Abstract
Introduction
Historically symptomatic AAAs were found to have intermediate mortality compared to asymptomatic and ruptured AAAs but, with wider EVAR use, a more recent study suggested mortality of symptomatic aneurysms were similar to asymptomatic AAAs. These prior studies were limited by small numbers. The purpose of this study is to evaluate the mortality and morbidity associated with symptomatic AAA repair in a large contemporary population.
Methods
All patients undergoing infrarenal AAA repair were identified in the 2011–2013 ACS-NSQIP, Vascular Surgery targeted module. We excluded acute conversions to open repair and those for whom the surgical indication was embolization, dissection, thrombosis, or not documented. We compared 30-day mortality and major adverse events (MAE) for asymptomatic, symptomatic, and ruptured AAA repair, stratified by EVAR and open repair, with univariate analysis and multivariable logistic regression.
Results
5502 infrarenal AAAs were identified, 4495 asymptomatic (830 open repair, 3665 [82%] EVAR), 455 symptomatic (143 open, 312 [69%] EVAR), and 552 ruptured aneurysms (263 open, 289 [52%] EVAR). Aneurysm diameter was similar between asymptomatic and symptomatic AAAs, when stratified by procedure type, but larger for ruptured aneurysms (EVAR symptomatic 5.8cm ±1.6 vs. ruptured 7.5cm ±2.0, P<.001; open repair symptomatic 6.4cm ±1.9 vs. ruptured 8.0cm ±1.9, P<.001). The proportion of females was similar in symptomatic and ruptured AAA (27% vs. 23%, P=.14, respectively), but lower in asymptomatic AAA (20%, P<.001). Symptomatic AAAs had intermediate 30-day mortality compared to asymptomatic and ruptured aneurysms after both EVAR (asymptomatic 1.4% vs. symptomatic 3.8%, P=.001; symptomatic vs. 22% ruptured, P<.001) and open repair (asymptomatic 4.3% vs. symptomatic 7.7% , P=.08; symptomatic vs. 57% ruptured, P<.001). After adjustment for age, gender, repair type, dialysis dependence, and history of severe COPD, patients undergoing repair of symptomatic AAAs were twice as likely to die within 30-days compared to those with asymptomatic aneurysms (OR 2.1, 95%CI 1.3–3.5). When stratified by repair type the effect size and direction of the odds ratios were similar (EVAR OR 2.4, CI 1.2–4.7; open repair OR 1.8, CI 0.86–3.9), although not significant for open repair. Patients with ruptured aneurysms had a sevenfold increased risk of 30-day mortality compared to symptomatic patients (OR 6.5, CI 4.1–10.6).
Conclusion
Patients with symptomatic AAAs had a two-fold increased risk of perioperative mortality, compared to asymptomatic aneurysms undergoing repair. Furthermore, patients with ruptured aneurysms have a seven-fold increased risk of mortality compared to symptomatic aneurysms.
Introduction
The 30-day mortality rate for abdominal aortic aneurysm (AAA) repair can range from approximately 1% to over 70% depending on whether the aneurysm is intact, symptomatic, or ruptured.[1–6] 3% to 15% of treated aneurysms have been described as symptomatic in prior studies.[7–10] Symptomatic abdominal aortic aneurysms present with symptoms of abdominal or back pain, often associated with tenderness to palpation of the aneurysm itself, and are thought to represent an intermediate risk group between elective and ruptured aneurysms.
Historically, many single institution studies showed that patients with symptomatic AAAs had higher rates of mortality and major adverse events compared to asymptomatic AAA repairs.[7, 10–14] However, most of these studies predated the wide use of EVAR and had small numbers of symptomatic AAAs. De Martino et al, using a contemporary clinical registry, the Vascular Study Group of New England (VSGNE), from 2003–2009, showed that there was no difference in in-hospital mortality between symptomatic and elective infrarenal AAA repairs, when stratified by procedure type.[8] This study had the largest cohort of symptomatic AAAs treated with EVAR at the time. Prior to this study, Cambria et al. reported that deferral of operation to medically optimize the patient and ensure appropriate staff are available, instead of immediate repair within the first 4 hours, improved outcomes for the symptomatic AAAs.[7] This led to an increased focus on preoperative management of the symptomatic patient and was thought to contribute to the lack of difference in perioperative mortality between elective and symptomatic patients in De Martino’s study. However, many still believe that symptomatic AAAs continue to have an intermediate operative mortality risk in the short-term but there have been no studies with a current-practice distribution of EVAR and open repair and an adequate number of symptomatic AAA patients to address this ongoing question.
The purpose of this study was to analyze the differences in mortality and morbidity between patients with symptomatic AAAs compared to both asymptomatic and ruptured aneurysms in a contemporary population where EVAR was the preferred treatment modality for elective repair.
Methods
Dataset
Using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) Vascular Surgery targeted module from 2011–2013, we identified all patients undergoing endovascular (EVAR) and open AAA repair. The NSQIP vascular targeted module is an extension of the original NSQIP with 72 participating hospitals in the AAA module as of 2013. It is a multi-institutional collaboration that continues to collect all the preoperative, intraoperative, and 30-day outcomes that were contained in the original NSQIP as well as further clinical detail selected by vascular surgeons in an effort to better risk adjust and determine best practices. Trained clinical nurse reviewers complete all data collection, and each hospital has a surgeon champion, available to answer any questions related to data entry for cases submitted. Additional information on the NSQIP is available at www.facs.org/quality-programs/acs-nsqip.
Patients and Cohorts
All 6703 patients undergoing AAA repair in the targeted NSQIP were identified. For direct comparison to prior studies the primary analysis of this paper focused on repair of infrarenal aneurysms, as identified by proximal aneurysm extent. Juxtarenal aneurysms were included in the analysis with adjustment in multivariable analysis. A subset of patients who were documented to have infrarenal aneurysm extent yet had a suprarenal clamp position were reclassified as juxtarenal. All those with a proximal aneurysm extent listed as pararenal, suprarenal, or Type IV thoracoabdominal were excluded from the analysis. Patients with no documented proximal aneurysm extent or operative indication were excluded (n= 439 and n= 81 respectively). Patients with an operative indication of dissection, thrombosis, or embolization or those undergoing conversion from EVAR to open repair (n=33) were also excluded. Patients with symptomatic AAAs were defined as those without evidence of rupture but presenting with abdominal or back pain, or symptoms from local compression by the aneurysm causing early satiety, hydronephrosis, or deep venous thrombosis. Ruptured aneurysms were divided into 2 groups based on hemodynamic status: hypotensive (defined as systolic blood pressure <90mmHg or drop in systolic blood pressure of >40mmHg from baseline or need for pressors preoperatively), and non-hypotensive. The asymptomatic non-ruptured group consisted of those with a surgical indication for repair listed as diameter, prior open repair with unsatisfactory result, or prior endovascular repair with unsatisfactory result. The latter two indications were accepted because it was thought likely that the symptomatic and rupture groups contained some of these patients as well, as only one indication can be entered per patient.
All variable definitions captured by the NSQIP can be found at www.facs.org/quality-programs/acs-nsqip. New or aggregate variables used in this analysis included, obesity, defined as a body mass index >30, and a binary variable for diabetes mellitus, defined as both insulin and non-insulin dependent diabetes. For EVAR, percutaneous access included attempted but failed percutaneous access attempts. We consolidated the main body devices analyzed and created an “Other” group that included Cook Zenith Fenestrated (1.9%), Cook Zenith Renu (1.4%), Lombard Aorfix (<0.1%), Medtronic Aneurx (0.2%), Medtronic Talent (0.6%), Trivascular Ovation (0.9%), and other (4.1%). 10% of patients were missing data on lower extremity revascularization but these patients were considered as not having revascularization in our analysis. Time from admission to operation was recorded in days with day 0 representing operation on day of admission. We identified patients undergoing surgery after the day of admission to highlight the number of symptomatic patients who have a delay in their repair since this has been shown to affect outcomes in prior literature.[7] Operative details and outcomes were presented for EVAR and open repair separately.
All outcomes were within 30 days of the index operation. A major adverse event was defined as a myocardial infarction (diagnosed as new Q waves on ECG and documentation stating diagnosis of MI), intraoperative cardiac arrest, pneumonia, prolonged intubation (defined as >48 hours), worsening renal function (defined as a rise in creatinine of >2.0mg/dl or new requirement for dialysis), bowel ischemia as stated in the medical record whether intervention was necessary or not, lower extremity ischemia requiring intervention, or subsequent rupture after repair.
Statistical Analysis
Continuous variables were presented as mean ± standard deviation, or as median and interquartile range based on distribution. Categorical variables were presented as counts and percentages. Univariate differences between cohorts were assessed using χ2 and Fisher’s exact tests for categorical variables and Student’s t-test and Mann Whitney U test for continuous variables, where appropriate. Comparisons were made between asymptomatic and symptomatic AAAs and symptomatic to ruptured AAAs, stratified by repair type. To identify independent risk factors for 30-day mortality and major adverse events we used purposeful selection, which utilizes both univariate analysis and previously identified predictors for the endpoint of interest, to fill the multivariable model for the comparison of asymptomatic to symptomatic and symptomatic to ruptured AAAs.[15] Certain variables, such as emergency repair and aneurysm diameter were not included in the model as they were collinear with symptomatic aneurysms. We listed the Hosmer and Lemeshow statistic for all steps of model optimization to support the stability of our model given the limited number of total events. P-value < .10 on univariate analysis was used for inclusion into each model. All tests were two-sided and significance was considered when P-value was < .05. IBM SPSS Statistics version 22.0 (IBM Inc., Chicago, IL) was used for all analysis. Permission to use deidentified data from the NSQIP, without the need for informed consent, was obtained from the Institutional Review Board at Beth Israel Deaconess Medical Center.
Results
From a total of 6703 patients undergoing AAA repair in the vascular targeted NSQIP from 2011–2013, we excluded 516 (7.7% of total) pararenal/suprarenal aneurysms, 439 (6.5%) patients without documentation of aneurysm extent, 213 (3.2%) for indication of dissection, embolization, thrombosis, or no documentation, and 33 (0.5%) for acute conversion from EVAR to open repair. This left 5502 patients undergoing repair of infrarenal (85%, 92% EVAR) or juxtarenal (15%; 20% EVAR) aneurysms. The final cohort included 4495 asymptomatic patients (82% EVAR), 455 symptomatic patients (69% EVAR), and 552 ruptured patients (52% EVAR). Within the asymptomatic group there were 138 (3.1%) patients with prior unsatisfactory endovascular repair (72% EVAR) and 19 (0.4%) with unsatisfactory open repair (79% EVAR).
Patient Characteristics
Asymptomatic vs. Symptomatic
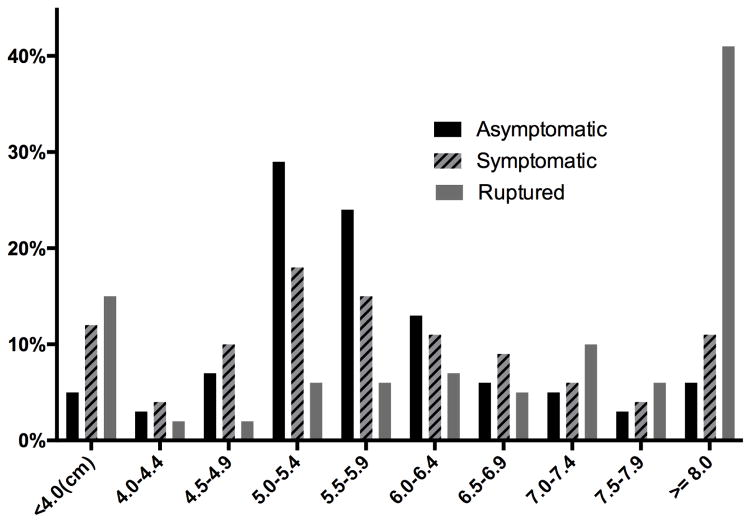
Symptomatic patients in general were younger (mean 72.6 SD ±10.1 vs. 73.6 ±8.6, p=.01), and less likely to be white (80% vs. 87%, P < .001), male (73% vs. 80%, P < .001), or obese (25% vs. 32%, P = .01)(Table I). They were more likely to be current smokers (43% vs. 32%, P < .001), have preoperative acute renal failure (0.9% vs. 0.2%, P = .03) or hemodialysis (2.6% vs. 0.8%, P < .001), and have a preoperative transfusion (3.1% vs. 0.9%, P < .001). Patients with symptomatic AAAs had larger mean aneurysm diameter overall compared to asymptomatic aneurysms (asymptomatic 5.8 SD ±1.2 vs. 6.0 ±1.7, P = .045). Figure 1 illustrates a higher proportion of asymptomatic AAA repairs occurring between 5.0–5.9cm compared to symptomatic AAAs, which coincides with guidelines for elective repair in this group. As expected there was a higher proportion of symptomatic patients listed as emergent compared to asymptomatic patients (EVAR 26% vs. 2.1% P < .001; open repair 39% vs. 3.0%, P < .001). Symptomatic patients were more likely to have surgery deferred and to not undergo surgical repair on the same calendar day as admission (EVAR 44% vs. 12%, P < .001; open repair 42% vs. 23%, P < .001), which we hypothesized to represent time spent medically optimizing the patient and avoiding off-hour operations, although a delay in diagnosis of a symptomatic aneurysm could also be contributing. Out of the 198 symptomatic patients who underwent repair after the day of admission 50% were operated on the next calendar day and 79% were operated on within three calendar days of admission.
Table I.
Preoperative Characteristics
| Asymptomatic, %(n) | P-value Asymptomatic vs. Symptomatic | Symptomatic, %(n) | P-value Symptomatic vs. Ruptured | Ruptured, %(n) | ||||
|---|---|---|---|---|---|---|---|---|
| N | 4495 | 455 | 552 | |||||
| EVAR | 82 | (3665) | <.001 | 69 | (312) | <.001 | 52 | (289) |
| Age (years, mean ±SD) | 73.6 | ±8.6 | .01 | 72.6 | ±10.1 | .30 | 73.3 | ±10.1 |
| White | 87 | (3887) | <.001 | 80 | (366) | .62 | 79 | (437) |
| Male | 80 | (3604) | <.001 | 73 | (330) | .14 | 77 | (423) |
| Diabetes | 16 | (710) | .01 | 11 | (52) | .44 | 13.0 | (72) |
| Current Smoker | 32 | (1423) | <.001 | 43 | (197) | .06 | 38 | (207) |
| Obesity (BMI >30) | 32 | (1408) | .01 | 35 | (109) | .03 | 32 | (137) |
| Dyspnea at rest or exertion | 19 | (851) | .83 | 19 | (88) | <.001 | 9.6 | (53) |
| Preoperative Intubation | 0.1 | (5) | .13 | 0.4 | (2) | <.001 | 11 | (60) |
| Hypertension | 82 | (3667) | .20 | 79 | (360) | .002 | 71 | (390) |
| History of | ||||||||
| Severe COPD | 19 | (837) | .10 | 22 | (99) | .48 | 20 | (110) |
| CHF exacerbation | 1.7 | (75) | .26 | 2.4 | (11) | .80 | 2.2 | (12) |
| Dependent functional status | 2.8 | (127) | .19 | 1.8 | (8) | .001 | 5.8 | (31) |
| ASA class > III | 24 | (1097) | <.001 | 43 | (195) | <.001 | 86 | (469) |
| Creatinine >1.78 mg/dla | 5.1 | (225) | .96 | 5.2 | (23) | <.001 | 17 | (85) |
| Acute renal failure pre-op | 0.2 | (9) | .03 | 0.9 | (4) | .21 | 1.8 | (10) |
| On Dialysis pre-op | 0.8 | (35) | <.001 | 2.6 | (12) | .50 | 2.0 | (11) |
| Open wound/wound infection | 1.0 | (47) | .17 | 1.8 | (8) | .70 | 1.4 | (8) |
| Transfusion | 0.9 | (40) | <.001 | 3.1 | (14) | <.001 | 26 | (146) |
| Prior Open Abd. Surgery | 23 | (979) | .39 | 25 | (104) | .13 | 21 | (100) |
| Aneurysm Diameter, cm (mean, SD) | 5.8 | ±1.2 | .045 | 6.0 | ±1.7 | <.001 | 7.7 | ±2.0 |
| Aneurysm Diameter, cm (median, IQR) | 5.5 | (5.1–6.2) | .19 | 5.7 | (5.0–6.8) | <.001 | 7.7 | (6.3–9.0) |
COPD=Chronic obstrucive pulmonary disease, CHF= Congestive heart failure
Patients on dialysis at baseline are not counted in those with elevated baseline Creatinine
Figure 1.
AAA diameter at time of repair as a percentage of all patients within each indication
Symptomatic vs. Ruptured
When compared to those with ruptured aneurysms, symptomatic patients were less likely to be obese (25% vs. 32%, P = .03), intubated prior to the OR (0.4% vs. 11%, P < .001), have dependent baseline functional status (1.8% vs. 5.8%, P = .001), preoperative creatinine elevation (5.2% vs. 17%, P = .001), preoperative transfusion (3.1% vs. 26%, P < .001), ASA score of 4 or 5 (43% vs. 86 %, P < .001)(Table I). Symptomatic patients were more likely to have preoperative dyspnea on exertion (19% vs. 10%, P < .001) and hypertension (79% vs. 71%, P < .001). Aneurysm diameter was significantly smaller in symptomatic patients (6.0 ±1.7 vs. 7.7 ±2.0, P < .001), (Figure 1). As expected, there was a lower proportion of emergent cases amongst the symptomatic patients compared to those with rupture (EVAR 26% vs. 88%, P < .001; open repair 39% vs. 92%, P < .001), and greater deferment of cases to the following days after admission (EVAR 44% vs. 13%, P < .001; open repair 42% vs. 12%, P < .001).
Operative details
EVAR
The use of EVAR was highest in asymptomatic patients followed by symptomatic patients (asymptomatic 82% vs. symptomatic 69%, P < .001), and lowest in ruptures (52%, P < .001)(Table II). Comparing EVAR for asymptomatic and symptomatic presentations, symptomatic patients were less likely to have a percutaneous attempt for access (20% vs. 27%, P = .01), had longer operative times (median 140 minutes [Inter-quartile range 110–178] vs. 133 [102–175], P = .02), and were more likely to require a concomitant access vessel conduit or repair (11% vs. 7.4%, P = .04).
Table II.
Operative details for EVAR
| Asymptomatic, %(n) | P-value Asymptomatic vs.Symptomatic | Symptomatic, %(n) | P-value Symptomatic vs. Ruptured | Ruptured, %(n) | ||||
|---|---|---|---|---|---|---|---|---|
| N | 3665 | 312 | 289 | |||||
| Emergent case | 2.1 | (76) | <.001 | 26 | (82) | <.001 | 88 | (253) |
| Distal Extenta | .12 | .32 | ||||||
| Aortic | 47 | (1499) | 45 | (111) | 38 | (85) | ||
| Common Iliac | 40 | (1280) | 39 | (96) | 42 | (94) | ||
| External Iliac | 5.7 | (183) | 5.2 | (13) | 8.6 | (19) | ||
| Internal Iliac | 7.4 | (237) | 12 | (29) | 11 | (24) | ||
| Operation after day of admission | 12 | (446) | <.001 | 44 | (138) | <.001 | 13 | (36) |
| Percutaneous Accessb | 27 | (972) | .01 | 20 | (61) | .34 | 23 | (66) |
| Juxtarenal Aneurysm | 4.7 | (171) | .91 | 4.8 | (15) | .27 | 6.9 | (20) |
| Main Body Device | .44 | .004 | ||||||
| Cook Zenith | 21 | (752) | 18 | (56) | 24 | (70) | ||
| Endologix Powerlink | 7.4 | (270) | 9.3 | (29) | 3.5 | (10) | ||
| Gore Excluder | 33 | (1206) | 36 | (113) | 38 | (108) | ||
| Medtronic Endurant | 30 | (1088) | 28 | (88) | 22 | (64) | ||
| Otherc | 8.9 | (325) | 8.0 | (25) | 12 | (35) | ||
| Concommitant Procedures | ||||||||
| Access Vessel Conduit/Repair | 7.4 | (271) | .04 | 11 | (33) | .01 | 18 | (52) |
| Hypogastric Embolization | 6.6 | (241) | .91 | 6.4 | (20) | .23 | 9.0 | (26) |
| Lower Ext. Revascularizationa | 3.5 | (129) | .08 | 5.4 | (312) | .12 | 8.7 | (25) |
| Aortic Bare Metal Stent | 2.1 | (78) | .89 | 2.2 | (7) | .55 | 1.4 | (4) |
| Iliac Bare Metal Stent | 3.4 | (124) | .87 | 3.2 | (10) | .86 | 3.5 | (10) |
Patients missing data (12.7%) for concomitant lower extremity revascularization, defined as any bypass or stent to an infrainguinal artery, coded as “No” because unlikely to have had the procedure and not be captured. The 12.7% missing data from distal extent were kept as missing.
Attempted percutaneous access counted as percutaneous
Other main body device includes: Cook Zenith Fenestrated and Renu, Lombard Aorfix, Medtronic Aneurx, Medtronic Talent, Not documented, Other, Trivascular Ovation
When comparing symptomatic to ruptured EVAR, symptomatic EVAR cases had shorter operative times (140 [110–178] vs. 157 [116–205], P = .01) and were less likely to have an access vessel conduit or repair (11% vs. 18%, P = .01)(Table II). There was a difference in main body devices used between groups. Excluder was the most common device used for asymptomatic, symptomatic, and ruptured aneurysms. Excluder was followed by Endurant then Zenith for asymptomatic and symptomatic aneurysms, but was followed by Zenith then Endurant for ruptures.
Open Repair
Comparing symptomatic to asymptomatic patients there was no difference in operative time (246 [173–290] vs. 232 [178–302], P = 0.8 respectively), distal aneurysm extent, aneurysm diameter, proportion of juxtarenal aneurysms, or concomitant procedures performed (Table III).
Table III.
Operative details for Open Repair
| Asymptomatic, %(n) | P-value Asymptomatic vs. Symptomatic | Symptomatic, %(n) | P-value Symptomatic vs. Ruptured | Ruptured, %(n) | ||||
|---|---|---|---|---|---|---|---|---|
| N | 830 | 143 | 263 | |||||
| Emergent case | 3.0 | (25) | <.001 | 39 | (56) | <.001 | 92 | (241) |
| Distal Extent | .83 | .72 | ||||||
| Aortic | 48 | (366) | 46 | (60) | 50 | (114) | ||
| Common Iliac | 43 | (330) | 47 | (61) | 41 | (94) | ||
| External Iliac | 5.2 | (40) | 3.8 | (5) | 3.1 | (7) | ||
| Internal Iliac | 3.5 | (27) | 3.8 | (5) | 5.3 | (12) | ||
| Operation after day of admission | 23 | (187) | <.001 | 42 | (60) | <.001 | 12 | (32) |
| Retroperitoneal approach | 25 | (205) | .82 | 26 | (37) | .002 | 14 | (35) |
| Juxtarenal Aneurysm | 55 | (432) | .09 | 47 | (64) | .08 | 56 | (141) |
| Concomitant Procedures | ||||||||
| Lower Ext. Revascularization | 7.6 | (63) | .58 | 6.3 | (9) | .32 | 9.1 | (24) |
| Non-arterial abd. repair/excision | 2.5 | (21) | .51 | 3.5 | (5) | .21 | 6.5 | (17) |
When comparing symptomatic to ruptured open repairs there was no difference in operative time (246 [173–290] vs. 235 [178–296], P = 0.9 respectively), distal aneurysm extent, proportion of juxtarenal aneurysms, or concomitant procedures performed (Table III). A retroperitoneal approach was more commonly used in symptomatic patients compared to those with rupture (26% vs. 14%, P = .01).
30-day Outcomes
Symptomatic vs. Asymptomatic
Mortality
The overall 30-day mortality rate was higher in symptomatic patients (5.1% vs. 1.9%, P < .001). For EVAR, symptomatic patients had a higher 30-day mortality rate (3.8% vs. 1.4%, P = .001) compared to asymptomatic patients (Table IVa). For open repair the mortality difference did not reach statistical significance (7.7% vs. 4.3%, P = .08)(Table IVb). There was also no difference in 30-day mortality for patients with symptomatic aneurysms whose surgery was not performed on day of admission (EVAR- day of admission 3.4% vs. not on day of admission 4.3%, P = .68 ; open repair 8.4 vs. 6.7, P = .76).
Table IVa.
30-day Outcomes for EVAR
| Asymptomatic, %(n) | P-value Asymptomatic vs. Symptomatic | Symptomatic,%(n) | Symptomatic vs. Ruptured | Ruptured, %(n) | ||||
|---|---|---|---|---|---|---|---|---|
| N | 3665 | 312 | 289 | |||||
| 30-day Mortality | 1.4 | (50) | .001 | 3.8 | (12) | <.001 | 22 | (62) |
| Major Adverse Event | 3.7 | (136) | <.001 | 9.3 | (29) | <.001 | 31 | (89) |
| Return to Operating Room | 3.7 | (136) | .49 | 4.5 | (14) | <.001 | 13 | (38) |
| Bleeding | 9.9 | (364) | <.001 | 18 | (57) | <.001 | 65 | (189) |
| Cardiac Arrest with CPR | 0.4 | (14) | <.001 | 1.9 | (6) | <.001 | 8.7 | (25) |
| Myocardial Infarction | 1.2 | (45) | .02 | 2.9 | (9) | .05 | 6.2 | (18) |
| Prolonged Ventilation (> 48hours) | 0.7 | (27) | <.001 | 2.9 | (9) | <.001 | 18 | (53) |
| Acute Kidney Injury | 0.3 | (12) | .11 | 1.0 | (3) | .08 | 3.1 | (9) |
| New Dialysis | 0.5 | (18) | 1.0 | 0.3 | (1) | <.001 | 10 | (29) |
| Post-op UTI | 1.1 | (39) | .77 | 0.6 | (2) | .12 | 2.1 | (6) |
| Surgical Site Infection | 1.7 | (61) | 1.0 | 1.6 | (5) | .02 | 4.8 | (14) |
| Pulmonary Embolism | 0.2 | (7) | .48 | 0.3 | (1) | .36 | 1.0 | (3) |
| DVT | 0.4 | (16) | .65 | 0.6 | (2) | .02 | 3.5 | (10) |
| Lower Extremity Ischemia | 1.2 | (44) | 1.0 | 1.0 | (3) | .08 | 3.1 | (9) |
| Rupture of Aneurysm after repair | 0.1 | (4) | 1.0 | 0.0 | (0) | <.001 | 4.8 | (14) |
| Ischemic Colitis | 0.5 | (17) | .20 | 1.0 | (3) | <.001 | 8.3 | (24) |
| Length of Stay (days, median (IQR))a | 2 | (1–3) | <.001 | 3 | (2–6) | <.001 | 7 | (4–11) |
| Readmissiona | 7.7 | (276) | .03 | 11 | (34) | .49 | 9.4 | (22) |
Bleeding defined as transfusion of 1 or more units of red blood cells from surgical start up to and including 72 hours post-op; Acute kidney injury defined as increase in Creatinine > 2mg/dl from pre-op; Surgical site infection includes superficial, deep, and organ space infection; Ischemic colitis identified by presence of diagnosis on discharge summary or endoscopy for the purpose of diagnosis; UTI=Urinary tract infection (excluded patients with UTI pre-op); DVT=deep venous thrombosis
Excluded patients who died in-hospital; Also data gathered from combination of historical readmission variable and more current one
Table IVb.
30-day Outcomes for Open Repair
| Asymptomatic, %(n) | P-value Asymptomatic vs. Symptomatic | Symptomatic, %(n) | Symptomatic vs. Ruptured | Ruptured, %(n) | ||||
|---|---|---|---|---|---|---|---|---|
| N | 830 | 143 | 263 | |||||
| 30-day Mortality | 4.3 | (36) | .08 | 7.7 | (11) | <.001 | 34 | (89) |
| Major Adverse Event | 20 | (165) | .64 | 18 | (26) | <.001 | 57 | (149) |
| Return to Operating Room | 10 | (86) | .47 | 8.4 | (12) | .001 | 22 | (57) |
| Bleeding | 73 | (602) | .42 | 69 | (99) | <.001 | 93 | (244) |
| Cardiac Arrest with CPR | 2.7 | (22) | .79 | 2.8 | (4) | .001 | 13 | (33) |
| Myocardial Infarction | 2.9 | (24) | 1.0 | 2.8 | (4) | .11 | 6.8 | (18) |
| Prolonged Ventilation (> 48hours) | 11 | (91) | .75 | 12 | (17) | <.001 | 43 | (112) |
| Acute Kidney Injury | 1.9 | (16) | 1.0 | 1.4 | (2) | .15 | 4.6 | (12) |
| New Dialysis | 4.0 | (33) | .50 | 2.8 | (4) | <.001 | 16 | (42) |
| Post-op UTI | 1.9 | (16) | .67 | 1.4 | (2) | .04 | 5.7 | (15) |
| Surgical Site Infection | 3.4 | (28) | .30 | 1.4 | (2) | .34 | 3.4 | (9) |
| Pulmonary Embolism | 0.5 | (4) | .55 | 0.7 | (1) | .66 | 1.5 | (4) |
| DVT | 2.3 | (19) | .89 | 2.1 | (3) | .13 | 5.7 | (15) |
| Lower Extremity Ischemia | 2.5 | (21) | 1.0 | 2.1 | (3) | .04 | 7.2 | (19) |
| Rupture of Aneurysm after repair | 0.6 | (3) | .30 | 1.4 | (2) | .002 | 9.1 | (24) |
| Ischemic Colitis | 3.9 | (32) | .46 | 2.1 | (3) | .001 | 11 | (29) |
| Length of Stay (days, median (IQR))a | 7 | (6–10) | .14 | 8 | (6–12) | <.001 | 13 | (9–23) |
| Readmissiona | 7.1 | (56) | .81 | 7.7 | (10) | .94 | 7.5 | (13) |
Excluded patients who died in-hospital
Morbidity
The rate of major adverse events was higher for symptomatic, compared to asymptomatic, patients after EVAR (9.3% vs. 3.7%, P < .001)(Table IVa). However, no significant difference was seen following open repair (19% vs. 20%, P = .64)(Table IVb). After EVAR, rates of bleeding, myocardial infarction, cardiac arrest, and prolonged intubation were also higher in symptomatic patients. Among those surviving through hospital discharge, symptomatic patients undergoing EVAR had a longer length of stay than asymptomatic patients (3 days [2–6] vs. 2 [1–3], P < .001). After open repair there were no differences in peri-operative morbidity or length of stay.
Symptomatic vs. Ruptured
Mortality
As expected, patients with symptomatic aneurysms had a lower 30-day mortality rate than those with ruptured aneurysms (5.1% vs. 27%, P < .001; OR 0.14, 95% CI 0.1–0.22). When stratified by type of repair, mortality was lower in the symptomatic group for both EVAR (3.8% vs. 22%, P < .001)(Table IVa) and open repair (7.7% vs. 34%, P < .001)(Table IVb).
Morbidity
Symptomatic patients had lower major adverse event rates compared to ruptured patients (EVAR 9.3% vs. 31%, P < .001 and open repair 18% vs. 57%, P < .001)(Table IVa and IVb respectively). Symptomatic patients also had a lower rate of bowel ischemia (EVAR 1.0% vs. 8.3%, P < .001; Open 2.1% vs. 11%, P = .001) and subsequent rupture after repair (EVAR 0.0% vs. 4.8%, P < .001; Open 1.4% vs. 9.1%, P = .002).
Multivariable Models
After adjustment symptomatic patients had twice the operative mortality compared to asymptomatic patients (OR 2.1, 95% CI 1.3–3.5)(Table V). Additional predictors included increasing age, female sex, open repair (vs. EVAR), history of severe COPD, and on dialysis preoperatively. When stratified by procedure, this same model showed an increased risk for mortality after EVAR (OR 2.4, CI 1.2–4.7) and a similar effect size and direction for open repair (OR 1.8, CI 0.86–3.9), although not significant in the open repair group. Since method of repair may be influenced by presence of symptoms we ran the overall model without adjusting for this and found a similar risk of 30-day mortality associated with symptomatic aneurysms (OR 2.3, CI 1.4–3.8). Symptomatic aneurysm was independently predictive of major adverse events as well (OR 1.5, CI 1.07–2.08)(Table VI).
Table V.
Independent Predictors of 30-day Mortality in Elective and Symptomatic AAAs
| OR | 95% CI | P-value | |
|---|---|---|---|
| Symptomatic aneurysm | 2.14 | 1.3–3.3 | .003 |
| EVAR (vs. open) | 0.40 | 0.2–0.7 | .001 |
| Age increase (by decade) | 1.81 | 1.4–2.3 | <.001 |
| Female | 1.83 | 1.2–2.8 | .004 |
| Dialysis dependent | 8.28 | 3.2–21.2 | <.001 |
| Hx of severe COPD | 1.85 | 1.2–2.8 | .01 |
| Juxtarenal aneurysm | 1.68 | 0.99–2.9 | .06 |
Initial model also included CHF episode within prior 30 days and elevated baseline creatinine >1.78mg/dl but not on dialysis Hosmer and Lemeshow test 0.52 (> 0.51 throughout all steps of model optimization)
EVAR repairs only: Symptomatic aneurysm OR 2.42 (CI 1.2–4.7, P=.01)
Open repairs only: Symptomatic aneurysm OR 1.83 (CI 0.9–3.9, P=.12)
Table VI.
Independent Predictors of MAE in Elective and Symptomatic AAAs
| OR | 95% CI | P-value | |
|---|---|---|---|
| Symptomatic aneurysm | 1.49 | 1.07–2.08 | .02 |
| EVAR (vs. open) | 0.23 | 0.17–0.30 | <.001 |
| Age increase (by decade) | 1.25 | 1.07–1.44 | .004 |
| Female | 1.51 | 1.17–1.95 | .002 |
| Current smoker | 1.24 | 0.97–1.60 | .09 |
| Baseline Cr > 1.78 mg/dl | 2.14 | 1.63–2.82 | <.001 |
| Pre-op Wound | 2.48 | 1.11–5.51 | .03 |
| Pre-op Transfusion | 2.37 | 1.15–4.88 | .02 |
| Juxtarenal aneurysm | 1.64 | 1.22–2.20 | .001 |
Also adjusted for diabetes, CHF, pre-op dialysis
Hosmer and Lemeshow test .13 (> .13 throughout all steps)
EVAR repairs only: Symptomatic aneurysm OR 2.42 (CI 1.57–3.74, P < .001)
Open repairs only: Symptomatic aneurysm OR 0.91 (CI 0.56–1.48, P = .70)
After similar adjustment for age, repair type, history of congestive heart failure, history of COPD, dialysis dependence, and juxtarenal aneurysms, ruptured aneurysms were at a 7-fold increased risk of 30-day mortality compared to symptomatic aneurysms (OR 6.5,CI 4.1–10.6) and 5-fold increased risk of a major adverse event (OR 5.1, CI 3.6–7.2).
Discussion
In this large contemporary series of symptomatic AAAs we found that symptomatic patients have a 2-fold increased risk of 30-day mortality compared to asymptomatic patients. Comparing ruptured and symptomatic patients we also found those with rupture have a 7-fold increased risk of 30-day mortality.
The distribution of symptomatic aneurysms in our study, 8.3%, lies well within the incidence previously reported in the literature, of 3% to 15%.[7–10] Many of the studies on symptomatic AAA repairs are outdated and under-represent the contemporary utilization of EVAR. We found that 69% of patients with symptomatic infrarenal aneurysms had EVAR in the NSQIP from 2011–13, which is quite different from the majority of prior studies on this topic in which open surgery was primarily or solely used.[14, 16–18] Studies that reported higher proportions of EVAR repair for symptomatic AAAs were limited by low numbers and were from single centers.[19, 20] From the Vascular Study Group of New England (VSGNE) in 2010, De Martino et al reported that 38% of symptomatic AAA repairs were completed using EVAR (60 EVARs of 156 symptomatic AAAs).[8] They found no difference in in-hospital mortality between asymptomatic and symptomatic infrarenal aneurysm repairs, for EVAR (asymptomatic 0.4% and symptomatic 0.0%) and open repair (asymptomatic 2.9% and symptomatic 2.1%). Their study, however, was limited both by smaller numbers and the ability to detect only inhospital-mortality rather than 30-day. However, over 1- and 4-years they did show reduced survival in symptomatic compared to asymptomatic patients. We have previously demonstrated that in-hospital mortality misses a substantial number of post discharge deaths that occur within 30 days, particularly after EVAR.[21] We found a significant difference in the larger EVAR subgroup (n=312) but not in the open repair subgroup (n=143), likely due to the smaller number of patients. Given the similar magnitude and direction of the effect size (odds ratio) in both the open and EVAR subgroups, it is reasonable to make the general statement from our larger multivariable model, that includes procedure type, that repair of symptomatic AAA is associated with twice the operative mortality compared to asymptomatic AAA repair. Subsequent to the VSGNE study, the ENGAGE registry for Endurant post-market surveillance reported similar 30-day mortality in 185 symptomatic AAAs compared to 1015 asymptomatic AAAs (0.5% vs. 1.5% respectively, p=.31).[9] However, it is difficult to compare real-world results from the NSQIP to a post-marketing surveillance study where most patients met strict eligibility criteria and received the same endograft. Our 30-day mortality rate for patients with asymptomatic aneurysms undergoing EVAR or open repair were consistent with rates previously reported for the NSQIP.[22]
Cambria et al reviewed the Mayo Clinic experience with symptomatic AAA and highlighted the importance of preoperative optimization of patients presenting with symptomatic AAA.[7] In that analysis patients with symptomatic AAAs undergoing operation within the first 4 hours of admission accounted for all deaths compared to those with surgery delayed either 4–24 hours or 24 hours to 7 days. The authors recommended delay to optimize fluid and electrolyte status, evaluation and limited preoperative improvement of cardiac and pulmonary status when necessary, and semi-elective repair when an experienced operating room staff was available. Unfortunately, the NSQIP does not track time from admission to operation in hours but instead by days. We were able to show that 42% of open repair and 44% of EVAR treated symptomatic patients underwent surgery at least one calendar day after the day of admission. We believe this is a surrogate for surgeons choosing to not operate on symptomatic AAAs emergently but allowing optimization and semi-elective repair as advocated by the Mayo Clinic group. We did not find a benefit to delayed surgery, but this may reflect our inability to quantify delay in hours rather than calendar days.
Symptomatic patients had higher rates of major adverse events after EVAR as well, when compared to asymptomatic patients, similar to VSGNE, where major adverse events were found to be approximately 7% and 28% after EVAR in asymptomatic and symptomatic patients respectively.[8]
Similar to prior studies, we had a higher proportion of females in the symptomatic group compared to the asymptomatic but there was no difference between symptomatic and ruptured.[7–9, 23] The reason for this remains unclear from this analysis; however, our previous work has shown that women are being repaired at relatively larger aneurysm sizes when diameter is indexed to body size.[24] Patients presenting with symptomatic or ruptured AAA were also more likely to be non-white. This could be from issues related to unequal access to care, screening, or differences in natural history of aneurysm disease between different races. Unfortunately further delineation between races could not be adequately assessed due to small numbers.
There was a difference in main body device preference between ruptured aneurysm repair and symptomatic/asymptomatic AAAs, with higher rates of the Cook Zenith and “other” devices being used compared to elective utilization, although the Gore Excluder was the most commonly used device for all 3 groups. Whether this is due to surgeon preference related to indication or what is available on the shelf for the more emergent situations is not clear from this analysis.
This study has several limitations. It was a retrospective analysis of a large clinical dataset. Also, despite the large number of symptomatic AAAs our multivariable models were limited by the number of total events. In addition, only one surgical indication could be chosen for recording purposes in the targeted NSQIP and because of this we could not identify the proportion of patients in the symptomatic and ruptured groups who had prior unsuccessful EVAR or open repair. However, those with prior unsuccessful aneurysm repair represented a very small percentage of the asymptomatic group, where it could be identified, and were unlikely to influence the results of this analysis. In addition, the definition of ruptured and symptomatic aneurysms are taken directly from the surgeon’s operative note and we believe the larger than expected proportion of non-emergent ruptured aneurysms is likely from miscoding of the emergent status, and may also include some contained ruptures that for undocumented reasons were not repaired emergently. We expected and confirmed that some small aneurysms were being repaired for symptoms but NSQIP lacks data for other potential reasons for repair of small AAA including rapid growth, large concurrent iliac aneurysm, saccular shape, pseudoaneurysm, infected aneurysm, or strong family history of rupture. Similarly, rupture of small AAA could be a result of the above factors as well. Finally, patients could be reported as symptomatic if their aneurysm caused local compression symptoms; this subgroup of symptomatic patients is presumably not at risk for imminent rupture but we could not differentiate them from the patients presenting with pain. However, inclusion of these patients would likely lower the mortality in this group.
Conclusion
In this large contemporary study of symptomatic AAA patients, in which the majority were treated with EVAR, we found that symptomatic patients have twice the perioperative mortality compared to asymptomatic patients. Despite this we also find a reduction in perioperative mortality for symptomatic aneurysms compared to prior reports where the majority were treated by open repair, and believe this supports an EVAR-first approach for symptomatic aneurysms with suitable anatomy.
Acknowledgments
Supported by grant 5R01HL105453-03 from the NHLBI and the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734.
Footnotes
Accepted for a Mini Presentation at the Society for Clinical Vascular Surgery (SCVS) 44th Annual Symposium, March 12–16, 2016, Las Vegas, Nevada
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schermerhorn ML, O'Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358(5):464–74. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- 2.Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG participants Et. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364(9437):843–8. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- 3.Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351(16):1607–18. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 4.Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Jr, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302(14):1535–42. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- 5.Reimerink JJ, van der Laan MJ, Koelemay MJ, Balm R, Legemate DA. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100(11):1405–13. doi: 10.1002/bjs.9235. [DOI] [PubMed] [Google Scholar]
- 6.Mani K, Lees T, Beiles B, Jensen LP, Venermo M, Simo G, et al. Treatment of abdominal aortic aneurysm in nine countries 2005–2009: a vascunet report. Eur J Vasc Endovasc Surg. 2011;42(5):598–607. doi: 10.1016/j.ejvs.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Cambria RA, Gloviczki P, Stanson AW, Cherry KJ, Jr, Hallett JW, Jr, Bower TC, et al. Symptomatic, nonruptured abdominal aortic aneurysms: are emergent operations necessary? Ann Vasc Surg. 1994;8(2):121–6. doi: 10.1007/BF02018859. [DOI] [PubMed] [Google Scholar]
- 8.De Martino RR, Nolan BW, Goodney PP, Chang CK, Schanzer A, Cambria R, et al. Outcomes of symptomatic abdominal aortic aneurysm repair. J Vasc Surg. 2010;52(1):5–12 e1. doi: 10.1016/j.jvs.2010.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stokmans RA, Teijink JA, Cuypers PW, Riambau V, van Sambeek MR. No differences in perioperative outcome between symptomatic and asymptomatic AAAs after EVAR: an analysis from the ENGAGE Registry. Eur J Vasc Endovasc Surg. 2012;43(6):667–73. doi: 10.1016/j.ejvs.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan CA, Rohrer MJ, Cutler BS. Clinical management of the symptomatic but unruptured abdominal aortic aneurysm. J Vasc Surg. 1990;11(6):799–803. [PubMed] [Google Scholar]
- 11.Leo E, Biancari F, Kechagias A, Ylonen K, Rainio P, Romsi P, et al. Outcome after emergency repair of symptomatic, unruptured abdominal aortic aneurysm: results in 42 patients and review of the literature. Scand Cardiovasc J. 2005;39(1–2):91–5. doi: 10.1080/14017430410016422. [DOI] [PubMed] [Google Scholar]
- 12.Antonello M, Lepidi S, Kechagias A, Frigatti P, Tripepi A, Biancari F, et al. Glasgow aneurysm score predicts the outcome after emergency open repair of symptomatic, unruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2007;33(3):272–6. doi: 10.1016/j.ejvs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Johnson G, Jr, McDevitt NB, Proctor HJ, Mandel SR, Peacock JB. Emergent or elective operation for symptomatic abdominal aortic aneurysm. Arch Surg. 1980;115(1):51–3. doi: 10.1001/archsurg.1980.01380010043008. [DOI] [PubMed] [Google Scholar]
- 14.Bradbury AW, Adam DJ, Makhdoomi KR, Stuart WP, Murie JA, Jenkins AM, et al. A 21-year experience of abdominal aortic aneurysm operations in Edinburgh. Br J Surg. 1998;85(5):645–7. doi: 10.1046/j.1365-2168.1998.00695.x. [DOI] [PubMed] [Google Scholar]
- 15.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darling C, 3rd, Shah DM, Chang BB, Paty PS, Leather RP. Current status of the use of retroperitoneal approach for reconstructions of the aorta and its branches. Ann Surg. 1996;224(4):501–6. doi: 10.1097/00000658-199610000-00008. discussion 506–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantonen I, Lepantalo M, Salenius JP, Matzke S, Luther M, Ylonen K. Mortality in abdominal aortic aneurysm surgery--the effect of hospital volume, patient mix and surgeon's case load. Eur J Vasc Endovasc Surg. 1997;14(5):375–9. doi: 10.1016/s1078-5884(97)80287-0. [DOI] [PubMed] [Google Scholar]
- 18.Olsen PS, Schroeder T, Agerskov K, Roder O, Sorensen S, Perko M, et al. Surgery for abdominal aortic aneurysms. A survey of 656 patients. J Cardiovasc Surg (Torino) 1991;32(5):636–42. [PubMed] [Google Scholar]
- 19.Franks S, Lloyd G, Fishwick G, Bown M, Sayers R. Endovascular treatment of ruptured and symptomatic abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2006;31(4):345–50. doi: 10.1016/j.ejvs.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 20.Oranen BI, Bos WT, Verhoeven EL, Tielliu IF, Zeebregts CJ, Prins TR, et al. Is emergency endovascular aneurysm repair associated with higher secondary intervention risk at mid-term follow-up? J Vasc Surg. 2006;44(6):1156–1161. doi: 10.1016/j.jvs.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 21.Schermerhorn ML, Giles KA, Sachs T, Bensley RP, O'Malley AJ, Cotterill P, et al. Defining perioperative mortality after open and endovascular aortic aneurysm repair in the US Medicare population. J Am Coll Surg. 2011;212(3):349–55. doi: 10.1016/j.jamcollsurg.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malas M, Arhuidese I, Qazi U, Black J, Perler B, Freischlag JA. Perioperative mortality following repair of abdominal aortic aneurysms: application of a randomized clinical trial to real-world practice using a validated nationwide data set. JAMA Surg. 2014;149(12):1260–5. doi: 10.1001/jamasurg.2014.275. [DOI] [PubMed] [Google Scholar]
- 23.Sayers RD, Thompson MM, Nasim A, Healey P, Taub N, Bell PR. Surgical management of 671 abdominal aortic aneurysms: a 13 year review from a single centre. Eur J Vasc Endovasc Surg. 1997;13(3):322–7. doi: 10.1016/s1078-5884(97)80105-0. [DOI] [PubMed] [Google Scholar]
- 24.Lo RC, Lu B, Fokkema MT, Conrad M, Patel VI, Fillinger M, et al. Relative importance of aneurysm diameter and body size for predicting abdominal aortic aneurysm rupture in men and women. J Vasc Surg. 2014;59(5):1209–16. doi: 10.1016/j.jvs.2013.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]