extracted from the tissues using the RNA pure tissue kit (Beijing Kangwei Shiji Biotechnology, Co., Ltd., Beijing, China) according to the manufacturer's instructions. Total RNA amounts and purity were determined by UV spectrophotometry. Primers for β-catenin, TCF-4 and sclerostin were designed and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) (Table I). Total RNA was reverse transcribed into cDNA using the Super RT cDNA kit (Beijing Kangwei Shiji Biotechnology, Co., Ltd.), according to the manufacturer's instructions. Quantitative PCR (qPCR) reactions (25 µl) were composed of cDNA (0.5 µl), SYBR-GreenER qPCR SuperMix Universal (Beijing Kangwei Shiji Biotechnolo gy, Co., Ltd.) (12.5 µl), forward and reverse primers (0.5 µl each) and diethyl-pyrocarbonate-treated water (11 µl). PCR was carried out at 50°C (20 min), 95°C (10 min) and 40 cycles at 95°C (15 sec) and 60°C (60 sec). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used as an internal reference; the Ct value of GAPDH in early OA served as a control. Finally, the relative mRNA levels of target genes were assessed using the 2−ΔΔCt method.
Table I.
Sequences of primers used for RT-qPCR.
| Gene | Sequence (5′→3′) | Product size (bp) |
|---|---|---|
| β-catenin | TGGTGACAGGGAAGACATCA | 20 |
| CCATAGTGAAGGCGAACTGC | 20 | |
| TCF-4 | CTCCGATGTCCACTTTCCAT | 20 |
| CGCTTCCTCTATTTGCCATT | 20 | |
| Sclerostin | TTCTCCTTCGGGACCTCAAT | 20 |
| TCTCTCACCTCTGCCCATT | 19 | |
| GAPDH | ACAACTTTGGTATCGTGGAAGG | 22 |
| GCCATCACGCCACAGTTTC | 19 |
TCF-4, transcription factor-4; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Western blot analysis
Five samples from each group were examined to determine protein levels by western blot analysis. In brief, the subchondral bone specimens were thawed, washed with pre-chilled distilled water (to remove bone marrow cavity contents), weighed and homogenized in liquid nitrogen. Total protein was extracted using the total protein sample kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's instructions, and the protein concentration was determined using the bicinchoninic acid (BCA) method. Subsequently, proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto a polyvinylidene fluoride membrane at 300 mA for 90 min. After 3 washes in TBST (5 min each time), the membrane was blocked in Tris-buffered saline-Tween-20 (TBST) containing 5% non-fat milk for 1 h, and treated with rabbit anti-human monoclonal antibodies raised against β-catenin (1:500), TCF-4 (1:1,000; ab185736), sclerostin (1:300), or β-actin (1:2,000; ab8227) (all from Abcam) in TBST at 4°C overnight. After 3 washes in TBST, the membrane was incubated with HRP-conjugated goat anti-rabbit IgG (1:2,000; Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd.) at room temperature for 1 h. Visualization was carried out by electrochemiluminescence, with the protein bands revealed on an ALS4000 gel image analysis system (GE Healthcare Life Sciences, Logan, UT, USA). Quantity One software (Bio-Rad Laboratories, Inc.) was employed to assess the protein bands, with target protein expression normalized to β-actin levels.
Statistical analysis
Statistical analysis was performed with SPSS version 20.0 software (IBM, Chicago, IL, USA). Data are the means ± standard deviation (SD), and were compared by one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls (SNK) test. Pearson's correlation analysis was employed to evaluate the associations of the protein and gene expression levels with the Mankin scores. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Macroscopic observations
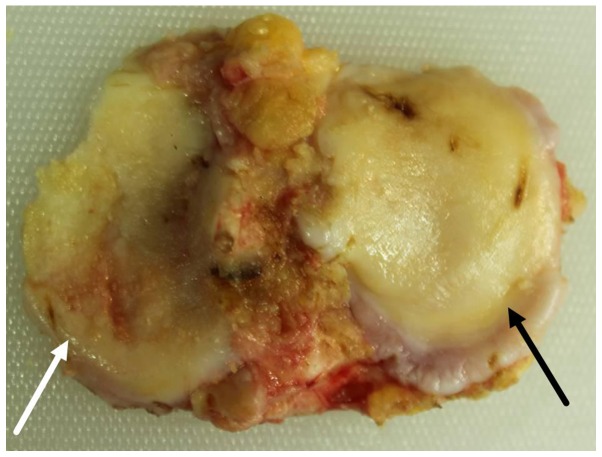
The medial and lateral tibial plateaus exhibited distinct degrees of degeneration. Patients with varus and valgus deformities, presented with severe degeneration of the medial and lateral tibial plateau, respectively. The tibial plateau samples with severe degeneration exhibited a coarse surface, giant fractures, extensive malacia, an altered cartilage thickness (in severe cases), ivory-like subchondral bone and multiple osteophytes at the edges. The tibial plateau samples with mild degeneration were relatively plain, but exhibited superficial ulcers, malacia, superficial fractures and a few osteophytes at the edges (Fig. 1).
Figure 1.
Macroscopic observations. Specimen with osteoarthritis (OA) and genu varum tibial plateau. White arrow indicates severe cartilage degeneration, while the black arrow indicates mild cartilage degeneration.
Safranin O/Fast Green staining
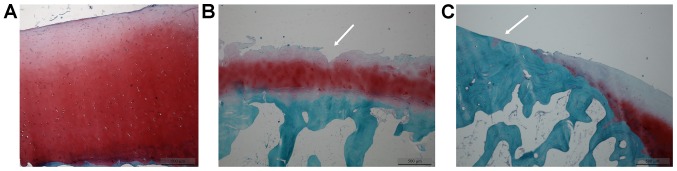
In the early-stage samples, the cartilage layer did not exhibit any fractures; the chondrocytes had a normal size and morphology, with the cartilage matrix evenly stained (Fig. 2A). In the intermediate-stage samples, vertical fractures were observed deep in the cartilage layer (Fig. 2B, white arrow), with an abnormal chondrocyte morphology and arrangement; in addition the proliferation and clustering of chondrocytes was evident, with the cartilage matrix lightly stained (Fig. 2B). In the late-stage samples, ivory-like subchondral bone (Fig. 2C, white arrow) was observed; the chondrocytes had an abnormal morphology and had becom swollen, and were reduced in number; the cartilage matrix was not stained (Fig. 2C). Safranin O/Fast green staining grading scores were calculated according to the Mankin scoring system (Table II).
Figure 2.
Tibial plateau degeneration (Safranin O/Fast Green staining; magnification, ×4). Arrows indicate positively stained cells. (A) Early-stage sample; (B) intermediate-stage sample; (C) late-stage sample.
Table II.
Structural parameters of cartilage and subchondral bone at different OA stages (mean ± SD).
| Groups | Mankin | TAC (µm) | SCP (µm) | BV/TV (%) |
|---|---|---|---|---|
| Early stage (n=15) | 3.3±1.2 | 2343.5±434.8 | 218.4±56.3 | 21.9±6.4 |
| Intermediate stage (n=13) | 7.7±0.9 | 1158.2±477.8a | 433.4±208.9a | 35.3±12.2a |
| Late stage (n=17) | 13.0±1.2 | 154.4±40.5a,b | 1223.2±291.4a,b | 49.7±5.0a,b |
P<0.05 vs. early stage;
P<0.05 vs. intermediate stage. OA, osteoarthritis; TAC, total articular cartilage; SCP, subchondral bone plate; BV/TV, trabecular bone volume.
Cartilage and subchondral bone structural parameters
The TAC levels were reduced, while SCP thickness and the BV/TV were increased with the increasing severity of OA. Compared with the values obtained from the early-stage samples, the TAC levels were decreased and SCP thickness and the BV/TV were increased in the intermediate- and late-stage samples, exhibiting statistically significant differences (P<0.05). Compared with the values obtained from the intermediate-stage samples, the TAC levels were decreased, and SCP thickness and the BV/TV were increased in the late-stage samples, with statistically significant differences (P<0.05) (Table II).
β-catenin and sclerostin levels detected by immunohistochemistry
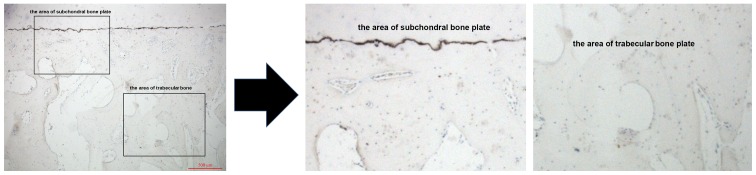
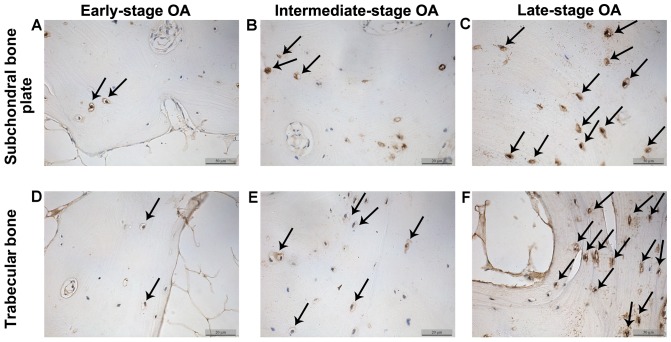
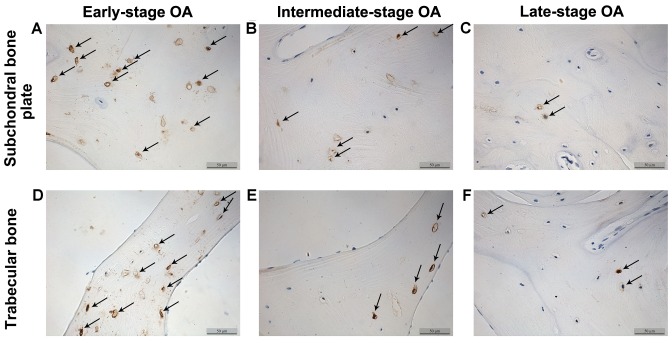
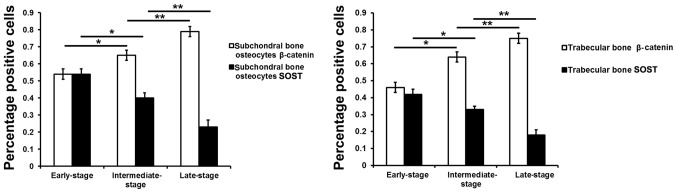
The β-catenin and sclerostin levels were observed in the subchondral bone plate and trabecular bone based on the corresponding positions (Fig. 3). β-catenin was expressed mainly in the cytoplasm and nucleus of the osteocytes, while sclerostin was found mainly in osteocytes and in the surrounding lacunae (Figs. 4 and 5). In the early-stage samples, weak β-catenin signals were observed in the bone cells, only in the subchondral bone plate (Fig. 4A and D). Strong positive sclerostin signals were observed in the bone cells, in the subchondral bone plate and trabecular bone (Fig. 5A and D). In the intermediate-stage samples, β-catenin exhibited a higher expression compared with the early-stage samples; the subchondral bone plate and trabecular bone exhibited partial expression (Fig. 4B and E). Sclerostin expression was weaker, with little expression in the subchondral bone plate and trabecular bone (Fig. 5B and E). In the late-stage samples, β-catenin exhibited strong signals in bone cells, both in the subchondral bone plate and trabecular bone (Fig. 4C and F). Sclerostin exhibited a low expression in bone cells, only in the subchondral bone plate (Fig. 5C and F). Fig. 6 represents the results of the quantification of the immunohistochemistry data.
Figure 3.
Immunohistochemistry of subchondral bone plate and trabecular bone.
Figure 4.
β-catenin expression in subchondral bone osteocytes and trabecular bone (magnification, ×200). Arrows indicate positive cells. OA, osteoarthritis.
Figure 5.
Sclerostin expression in subchondral bone osteocytes and trabecular bone (magnification, ×200). Arrows indicate positive cells. OA, osteoarthritis
Figure 6.
Ratios of β-catenin- and sclerostin-positive osteocytes in different stages of osteoarthritis. *P<0.05, intermediate-stage group vs. early-stage group; **P<0.05, intermediate-stage group vs. late-stage group. SOST, sclerostin.
Correlation of β-catenin and sclerostin levels in osteocytes with Mankin scores
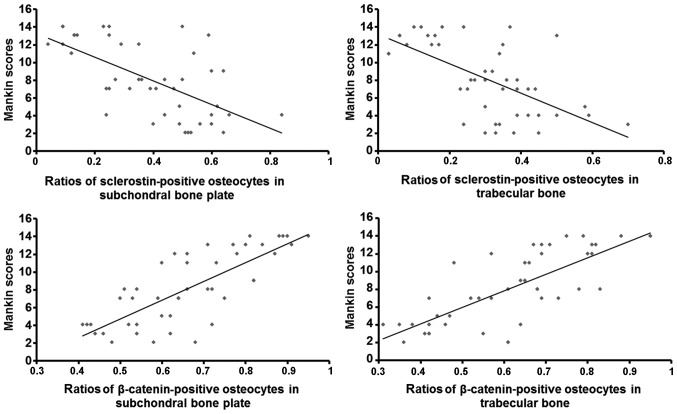
Pearson's correlation analysis revealed that the ratios of β-catenin-positive osteocytes in the subchondral bone plate (r=0.775; P<0.01) and trabecular bone (r=0.769; P<0.01) positively correlated with the Mankin scores, suggesting that β-catenin protein expression in subchondral bone was positively associated with the severity of degeneration (Fig. 7, bottom panels). Furthermore, the ratios of sclerostin-positive osteocytes in the subchondral bone plate (r=0.632; P<0.01) and trabecular bone (r=0.620; P<0.01) negatively correlated with the Mankin scores, indicating that sclerostin expression in subchondral bone was negatively associated with the severity of degeneration (Fig. 7, top panels).
Figure 7.
Linear regression analysis of β-catenin and sclerostin levels vs. Mankin scores. *P<0.05, early-stage group vs. intermediate-stage group; **P<0.05, intermediate-stage group vs. late-stage group.
β-catenin, TCF-4 and sclerostin mRNA levels in subchondral bone
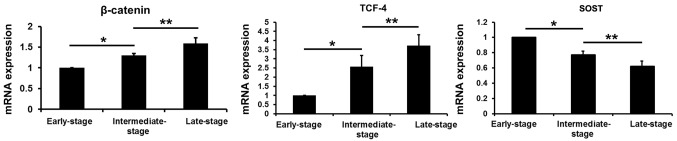
Compared with the values obtained from the early-stage samples, the mRNA levels of β-catenin and TCF-4 were increased in the intermediate- and late-stage samples (P<0.05), while the sclerostin levels were decreased (P<0.05). Compared with the values obtained from the intermediate-stage samples, the β-catenin and TCF-4 mRNA levels were increased in the late-stage samples (P<0.05), and the sclerostin levels were decreased (P<0.05) (Fig. 8).
Figure 8.
mRNA levels of β-catenin, transcription factor-4 (TCF-4) and SOST in subchondral bone at different stages of osteoarthritis (OA). SOST, sclerostin.*P<0.05, early-stage group vs. intermediate-stage group; **P<0.05, intermediate-stage group: late-stage group.
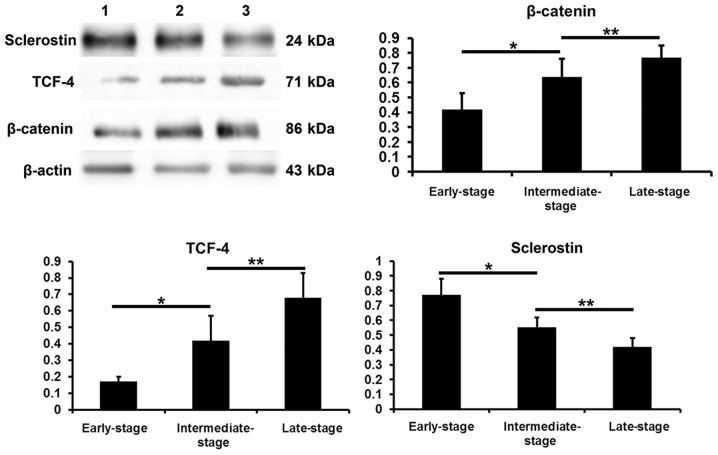
β-catenin, TCF-4 and sclerostin protein levels in subchondral bone determined by western blot analysis
Compared with the values obtained from the early-stage samples, the β-catenin and TCF-4 protein levels were increased in the intermediate- and late-stages samples (P<0.05), while the sclerostin levels were decreased (P<0.05). Compared with the intermediate-stage samples, the β-catenin and TCF-4 protein levels were increased in the late-stage samples (P<0.05), while the sclerostin levels were decreased (P<0.05) (Fig. 9).
Figure 9.
β-catenin, transcription factor-4 (TCF-4) and sclerostin protein levels in subchondral bone samples of different groups. *P<0.05, early-stage group vs. intermediate-stage group; **P<0.05, intermediate-stage group: late-stage group.
Discussion
During the development of OA, the subchondral bone is pathologically characterized by an abnormally increased bone turnover. Bone resorption overwhelms osteogenesis, resulting in transient osteoporosis in early-stage OA. With disease progression, compensatory bone formation is observed, with bone formation greater than resorption, finally resulting in increased bone mass and bone sclerosis. The abnormally high bone turnover yields new bones with incomplete mineralization and the loss of cartilage protection; this results in cartilage degeneration, accelerating the process of OA (1). Of note, matrix metalloproteinases may enter the deep cartilage through new blood vessels and articular calcified cartilage, resulting in the abnormal metabolism of cartilage cells, and inducing cartilage degeneration and matrix degradation (2,13).
Osteogenesis increases as a compensation and overwhelms bone resorption, resulting in increased bone mass and bone sclerosis in intermediate- and late-stage OA (14–17). The OPG/RANKL/RANK system is an important downstream signaling pathway that regulates the balance between bone resorption and osteogenesis. In early-stage OA, the OPG/RANKL ratio decreases, and OA is characterized by increased bone resorption and transient osteoporosis. The progression of OA can increase the OPG/RANKL ratio, characterized by enhanced osteogenesis and bone sclerosis. OPG and RANKL are secreted mainly by bone osteocytes and osteoblasts, and may both constitute important factors in determining osteoclast generation and activity (18), as well as subchondral bone turnover. Thus, the regulation of osteocyte and osteoblast differentiation, proliferation and apoptosis is considered a key target in therapeutic attempts to modulate bone metabolism.
The Wnt/β-catenin signaling is mainly involved in cell proliferation, differentiation and apoptosis. In recent years, studies have demonstrated that Wnt/β-catenin signaling plays an important role in maintaining normal osteocyte and osteoblast function, and bone metabolism. The following findings have been demonstrated: i) as regards bone formation, Wnt/β-catenin signaling restrains mesenchymal stem cells from differentiating into adipocytes, and promotes osteoblast differentiation, proliferation and maturation (3); in addition, Wnt/β-catenin signaling inhibits osteoblast apoptosis (4). ii) As regards bone absorption, Wnt/β-catenin signaling promotes OPG secretion and inhibits RANKL expression in osteoblasts (5), thus reducing the RANKL/OPG ratio and inhibiting bone resorption. iii) Wnt/β-catenin signaling inhibits bone cell apoptosis (19), activating downstream target genes, and regulating bone metabolism; however, the regulatory mechanisms of action of the Wnt/β-catenin pathway signaling require further, more in-depth investigations. Thus, the activation of Wnt/β-catenin signaling reduces bone resorption and increases bone formation. β-catenin is a classic hub molecule in the Wnt/β-catenin signaling pathway. It is distributed in the cell membrane, cytoplasm and nucleus (19), and its expression is associated with the activation of the Wnt/β-catenin signaling pathway. TCF-4, a transcription factor, and serves as an active bridge connecting upstream and downstream effectors; it has been shown to promote cell apoptosis (20). Sclerostin is specifically secreted by osteocytes and may negatively regulate bone mass (7,8,21,22). Generally, after osteocytes are embedded in the bone matrix, they begin to secrete sclerostin, which then affects osteocytes in an autocrine manner or osteoblasts via a paracrine mechanism (23). Sclerostin binds to the co-receptor, LRP5/6, on the cell membrane to inhibit the Wnt/β-catenin signaling pathway (9,10). There is evidence to indicate that sclerostin, as a mechanics sensitive protein, may bridge the mechanical sensation and osteogenesis (24–26).
In the present study, fresh medial or lateral tibial plateau samples were collected from patients with primary OA during total knee arthroplasty and scored according to the Mankin scoring system. Subsequently, the structural parameters of the cartilage and subchondral bone were measured. Of note, the TAC levels decreased, while SCP thickness and the BV/TV increased with the progression of OA, corroborating previous studies (16). These findings suggest abnormal subchondral bone reconstruction, excessive osteogenesis, and increased bone mass and sclerosis during OA progression, as reported in previous studies (27–29). In addition, immunohistochemistry revealed more β-catenin-positive and less sclerostin-positive cells with the increasing Mankin scores. As shown by western blot analysis and RT-qPCR, the β-catenin and TCF-4 levels increased, while the sclerostin levels decreased with the increasing Mankin scores, both at the gene and protein levels.
Our findings demonstrated that the expression levels of key Wnt/β-catenin signaling components (specifically, β-catenin and TCF-4) increased with the increasing OA pathological stage, and were positively associated with the severity of degeneration. However, the levels of sclerostin, an antagonist of the Wnt/β-catenin signaling pathway, were reduced with the increasing pathological stage of OA, and negatively correlated with the severity of degeneration.
Thus, we hypothesized that during the progression of OA, sclerostin expression is reduced as a result of multiple factors that activate the Wnt/β-catenin signaling pathway, thus increasing osteogenesis and reducing bone resorption. This cascade of signaling events may result in osteogenesis overwhelming bone resorption, a condition characterized by increased bone mass and sclerosis, which promotes the progression of OA.
Wnt signaling is considered a therapeutic target for diseases characterized by abnormal bone density. However, the activation of Wnt signaling is also closely related to tumorigenesis. Thus, the targeted regulation of the Wnt signaling pathway in bone tissues is key in modulating bone reconstruction (30). In adults, sclerostin is specifically expressed in bone tissues, and systemic modulation may affect Wnt/β-catenin signaling in bone tissues, but apparently has no impact on other tissues (7,8,21).
A sclerostin monoclonal antibody has been used in the treatment of post-menopausal osteoporosis and other diseases in animal experiments and clinical trials, with favorable results (31). Although therapy targeting sclerostin for the treatment of bone density-related diseases (such as post-menopausal osteoporosis) has achieved effective results, whether this strategy would play an important role in OA prevention and therapy remains unclear. If safe and effective, therapy targeting sclerostin may significantly aid in the prevention and treatment of OA, thereby yielding health, social and economic benefits. Our results revealed a reduced sclerostin expression in the subchondral bone and the activation of the Wnt/β-catenin signaling pathway during the progression of OA.
Based on these findings, the inhibition of osteogenesis may serve as a therapeutic strategy for the treatment of OA. In future studies, the systemic or intra-articular administration of recombinant sclerostin may be employed to increase sclerostin expression in the subchondral bone of patients with OA. Other studies are also required to address abnormal osteogenesis, subchondral bone and OA progression to assess the efficacy and safety of such therapies.
References
- 1.Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res Ther. 2009;11:203. doi: 10.1186/ar2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Neogi T. Osteoarthritis: is it a disease of cartilage or of bone? Arthritis Rheum. 2004;50:341–344. doi: 10.1002/art.20051. [DOI] [PubMed] [Google Scholar]
- 3.Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J. Loss of wnt/β-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res. 2012;27:2344–2358. doi: 10.1002/jbmr.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen B, Li XD, Liu DX, Wang H, Xie P, Liu ZY, Hou GQ, Chang B, Du SX. Canonical Wnt signaling is required for Panax notoginseng saponin-mediated attenuation of the RANKL/OPG ratio in bone marrow stromal cells during osteogenic differentiation. Phytomedicine. 2012;19:1029–1034. doi: 10.1016/j.phymed.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 6.Moester MJ, Papapoulos SE, Löwik CW, van Bezooijen RL. Sclerostin: current knowledge and future perspectives. Calcif Tissue Int. 2010;87:99–107. doi: 10.1007/s00223-010-9372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 10.Semënov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 11.Altman RD. Criteria for classification of clinical osteoarthritis. J Rheumatol Suppl. 1991;27:10–12. [PubMed] [Google Scholar]
- 12.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Bobinac D, Spanjol J, Zoricic S, Maric I. Changes in articular cartilage and subchondral bone histomorphometry in osteoarthritic knee joints in humans. Bone. 2003;32:284–290. doi: 10.1016/S8756-3282(02)00982-1. [DOI] [PubMed] [Google Scholar]
- 14.Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthro sis. Osteoarthritis Cartilage. 2004;12(Suppl A):S20–30. doi: 10.1016/j.joca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Prasadam I, van Gennip S, Friis T, Shi W, Crawford R, Xiao Y. ERK-1/2 and p38 in the regulation of hypertrophic changes of normal articular cartilage chondrocytes induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum. 2010;62:1349–1360. doi: 10.1002/art.27397. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by interleukin-6, -1beta and oncostatin M pre-treated non-sclerotic osteoblasts. Osteoarthritis Cartilage. 2005;13:979–987. doi: 10.1016/j.joca.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Karsdal MA, Leeming DJ, Dam EB, Henriksen K, Alexandersen P, Pastoureau P, Altman RD, Christiansen C. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthritis Cartilage. 2008;16:638–646. doi: 10.1016/j.joca.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Trouvin AP, Goëb V. Receptor activator of nuclear factor-κB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin Interv Aging. 2010;5:345–354. doi: 10.2147/CIA.S10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodine PV. Wnt signaling control of bone cell apoptosis. Cell Res. 2008;18:248–253. doi: 10.1038/cr.2008.13. [DOI] [PubMed] [Google Scholar]
- 20.Ma B, Zhong L, van Blitterswijk CA, Post JN, Karperien M. T cell factor 4 is a pro-catabolic and apoptotic factor in human articular chondrocytes by potentiating nuclear factor κB signaling. J Biol Chem. 2013;288:17552–17558. doi: 10.1074/jbc.M113.453985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Bezooijen RL, Bronckers AL, Gortzak RA, Hogendoorn PC, van der Wee-Pals L, Balemans W, Oostenbroek HJ, Van Hul W, Hamersma H, Dikkers FG, et al. Sclerostin in mineralized matrices and van Buchem disease. J Dent Res. 2009;88:569–574. doi: 10.1177/0022034509338340. [DOI] [PubMed] [Google Scholar]
- 23.Krause C, Korchynskyi O, de Rooij K, Weidauer SE, de Gorter DJ, van Bezooijen RL, Hatsell S, Economides AN, Mueller TD, Löwik CW, ten Dijke P. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem. 2010;285:41614–41626. doi: 10.1074/jbc.M110.153890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 25.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 26.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burr DB. The importance of subchondral bone in the progression of osteoarthritis. J Rheumatol Suppl. 2004;70:77–80. [PubMed] [Google Scholar]
- 28.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 29.Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18:419–433. doi: 10.1007/s00167-010-1054-z. [DOI] [PubMed] [Google Scholar]
- 30.Funck-Brentano T, Bouaziz W, Marty C, Geoffroy V, Hay E, Cohen-Solal M. Dkk-1-mediated inhibition of Wnt signaling in bone ameliorates osteoarthritis in mice. Arthritis Rheumatol. 2014;66:3028–3039. doi: 10.1002/art.38799. [DOI] [PubMed] [Google Scholar]
- 31.Ominsky M, Warmington K, Asuncion F, Tan H, Grisanti M, Geng Z, Stephens T, Henry A, Lawson A, Lightwood D, et al. Sclerostin monoclonal antibody treatment increases bone strength in aged osteopenic ovariectomized rats. J Bone Miner Res. 2006;21(Suppl 1):S44. [Google Scholar]