Abstract
Vascular-targeted nanocarriers are an attractive option for the treatment of a number of cardiovascular diseases, as they allow for more specific delivery and increased efficacy of many small molecule drugs. However, immune clearance, limited cellular uptake, and particle-cell dynamics in blood flow can hinder nanocarrier efficacy in many applications. This review aims to investigate successful strategies for the use of vascular-targeted nanocarriers in the treatment of cardiovascular diseases such as atherosclerosis. In particular, the review will highlight strategies employed for actively targeting the components of the atherosclerotic plaque, including endothelial cells, macrophages, and platelets and passive targeting via endothelial permeability, as well as design specifications (such as size, shape, and density) aimed at enhancing the ability of nanocarriers to reach the vascular wall.
Introduction
Targeted nanocarriers (NCs) for drug delivery remain an attractive option for improving the efficacy of a number of drug molecules. In principle, targeted NCs are drug-loaded, nanoscale particles with surface-linked targeting moieties that are able to safely navigate the vasculature and bind to a specific target, where they are able to release their drug payload for a local therapeutic effect. This disease site drug release, rather than a systemic release, increases drug efficacy and decreases systemic side effects. In many cases, NCs are targeted specifically to the vascular wall due to the intimate involvement of the cells of the vascular wall in the pathogenesis of a variety of diseases. However, like any strategy, there are limitations to the use of targeted NCs in drug delivery. For one, nanocarriers may undergo phagocytosis by immune cells, may be cleared by the liver, or may be unable to marginate (travel) to the vascular wall to reach their targets as effectively as micron-sized carriers. (See Sidebar: The Margination Problem, Figure 1).1 Furthermore, it is often not enough to simply reach a target on the vascular side; in order to be effective, it is frequently necessary for NCs to cross the endothelium to allow the drug payload to be delivered directly to the tissue. Given the variety of challenges present in achieving a successful targeted drug delivery system, there are a number of decisions to be made in the design process. This review will highlight successful strategies for design of vascular-targeted NCs (and, to some extent, microcarriers) focused on enhanced margination, avoidance of immune clearance, and transmigration from the vascular wall to the diseased tissue space with specific focus on atherosclerosis and other cardiovascular diseases.
Sidebar title: The Margination Problem.
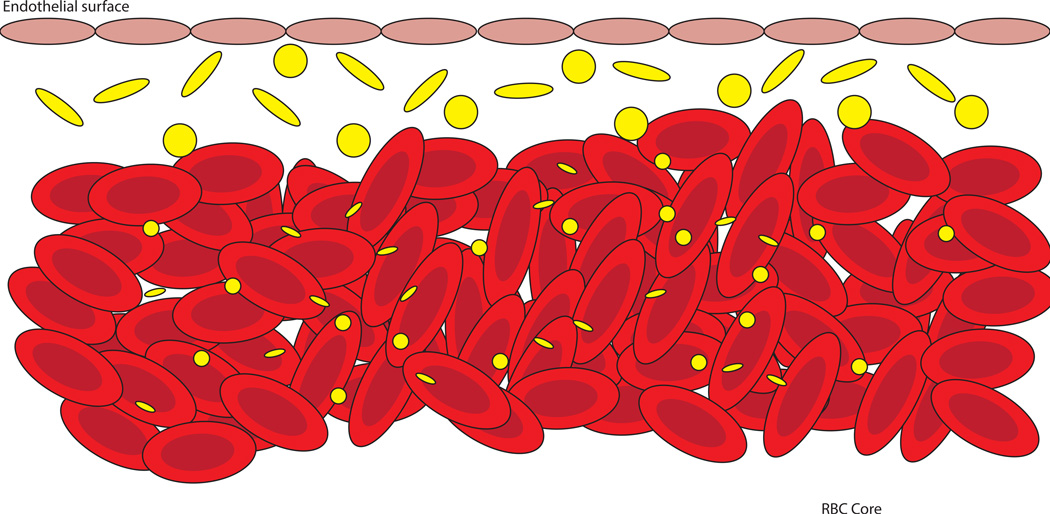
The fluid mechanics of blood flow (termed hemodynamics) are quite complex, due to the heterogeneous nature of blood. Interactions between blood cells create some interesting effects. Due to the laminar flow profile and the physical properties of red blood cells (RBCs), a “red cell core” is formed along the centerline of blood flow, with very few red cells flowing close to the endothelial surface. Nanocarriers often become trapped within this core, unable to travel (marginate) to the vascular wall, and there is evidence that suggests that smaller particles are more prone to inhibited margination.1 Thus, researchers designing vascular-targeted carriers should be wary of the assumption that smaller particles will always perform better due to increased transmigration ability. See Figure 1.
Figure 1.
Particle-cell dynamics in blood flow and their influence on margination. Nanosized carriers, both spherical and rod-like, are more likely to become entrapped in the RBC core, and are unable to effectively marginate to the vascular wall. Larger particles, 1–2µm in size, are pushed toward the cell-free layer, where they are able to interact with the endothelium. Rod-like microparticles may exhibit enhanced margination due to an increased drift force and tumbling motion in flow.
Nanoparticles for Vascular-Targeting in Atherosclerotic Plaques
Overview of Atherosclerotic Pathogenesis
Cardiovascular disease (CVD) is the leading cause of death worldwide. Atherosclerosis is the most common type of CVD, often resulting in a stroke or heart attack. It is characterized by the progressive formation and rupture of plaques in the arterial wall associated with inflammation and endothelial dysfunction.2 These plaques often originate as a result of injury to the endothelium, which can be caused by risk factors such as hypertension (high blood pressure) and smoking.3 Once injured, the ECs begin exhibiting an inflammatory phenotype, recruiting macrophages to the area and expressing endothelial adhesion molecules. As chemokines (such as monocyte chemoattractant protein-1 and macrophage colony-stimulating factor) are produced, monocytes and leukocytes bind to the damaged endothelium and extravasate into the tissue space. Monocytes then differentiate into macrophages, professional phagocytes recruited to take up excess lipids accumulating at the injury site. The atherosclerotic plaque itself consist of lipids, inflamed endothelial cells, macrophages, foam cells (resulting from macrophage uptake of oxidized LDL), vascular smooth muscle cells (which further recruit leukocytes and increase endothelial dysfunction), and several other components that accumulate over time, leading to calcification and thrombus formation. The accumulated lipids are oxidized by a number of enzymes, leading to tissue damage, release of pro-inflammatory chemokines, and hence increased leukocyte and platelet adhesion via upregulated/overexpressed adhesion molecules.
Throughout the disease progression, numerous atherosclerotic biomarkers and characteristics can aid in vascular-targeted drug delivery. Several studies have evaluated the ability of drug carriers to deliver imaging agents and therapeutics to the vascular tissue in atherosclerosis. Macrophages and endothelial cells have served as the two main cell targets because of their specific expression of inflammatory molecules (such as E-selectin, P-selectin, ICAM, and VCAM-1) and attributes (e.g. “leaky” vasculature exhibited by endothelial cells). Table 1 provides a brief summary of many of the targeting methods discussed in this review, along with key references utilizing these targeting schemes.
Table 1.
Summary of Targeting Schemes and their Advantages and Disadvantages
| Endothelial Targets | ||||
|---|---|---|---|---|
| Method | Type | Advantages | Disadvantages | Ref. |
| Enhanced Vascular Permeability |
Passive |
|
|
6, 7 |
| Magnetically Guided Nanoparticles |
Pseudo- passive |
|
|
16–21 |
| Shear-Induced Targeting |
Passive |
|
|
24, 25 |
| E-Selectin | Active |
|
|
27–29 |
| ICAM-1 | Active |
|
|
27, 28, 34 |
| VCAM-1 | Active |
|
|
30, 31 |
| Other Targeting Schemes for Vascular Targeted Delivery | ||||
| Macrophage Scavenger Receptors (SR- A, CD36) |
Active |
|
|
37–44 |
| Platelet P- Selectin |
Active |
|
|
47 |
| Multi-targeted nanocarriers |
Active |
|
|
49–57 |
| Collagen | Active |
|
|
58, 59 |
“Passive” targeting of cardiovascular disease sites
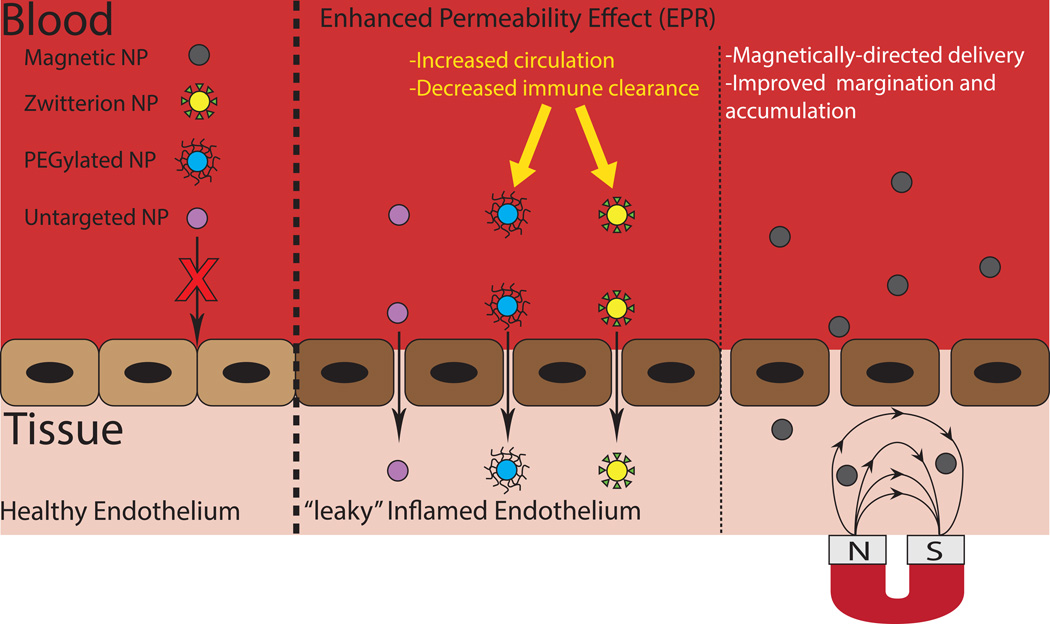
Enhanced Vascular Permeability
One main mechanism exploited for passive targeting of diseased vessels is through the enhanced permeability of the endothelium in inflammation. This effect, long investigated in the context of cancer therapy, is assumed to be present to some extent in atherosclerosis due to the prominent involvement of inflammation in disease progression. In addition, the intima of the plaque is reported to contain leaky microvessels rapidly formed due to the growing atherosclerotic plaque.4,5 This combination of increased vascular permeability and leaky microvessels in theory should allow NCs to permeate the vascular wall and accumulate within the diseased tissue. However, only limited works have explored this possibility. Kim et al. examined the influence of endothelial permeability on lipid-polymer hybrid nanoparticle translocation using an endothelialized microfluidic device and an in vivo atherosclerotic rabbit model. The permeability of the endothelium in vitro was modified through TNF-α stimulation and varying shear stress. The authors find that increases in nanoparticle translocation correlated with enhanced permeability.6 Similarly, Lobatto et al. conducted in vivo and ex vivo atherosclerotic rabbit experiments to elucidate the destination of long-circulating liposomal nanoparticles7 and found liposomal nanoparticles to accumulate within plaques due to enhanced permeability. From tracking liposomal nanoparticles over time, it was determined that they enter through the luminal side and disperse throughout the plaque. However, Kim et al. and Lobatto et al. only examined one specific size each (70nm and 123nm, respectively), so it would be important to understand the effect of particle size on these results, as it has been established that size has a great impact on carrier transport across the endothelium.
One critical issue when relying on passive accumulation due to leaky endothelium/microvessels is the clearance of NCs before exposure to diseased tissue. In this context, developers of NCs have employed poly (ethylene glycol) (PEG) in particle design. PEG is a highly flexible, hydrophilic polymer that when grafted onto a surface creates a hydration layer that effectively reduce protein adsorption onto the surface, which is a critical component of immune recognition and clearance – hence PEG increases circulation time.8 Applying this principle, Zhang et al. fabricated nanoparticles consisting of poly (lactic-co-glycolic acid) (PLGA) and PEG chains that encapsulated a liver X receptor (LXR) agonist aimed to target macrophages within atherosclerotic plaques to control LXR gene expression. The LXR gene in macrophages is responsible for regulating inflammation and lipid metabolism.9 The objective of this study was to promote gene expression within plaques but not in the liver, which was the main drawback of free LXR agonist leading to hepatic steatosis. Ultimately, the PLGA-PEG nanoparticles encapsulating the LXR agonist were able to reduce the amount of macrophages in plaques by about 50% without increasing the concentration of lipids in the liver and plasma compared to free LXR agonist.9 Another study used PEG-coated ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles as an imaging agent for areas of inflammation, particularly atherosclerotic plaques.10 They found that PEGylation allowed their particles to evade immune clearance such that they were able to accurately detect plaque formation.
It is important to note that shape may be combined with PEGylation to further enhance long circulation. Specifically, Geng et al. showed that worm micelles constructed from PEG-based diblock copolymer amphiphiles exhibit long circulation and enhance tumor localization largely due slower action of tissue macrophages.11 However, two key issues have emerged in recent years with the use of PEG. For one, the presence of PEG on NCs has been shown to interfere with cellular internalization. Secondly, there is evidence of immune response to PEG where a PEG-specific antibody is generated upon the first introduction of PEG NCs in vivo, which leads to rapid clearance of the particles upon repeat dosing. A comprehensive review of these issues and ways to circumvent can be found in work by Amoozgar and Yeo.12
In search for a more effective alternative to PEG coatings, researchers have begun to look toward zwitterionic coatings to protect particles from protein adsorption and immune clearance. Zwitterionic materials are able to strongly bind water molecules via electrostatic interactions, creating high degrees of hydration and thus protecting the surface from protein adsorption.13 To date, NCs coated in zwitterionic materials have been investigated to determine their circulation time and in vivo clearance. Xiao et al. prepared magnetite particles coated with both polyacrylic acid (PAA) and zwitterionic coating comprised of PAA reacted with 3-(dimethylamino)propylamine (DEAPA) designed to function as MRI blood pool contrast agents.14 The zwitterionic particles exhibited greatly reduced macrophage uptake as well as a greater-than-threefold increase in circulation half-life. Similarly, Sun et al. developed quantum dots with a zwitterionic coating, and compared their biodistribution, uptake, and circulation times with similar PEGylated quantum dots.15 They found that the zwitterionic coating resulted in a much longer circulation half-life (t1/2 = 21 ± 1hr for the zwitterionic coating compared to t1/2 = 6.4 ± 0.5 minutes for the PEG coating), and greatly decreased accumulation in the liver and spleen. The increased circulation time of zwitterion-coated NCs could prove to be very useful in the design of NCs for the treatment of atherosclerosis, as it could result in greater accumulation in plaques, but studies evaluating their effectiveness in this context are yet to be done. A comprehensive study of the zwitterion coating on nanoparticle degradation and drug release from typical biomaterials, such as PLGA, would also be necessary for these novel coatings to eventually translate to widespread clinical application (Figure 2).
Figure 2.
Passive targeting approach for cardiovascular diseases. Inflammation and endothelial dysfunction are characteristics demonstrated by damaged vascular tissues. Here are displayed four types of nanoparticles that take advantage of the “Enhanced Permeability Effect (EPR).” One drawback of this approach is that the particle may become cleared before reaching the diseased endothelium. The addition of PEG and zwitterions to the surface of the particles has shown to decrease plasma protein adsorption increasing circulation time and decrease clearance. The third approach depicted is the application of a magnetic field used to direct nanoparticles throughout the body.
Magnetically Guided Nanoparticles
One interesting “pseudo-passive” targeting approach that can potentially take advantage of the enhanced vascular permeability effect, perhaps more fully, is the use of magnetically guided NCs. In theory, the use of magnetic nanoparticles allows for the application of an external magnetic field, which can guide nanoparticles specifically to the disease site, improving margination and accumulation within diseased tissue.16 This strategy has previously been studied extensively in the context of tumor targeting and recent evidence suggests that this strategy can prove to be beneficial in cardiovascular disease as well. Simulations by Freund et al. have shown that applying a magnetic force both parallel and perpendicular to the blood vessel will attract magnetic particles to the vessel wall. The external magnetic field assists in transporting particles from the cell-free layer, which lacks red blood cells and, thus, margination-inducing collisions, to the vessel wall.17 This increased margination toward the vessel wall can allow for increased accumulation within leaky vasculature, or increase the amount of time for a given particle to bind to its target. Such simulations are backed up by experimental evidence, as well. Chorny et al. used magnetic nanoparticles made by controlled precipitation of calcium oleate in the presence of magnetic-based ferrofluid for the delivery of antioxidant enzymes to endothelial cells in vitro.18 The results of the study showed that the internalization of the nanoparticles by HUVEC cells increased from 3–8% for the cells treated with particles without the presence of the magnetic field to 46–71% for the case of cells under the exposure of the magnetic field. Fluorescent microscopy also validated the hypothesis of increased cell uptake of nanoparticles in the presence of the magnetic field. The therapeutic effect of these particles was studied by quantification of recovery of HUVEC cells after exposure to 10 mM hydrogen peroxide for 5 h. Their results indicated average recovery of 62% for cells treated with magnetic particles under the exposure of magnetic field while the recovery in the absence of the magnetic field remained under 20%. Another study by Chorny et al. used zinc oleate-based magnetic nanoparticles for gene delivery to stented arteries.19 Their results indicated that the gene expression in the rat carotid model of stent angioplasty was 8–14 fold higher in magnetically treated animals in comparison to non-magnetic controls even 9 days after the treatment. Mannell et al. used perfluorocarbon-filled lipid microbubbles associated with iron magnetic particles for gene delivery to the endothelium.20 These microbubbles were directed toward the target site using an external magnetic field. Their results also demonstrated increased uptake of nanoparticles by HMEC endothelial cells after exposure to the external magnetic field. Forbes et al. have proposed the idea of developing stent modified with magnetic coating for implanting at the disease sites to capture magnetic nanoparticles.21 These stents can be activated with a magnetic field to capture magnetic nanoparticles. In this study, the stainless steel stents were coated with cobalt nickel alloy. In vivo experiments using a rat model indicated the magnetic nanoparticles preferentially accumulated in the region around the implanted stent. Utilizing these stents can be a practical method for introducing the magnetic field in the diseased site for transition to the in vivo systems.
A recent computational study by Chandramouli et al. has introduced the idea of using super paramagnetic iron oxide particles to destroy atherosclerotic plaques via hyperthermia.22 These magnetic particles can be guided to the plaque site using an external magnetic field, and then heated by rapid switching of the magnetic field, creating a spin on the particles. This spinning will give off heat, reducing the hardness of the plaque due to its thermal expansion, which can consecutively lead to abrasion of the plaque. Their calculations demonstrated an increase in the plaque removal rate by increasing particle mass flow rate and velocity. However, such abrasion of plaque in practice could result in an increased risk of stroke as the exposed plaque material can trigger the clotting cascade.23
Shear-Induced Targeting
In advanced atherosclerosis, the affected vessels can become stenosed due to outward (toward the lumen) growth of the intima. This stenosis, or narrowing of the blood vessel, results in increased blood shear stress over the plaque due to increased blood velocity. This unique high shear, and in many cases disturbed, flow environment creates an opportunity for shear sensitive NCs. Taking advantage of this effect, Home et al. designed “lenticular” (lentil-shaped) liposomes which were found to deform in response to high shear, releasing their drug cargo.24 These liposomes specifically responded to the high-shear environment found in highly occluded atherosclerotic vessels in an in vitro environment, releasing their cargo at these sites. However, it is worth nothing that experiments in this study were performed in a buffer solution (reasonably, for the sake of simplicity), and further trials in human blood and in an in vivo environment would yield more information about the efficacy of such carriers. Employing a different strategy, Korin et al. developed shear-activated nanotherapeutics (SA-NTs), micron-scale aggregates of NCs which become disaggregated under high shear conditions.25 These SA-NTs were shown to disaggregate when entering highly-occluded vessels both in vitro and in vivo. Further, the SA-NTs were loaded with a fibrolytic drug to explore their effectiveness in treatment of thrombosis, and greatly improved the survival time for mice with induced thrombosis.
Overall, it is clear that enhanced permeability is present in some capacity in atherosclerosis though likely not as robust as has been described in cancer, and there may be methods for taking advantage of this phenomenon more fully. As material design becomes more advanced and the interaction between particular materials and blood cells is more fully understood, researchers will have a wide array of materials to choose from when designing drug carriers, with varying degrees of protection from clearance. Aside from further investigation of zwitterionic materials, researchers can also consider masking their carriers by mimicking various cells, such as platelets, as shown in a recent study by Hu et al.26 The authors directly coated PLGA nanoparticles with a platelet plasma membrane, which they described as “cloaking.” The platelet membrane-cloaked PLGA nanoparticles (PNPs) demonstrated decreased macrophage internalization and complement activation, preventing their recognition by the body’s immune response. Further, the use of “guiding” via magnetic fields can assist in localizing drug carriers to the site of disease, increasing the accumulation of NCs in the diseased tissue.
Inflammatory biomarkers for active targeting of specific cells
Although a number of studies have successfully explored the use of “passively” targeted NCs, there is great interest in increasing drug carrier specificity and efficacy through the use of “actively” targeted NCs, which are able to preferentially bind to specific cellular markers present in diseased states. Such design strategies are discussed in detail below.
Endothelial cells
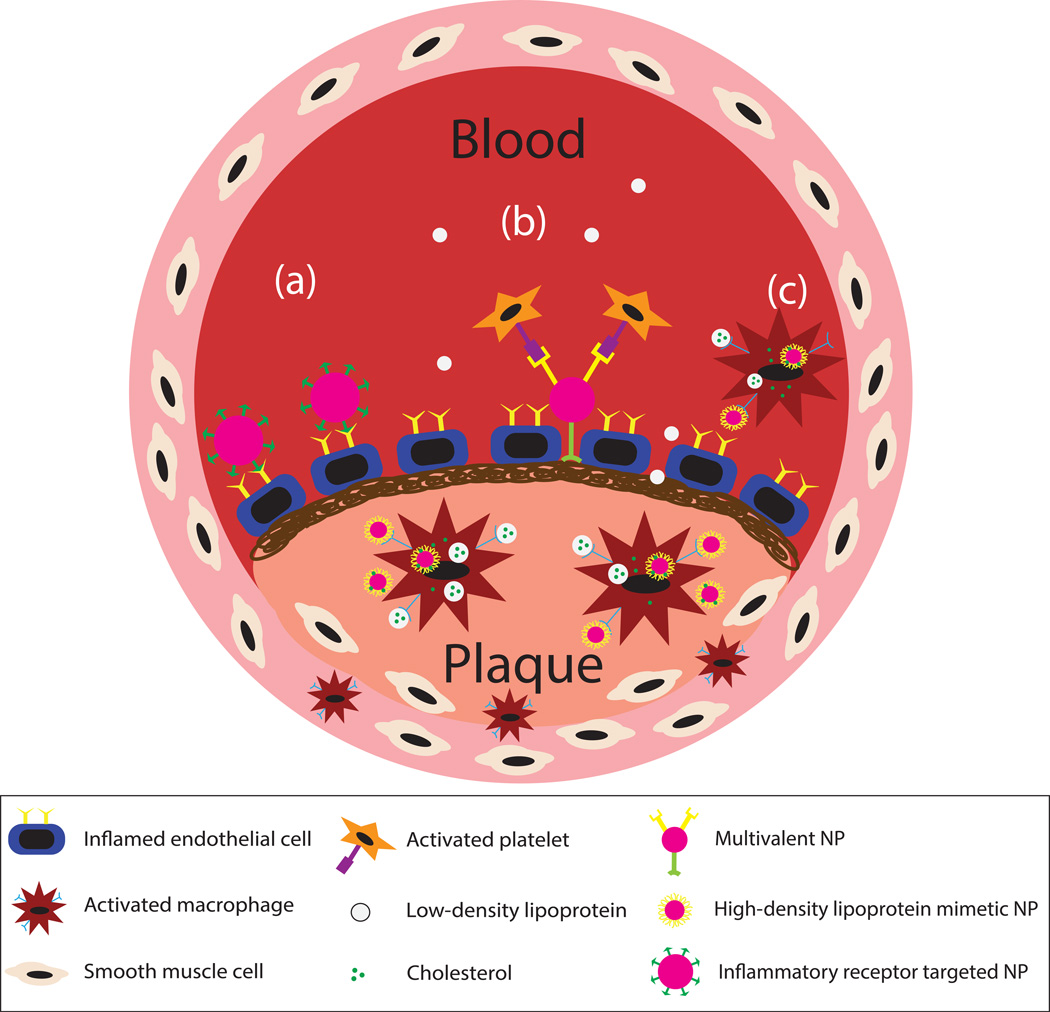
Inflammation is an immune response that becomes unregulated and exaggerated during atherosclerosis. Specifically, lipid accumulation promotes the overexpression of adhesion receptors by endothelial cells that leads to the recruitment of platelets, leukocytes, and smooth muscle cells.3 Therefore, decorating the nanoparticle surface with ligands/antibodies specific for an overexpressed inflammatory molecule in atherosclerosis has been widely implemented as a targeting approach (see Figure 3). In general, ECs are a highly attractive target for NCs in vascular diseases as they are directly accessible from the bloodstream, rather than from within the forming atherosclerotic plaque. We have extensively evaluated the adhesion of drug carriers to inflamed HUVECs targeting E-selectin, ICAM-1, and VCAM-1 adhesion receptors involved in leukocyte adhesion and transmigration. Decorating the surface of nanoparticles with sialyl Lewisa (sLea) targeting ligand or anti-ICAM-1 antibody showed that nanoparticles are able to bind activated human umbilical vein endothelial cells (HUVECs) in in vitro parallel plate flow chamber experiments but have difficulty marginating and localizing to the endothelial surface compared to microparticles.27 To improve the adhesion of nanoparticles, we examined the effect of PEGylation using the two receptor-ligand targeting mechanisms. It was interesting to find that PEGylation improved the adhesion of aICAM-1 nanoparticles but did not improve the performance of sLea polystyrene.28,29 This discrepancy in the impact of PEG chains on particle adhesion was linked to the receptor-ligand kinetics, where the presence of PEG improved particle binding for ligand systems with weak kinetics. As such, it is important to understand the capability of receptor-ligand systems when targeting specific cells, especially since expression of surface receptors can vary throughout disease stages.
Figure 3.
Depiction of atherosclerotic plaque and nanoparticle active targeting mechanisms. During atherosclerosis, the endothelial cells become inflamed as low-density lipoproteins (LDLs) accumulate. LDLs are oxidized and taken up by macrophages, which then differentiate into foam cells. Inflammatory molecules are released promoting leukocyte and platelet adhesion leading to smooth muscle cell recruitment. Shown are three active targeting mechanisms: (a) targeting of inflammatory surface receptors, (b) multivalent targeting of exposed collagen (post-surgical intervention) and platelets, and (c) high-density lipoprotein mimetic targeting utilizing the reverse cholesterol transport mechanism. NP=nanoparticle.
Yang et al. decorated the surface of silica nanoparticles with anti-VCAM-1 monoclonal antibody. The study demonstrated VCAM-1 targeted nanoparticles were able to bind to sites of inflammation and were taken up by ECs.30 In a separate study by Calin et al., a peptide specific for the VCAM-1 receptor was attached to the surface of pegylated target-sensitive liposomes (TSLs) encapsulating a CCR2 agonist, which become destabilized when binding to target.31 VCAM-1 targeted TSLs were able to specifically bind to activated ECs and immobilized VCAM-1 in vitro. These results were conducted mainly in a static environment and neglected physiological blood flow conditions that could potentially affect liposome stability. In situ studies with ApoE −/− mice demonstrated that VCAM-1 targeted TSLs were able to efficiently bind to atherosclerotic aortas compared to non-targeted TSLs.31
The persistent presence of blood flow over the endothelium in healthy vessels means there is constant presence of flow-induced shear at the vascular wall, which has a significant impact on the adhesion dynamics of nanoparticles. As previously alluded to, the blood shear for plaques in different vessels can vary depending on the presence and extent of stenosis in the vessels. As such, there have been a number of studies exploring the effects of shear stress on the targeting and uptake efficiency of vascular-targeted NCs. Shear stress naturally results in a negative impact on the ability of targeted NCs to bind to the inflamed endothelium due to drag forces on the particle competing with binding forces,32 but can also impact the phenotype and function of endothelial cells. Malek et al. reported calcium- and tyrosine kinase-dependent endothelial cell shape change and F-actin filament rearrangement in response to shear flow.33 These cellular changes also appear to affect the uptake of targeted NCs via CAM-mediated endocytosis. Bhowmick et al. demonstrated that flow-conditioned endothelial cells exhibit delayed CAM-mediated endocytosis of NCs targeted to ICAM-1, and hypothesized that this delayed uptake is related to the rearrangement of the actin cytoskeleton of the ECs.34 Similarly, Han et al. demonstrated that flow-conditioned ECs exhibited reduced uptake of PECAM-1 targeted NCs, and that this reduced uptake was linked to the rearrangement of actin stress fibers in the ECs.35 Perhaps more interestingly, this same study showed that acute shear stress stimulates the uptake of PECAM-1 targeted NCs, while chronic shear stress (under which the ECs become elongated) decreases uptake, suggesting that acute shear stress likely stimulates a separate mechanism of NC internalization. A follow-up study confirmed that this effect involves mechanosensing of shear stress in cholesterol-rich regions of the plasmalemma, and also suggested the importance of both RhoA/ROCK signalling pathways and Src family kinases.36
Macrophages
Macrophages are an important component in atherosclerosis disease progression because of their role in oxidized lipid uptake leading to their differentiation into foam cells, which secrete inflammatory cytokines maintaining the inflammatory disease state. Petersen et al. describe amphiphilic nanoparticles that have the potential to inhibit lipid uptake by macrophages and preventing their differentiation into foam cells. The authors fabricated nanoparticles comprised of a mucic acid core and mucic acid- PEG shell.37 The mucic acid component was used to target scavenger receptors on macrophages, such as SR-A and CD36, which normally bind lipids. The amphiphilic nanoparticles had the ability to inhibit lipid uptake by competitively binding receptors and decreasing scavenger receptor surface expression.37 Iverson et al. also used mucic acid nanoparticles to target scavenger receptors of inflammatory macrophages. The macromolecules lowered oxidized low density lipoprotein (oxLDL) accumulation by 88%. In vivo experiments in rats demonstrated a decrease in the cholesterol accumulation and macrophage retention by usage of mucic acid nanoparticles.38 Another recent study by Bagalkot et al. utilized hybrid lipid-latex (LiLa) nanoparticles to target M1 inflammatory macrophages. Their results suggested that LiLa nanoparticles can preferably target inflammatory macrophages in atherosclerosis and obesity models39 and demonstrated that macrophages are a potential target for treating and detecting inflammatory diseases.
High-density lipoprotein (HDL) helps move cholesterol from macrophages to the liver through a reverse cholesterol transport mechanism.40 Despite the poor understanding of this reverse transport process, the resulting protection against atherosclerosis has inspired nanoparticle mimics for therapy in this disease. HDL contains a lipid hydrophobic core coated with phospholipids and apolipoproteins (ApoA-I or Apo-II). Sanchez-Gaytan et al. incorporated this phenomenon into their particle design by fabricating HDL-PLGA nanoparticles. The nanoparticles consisted of a PLGA core coated with phospholipids and apolipoproteins (ApoA-I). The HDL-PLGA nanoparticles interacted preferentially with macrophages in incubation assays and were found to localize with macrophages with in atherosclerotic aortas.40 Duivenvoorden et al. produced a statin-loaded reconstituted HDL nanoparticle displaying loss of cell viability in macrophages through decreased proliferation and non-apoptotic cell death.41 In vitro and in vivo apoE-KO mice studies demonstrated an accumulation of statin-loaded rHDL nanoparticles within plaque macrophages, which was attributed to the enhanced permeability of the diseased tissue. The authors also mentioned the possibility of rHDL interacting with scavenger receptor B1 and other components employed by native HDL in cholesterol transport or macrophage phagocytosis as alternative pathways for accumulation within atherosclerotic plaque.
Many groups have also explored the use of nanoparticle systems for targeting macrophages to deliver contrast agents for plaque detection. Early detection of atherosclerotic plaques can allow clinicians to deliver therapeutics before plaque rupture and thrombus formation. Dextran sulfate, another ligand for SR-A, was used to coat iron oxide nanoparticles for binding macrophages with the goal of delivering a contrast agent for imaging within the plaque, i.e. macrophages internalize bound contrast-loaded nanoparticles and migrate into the plaque.42 Nie et al. specifically targeted the CD36 receptor in both mouse and human macrophages using nanovesicles decorated with oxidized phosphatidylcholine.43 The targeted nanovesicles demonstrated higher binding and uptake by macrophages in vitro. The in vivo studies carried out in atherosclerotic (LDLr −/−) mice provided evidence that the targeted nanovesicles were co-localized with intima macrophages specifically through the CD36 receptor. This approach allows for the imaging of atherosclerotic plaques to evaluate the accumulation and distribution of macrophages.43 Marrache et al. developed high density lipoprotein (HDL)-mimicking nanoparticles to target macrophage apoptosis for delivering contrast agents for detection of vulnerable plaques.44 These nanoparticles were made up of a core comprised of biodegradable PLGA, cholesteryl oleate and a phospholipid bilayer and were decorated with triphenylphosphonium(TPP), a cation for detecting the collapse of mitochondrial membrane during apoptosis. Fluorescent microscopy, flow cytometry data and in vivo experiments in rats showed the success of these particles for detecting apoptotic macrophages in comparison to non-targeted particles. These particles have also the potential to enhance atheroprotection by delivering HDL to macrophages.44 Spyropoulos-Antonakalis et al. used polyamidoamine dendimers for delivery of ZnPc photosensitizing (PS) molecules for photodynamic therapy of cardiovascular disease to macrophages responsible for atheromatous plaque growth.45 Irradiation of the atheromatous plaque at specific wavelength activates PS molecules inducing cell necrosis and inhibiting activity of the macrophage cells responsible for plaque growth.
In summary, all mentioned studies demonstrate the importance of macrophages as a potential target for detection and treatment of cardiovascular inflammatory disease specifically in atherosclerosis. However, it is yet to be understood how researchers may take full advantage of macrophages’ natural functions (phagocytosis of excess LDL, as well as other immune cells) in order to more effectively design therapies for cardiovascular disease. In particular, it remains unclear whether therapeutics should be directed toward the macrophages themselves, i.e. reducing their accumulation, or whether macrophages should serve as a microscale delivery vehicle for nanoparticles containing drugs directed towards other critical components of the atherosclerotic plaque. In the case of the latter, careful choice of the specific therapeutics to be delivered and the NC (type/design) would be required to ensure drug with retained activity can be released by macrophages into the plaque.
Platelets
Platelets experience increased adhesion to the vascular wall in atherosclerosis due to the tissue damage conferred by oxidized lipids, which propagates the inflammatory process and eventually lead to recruitment of smooth muscle cells (SMCs).3 This prominence of platelets in the progression of atherosclerosis makes platelet targeting a potentially attractive option for delivering therapeutics and imaging agents.
One targeting approach involving platelets is to directly bind nanoparticles to platelet surface receptors. Jacobin-Valat et al. fabricated Versatile Ultrasmall SuperParamagnetic Iron Oxide (VUSPIO) nanoparticles conjugated to recombinant human antibody targeting activated platelets, rIgG4 TEG4.46 The specificity of rIgG4 TEG4 for the αIIbβ3-complex, present on platelet membrane, was examined using immunohistochemistry on murine and human atheromas, which proved to be similar to murine antibody, AP-2, that binds αIIb and β3 glycoproteins. Ex vivo ApoE−/− mice experiments demonstrated targeted VUSPIO nanoparticles localized within atherosclerotic vessel wall. The study also pointed out that platelets co-localized in areas rich in macrophages. A previous study by Jacobin-Valat incorporated an anti-human P-selectin antibody to the VUSPIO surface; in this case, the nanoparticles accumulated on the surface of and within activated platelets.47 Bachalet-Violette et al. targeted P-selectin by coating superparamagnetic iron oxide (SPIO) nanoparticles with fuciodan, a sulfated polysaccharide that binds P-selectin. Fucoidan-SPIO nanoparticles were able to specifically bind activated platelets in citrated whole blood conditions.48 Several of these studies combined imaging techniques, such as the use of iron oxide nanoparticles, and ligands specific for activated platelet surface receptors to image atherosclerotic lesions with MRI approaches. However, the potential flaw with these studies is the lack of concrete evidence of the specificity of targeted nanoparticles to platelets rather the endothelium, which also express P-selectin. In general, the platelet targeting approach in atherosclerosis is supported by very loose evidence of platelet-leukocyte interaction in atherosclerosis. As such, this approach of platelet targeted NCs may only be useful in cases of plaque rupture where extracellular material is exposed and facility platelet adhesion.
Multi-targeted nanocarriers
One new technique is the design of nanoparticles that exhibit multiple inflammatory cell characteristics. For instance, Anselmo et al. developed nanoparticles that mimic the discoidal shape, flexibility, and receptor surface expression of platelets. The nanoparticles were fabricated with PAH and BSA bilayers, where PAMAM dendrimers conjugated to three different targeting moieties were reacted to the surface.49 The three moieties included a collagen binding peptide, von Willebrand Factor (VWF) binding peptide, and a linear fibrinogen-mimetic peptide targeted to collagen, secreted VWF by endothelium, and integrin GPIIb-IIIa on activated platelets, respectively. The multivalency of these particles allowed them to interact with the injured endothelium and activate circulating platelets causing them to accumulate.49 Hyaluronic acid (HA) is a molecule that specifically binds to stabilin-2 and CD44 receptors used by Lee et al to formulate HA nanoparticles.50 Stabilin-2 has been shown to be overexpressed by macrophages, smooth muscle cells, and endothelial cells, while CD44 is expressed by activated macrophages found in plaques. The study demonstrated that HA nanoparticles were capable of targeting atherosclerotic plaques through receptor-mediated endocytosis, specifically localizing in areas of stabilin-2 and CD44 overexpression. These two examples demonstrate that potential of targeting multiple cells, which could improve localization to atherosclerotic tissues due to the collection of cells involved.
The dynamics of leukocyte binding are dependent on the interaction of ligands with multiple receptors. Therefore, several studies have focused on targeting multiple receptors as an efficient approach to increase the targeting efficiency of drug carriers. Eniola et al. used leukocyte-mimicking particles functionalized with sialyl LewisX (sLeX), and an antibody against ICAM-1, anti-ICAM-1 for simultaneous targeting of selectin and intercellular cell adhesion molecule-1 receptors.51 Their results demonstrated that only dual-targeted nanoparticles exhibit tight binding to the substrate under flow conditions as they mimic leukocyte binding dynamics more closely.51 Another study by Rafat et al. using polyvinyl alcohol hydrogels showed that conjugation of these hydrogels with anti-VCAM1 and anti-E-Selectin in a 1:1 ratio resulted in increased adhesion to HUVECs compared to the case where anti-VCAM1, or anti-E-Selectin were used alone.52 McAteer et al. developed dual targeted microparticles of iron oxide (MPIO) mimicking leukocyte adhesion by targeting both vascular cell adhesion molecule-1 (VCAM-1) and P-selectin in a mouse model of atherosclerosis. Their data showed that dual-targeted nanoparticles had 5–7 fold higher in vitro binding and 3–5 fold higher in vivo binding to endothelial cells than P-selectin-MPIO or VCAM-MPIO alone.53 Another study used PLGA nanoparticles conjugated with PEG, glycoprotein 1b (Gb1b) and trans-activating transcriptional (TAT) peptide to target P-selectin and von Willebrand Factor of inflamed endothelial cells.54 Their results indicated increased binding to human aortic endothelial cells under shear flow from 40% for particles conjugated with single ligand to 70% for multi-ligand particles. Studying intima stenosis inhibition of the dextran-loaded particles also indicated that dual-targeted nanoparticles can result in equivalent stenosis inhibition at much lower concentrations than free dextran solution.54 A recent study by Papademetriou et al. compared the efficiency of single, double and triple targeted polystyrene particles functionalized with intercellular (ICAM-1), platelet-endothelial (PECAM-1), and/or vascular (VCAM-1) cell adhesion molecules. ICAM-1 and PECAM-1 utilize CAM-mediated endocytosis and VCAM-1 is active in clathrin-mediated endocytosis pathways. Their results demonstrated that triple targeted nanoparticles enhanced selectivity in disease conditions, internalization by cells, and in vivo targeting efficiency.55
Another recent technique called collaborative enhancement of antibody binding utilizes the increased binding of antibodies (Abs) to epitopes of platelet cell adhesion molecule-1 (PECAM-1) by pairing them with a second antibody binding to a different adjacent epitope.56 This phenomenon is believed to occur because of the conformational and accessibility change of one epitope as a result of the attachment of another distinct epitope.56 Chacko et al. have shown that this paired interaction between Abs coated on polystyrene NCs targeting distinct adjacent epitopes of PECAM will increase the binding of NCs and the effect is even more pronounced on multivalent carriers than free Abs.57 Pre-treatment with Ab2h anti-PECAM Ab enhanced the in vitro binding of Ab1h/NC to endothelial cells by this paired interaction specifically at high Ab1h densities and under flow conditions. In vivo studies in mice also showed that the presence of Ab2m antibody enhances pulmonary uptake of Ab1m/125I-NC by 5.4 times. The reversed effect was also observed showing doubled Ab2m/125I-NC binding in the presence of Ab1m.57 These studies show the potential of this newly introduced technique to improve the binding of drug carriers.
All of the studies above show that targeting more than one receptor or cell type simultaneously improves the therapeutic performance of particles either by making them adhere more tightly to their target cells or giving them the ability to bind to different cells which are involved in a diseased state. Further, a full understanding of the microenvironment and phenotype associated with a particular cardiovascular disease allows researchers to attach ligands for the key markers associated with said disease, allowing for much more selective and specific targeting. It is also important for researchers to understand possible spatial/steric limitations to multi-targeted approaches, as specific markers could be expressed either too close to one another or too far away from one another to allow multi-targeting to make a difference. This approach, of course, can lead to reduced systemic side effects for therapeutics and reduced background signal for imaging agents. It would be prudent for researchers to design their particulate carriers using a variety of ligands with a range of ligand ratios, in order to optimize the effectiveness of their carrier.
Targeting extracellular matrix components, proteins, and other cell types
Oftentimes, when stents are surgically placed in order to unblock an occluded atherosclerotic artery, the endothelium becomes damaged, resulting in the exposure of collagen (the major constituent of the extracellular matrix), which then allows for platelets to accumulate and potentially form dangerous clots or result in restenosis.58 McMasters and Panitch examined the ability of collagen-directed thermosensitive nanoparticles to prevent platelet adhesion and activation following stent placement. The authors incubated platelets and collagen-targeted nanoparticles in plasma over a collagen-coated plate. Measuring the concentrations of two platelet activation factors released, it was observed that collagen-targeted nanoparticles inhibited platelet activation by about 60%.58 This study displays a competitive inhibition where the targeted nanoparticles bind the collagen before the platelets are able to, reducing platelet activation. It is important to note, however, such collagen targeting nanoparticles can only be used prophylactically in clinical situation where denudation of the endothelium is anticipated, e.g. in stent placement. Kamaly et al. employed the concept of targeting collagen exposed on inflamed vascular sites to deliver an anti-inflammatory peptide to reduce the risk of continued inflammation and restenosis.59 The nanoparticles encapsulating the anti-inflammatory peptide consisted of PLGA-PEG chains with collagen IV targeting ligand, conjugated to the end of the PEG chains. The study demonstrated that these nanoparticles had the capability to improve resolution and reduce inflammation in in vivo mouse models of peritonitis and hind-limb ischemia reperfusion, respectively.59 As previously discussed in the context of creating long-circulating particles, Hu et al. coated PLGA nanoparticles (PNPs) with a platelet plasma membrane as a “cloaking” system.26 These PNPs also showed preferential adhesion to sites of diseased vasculature where collagen was exposed. Another protein found at vascular injury sites is fibrin, which is shown to accumulate after an atherosclerotic plaque is removed, promoting restenosis.60 Gu et al. studied the targeting ability of fibrin-targeted layered double hydroxide (LDH) nanoparticles conjugated to low molecular weight heparin (LMWH) to arterial injuries of rats. A comparison between injured and uninjured arteries demonstrated that fibrin-targeted LDH nanoparticles successfully bound at injury sites and delivered LMWH, which reduced luminal loss and thrombotic occlusion.60 Other components involved in atherosclerosis treatment are high-density lipoproteins and statins, which are currently the gold standard for preventing or decelerating atherosclerosis.2 As discussed above, there are numerous biomarkers presented in atherosclerosis that can be taken advantage of when designing targeted drug delivery systems. Further, many groups are finding success by taking inspiration from the body’s natural processes, designing their carriers to mimic blood components or take advantage of the natural function of cells to deliver their drug carriers to the target site. While the choice of targeting strategy is highly important to the design of effective VTCs, NC physical properties such as size, shape, and density heavily influence the effectiveness of particle drug carriers in traveling and binding to the vascular endothelium. These effects are discussed in detail below.
Effect of Carrier Physical Properties on Vascular Wall Margination and Targeting Efficiency
Intravenously administered vascular-targeted NCs will interact with cells in the bloodstream en route to their target destination, and must effectively marginate, or localize, to the vascular wall and bind in order to reach the disease site. In the absence of effective margination, vascular-targeted NCs are rendered useless. There are a number of factors that influence the ability of particles to marginate to the vascular wall: particle size and shape, as well as the size of the cell-free layer all have an effect on the margination efficiency of NC systems.1,61–63
Effect of Carrier Size on Margination and Targeting Efficiency
A number of works have investigated the influence of carrier size on margination efficiency.63–67 Charoenphol et al. used parallel plate flow chamber (PPFC) assays to demonstrate that spherical, vascular-targeted particles in the 2–5µm diameter size range adhere with much greater efficiency than their nano-sized counterparts, likely due to preferential collision dynamics in blood flow.63 This result was replicated in a follow-up in vivo study, where targeted particles of varying sizes were intravenously injected into atherosclerotic mice; again, the group found that 2µm particles outperformed nanoparticles in reaching and binding to the endothelial cell lining of the aorta in these animals. Namdee et al. later showed in parallel plate flow chamber experiments that poor nanoparticle adhesion is directly linked to poor margination, where nano-sized particles instead become entrapped in the red blood cell core (see Sidebar 1), and that a disproportionally high concentration of NCs would be required to achieve the same adhesion as micron-sized particles.68 This result was replicated by Lee et al.69 and Müller et al.64,70, both computationally and experimentally. It is important to note, however, that Charoenphol et al. also demonstrated reduced adhesion efficiency for larger particles under high shear conditions, due to the increased shear forces acting on a larger particle at the vascular wall.
Some recent works have shown an opposite trend. Patil et al. observed a decrease in particle adhesion in high-shear flow with increasing particle diameter from 5µm to 20µm.71 Toy et al. observed reduced margination efficiency with increased sized in a series of microfluidic in vitro experiments with particles ranging in size from 60nm to 130nm66, and Jurney et al. saw a similar trend for particles ranging in size from 60nm to 970nm.65 These works seem to suggest that larger, heavier particles are more likely to settle due to gravitational forces, and that increasing shear forces acting on larger particles greatly reduce their adhesion efficiency. However, all of these works examined margination and adhesion in buffer flow, rather than human blood flow. It is now known that collisions between red blood cells and particulate carriers greatly influence particle margination63,72, and this must be considered when designing vascular-targeted NCs.
Effect of Carrier Shape on Margination and Targeting Efficiency
In recent years, groups have begun to explore shape as a key property in drug carrier design.73 It is long known that differently shaped particles behave differently under flow conditions; thus, a number of groups have investigated how shape might influence the ability of particles to marginate in blood flow.61,66,74,75 Thompson et al. compared spherical carriers to ellipsoidal (rod-shaped) particles of varying aspect ratios in in vitro parallel plate flow chamber assays using human blood and in vivo in ApoE−/− mice and found that rod-like particles with equivalent spherical diameter (ESD) greater than 1µm outperformed their spherical counterparts.61,76 However, rod-like particles with ESD less than 1µm exhibited little to no improvement in margination and adhesion efficiency relative to equivalent spheres. This suggests that, below a certain size, particles of all shapes are unable to effectively marginate to the vascular wall, but above a certain threshold, elongated particles may be able to marginate and adhere more effectively. Similarly, Wen et al. investigated the ability of icosahedral particles (approximating spheres) and rod-like particles loaded with imaging agents to accumulate at sites of thrombus formation, finding that the rod-like particles were much more efficient in this regard.75 Further, Toy et al. showed that rod-shaped nanoparticles outperformed spherical nanoparticles in in vitro adhesion assays performed in buffer flow.66 The fact that red blood cells were absent in assays from the Toy work highlights the critical function of particle-cell dynamics in margination, as discussed earlier.
Computational studies have also explored the effect of carrier shape on margination and adhesion efficiency. Vahidkhah et al. constructed a 3D computational fluid dynamics model to simulate margination, wall contact, and adhesion of particulate carriers of varying shapes, including spherical, oblate, and prolate particles.74 Interestingly, their study found that shape influences margination and adhesion efficiency differently. The oblate particles were most effective at marginating toward the vascular wall, due to their increased collision frequency with RBCs and rotation in flow. However, once present in the cell-free layer close to the vascular wall, prolate particles were most likely to make contact with the wall due to their elongated shape. Finally, oblate particles were most effective at forming firm adhesive contact, due to a large contact surface area. This work strengthens and supports previous experimental studies and sheds some light on the various effects at play in blood flow.
Although only a handful of works are highlighted here in order to provide some context, many more studies have been performed to elucidate the effect of shape on particle margination and adhesion efficiency. For a more in-depth review on the effects of particle shape on carrier effectiveness, see Fish et al.77
Effect of Carrier Density on Margination and Targeting Efficiency
To date, the bulk of NCs used for vascular-targeting are essentially neutrally buoyant or only slightly denser than blood, including liposomes and most biodegradable polymeric NCs. Recently, Thompson et al. reported density as a potential avenue for enhancing vascular wall margination of NCs in human blood flow, where silica nanoparticles with twice (~2 g mL−1) the density of blood were shown to exhibit > 3 folds higher adhesion to vascular wall endothelial cells compared to neutrally buoyant polystyrene nanoparticles of the same size and targeting ligand in in vitro assays with human blood flow.78 The authors show via confocal microscopy that this enhanced performance by silica nanoparticles was due to the presence of red blood cells promoting enhanced margination for silica relative to buffer flow while the density neutral polystyrene exhibit negative margination in the presence of red blood cells. Interestingly, titania nanoparticles of the same size and targeting characteristics but with four times (~3.9 g mL−1) the density of blood did not marginate differently from polystyrene ones, suggesting that their perhaps is an optimal particle density for which interaction with cells in blood, particularly red blood cells, results in maximum displacement to the vascular wall. However, in a study by Toy et al., particle density was found to have a negative impact on margination, where less dense nanoparticles exhibited significantly higher margination than dense ones in simple buffer flow, which was attributed to larger particles carrying a larger momentum in flow. Again, the key difference between the work by Toy et al. and Thompson et al. is the presence of red blood cells, highlighting the importance of cell-particle collision in margination in blood flow.66 More work would need to be done, however, to fully flesh out the utility of density to enhance the margination of NCs. One limitation to this is the limited availability of nanoparticles with a wide range of density to explore. In this regard, computational modeling might be beneficial for bridging the gap. Another potential limitation to use of density as a parameter for vascular-targeting of NCs is the fact that the bulk of the biocompatible materials available for construct of dense NCs, including iron oxide (~5 g mL−1) and gold (~19 g mL−1), are not conducive to drug loading.
Conclusion
Many of the successful strategies used by researchers designing vascular-targeted NCs involve designing bio-mimetic carriers, or carriers that take advantage of the natural function of native cells in the disease state. The successful use of such tactics highlights the need for a deeper understanding of the physiology present in cardiovascular disease. Taking inspiration from nature, we can design more effective drug carriers that will result in a larger therapeutic benefit in cardiovascular disease. This “reverse-engineering” of physiological systems in order to produce a therapeutic benefit is a common, emerging theme in the biological sciences, and could prove to be very useful in carrier design. It is crucial for researchers to consider both the physical properties of VTCs and the effectiveness of various targeting schemes when designing VTCs, which presents quite the challenge for researchers. However, emerging technologies from the materials science realm are providing more control over carrier physical properties, but it can still be quite difficult to achieve a particle drug carrier with optimized size, shape, and density, as well as readily available groups for conjugate chemistry. This is most clear in the discussed importance of the physical characteristics of the NC in vascular targeting; NCs must be small enough to navigate the vasculature and readily transmigrate across biological barriers, but very small NCs have been shown to exhibit difficulties in marginating from the center of blood flow to the vascular wall.
Another factor to keep in mind is the potential clinical utility for a particular drug carrier design. In this context, it is beneficial to consider the utility of nanoscale drug carriers in chronic versus acute conditions, for instance. While it is potentially possible to deliver a number of therapeutic agents for treating chronic conditions via vascular targeted carriers, researchers must consider the potential physiological impact of repeated or continuous injections of a particular drug carrier, and whether there are any negative consequences that outweigh therapeutic benefit. These considerations are discussed in more detail in a recent review by Howard et al.81
Additionally, through examination of recent literature, a number of important aspects of the design of vascular-targeted NCs for cardiovascular disease come to light. In particular, carrier parameters such as carrier size, shape, and material must be considered in order to design a carrier that effectively marginates from the red cell core to the vascular wall and binds to its target. These parameters are critical for the successful use of vascular-targeted NCs.
Further, NCs will always have to combat immunological clearance mechanisms (such as phagocytosis by white blood cells), which may be mitigated to some extent by the use of protective coatings such as PEG or zwitterionic coatings. Finally, there are recently highlighted limitations in the animal models used to evaluate the efficacy of vascular-targeted NCs as therapeutics. Our group has shown that vascular-targeted carriers can perform very differently in a mouse versus a human due to the differences in hemodynamics between humans and animals.82 Furthermore, differences in plasma proteins between animal models are now known to have varying impacts on the performance of targeted NCs.83 In particular, it seems that commonly-used mouse models may not accurately capture the interactions between NCs and plasma proteins in humans, while porcine models may provide a more complete picture. These limitations, together, create a balance of factors that is very important to consider in the design of novel targeted NCs.
Acknowledgments
This work was funded in part by an NSF Fellowship (W. J. K) and an NIH grant R01 HL115138 (O.E.A.).
Footnotes
Further Reading/Resources
Alvine, Travis D, Knopick, Peter L, Nilles, Matthew L, and Bradley, David S(Sep 2015) Inflammatory Mediators. In: eLS. John Wiley & Sons Ltd, Chichester. http://www.els.net [doi: 10.1002/9780470015902.a0000945.pub2]
Kumar, Rakesh K, and Wakefield, Denis(Apr 2015) Inflammation: Chronic. In: eLS. John Wiley & Sons Ltd, Chichester. http://www.els.net [doi: 10.1002/9780470015902.a0000944.pub4]
References
- 1.Gentile F, Curcio A, Indolfi C, Ferrari M, Decuzzi P. The margination propensity of spherical particles for vascular targeting in the microcirculation. J Nanobiotechnology. 2008;6:9. doi: 10.1186/1477-3155-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whayne TF. Atherosclerosis: current status of prevention and treatment. Int J Angiol. 2011;20(4):213–222. doi: 10.1055/s-0031-1295520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang T, Palucci D, Law K, Yanagawa B, Yam J, Butany J. Atherosclerosis: pathogenesis and pathology. Diagnostic Histopathol. 2012;18(11):461–467. [Google Scholar]
- 4.Crielaard BJ, Lammers T, Schiffelers RM, Storm G. Drug targeting systems for inflammatory disease: One for all, all for one. J Control Release. 2012;161(2):225–234. doi: 10.1016/j.jconrel.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Lobatto ME, Fuster V, Fayad Za, Mulder WJM. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat Rev Drug Discov. 2011;10(11):835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y, Lobatto ME, Kawahara T, et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc Natl Acad Sci U S A. 2014;111(3):1078–1083. doi: 10.1073/pnas.1322725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobatto ME, Calcagno C, Millon A, et al. Atherosclerotic plaque targeting mechanism of long-circulating nanoparticles established by multimodal imaging. ACS Nano. 2015;9(2):1837–1847. doi: 10.1021/nn506750r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6(4):715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X-Q, Even-Or O, Xu X, et al. Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis. Adv Healthc Mater. 2015;4(2):228–236. doi: 10.1002/adhm.201400337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen S, Liu D-F, Cui Y, et al. In vivo MRI detection of carotid atherosclerotic lesions and kidney inflammation in ApoE-deficient mice by using LOX-1 targeted iron nanoparticles. Nanomedicine. 2014;10(3):639–649. doi: 10.1016/j.nano.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Geng YaN, Dalhaimer P, Cai S, Tsai R, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology. 2012;4(2):219–233. doi: 10.1002/wnan.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang S, Cao Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv Mater. 2010;22(9):920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 14.Xiao W, Lin J, Li M, et al. Prolonged in vivo circulation time by zwitterionic modification of magnetite nanoparticles for blood pool contrast agents. Contrast Media Mol Imaging. 2012;7(3):320–327. doi: 10.1002/cmmi.501. [DOI] [PubMed] [Google Scholar]
- 15.Sun M, Hoffman D, Sundaresan G, Yang L, Lamichhane N, Zweit J. Synthesis and characterization of intrinsically radio-labeled quantum dots for bimodal detection. Am J Nucl Med Mol Imaging. 2012;2(2):122–135. [PMC free article] [PubMed] [Google Scholar]
- 16.Prijic S, Sersa G. Magnetic nanoparticles as targeted delivery systems in oncology Magnetic nanoparticles. 2011;45(1):1–16. doi: 10.2478/v10019-011-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freund JB, Shapiro B. Transport of particles by magnetic forces and cellular blood flow in a model microvessel. Phys Fluids. 2012;24(051904) [Google Scholar]
- 18.Chorny M, Hood E, Levy RJ, Muzykantov VR. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J Control Release. 2010;146(1):144–151. doi: 10.1016/j.jconrel.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chorny M, Fishbein I, Tengood JE, Adamo RF, Alferiev IS, Levy RJ. Site-specific gene delivery to stented arteries using magnetically guided zinc oleate-based nanoparticles loaded with adenoviral vectors. FASEB J. 2013;27(6):2198–2206. doi: 10.1096/fj.12-224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannell H, Pircher J, Räthel T, et al. Targeted Endothelial Gene Delivery by Ultrasonic Destruction of Magnetic Microbubbles Carrying Lentiviral Vectors. Pharm Res. 2012;29(5):1282–1294. doi: 10.1007/s11095-012-0678-8. [DOI] [PubMed] [Google Scholar]
- 21.Forbes ZG, Yellen BB, Halverson DS, Fridman G, Barbee KA, Friedman G. Validation of High Gradient Magnetic Field Based Drug Delivery to Magnetizable Implants Under Flow. IEEE Trans Biomed Eng. 2008;55(2):643–649. doi: 10.1109/TBME.2007.899347. [DOI] [PubMed] [Google Scholar]
- 22.Chandramouli S, Sanjana S, Swathi S. Use of Super Paramagnetic Iron-Oxide Nanoparticles in the Treatment of Atherosclerosis. IFBME Proceedings. :67–70. [Google Scholar]
- 23.Spronk HM, van der Voort D, Ten Cate H. Blood coagulation and the risk of atherothrombosis: a complex relationship. Thromb J. 2004;2(1):12. doi: 10.1186/1477-9560-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holme MN, Fedotenko IA, Abegg D, et al. Shear-stress sensitive lenticular vesicles for targeted drug delivery. Nat Nanotechnol. 2012;7(8):536–543. doi: 10.1038/nnano.2012.84. [DOI] [PubMed] [Google Scholar]
- 25.Korin N, Kanapathipillai M, Matthews BD, et al. Shear-Activated Nanotherapeutics for Drug Targeting to Obstructed Blood Vessels. Science. 2012;337:738–743. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- 26.Hu C-MJ, Fang RH, Wang K-C, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charoenphol P, Mocherla S, Bouis D, Namdee K, Pinsky DJ, Eniola-Adefeso O. Targeting therapeutics to the vascular wall in atherosclerosis--carrier size matters. Atherosclerosis. 2011;217(2):364–370. doi: 10.1016/j.atherosclerosis.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Onyskiw PJ, Eniola-Adefeso O. Effect of PEGylation on ligand-based targeting of drug carriers to the vascular wall in blood flow. Langmuir. 2013;29(35):11127–11134. doi: 10.1021/la402182j. [DOI] [PubMed] [Google Scholar]
- 29.Sobczynski DJ, Charoenphol P, Heslinga MJ, et al. Plasma protein corona modulates the vascular wall interaction of drug carriers in a material and donor specific manner. PLoS One. 2014;9(9):e107408. doi: 10.1371/journal.pone.0107408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H, Zhao F, Li Y, et al. VCAM-1-targeted core/shell nanoparticles for selective adhesion and delivery to endothelial cells with lipopolysaccharide-induced inflammation under shear flow and cellular magnetic resonance imaging in vitro. Int J Nanomedicine. 2013;8:1897–1906. doi: 10.2147/IJN.S44997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calin M, Stan D, Schlesinger M, et al. VCAM-1 directed target-sensitive liposomes carrying CCR2 antagonists bind to activated endothelium and reduce adhesion and transmigration of monocytes. Eur J Pharm Biopharm. 2015;89:18–29. doi: 10.1016/j.ejpb.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Calderon AJ, Muzykantov V, Muro S, Eckmann DM. Flow dynamics, binding and detachment of spherical carriers targeted to ICAM-1 on endothelial cells. Biorheology. 2009;46(4):323–341. doi: 10.3233/BIR-2009-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malek aM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci. 1996;109:713–726. doi: 10.1242/jcs.109.4.713. [DOI] [PubMed] [Google Scholar]
- 34.Bhowmick T, Berk E, Cui X, Muzykantov V, Muro S. Effect of flow on endothelial endocytosis of nanocarriers targeted to ICAM-1. J Control Release. 2012;157(3):485–492. doi: 10.1016/j.jconrel.2011.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han J, Zern BJ, Shuvaev VV, Davies PF, Muro S, Muzykantov V. Acute and chronic shear stress differently regulate endothelial internalization of nanocarriers targeted to platelet-endothelial cell adhesion molecule-1. ACS Nano. 2012;6(10):8824–8836. doi: 10.1021/nn302687n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han J, Shuvaev VV, Davies PF, Eckmann DM, Muro S, Muzykantov VR. Flow shear stress differentially regulates endothelial uptake of nanocarriers targeted to distinct epitopes of PECAM-1. J Control Release. 2015;210:39–47. doi: 10.1016/j.jconrel.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen LK, York AW, Lewis DR. Amphiphilic Nanoparticles Repress Macrophage Atherogenesis: Novel Core/Shell Designs for Scavenger Receptor Targeting and Down-Regulation. 2014;11(8):2815–2824. doi: 10.1021/mp500188g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iverson NM, Plourde NM, Sparks SM, et al. Biomaterials Dual use of amphiphilic macromolecules as cholesterol efflux triggers and inhibitors of macrophage athero-inflammation. 2011;32:8319–8327. doi: 10.1016/j.biomaterials.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagalkot V, Badgeley MA, Kampfrath T, Deiuliis JA, Rajagopalan S, Maiseyeu A. Hybrid Nanoparticles Improve Targeting to Inflammatory Macrophages Through Phagocytic Signals. J Control Release. 2015;217:243–255. doi: 10.1016/j.jconrel.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Gaytan BL, Fay F, Lobatto ME, et al. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug Chem. 2015;26(3):443–451. doi: 10.1021/bc500517k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duivenvoorden R, Tang J, Cormode DP, et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tu C, Ng TSC, Sohi HK, et al. Receptor-targeted iron oxide nanoparticles for molecular MR imaging of inflamed atherosclerotic plaques. Biomaterials. 2011;32(29):7209–7216. doi: 10.1016/j.biomaterials.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nie S, Zhang J, Martinez-Zaguilan R, et al. Detection of atherosclerotic lesions and intimal macrophages using CD36-targeted nanovesicles. J Control Release. 2015;220(Pt A):61–70. doi: 10.1016/j.jconrel.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marrachea S, Dhar S. Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc Natl Acad Sci. 2013;110(23):9445–9450. doi: 10.1073/pnas.1301929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spyropoulos-Antonakakis N, Sarantopoulou E, Trohopoulos PN, et al. Selective aggregation of PAMAM dendrimer nanocarriers and PAMAM/ZnPc nanodrugs on human atheromatous carotid tissues: a photodynamic therapy for atherosclerosis. Nanoscale Res Lett. 2015;10(210) doi: 10.1186/s11671-015-0904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobin-Valat M-J, Laroche-Traineau J, Larivière M, et al. Nanoparticles functionalised with an anti-platelet human antibody for in vivo detection of atherosclerotic plaque by magnetic resonance imaging. Nanomedicine. 2015;11(4):927–937. doi: 10.1016/j.nano.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Jacobin-Valat M-J, Deramchia K, Mornet S, et al. MRI of inducible P-selectin expression in human activated platelets involved in the early stages of atherosclerosis. NMR Biomed. 2011;24(4):413–424. doi: 10.1002/nbm.1606. [DOI] [PubMed] [Google Scholar]
- 48.Bachelet-Violette L, Silva AKA, Maire M, et al. Strong and specific interaction of ultra small superparamagnetic iron oxide nanoparticles and human activated platelets mediated by fucoidan coating. RSC Adv. 2014;4(10):4864. [Google Scholar]
- 49.Anselmo AC, Modery-Pawlowski CL, Menegatti S, et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano. 2014;8(11):11243–11253. doi: 10.1021/nn503732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee GY, Kim J-H, Choi KY, et al. Hyaluronic acid nanoparticles for active targeting atherosclerosis. Biomaterials. 2015;53:341–348. doi: 10.1016/j.biomaterials.2015.02.089. [DOI] [PubMed] [Google Scholar]
- 51.Eniola AO, Hammer DA. In vitro characterization of leukocyte mimetic for targeting therapeutics to the endothelium using two receptors. 2005;26:7136–7144. doi: 10.1016/j.biomaterials.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Rafat M, Rotenstein LS, You J, Auguste DT. Biomaterials Dual functionalized PVA hydrogels that adhere endothelial cells synergistically. Biomaterials. 2012;33(15):3880–3886. doi: 10.1016/j.biomaterials.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Mcateer MA, Schneider JE, Ali ZA, et al. Magnetic Resonance Imaging of Endothelial Adhesion Molecules in Mouse Atherosclerosis Using Dual-Targeted Microparticles of Iron Oxide. 2007:77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H, Kona S, Su L, Tsai Y. Multi-Ligand Poly (L -Lactic- co -Glycolic Acid) Nanoparticles Inhibit Activation of Endothelial Cells. 2013:570–578. doi: 10.1007/s12265-013-9460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papademetriou I, Tsinas Z, Hsu J, Muro S. Combination-targeting to multiple endothelial cell adhesion molecules modulates binding, endocytosis, and in vivo biodistribution of drug nanocarriers and their therapeutic cargoes. J Control Release. 2014;188:87–98. doi: 10.1016/j.jconrel.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chacko A, Nayak M, Greineder CF, Delisser HM, Muzykantov VR. Collaborative Enhancement of Antibody Binding to Distinct PECAM-1 Epitopes Modulates Endothelial Targeting. 2012;7(4) doi: 10.1371/journal.pone.0034958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chacko A, Han J, Greineder CF, et al. Collaborative Enhancement of Endothelial Targeting of Nanocarriers by Modulating Platelet-Endothelial Cell Engagement. ACS Nano. 2015;9(7):6785–6793. doi: 10.1021/nn505672x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McMasters J, Panitch A. Prevention of Collagen-Induced Platelet Binding and Activation by Thermosensitive Nanoparticles. AAPS J. 2015;17(5):1117–1125. doi: 10.1208/s12248-015-9794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamaly N, Fredman G, Subramanian M, et al. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci U S A. 2013;110(16):6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu Z, Rolfe BE, Xu ZP, Campbell JH, Lu GQM, Thomas AC. Antibody-targeted drug delivery to injured arteries using layered double hydroxide nanoparticles. Adv Healthc Mater. 2012;1(5):669–673. doi: 10.1002/adhm.201200069. [DOI] [PubMed] [Google Scholar]
- 61.Thompson AJ, Mastria EM, Eniola-Adefeso O. The margination propensity of ellipsoidal micro/nanoparticles to the endothelium in human blood flow. Biomaterials. 2013;34(23):5863–5871. doi: 10.1016/j.biomaterials.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Kumar A, Graham MD. Mechanism of margination in confined flows of blood and other multicomponent suspensions. Phys Rev Lett. 2012;109(10):1–5. doi: 10.1103/PhysRevLett.109.108102. [DOI] [PubMed] [Google Scholar]
- 63.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31(6):1392–1402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Müller K, Fedosov DA, Gompper G. Understanding particle margination in blood flow – A step toward optimized drug delivery systems. Med Eng Phys. 2015:1–9. doi: 10.1016/j.medengphy.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Jurney P, Agarwal R, Singh V, Roy K, Sreenivasan SV, Shi L. The Effect of Nanoparticle Size on Margination and Adhesion Propensity in Artificial Micro-Capillaries; Proceedings of the Asme Micro/Nanoscale Heat and Mass Transfer International Conference, 2012; 2012. pp. 109–115. [Google Scholar]
- 66.Toy R, Hayden E, Shoup C, Baskaran H, Karathanasis E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology. 2011;22(11):115101. doi: 10.1088/0957-4484/22/11/115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Charoenphol P, Mocherla S, Bouis D, Namdee K, Pinsky DJ, Eniola-Adefeso O. Targeting therapeutics to the vascular wall in atherosclerosis--carrier size matters. Atherosclerosis. 2011;217(2):364–370. doi: 10.1016/j.atherosclerosis.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 68.Namdee K, Thompson AJ, Charoenphol P, Eniola-Adefeso O. Margination propensity of vascular-targeted spheres from blood flow in a microfluidic model of human microvessels. Langmuir. 2013;29(8):2530–2535. doi: 10.1021/la304746p. [DOI] [PubMed] [Google Scholar]
- 69.Lee T-R, Choi M, Kopacz AM, Yun S-H, Liu WK, Decuzzi P. On the near-wall accumulation of injectable particles in the microcirculation: smaller is not better. Sci Rep. 2013;3:2079. doi: 10.1038/srep02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Müller K, Fedosov Da, Gompper G. Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci Rep. 2014;4(4871) doi: 10.1038/srep04871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shinde Patil VR, Campbell CJ, Yun YH, Slack SM, Goetz DJ. Particle diameter influences adhesion under flow. Biophys J. 2001;80(4):1733–1743. doi: 10.1016/s0006-3495(01)76144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar A, Graham MD. Mechanism of Margination in Confined Flows of Blood and Other Multicomponent Suspensions. Phys Rev Lett. 2012;109(10):108102. doi: 10.1103/PhysRevLett.109.108102. [DOI] [PubMed] [Google Scholar]
- 73.Champion Ja, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc Natl Acad Sci U S A. 2007;104(29):11901–11904. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vahidkhah K, Bagchi P. Microparticle shape effects on margination, near-wall dynamics and adhesion in a three-dimensional simulation of red blood cell suspension. Soft Matter. 2015;11(11):2097–2109. doi: 10.1039/c4sm02686a. [DOI] [PubMed] [Google Scholar]
- 75.Wen AM, Wang Y, Jiang K, et al. Shaping bio-inspired nanotechnologies to target thrombosis for dual optical-magnetic resonance imaging. J Mater Chem B. 2015;3(29):6037–6045. doi: 10.1039/C5TB00879D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Namdee K, Thompson AJ, Golinski A, Mocherla S, Bouis D, Eniola-Adefeso O. In vivo evaluation of vascular-targeted spheroidal microparticles for imaging and drug delivery application in atherosclerosis. Atherosclerosis. 2014;237(1):279–286. doi: 10.1016/j.atherosclerosis.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 77.Fish MB, Thompson AJ, Fromen Ca, Eniola-Adefeso O. Emergence and Utility of Nonspherical Particles in Biomedicine. Ind Eng Chem Res. 2015;54(16):4043–4059. doi: 10.1021/ie504452j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson AJ, Eniola-Adefeso O. Dense nanoparticles exhibit enhanced vascular wall targeting over neutrally buoyant nanoparticles in human blood flow. Acta Biomater. 2015 doi: 10.1016/j.actbio.2015.04.005. In Press. [DOI] [PubMed] [Google Scholar]
- 79.Avula UMR, Yoon HK, Lee CH, et al. Cell-selective arrhythmia ablation for photomodulation of heart rhythm. Sci Transl Med. 2015;7(311):311ra172. doi: 10.1126/scitranslmed.aab3665. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen MM, Carlini AS, Chien MP, et al. Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Adv Mater. 2015;27(37):5547–5552. doi: 10.1002/adma.201502003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Howard MD, Hood ED, Zern B, Shuvaev VV, Grosser T, Muzykantov VR. Nanocarriers for vascular delivery of anti-inflammatory agents. Annu Rev Pharmacol Toxicol. 2014;54:205–226. doi: 10.1146/annurev-pharmtox-011613-140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Namdee K, Carrasco-Teja M, Fish MB, Charoenphol P, Eniola-Adefeso O. Effect of Variation in hemorheology between human and animal blood on the binding efficacy of vascular-targeted carriers. Sci Rep. 2015;5(February):11631. doi: 10.1038/srep11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Namdee K, Sobczynski DJ, Onyskiw PJ, Eniola-Adefeso O. Differential Impact of Plasma Proteins on the Adhesion Efficiency of Vascular-Targeted Carriers (VTCs) in Blood of Common Laboratory Animals. Bioconjug Chem. 2015 doi: 10.1021/acs.bioconjchem.5b00474. acs.bioconjchem.5b00474. [DOI] [PMC free article] [PubMed] [Google Scholar]