Abstract
Objective
Combination of β1-adrenergic receptor (AR) blockade and β2-AR activation might be a potential novel therapy for treating heart failure. However, use of β-AR agonists and/or antagonists in the clinical setting is controversial because of the lack of information on cardiac inotropic or chronotropic regulation by AR signaling.
Methods
In this study, we performed hemodynamic evaluation by examining force frequency response (FFR), Frank-Starling relationship, and response to a non-selective β-AR agonist (isoproterenol) in hearts isolated from 6-month-old transgenic (TG) mice overexpressing β1- and β2-ARs (β1- and β2-AR TG mice, respectively).
Results
Cardiac physiologic consequences of β1- and β2-AR overexpression resulted in similar maximal response to isoproterenol and faster temporary decline of positive inotropic response in β2-AR TG mice. β1-AR TG mice showed a pronounced negative limb of FFR, whereas β2-AR TG mice showed high stimulation frequencies with low contractile depression during FFR. In contrast, Frank-Starling relationship was equally enhanced in both β1- and β2-AR TG mice.
Conclusion
Hemodynamic evaluation performed in the present showed a difference in β1- and β2-AR signaling, which may be due to the difference in the desensitization of β1- and β2-ARs.
Keywords: Adrenergic receptors, Transgenic mice, Isoproterenol, Inotropic, Chronotropic
INTRODUCTION
β1- and β2-adrenergic receptors (ARs), expressed on cardiomyocytes, participate in catecholamine-mediated enhancement of cardiac inotropic or chronotropic responses [1-3]. Broad therapeutic spectrum of β2-AR agonists is the rationale for combining selective β1-AR blockade and moderate β2-AR activation as potential novel therapy for preventing or treating the loss of ventricular function, and for improving adrenergic signaling and responsiveness during heart failure [4-7]. However, the use of β2-AR agonists for treating heart failure symptoms is controversial because of concerns associated with their efficacy, regulation of receptor signaling, and potential adverse effects [8-11]. Limited number of studies have assessed therapeutic targeting of β2-AR compared with that of β1-AR by using force frequency response (FFR), myofibril length-dependent mechanisms (Frank-Starling relationship), and receptor systems regulating cardiac inotropes in normal and failing hearts.
Therefore, in the present study, we examined the specific contribution of β1- and β2-ARs to intrinsic cardiac regulatory mechanisms. We developed transgenic (TG) mice by using a previously described method [12]; performed hemodynamic evaluation, including FFR and Frank-Starling relationship assessment; and examined response to a β-AR agonist (isoproterenol) by using the hearts isolated from TG mice with comparable levels of β1- and β2-AR overexpression.
METHODS
TG mice
TG mice overexpressing cardiac-specific β1- and β2-ARs (β1- and β2-AR TG mice) were developed, as described previously [1,2]. Briefly, wild-type human β1- and β2-AR cDNA was ligated to the SalI site (exon 3) of a full-length 5.5-kb α-myosin heavy chain promoter. The linearized constructs were injected into the male pronuclei of fertilized FVB/N mouse oocytes, and the oocytes were implanted into the oviducts of pseudopregnant female mice. Genomic DNA isolated from mouse tail-cuts was screened for the transgenes by performing targeted PCR with one primer against the α-myosin heavy chain promoter and one primer against the TG cDNA. β1- and β2-AR TG mice were examined at 6 months of age when they developed phenotypes independent of the confounding effects of cardiac growth and of the changes in the functional coupling of β-ARs. Procedures for animal studies were approved by the Institutional Animal Care and Use Committee of the University of Maryland Baltimore.
Isolated work-performing hearts
Experimental conditions used for heart preparations have been described previously [2]. Mice were anesthetized by intraperitoneally injecting 100 mg/kg sodium Nembutal and 1.5 units heparin to prevent microthrombus formation (n=15/group). The heart and aorta were attached to a 20-gauge cannula, and temporary retrograde perfusion was performed using oxygenated Krebs-Henseleit solution (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 0.5 mM Na-EDTA, 25 mM NaHCO3, 1.2 mM KH2PO4, and 11 mM glucose saturated with 95% O2 and 5% CO2). A polyethelene-50 catheter was inserted into the apex of the left ventricle to measure intraventricular pressure. The pulmonary vein was connected to another cannula, and antegrade perfusion was performed using a basal workload of 300 mmHg mL/min (6 mL venous return and 50 mmHg mean aortic pressure). The hearts were equilibrated for 20 minutes. Atrial pressure was monitored using the sidearm of the left atrial cannula, and left ventricular pressure signals were digitized at 1 kHz and were analyzed offline by using BioBench software (National Instruments, Austin, TX, USA). The first positive and negative derivatives of the left intraventricular pressure curve (maximal rate pressure development [+dP/dt] and maximal rate pressure decline [-dP/dt]), duration of contraction and relaxation (time to peak pressure [TPP]), and time to half relaxation were calculated. TPP and time to half relaxation were normalized using peak systolic pressure and half relaxation time, respectively, because they depended upon the extent of pressure development. Peak pressure for normalizing TPP was calculated by subtracting end diastolic pressure from systolic pressure. Half relaxation pressure for normalizing time to half relaxation was calculated using the following formula: (systolic pressure–diastolic pressure)/2.
FFR was measured by pacing the hearts with electrodes connected to aortic and venous return cannulae with a Grass SD9 stimulator (Grass Instruments, West Warwick, RI, USA). A primary-phase negative FFR [13] was induced over a low-frequency range of 1 to 3 Hz and was not used for performing assessments in the present study. The hearts were stimulated from 4 to 12 Hz, with increments of 60 beats/min, to induce FFR over a frequency range similar to the physiological heart rate [13]. These stimulation frequencies induced secondary-phase positive and negative FFRs that were used for analyzing frequency-dependent changes in cardiac +dP/dt and -dP/dt.
Frank-Starling curves were generated by altering ventricular afterloads through a graded aortic flow constriction. Pressure loading was performed by increasing afterloads (aortic resistance) until contractility was no longer elevated and by keeping venous return constant (6 mL/min). Cardiac work at different aortic resistances was calculated and was expressed as mmHg mL/min.
Drug infusion
Cardiac responses to the infusion of a nonselective β-AR agonist isoproterenol (Sigma-Aldrich Co., Saint Louis, MO, USA) were determined after assessing baseline cardiac responses to 10-7 M isoproterenol. Stimulation of β1- and β2-ARs with isoproterenol produced various inotropic and chronotropic responses whose magnitudes were approximately saturated after the infusion of 10-7 M isoproterenol. After determining the maximum response, time courses of pressure-derived parameters (+dP/dt and -dP/dt) were analyzed over 40 minutes, with 5-minute intervals.
Statistical analysis
All data are presented as mean±standard error. Statistical significance of dP/dt was estimated using one- and two-way analysis of variance and repeated measures with IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). Differences among groups at specific time points, stimulation frequencies (Hz), and pressure (mmHg) were assessed by performing one-way analysis of variance and post hoc Bonferroni test. P<0.05 was considered statistically significant.
RESULTS
Myocardial hypertrophy and physiological function
β1- and β2-AR TG mice showed higher heart/body weight ratios than wild-type mice (3.91±0.17 and 3.78±0.14, respectively, vs. 3.61±0.07 mg/g; P<0.05); however, this difference was not statistically significant. Myocardial cell diameter showed the same trend as the heart/body weight ratios, with β1- and β2-AR TG mice showing greater myocardial diameter than wild-type mice; however, this difference was also not statistically significant (data not shown). Table 1 shows that cardiac-specific overexpression of both β1- and β2-AR enhanced cardiac function in TG mice. Cardiac contractility and relaxation and heart rate in β1- and β2-AR TG mice were significantly higher than those in wild-type mice. No significant differences were observed between cardiac parameters of β1- and β2-AR TG mice; however, these parameters were slightly improved in β2-AR TG mice compared with those in β1- AR TG mice.
Table 1.
Baseline hemodynamic parameters of the isolated work-performing hearts of wild-type mice and β1- and β2-AR-overexpressing TG mice
| Wild-type mice (n=5) | β1-AR TG mice (n=5) | β2-AR TG mice (n=5) | |
|---|---|---|---|
| SP (mmHg) | 132.0±4.5 | 158.9±9.0* | 167.0±19* |
| DP (mmHg) | -7.2±3.2 | -32.0±2.4 | -38.2±6.7 |
| EDP (mmHg) | 6.4±2.1 | 1.3±1.0* | 3.7±2.7* |
| +dP/dt (mmHg/sec) | 3,863±85 | 5,718±594* | 5,901±749* |
| -dP/dt (mmHg/sec) | 2,852±272 | 5,085±603* | 5,149±342* |
| HR | 259±8 | 347±12* | 335±12* |
| TPP (ms/mmHg) | 0.41±0.04 | 0.26±0.02* | 0.30±0.03* |
| TR1/2 (ms/mmHg) | 0.65±0.03 | 0.44±0.04* | 0.46±0.06* |
Values are presented as mean±standard error.
AR, adrenergic receptor; TG, transgenic; SP, left ventricular systolic pressure; DP, left ventricular diastolic pressure; EDP, left ventricular end diastolic pressure; +dP/dt, maximal rate pressure development; -dP/dt, maximal rate pressure decline; TPP, time to peak pressure (normalized to peak pressure); TR1/2, half relaxation pressure (normalized to half relaxation pressure).
P<0.05, β1- versus β2-AR TG mice versus wild-type mice.
AR subtype-specific time-dependent effect of the β-AR agonist on the inotropic responses of the isolated work-performing hearts
Baseline cardiac contractility and relaxation and heart rate were similar between β1- and β2-AR TG mice (Table 1). Stimulation of β1- and β2-AR TG mice with the β-AR agonist isoproterenol (10-7 M) enhanced cardiac contractility and relaxation. Both β1- and β2-AR TG mice showed similar increases in +dP/dt and -dP/dt after maximum isoproterenol stimulation compared with wild-type mice (Fig. 1A). Next, we analyzed inotropic responses over 30 minutes and observed that time-dependent return to basal contraction rate increased in the hearts of β2-AR TG mice compared with that in the hearts of wild-type mice and β1-AR TG mice (Fig. 1B, C).
Fig. 1.
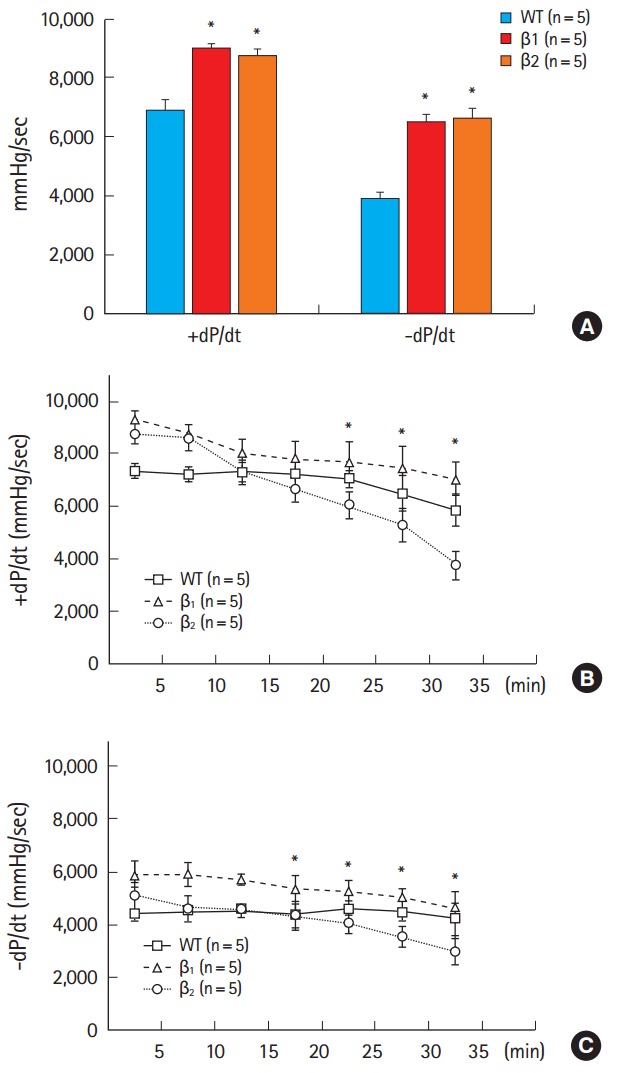
(A) Maximum inotropic and lusitropic responses to isoproterenol in wild-type (WT) mice and β1- and β2-adrenergic receptor (AR) transgenic (TG) mice. All the measurements were obtained under maximal responses after infusion of 10-7 M isoproterenol. *P<0.05, β1- and β2-AR TG mice versus WT mice. (B,C) The time course of left ventricular +dP/dt and -dP/dt responses in β1- and β2-AR TG mice to 10-7 M isoproterenol infusion. Inotropic responses in β1-AR TG mice were higher than those in β2-AR TG mice. *P<0.05, β1- versus β2-AR TG mice. Results are presented as mean±standard error.
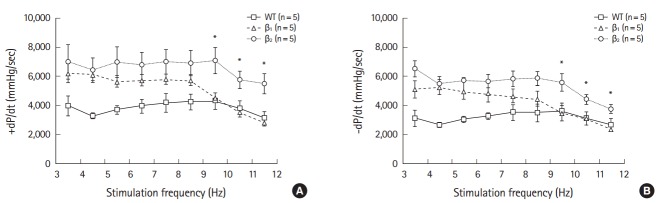
FFR and response to loading of isolated work-performing hearts
Data obtained using different frequencies (4 to 11 Hz) for inducing the secondary phase are shown in Fig. 2. β1- and β2-AR TG mice showed a flattened secondary phase at frequencies 4 to 9 Hz, with a critical decline observed at the limb of 9 Hz. Negative FFR of the isolated work-performing hearts was induced at a high-frequency range (9 to 12 Hz). Differences between the hearts of β1- and β2-AR TG mice were observed in the secondary-phase negative FFR. β2-AR TG mice showed enhanced contractility (+dP/dt) and relaxation (-dP/dt) indices at frequencies that induced the contractile depression of the heart. Although no differences were observed in +dP/dt and -dP/dt among mice in the three groups at the initial frequency of 4 Hz, β2-AR TG mice showed significantly higher +dP/dt than β1-AR TG and wild-type mice (4,665±384, 2,805±245 mmHg/sec, P<0.05).
Fig. 2.
Force frequency response (FFR) of the work-performing hearts of mice at pacing rates of 4 to 12 Hz (secondary-phase positive and negative FFR). Compared with wild-type (WT) mice, +dP/dt (A) and -dP/dt (B) in β1- and β2-adrenergic receptor (AR) transgenic (TG) mice augmented over a range of frequencies of positive FFR (4 to 9 Hz). The β2-AR TG mice demonstrate an augmented contractility and relaxation over the positive and negative phases of the FFR at stimulation frequencies from 4 to 14 Hz in the WT, and β1- and β2-AR TG mice. *P<0.05, β1- versus β2-AR TG mice.
Frank-Starling
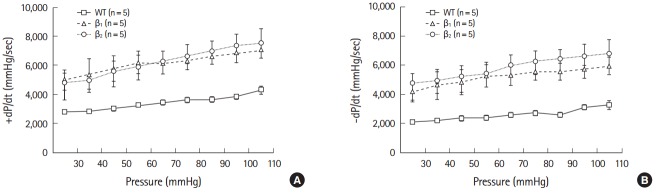
The capacity of the ventricle to adjust the force of contraction as a function of cardiac load is called Frank-Starling mechanism. Cardiac minute work varied from 50 mmHg mL/min to the maximal level of mean aortic pressure that was generated at a given venous return of 6 mL/min to determine the extent to which β1- and β2-AR TG mice could be subjected to increasing workload. The slope of the initial part of the Frank-Starling left ventricular functional curve (range, 0 to 250 mmHg mL/min) was calculated by performing linear regression analysis. This slope reflected the changes in myofibril length-dependent activation, and its γ-intercept indicated the contractile status under low cardiac load [13]. Wild-type mice and both β1- and β2-AR TG mice showed a strong positive correlation among +dP/dt, -dP/dt, and cardiac work (Fig. 3). At all workloads, the hearts of wild-type mice showed lower absolute values of +dP/dt and -dP/dt than those of β1- and β2-AR TG mice. The slope of workload response was also higher for both β1- and β2-AR TG mice than for wild-type mice, reflecting steeper functional response to high workloads (intercepts [+dP/dt]: β1-AR TG mice, 4,168±450; β2-AR TG mice, 4,123±266; wild-type mice, 1,778±158; slopes: β1-AR TG mice, 27.6±7.1; β2-AR TG mice, 22.9±6.2; wild-type mice, 12.5±2.5; intercepts [-dP/dt]: β1-AR TG mice, 4,027±604; β2-AR TG mice, 4,580±635; wild-type mice, 2,367±126; slopes (-dP/dt): β1-AR TG mice, 36.8 ±9.2; β2-AR TG mice, 28.8±6.9; wild-type mice, 16.5±2.3).
Fig. 3.
Responses of the isolated work-performing hearts to preload over a range of cardiac work from 100 to 600 mmHg/mL min. Baseline recordings were obtained under similar conditions: mean aortic pressure (afterload, 50 mmHg) and venous return (preload, 6 mL/min; left ventricular minute work 300 mmHg/mL min. +dP/dt (A) and -dP/dt (B) of experimental groups were plotted against gradually increasing afterloads at a constant venous return. In both β1- and β2-adrenergic receptor transgenic mice, +dP/dt and -dP/dt increased at different cardiac workloads, indicating augmented contractility at low and high cardiac workloads.
DISCUSSION
In this study, we examined the accelerated temporal decline in inotropic cardiac response after acute infusion of isoproterenol, a nonselective β-AR agonist, in β2-AR TG mice. Moreover, we compared the functional effects of β1- and β2-AR overexpression in the hearts of TG mice and established its physiological effects based on the differences in AR signaling.
Petrashevskaya et al. [12] reported that inotropic stimulation mediated by β1- and β2-ARs decreased in 2-month-old TG mice after long-term exposure to β-AR agonists, which was similar to that observed in the present study. They also showed that faster functional desensitization in response to acute agonist stimulation in 2-month-old β2-AR TG mice did not salvage the loss of agonist responsiveness in later life, which was similar to that in 2-month-old β1-AR TG mice. In the present study, 6-month-old β2-AR TG mice showed rapid functional desensitization of ARs compared with 6-month-old β1-AR TG mice. These findings suggested that compared with β1-AR overexpression, the effect of β2-AR overexpression was bifurcated at the level of Gi proteins, with more prominent Gi2 upregulation in β2-AR TG mice, indicating that Gi2 contributed to the prolonged survival of and delayed cardiac pathology in β2-AR TG mice [14]. However, downstream signaling effectors connecting the β2-AR/Gi2 axis to cardiac protection have not been established. Potentially, it may mitigate the deleterious effects of catecholamine signaling and contribute to different aspects of protective changes associated with β2-AR/Gi coupling or may decrease cardiac responsiveness to various Gq protein-related pro-growth factors.
FFR as well as the Frank-Starling mechanism are essential for adjusting cardiac contractile function to hemodynamic needs [8,13].
In the present study, we observed that the effect of β1- and β2-AR overexpression differed at the negative descending limb of the secondary FFR. At higher frequencies (9 to 12 Hz), β2-AR TG mice showed less inotropic depression than β1-AR TG mice, resulting in a secondary-phase negative FFR. Endoh [13] reported that an enhanced positive limb of FFR was observed upon acute activation of ARs. However, this was not detected in the hearts of both β1- and β2-AR TG mice in the present study.
In contrast, the Frank-Starling curves, which primarily reflect myofibril length-dependent changes in Ca2+ sensitivity of myofibrillar force, were steeper for both β1- and β2-AR TG mice in the present study. Protein kinase A (PKA) mediates the acute effects of the phosphorylation of troponin I and troponin C, with different effects on the sarcomere length dependence of Ca2+ sensitivity [15-17]. Myofibril length-dependent changes in Ca2+ sensitivity were unchanged in both normal and failing cardiomyocytes after acute incubation with PKA, indicating that PKA-mediated phosphorylation was not involved in sarcomere length-dependent force development in the failing heart [17]. Long-term activation of both β1-and β2-ARs enhances cardiac function during acute increases in afterload, which is partly mediated by the Frank-Starling mechanism [17]. Thus, both β1-and β2-ARs may contribute to more efficient Ca2+-myofibril interaction, actin- and myosin-binding protein C phosphorylation, and steeper ventricular function curves.
Together, these results indicated that both β1- and β2-AR TG mice showed enhanced maximal response to the β-AR agonist. However, inotropic support was significantly downregulated in the hearts of β2-AR TG mice after long-term exposure to the β-AR agonist, which may have contributed to the accelerated functional desensitization of β2-AR. Cardiac contractility (+dP/dt) and relaxation (-dP/dt) were higher in β2-AR TG mice at stimulation frequencies. Frank-Starling responses were steeper in both β1- and β2-AR TG mice. Thus, hemodynamic evaluation performed in the present study indicated a difference in β1- and β2-AR signaling and indicated that this difference was caused by the differential desensitization of β2- and β1-ARs. Moreover, our results provided evidence that selective β1-AR blockade and β2-AR activation may be a novel therapy for treating heart failure.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2057263). Petra-shevskaya Natalia was supported by an NIH grant HL077101 awarded to S. Liggett.
Capsule Summary
What is already known
Combination of β1-adrenergic receptor (AR) blockade and β2-AR activation is a potential novel therapy for treating heart failure. However, use of β-AR agonists and/or antagonists in the clinical setting is controversial because of the lack of information on cardiac inotropic or chronotropic regulation by AR signaling.
What is new in the current study
Results of hemodynamic evaluation performed in the present study showed a difference between β1- and β2-AR signaling, which may be because of a difference in the desensitization of β1- and β2-ARs.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Liggett SB, Tepe NM, Lorenz JN, et al. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707–14. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 2.Mialet Perez J, Rathz DA, Petrashevskaya NN, et al. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9:1300–5. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 3.Xiao RP, Zhu W, Zheng M, et al. Subtype-specific beta-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol Sci. 2004;25:358–65. doi: 10.1016/j.tips.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–90. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 5.Dorn GW, 2nd, Tepe NM, Lorenz JN, Koch WJ, Liggett SB. Low- and high-level transgenic expression of beta2-adrenergic receptors differentially affect cardiac hypertrophy and function in Galphaq-overexpressing mice. Proc Natl Acad Sci U S A. 1999;96:6400–5. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du XJ, Gao XM, Jennings GL, Dart AM, Woodcock EA. Preserved ventricular contractility in infarcted mouse heart overexpressing beta(2)-adrenergic receptors. Am J Physiol Heart Circ Physiol. 2000;279:H2456–63. doi: 10.1152/ajpheart.2000.279.5.H2456. [DOI] [PubMed] [Google Scholar]
- 7.Shah AS, Lilly RE, Kypson AP, et al. Intracoronary adenovirus-mediated delivery and overexpression of the beta(2)-adrenergic receptor in the heart: prospects for molecular ventricular assistance. Circulation. 2000;101:408–14. doi: 10.1161/01.cir.101.4.408. [DOI] [PubMed] [Google Scholar]
- 8.Brodde OE, Bruck H, Leineweber K. Cardiac adrenoceptors: physiological and pathophysiological relevance. J Pharmacol Sci. 2006;100:323–37. doi: 10.1254/jphs.crj06001x. [DOI] [PubMed] [Google Scholar]
- 9.Du XJ, Autelitano DJ, Dilley RJ, Wang B, Dart AM, Woodcock EA. beta(2)-adrenergic receptor overexpression exacerbates development of heart failure after aortic stenosis. Circulation. 2000;101:71–7. doi: 10.1161/01.cir.101.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Vinge LE, Raake PW, Koch WJ. Gene therapy in heart failure. Circ Res. 2008;102:1458–70. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo B, Lemaire A, Mangmool S, et al. Beta1-adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;297:H1377–86. doi: 10.1152/ajpheart.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrashevskaya N, Gaume BR, Mihlbachler KA, Dorn GW, 2nd, Liggett SB. Bitransgenesis with beta(2)-adrenergic receptors or adenylyl cyclase fails to improve beta(1)-adrenergic receptor cardiomyopathy. Clin Transl Sci. 2008;1:221–7. doi: 10.1111/j.1752-8062.2008.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol. 2004;500:73–86. doi: 10.1016/j.ejphar.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Foerster K, Groner F, Matthes J, Koch WJ, Birnbaumer L, Herzig S. Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through beta 2-adrenoceptors. Proc Natl Acad Sci U S A. 2003;100:14475–80. doi: 10.1073/pnas.1936026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanft LM, McDonald KS. Sarcomere length dependence of power output is increased after PKA treatment in rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2009;296:H1524–31. doi: 10.1152/ajpheart.00864.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komukai K, Kurihara S. Length dependence of Ca(2+)-tension relationship in aequorin-injected ferret papillary muscles. Am J Physiol. 1997;273(3 Pt 2):H1068–74. doi: 10.1152/ajpheart.1997.273.3.H1068. [DOI] [PubMed] [Google Scholar]
- 17.van der Velden J, de Jong JW, Owen VJ, Burton PB, Stienen GJ. Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovasc Res. 2000;46:487–95. doi: 10.1016/s0008-6363(00)00050-x. [DOI] [PubMed] [Google Scholar]