Abstract
Background:
Elbow arthroplasty is the treatment of choice for end-stage rheumatoid arthritis (RA). The purpose of this study was to determine the long-term outcome of a linked semiconstrained elbow arthroplasty implant design in patients with RA.
Methods:
Between 1982 and 2006, 461 primary total elbow arthroplasties using the Coonrad-Morrey prosthesis were performed in 387 patients with RA. Fifty-five of the arthroplasties were performed to treat concurrent traumatic or posttraumatic conditions. There were 305 women (365 elbows, 79%) and 82 men (96 elbows, 21%). Ten patients (10 elbows) were lost to follow-up, 9 patients (10 elbows) died, and 6 patients (6 elbows) underwent revision surgery within the first 2 years. For the 435 elbows (362 patients, 94%) with a minimum of 2 years of follow-up, the median follow-up was 10 years (range, 2 to 30 years).
Results:
At the most recent follow-up, 49 (11%) of the elbows had undergone component revision or removal (deep infection, 10 elbows; and mechanical failure, 39 elbows). Eight additional elbows were considered to have radiographic evidence of loosening. For surviving implants followed for a minimum of 2 years, the median Mayo Elbow Performance Score (MEPS) was 90 points. Bushing wear was identified in 71 (23%) of the surviving elbows with a minimum of 2 years of radiographic follow-up; however, only 2% of the elbows had been revised for isolated bushing wear. The rate of survivorship free of implant revision or removal for any reason was 92% (95% confidence interval [CI] = 88% to 94%) at 10 years, 83% (95% CI = 77% to 88%) at 15 years, and 68% (95% CI = 56% to 78%) at 20 years. The survivorship at 20 years was 88% (95% CI = 83% to 92%) with revision due to aseptic loosening as the end point and 89% (95% CI = 77% to 95%) with isolated bushing exchange as the end point. Risk factors for implant revision for any cause included male sex, a history of concomitant traumatic pathology, and implantation of an ulnar component with a polymethylmethacrylate surface finish.
Conclusions:
Elbow arthroplasty using a cemented linked semiconstrained elbow arthroplasty provides satisfactory clinical results in the treatment of RA with a reasonable rate of survivorship free of mechanical failure at 20 years. Although bushing wear was identified on radiographs in approximately one-fourth of the patients, revision for isolated bushing wear was uncommon.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Rheumatoid arthritis (RA) is a chronic inflammatory disease of unknown etiology marked by a symmetric, peripheral polyarthritis. It is the most common form of chronic inflammatory arthritis (0.5% to 1% of the adult population worldwide)1 and often results in joint damage and physical disability, in addition to a variety of extra-articular manifestations. Involvement of the elbow joint is reported in 20% to 65% of patients with RA1. Elbow arthroplasty is now accepted as the treatment of choice for patients with end-stage rheumatoid elbow involvement2,3. Various authors have reported on the short, mid, and long-term outcomes of elbow arthroplasty for RA using various implant designs4-11.
At our institution, for a number of years, the majority of elbow arthroplasties for RA were performed using the same type of linked semiconstrained elbow implant. Some features of the implant, such as the surface finish and the materials used for the bearing surfaces, changed over time, but other design features remained similar. In a previous study, Gill and Morrey reported a 92% 10-year survival rate in a cohort of 78 elbow arthroplasties performed for RA using the Coonrad-Morrey implant (Zimmer), 46 of which had at least 10 years of follow-up11. Few additional studies have reported on the long-term outcome of this elbow arthroplasty implant.
In this study, we aimed to report on the long-term outcome of total elbow arthroplasty performed with use of the Coonrad-Morrey implant at a single institution in patients with RA.
Materials and Methods
Patients
Between 1982 and 2006, 461 primary total elbow arthroplasties with use of the Coonrad-Morrey prosthesis were performed at our institution in 387 patients with RA. The diagnosis of RA was confirmed through a comprehensive review of the medical record. Of the 461 arthroplasties, 55 were performed to treat concurrent acute traumatic or posttraumatic conditions, including superimposed posttraumatic arthritis (10 elbows), distal humeral fracture or nonunion (40 elbows), olecranon nonunion (4 elbows), or a combined distal humeral/olecranon nonunion (1 elbow). Table I summarizes the number of arthroplasties performed per year that were included in the study.
TABLE I.
The Included Elbow Arthroplasties Per Year
| Year | No. of Arthroplasties |
| 1982 | 8 |
| 1983 | 12 |
| 1984 | 16 |
| 1985 | 7 |
| 1986 | 19 |
| 1987 | 14 |
| 1988 | 11 |
| 1989 | 23 |
| 1990 | 31 |
| 1991 | 23 |
| 1992 | 28 |
| 1993 | 27 |
| 1994 | 23 |
| 1995 | 25 |
| 1996 | 22 |
| 1997 | 28 |
| 1998 | 16 |
| 1999 | 18 |
| 2000 | 23 |
| 2001 | 10 |
| 2002 | 16 |
| 2003 | 14 |
| 2004 | 18 |
| 2005 | 19 |
| 2006 | 10 |
There were 305 women (365 elbows, 79%) and 82 men (96 elbows, 21%) with a mean age (and standard deviation) at the time of the index arthroplasty of 64 ± 11 years and a mean body mass index (BMI) of 25 ± 6 kg/m2 (n = 456). Only 67 (15%) of the arthroplasties were performed in patients with a BMI of ≥30 kg/m2. Seventy-four patients had both elbows replaced; of these, 15 patients (30 elbows) had simultaneous bilateral elbow arthroplasty.
Implants and Procedures
All patients received a Coonrad-Morrey linked semiconstrained elbow arthroplasty prosthesis (Zimmer). The technique for implantation of this prosthesis has been described previously11. Antibiotic-loaded polymethylmethacrylate (PMMA) was used in all but 4 uncemented replacements. The length of the humeral component was 4 inches (10.2 cm) in 304 elbows, 6 inches (15.2 cm) in 153 elbows, and 8 inches (20.3 cm) in 4 elbows. There were no custom humeral components; 9 procedures were performed using a custom ulnar component.
The surface finish of the ulnar component of this implant system was modified over time12. Thirty-six ulnar components had an early (first-generation) plasma spray coating, 157 had a beaded porous coating, 140 were precoated with PMMA, and 128 had a modern plasma spray coating. The mechanism used for component linking was modified in 1999; the first-generation mechanism included a titanium pin and a locking ring, whereas the more modern mechanism included 2 cobalt-chromium interlocking pins.
Evaluation
The patients were followed prospectively using a joint registry database for data management. This database includes preoperative clinical information as well as follow-up clinical information recorded at regular intervals after the index procedure (2 years, 5 years, 10 years, and every 5 years thereafter). At each follow-up interval, patients are queried regarding pain, motion, stability, activities of daily living, complications, and reoperations. The information may be collected during a follow-up appointment or through the use of a mailed or telephone questionnaire. Radiographs are also obtained at the same time points.
For the purpose of this study, a researcher independent of the treating surgeons retrospectively collected information from the database and reviewed all patient charts to record pain, motion, scores as assessed with the Mayo Elbow Performance Score (MEPS), complications, and reoperations. Radiographs obtained prior to the index procedure, at 3 months postoperatively, and at the most recent follow-up were reviewed to determine implant fixation and polyethylene wear. Of note, wear was not routinely assessed with fluoroscopically positioned radiographs or stress fluoroscopy.
The duration of clinical follow-up was calculated from the time of the index arthroplasty until either the most recent patient contact or reoperation involving exchange or removal of any component (implant revision or removal). Of the 461 consecutive arthroplasties performed in the study period, 10 (2%) of the elbows (10 patients) were lost to follow-up, 10 were in patients who died within the first 2 years after surgery (9 patients, 2 patients in the first year and 7 in the second year), and 6 elbows (6 patients) underwent revision surgery within the first 2 years. The remaining 435 elbows were followed for a minimum of 2 years. The median clinical follow-up time for all 461 elbows was 9 years (range, 0 to 30 years). For the 435 elbows (362 patients) with a minimum follow-up of 2 years (94% of the initial cohort of elbows), the median follow-up was 10 years (range, 2 to 30 years).
Statistical Analysis
Continuous variables are shown as the mean and standard deviation, the mean with the range, the median with the range, or the median with the interquartile range (IQR). Categorical variables are expressed as the number with the proportion. A Kaplan-Meier analysis was utilized to determine the implant survivorship rates and pointwise 95% confidence intervals (CIs) for the whole prosthesis and the separate ulnar, humeral, and bushing components, with the end points defined as revision or resection for any reason, revision or resection for mechanical failure, revision or resection for aseptic component loosening, revision for isolated bushing exchange, and revision or resection for deep infection13. The category of revision for mechanical failure includes component aseptic loosening, periprosthetic bone fracture in the presence of aseptic loosening, component fracture, and/or bushing wear.
A multivariate Cox hazard model was created to determine the risks of revision for any reason and included sex, age, BMI (nonobese patients with a BMI of <30 kg/m2 and obese patients with a BMI of ≥30 kg/m2), prior elbow trauma, prior elbow surgery, and year of replacement surgery as variables. A univariate Cox model was also created evaluating the effect of the ulnar surface finish type on the risks of ulnar component revision for mechanical failure. All statistical tests were 2-sided, and the level of significance was set at p < 0.05.
Results
Revision Surgery and Component Removal
At the most recent follow-up, 49 (11%) of the elbows had undergone component revision or implant removal (Table II). Deep infection was treated with implant removal or revision in 10 elbows at a median follow-up of 4 years (range, 0.1 to 20 years). The remaining 39 elbows were revised because of mechanical failure at a median follow-up of 7 years (range, 0.9 to 21 years). Ulnar component failure was the main reason for revision in 24 elbows. Humeral component failure was the main reason for revision in 5 elbows. One elbow underwent revision for combined humeral and ulnar loosening. The remaining 9 (2%) of the elbows underwent bushing exchange for wear, with retention of the ulnar and humeral components. Of the 6 elbows revised during the first 2 years after implantation, 3 underwent prosthesis removal because of infection and 3 underwent revision for aseptic loosening.
TABLE II.
Surgical Revision Procedures with Prosthesis Exchange or Removal in Patients Who Underwent Primary Total Elbow Arthroplasty for RA
| Description | No. of Elbows |
| Mechanical failure | |
| Bushing exchange | 9 |
| Ulnar component | |
| Aseptic loosening | 8 |
| Aseptic loosening and component fracture | 3 |
| Aseptic loosening and periprosthetic fracture | 8* |
| Aseptic loosening, component fracture, and periprosthetic fracture | 4* |
| Component fracture | 1 |
| Humeral component | |
| Aseptic loosening | 3† |
| Aseptic loosening and periprosthetic fracture | 2† |
| Humeral and ulnar components | |
| Humeral and ulnar aseptic loosening and humeral component fracture | 1 |
| Deep infection | |
| Humeral and ulnar components | 9 |
| Humeral component | 1 |
Two total elbow arthroplasty revisions required bone-graft struts for construct augmentation.
One total elbow arthroplasty revision required a bone-graft strut for construct augmentation.
Survivorship Analysis
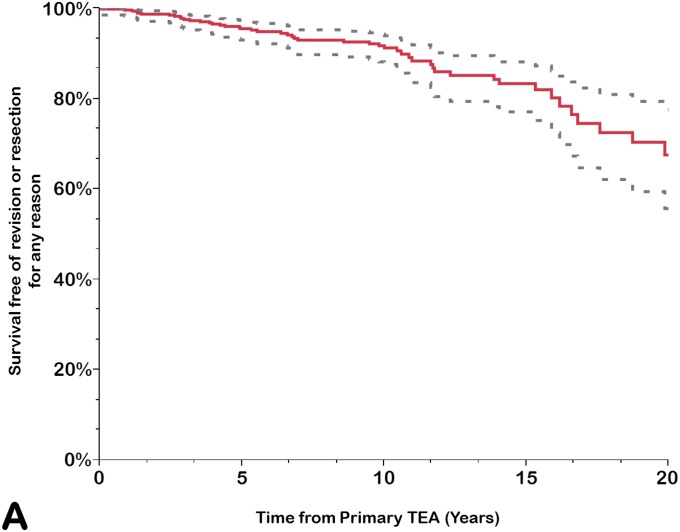
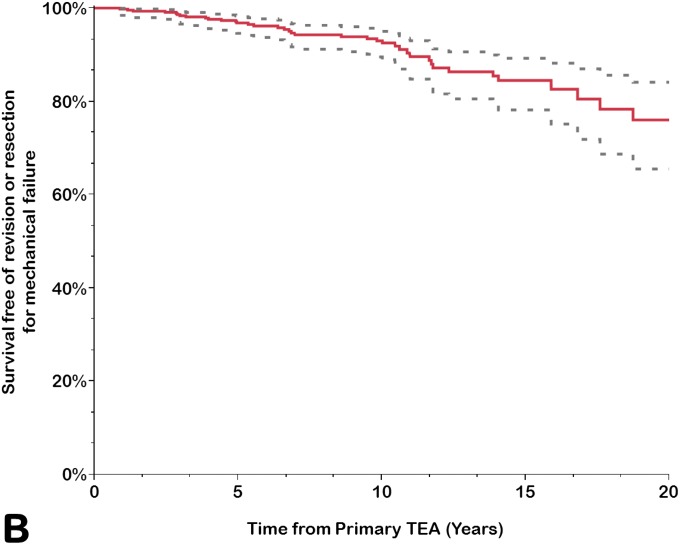
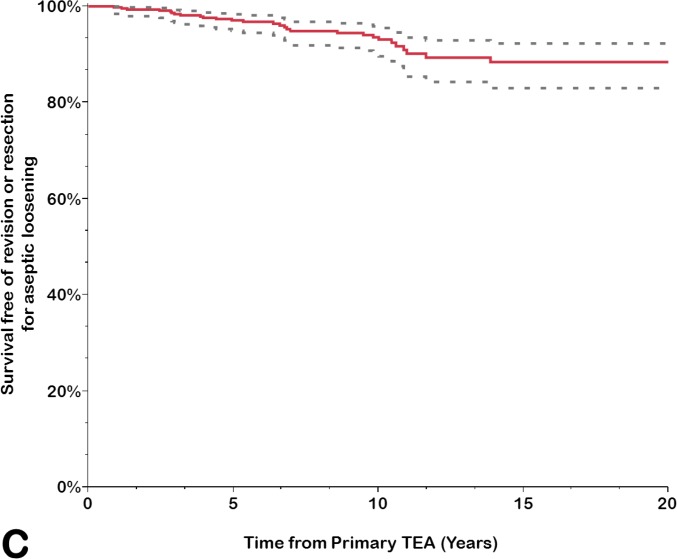
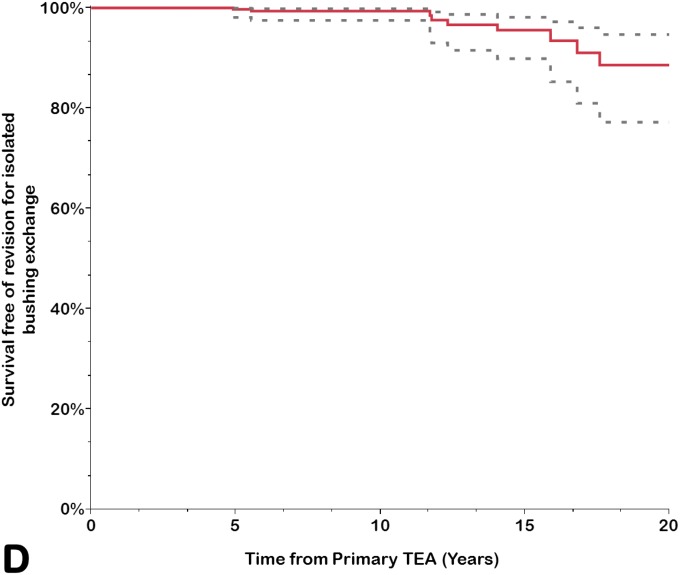
Implant survivorship free of component revision or removal for any reason was calculated for the prosthesis as a whole (any component, including revision for isolated bushing exchange) and for the ulnar and humeral components separately (Table III). The rate of survivorship free of revision or removal for any reason was 92% (95% CI = 88% to 94%) at 10 years, 83% (95% CI = 77% to 88%) at 15 years, and 68% (95% CI = 56% to 78%) at 20 years. Using component revision for aseptic loosening as an end point, the survivorship rate at 20 years was 88% (95% CI = 83% to 92%); it was 99% (95% CI = 97% to 99%) for the humeral component and 89% (95% CI = 84% to 93%) for the ulnar component. Using isolated bushing exchange as an end point, the rate of implant survivorship at 20 years was 89% (95% CI = 77% to 95%). Overall, approximately one-third of the arthroplasties had been removed or revised for any reason at 20 years (Figs. 1-A through 1-D).
TABLE III.
Estimated Total Elbow Arthroplasty Implant Survivorship Rates (%) by End Point*
| Any Reason | Ulnar Component Loosening | Humeral Component Loosening | Mechanical Failure | Aseptic Loosening | Isolated Bushing Exchange | Deep Infection | |
| No. of events | 49 | 34 | 16 | 39 | 29 | 9 | 10 |
| Time point | |||||||
| 2 yr | 99 (97-99) | 99 (98-100) | 99 (98-100) | 99 (98-100) | 99 (98-100) | 100 | 99 (98-100) |
| 5 yr | 95 (93-97) | 97 (95-98) | 97 (95-99) | 97 (95-98) | 97 (95-98) | 99.7 (98-100) | 99 (97-99) |
| 10 yr | 92 (88-94) | 93 (90-96) | 97 (95-99) | 93 (90-95) | 94 (90-96) | 99.4 (98-100) | 99 (97-99) |
| 15 yr | 83 (77-88) | 88 (83-92) | 97 (95-99) | 84 (78-89) | 88 (83-92) | 96 (90-98) | 99 (97-99) |
| 20 yr | 68 (56-78) | 77 (65-86) | 88 (75-95) | 76 (66-84) | 88 (83-92) | 89 (77-95) | 89 (75-95) |
The values are given as the Kaplan-Meier survival estimate, with the pointwise 95% CI in parentheses. All surgical revision procedures with prosthesis exchange or removal during the study period were included in the analyses.
Figs. 1-A through 1-D.
Kaplan-Meier curves for all primary total elbow arthroplasties (TEAs) for RA included in this study, showing the survivorship estimates (solid line) and the pointwise 95% confidence intervals (dashed lines) using revision or resection for any reason (Fig. 1-A), revision or resection for mechanical failure (Fig. 1-B), revision or resection for aseptic loosening (Fig. 1-C), and revision for isolated bushing exchange (Fig. 1-D) as the end points.
Other Reoperations and Complications
In this study cohort, the 90-day mortality rate was 0.3% for patients (1 of 387 patients) and 0.2% for procedures (1 of 461 arthroplasties). Other complications are summarized in Table IV. A total of 95 elbows (88 patients) underwent at least 1 additional procedure after the index arthroplasty (median, 1 procedure; range, 1 to 8 procedures). Fifty-seven elbows underwent only 1 additional procedure, 20 elbows underwent 2, 9 elbows underwent 3, 6 elbows underwent 4, and 1 elbow each underwent 6, 7, and 8 additional procedures, respectively (Table V). As mentioned previously, 49 elbows underwent a reoperation involving component revision or removal. Of the remaining 46 elbows that underwent a reoperation with component retention, 26 underwent ≥1 irrigation and debridement procedure for deep infection. Thus, the overall rate of deep infection requiring reoperation was 7.8%.
TABLE IV.
Complications After Primary Total Elbow Arthroplasty for RA*
| Description | No. (%) of Total Elbow Arthroplasties |
| Pulmonary embolism | 1 (0.2%) |
| 90-day periop. mortality | 1 (0.2%) |
| Nerve injury | 1 (0.2%) |
| Extensor mechanism dysfunction | 6 (1%) |
| Bone fracture | |
| Intraop. humeral fracture | 13 (3%) |
| Intraop. ulnar fracture | 5 (1%) |
| Postop. humeral fracture | 7 (2%) |
| Postop. ulnar fracture | 21 (5%) |
Includes all complications that occurred during the study period and by the time of most recent follow-up, whether treated operatively or conservatively.
TABLE V.
Reoperations with No Prosthesis Exchange or Removal in Patients Who Had Primary Total Elbow Arthroplasty for RA*
| Description | No. of Total Elbow Arthroplasties |
| Irrigation and debridement | 30 |
| Skin flap coverage | 6 |
| Extensor mechanism repair | 5 |
| Ectopic bone and heterotopic ossification removal | 2 |
| Open reduction and internal fixation for a fracture | |
| Ulna | 2 |
| Humerus | 2 |
| Hardware removal | 6 |
| Manipulation under anesthesia | 2 |
| Skin rheumatoid nodule removal | 7 |
| Bursitis | 4 |
| Synovectomy | 1 |
| Skin granuloma removal | 1 |
Multiple reoperations with the same or different indications were performed in some elbows. Some reoperations were performed in elbows that eventually required additional surgery with implant exchange or removal.
Pain and Functional Outcomes
Subscores for calculating the MEPS were available for 327 (85%) of the 386 elbow arthroplasties with a minimum follow-up of 2 years and no implant revision or removal. At the most recent follow-up, the median MEPS was 90 points (IQR, 80 to 100 points). Pain was graded as mild or absent in 285 (87%) of the elbows. The latest arc of motion measurements showed a mean of 20° for extension (range, 0° to 90°) and a mean of 135° for flexion (range, 50° to 155°), with a flexion-extension arc of >100° in 234 (72%) of the elbows. The median MEPS function subscore was 25 points (IQR, 20 to 25 points), with full performance of activities of daily living (i.e., combing the hair, feeding oneself, personal hygiene, and putting on pants and shoes) noted for 204 of the elbows.
Radiographic Outcomes
There were 309 elbows (265 patients) with adequate radiographs at a minimum of 2 years after the index arthroplasty; these represent 80% of the 386 elbows (334 patients) with a minimum clinical follow-up of 2 years and no implant revision or removal. The median radiographic follow-up for these 309 elbows was 6 years (range, 2 to 26 years). Radiographic follow-up was <2 years for 106 elbows, between 2 and 5 years for 109 elbows, between 5 and 10 years for 125 elbows, between 10 and 15 years for 77 elbows, between 15 and 20 years for 35 elbows, and >20 years for 9 elbows.
At the most recent follow-up, 301 of the total elbow arthroplasties had no radiolucency or minor radiolucency (none, type I or type II) around both components, 6 around the humeral component only, and 1 around the ulnar component only (Figs. 2-A and 2-B)11. One total elbow arthroplasty had major radiolucency (type IV or V) around both components, 1 around the humeral component only, and 6 around the ulnar component only, with an ulnar component fracture in 1 elbow. These 8 elbows were considered to have radiographic evidence of loosening but had not been revised at the most recent follow-up.
Figs. 2-A and 2-B.
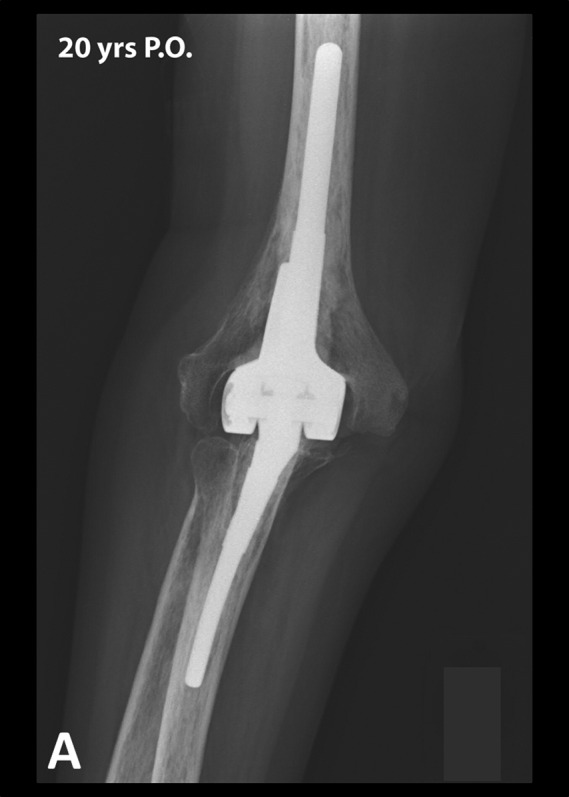
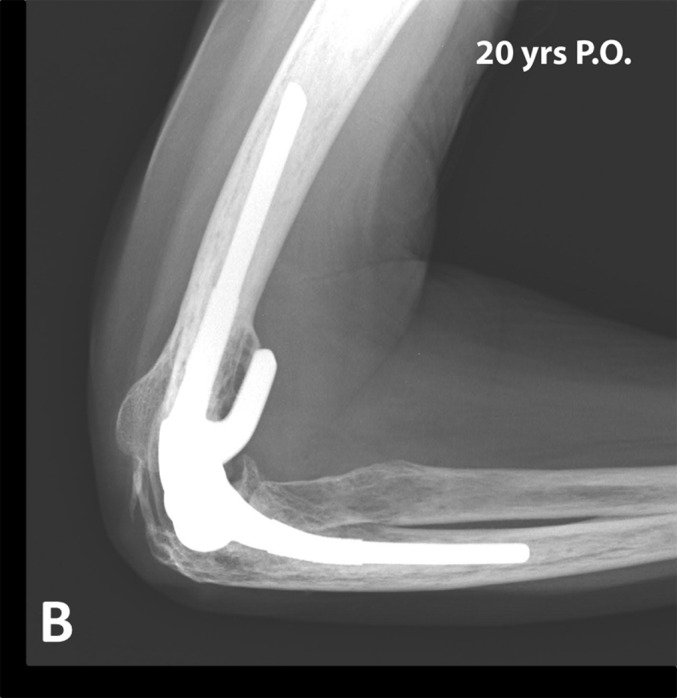
Anteroposterior (Fig. 2-A) and lateral (Fig. 2-B) radiographs 20 years after total elbow arthroplasty performed in a 41-year-old female patient. Note the absence of radiolucent lines or substantial wear. This patient reported no pain and had an excellent MEPS. P.O. = postoperative.
The bone graft placed behind the flange of the humeral component was considered radiographically to be incorporated in 274 elbows and partially or completely resorbed in 35 elbows. Bushing wear, defined as an intersection angle of >7° between the humeral and the ulnar component, was identified on the most recent anteroposterior radiograph in 71 elbows (23% of the surviving elbows with a minimum of 2 years of radiographic follow-up) (Figs. 3-A and 3-B).
Figs. 3-A and 3-B.
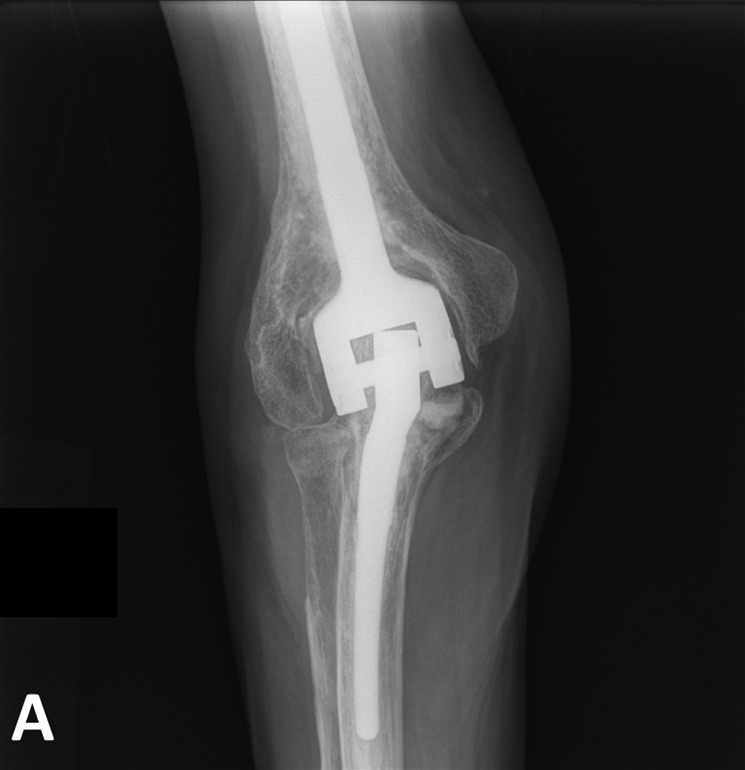
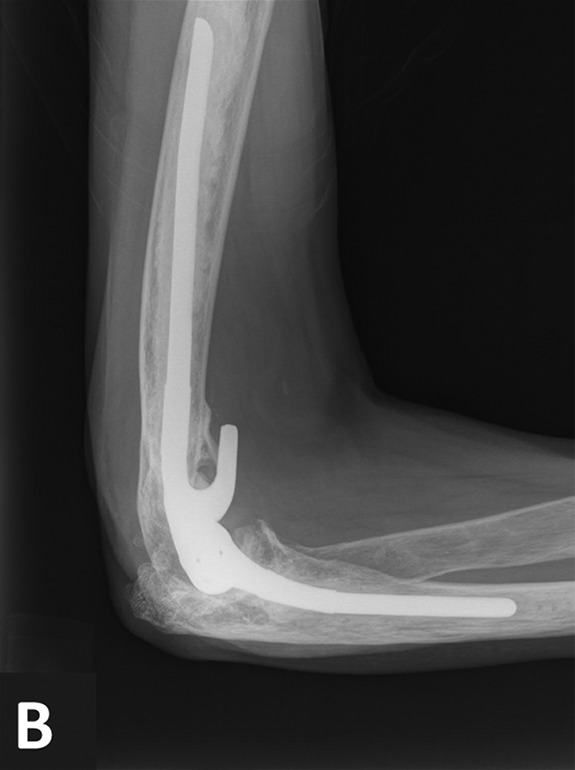
Anteroposterior (Fig. 3-A) and lateral (Fig. 3-B) radiographs 21 years after a total elbow arthroplasty performed in a 50-year-old male patient with RA. Note the presence of polyethylene wear.
The results of a subset of elbows followed for a minimum of 10 years are presented in the Appendix.
Risk Factors for Revision or Component Removal
Men had a significantly greater risk of revision compared with women (hazard ratio [HR] = 2.49; 95% CI = 1.32 to 4.55; p = 0.006). In addition, patients with concomitant trauma or posttraumatic sequelae had a significantly greater risk of revision compared with those who did not have a history of elbow trauma (HR = 2.46; 95% CI = 1.12 to 4.97; p = 0.026). The remaining variables analyzed did not achieve significance.
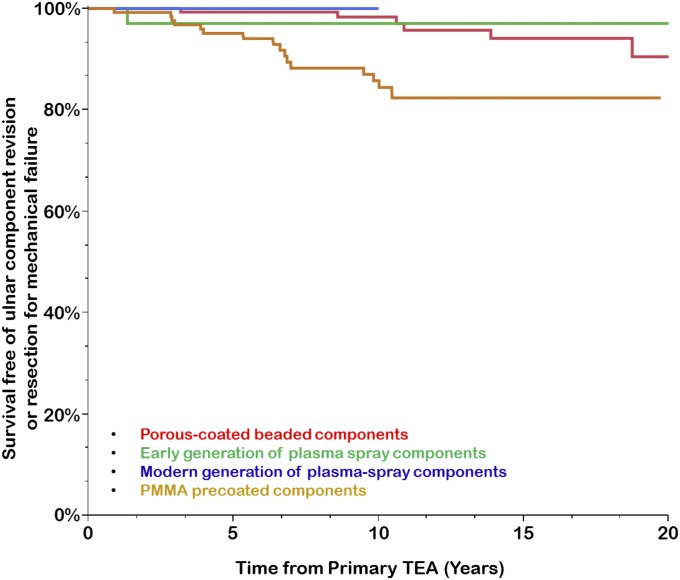
The effect of the ulnar-surface finish type on the risk of ulnar component revision for mechanical failure was assessed using a univariate analysis. Four surface finishes were compared: early (first-generation) plasma spray coating, beaded porous coating, PMMA precoating, and modern plasma spray coating. Compared with the component with the modern plasma spray, the PMMA precoated component was associated with a significantly greater risk of ulnar component revision for mechanical failure (HR = 4.57; 95% CI = 1.27 to 29.23; p = 0.017), whereas the component with the early plasma spray (HR = 1.05; 95% CI = 0.05 to 11.28; p = 0.97) and the porous-coated beaded component were not (HR = 1.06; 95% CI = 0.23 to 7.46; p = 0.94). The PMMA precoated component also showed significantly greater risk of ulnar component revision for mechanical failure compared with the porous-coated beaded component (HR = 4.30; 95% CI = 1.73 to 12.28; p = 0.0013); however, this increase in risk was not statistically detectable when the PMMA precoated component was compared with the component with the early generation of plasma spray (HR = 4.34; 95% CI = 0.87 to 79.11; p = 0.08) (Fig. 4).
Fig. 4.
Kaplan-Meier curves for primary total elbow arthroplasty (TEA) performed for RA, showing the survivorship estimates for survival free of ulnar component revision or resection for mechanical failure according to the type of surface finish of the ulnar component.
Discussion
The results of our study seem to indicate that total elbow arthroplasty with a very commonly used linked semiconstrained implant design provided satisfactory long-term outcomes in a large cohort of patients with RA. Deep infection, aseptic loosening of the ulnar component, and polyethylene wear were the main reasons for failure. The overall rate of infection requiring revision or reoperation was nearly 8%. Ulnar component revision was the main reason for revision in 24 of the 39 elbows revised for mechanical failure. Bushing wear was identified in almost 1 of every 4 surviving elbows with a minimum of 2 years of radiographic follow-up.
Deep infection required implant removal or revision in 10 (2.3%) of the elbows. However, the overall infection rate was much higher (7.8%), with 26 additional elbows requiring debridement and component retention for deep infection. The relatively high rate of infection following elbow arthroplasty compared with the replacement of other joints needs to be kept in mind when counseling patients with RA.
Mechanical failure of the ulna was more common for this particular implant. It was particularly high with the PMMA precoated ulnar component. A previous study has noted the high failure rate of this surface finish, in which component debonding from cement led to florid osteolysis and sometimes periprosthetic fracture12. This seems to have been resolved with the current plasma spray surface finish. This finding emphasizes the fact that sometimes a single change in component design can lead to an unexpected change in failure rates. Overall, however, cement fixation of the Coonrad-Morrey implant provided long-lasting fixation, as reflected by a 20-year rate of survivorship free of revision due to aseptic loosening of 88%.
Polyethylene wear is difficult to assess accurately after elbow arthroplasty14-18. In the absence of stress radiographs in varus and valgus, wear can be underestimated. In this study, we assessed wear by measuring the angle between the ulnar and humeral components on the anteroposterior radiograph. Polyethylene wear was identified in approximately one-fourth of the surviving elbows with adequate radiographic follow-up of at least 2 years. Only 9 (2%) of the elbows were revised specifically to exchange the polyethylene bushing, but polyethylene wear was also noted at the time of revision surgery in most elbows revised for loosening. This finding supports recent efforts by various designers of modern implants to develop components with better wear performance.
Our study had a number of limitations. Clinical and radiographic information was not available for all patients with a minimum of 2 years of follow-up; adequate radiographs at the most recent follow-up were not available for approximately 20% of the elbows with no history of implant revision or removal. Thus, our study may underestimate the rate of radiographic asymptomatic loosening or polyethylene wear. In addition, the evolution of implant design had a major effect on the overall survivorship. Information on triceps function after arthroplasty or sensory changes in the ulnar nerve distribution were not fully captured in earlier medical records, preventing us from accurately analyzing these aspects of outcome. Lastly, polyethylene wear was not measured on standardized stress radiographs.
Nonetheless, this study also had a number of strengths, including, to our knowledge, the largest experience yet reported in the literature of a continuous series, without exclusion of any elbow operated on, since this particular implant was introduced; clinical and radiographic surveillance of up to 30 years; accurate capture of reoperations and complications; and detailed radiographic evaluation.
It is important to emphasize that the characteristics of patients with RA have changed over time1,2. Currently, patients with RA are less affected by their disease because of the introduction of biologic disease-modifying drugs. It is possible that more recent patients with RA are more active than most of the patients included in this study19. Thus, the results of our study should be interpreted with caution, since more active patients with RA might be prone to a higher implant failure rate as seen in patients with posttraumatic arthritis20.
It is also important to realize that over the last 2 decades, the indications for elbow arthroplasty have been expanded to patients without inflammatory arthritis. Patients with acute distal humeral fractures and nonunions, as well as those with posttraumatic osteoarthritis, represent very different patient populations compared with those with inflammatory arthritis. Lessons learned from our experience with elbow arthroplasty for RA may be used only to some extent to improve the outcome of the procedure across other indications. We have learned that some aspects of implant design that may have been introduced in an effort to improve performance can actually result in higher failure rates, making it important to be careful when selecting implants and introducing new design modifications. We have also learned that, with the correct stem design and surface finish, stem loosening becomes less of a problem, and polyethylene wear becomes the “weak link”. Finally, we have learned that non-implant-related complications, such as ulnar neuritis, triceps insufficiency, fractures, and infection, compromise the clinical outcome of the procedure in patients with surviving implants, and that once a patient develops a complication needing reoperation, it sets the stage for possible additional reoperations.
In conclusion, elbow arthroplasty involving the use of a linked semiconstrained cemented implant provided good clinical results in patients with RA. In this large cohort of patients followed for up to 30 years, the overall revision rate for implant revision or removal was 11%, leading to a 15-year survival rate of 83% and a 20-year survival rate of 68%. Importantly, failures gradually increased with follow-up, a finding that has substantial implications when considering the treatment of younger, more active patients. Most fixation failures were attributed to a change in the surface finish of the ulnar component. The decrease in survival between 15 and 20 years seems to be attributed mostly to polyethylene wear. These results will hopefully be of use to counsel patients with RA about the expected long-term performance of elbow arthroplasty and to determine whether newer implant designs truly translate into improved implant longevity.
Appendix
An appendix describing the subset of elbows followed for a minimum of 10 years is available with the online version of this article as a data supplement at jbjs.org.
Footnotes
Investigation performed at the Department of Orthopedic Surgery, Mayo Clinic, Rochester, Minnesota
Disclosure: This study was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). These funds were used to pay for statistical services. In addition, one of the authors (B.F.M.) reports royalties received from Zimmer, the manufacturer of the prosthesis used in this study. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work; “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work; and “yes” to indicate that the author had other relationships or activities that could be perceived to influence, or have the potential to influence, what was written in this work.
Disclaimer: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
References
- 1.Studer A, Athwal GS. Rheumatoid arthritis of the elbow. Hand Clin. 2011. May;27(2):139-50, v. Epub 2011 Apr 20. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Sotelo J, Ramsey ML, King GJ, Morrey BF. Elbow arthroplasty: lessons learned from the past and directions for the future. Instr Course Lect. 2011;60:157-69. Epub 2011 May 11. [PubMed] [Google Scholar]
- 3.Sanchez-Sotelo J. Total elbow arthroplasty. Open Orthop J. 2011;5:115-23. Epub 2011 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukka S, Berg G, Hassany HR, Koye AK, Sjödén G, Sayed-Noor AS. Semiconstrained total elbow arthroplasty for rheumatoid arthritis patients: clinical and radiological results of 1-8 years follow-up. Arch Orthop Trauma Surg. 2015. May;135(5):595-600. Epub 2015 Mar 4. [DOI] [PubMed] [Google Scholar]
- 5.Cross MB, Cicalese E, Nam D, McArthur BA, Lipman JD, Figgie MP. Results of custom-fit, noncemented, semiconstrained total elbow arthroplasty for inflammatory arthritis at an average of eighteen years of follow-up. J Shoulder Elbow Surg. 2014. September;23(9):1368-73. Epub 2014 May 14. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi F, Draviaraj KP, Stanley D. The Kudo 5 total elbow replacement in the treatment of the rheumatoid elbow: results at a minimum of ten years. J Bone Joint Surg Br. 2010. October;92(10):1416-21. Epub 2010 Oct 5. [DOI] [PubMed] [Google Scholar]
- 7.Prasad N, Dent C. Outcome of total elbow replacement for rheumatoid arthritis: single surgeon’s series with Souter-Strathclyde and Coonrad-Morrey prosthesis. J Shoulder Elbow Surg. 2010. April;19(3):376-83. Epub 2010 Jan 13. [DOI] [PubMed] [Google Scholar]
- 8.Kleinlugtenbelt IV, Bakx PA, Huij J. Instrumented bone preserving elbow prosthesis in rheumatoid arthritis: 2-8 year follow-up. J Shoulder Elbow Surg. 2010. September;19(6):923-8. Epub 2010 Aug 18. [DOI] [PubMed] [Google Scholar]
- 9.Ikävalko M, Tiihonen R, Skyttä ET, Belt EA. Long-term survival of the Souter-Strathclyde total elbow replacement in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2010. May;92(5):656-60. Epub 2010 May 4. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe F, Zwillich SH. The long-term outcomes of rheumatoid arthritis: a 23-year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis. Arthritis Rheum. 1998. June;41(6):1072-82. Epub 1998 Jun 17. [DOI] [PubMed] [Google Scholar]
- 11.Gill DR, Morrey BF. The Coonrad-Morrey total elbow arthroplasty in patients who have rheumatoid arthritis. A ten to fifteen-year follow-up study. J Bone Joint Surg Am. 1998. September;80(9):1327-35. Epub 1998 Oct 6. [DOI] [PubMed] [Google Scholar]
- 12.Jeon IH, Morrey BF, Sanchez-Sotelo J. Ulnar component surface finish influenced the outcome of primary Coonrad-Morrey total elbow arthroplasty. J Shoulder Elbow Surg. 2012. September;21(9):1229-35. Epub 2011 Nov 21. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958. June;53(282):457-81. [Google Scholar]
- 14.Sanchez-Sotelo J, Morrey BF. Total elbow arthroplasty. J Am Acad Orthop Surg. 2011. February;19(2):121-5. Epub 2011 Feb 5. [DOI] [PubMed] [Google Scholar]
- 15.Day JS, Baxter RM, Ramsey ML, Morrey BF, Connor PM, Kurtz SM, Steinbeck MJ. Characterization of wear debris in total elbow arthroplasty. J Shoulder Elbow Surg. 2013. July;22(7):924-31. Epub 2013 Apr 10. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg SH, Urban RM, Jacobs JJ, King GJ, O’Driscoll SW, Cohen MS. Modes of wear after semiconstrained total elbow arthroplasty. J Bone Joint Surg Am. 2008. March;90(3):609-19. Epub 2008 Mar 4. [DOI] [PubMed] [Google Scholar]
- 17.Lee BP, Adams RA, Morrey BF. Polyethylene wear after total elbow arthroplasty. J Bone Joint Surg Am. 2005. May;87(5):1080-7. Epub 2005 May 4. [DOI] [PubMed] [Google Scholar]
- 18.Baghdadi YM, Jacobson JA, Duquin TR, Larson DR, Morrey BF, Sanchez-Sotelo J. The outcome of total elbow arthroplasty in juvenile idiopathic arthritis (juvenile rheumatoid arthritis) patients. J Shoulder Elbow Surg. 2014 Jun 4 Sep;23(9):1374-80. Epub 2014 Jun 4. [DOI] [PubMed]
- 19.Barlow JD, Morrey BF, O’Driscoll SW, Steinmann SP, Sanchez-Sotelo J. Activities after total elbow arthroplasty. J Shoulder Elbow Surg. 2013. June;22(6):787-91. Epub 2013 Mar 13. [DOI] [PubMed] [Google Scholar]
- 20.Throckmorton T, Zarkadas P, Sanchez-Sotelo J, Morrey B. Failure patterns after linked semiconstrained total elbow arthroplasty for posttraumatic arthritis. J Bone Joint Surg Am. 2010. June;92(6):1432-41. Epub 2010 Jun 3. [DOI] [PubMed] [Google Scholar]