Abstract
IMPORTANCE
Long-acting, injectable, second-generation antipsychotic medication has tremendous potential to bring clinical stability to persons with schizophrenia. However, long-acting medications are rarely used following a first episode of schizophrenia.
OBJECTIVE
To compare the clinical efficacy of the long-acting injectable formulation of risperidone with the oral formulation in the early course of schizophrenia.
DESIGN, SETTING, AND PARTICIPANTS
A randomized clinical trial performed at a university-based research clinic, between 2005 and 2012. Eighty-six patients with recent onset of schizophrenia were randomized to receive long-acting injectable risperidone or oral risperidone. Half of each group was simultaneously randomized to receive cognitive remediation to improve cognitive functioning or healthy-behaviors training to improve lifestyle habits and well-being. An intent-to-treat analysis was performed between October 4, 2012, and November 12, 2014.
INTERVENTIONS
A 12-month trial comparing the long-acting injectable vs oral risperidone and cognitive remediation vs healthy-behaviors training.
MAIN OUTCOMES AND MEASURES
Psychotic relapse and control of breakthrough psychotic symptoms.
RESULTS
Of the 86 patients randomized, 3 refused treatment in the long-acting injectable risperidone group. The psychotic exacerbation and/or relapse rate was lower for the long-acting risperidone group compared with the oral group (5% vs 33%; χ21 = 11.1; P < .001; relative risk reduction, 84.7%). Long-acting injectable risperidone better controlled mean levels of hallucinations and delusions throughout follow-up (β = −0.30; t68 = −2.6, P = .01). The cognitive remediation and healthy-behaviors training groups did not differ significantly regarding psychotic relapse, psychotic symptom control, or hospitalization rates, and there were no significant interactions between the 2 medications and the 2 psychosocial treatments. Discontinuations owing to inadequate clinical response were more common in the oral group than in the long-acting risperidone group (χ21 = 6.1; P = .01). Adherence to oral risperidone did not appear to differ before randomization but was better for the long-acting risperidone group compared with the oral group (t80 = 5.3; P < .001). Medication adherence was associated with prevention of exacerbation and/or relapse (χ21 =11.1; P = .003) and control of breakthrough psychotic symptoms (β = 0.2; t79 = 2.1; P = .04).
CONCLUSIONS AND RELEVANCE
The use of long-acting injectable risperidone after a first episode of schizophrenia has notable advantages for clinical outcomes. The key clinical advantages are apparently owing to the more consistent administration of the long-acting injectable. Such formulations should be offered earlier in the course of illness.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00333177
An earlier study1 found that nonadherence with oral risperidone strongly predicted the return of psychotic symptoms in patients with a first episode of schizophrenia. Oral antipsychotic medication requires daily recommitment to treatment, which is hampered by patients’ poor awareness of the need for continued treatment. Long-acting injectable antipsychotic medication has the potential to control psychotic symptoms substantially better than short-acting medication.2–4 However, randomized clinical trials (RCTs) of long-acting injectable antipsychotics have generally not been convincing in this regard.5–12 Long-acting injectable antipsychotics are typically not used in the period following a first psychotic episode, instead being reserved for patients with demonstrated medication nonadherence. Ironically, when adherent, patients with a recent onset of schizophrenia usually have a more robust response to antipsychotic medications. Thus, given their frequent nonadherence with oral antipsychotics, recent-onset patients may show the clearest advantages of treatment with long-acting injectable antipsychotics.
We compared the clinical efficacy of long-acting injectable risperidone with the oral form of risperidone in a 12-month RCT of patients with a recent onset of schizophrenia. Two other studies of long-acting injectable risperidone in first-episode patients found no lasting advantage for adherence or psychotic symptom control.5,13 However, neither study examined the same second-generation antipsychotic molecule, risperidone, in the oral and long-acting injectable forms, but instead continued giving patients in the oral comparator condition whatever second-generation oral antipsychotic they had already been prescribed. This would give an advantage to the oral-medication condition because the patients and psychiatrists might have settled on particular medications and dosages after a period of individualized trial-and-error attempts to find effective treatment. This study is the first direct comparison, to our knowledge, of oral vs long-acting injectable versions of the same second-generation antipsychotic molecule, risperidone, in patients with a recent first episode of schizophrenia.
Methods
Participants
The sample consisted of 83 individuals recruited from Los Angeles–area psychiatric hospitals and clinics, outpatient treatment facilities, and private psychiatry practices. Participants were not recruited or referred to us based on their level of previous medication adherence. All study participants received out patient psychiatric treatment at the UCLA Aftercare Research Program between March 16, 2005, and September 27, 2012.14–16 Typically, the outpatient psychiatric treatment involved clinic visits 1 to 2 days per week. This study was approved by the UCLA Institutional Review Board, and all participants gave written informed consent. The complete study protocol appears in Supplement 1.
Study Inclusion and Exclusion Criteria
Entry diagnostic criteria were (1) a recent onset of psychotic illness, with the beginning of the first major psychotic episode within the past 2 years, and (2) a diagnosis using the DSM-IV of schizophrenia, schizoaffective disorder, depressed type, or schizophreniform disorder. Additional criteria can be found in the eAppendix in Supplement 2.
Randomized Treatment With Oral or Long-Acting Injectable Risperidone
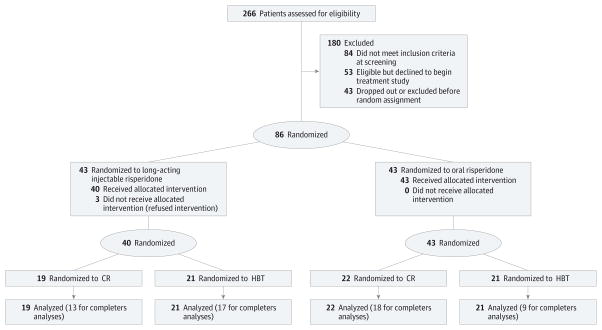
All participants were provided treatment with antipsychotic medication, regular psychiatrist visits, and weekly individual case management by master’s and doctoral degree–level therapists. After a brief lead-in period of oral risperidone as the sole antipsychotic medication for at least 3 weeks, participants participated in baseline assessments and then were randomly assigned to continued treatment with oral risperidone or to long-acting injectable risperidone. Participants were also simultaneously randomly assigned to receive cognitive remediation (CR) or healthy-behaviors training (HBT) in a fully crossed 2 × 2 design, with the intention of improving cognitive functioning in the CR group and lifestyle habits and well-being in the HBT group. See Figure 1 for the recruitment and enrollment flowchart. For procedures regarding measurement of medication nonadherence, see the Methods section in the eAppendix in Supplement 2.
Figure 1. CONSORT Flow Diagram of Progress Through the Randomized Controlled Trial Phases.
CR indicates cognitive remediation; HBT, healthy-behaviors training.
Randomized Psychosocial Treatment: CR or HBT
The patients were randomly assigned to receive either CR or HBT fully crossed with the medication conditions (2 × 2 design). Patients in CR participated in a computer-assisted cognitive training program for 6 months, 2 hours per week, followed by 1 hour per week for another 6 months, as described elsewhere.16 The participants in HBT were instructed in relaxation training, developing healthy eating habits, and light exercise for an equal number of hours per week, rotating the topics every 3 weeks.17,18 An employment specialist worked with patients in both conditions to assist in finding employment or returning to school using the Individual Placement and Support model.14 See the Methods section in the eAppendix in Supplement 2 for additional details.
Primary Outcome: Psychotic Exacerbation and/or Relapse
The expanded 24-item version of the Brief Psychiatric Rating Scale (BPRS)19 was used to rate patients every 2 weeks, with each item rated from 1 (not present) to 7 (extremely severe). Exacerbation and/or relapse was identified based on increases in the BPRS items unusual thought content, hallucinations, or conceptual disorganization using computer scoring algorithms.20 The 3 types of psychotic symptom return, remission followed by relapse, remission followed by significant exacerbation, or persisting symptoms followed by significant exacerbation, did not consider other aspects of outcome, such as hospitalization or role functioning. See the Methods section in the eAppendix in Supplement 2 for additional details.
Secondary Outcomes
Psychiatric Hospitalizations
Psychiatric hospitalizations that occurred during the follow-up period were examined independently of symptom return. Hospitalizations often occurred owing to suicidality, aggression, or agitation rather than psychotic symptoms.
Psychotic Symptom Control
The proportion of the follow-up period during which BPRS hallucinations and unusual thought content rated less than 4 on the BPRS (ie, the nonpathological range) was used as the index of psychotic symptom control. See the Methods section in the eAppendix in Supplement 2 for additional details.
Drug Discontinuation
We tallied the clinical decisions to discontinue either oral or long-acting injectable risperidone that were owing to lack of clinical efficacy, intolerable adverse effects, and patients’ decisions to withdraw from the program.
Statistical Analysis
Our analyses were performed between October 4, 2012, and November 12, 2014. The primary analyses were conducted in an intent-to-treat analysis with all 83 patients who began the RCT, including 5 who discontinued treatment before 8 weeks and another 21 who dropped out or switched antipsychotic medication before completing the 12-month study. Psychotic symptoms were not examined after the end of the study. The mean (SD) length of follow-up (either completing the 12-month protocol or discontinuing for any reason) was 10.2 (98.4) months (309 days; range, 70–365). Secondary analyses were conducted with the 57 patients (oral risperidone group, 27 patients; long-acting injectable risperidone group, 30 patients) who completed the 12-month medication protocol (eAppendix in Supplement 2). Because longer initial stabilization periods might lead to greater clinical stability at baseline, the length of the initial stabilization period was included as a covariate in all analyses. No adjustment was made for multiple comparisons because exacerbation and/or relapse was the only primary outcome.
Results
Sample Characteristics
The participants had an age level, educational level, and sex distribution typical of individuals with a first episode of psychosis (Table 1). The modal dosage of oral risperidone was 2 mg/d, although a minority of participants needed substantially higher dosages, raising the mean (SD) dosage to 3.6 (1.9) mg/d (range, 1.0–7.5 mg/d). The modal dosage for long-acting injectable risperidone was 25 mg every 2 weeks (mean [SD], 26.3 [0.15] mg; range, 12.5–37.5 mg). All patients in the long-acting injectable risperidone group first received a minimum of 2 sequential 25-mg injections. See eAppendix in Supplement 2 for additional details.
Table 1.
Demographic and Clinical Characteristics of 83 Study Patients
| Characteristic | Oral Risperidonea | Long-Acting Injectable Risperidonea | Totala |
|---|---|---|---|
| Age, mean (SD), y | 21.1 (3.2) | 21.9 (3.8) | 21.5 (3.5) |
| Educational level, mean (SD), y | 12.4 (1.5) | 12.6 (2.0) | 12.5 (1.7) |
| Parental educational level, mean (SD), y | 13.5 (4.1) | 14.4 (3.5) | 14.0 (3.8) |
| Time since psychosis onset, mean (SD), mo | 7.9 (6.6) | 6.9 (6.8) | 7.4 (6.9) |
| Male sex | 79 | 78 | 78 |
| Marital status | |||
| Single | 96 | 96 | 96 |
| Married | 4 | 4 | 4 |
| Separated/divorced | 0 | 0 | 0 |
| Race | |||
| White | 54 | 45 | 49 |
| Asian | 9 | 13 | 11 |
| Native American | 7 | 2 | 5 |
| African American | 21 | 35 | 28 |
| Pacific Islander | 2 | 0 | 1 |
| Mixed | 7 | 5 | 6 |
| Ethnicity | |||
| Hispanic | 46 | 38 | 42 |
| Non-Hispanic | 54 | 62 | 58 |
| Diagnosis | |||
| Schizophrenia | 61 | 50 | 55 |
| Schizoaffective disorder | 9 | 15 | 12 |
| Schizophreniform disorder | 30 | 35 | 33 |
| BPRS rating, mean (SD) | |||
| Thought disturbance factor21 at entry | 3.5 (1.6) | 3.8 (1.5) | 3.6 (1.5) |
| Withdrawal-retardation factor at entry | 2.0 (1.1) | 2.3 (0.8) | 2.1 (1.0) |
| Thought disturbance factor at randomization | 2.2 (1.2) | 1.9 (1.0) | 2.1 (1.1) |
| Withdrawal-retardation factor at randomization | 1.8 (0.8) | 1.9 (0.9) | 1.9 (0.9) |
Abbreviation: BPRS, Brief Psychiatric Rating scale.
Values are presented as the percentage of patients unless otherwise indicated. No difference between groups was significant at P < .05.
Acceptance of Injectable Medication
No participants who had consented to the protocol at study entry subsequently dropped out before randomization because of concern about the possibility of being assigned to receive injectable medication. Only 3 of 43 patients (7.0%) who were randomly assigned to the long-acting injectable risperidone group refused to continue treatment at the time of randomization.
Effects of the Psychosocial Treatment Condition
For all analyses involving the comparison of oral vs long-acting injectable risperidone, the effects of the psychosocial treatment (CR vs HBT) and the interaction of the medication condition and psychosocial treatment were entered as additional predictor variables in a stepwise manner after the basic medication-condition variable (or medication adherence–level measure).
Primary Outcome: Psychotic Exacerbation and/or Relapse
Sixteen of 83 patients (19%) had a return of psychotic symptoms that met the exacerbation and/or relapse criteria during the 12-month follow-up period. We tested the combined effects of the medication group and psychosocial intervention on the return of psychotic symptoms using hierarchical log-linear models, first the 3-way interaction between the 3 variables of interest (medication × psychosocial × relapse/no relapse) and then, if the 3-way interaction was not significant, all 2-way interactions among the variables. There was no significant 3-way interaction among the psychosocial condition, medication condition, and relapse (P = .88). The rate of return of psychosis was comparable in the 2 psychosocial treatments: 9 of 41 (22%) in the CR group and 7 of 42 (17%) in the HBT group (χ21 = 0.3; P = .60). There was a strong advantage of long-acting injectable risperidone for psychotic exacerbation and/or relapse; 2 of 40 patients (5%) in the long-acting injectable risperidone group experienced a psychotic exacerbation and/or relapse compared with 14 of 43 (33%) in the oral group (χ21 = 11.1; P < .001). The relative risk reduction for long-acting injectable compared with oral risperidone was 84.7%.
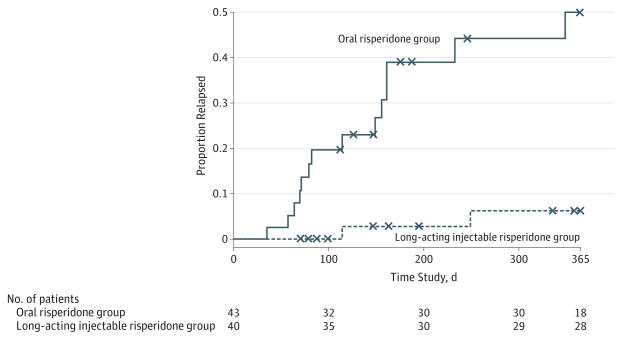
We examined the rate of exacerbation and/or relapse over time in a Cox proportional hazards regression survival analysis. There were no significant psychosocial (P = .76) or psychosocial × medication group (P = .83) effects. Therefore, we examined the medication main effect in the more parsimonious model without these terms. The risk of exacerbation and/or relapse over time was significantly lower for long-acting injectable risperidone than for oral risperidone (β = 2.2; Wald = 8.2; df=1; P < .004; meantime to relapse, 298.5 vs 218.6 days, respectively) (Figure 2). Twelve of the 14 relapses (86%) among patients who received oral risperidone occurred during the first 6 months following randomization (mean [SD], 127.1 [84.4] days; range, 35–349), whereas the 2 relapses among patients who received long-acting injectable risperidone occurred at approximately 4 and 8 months following initiation of treatment (mean [SD], 182.0 [96.2] days).
Figure 2. Time to First Psychotic Exacerbation and/or Relapse as a Function of Form of Medication Administration in 83 Patients.
The risk of exacerbation and/or relapse over time was significantly lower for the long-acting injectable risperidone group than for the oral risperidone group. x Indicates censored data.
See the eAppendix in Supplement 2 for additional details of participants who completed the 12-month medication study (completers).
Secondary Outcomes
Psychiatric Hospitalizations
The proportion of patients who required psychiatric hospitalization was significantly lower for long-acting injectable risperidone (2 of 40 [5.0%]) than for oral risperidone (8 of 43 [18.6%]; ; P = .05, hierarchical log-linear model). Cumulative hospital days were a mean of 1.3 days less for the long-acting injectable risperidone group (0.5 days) compared with the oral risperidone group (1.8 days) (t68 = 1.9; P = .07). See the eAppendix in Supplement 2 for additional details regarding hospitalizations.
Control of Psychotic Symptoms During Follow-up
There were no significant effects of the psychosocial condition or interaction effects between the psychosocial and medication conditions, so the main effects of the medication condition are reported here. Long-acting injectable risperidone was significantly associated with lower mean levels of BPRS hallucinations and unusual thought content during the follow-up period (β = −0.30; t68 = −2.6; P = .01). See the eAppendix in Supplement 2 for additional details.
Drug Discontinuation
The long-acting injectable and oral risperidone groups did not appear to differ in the mean length of the follow-up assessment period (mean [SD], 308 [108.3] days vs 277 [123.5] days; t81 = −1.2; P = .24). There was a significant interaction between the medication and psychosocial conditions (hierarchical log-linear model, χ21 = 7.2; P = .007), reflecting a higher rate of discontinuation for any reason for patients in the HBT condition who were prescribed oral risperidone. The rate of early treatment discontinuation owing to intolerable adverse effects did not appear to differ between the long-acting injectable and oral risperidone groups (10% vs 21%; χ21 = 2.1; P = .14). However, fewer patients who were treated with long-acting injectable risperidone discontinued therapy (1 of 40 [2.5%]) because of inadequate treatment response than those who received oral risperidone (7 of 42 [17%]; hierarchical log-linear model, ; P = .01). There was no significant medication by psychosocial treatment condition interaction effect (P = .33), but fewer patients who received CR (0 of 40 [0%]) discontinued therapy owing to inadequate treatment response than those who received HBT (8 of 42 [19%]) (psychosocial main effect, χ21 = 12.4; P < .001). See the eAppendix in Supplement 2 and Table 2 for additional details regarding discontinuation and adverse effects.
Table 2.
Adverse Effects of Oral vs Long-Acting Injectable Risperidone
| Adverse Effect Variable | Oral Risperidonea
|
Long-Acting Injectable Risperidonea
|
||
|---|---|---|---|---|
| Baseline | End Point | Baseline | End Point | |
| Akathisia | ||||
|
| ||||
| Severity, mean (SD)b | 0.2 (0.8) | 0.4 (0.8) | 0.3 (0.5) | 0.2 (0.4) |
|
| ||||
| Proportion rated ≥2, % | 5.9 | 3.3 | 0 | 0 |
|
| ||||
| Proportion rated ≥3, % | 2.9 | 3.3 | 0 | 0 |
|
| ||||
| Involuntary movements | ||||
|
| ||||
| Severity, mean (SD)c | 0.2 (0.6) | 0.1 (0.4) | 0.0 (0.0) | 0.03 (0.2) |
|
| ||||
| Proportion rated ≥2 on any item, % | 5.9 | 10.0 | 0 | 0 |
|
| ||||
| Proportion rated ≥3 on any item, % | 0 | 3.3 | 0 | 0 |
|
| ||||
| BMI, mean (SD) | 27.0 (5.5) | 28.3 (6.7) | 28.8 (5.1) | 30.8 (6.1) |
|
| ||||
| Total cholesterol level, mean (SD) | 179.7 (5.8) | 171.9 (7.0) | 179.4 (5.9) | 177.9 (6.6) |
|
| ||||
| Hemoglobin A1c level, mean (SD) | 5.3 (0.3) | 5.3 (0.3) | 5.4 (0.7) | 5.7 (1.3) |
|
| ||||
| Prolactin level, mean (SD) | 57.3 (41.7) | 41.6 (22.8) | 56.8 (36.3) | 36.0 (18.6) |
|
| ||||
| Blood pressure, mean (SD), mm Hg | ||||
|
| ||||
| Systolic | 111.1 (10.0) | 117.2 (11.6) | 112.7 (12.3) | 119.2 (11.4) |
|
| ||||
| Diastolic | 70.9 (7.2) | 72.5 (11.5) | 71.9 (8.5) | 75.9 (7.1) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
SI conversion factors: To convert total cholesterol to millimoles per liter, multiply by 0.0259; hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01; prolactin to picomoles per liter, multiply by 43.478.
No change in score differences between groups were significant at P < .05.
The severity item of the Barnes Rating Scale for Akathisia is rated on a scale from 0 (absent) to 5 (severe akathisia).
The Abnormal Involuntary Movement Scale items are rated on a scale of 0 (none) to 4 (severe).
Effect of Injectable Risperidone on Medication Adherence
Adherence to oral risperidone treatment before randomization did not appear to differ between the 2 treatment groups (mean for long-acting injectable risperidone, 2.1 vs 2.0 for oral risperidone [on a scale of 1 to 5, in which 1 indicates perfect adherence and 5, complete nonadherence]; t73 = 0.58; P = .56). However, treatment with long-acting injectable risperidone led to significantly better medication adherence averaged over the follow-up period (mean [SD], 1.1 [0.5]) compared with oral risperidone (mean [SD], 1.9 [0.8]; t80 = 5.3; P < .001) despite our best efforts to engage the patients receiving oral medication and maximize their adherence. Ninety-five percent of the patients in the long-acting injectable risperidone group had excellent levels of adherence (<1.5 on a scale of 1 to 5, in which 1 indicates perfect adherence and 5, complete nonadherence) averaged over the follow-up period, whereas only 33% of the patients who received oral risperidone had excellent levels. Medication adherence during the initial 6 months following randomization was highly correlated with the level of adherence during the subsequent 6-month period (r = 0.77; P < .001; n = 67).
Primary Outcome: Psychotic Exacerbation and/or Relapse
The measure of adherence across the 2 medication groups was significantly associated with proportionally fewer positive psychotic symptom exacerbations and/or relapses during the 12-month follow-up period (logistic regression β = 1.1; Wald = 9.1; df = 1; P = .003). When the medication group assignment was entered into the logistic regression after the adherence variable, the main effect of the medication condition was statistically significant (β = −1.9; Wald = 4.3; df=1; P =.04). This result suggests that the effect of the medication conditions assignment (oral vs long-acting injectable risperidone) on relapse cannot entirely account for differences in adherence in the 2 conditions when patients who did not complete the medication protocol were included in the analyses.
Similarly, there were no significant psychosocial (P = .90) or psychosocial × adherence (P = .70) effects on the likelihood of exacerbation and/or relapse over time in a survival analysis. Therefore, we examined the medication main effect of adherence on exacerbation and/or relapse, which showed that the time without exacerbation and/or relapse was significantly associated with adherence (β = 1.0; Wald = 12.3; df = 1; P < .001). See the eAppendix in Supplement 2 for additional details.
Secondary Outcomes
Psychiatric Hospitalizations
There were no significant effects of the psychosocial conditions or interactions between the psychosocial and medication adherence. Medication adherence significantly predicted the need for psychiatric hospitalization (logistic regression β = 1.1; Wald = 7.4; df = 1; P = .007). The analyses with study completers produced similar results (logistic regression β = 1.3; Wald = 5.7; df = 1; P = .02).
Control of Psychotic Symptoms During Follow-up
No significant effects of the psychosocial condition or interactions between the psychosocial condition and medication adherence were observed. Across both medication groups, patients with better medication adherence had greater total time with hallucinations and unusual thought content in the nonpathological range (ie, <4 on the BPRS; multiple regression analysis β = 0.2; t79 = 2.1; P = .04). Medication adherence was also associated with lower mean levels of these 2 symptoms (β = 0.3; t67 = 2.1; P = .04). Entering the medication group assignment after the medication adherence variable in the regression analyses for protocol completers resulted in nonsignificant effects for the medication group assignment for control of breakthrough hallucinations and unusual thought content (β = −.04; t78 = −0.3; P = .76) and for mean psychotic symptom level (β = −0.2; t66 = −1.3; P = .21), suggesting that the advantage for long-acting injectable risperidone was attributable to greater adherence. See the eAppendix in Supplement 2 for additional details on the protocol completers as well as relationships of plasma medication levels to outcome measures.
Discussion
The advantages of long-acting injectable risperidone for psychotic symptom control demonstrate that it is an excellent choice of antipsychotic medication for first-episode patients. We find that long-acting injectable antipsychotic medication is readily accepted by patients with a first episode of schizophrenia and that adherence to long-acting injectable risperidone therapy surpasses that of oral risperidone. Furthermore, long-acting injectable risperidone conferred a number of advantages over oral risperidone for relapse prevention and psychotic symptom control. There was a striking 6-fold difference in 12-month relapse rates between the 2 medication groups. Despite the greater adherence to long-acting injectable risperidone therapy, the occurrence of adverse effects and treatment discontinuation owing to adverse effects appeared similar to that of oral risperidone. Discontinuation of long-acting injectable risperidone therapy owing to inadequate clinical response was rare. The 2 psychosocial interventions, CR and HBT, did not differ significantly from each other in control or prevention of the return of psychotic symptoms or rate of hospitalization.
These findings, which clearly favor the long-acting injectable form of risperidone, conflict with a number of recent studies of patients with chronic schizophrenia that showed no advantage for relapse prevention with long-acting injectable medications compared with oral antipsychotics.12 The key difference is the focus, in this study, on patients with a recent first episode of schizophrenia. Poor awareness of having a psychotic disorder accompanied by poor awareness of the need for medication is the norm in this early phase. Furthermore, individuals with a single episode of schizophrenia who have responded well to antipsychotic medication, even if they understand that they have a mental disorder, very often doubt whether medication continues to be necessary. Often they become nonadherent, either overtly or covertly. Nonadherence to an oral medication regimen is often difficult to detect. The nonadherence to oral risperidone therapy occurred despite our vigorous efforts to keep all patients adherent, including monitoring of medication adherence using frequent pill counts, blood tests, and the Medication Event Monitoring System. In contrast, nonadherence to long-acting injectable risperidone therapy is obvious and readily actionable, as seen in the nearly perfect adherence in this sample.
Two other studies of long-acting injectable risperidone in first-episode patients compared long-acting injectable risperidone with any second-generation oral antipsychotic medication and found no lasting advantage of long-acting injectable risperidone for adherence or psychotic symptom control.5,13 Neither study provided the frequent clinic visits and the rich offerings of psychosocial services that were present in this study. One study,5 as noted by the authors, was underpowered to detect clinical outcome differences between the treatment groups and did not identify this as a study focus. Furthermore, neither study examined the same second-generation antipsychotic molecule, risperidone, in the oral and long-acting injectable forms, but instead allowed participants in the oral medication group to continue taking whatever second-generation antipsychotic they had already been taking. It is expected that patients who continued to take oral medication with which they had already been clinically stabilized would be more likely to remain stable, possibly reducing group differences compared with patients who received long-acting injectable risperidone. This result is likely owing to both prior trial-and-error selection of an effective antipsychotic for a given patient and avoidance of any disruptive effects of switching antipsychotics. In contrast, participants in the current RCT were all prescribed oral risperidone at study entry. Individuals who had been receiving other antipsychotic medications were switched to treatment with oral risperidone at that time.
Few patients refused to enter this RCT because of the possibility of being assigned to long-acting injectable risperidone, and few patients refused long-acting injectable risperidone after random assignment. In contrast to most previous studies, participants were offered transportation, if needed, and comprehensive case management and enhanced psychosocial services as part of the simultaneous comparison of CR and HBT. Perhaps the enriched psychosocial treatment environment, with strong support for return to work or school, was a further incentive to accept assignment to the long-acting injectable risperidone group.
In general, our findings are consistent with the supposition that the superiority of long-acting injectable risperidone for psychotic symptom control is likely owing to greater adherence. The benefits of long-acting injectable risperidone include more stable levels of medication in the blood, but secondary analyses suggested that the advantage of long-acting injectable risperidone for psychotic symptom control was accounted for by superior medication adherence.
This RCT also differed from most previous studies in that it did not combine psychiatric hospitalization and return of positive psychotic symptoms into a single end point. Focusing on the return of psychotic symptoms might have enhanced sensitivity to the antipsychotic effects of long-acting injectable vs the oral medication. In this sample, most psychotic relapses and exacerbations were treated on an outpatient basis, while most hospital admissions were owing to suicidality. We focused on psychotic symptoms because they are the primary target of antipsychotic medication.
We excluded individuals with substantial substance abuse because our focus was on the efficacy of long-acting injectable risperidone after a first episode of psychosis that was clearly due to schizophrenia rather than a drug-induced psychosis. Although substance abuse that triggered the psychosis was an eligibility exclusion, some individuals had lower levels of substance use (particularly cannabis use) before entry or developed substance abuse after study entry. Nonetheless, our findings might not be generalizable to persons with schizophrenia who also have notable substance abuse. Furthermore, prescribing everyone in the comparator group the same oral antipsychotic medication (and one that is molecularly identical to the injectable medication group) is a methodological advantage for examining the efficacy of long-acting injectable risperidone but not for examining the effectiveness because it limits the generalizability of the findings. Another limitation is that symptom raters were not blinded to treatment condition. However, many steps were used to ensure symptom data integrity. It is possible that the advantages of long-acting injectable risperidone over oral risperidone might not fully generalize to clinical settings that do not provide accompanying enriched psychosocial treatment programs. The close monitoring and more intensive psychosocial treatment in this tightly controlled RCT might have reduced relapse rates in both groups, allowing the advantage of long-acting injectable antipsychotics to be more sensitively detected owing to the larger odds ratio when rates are relatively low.
Conclusions
Long-acting injectable antipsychotic medication was well accepted by patients with a recent first episode of schizophrenia. Long-acting injectable medication led to better medication adherence, greater relapse prevention, and better psychotic symptom control than oral antipsychotic medication. The superiority of long-acting injectable risperidone extends beyond preventing psychotic symptom return. Use of long-acting injectable risperidone in our first-episode patients also led to better maintenance of intracortical myelination22 as well as improved cognitive functioning.23 If this trifecta of improved psychotic symptom control, cognition, and intracortical myelination can be replicated in longer longitudinal studies of patients with a first episode of schizophrenia, it would suggest that the use of long-acting injectable antipsychotics early in schizophrenia can modify the trajectory of the disorder and lead to better long-term outcomes. This possibility would be a “game changer” for the field.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by grants MH037705 (clinicalTrials.gov Identifier: NCT00333177) and MH066286 (clinicalTrials.gov Identifier: NCT00330551) from the National Institute of Mental Health (Dr Nuechterlein) and supplementary funding and medication was provided by Janssen Scientific Affairs, LLC.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, or Janssen Scientific Affairs, LLC.
Additional Contributions: We thank the patients for their participation in this longitudinal study. Walter Dunn, MD, PhD, Department of Psychiatry and Behavioral Sciences, UCLA, commented on the analysis of plasma assays. Kimberly Baldwin, MFT, Rosemary Collier, MA, Nicole R. DeTore, MA, Yurika Sturdevant, PsyD, and Luana Turner, PsyD, UCLA Aftercare Research Program, served as case managers. Elisha Agee, Elizabeth Arreola, BS, Manjot Bains, Miriam Barillas, BS, Ashton Christian, Kassandra Coronel, Liset Cristino Crespin, BS, Jing Gong, Angie Sung Hyun Lim, Sabiha Kaiser, Steven Kwong, Lilian Medina, BS, Gabriella Pasqual, Leila Sims, Gabriel Swerdlow, John Tran, Andres Victoria, BS, Yejin Yoo, and Liang Zhu rated medication adherence. Mss Arreola, Barillas, Cristino Crespin, and Medina and Mr Victoria were paid research assistants. No others were financially compensated.
Supplemental content at jamapsychiatry.com
Author Contributions: Drs Subotnik and Nuechterlein had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Subotnik, Casaus, Marder, Nuechterlein.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Subotnik, Hellemann, Nuechterlein.
Critical revision of the manuscript for important intellectual content: Subotnik, Casaus, Ventura, Luo, Gretchen-Doorly, Marder, Nuechterlein.
Statistical analysis: Subotnik, Hellemann, Nuechterlein.
Obtained funding: Subotnik, Ventura, Nuechterlein.
Administrative, technical, or material support: Subotnik, Casaus, Ventura, Luo, Gretchen-Doorly, Nuechterlein.
Study supervision: Subotnik, Casaus, Ventura, Luo, Marder, Nuechterlein.
Conflict of Interest Disclosures: Dr Subotnik reports serving as a consultant to Janssen Scientific Affairs, LLC; being on the speaker’s bureau for Otsuka America Pharmaceutical, Inc; and receiving research support from Janssen Scientific Affairs, LLC, and Genentech Inc, through grants to Drs Nuechterlein and Ventura. Dr Ventura reports serving as a consultant to Posit Science Corporation and Boehringer-Ingelheim GmbH and receiving research support from Janssen Scientific Affairs, LLC, Posit Science Corporation, and Genentech Inc. Dr Marder reports receiving consulting fees from AbbVie, Pfizer, Lundbeck, Boeringer Ingelheim GmbH, Shire Plc, Roche, Genentech Inc, Takeda Pharmaceutical Company Ltd, Jazz Pharmaceuticals, Otsuka America Pharmaceutical, Inc, Targacept, and FORUM Pharmaceuticals and grant support from Amgen, Synchroneuron, and Sunovion. Dr Nuechterlein reports serving as a consultant to Genentech Inc, Janssen Scientific Affairs, LLC, Otsuka America Pharmaceutical, Inc, and Janssen-Cilag and receiving support from Janssen Scientific Affairs, LLC, Posit Science Corporation, and Genentech Inc. No other disclosures were reported.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286–292. doi: 10.1176/appi.ajp.2010.09010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emsley R, Oosthuizen P, Koen L, Niehaus DJ, Medori R, Rabinowitz J. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378–2386. doi: 10.1016/j.clinthera.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia: a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127(1–3):83–92. doi: 10.1016/j.schres.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Lasser RA, Bossie CA, Gharabawi GM, Kane JM. Remission in schizophrenia: results from a 1-year study of long-acting risperidone injection. Schizophr Res. 2005;77(2–3):215–227. doi: 10.1016/j.schres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Weiden PJ, Schooler NR, Weedon JC, Elmouchtari A, Sunakawa-McMillan A. Maintenance treatment with long-acting injectable risperidone in first-episode schizophrenia: a randomized effectiveness study. J Clin Psychiatry. 2012;73(9):1224–1233. doi: 10.4088/JCP.11m06905. [DOI] [PubMed] [Google Scholar]
- 6.Rosenheck RA, Krystal JH, Lew R, et al. CSP555 Research Group. Long-acting risperidone and oral antipsychotics in unstable schizophrenia. N Engl J Med. 2011;364(9):842–851. doi: 10.1056/NEJMoa1005987. [DOI] [PubMed] [Google Scholar]
- 7.Macfadden W, Ma YW, Thomas Haskins J, Bossie CA, Alphs L. A prospective study comparing the long-term effectiveness of injectable risperidone long-acting therapy and oral aripiprazole in patients with schizophrenia. Psychiatry (Edgmont) 2010;7(11):23–31. [PMC free article] [PubMed] [Google Scholar]
- 8.Keks NA, Ingham M, Khan A, Karcher K. Long-acting injectable risperidone v. olanzapine tablets for schizophrenia or schizoaffective disorder: randomised, controlled, open-label study. Br J Psychiatry. 2007;191:131–139. doi: 10.1192/bjp.bp.105.017020. [DOI] [PubMed] [Google Scholar]
- 9.Kane JM, Detke HC, Naber D, et al. Olanzapine long-acting injection: a 24-week, randomized, double-blind trial of maintenance treatment in patients with schizophrenia. Am J Psychiatry. 2010;167(2):181–189. doi: 10.1176/appi.ajp.2009.07081221. [DOI] [PubMed] [Google Scholar]
- 10.Gaebel W, Schreiner A, Bergmans P, et al. Relapse prevention in schizophrenia and schizoaffective disorder with risperidone long-acting injectable vs quetiapine: results of a long-term, open-label, randomized clinical trial. Neuropsychopharmacology. 2010;35(12):2367–2377. doi: 10.1038/npp.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai YM, Ting Chen T, Chen JY, et al. Equivalent switching dose from oral risperidone to risperidone long-acting injection: a 48-week randomized, prospective, single-blind pharmacokinetic study. J Clin Psychiatry. 2007;68(8):1218–1225. doi: 10.4088/jcp.v68n0808. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192–213. doi: 10.1093/schbul/sbs150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malla A, Chue P, Jordan G, et al. An exploratory open-label randomized trial comparing risperidone long acting injectable (RLAI) with oral antipsychotic medication in the treatment of early psychosis. Clin Schizophr Relat Psychoses. 2013;17:1–26. doi: 10.3371/CSRP.MACH.061213. [DOI] [PubMed] [Google Scholar]
- 14.Nuechterlein KH, Subotnik KL, Turner LR, Ventura J, Becker DR, Drake RE. Individual placement and support for individuals with recent-onset schizophrenia: integrating supported education and supported employment. Psychiatr Rehabil J. 2008;31(4):340–349. doi: 10.2975/31.4.2008.340.349. [DOI] [PubMed] [Google Scholar]
- 15.Nuechterlein KH, Dawson ME, Gitlin M, et al. Developmental processes in schizophrenic disorders: longitudinal studies of vulnerability and stress. Schizophr Bull. 1992;18(3):387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- 16.Nuechterlein KH, Ventura J, Subotnik KL, Hayata JN, Medalia A, Bell MD. Developing a cognitive training strategy for first-episode schizophrenia: integrating bottom-up and top-down approaches. Am J Psychiatr Rehabil. 2014;17(3):225–253. doi: 10.1080/15487768.2014.935674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gretchen-Doorly D, Subotnik KL, Ventura J, Nuechterlein KH. Comparing Healthy Lifestyle Training and Cognitive Remediation in First-Episode Schizophrenia Patients: Six-Month Health Attitude and Independent Living Skills Outcomes. Ann Arbor, MI: Society for Research in Psychopathology; 2012. [Google Scholar]
- 18.Gretchen-Doorly D, Subotnik KL, Kite RE, Alarcon E, Nuechterlein KH. Examining the impact of a wellness-based group intervention for recent-onset schizophrenia patients: preliminary results from an ongoing trial. Paper presented at: The International Congress on Schizophrenia Research; March 20, 2009; San Diego, CA. [Google Scholar]
- 19.Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. Int J Methods Psychiatr Res. 1993;3:227–243. [Google Scholar]
- 20.Nuechterlein KH, Miklowitz DJ, Ventura J, Gitlin MJ, Stoddard M, Lukoff D. Classifying episodes in schizophrenia and bipolar disorder: criteria for relapse and remission applied to recent-onset samples. Psychiatry Res. 2006;144(2–3):153–166. doi: 10.1016/j.psychres.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Overall JE, Hollister LE, Pichot P. Major psychiatric disorders: a four-dimensional model. Arch Gen Psychiatry. 1967;16(2):146–151. doi: 10.1001/archpsyc.1967.01730200014003. [DOI] [PubMed] [Google Scholar]
- 22.Bartzokis G, Lu PH, Raven EP, et al. Impact on intracortical myelination trajectory of long acting injection versus oral risperidone in first-episode schizophrenia. Schizophr Res. 2012;140(1–3):122–128. doi: 10.1016/j.schres.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuechterlein KH, Subotnik KL, Ventura J, et al. Long-acting injectable risperidone and medication adherence enhance cognition and work functioning after a first psychotic episode. Paper presented at: 14th International Congress on Schizophrenia Research; April 24, 2013; Orlando, FL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.