Abstract
Objectives
Early appendectomy is inversely associated with the development of ulcerative colitis (UC). However, the impact of appendectomy on the clinical course of UC is controversial, generally favoring a milder disease course. We aim to describe the effect appendectomy has on the disease course of UC with focus on the timing of appendectomy in relation to UC diagnosis.
Design
Using the National Institute of Diabetes and Digestive and Kidney Diseases IBD Genetics Consortium database of UC patients, the risk of colectomy was compared between patients who did and did not undergo appendectomy. In addition, we performed a meta-analysis of studies which examined the association between appendectomy and colectomy.
Results
2980 UC patients were initially included. 111 (4.4%) UC patients had an appendectomy; of which 63 were performed prior to UC diagnosis, and 48 after diagnosis. In multivariable analysis, appendectomy performed at any time was an independent risk factor for colectomy (odds ratio [OR] 1.9, 95% confidence interval [CI] 1.1 – 3.1), with appendectomy performed after UC diagnosis most strongly associated with colectomy (OR: 2.2; 95% CI 1.1 – 4.5). An updated meta-analysis showed appendectomy performed either prior to or after UC diagnosis had no effect on colectomy rates.
Conclusions
Appendectomy performed at any time in relation to UC diagnosis was not associated with a decrease in severity of disease. In fact, appendectomy after UC diagnosis may be associated with a higher risk of colectomy. These findings question the proposed use of appendectomy as treatment for UC.
Keywords: Ulcerative colitis, appendectomy, colectomy, ulcerative colitis disease severity
INTRODUCTION
Ulcerative colitis (UC) is a relapsing and remitting chronic inflammatory condition confined to the mucosal layer of the colon. The pathogenesis is believed to be multifactorial including both genetic and environmental factors.1-3 Residence in a Western industrialized country, antibiotic use, 4,5 gastrointestinal infections, 6-9 and non-steroidal anti-inflammatory drugs 10,11 have been implicated as possible risk factors for the development of UC. Conversely, smoking 12-14 and appendectomy 5,15-22 have been negatively associated with the development of UC.
Appendectomy is believed to alter the immune response profile in favor of suppressor T cells by decreasing the number of helper T cells and therefore decreasing interleukin 4 expression, a cytokine mediator in UC pathogenesis. 22-25 Appendectomies performed in young T-cell receptor-alpha mutant mice, an animal model of UC, suppressed the development of colitis. 24 Multiple observational studies in humans have also demonstrated an inverse relationship between appendectomy and the development of UC; however, studies evaluating the effect on UC severity, such as requiring a colectomy, have been conflicting. Some have shown a less severe disease course defined as either fewer relapses, a decrease in immunosuppressant requirements or a decrease in colectomy rates in UC patients who underwent appendectomy. 17,18,26,27 Given its potentially protective role, appendectomy has been proposed as a treatment for UC and even as primary prevention for genetically susceptible individuals,17 with several positive case reports. 28-33 In contrast, other studies have demonstrated no difference in colectomy rates or need for immunosuppressants. 16,34 A systematic review from 2012 was not able to determine if appendectomy was helpful, harmful or neutral on the disease course of ulcerative colitis since the included studies had heterogeneous outcomes including hospitalization, escalation of medication and colectomy.35
Since the appendix may play a role in modulating the development and disease course of UC, further investigation is needed to determine the effects appendectomy may have on UC disease course.
Our study had three aims. First, we evaluated whether appendectomy, performed at any time in relation to UC diagnosis, was negatively associated with severe UC, as defined by the need for colectomy. Second, we determined whether the timing of the appendectomy (i.e. prior to or after UC diagnosis) was associated with colectomy rates. Finally, given the discrepancy of findings in prior literature, we performed an updated systematic review and meta-analysis to determine the effect of appendectomy, including the timing of appendectomy, on colectomy rates in UC patients.
METHODS
Population
We queried the phenotype database of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Inflammatory Bowel Disease Genetics Consortium. This large database includes over 9,000 participants with inflammatory bowel disease (IBD) and unaffected, non-IBD controls. Participants were recruited from the United States, Puerto Rico and Canada for genetic research studies. Phenotyping of IBD participants was performed according to a validated phenotype operating manual. 36 The validity of the phenotype classification using the definitions and procedures outlined in the manual has been previously described. 36
We included all patients with a confirmed diagnosis of UC from January 1, 2003 to November 30, 2013. Briefly, UC was defined as superficial inflammation and/or ulceration of the colon which is continuous from the rectum extending proximally without any skip lesions and without inflammation of the small intestines. Patients with Crohn's disease (CD) or IBD undetermined type and participants with incomplete data on surgical or appendectomy history were excluded. UC patients with incomplete data who were excluded had no significant differences compared to those UC patients with complete data who were included.
Patient Data
Data recorded for each UC patient included demographic background (sex, birth year, race, and ethnicity), smoking status, first-degree relative with IBD, date of UC diagnosis, disease duration (time from diagnosis until most recently available medical records), disease extent, surgical history and the presence of any extra-intestinal manifestations (EIMs), including any joint involvement, erythema nodosum, pyoderma, uveitis, episcleritis, undiagnosed ocular inflammation and primary sclerosing cholangitis. Smoking history was divided into never, former, and current smoker at the time of UC diagnosis. Disease extent was categorized as having macroscopic evidence of inflammatory disease in the rectum (proctitis), up to the splenic flexure (left-sided), or beyond the splenic flexure (extensive). If a patient underwent colectomy, the indication was recorded as either colorectal dysplasia or cancer, acute fulminant UC, or chronic UC. Dates of appendectomy and colectomy were reported only as years and therefore any appendectomy that occurred the same year as the colectomy was included in the noappendectomy group as it was assumed that the appendix was removed during colectomy.
Statistical Analysis
Categorical variables were compared using the Chi square or Fisher test, where appropriate. Continuous variables were compared using the Student t test. Two variables had more than 3% missing data (peri-appendiceal inflammation, 27%; surgery for dysplasia, 83%) and were therefore excluded from analysis. Missing data in the included variables were otherwise handled by model-wise deletion and excluded from the analysis. Univariable and multivariable logistic regression were used to estimate the relative odds of colectomy according to prior appendectomy status, while adjusting for potential confounders, including age at diagnosis, sex, race, smoking, family history of IBD, disease extent, presence of EIMs, and disease duration. Use of Cox regression was not appropriate with the available data due to failing the proportional hazards test based on Schoenfeld residuals. Disease duration was measured as the time from disease onset to colectomy or most recent data collection, and binned as pre-defined durations of 0-5, 6-10, 11-20, and >20 years. Subgroup analyses included a similar evaluation of colectomy rates in two groups: UC patients with pre- and post-diagnosis appendectomy. Kaplan-Meier analysis was performed to evaluate colectomy-free survival over time. The log-rank test was used to compare colectomy-free survival between the two groups with or without prior appendectomy. A two-sided p value of < 0.05 was considered statistically significant. All statistical analyses were performed using Stata 12.1 (StataCorp, College Station, Texas).
This retrospective study with de-identified data is exempt from review by the Johns Hopkins Institutional Review Board (IRB).
Systematic Review and Meta-analysis
PubMed and EMBASE electronic databases were searched through May 20, 2015 for both UC and appendectomy using free text and MeSH or Emtree terms (Supplemental Table 1). Two independent reviewers screened the titles and abstracts for inclusion with adjudication by a third reviewer. An internal protocol was written to extract pertinent information.
Studies evaluating the effect of appendectomy on colectomy rates in ulcerative colitis patients were included. Appendectomies performed both prior and after UC diagnosis were included, although they were separated for independent analyses. Studies that reported other effects of appendectomy such as medication escalation/de-escalation, hospitalizations, and clinical disease activity were excluded unless colectomy was reported.
The cases were defined as UC patients that underwent appendectomy and the controls were defined as UC patients that did not undergo appendectomy. The outcome of interest was colectomy rate. The timing of appendectomy – any time, prior to UC diagnosis and after UC diagnosis – were analyzed separately as a secondary aim to determine if timing of appendectomy had an effect on disease severity, defined as colectomy, in UC patients.
The data extracted from each study included the total number of UC patients included, the number of UC patients that underwent appendectomy and the number that did not. The colectomy rates were recorded for each group of patients and 2 × 2 tables were created for each study. The odds ratio (OR) was calculated for each individual study. Between-study heterogeneity was assessed by Q-statistic and quantified by I2. In the presence of statistical heterogeneity, evaluation of clinical characteristics of the studies, or leaving each study out, did not change the overall inference of the pooled estimate, thus a decision was made to present the overall odds ratio. Publication bias was assessed by using the Harbord test.
RESULTS
Patient Characteristics
There were 2,980 patient with UC initially included in our study. Of the 2,714 patients that had complete data on appendectomy and colectomy history including date of surgery, 111 (4%) had an appendectomy.
Table 1 compares patient characteristics between UC patients who did (n=111) and did not (n=2603) undergo appendectomy. The mean age of UC onset was delayed in patients who underwent appendectomy prior to UC diagnosis (41.8 years (standard deviation [sd] = 15.4) vs. 30.8 (14.5) years, p < 0.01). Sex, smoking status, family history of IBD, extent of disease, peri-appendiceal inflammation, and presence of EIMs were not statistically different between the two groups. UC patients with appendectomy (prior to or after UC diagnosis) had a higher rate of surgery for chronic UC, compared with UC patients without appendectomy (21.8% vs. 14.0%, p = 0.02).
Table 1.
UC Patient Characteristics
| Appendectomy (n = 111) | No Appendectomy (n = 2603) | p - value | |
|---|---|---|---|
| Age at diagnosis (mean year, SD) | 36.5 (15.9) | 30.8 (14.5) | <0.01 |
| Female | 62/111 (55.9%) | 1283/2603 (49.3%) | 0.18 |
| Caucasian Race | 89/111 (80.1%) | 1995/2600 (76.7%) | 0.40 |
| Smoking at UC diagnosis | 0.40 | ||
| Never | 74/110 (67.3%) | 1862/2564 (72.6%) | |
| Former | 23/110 (20.9%) | 479/2564 (18.7%) | |
| Current | 13/110 (11.8%) | 223/2564 (8.7%) | |
| 1st degree Relative with IBD | 20/110 (18.2%) | 427/2544 (16.8%) | 0.70 |
| Disease Location | 0.94 | ||
| Proctitis | 10/107 (9.3%) | 238/2566 (9.3%) | |
| Left-sided | 35/104 (33.7%) | 789/2543 (31.0%) | |
| Extensive | 64/101 (63.4%) | 1584/2442 (64.9%) | |
| Extra-intestinal Manifestations | 27/111 (24.3%) | 537/2603 (20.6%) | 0.35 |
| Colectomy for acute UC | 2/110 (1.8%) | 62/2593 (2.4%) | 0.70 |
| Colectomy for chronic UC | 24/110 (21.8%) | 362/2593 (14.0%) | 0.02 |
| Disease Duration, years (mean, SD) | 10.74 (9.7) | 8.71 (9.1) | 0.02 |
Aim 1: Does appendectomy, independent of the timing with UC diagnosis, associate with UC disease severity?
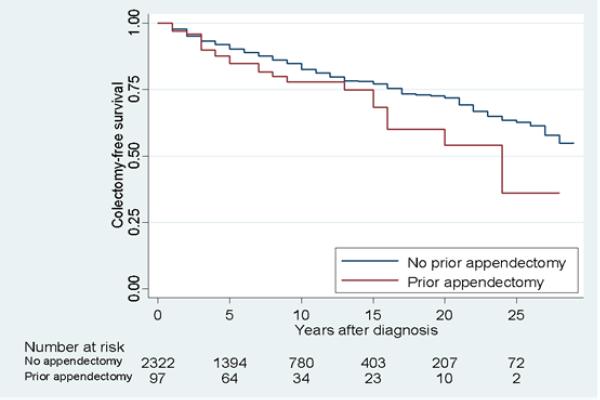
Appendectomy performed at any time was found to be associated with an increased risk of colectomy, (odds ratio [OR] 1.65, 95% confidence interval [CI] 1.05 – 2.57, p = 0.03). In the multivariable analysis adjusting for sex, race, smoking status, family history of IBD, presence of EIMs, disease duration and disease extent, appendectomy remained an independent risk factor for colectomy (odds ratio [OR] 1.87, 95% confidence interval [CI] 1.12 – 3.14, p=0.02), Table 2. This observation was confirmed over time in the appendectomy versus non-appendectomy UC patients (Figure 1).
Table 2.
Appendectomy and Extensive Disease are Independently Associated with a Higher Rate of Colectomy
| Univariable Analysis | Multivariable Analysis* | |||
|---|---|---|---|---|
| OR (95% CL) | P - value | OR (95% CL) | P - value | |
| Appendectomy | 1.65 (1.05 – 2.57) | 0.03 | 1.87 (1.12 – 3.14) | 0.02 |
| Female | 0.81 (0.67 – 0.98) | 0.03 | 0.95 (0.75 – 1.20) | 0.68 |
| Non-White Race | 0.96 (0.76 – 1.21) | 0.73 | 0.99 (0.75 – 1.32) | 0.96 |
| Smoking | ||||
| Never | 1.00 (reference) | 1.00 (reference) | ||
| Former | 1.07 (0.83 -1.37) | 0.61 | 1.44 (1.05 – 1.98) | 0.03 |
| Current | 0.96 (0.67 – 1.35) | 0.80 | 1.01 (0.66 – 1.53) | 0.97 |
| 1st Degree Relative with IBD | 1.26 (0.98 – 1.62) | 0.07 | 1.37 (1.01 – 1.86) | 0.05 |
| Age at Diagnosis (decade) | 0.93 (0.87 – 0.99) | 0.03 | 0.92 (0.85 – 1.01) | 0.85 |
| Disease Location | ||||
| Proctitis | 1.00 (reference) | 1.00 (reference) | ||
| Left-sided | 1.07 (0.61 – 1.90) | 0.81 | 1.04 (0.52 – 2.08) | 0.90 |
| Extensive | 4.28 (2.55 – 7.20) | <0.01 | 4.58 (2.44 – 8.58) | <0.01 |
| EIM | 1.71 (1.37 – 2.13) | <0.01 | 1.73 (1.33 – 2.25) | <0.01 |
| Disease Duration | ||||
| 0 to 5 | 1.00 (reference) | 1.00 (reference) | ||
| 6 to 10 | 0.82 (0.62 – 1.10) | 0.19 | 0.93 (0.68 – 1.27) | 0.63 |
| 11 to 20 | 0.72 (0.55 – 0.94) | 0.02 | 0.74 (0.55 – 0.99) | 0.05 |
| > 20 | 0.57 (0.39 – 0.82) | <0.01 | 0.54 (0.36 – 0.82) | <0.01 |
Adjusted for: Appendectomy, sex, race, smoking status, family history of IBD, age at IBD diagnosis, disease location, presence of extra-intestinal manifestations, and disease duration.
Figure 1.
Appendectomy is associated with a higher rate of colectomy independent of disease extent. Log – rank p = 0.05
A sub-analysis was performed on UC patients that underwent early appendectomy (defined as prior to age 20; Supplementary Table 2). There were 28 total UC patients that underwent early appendectomy. Compared to UC patients that did not undergo appendectomy, there was no difference in rate of colectomy in univariate (OR 1.05, 95% CI 0.39 – 2.83) or multivariate analysis (OR 0.75, 95% CI 0.20 – 2.90).
Aim two: Does timing of appendectomy with respect to UC diagnosis affect severity?
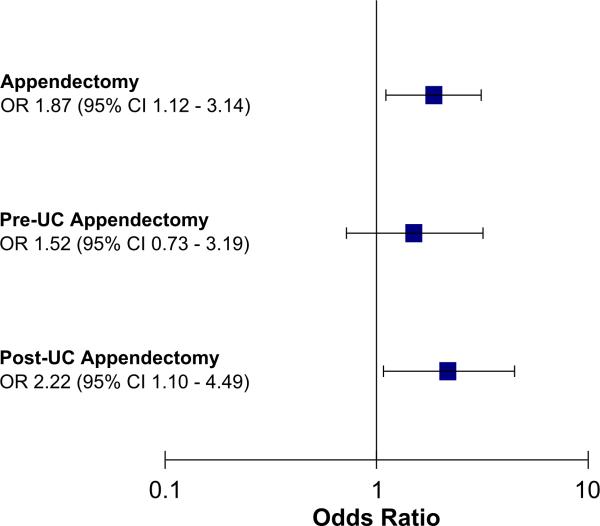
To evaluate how timing of appendectomy affects the disease course, we examined the 111 UC patients who underwent appendectomy. Of these patients, 63 had an appendectomy prior to UC diagnosis and 48 after UC diagnosis. A subgroup analysis (adjusted for: age, sex, race, smoking status, family history of IBD, disease extent, presence of EIMs and disease duration) was performed and found that appendectomy after UC diagnosis remained significantly associated with colectomy (OR 2.22, 95% CI 1.10 – 4.49, p=0.03), while an appendectomy performed before UC diagnosis was not significantly associated with colectomy (OR 1.52, 95% CI 0.73 – 3.19, p=0.27; Figure 2). Our results suggest that the association with appendectomy regardless of the timing of UC diagnosis was mainly driven by appendectomy after UC diagnosis and not prior to diagnosis.
Figure 2.
Post – UC Appendectomy is associated with a higher risk of colectomy. Adjusted for sex, race, smoking status, first degree relative with IBD, age at diagnosis of IBD, disease extent, presence of extra-intestinal manifestations, disease duration.
Other Predictors of Colectomy
In univariable analyses, colectomy was associated with male sex, earlier age at UC diagnosis, extensive disease, and the presence of one or more EIMs (Table 2). In contrast, race, tobacco use and family history of IBD were not. Longer disease duration was inversely associated with the risk of undergoing colectomy.
In multivariable analyses, extensive disease (OR 4.58, 95% CI 2.44 – 8.58, p<0.01), one or more EIM (OR 1.72, 95% CI 1.33 – 2.2, p < 0.001), and former but not current smoking (OR 1.44, 95% CI 1.05 – 1.98, p=0.03) were independent risk factors for colectomy.
Primary sclerosing cholangitis (PSC) was not associated with a higher rate of overall colectomy in our UC cohort; however PSC was associated with a higher rate of colectomy for dysplasia. Sensitivity analysis was performed to make certain this unique subset of patients were not skewing our data. All UC patients with colectomy for dysplasia were first excluded and the outcome data was not changed (Supplemental table 3). Secondly, all PSC patients were excluded from analysis and again the data remained the same with appendectomy associated with colectomy changed (Supplemental table 4).
Aim 3: Updated Systematic Review and Meta-analysis of appendectomy and appendectomy timing on UC disease severity
In order to examine whether our results were consistent with prior data and to perform a meta-analysis, we conducted a comprehensive review of the literature. The PubMed and EMBASE search resulted in 316 and 608 potential titles respectively. After title and abstract review, a total of 8 studies, including ours, met inclusion criteria including our current study. 16,17,26,27,34,37,38 One study was excluded since the primary outcome of interest was time to event. 17
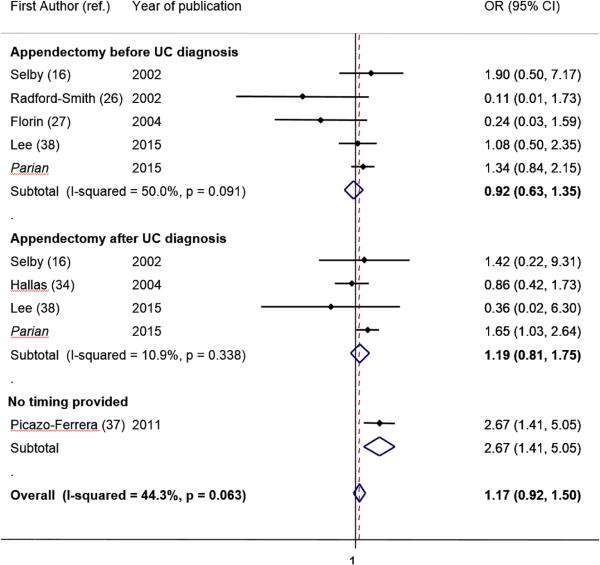
Seven studies including our study 16,26,27,34,37,38 [7280 patients] examined the overall effect of appendectomy on the rate of colectomy in UC patients (Table 3; Figure 3). Overall, UC patients who underwent appendectomy at any time in relation to UC diagnosis did not have a decreased rate of colectomy compared to UC patients without appendectomy, (OR 1.17; 95% CI: 0.92 – 1.50, I2 = 44.3%).
Table 3.
Meta-analysis included studies
| Country | Total # UC patients | Extensive disease | Timing of appendectomy provided? | Total colectomy | Total appendectomies | Tertiary referral center? (Y/N) | |
|---|---|---|---|---|---|---|---|
| Parian, 2015 | USA | 2673 | 64% | Yes | 450 | 111 | Y |
| Lee, 2014 | Korea | 2648 | 25% | Yes | 218 | 106 | Y |
| Hallas, 2004 | Denmark | 1010 | N/A | Yes | 51 | 202 | N |
| Florin, 2004 | Australia | 285 | 52% | No | 68 | 17 | Y |
| Selby, 2002 | Australia | 259 | 38% | Yes | 24 | 20 | Y |
| Radford-Smith, 2002 | Australia | 301 | 44% | No | 60 | 21 | Y |
| Picazzo-Ferrera, 2011 | Mexico | 104 | 49% | No | 28 | 38 | N/A |
Figure 3.
Meta-analysis of studies examining the effect of appendectomy on colectomy rates in UC patients.
Six studies, including ours, specifically evaluated the timing of appendectomy (prior to or after UC diagnosis). Among those patients that underwent appendectomy before UC diagnosis (180 patients), there was no association between prior surgery and colectomy (OR: 0.92, 95% CI: 0.63, 1.35, I2=50%.) Analysis of patients that underwent appendectomy after UC diagnosis (4134 patients did not show any protection from colectomy (OR 1.19; 95% CI: 0.81 – 1.75, I2 = 10.9%) (Figure 3). In both analyses (overall appendectomy and timing of appendectomy), none of the studies was highly influential and/or showed clinical heterogeneity. The Harbord test showed no evidence of publication bias (p = 0.49), (Supplementary Figure 1). Removing each study at a time, did not change significantly the inference of the meta-analysis (Supplementary Figure 2).
DISCUSSION
The results of our large, multi-center study demonstrate that appendectomy, regardless of the timing with respect to UC diagnosis does NOT decrease the severity of UC disease course, defined as the need for total colectomy, when compared to UC patients who did not undergo appendectomy. In fact, our results suggest that performing appendectomy after UC diagnosis may be harmful, as it showed a 2.2-fold increased risk of colectomy. Our updated systematic review and meta-analysis confirmed our findings and showed appendectomy had no effects on colectomy rates in UC patients.
Appendectomy and UC Disease Severity
Previously published studies have suggested that appendectomy may predict a milder disease course for patients with UC. Naganuma et al. compared 325 UC patients, 21 of whom underwent appendectomy prior to the diagnosis of UC and 304 who did not undergo appendectomy.18 Patients with a prior appendectomy had fewer relapses, (57% vs. 79%; p < 0.05) and were less likely to have extensive colitis (38% vs. 51%). Notably, this study had a small sample of UC patients that underwent appendectomy, and the timing of the appendectomy was not specifically evaluated. 18 Radford-Smith et al. studied 307 UC patients from the Brisbane IBD Research Group, 21 of whom had an appendectomy, and found a lower rate of colectomy in patients with a prior appendectomy (0% vs. 21%, p = 0.02) as well as a decreased need for immunosuppression (5% vs. 25%, p = 0.04). 26 However, disease extent and disease duration were not controlled for which are known predictors of colectomy in UC.26 A study by Florin et al. also using the Brisbane database over the same period of time demonstrated a decreased need for colectomy or immunosuppression for severe UC in patients with prior appendectomy (6% vs. 25%, p = 0.004). It should be noted that neither outcome on its own (colectomy or immunosuppression) was statistically significant and the number of UC patients with appendectomy was quite small (19).27
Appendectomy has even been proposed as a treatment modality for UC. 28-30,33,39 There have been several case reports 33,39 including one by Okazaki et al. describing a case of mild UC proctitis “cured” by appendectomy after 3 years of follow up. 33 The largest case series by Bolin et al. in 2009 studied 30 patients with ulcerative proctitis who underwent an appendectomy for treatment of their disease. Almost all patients (90%) had some improvement in symptoms, with 40% of patients achieving complete resolution of symptoms. 30 Our findings call into question the use appendectomy in the treatment of UC. These small case reports and case series may simply be publication bias and not indicative of the true outcomes of appendectomy in UC patients.
Our study yielded a different conclusion: appendectomy was associated with a higher rate of colectomy in UC patients in a multivariable analysis and was independent of all other factors studied including extent of disease and follow-up time. Notably, not all studies have detected a milder course in UC patients who have undergone appendectomy. In a recent study, Lee et al. found no difference in colectomy rates in Korean UC patients with and without appendectomy.38 A study from Mexico found a higher colectomy rate in UC patients that underwent appendectomy although confounding factors were not controlled and timing of appendectomy was not reported. 37 Hallas et al. studied 202 UC patients who had undergone an appendectomy after UC diagnosis and found no difference in colectomy rates. 34
There are a number of possible explanations as to why appendectomy may or may not influence disease course. Previous studies suggest the reason appendectomy is inversely related to the development of UC is due to the presence of appendicitis and not simply the removal of the appendix. 20,40,41 Therefore, the removal of a healthy appendix may have no role in the clinical course of UC. Additionally, the appendix may have a role in the pathogenesis and development of UC, but not have a role in the subsequent clinical course once UC does develop. Patient factors may explain why our results contradict other studies, since the majority of our patients had extensive disease and a mean disease duration over 10 years. However, a sub-analysis of UC patients with only proctitis found that appendectomy did not decrease colectomy rate (data available upon request). Lastly, perhaps the timing of appendectomy could be an influential factor in the clinical course of UC.
Timing of Appendectomy
Early appendectomy has been shown to protect against the development of UC, thus supporting a role of the appendix in the pathogenesis of UC. 20 However, appendectomy after age 20 do not confer protection, 20 suggesting that cells involved in the development of UC may expand beyond the appendix to other lymphoid tissue after this age. 24 Since the age of appendectomy may be an important factor, a sub-analysis of our data examining only those patients who underwent an appendectomy prior to age 20 was performed (Supplementary Table 2). Colectomy rates were still not decreased in UC patients who underwent early appendectomy compared to UC patients that did not undergo any appendectomy.
In our study, patients who underwent appendectomy prior to UC diagnosis had a significantly later onset of disease compared to UC patients who did not undergo appendectomy; however, we did not observe a decreased risk of colectomy in this subgroup. Radford-Smith et al. 26 and Selby et al. 16 reported similar findings of delay in disease presentation in patients with prior appendectomy further supporting the theory that the appendix plays a part in the pathogenesis of UC, but does not necessarily play a role in the clinical course once UC develops.
We noted that appendectomy performed after UC diagnosis was significantly associated with a 2.2-fold increased colectomy rate compared to UC patients who did not have an appendectomy, whereas, appendectomy performed prior to UC diagnosis did not affect the rate of colectomy.
The mechanism behind increased colectomy rates in UC patients who underwent appendectomy after the UC diagnosis is unclear. Appendectomy in this already altered immunologic environment may act as a trigger for more severe disease. There may also be proinflammatory changes in the microbiome that occur after appendectomy that have yet to be explained. Finally, it is possible that the development of appendicitis after UC diagnosis is a surrogate of already existing active disease. Counter to this argument, we found no difference in rates of pancolitis or peri-appendiceal inflammation between UC patients who did or did not undergo appendectomy.
Other Predictors of Colectomy
Extensive disease 42-45 and the presence of one or more EIMs 43,44,46, (both parameters of more advanced disease) were also associated with a higher risk for total colectomy. In our cohort of patients, 64% had extensive disease which can be attributed to the tertiary and quaternary centers included in the NIDDK IBD genetics consortium. Other studies evaluating the effect of appendectomy on disease severity had lower rates of extensive disease. Therefore, disease extent was included in the multivariate analysis in examining the effect of appendectomy on colectomy rates. After controlling for disease extent and the presence of EIMs, appendectomy remained significantly associated an increased risk for total colectomy.
Systematic Review & Meta-analysis
Despite multiple studies investigating the role of appendectomy on UC disease severity, there was no consensus. Each study targeted different outcomes and had varied limitations. A prior systematic review was performed but did not narrow in on one outcome and due to clinical heterogeneity no meta-analysis could be conducted. 35 The authors concluded that there are inconsistent data but most studies suggest a beneficial effect of appendectomy on the course of UC. 35 Recently, Sahami et al. published a review which used some of the tools used in systematic reviews and meta-analyses, however, it is not, strictly speaking “a systematic review and meta-analysis” as it lacked core elements of meta-analyses (e.g. assessment of statistical heterogeneity, publication bias). 47 Compared to Sahami et al. we focused our search on the effect appendectomy had on rates of colectomy and applied standardized tools in pooled analyses. (Moher D et al. The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement available at http://www.prisma-statement.org/ Supplementary Table 5).
Our updated systematic review with meta-analysis found, in contrast, no benefit of appendectomy on disease severity of UC measured by colectomy rates. In our meta-analysis, neither appendectomy prior to nor after UC diagnosis protected against severe disease. Based on an alpha error equal to 0.05, a power of 0.8, and fixed parameters based on each of the individual studies of the meta-analyses we further conclude that, overall, the strength of the association between timing of appendectomy and the risk of total colectomy is absent or at most weak (Supplementary Table 6).
Our study has several strengths including the large sample size and the inclusion of multiple centers across several countries which increases the external validity and generalizability of our findings. The patients were also well phenotyped following pre-defined protocols which minimized risk of information bias. Furthermore, the collection of numerous variables into our primary analyses, allowed the multivariate adjustment to control for potential confounders. As a result of our large sample size and comparatively large number of UC patients with an appendectomy we were able to separately analyze patients with appendectomy prior to UC diagnosis from those with appendectomy after UC diagnosis, whereas the majority of other studies did not. This may be an important distinction to make; given the associated immunologic changes after the development of UC, appendectomy may no longer be protective. In order to overcome previous meta-analyses and address inconsistent results while increasing power to detect associations, we conducted a meta-analysis which showed that either overall appendectomy or timing of appendectomy did not influence the association with severity of UC as defined by rates of colectomy.
Our study has several limitations. The clinical scenario leading up to the appendectomy and the presence or absence of appendicitis on pathology was not known. Unfortunately, as a limitation of a retrospective database analysis we do not have access to information related to medication use. All sites that participated in the NIDDK IBD Genetics Consortium were tertiary and quaternary institutions and believed to have similar availability of medications such as biologics and immunomodulators. The study by Lee et al. included the need for steroids, biologics or immunomodulators and found no difference in between UC patients with or without appendectomy.38 Selby et al. also evaluated the need for immunomodulator use and found no difference between UC patients who did or did not have an appendectomy.16 Radford-Smith et al. did find a decreased need for immunomodulators in UC patients who had a prior appendectomy as well as a decreased need for colectomy. However, Radford-Smith's study did not control for disease duration and disease extent which are known to be important independent predictors for colectomy.26 Nevertheless, these data, along with pathological data on appendix should be incorporated on prospective studies to better understand whether these characteristics mediates the association between appendectomy and colectomy.
Additionally, the database did not contain information on disease severity or hospitalization and therefore colectomy is used as a surrogate for severity; nevertheless, we think that colectomy is a valid measure of disease severity as it has been shown in different studies. 16,17,26,27,34,37,38 Other factors were also analyzed, including the co-existence of primary sclerosing cholangitis, since PSC is an established risk factor for dysplasia and colectomy. When we excluded the 32 patients who underwent colectomy for dysplasia or 93 patients who had PSC in our cohort, the positive association between appendectomy and total colectomy did not change (Supplementary Tables 3 and 4). As with most large databases, there were incomplete data on some of the patients requiring exclusion of these patients, although this was quite minimal and the patients excluded had similar demographics and are not believed to be a skewed population.
In conclusion, in this large observational study with an updated meta-analysis, we found that appendectomy performed at any time in relation to UC diagnosis was not associated with a decrease in severity of disease. In fact, appendectomy after UC diagnosis may be associated with a higher risk of colectomy. Therefore, based on current observational data, appendectomy as a treatment for UC is not recommended. There is an ongoing prospective trial (ACCURE) investigating the effect of appendectomy on the clinical course of ulcerative colitis. 48 Until then, the current evidence does not support the therapeutic role of appendectomy in severe UC.
Supplementary Material
What is already known about this subject?
Early appendectomy is inversely associated with the development of ulcerative colitis (UC) and may delay the onset of UC.
Appendectomy may reduce the risk for colectomy in UC patients and has been explored as a treatment for UC, however, this is based on observational studies which shows conflicting evidence in the association between appendectomy and the need for total colectomy
What are the new findings?
Based on our findings and meta-analysis, appendectomy does not decrease the need for colectomy in UC patients
If performed after UC diagnosis, appendectomy may increase the risk for colectomy
How might it impact on clinical practice in the foreseeable future?
Several previously published observational studies suggest that UC can be treated with appendectomy. Our data and subsequent meta-analysis show that appendectomy does not protect against colectomy. Therefore, our findings do not support the practice of appendectomy in the treatment of UC to prevent the need for total colectomy
Acknowledgments
Grant support: This study was performed independently of any financial support.
Abbreviations
- CI
Confidence internal
- EIM
extra-intestinal manifestation
- IBD
inflammatory bowel disease
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- OR
odds ratio
- sd
standard deviation
- UC
ulcerative colitis
Footnotes
Author Contributions:
Joyce Koh – Study concept and design, critical revision of the manuscript. This author has approved the final draft for submission.
Steven R. Brant – Acquisition of data, critical revision of the manuscript, study supervision. This author has approved the final draft for submission.
Alain Bitton – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
Judy H. Cho – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
Richard H. Duerr – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
Dermot P. McGovern – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
Deborah D. Proctor – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
Miguel Regueiro – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
John D. Rioux – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
Phil Schumm – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
Kent D. Taylor – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
Mark S. Silverberg – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
A. Hillary Steinhart – Acquisition of data and critical revision of the manuscript. This author has approved the final draft for submission.
Disclosures:
Hillary Steinhart: Janssen - Advisory Board, Honorarium (for lectures) Abbvie - Advisory Board, Honorarium (for lectures), Grant support Takeda - Advisory Board, Honorarium (for lectures)
The remaining authors have no disclosures to report.
References
- 1.Orholm M, Munkholm P, Langholz E, et al. Familial occurrence of inflammatory bowel disease. N Engl J Med. 1991;324(2):84–8. doi: 10.1056/NEJM199101103240203. [DOI] [PubMed] [Google Scholar]
- 2.Binder V, Orholm M. Familial occurrence and inheritance studies in inflammatory bowel disease. Neth J Med. 1996;48(2):53–6. doi: 10.1016/0300-2977(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 3.Tysk C, Lindberg E, Jarnerot G, et al. Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988;29(7):990–6. doi: 10.1136/gut.29.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics and new diagnoses of Crohn's disease and ulcerative colitis. Am J Gastroenterol. 2011;106(12):2133–42. doi: 10.1038/ajg.2011.304. [DOI] [PubMed] [Google Scholar]
- 5.Gilat T, Hacohen D, Lilos P, et al. Childhood factors in ulcerative colitis and Crohn's disease. An international cooperative study. Scand J Gastroenterol. 1987;22(8):1009–24. doi: 10.3109/00365528708991950. [DOI] [PubMed] [Google Scholar]
- 6.Porter CK, Tribble DR, Aliaga PA, et al. Infectious gastroenteritis and risk of developing inflammatory bowel disease. Gastroenterology. 2008;135(3):781–6. doi: 10.1053/j.gastro.2008.05.081. [DOI] [PubMed] [Google Scholar]
- 7.Garcia Rodriguez LA, Ruigomez A, Panes J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology. 2006;130(6):1588–94. doi: 10.1053/j.gastro.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Gradel KO, Nielsen HL, Schonheyder HC, et al. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137(2):495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Serrano P, Perez-Calle JL, Perez-Fernandez MT, et al. Environmental risk factors in inflammatory bowel diseases. Investigating the hygiene hypothesis: a Spanish case-control study. Scand J Gastroenterol. 2010;45(12):1464–71. doi: 10.3109/00365521.2010.510575. [DOI] [PubMed] [Google Scholar]
- 10.Tanner AR, Raghunath AS. Colonic inflammation and nonsteroidal anti-inflammatory drug administration. An assessment of the frequency of the problem. Digestion. 1988;41(2):116–20. doi: 10.1159/000199740. [DOI] [PubMed] [Google Scholar]
- 11.Ananthakrishnan AN, Higuchi LM, Huang ES, et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med. 2012;156(5):350–9. doi: 10.1059/0003-4819-156-5-201203060-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higuchi LM, Khalili H, Chan AT, et al. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol. 2012;107(9):1399–406. doi: 10.1038/ajg.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaugerie L, Massot N, Carbonnel F, et al. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol. 2001;96(7):2113–6. doi: 10.1111/j.1572-0241.2001.03944.x. [DOI] [PubMed] [Google Scholar]
- 14.Boyko EJ, Perera DR, Koepsell TD, et al. Effects of cigarette smoking on the clinical course of ulcerative colitis. Scand J Gastroenterol. 1988;23(9):1147–52. doi: 10.3109/00365528809090183. [DOI] [PubMed] [Google Scholar]
- 15.Loftus EV., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 16.Selby WS, Griffin S, Abraham N, et al. Appendectomy protects against the development of ulcerative colitis but does not affect its course. Am J Gastroenterol. 2002;97(11):2834–8. doi: 10.1111/j.1572-0241.2002.07049.x. [DOI] [PubMed] [Google Scholar]
- 17.Cosnes J, Carbonnel F, Beaugerie L, et al. Effects of appendicectomy on the course of ulcerative colitis. Gut. 2002;51(6):803–7. doi: 10.1136/gut.51.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naganuma M, Iizuka B, Torii A, et al. Appendectomy protects against the development of ulcerative colitis and reduces its recurrence: results of a multicenter case-controlled study in Japan. Am J Gastroenterol. 2001;96(4):1123–6. doi: 10.1111/j.1572-0241.2001.03757.x. [DOI] [PubMed] [Google Scholar]
- 19.Schattner A. Appendicectomy in ulcerative colitis. Lancet. 1999;353(9153):674. doi: 10.1016/S0140-6736(05)75471-4. [DOI] [PubMed] [Google Scholar]
- 20.Andersson RE, Olaison G, Tysk C, et al. Appendectomy and protection against ulcerative colitis. N Engl J Med. 2001;344(11):808–14. doi: 10.1056/NEJM200103153441104. [DOI] [PubMed] [Google Scholar]
- 21.Russel MG, Dorant E, Brummer RJ, et al. Appendectomy and the risk of developing ulcerative colitis or Crohn's disease: results of a large case-control study. South Limburg Inflammatory Bowel Disease Study Group. Gastroenterology. 1997;113(2):377–82. doi: 10.1053/gast.1997.v113.pm9247453. [DOI] [PubMed] [Google Scholar]
- 22.Rutgeerts P, D'Haens G, Hiele M, et al. Appendectomy protects against ulcerative colitis. Gastroenterology. 1994;106(5):1251–3. doi: 10.1016/0016-5085(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita M, Takakuwa H, Matsubayashi Y, et al. Appendix is a priming site in the development of ulcerative colitis. World J Gastroenterol. 2005;11(31):4869–74. doi: 10.3748/wjg.v11.i31.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizoguchi A, Mizoguchi E, Chiba C, et al. Role of appendix in the development of inflammatory bowel disease in TCR-alpha mutant mice. J Exp Med. 1996;184(2):707–15. doi: 10.1084/jem.184.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rachmilewitz D, Karmeli F, Takabayashi K, et al. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002;122(5):1428–41. doi: 10.1053/gast.2002.32994. [DOI] [PubMed] [Google Scholar]
- 26.Radford-Smith GL, Edwards JE, Purdie DM, et al. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn's disease. Gut. 2002;51(6):808–13. doi: 10.1136/gut.51.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Florin TH, Pandeya N, Radford-Smith GL. Epidemiology of appendicectomy in primary sclerosing cholangitis and ulcerative colitis: its influence on the clinical behaviour of these diseases. Gut. 2004;53(7):973–9. doi: 10.1136/gut.2003.036483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bageacu S, Coatmeur O, Lemaitre JP, et al. Appendicectomy as a potential therapy for refractory ulcerative proctitis. Aliment Pharmacol Ther. 2011;34(2):257–8. doi: 10.1111/j.1365-2036.2011.04705.x. [DOI] [PubMed] [Google Scholar]
- 29.Jarnerot G, Andersson M, Franzen L. Laparoscopic appendectomy in patients with refractory ulcerative colitis. Gastroenterology. 2001;120(6):1562–3. doi: 10.1053/gast.2001.24508. [DOI] [PubMed] [Google Scholar]
- 30.Bolin TD, Wong S, Crouch R, et al. Appendicectomy as a therapy for ulcerative proctitis. Am J Gastroenterol. 2009;104(10):2476–82. doi: 10.1038/ajg.2009.388. [DOI] [PubMed] [Google Scholar]
- 31.Jo Y, Matsumoto T, Yada S, et al. Histological and immunological features of appendix in patients with ulcerative colitis. Dig Dis Sci. 2003;48(1):99–108. doi: 10.1023/a:1021742616794. [DOI] [PubMed] [Google Scholar]
- 32.Kim SW, Lee ST, Lee SO, et al. [Clinical improvement of severe ulcerative colitis after incidental appendectomy: a case report]. Korean J Gastroenterol. 2006;47(6):463–6. [PubMed] [Google Scholar]
- 33.Okazaki K, Onodera H, Watanabe N, et al. A patient with improvement of ulcerative colitis after appendectomy. Gastroenterology. 2000;119(2):502–6. doi: 10.1053/gast.2000.9368. [DOI] [PubMed] [Google Scholar]
- 34.Hallas J, Gaist D, Vach W, et al. Appendicectomy has no beneficial effect on admission rates in patients with ulcerative colitis. Gut. 2004;53(3):351–4. doi: 10.1136/gut.2003.016915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardenbroek TJ, Eshuis EJ, Ponsioen CI, et al. The effect of appendectomy on the course of ulcerative colitis: a systematic review. Colorectal Dis. 2012;14(5):545–53. doi: 10.1111/j.1463-1318.2011.02600.x. [DOI] [PubMed] [Google Scholar]
- 36.Dassopoulos T, Nguyen GC, Bitton A, et al. Assessment of reliability and validity of IBD phenotyping within the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) IBD Genetics Consortium (IBDGC). Inflamm Bowel Dis. 2007;13(8):975–83. doi: 10.1002/ibd.20144. [DOI] [PubMed] [Google Scholar]
- 37.Picazo-Ferrera K, Bustamante-Quan Y, Santiago-Hernandez J, et al. [The role of appendectomy in the ulcerative colitis in Mexico]. Rev Gastroenterol Mex. 2011;76(4):316–21. [PubMed] [Google Scholar]
- 38.Lee HS, Park SH, Yang SK, et al. Appendectomy and the clinical course of ulcerative colitis: a retrospective cohort study and a nested case-control study from Korea. J Gastroenterol Hepatol. 2015;30(3):470–7. doi: 10.1111/jgh.12707. [DOI] [PubMed] [Google Scholar]
- 39.Noh CH, Cheung DY, Kim TH, et al. [Remission of ulcerative colitis after appendectomy: a case report]. Korean J Gastroenterol. 2010;56(3):201–4. doi: 10.4166/kjg.2010.56.3.201. [DOI] [PubMed] [Google Scholar]
- 40.Smithson JE, Radford-Smith G, Jewell GP. Appendectomy and tonsillectomy in patients with inflammatory bowel disease. J Clin Gastroenterol. 1995;21(4):283–6. doi: 10.1097/00004836-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Duggan AE, Usmani I, Neal KR, et al. Appendicectomy, childhood hygiene, Helicobacter pylori status, and risk of inflammatory bowel disease: a case control study. Gut. 1998;43(4):494–8. doi: 10.1136/gut.43.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sicilia B, Vicente R, Arroyo MT, et al. [Ulcerative pancolitis predicts the need for colectomy: study of an incident cohort of patients with ulcerative colitis in Aragon (Spain)]. Gastroenterol Hepatol. 2005;28(2):55–9. doi: 10.1157/13070700. [DOI] [PubMed] [Google Scholar]
- 43.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38(6):1137–46. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- 44.Gower-Rousseau C, Dauchet L, Vernier-Massouille G, et al. The natural history of pediatric ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 2009;104(8):2080–8. doi: 10.1038/ajg.2009.177. [DOI] [PubMed] [Google Scholar]
- 45.Leijonmarck CE, Persson PG, Hellers G. Factors affecting colectomy rate in ulcerative colitis: an epidemiologic study. Gut. 1990;31(3):329–33. doi: 10.1136/gut.31.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakatos L, Pandur T, David G, et al. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9(10):2300–7. doi: 10.3748/wjg.v9.i10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahami S, Kooij IA, Meijer SL, et al. The Link between the Appendix and Ulcerative Colitis: Clinical Relevance and Potential Immunological Mechanisms. Am J Gastroenterol. 2016;111(2):163–9. doi: 10.1038/ajg.2015.301. [DOI] [PubMed] [Google Scholar]
- 48.Gardenbroek TJ, Pinkney TD, Sahami S, et al. The ACCURE-trial: the effect of appendectomy on the clinical course of ulcerative colitis, a randomised international multicenter trial (NTR2883) and the ACCURE-UK trial: a randomised external pilot trial (ISRCTN56523019). BMC Surg. 2015;15:30. doi: 10.1186/s12893-015-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.