Summary
Background
Obesity is a heterogeneous condition, which includes a subset of individuals that can be classified as having metabolically healthy obesity (MHO), but there is no consensus on what constitutes MHO. Thus, the objective of the study is to examine the prevalence and predictors of prevalent MHO in adolescents using various definitions of MHO.
Methods
Cross-sectional data from the 1999–2010 National Health and Nutrition Examination Surveys were used. Participants included 316 male and 316 female adolescents aged 12–19 years with a BMI ≥ 95th percentile. Two definitions were used to define MHO. First, MHO was defined as having ≤ 1 metabolic syndrome criteria (excluding waist) and being free of type 2 diabetes, hypertension and dyslipidemia. Second, MHO was defined as being free of all metabolic syndrome criteria, insulin resistance and inflammation.
Results
The prevalence of MHO was 42% (male) and 74% (female) using the first definition and 7% (male) and 12% (female) using the second more conservative definition. Lower abdominal obesity (waist circumference) and lower insulin resistance predicted prevalent MHO in male and female adolescents for both definitions (p <0.01). Associations between dietary components and MHO were weak and inconsistent, while physical activity and inflammation were not associated with MHO in male and female adolescents for both definitions (p >0.05).
Conclusions
The prevalence of MHO in adolescents varied across definitions, with lower levels of abdominal obesity and insulin resistance as the most consistent predictors of prevalent MHO status.
Keywords: diet, insulin resistance, metabolic syndrome, physical activity
Introduction
Obesity in adolescents has tripled in the past three decades (1) and has been accompanied by a rise in the prevalence of metabolic risk factors (2). Despite the documented health risks, adolescent obesity is a heterogeneous condition wherein a subset does not appear to present with these risk factors (3), termed metabolically healthy obesity (MHO). There is wide variation in the reported prevalence of MHO range from 16% to 68% due to differences in how MHO is defined and conceptualized (3–8). The most common definitions used for MHO involve components of metabolic syndrome (3–6,8,9) and/or insulin resistance (4,7). However, the clinical appropriateness of these definitions is questionable given that individuals can still be classified as MHO if they have one clinically elevated metabolic risk factor in isolation. This means that individuals with isolated hypertriglyceridemia or hypertension could be classified as MHO following the framework allowing for one or two metabolic syndrome factors or using insulin resistance alone.
As most attempts to treat obesity have proven to have limited success (10), it may therefore be a more attainable goal for adolescents with obesity to achieve or maintain MHO. This is relevant given that some adolescents with MHO may mature to be healthy, non-obese adults or adults with MHO (11). The correlates of MHO have been debated, but some research has shown that metabolic differences between adolescents with MHO or without (metabolically unhealthy obesity, MUO) may be related to differences in lifestyle habits (4,5), degree of obesity (4,12), inflammation (12) or insulin resistance (12). To our knowledge, no researchers have used a large nationally representative sample to determine which of these factors is most important in predicting prevalent MHO in adolescents. The objective of this study was to (i) examine the prevalence of adolescents with MHO using two approaches and (ii) investigate the independent relationships between obesity, diet, physical activity, insulin resistance and inflammation and MHO status in male and female adolescents.
Methods
Data source and participants
Data from the 1999–2010 years of the National Health and Nutrition Examination Survey (NHANES), an ongoing cross-sectional and nationally representative study, were analyzed. Details of the survey methods are described elsewhere (13). The present study included NHANES participants ages 12 to 19 years with an age-specific and sex-specific BMI ≥ 95th percentile (n = 2370). Participants who were (i) pregnant (n = 47), (ii) taking insulin (n = 6), (iii) using tobacco or nicotine products within the past 5 days (n = 272) or (iv) fasted for less than 8 h (n = 438) were excluded. Individuals with variable outliers, implausible values or missing values for either waist circumference (WC; n = 63), tobacco use within the past 5 days (n = 178), moderate physical activity (MPA; n = 93), vigorous physical activity (VPA; n = 81), energy intake (n = 84), total fat intake (n = 88), saturated fat intake (n = 90), monounsaturated fat intake (n = 83), polyunsaturated fat intake (n = 93), carbohydrate intake (n = 83), protein intake (n = 85), fibre intake (n = 86), fasting triglycerides (n = 1383), fasting glucose (n = 1376), fasting insulin (n = 1385), fasting HDL-C (n = 228), high-sensitivity C-reactive protein (CRP; n = 220), poverty income ratio (n = 180), systolic blood pressure (SBP; n = 90) and diastolic blood pressure (DBP; n = 110) were also excluded from the analysis. The final sample included 316 male and 316 female adolescents with obesity (27% of the original sample).
Covariates
Information on age, sex, family size and household income was obtained via home interviews. Data were reported by a proxy for participants under the age of 16 years and were self-reported for participants aged 16 to 19 years. Family size and income were used to compute poverty income ratio, a measure of socioeconomic status calculated as family income divided by the Department of Health and Human Services’ poverty guidelines based on family size, year and state.
Obesity, diet and physical activity
Waist circumference was measured without clothing, to the nearest 0.1 cm, at the top of the iliac crest during minimal respiration (14). Normal WC was defined as having a WC ≤ 90th percentile for age and sex (15) or the adult cut-off if lower (male: 102 cm and female: 88 cm). Weight was assessed to the nearest 0.1 kg with a Toledo digital scale (Mettler-Toledo Inc, Columbus, OH, USA) while participants wore underwear, disposable paper gowns and foam slippers. Height was measured to the nearest 0.1 cm. BMI percentiles were calculated from weight and height measurements using an age-specific and sex-specific CDC growth chart macro (16).
Data on diet were obtained using a 24-h diet recall method from 1999 to 2002 (17). Participants were asked to list the volume of all foods and beverages consumed from midnight to midnight the day prior. Trained interviewers recorded this information using the USDA automated pass method. During the years 2003–2010, the average of the two 24-h diet recalls was used (17).
Data on physical activity were assessed using a questionnaire on the frequency and duration (in minutes) of all recreational MPA and VPA they had engaged in for at least 10 min continuously ‘in the past 30 days’ for NHANES 1999–2006 and ‘in a typical week’ for NHANES 2007–2010. Moderate-vigorous physical activity (MVPA) was calculated as the sum of MPA and VPA (min/week) (18).
Laboratory measures
Systolic blood pressure and DBP (mmHg) were recorded based on an average of two to three readings from a manual mercury sphygmomanometer. A fasting sample of blood was used to determine plasma glucose (mM), insulin (mU/L), CRP (mg/dL), HDL-C (mM) and triglycerides (mM); samples were processed, stored and shipped to appropriate laboratories for analysis. Fasting plasma glucose was measured using hexokinase methods. Plasma insulin was measured using the radioimmunoassay double-antibody batch method (1999–2002), the two-site immunoenzymatic assay (2003–2005) and the human insulin immunoassay method (ELISA) (2005–2010). Fasting plasma glucose and insulin were used to calculate the homeostatic model assessment of insulin resistance (19). Blood CRP was quantified with a Behring Nephelometer. Serum triglycerides were assessed enzymatically using a series of coupled reactions (1999–2006) and a two-reagent end-point reaction (2007–2010). HDL-C levels were assessed using the heparin-Mn precipitation method (1999–2001) and the direct HDL method (2003–2010). Triglyceride and HDL-C levels were measured using a Hitachi 704 Analyzer (1999–2004), Roche Hitachi 717 in 2005, Roche Hitachi 717 and 912 in 2006, and a Roche Modular P chemistry analyzer (2007–2010). Details of all NHANES laboratory protocol and procedures are available elsewhere (20).
Definition of metabolically healthy obesity
Two methods were used to define MHO, which reflect a modified version of those most commonly used and a more stringent definition of healthy (3–9).
The first definition uses the metabolic syndrome framework (excluding WC) but also excludes individuals who would be diagnosed with a chronic condition from being categorized as ‘healthy’:
-
≤ 1 pre-clinical or metabolic syndrome criteria (21,22):
Triglycerides ≥ 1.24 mmol/L
Glucose ≥ 5.55 mmol/L (21)
Systolic or diastolic blood pressure (SBP or DBP) ≥ 90th percentile for sex, age and height for youth under 18 and ≥ 130/85 mmHg for 18 and 19 year olds
HDL-C ≤ 1.04 mmol/L
-
no clinically diagnosable levels (21,23) of:
Hypertriglyceremia – fasting plasma triglyceride (≥ 1.47 mmol/L) or lipid medication
Type 2 diabetes – glucose (≥ 7.0 mmol/L) or diabetes medication
Hypoalphalipoproteinemia – HDL-C
Hypertension – SBP or DBP ≥ 95th percentile for sex, age and height for youth under 18 years, and ≥ 140/90 mmHg for 18 and 19 year olds, or medication for hypertension.
The second definition is even more stringent as further precludes anyone with any of the following from being categorized as MHO:
Statistical analysis
All statistical analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC, USA) with a significance level of p <0.05. Analyses were stratified by sex. Due to the complex sampling design of NHANES, sample weights were applied to make the studied sample representative of the US adolescent population. Descriptive data are presented as means ± standard errors, and differences between MHO and MUO groups were determined using independent samples t-tests. In multivariable analyses, predictors were standardized using standard error to allow for comparisons between variables. Logistic regressions adjusted for age and socioeconomic status were performed on the standard error of each independent variable using Proc Surveylogistic. Odds ratio and 95% confidence interval (CI) were then used to compute prevalent risk (PR) and 95% CI (25). The most significant predictor of MHO from each variable category (obesity, diet, physical activity, and laboratory measure – primary MHO definition only) was then entered into a mutually adjusted model with further adjustment for age and socioeconomic status.
Results
Prevalence of metabolically healthy obesity
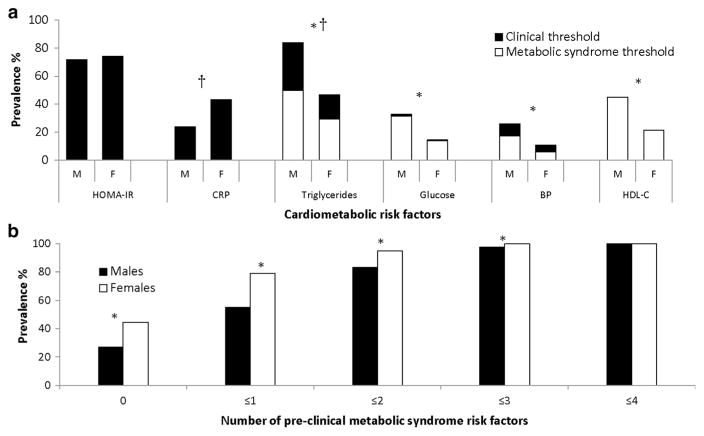
Figure 1a displays the proportion of adolescents with each metabolic risk factor, and Fig. 1b displays the number of pre-clinical metabolic syndrome risk factors (triglycerides, glucose, HDL-C and BP) in the study sample. The percentage of adolescents free of pre-clinical metabolic syndrome risk factors was 27% in male and 45% in female adolescents, and the proportion of adolescents with ≤ 1 pre-clinical metabolic syndrome risk factors was 55% in male and 79% in female adolescents (Fig. 1b). After excluding individuals with clinically diagnosable levels of metabolic syndrome risk factors, the prevalence of MHO was decreased to 42% in male and 74% in female adolescents (first definition).
Figure 1.
(a, b) Prevalence of cardiometabolic risk factors in male and female adolescents. Clinical thresholds: HOMA-IR ≥ 3.16, CRP ≥ 3.0 mg/L, triglycerides ≥ 1.47 mmol/L, glucose ≥ 7.0 mmol/L and BP (currently taking medication for hypertension or SBP or DBP ≥ 95th percentile for sex, age and height for youth under 18, and ≥ 140/90 mmHg for 18 and 19 year olds). Pre-clinical or metabolic syndrome thresholds: triglycerides ≥ 1.24 mmol/L, glucose ≥ 5.55 mmol/L, BP (SBP or DBP ≥ 90th percentile for sex, age and height for youth under 18, and ≥ 130/85 mmHg for 18 and 19 year olds), HDL-C ≤ 1.04 mmol/L. Abbreviations: M, male; F, female; HOMA-IR, homeostatic model assessment of insulin resistance; CRP, C-reactive protein; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol. †Significant sex difference in the prevalence of clinically elevated risk factors (p <0.05). *Significant sex difference in the prevalence of metabolic syndrome risk factors (p <0.05).
Characteristics of the study sample by sex and metabolic health status are presented in Table 1. Using the first definition of MHO, MHO male adolescents were younger, of a lower socioeconomic status, less obese, ate more total fat, saturated fat, monounsaturated fat, protein, fibre and cholesterol and were less insulin resistant than MUO male adolescents. In female adolescents, MHO and MUO only differed by insulin resistance. According to the second definition, the prevalence of MHO was 7% in male and 12% in female adolescents. MHO male adolescents were of a higher socioeconomic status, less obese, reported a lower fibre intake and participated in more MPA and VPA than MUO male adolescents. MHO female adolescents had a higher socioeconomic status, lower WC and participated in less VPA than MUO female adolescents.
Table 1.
Descriptive characteristics of MHO and MUO adolescents by sex and metabolic health status
| Male (n = 316)
|
Female (n = 316)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| First definition
|
Second definition
|
First definition
|
Second definition
|
|||||
| MHO (n = 142) | MUO (n = 174) | MHO (n = 21) | MUO (n = 295) | MHO (n = 214) | MUO (n = 102) | MHO (n = 27) | MUO (n = 289) | |
| Age (y) | 14.9 ± 0.2 | 15.5 ± 0.2* | 15.3 ± 0.2 | 15.2 ± 0.1 | 15.0 ± 0.3 | 15.0 ± 0.3 | 14.9 ± 0.4 | 15.0 ± 0.2 |
| PIR | 2.3 ± 0.2 | 2.6 ± 0.1* | 3.1 ± 0.3 | 2.4 ± 0.1** | 2.4 ± 0.1 | 2.3 ± 0.2 | 3.1 ± 0.3 | 2.2 ± 0.1** |
| Obesity | ||||||||
| BMI percentile | 97.6 ± 0.1 | 98.3 ± 0.1** | 97.4 ± 0.2 | 98.1 ± 0.1** | 97.4 ± 0.1 | 97.1 ± 0.2 | 97.1 ± 0.2 | 97.6 ± 0.1 |
| WC (cm) | 100.2 ± 0.9 | 108.1 ± 1.3** | 96.2 ± 1.1 | 105.4 ± 0.7* | 100.7 ± 0.8 | 104.5 ± 1.6 | 95.7 ± 0.6 | 102.5 ± 0.8** |
| Diet | ||||||||
| Energy (kcal/d) | 2334 ± 83 | 2138 ± 93 | 2210 ± 105 | 2221 ± 66 | 1674 ± 58 | 1738 ± 78 | 1693 ± 213 | 1690 ± 42 |
| Total fat (g/d) | 87.8 ± 4.1 | 77.4 ± 3.8* | 82.4 ± 4.5 | 81.7 ± 3.1 | 63.2 ± 2.8 | 64.3 ± 3.8 | 65.7 ± 11.4 | 63.2 ± 2.1 |
| Saturated fat (g/d) | 29.9 ± 1.3 | 26.5 ± 1.3* | 29.8 ± 1.4 | 27.8 ± 1.0 | 21.5 ± 1.2 | 23.1 ± 1.4 | 20.8 ± 4.4 | 22.1 ± 0.8 |
| Monounsaturated fat (g/d) | 32.9 ± 1.6 | 28.7 ± 1.4* | 30.0 ± 1.9 | 30.5 ± 1.1 | 23.5 ± 1.1 | 24.2 ± 1.5 | 26.0 ± 4.0 | 23.4 ± 0.8 |
| Polyunsaturated fat (g/d) | 17.4 ± 1.1 | 16.0 ± 1.1 | 15.3 ± 0.9 | 16.7 ± 0.9 | 13.1 ± 0.6 | 11.8 ± 0.9 | 14.1 ± 2.5 | 12.6 ± 0.5 |
| Carbohydrates (g/d) | 298.7 ± 10.3 | 286.4 ± 13.0 | 285.9 ± 14.8 | 292.0 ± 8.6 | 223.0 ± 6.9 | 232.9 ± 12.6 | 229.8 ± 23.1 | 225.1 ± 5.4 |
| Protein (g/d) | 90.1 ± 3.0 | 79.0 ± 3.6** | 85.6 ± 4.3 | 83.5 ± 2.7 | 57.6 ± 2.3 | 60.4 ± 2.9 | 51.3 ± 7.7 | 59.3 ± 1.6 |
| Fibre (g/d) | 14.1 ± 0.6 | 12.6 ± 0.5* | 11.6 ± 1.0 | 13.3 ± 0.4* | 10.4 ± 0.4 | 10.6 ± 0.8 | 10.2 ± 1.9 | 10.5 ± 0.3 |
| Cholesterol (mg/d) | 299 ± 18 | 258 ± 15* | 283 ± 32 | 275 ± 14 | 188 ± 13 | 204 ± 17 | 157 ± 39 | 197 ± 11 |
| Physical activity | ||||||||
| MPA (min/week) | 192 ± 20 | 189 ± 41 | 270 ± 86 | 184 ± 27** | 135 ± 16 | 124 ± 29 | 164 ± 73 | 128 ± 14 |
| VPA (min/week) | 314 ± 34 | 244 ± 25 | 324 ± 55 | 269 ± 21* | 171 ± 24 | 140 ± 27 | 55 ± 34 | 177 ± 19** |
| Health measures | ||||||||
| HOMA-IR | 4.5 ± 0.2 | 6.8 ± 0.7** | — | — | 4.4 ± 0.2 | 6.6 ± 0.5** | — | — |
| Inflammation (mg/L) | 0.3 ± 0.0 | 0.2 ± 0.0 | — | — | 0.4 ± 0.0 | 0.4 ± 0.1 | — | — |
Data are presented as mean ± standard errors.
MHO, metabolically healthy obesity; MUO, metabolically unhealthy obesity; PIR, poverty income ratio (socioeconomic status); WC, waist circumference; MPA, moderate physical activity; VPA, vigorous physical activity.
Variable not included in MHO definition.
MHO–MUO difference significant at:
p < 0.05;
p < 0.01.
Predictors of metabolically healthy obesity (unadjusted analyses)
First definition
In male adolescents, BMI percentile and WC were negatively associated with MHO, and lower carbohydrate intake, higher protein intake and higher cholesterol intake were associated with MHO (Table 2). Physical activity did not predict MHO. Lower insulin resistance but not inflammation was associated with MHO. In female adolescents, BMI percentile was not associated with MHO and, of the dietary components, only a higher polyunsaturated fat intake predicted MHO. Physical activity was not associated with MHO. Lower insulin resistance but not inflammation was associated with MHO.
Table 2.
Associations between obesity, diet, physical activity and laboratory measures with risk of prevalent MHO
| SE | Male
|
SE | Female
|
|||
|---|---|---|---|---|---|---|
| First definition
|
Second definition
|
First definition
|
Second definition
|
|||
| PR of MHO (95% CI) | PR of MHO (95% CI) | PR of MHO (95% CI) | PR of MHO (95% CI) | |||
| Obesity | ||||||
| BMI percentile | 0.1 | 0.97 (0.96–0.99)** | 0.96 (0.94–0.98)** | 0.1 | 0.98 (0.95–1.01) | 0.98 (0.95–1.01) |
| WC (cm) | 0.7 | 0.92 (0.88–0.96)** | 0.92 (0.88–0.96)** | 0.7 | 0.98 (0.96–1.00) | 0.95 (0.93–0.98)** |
| Diet | ||||||
| Energy (kcal) | 63 | 1.02 (1.00–1.04) | 1.00 (0.98–1.01) | 46 | 0.99 (0.98–1.01) | 1.00 (0.96–1.03) |
| Total fat (g)a | 2.9 | 1.02 (0.99–1.05) | 1.01 (0.97–1.10) | 2.3 | 1.02 (0.97–1.07) | 1.03 (0.97–1.09) |
| Saturated fat (g)a | 1.0 | 1.02 (0.99–1.04) | 1.04 (1.01–1.08)** | 0.9 | 0.98 (0.93–1.03) | 0.97 (0.90–1.04) |
| Monounsaturated fat (g)a | 1.1 | 1.02 (0.99–1.06) | 1.00 (0.96–1.04) | 0.9 | 1.00 (0.96–1.05) | 1.05 (1.01–1.10)* |
| Polyunsaturated fat (g)a | 0.8 | 1.00 (0.97–1.02) | 0.99 (0.97–1.00) | 0.5 | 1.05 (1.01–1.08)** | 1.04 (1.00–1.08) |
| Carbohydrates (g)b | 8.2 | 0.96 (0.92–1.00)* | 0.99 (0.95–1.02) | 5.5 | 1.00 (0.95–1.04) | 1.01 (0.96–1.07) |
| Protein (g)a | 2.5 | 1.03 (1.00–1.05)* | 1.01 (0.97–1.05) | 1.8 | 0.99 (0.96–1.03) | 0.94 (0.86–1.02) |
| Fibre (g)a | 0.4 | 1.00 (0.99–1.04) | 0.98 (0.95–1.01) | 0.4 | 1.00 (0.97–1.03) | 0.99 (0.95–1.03) |
| Cholesterol (mg)a | 13.0 | 1.01 (1.00–1.03)* | 1.01 (0.98–1.04) | 11.0 | 0.99 (0.97–1.02) | 0.97 (0.91–1.03) |
| Physical activity | ||||||
| MPA (min/week) | 27 | 1.00 (0.99–1.02) | 1.01 (0.98–1.04) | 14 | 1.00 (0.98–1.03) | 1.01 (0.97–1.06) |
| VPA (min/week) | 19 | 1.01 (1.00–1.02) | 1.01 (0.99–1.02) | 18 | 1.01 (0.99–1.03) | 0.93 (0.83–1.04) |
| MVPA (min/week) | 35 | 1.01 (1.00–1.02) | 1.01 (0.99–1.03) | 22 | 1.01 (0.99–1.02) | 0.98 (0.94–1.03) |
| Laboratory measures | ||||||
| Insulin resistance | 0.4 | 0.91 (0.88–0.95)** | — | 0.2 | 0.95 (0.92–0.97)** | — |
| Inflammation (mg/L) | 0.0 | 1.00 (0.99–1.02) | — | 0.0 | 0.99 (0.97–1.01) | — |
Prevalent risk (PR) is presented per unit SE and adjusted for age and PIR (socioeconomic status).
MHO, metabolically healthy obesity; MUO, metabolically unhealthy obesity; PR, prevalent risk; CI, confidence intervals; SD, standard error; WC, waist circumference; MPA, moderate physical activity; VPA, vigorous physical activity; MVPA, moderate-vigorous physical activity.
Variable not included in MHO definition.
Adjusted for energy intake.
Adjusted for energy and fibre intakes.
PR significant at:
p < 0.05;
p < 0.01
Second definition
In male adolescents, BMI percentile and WC were negatively associated with MHO (Table 2). Of the dietary components, only saturated fat intake was positively associated with MHO. Physical activity was not associated with MHO. In female adolescents, lower WC and higher monounsaturated fat intake were associated with the MHO. Physical activity was not associated with MHO.
Predictors of metabolically healthy obesity (adjusted analyses)
To determine the independent associations between obesity, diet, physical activity and laboratory measures with MHO, the most significant obesity, diet, physical activity and laboratory correlates of MHO were entered into a mutually adjusted model with further adjustment for age and socioeconomic status.
First definition
In male adolescents, the model included WC, carbohydrate intake, VPA and insulin resistance, of which only WC [PR{95%} = 0.97{0.95–0.99}] and insulin resistance [PR{95%} = 0.94{0.91–0.97}] remained significantly associated with MHO. In female adolescents, the mutually adjusted model for the first definition of MHO included WC, polyunsaturated fat, MVPA and insulin resistance, of which higher polyunsaturated fat intake [PR{95%} = 1.02{1.00–1.04}] and lower insulin resistance [PR{95%} = 0.95{0.92–0.98}] remained independently associated with MHO.
Second definition
The model in male adolescents included WC, saturated fat and MVPA, of which WC [PR{95%} = 0.92 {0.88–0.96}] remained negatively associated with MHO. The model in female adolescents included WC, monounsaturated fat and VPA, of which only WC remained associated with MHO [PR{95%} = 0.95{0.93–0.97}].
Discussion
The present study investigated the prevalence and predictors of MHO in a nationally representative sample of adolescents with obesity. We demonstrated that 42% of male and 74% female adolescents were classified as MHO using the most commonly used definition, while only 7% of male and 12% of female adolescents with obesity could be classified as MHO using our more conservative definition (i.e. free of all metabolic syndrome risk factors, insulin resistance and inflammation). Lower WC and insulin resistance levels were the most consistent predictors of MHO in both male and female adolescents. Overall, no individual dietary components were consistently related to MHO, and physical activity did not predict MHO.
Previous studies reporting that the prevalence of MHO varies between 16% and 68% have used different approaches but are generally use components of the metabolic syndrome (3–6,8,9) and/or insulin resistance (4,7). These definitions vary in the number of criteria and cut-offs used to define MHO and may be problematic given that individuals with isolated type 2 diabetes, hypertension or dyslipidemia may be considered ‘healthy’ using these frameworks. Our first definition precludes individuals with any of these conditions even if they have only one metabolic syndrome criteria, and our second definition requires individuals to be free of all metabolic syndrome criteria, insulin resistance and inflammation. Using this more conservative second definition, we demonstrate that the prevalence may be even lower (7–12%) than what is typically reported. Further, the inclusion of additional metabolic criteria that are not normally assessed such as adiponectin, resistin, apolipoproteins or euglycemic–hyperinsulinemic clamp measured insulin sensitivity may further reduce the prevalence of MHO. Currently, which definition is more indicative of healthy and the longer term clinical implications for each ‘healthy’ phenotype are is unclear.
The aetiology of metabolic health has been debated with some arguing that insulin resistance is the central feature, while others suggest that obesity is the primary factor (2). The degree of obesity is one of the most consistent predictors of MHO in adolescents (4,5,12), which may suggest that MHO may not truly exist at higher levels of obesity. We observe in accordance with the literature (26) that abdominal obesity is a stronger correlate of MHO as compared with overall obesity as assessed by BMI percentile. This demonstrates the importance of assessing obesity phenotype as opposed to just weight relative to height when assessing health risk. Insulin resistance is not examined as frequently but is also identified as an important predictor of MHO (6,7,12). Our findings indicated that both abdominal obesity and insulin resistance were predictors of MHO in obese adolescents, independent of lifestyle factors. Insulin resistance, however, was observed to be more closely associated with MHO than obesity, especially in female adolescents. Within our MHO youth (first definition), we observe that although 31% were free of insulin resistance, only 7% had a normal WC and only 4% had both insulin sensitivity and a normal WC (data not shown). Both insulin resistance and obesity are modifiable by lifestyle changes; however, in our sample, the associations between dietary components and metabolic health in youth were relatively small and inconsistent. Nevertheless, this is consistent with the MHO literature in which only one previous study has reported a negative (albeit small) association between dietary fat intake and MHO status in youth (4) but is more consistently related with insulin resistance (27). Conversely, we and Camhi et al. (9) both observe the unexpected finding that saturated fat intake is positively associated with prevalent MHO status in male adolescents using our second definition. Given the widely established link between saturated fat intake and dyslipidemia, these observations may be due to reverse causation, wherein Metabolically Abnormal Obesity (MAO) are attempting to improve their diet in response to their elevated health risk profile. However, saturated fat was not associated with metabolic status in our fully adjusted model. Conversely, polyunsaturated fat remained independently associated with MHO in female adolescents. Clearly, further study is needed to confirm how diet relates with MHO status. Similarly, the relationship between physical activity and MHO status in youth is less clear as only three studies have investigated this relationship. One of these studies reported a positive association (4) between physical activity and MHO, whereas the study by Camhi et al. (3) and the present study reported no association. The absence of an association between physical activity and MHO in the present study may be explained by the high prevalence of adolescents participating in at least one bout of physical activity per week (87%) as most of the health benefits are obtained when transitioning from a state of inactivity to engaging in any activity (28). Alternatively, factors such as musculoskeletal or cardiorespiratory fitness or sedentary behaviour were not available but may provide added insight as to why some youth with obesity develop metabolic aberrations versus those who do not.
We acknowledge that there are several limitations to consider in our research. First, data on diet and physical activity are subject to self-reporting biases (e.g. under-reporting is common in obese and adolescent populations (29)). However, this should not affect the integrity of our results, unless MHO and MUO adolescents reported these behaviours differently. Further, we do not have measures of pubertal status, which has known effects on metabolic health (30). This study is cross-sectional in design, and thus, causality cannot be inferred from the associations observed. Our sample of MHO was relatively small using the second definition and thus increases the possibility that our observations are due to chance. This may explain why we observe some unexpected findings such as a positive association between saturated fat and MHO that need to be confirmed with additional study. Nevertheless, this study has used a sample that is representative of the US adolescent population and clearly illustrates that the prevalence of MHO can vary widely depending on the criteria used.
In conclusion, this study demonstrated that 7% to 74% of youth with obesity are metabolically healthy depending on the definition used. The likelihood of being in the MHO group decreased among adolescents with higher levels of abdominal obesity and insulin resistance. We showed that diet, physical activity and inflammation were not consistent predictors of MHO. Our findings highlight the need for effective strategies for adolescents with obesity to limit the severity of obesity and manage insulin resistance to achieve and maintain health.
Acknowledgments
Funding Sources
J. L. K. received funding from the Canadian Institutes of Health Research (no. 131594) and York University to conduct the study.
Footnotes
Conflict of Interest Statement
No conflict of interest was declared.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Steinberger J, Daniels SR, Eckel RH, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 3.Camhi SM, Waring ME, Sisson SB, Hayman LL, Must A. Physical activity and screen time in metabolically healthy obese phenotypes in adolescents and adults. J Obes. 2013;2013:984613. doi: 10.1155/2013/984613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prince RL, Kuk JL, Ambler KA, Dhaliwal J, Ball GD. Predictors of metabolically healthy obesity in children. Diabetes Care. 2014;37:1462–8. doi: 10.2337/dc13-1697. [DOI] [PubMed] [Google Scholar]
- 5.Senechal M, Wicklow B, Wittmeier K, et al. Cardiorespiratory fitness and adiposity in metabolically healthy overweight and obese youth. Pediatrics. 2013;132:e85–92. doi: 10.1542/peds.2013-0296. [DOI] [PubMed] [Google Scholar]
- 6.Mangge H, Zelzer S, Puerstner P, et al. Uric acid best predicts metabolically unhealthy obesity with increased cardiovascular risk in youth and adults. Obesity (Silver Spring) 2013;21:E71–7. doi: 10.1002/oby.20061. [DOI] [PubMed] [Google Scholar]
- 7.Vukovic R, Mitrovic K, Milenkovic T, et al. Insulin-sensitive obese children display a favorable metabolic profile. Eur J Pediatr. 2013;172:201–6. doi: 10.1007/s00431-012-1867-5. [DOI] [PubMed] [Google Scholar]
- 8.Camhi SM, Katzmarzyk PT. Prevalence of cardiometabolic risk factor clustering and body mass index in adolescents. J Pediatr. 2011;159:303–7. doi: 10.1016/j.jpeds.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 9.Camhi SM, Whitney Evans E, Hayman LL, Lichtenstein AH, Must A. Healthy eating index and metabolically healthy obesity in U.S. adolescents and adults. Prev Med. 2015;77:23–7. doi: 10.1016/j.ypmed.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Tataranni PA. Treatment of obesity: should we target the individual or society? Curr Pharm Des. 2003;9:1151–63. doi: 10.2174/1381612033454946. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Chen W, Srinivasan SR, Xu J, Berenson GS. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol. 2012;176(Suppl 7):S142–9. doi: 10.1093/aje/kws236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weghuber D, Zelzer S, Stelzer I, et al. High risk vs. “metabolically healthy” phenotype in juvenile obesity – neck subcutaneous adipose tissue and serum uric acid are clinically relevant. Exp Clin Endocrinol Diabetes. 2013;121:384–90. doi: 10.1055/s-0033-1341440. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. [accessed June 2015];NHANES – continuous NHANES web tutorial – sample design [WWWdocument] 2013 http://www.cdc.gov/nchs/tutorials/nhanes/SurveyDesign/SampleDesign/intro.htm.
- 14.Centers for Disease Control and Prevention. [accessed June 2015];NHANES examination data – body measures [WWWdocument] 2015 http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Examination.
- 15.Katzmarzyk PT, Perusse L, Rao DC, Bouchard C. Familial risk of obesity and central adipose tissue distribution in the general Canadian population. Am J Epidemiol. 1999;149:933–942. doi: 10.1093/oxfordjournals.aje.a009737. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. [accessed June 2015];A SAS program for the 2000 CDC growth charts (ages 0 to <20 years) [WWWdocument] 2015 http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 17.Centers for Disease Control and Prevention. [accessed June 2015];NHANES dietary data [WWWdocument] 2015 http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Dietary.
- 18.Centers for Disease Control and Prevention. [accessed June 2015];NHANES questionnaire data – physical activity [WWWdocument] 2015 http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Questionnaire.
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. [accessed June 2015];NHANES laboratory data [WWWdocument] 2015 http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory.
- 21.Expert Panel on Integrated Guidelines for Cardiovascular, H, Risk Reduction in, C, Adolescents National Heart, L and Blood, I. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–56. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 23.Executive Summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115:e500–3. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 26.Bauer KW, Marcus MD, El ghormli L, Ogden CL, Foster GD. Cardio-metabolic risk screening among adolescents: understanding the utility of body mass index, waist circumference and waist to height ratio. Pediatric Obesity. 2014 doi: 10.1111/ijpo.267. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weigensberg MJ, Ball GDC, Shaibi GQ, Cruz ML, Gower BA, Goran MI. Dietary fat intake and insulin resistance in Black and White children. Obes Res. 2005;13:1630–7. doi: 10.1038/oby.2005.200. [DOI] [PubMed] [Google Scholar]
- 28.Powell KE, Paluch AE, Blair SN. Physical activity for health: what kind? How much? How intense? On top of what? Annu Rev Public Health. 2011;32:349–65. doi: 10.1146/annurev-publhealth-031210-101151. [DOI] [PubMed] [Google Scholar]
- 29.Hill RJ, Davies PS. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr. 2001;85:415–30. doi: 10.1079/bjn2000281. [DOI] [PubMed] [Google Scholar]
- 30.Tobisch B, Blatniczky L, Barkai L. Cardiometabolic risk factors and insulin resistance in obese children and adolescents: relation to puberty. Pediatric Obesity. 2015;10:37–44. doi: 10.1111/j.2047-6310.2013.00202.x. [DOI] [PubMed] [Google Scholar]